Abstract
The local recurrence rate of phyllodes tumors of the breast varies widely among different subtypes, and distant metastasis is associated with poor survival. This study aimed to identify factors that are predictive of local recurrence-free survival (LRFS), distant metastasis-free survival (DMFS), and overall survival (OS) in patients with phyllodes tumors of the breast. Clinical data of all patients with a phyllodes tumor of the breast (n = 192) treated at Sun Yat-sen University Cancer Center between March 1997 and December 2012 were reviewed. The Pearson χ2 test was used to investigate the relationship between clinical features of patients and histotypes of tumors. Univariate and multivariate Cox regression analyses were performed to identify factors that are predictive of LRFS, DMFS, and OS. In total, 31 (16.1%) patients developed local recurrence, and 12 (6.3%) developed distant metastasis. For the patients who developed local recurrence, the median age at the diagnosis of primary tumor was 33 years (range, 17-56 years), and the median size of primary tumor was 6.0 cm (range, 0.8-18 cm). For patients who developed distant metastasis, the median age at the diagnosis of primary tumor was 46 years (range, 24-68 years), and the median size of primary tumor was 5.0 cm (range, 0.8-18 cm). In univariate analysis, age, size, hemorrhage, and margin status were found to be predictive factors for LRFS (P = 0.009, 0.024, 0.004, and 0.001, respectively), whereas histotype, epithelial hyperplasia, margin status, and local recurrence were predictors of DMFS (P = 0.001, 0.007, 0.007, and < 0.001, respectively). In multivariate analysis, independent prognostic factors for LRFS included age [hazard ratio (HR) = 3.045, P = 0.005], tumor size (HR = 2.668, P = 0.013), histotype (HR = 1.715, P = 0.017), and margin status (HR = 4.530, P< 0.001). Histotype (DMFS: HR = 4.409, P = 0.002; OS: HR = 4.194, P = 0.003) and margin status (DMFS: HR = 2.581, P = 0.013; OS: HR = 2.507, P = 0.020) were independent predictors of both DMFS and OS. In this cohort, younger age, a larger tumor size, a higher tumor grade, and positive margins were associated with lower rates of LRFS. Histotype and margin status were found to be independent predictors of DMFS and OS.
Keywords: Phyllodes tumor, local recurrence, distant metastasis, overall survival
Phyllodes tumor is a rare neoplasm that accounts for 1% of all breast neoplasms in women[1],[2]. The median age at presentation ranges from 40 to 46 years[1],[3]–[5]. Phyllodes tumors generally present as oval-shaped breast tumors that are elastic and well separated from surrounding tissues. Phyllodes tumors are frequently large and occasionally reach up to 30-40 cm in diameter[6]–[9]. Approximately 15% of phyllodes tumors have a propensity to recur locally, whereas fewer tumors metastasize to distant sites[3],[10]–[13]. Metastases to the axillary lymph nodes are observed in fewer than 5% of all patients with phyllodes tumors[1],[2],[14]–[19]. Phyllodes tumors were initially described as “cystosarcoma phyllodes” by Johannes Müller in 1838, and these tumors have as many as 62 different synonyms. The current World Health Organization (WHO) classification was established in 2003[20]. Phyllodes tumors are composed of biphasic stromal and glandular elements. Based on histological characteristics that include the degree of stromal hypercellularity, mitoses and cytologic atypia, stromal overgrowth, and the nature of tumor borders, the WHO classification distinguishes 3 histological subtypes of phyllodes tumors: benign, borderline, and malignant[20], which account for 58.4%-74.6%, 15.0%-16.1%, and 9.3%-31% of all phyllodes tumors, respectively[3],[14],[21]. The behavior of most benign phyllodes tumors is similar to that of fibroadenomas, whereas malignant phyllodes tumors carry a significantly higher risk of recurrence and metastasis[3],[11],[21],[22]. The histological type of a phyllodes tumor is commonly accepted as the only independent prognostic factor[2],[3],[5],[10],[11],[14],[15],[23]. Surgical resection remains the most important treatment, as phyllodes tumors are not sensitive to either chemotherapy or radiotherapy. Tumor histology is an important prognostic factor and an important consideration in the choice of the surgical procedure [i.e., excision, wide local excision (WLE), or mastectomy]. However, a subset of patients with benign phyllodes tumors will develop local recurrence and even distant metastasis. Patients with phyllodes tumors with distant metastasis are incurable and have a poor prognosis; most will die within 3 years following the diagnosis of metastasis[3],[12],[24]. As the morbidity of phyllodes tumors is low, it is difficult to conduct prospective clinical trials to understand the biological behavior of and the best treatment for this disease. There is still no uniform agreement on criteria for prediction of the biological behavior of this tumor type, although the histopathologic classification has been used for decades. The recurrence rates and outcomes of the three subtypes vary widely among different reports, and the clinical courses of this disease are often inconsistent. It seems that histotype is not the only prognostic factor. Because most studies have been conducted in different cohorts of patients, it has been difficult to formulate any conclusions. Therefore, we performed this retrospective study to identify the predictive factors for local recurrence-free survival (LRFS), distant metastasis-free survival (DMFS), and overall survival (OS) in patients with phyllodes tumors of the breast.
Materials and Methods
Patients
All patients diagnosed with a phyllodes tumor at the Sun Yat-sen University Cancer Center between March 1997 and December 2012 whose pathologic slides and complete follow-up records were available were included in this study. Patients with other malignancies or severe cardiorespiratory, liver, or renal failure not attributable to the phyllodes tumor at the time of diagnosis were excluded. The following factors were assessed: age, tumor size, pathologic parameters (e.g., stromal hypercellularity, mitosis, stromal atypia, stromal overgrowth, borders, necrosis, hemorrhage, epithelial hyperplasia, the presence of tumor giant cells, and pathologic mitosis), histotype, sites of local recurrence, sites of distant metastasis, LRFS, DMFS, and OS.
The surgical approaches were classified into three categories: excision, WLE, and mastectomy. Excision refers to enucleation of the tumor, with involved or uninvolved margins < 1 cm; WLE means that the entire tumor was completely dissected, with clear margins ≥1 cm.
The histopathologic diagnoses of all cases were reassessed based on established histological criteria defined by the WHO Classification of Tumours of the Breast in 2012[25]. The margin status was determined as follows: a positive margin was defined if tumor cells were present at the surgical margin, a close margin was defined if tumor cells were present < 1 cm from the closest surgical margin, and a clear margin was defined if tumor cells were present >1 cm from the closest surgical margin.
Statistical analysis
Distant metastasis was defined as metastatic disease or advanced locoregional disease that extended by growth into the thoracic cavity and into the wide soft tissue outside of the breast. LRFS was defined from the date of surgery to the date of diagnosis of local recurrence. DMFS was defined from the date of surgery to the date of diagnosis of distant metastasis. OS was defined from the date of surgery to the date of death. Patients who survived were censored when the study ended.
Statistical analysis was performed using SPSS 17.0 software. The Pearson χ2 test was used to investigate the relationship between clinical features and tumor histotype. Survival curves were obtained using the Kaplan-Meier method based on the log-rank test[26]. Univariate and multivariate Cox regression analyses were performed to identify variables that were predictive of outcomes. A P value < 0.05 was considered significant.
Results
Patients
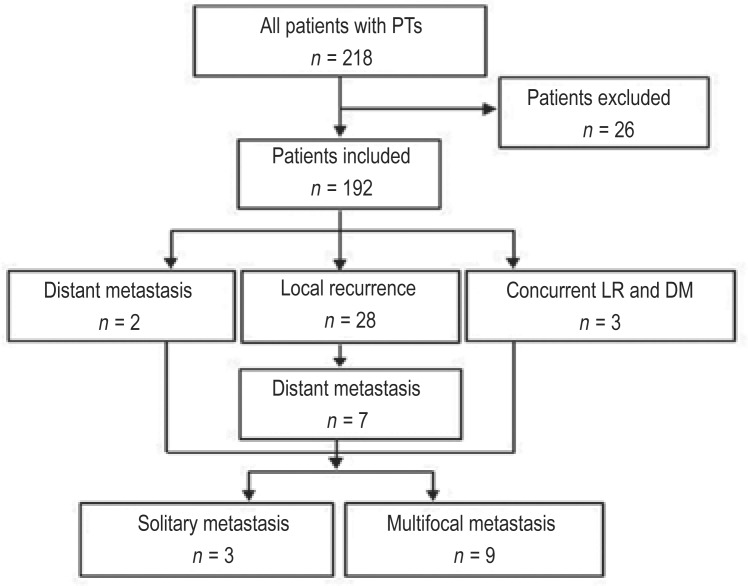
This study included a total of 192 patients (Figure 1). The median age of the patients with a phyllodes tumor at the time of diagnosis was 40 years (range, 11-77 years). When the patients were stratified by age, 63 patients were younger than 35 years, and 129 were 35 years of age or older. Tumor size was documented in 159 patients and ranged from 0.6 to 18.0 cm (mean, 5.4 cm; median, 4.0 cm); in the other 33 patients, excisional biopsy was performed prior to their referral to our institution, and thus, the sizes of those tumors were unavailable. Tumors were classified as benign in 80 patients, borderline in 63 patients, and malignant in 49 patients. The pathologic presentation of the three subtypes is shown in Figure 2. The median ages of the patients with benign, borderline, and malignant phyllodes tumors were 38.5, 41.0, and 41.0 years, respectively, and the median tumor sizes were 4.3, 4.0, and 5.0 cm, respectively. The tumor was located in the left breast in 104 (54.2%) patients, in the right breast in 87 (45.3%) patients, and in both breasts in 1 (0.5%) patient. In the Pearson χ2 test, there was no significant difference in age, tumor size, or laterality among the tumors of the three different histotypes (P = 0.351, 0.444, and 0.421, respectively).
Figure 1. The inclusion diagram of patients with phyllodes tumors of the breast.
Eighteen patients were excluded because of the lack of pathologic slides or complete follow-up records, and 8 were excluded because of concurrent diagnosis of other malignancies. Local recurrence (LR) occurred in 31 patients, and distant metastasis (DM) occurred in 12 patients.
Figure 2. The three subtypes of phyllodes tumors (HE ×100).
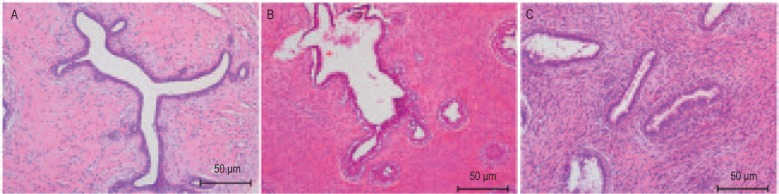
A, benign phyllodes tumor; B, borderline phyllodes tumor; C, malignant phyllodes tumor. According to the World Health Organization (WHO) classification criteria, benign tumors were identified when <5 mitoses/10 high-power fields (HPFs), pushing margins, minimal or moderate stromal overgrowth, and minimal stromal cellularity and atypia were observed; borderline phyllodes tumor were identified when 5-9 mitoses/10 HPFs, pushing or infiltrating margins, moderate stromal cellularity, atypia, and overgrowth were observed; and malignant phyllodes tumors were identified when >10 mitoses/10 HPFs, infiltrating margins, moderate to marked stromal cellularity, atypia, and overgrowth were observed.
Treatments
In total, 41 (21.4%) patients underwent excision (including 5 patients with positive margins and without salvage surgery); 104 (54.2%) patients underwent WLE (including 9 patients who underwent excisional biopsy at other hospitals and re-excision at our institution for the following indications: 1 borderline and 1 malignant phyllodes tumor with positive margins and 3 borderline and 4 malignant phyllodes tumors with close margins); 47 (24.4%) patients underwent mastectomy (including 12 patients who underwent excisional biopsy at other hospitals and mastectomy at our institution for the following indications: 2 borderline and 2 malignant phyllodes tumors with positive margins and 8 malignant phyllodes tumors with close margins). Axillary dissection was performed in 14 patients during the initial surgery, but no lymph node involvement was identified. In addition, no patients received adjuvant or neoadjuvant radiotherapy/chemotherapy for the treatment of their primary phyllodes tumors. Five patients received chemotherapy after recurrence or metastasis (1 for local recurrence and 4 for distant metastasis). No patients received radiotherapy after local recurrence or distant metastasis.
Local recurrence and distant metastasis
The clinical features of the 192 patients with local recurrence or distant metastasis of phyllodes tumors are summarized in Table 1.
Table 1. Clinical features of the 192 patients with local recurrence or distant metastasis of phyllodes tumors.
| Feature | Total [cases (%)] | LR [cases (%)] | P | DM [cases (%)] | P |
| Total | 192 | 31 (16.1) | 12 (6.3) | ||
| Age (years) | 0.015 | 0.781 | |||
| < 35 | 63 (32.8) | 16 (25.4) | 3 (5.0) | ||
| ≥35 | 129 (67.2) | 15 (11.6) | 9 (7.0) | ||
| Tumor size (cm) a | 0.016 | 0.787 | |||
| ≥5 | 100 (62.9) | 12 (12.0) | 6 (6.0) | ||
| > 5 | 59 (37.1) | 16 (27.1) | 5 (8.5) | ||
| Histotype | 0.139 | < 0.001 | |||
| Benign | 80 (41.7) | 9 (11.2) | 0 (0.0) | ||
| Borderline | 63 (32.8) | 10 (15.9) | 4 (6.3) | ||
| Malignant | 49 (25.5) | 12 (24.5) | 8 (16.3) | ||
| Margin status | <0.001 | 0.002 | |||
| Positive margin | 5 (2.6) | 3 (60.0) | 2 (40.0) | ||
| Close margin | 23 (14.0) | 14 (60.9) | 4 (17.4) | ||
| Clear margin | 164 (85.4) | 13 (7.9) | 6 (3.7) | ||
| Local recurrence | NA | < 0.001 | |||
| No | 161 (83.9) | NA | 2 (1.2) | ||
| Yes | 31 (16.1) | NA | 10 (32.3) |
a The tumor sizes of 33 patients were unavailable because excisional biopsy was performed at other hospitals prior to referral to our institution. LR, local recurrence; DM, distant metastasis; NA, not applicable.
The median follow-up time was 72.8 months (range, 7.1-171.5 months). Local recurrence occurred in 31 patients, and among these patients, the median age at the diagnosis of primary tumor was 33 years (range, 17-56 years), and the median primary tumor size was 6.0 cm (range, 0.8-18 cm). All patients with local recurrence underwent WLE or mastectomy as re-resection. No patients with benign phyllodes tumors developed distant metastasis. In all 31 patients with local recurrence, the LRFS ranged from 1.3 to 41.1 months (median, 12.6 months). Distant metastasis occurred in 12 patients: 3 were diagnosed with synchronous local recurrence, 7 developed distant metastasis 4.8-50.9 months after the diagnosis of local recurrence, 1 developed distant metastasis without local recurrence at 28.6 months after the first resection, and 1 developed pancreatic tail metastasis at the time of the diagnosis of primary phyllodes tumor. The primary phyllodes tumors were borderline in 4 patients and malignant in 8 patients. Metastases most commonly involved soft tissues (such as wide chest wall, back, and abdominal wall), the lungs, thoracic cavity, and pleura. Case 24 presented with a borderline phyllodes tumor of the right breast and a simultaneous pancreatic tail metastasis at her first diagnosis. She underwent a mastectomy and resection of the pancreatic tail and then received 6 cycles of chemotherapy (pirarubicin + ifosfamide + dacarbazine). She experienced 39.3 months of disease-free survival (DFS) until a second pancreatic metastasis occurred. This case was included in the OS analysis but not in the analyses of LRFS and DMFS. Case 50, who developed bilateral lung metastases, underwent wedge resection aided by video-assisted thoracoscopic surgery (VATS) of the left upper lobe metastasis and open wedge resection of the right upper lobe metastasis. The remaining 10 patients were not candidates for mastectomy after developing multiple metastases; 4 of them received salvage chemotherapy. All patients with distant metastasis died of the tumors. The clinical features and survival data of the 12 patients are summarized in Table 2.
Table 2. Clinical features of 12 patients with phyllodes tumors who had DM.
| Case No. | Agea (years) | Tumor size (cm) | Histotype | LR | LRFS (months) | DMFS (months) | Survival from DM (months) | OS (months) | Site of DM |
| 1 | 24 | 4.0 | Malignant | Yes | 9.1 | 60.0 | 16.5 | 76.5 | Soft tissue, thoracic cavity |
| 2 | 38 | 6.0 | Malignant | Yes | 25.2 | 45.1 | 10.5 | 55.6 | Soft tissue, thoracic cavity |
| 5 | 68 | 2.0 | Malignant | Yes | 11.4 | 19.4 | 12.0 | 32.1 | Bone, lung, liver, pleura |
| 8 | 54 | 6.0 | Malignant | Yes | 7.7 | 12.5 | 10.3 | 22.8 | Soft tissue, internal mammary lymph nodes |
| 24b | 53 | 7.0 | Borderline | No | 41.1 | 0b | 41.1 | 41.1 | Pancreas |
| 28 | 46 | 2.0 | Borderline | Yes | 23.3 | 23.3 | 9.6 | 32.9 | Soft tissue, thoracic cavity |
| 29 | 46 | 4.0 | Borderline | Yes | 5.7 | 5.7 | 8.2 | 14.0 | Lung |
| 30 | 24 | 0.8 | Borderline | Yes | 34.0 | 52.3 | 4.6 | 56.9 | Soft tissue, pleura |
| 45 | 42 | 18.0 | Malignant | Yes | 6.7 | 31.1 | 4.6 | 35.7 | Soft tissue, bone, thoracic cavity |
| 50 | 37 | 4.0 | Malignant | Yes | 11.0 | 38.9 | 41.1 | 80.0 | Lung |
| 53 | 31 | 7.0 | Malignant | Yes | 12.6 | 12.6 | 5.2 | 17.8 | Bone, lung, liver, pleura, soft tissue |
| 74 | 60 | NK | Malignant | No | 30.6 | 28.6 | 2.0 | 30.6 | Lung |
aAge at diagnosis of primary phyllodes tumor. bCase 24: pancreatic tail metastasis was detected at the diagnosis of primary phyllodes tumor. LRFS, local recurrence-free survival; DMFS, distant metastasis-free survival; OS, overall survival; NK, not known. Other abbreviations as in Table 1.
Survival
Twelve patients with distant metastasis died, whereas the other 180 patients survived. During the follow-up, the median LRFS, DMFS, and OS were 12.6 months (range, 1.3-171.5 months), 71.6 months (range, 5.7-171.5 months), and 72.8 months (range, 7.1-171.5 months), respectively.
Age and tumor size were predictive factors for LRFS in the univariate and multivariate analyses, but not for DMFS and OS (Tables 3 and 4). Histotype, margin, local recurrence, and distant metastasis were predictive factors for OS (Figure 3).
Table 3. Univariate analysis of clinicopathologic factors related to 5-year survival.
| Factor | Total (cases) | LRFS (%) | P | DMFSa (%) | P | OS (%) | P |
| Age (years) | 0.009 | 0.662 | 0.704 | ||||
| < 35 | 63 | 72.0 | 93.0 | 95.0 | |||
| ≥35 | 129 | 87.0 | 92.0 | 92.0 | |||
| Tumor size(cm)b | 0.024 | 0.692 | 0.666 | ||||
| ≥5 | 100 | 87.0 | 91.0 | 94.0 | |||
| > 5 | 59 | 70.0 | 90.0 | 89.0 | |||
| Histotype | 82.0 | 0.125 | 92.0 | 0.001 | 94.0 | 0.001 | |
| Benign | 80 | 88.0 | 100.0 | 100.0 | |||
| Borderline | 63 | 83.0 | 92.0 | 92.0 | |||
| Malignant | 49 | 74.0 | 79.0 | 84.0 | |||
| Borders | <0.001 | 0.001 | <0.001 | ||||
| Circumscribed | 157 | 89.0 | 96.0 | 97.0 | |||
| Infiltrated | 35 | 57.0 | 78.0 | 79.0 | |||
| Stromal cellularity | <0.001 | <0.001 | <0.001 | ||||
| Mild | 69 | 95.0 | 98.0 | 98.0 | |||
| Moderate | 102 | 82.0 | 94.0 | 93.0 | |||
| Marked | 21 | 21.0 | 40.0 | 65.0 | |||
| Stromal atypia | 0.009 | <0.001 | <0.001 | ||||
| Mild | 125 | 88.0 | 98.0 | 98.0 | |||
| Moderate | 47 | 77.0 | 87.0 | 90.0 | |||
| Marked | 20 | 59.0 | 66.0 | 73.0 | |||
| Mitosis (/10 HPFs) | 0.031 | 0.001 | 0.001 | ||||
| 0-4 | 92 | 91.0 | 99.0 | 99.0 | |||
| 5-9 | 74 | 74.0 | 90.0 | 92.0 | |||
| ≥10 | 26 | 79.0 | 75.0 | 78.0 | |||
| Stromal overgrowth | <0.001 | 0.001 | 0.001 | ||||
| Absent | 104 | 94.0 | 99.0 | 99.0 | |||
| Present | 88 | 69.0 | 84.0 | 86.0 | |||
| Necrosis | 0.432 | 0.118 | 0.089 | ||||
| Absent | 180 | 83.0 | 93.0 | 94.0 | |||
| Present | 12 | 72.0 | 81.0 | 79.0 | |||
| Hemorrhage | 0.004 | 0.073 | 0.062 | ||||
| Absent | 181 | 84.0 | 93.0 | 94.0 | |||
| Present | 11 | 16.0 | 79.0 | 79.0 | |||
| Epithelial hyperplasia | 0.989 | 0.007 | 0.003 | ||||
| Absent | 164 | 82.0 | 94.0 | ||||
| Present | 28 | 85.0 | 84.0 | ||||
| Tumor giant cells | 0.630 | 0.576 | 0.565 | ||||
| Absent | 182 | 82.0 | 92.0 | 94.0 | |||
| Present | 10 | 90.0 | 86.0 | 86.0 | |||
| Pathologic mitosis | 0.264 | 0.517 | 0.536 | ||||
| Absent | 185 | 82.0 | 92.0 | 93.0 | |||
| Present | 7 | 100.0 | 100.0 | 100.0 | |||
| Margin status | <0.001 | 0.002 | 0.001 | ||||
| Positive margin | 5 | 40.0 | 60.0 | 75.0 | |||
| Close margin | 23 | 49.0 | 81.0 | 86.0 | |||
| Clear margin | 164 | 91.0 | 95.0 | 95.0 | |||
| Local recurrence | NA | < 0.001 | < 0.001 | ||||
| No | 161 | NA | 99.0 | 98.0 | |||
| Yes | 31 | NA | 62.0 | 68.0 | |||
| Distant metastases | NA | NA | < 0.001 | ||||
| No | 180 | NA | NA | 100.0 | |||
| Yes | 12 | NA | NA | 17.0 |
Table 4. Multivariate Cox analysis of prognostic factors for survival.
| Factor | LRFS |
DMFS |
OS |
|||
| P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | |
| Age < 35 years | 0.005 | 3.045 (1.407-6.589) | NA | NA a | NA | NA a |
| Size > 5 cm | 0.013 | 2.668 (1.227-5.801) | NA | NA a | NA | NA a |
| Histotype | 0.017 | 1.715 (1.105-2.775) | 0.002 | 4.409 (1.692-11.488) | 0.003 | 4.194 (1.622-10.842) |
| Margin status | <0.001 | 4.530 (2.589-7.928) | 0.013 | 2.581 (1.223-5.451) | 0.020 | 2.507 (1.157-5.431) |
The factors that were analyzed in the multivariate Cox analysis included age, tumor size, histotype, margin status, the presence of tumor giant cells, pathologic mitosis, epithelial hyperplasia, hemorrhage, and necrosis. a Age and tumor size were not prognostic factors for DMFS and OS in the univariate Cox regression equation. HR: hazard ratio; 95% CI: 95% confidence interval. Other abbreviations as in Tables 1 and 2.
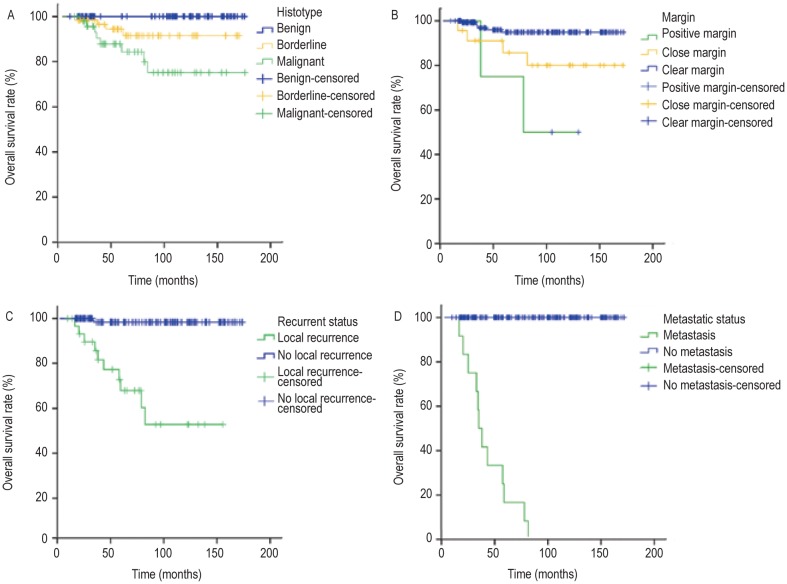
Figure 3. Overall survival (OS) curves of patients with phyllodes tumors stratified by histotype, margin, local recurrence, and distant metastasis.
A, patients with malignant phyllodes tumors had a higher OS rate than did patients with other subtypes of phyllodes tumors (P < 0.001). B, the presence of positive margins was a poor prognostic factor for OS (P < 0.001). C, patients who developed local recurrence had reduced survival compared with those who did not (P < 0.001). D, patients who developed distant metastasis also had reduced survival compared with those who did not (P < 0.001).
Discussion
In our cohort, younger patients were more likely to develop local recurrence. The median age was only 33 years in patients who developed local recurrence, whereas it was 40 years in the entire group, which is similar to previous reports[1],[3],[22],[24]. Patients younger than 35 years had a higher rate of local recurrence than did patients older than 35 years (univariate analysis, P = 0.015, Table 3; multivariate analysis, P = 0.005, Table 4), but the patients did not differ significantly with respect to DMFS and OS. In previous reports, the relationship between age and local recurrence was unclear, and the majority of reports have failed to show a significant association between age and either local recurrence or distant metastasis[3],[18],[21],[22],[24]. However, Spitaleri et al.[4] reported that among patients who presented with breast tumors, younger patients (<35 years of age) had a higher risk of phyllodes tumor-related events. Our data indicate that young age is a poor prognostic factor for local recurrence, but this does not apply to distant metastasis and OS. These findings may help us to distinguish the patients at high risk of developing local recurrence and may also imply that thorough surgery, such as WLE and mastectomy, should be performed on younger patients, especially patients with malignant phyllodes tumors. In addition, we should put high value on monitoring local recurrence in these patients. The reason why our finding was different from those of other studies may have been differences in the patients' race or in the numbers of patients or other reasons. Prospective randomized trials are needed to confirm this speculation.
In previous reports, the local recurrence rates of phyllodes tumors ranged from 12.2% to 32% (average, ∼15%), whereas 10% of patients developed distant metastases[3],[10]–[13]. Of the patients who developed distant metastasis, approximately 25% were diagnosed with malignant primary phyllodes tumors. Our results are consistent with what has been previously reported. In our cohort, the local recurrence rates were 11.2%, 15.9%, and 24.5% for the benign, borderline, and malignant subtypes, respectively, and the distant metastasis rates were 0%, 8%, and 16%, respectively. Most patients with distant metastasis had progressed from local recurrence (7/12). As shown in Table 2, soft tissue (7/12) was the most frequently affected site of distant metastases, followed by the lungs (5/12), thoracic cavity (4/12), bones (3/12), and pleura (3/12). Soft tissue lesions and thoracic cavity metastases likely arose from the extension of local recurrence into adjacent tissues. Thus, we believe that it is critical to reduce local recurrence to prevent distant metastasis.
Although histotype has been considered the most important prognostic factor for LRFS and DMFS, certain reports have suggested that it is less important than margin status for the prediction of local recurrence[1],[4],[12]. We found this concept to be the case in our patient cohort. According to a multivariate Cox analysis, the predictive factors for local recurrence were age, tumor size, histotype, and margin status. The HR for margin status (HR = 4.530) was the highest among the four factors and was much higher than the HR for histotype (HR = 1.715). The margin status was also an important prognostic factor for DMFS (HR = 4.767) and OS (HR = 4.941). The significance of margin status reflects the impact of treatment, especially adequate surgical resection, on patient outcomes. The importance of adequate local disease control to patient outcomes has been recognized for many years. In the latest National Comprehensive Cancer Network (NCCN) guidelines, Breast Cancer, Version 1.2014 (http://www.nccn.org)[27], it is clearly stated that the margin distance should be at least 1 cm for adequate surgical resection. In our cohort, patients in the close margin group and patients in the positive margin group had similar risks of local recurrence, which were significantly higher than the risk of local recurrence among patients in the clear margin group. Thus, whenever possible, an adequate margin should be obtained. In our analysis, we found tumor size to be a predictive factor for LRFS, but not for DMFS and OS. A larger tumor size may be associated with an increased risk of local recurrence because the size affects the ability of the surgeon to achieve adequate local control and may reflect the underlying tumor biology, underlying tumor aggressiveness, or metastatic potential. In the present study, the tumor histotype showed a significant association with LRFS, and several of the parameters, such as tumor borders, stromal cellularity, stromal atypia, stromal overgrowth, and mitosis, were significantly associated with local recurrence according to the univariate analysis. Because these parameters are considered during the classification of the histotype of phyllodes tumors, we did not include them in the multivariate analysis to minimize the collinearity of variables that were included in the final model. However, it appears that the stromal component significantly affects the biological behavior of phyllodes tumors, more than other cell types in the tumor do. The following features are found only in phyllodes tumors (especially in malignant phyllodes tumors) and are not observed in fibroadenomas: stromal hypercellularity, stromal overgrowth, stromal condensation around the ducts, and stromal infiltration into the surrounding breast parenchyma. Some pathologists even believe that the stromal component of the tumor might secondarily evoke glandular or ductal proliferation. Kim et al.[28] reported that the expression of stromal matrix metalloprotein-14 (MMP-14) was associated with a higher rate of recurrence (P < 0.05). These previously reported findings support the hypothesis that the stromal component plays an important role in the development of these diseases and in the promotion of local recurrence. Our findings indicate that although defined histotypes of phyllodes tumors based on pathologic features are important to predict the behavior of these tumors, the details of individual histological parameters should be provided in pathology reports. Our study is a retrospective study performed at a single center; further studies of prospective randomized trials at multiple cancer centers are needed to confirm our conclusions.
In summary, we found that age, size, histotype, and margin status are associated with LRFS. Tumor histotype and local recurrence are also important predictive factors for distant metastasis. The identification of these risk factors at the initial presentation of patients with phyllodes tumors and the selection of an adequate surgical procedure to ensure clear margins, followed by close monitoring, are all critical to successfully minimize the rates of local recurrence and distant metastasis and to improve outcomes. In patients with a single metastatic focus, surgical resection is a feasible approach to prolong survival.
Acknowledgments
We thank the Department of Pathology, Sun Yat-sen University Cancer Center for confirming the histopathologic diagnoses of the patients.
References
- 1.Belkacemi Y, Bousquet G, Marsiglia H, et al. Phyllodes tumor of the breast. Int J Radiat Oncol Biol Phys. 2008;70:492–500. doi: 10.1016/j.ijrobp.2007.06.059. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki-Uematsu S, Shiraishi K, Ito T, et al. Malignant phyllodes tumor composed almost exclusively of a fibrosarcomatous component: a case report and review of malignant phyllodes tumors with metastases. Breast Cancer. 2010;17:218–224. doi: 10.1007/s12282-009-0099-7. [DOI] [PubMed] [Google Scholar]
- 3.Tan PH, Jayabaskar T, Chuah KL, et al. Phyllodes tumors of the breast: the role of pathologic parameters. Am J Clin Pathol. 2005;123:529–540. doi: 10.1309/U6DV-BFM8-1MLJ-C1FN. [DOI] [PubMed] [Google Scholar]
- 4.Spitaleri G, Toesca A, Botteri E, et al. Breast phyllodes tumor: a review of literature and a single center retrospective series analysis. Crit Rev Oncol Hematol. 2013;88:427–436. doi: 10.1016/j.critrevonc.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Tse GM, Chaiwun B, Lau KM, et al. Endothelin-1 expression correlates with atypical histological features in mammary phyllodes tumours. J Clin Pathol. 2007;60:1051–1056. doi: 10.1136/jcp.2006.044354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gebrim LH, Bernardes JJR, Nazario AC, et al. Malignant phyllodes tumor in the right breast and invasive lobular carcinoma within fibroadenoma in the other: case report. Sao Paulo Med J. 2000;118:46–48. doi: 10.1590/S1516-31802000000200004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Roos WK, Kaye P, Dent DM. Factors leading to local recurrence or death after surgical resection of phyllodes tumours of the breast. Br J Surg. 1999;86:396–399. doi: 10.1046/j.1365-2168.1999.01035.x. [DOI] [PubMed] [Google Scholar]
- 8.Bennett IC, Khan A, De Freitas R, et al. Phyllodes tumours: a clinicopathological review of 30 cases. Aust N Z J Surg. 1992;62:628–633. doi: 10.1111/j.1445-2197.1992.tb07534.x. [DOI] [PubMed] [Google Scholar]
- 9.Reinfuss M, Mitus J, Duda K, et al. The treatment and prognosis of patients with phyllodes tumor of the breast: an analysis of 170 cases. Cancer. 1996;77:910–916. doi: 10.1002/(sici)1097-0142(19960301)77:5<910::aid-cncr16>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 10.Ben HJ, Damak T, Gamoudi A, et al. Phyllodes tumors of the breast: a case series of 106 patients. Am J Surg. 2006;192:141–147. doi: 10.1016/j.amjsurg.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Parker SJ, Harries SA. Phyllodes tumours. Postgrad Med J. 2001;77:428–435. doi: 10.1136/pmj.77.909.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asoglu O, Ugurlu MM, Blanchard K, et al. Risk factors for recurrence and death after primary surgical treatment of malignant phyllodes tumors. Ann Surg Oncol. 2004;11:1011–1017. doi: 10.1245/ASO.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Tan PH. 2005 Galloway Memorial Lecture: Breast phyllodes tumours—morphology and beyond. Ann Acad Med Singapore. 2005;34:671–677. [PubMed] [Google Scholar]
- 14.Karim RZ, Gerega SK, Yang YH, et al. Phyllodes tumours of the breast: a clinicopathological analysis of 65 cases from a single institution. Breast. 2009;18:165–170. doi: 10.1016/j.breast.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Niezabitowski A, Lackowska B, Rys J, et al. Prognostic evaluation of proliferative activity and DNA content in the phyllodes tumor of the breast: immunohistochemical and flow cytometric study of 118 cases. Breast Cancer Res Treat. 2001;65:77–85. doi: 10.1023/a:1006457304526. [DOI] [PubMed] [Google Scholar]
- 16.Tse GM, Lee CS, Kung FY, et al. Hormonal receptors expression in epithelial cells of mammary phyllodes tumors correlates with pathologic grade of the tumor: a multicenter study of 143 cases. Am J Clin Pathol. 2002;118:522–526. doi: 10.1309/D206-DLF8-WDNC-XJ8K. [DOI] [PubMed] [Google Scholar]
- 17.Tse GM, Lui PC, Lee CS, et al. Stromal expression of vascular endothelial growth factor correlates with tumor grade and microvessel density in mammary phyllodes tumors: a multicenter study of 185 cases. Hum Pathol. 2004;35:1053–1057. doi: 10.1016/j.humpath.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 18.Mangi AA, Smith BL, Gadd MA, et al. Surgical management of phyllodes tumors. Arch Surg. 1999;134:487–92. doi: 10.1001/archsurg.134.5.487. [DOI] [PubMed] [Google Scholar]
- 19.Harada S, Fujiwara H, Hisatsugu T, et al. Malignant cystosarcoma phyllodes with lymph node metastasis—a case report. Jpn J Surg. 1987;17:174–177. doi: 10.1007/BF02470594. [DOI] [PubMed] [Google Scholar]
- 20.Tavassoli FA, Devilee P, editors. World Health Organization Classification of Tumours. Lyon, France: IARC Press; 2003. Pathology and genetics of tumours of the breast and female genital organs; pp. 99–103. [Google Scholar]
- 21.Taira N, Takabatake D, Aogi K, et al. Phyllodes tumor of the breast: stromal overgrowth and histological classification are useful prognosis predictive factors for local recurrence in patients with a positive surgical margin. Jpn J Clin Oncol. 2007;37:730–736. doi: 10.1093/jjco/hym099. [DOI] [PubMed] [Google Scholar]
- 22.Abdalla HM, Sakr MA. Predictive factors of local recurrence and survival following primary surgical treatment of phyllodes tumors of the breast. J Egypt Natl Canc Inst. 2006;18:125–133. [PubMed] [Google Scholar]
- 23.Jacklin RK, Ridgway PF, Ziprin P, et al. Optimising preoperative diagnosis in phyllodes tumour of the breast. J Clin Pathol. 2006;59:454–459. doi: 10.1136/jcp.2005.025866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barrio AV, Clark BD, Goldberg JI, et al. Clinicopathologic features and long-term outcomes of 293 phyllodes tumors of the breast. Ann Surg Oncol. 2007;14:2961–2970. doi: 10.1245/s10434-007-9439-z. [DOI] [PubMed] [Google Scholar]
- 25.Lakhani SR, Ellis IO, Schnitt SJ, et al. WHO classification of tumours of the breast. 4th ed. Lyon, France: IARC Press; 2012. pp. 143–147. [Google Scholar]
- 26.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 27.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines), Breast Cancer, Version 1.2014, MS56. Available at: http://www.nccn.org.
- 28.Kim GE, Kim JH, Lee KH, et al. Stromal matrix metalloproteinase-14 expression correlates with the grade and biological behavior of mammary phyllodes tumors. Appl Immunohistochem Mol Morphol. 2012;20:298–303. doi: 10.1097/PAI.0b013e318235a132. [DOI] [PubMed] [Google Scholar]