Abstract
Purpose
This study aimed to confirm the utility of the European Organization for Research and Treatment of Cancer (EORTC) and the Spanish Urological Club for Oncological Treatment (CUETO) scoring systems and to determine which model is preferred as a prognostic model in Korean patients with non-muscle-invasive bladder cancer.
Materials and Methods
Between 1985 and 2011, 531 patients who were treated by transurethral resection of bladder cancer were retrospectively analyzed by use of the EORTC and CUETO models. Statistically, we performed Kaplan-Meier survival analysis; calculated Harrell's concordance index, receiver operating characteristic (ROC) curve, and cutoff values; and performed univariate and multivariate Cox proportional hazards regression analyses.
Results
For risk of recurrence, with the use of the EORTC model, all groups had statistically significant differences except between the group with a score of 0 and the group with a score of 1-4. With the use of the CUETO model, all groups differed significantly. For risk of progression, with the use of the EORTC model, significant differences were observed between all groups except between the group with a score of 2-6 and the group with a score of 7-13. With the use of the CUETO model, a significant difference was observed between the group with a score of 0 and the other groups. The concordance index of the EORTC and CUETO models was 0.759 and 0.836 for recurrence and 0.704 and 0.745 for progression, respectively. The area under the ROC curve for the EORTC and CUETO models was 0.832 and 0.894 for recurrence and 0.722 and 0.724 for progression, respectively.
Conclusions
Both scoring systems, especially the CUETO model, showed value in predicting recurrence and progression in Korean patients, which will help in individualizing treatment and follow-up schedules.
Keywords: Bladder cancer, EORTC, Progression, Recurrence
INTRODUCTION
Urothelial carcinoma accounts for more than 90% of bladder tumors, and about 70% of urothelial carcinomas are non-muscle-invasive bladder cancers (NMIBCs) at the time of the initial diagnosis [1]. Although NMIBC is usually not life-threatening in the early stage, more than half of these tumors will relapse and approximately 10% to 20% of these tumors will develop into muscle-invasive bladder tumors [2]. Although several studies have been performed, a single prognostic factor has not yet been identified because NMIBC is considered to be a heterogeneous disease.
To predict the risk of recurrence and progression, the European Organization for Research and Treatment of Cancer (EORTC) developed a simple scoring system that includes factors such as the number of tumors, tumor size, prior recurrence rate, cancer stage, presence of carcinoma in situ (CIS), and World Health Organization (WHO) grade on the basis of data from 2,596 patients with NMIBC [3]. To correct the overestimated risks of recurrence and progression owing to the low rate of Bacillus Calmette-Guerin (BCG) instillation, the Spanish Urological Club for Oncological Treatment (CUETO) proposed a modified model using gender, age, recurrent tumor, number of tumors, cancer stage, CIS, and WHO grade on the basis of data from 1,062 patients who were treated by BCG instillation [4]. These scoring systems can help in individualizing patients' follow-up schedules and in the decision making process for performing an early cystectomy.
The goal of this study was to confirm the utility of the EORTC and CUETO scoring systems in a Korean population.
MATERIALS AND METHODS
Between January 1985 and December 2011, 531 patients underwent transurethral resection of bladder tumor (TUR-BT) at Chung-Ang University Hospital owing to a histological diagnosis of NMIBC. We retrospectively analyzed the patients' medical records, which contained information on age, gender, prior recurrence rate, number of tumors, tumor size, cancer stage, presence of CIS, WHO grade, intravesical treatment, recurrence, and progression of bladder tumor. The follow-up period of the patients was checked from the first operation to the last cystoscopy procedure. The follow-up period had to be at least 1 year. Patients who were found to have advanced bladder tumors, ureteral tumors, or nonurothelial carcinoma at the first operation were excluded from the study. After pathologic confirmation, all patients underwent follow-up cystoscopies at the authors' clinic and received intravesical treatment when indicated. Patients who had a T1 tumor, grade 3, or no muscle lesion on pathology underwent repeat TUR-BT within 6 weeks. The Institutional Review Board approved this study.
We calculated the time to first recurrence (disease-free interval) as months to detect recurrence on cystoscopy after the diagnosis of bladder cancer. Patients alive without recurrence were censored at the time of the last available follow-up cystoscopy. We calculated the time to progression as months to detect muscle-invasive disease on pathological examination or metastasis on radiologic imaging after the diagnosis of bladder cancer. Patients alive without stage T2 or higher disease in the bladder were censored at the time of the last available follow-up cystoscopy. Twenty-four patients had undergone cystectomy or had experienced recurrence at the ureter or prostatic urethra after the first diagnosis of NMIBC. These patients were regarded as having progression. Cystoscopy was the main examination in the follow-up period. Urine cytology and computer tomography were used according to the European Association of Urology (EAU) guideline, and intravesical biopsy was performed for suspicious lesions. Finally, recurrence and progression were confirmed by histological examination.
The scores for risk of progression and recurrence were estimated by using the EORTC and CUETO models [3,4]. Cumulative incidence probabilities of recurrence and progression at 1 and 5 years were analyzed with 95% confidence intervals (95% CIs). The Kaplan-Meier survival analysis was used to assess recurrence and progression curves in both models. Harrell's concordance index was used to evaluate the discrimination ability of each model. Calibration ability was assessed by Hosmer-Lemeshow test. We assessed the predictive performance for recurrence and progression by using a receiver operating characteristic (ROC) curve of the EORTC score and CUETO score and calculated the cutoff values. Univariate and multivariate Cox proportional hazards regression analyses were used to identify the prognostic factors for recurrence and progression. A p-value of less than 0.05 was considered statistically significant. All statistical analyses were conducted by using IBM SPSS ver. 18.0 (IBM Co., Armonk, NY, USA), and R ver. 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
The basic characteristics of the patients are shown in Table 1 for comparison between the EORTC and CUETO models. The patients' mean age was 63.7 years (range, 22-93 years) at the time of diagnosis of bladder tumor, and the median follow-up duration was 58 months (range, 3-321 months). One hundred seventy-five patients (33.0%) had a relapse of bladder tumor within a mean follow-up of 19.0 months (range, 3-321 months). Forty-eight patients (9.0%) showed progression to muscle-invasive disease within a mean period of 33.6 months (range, 3-301 months). The 60- to 70-year-old age group was the largest (37.1%), and 88.7% of the patients were male. Primary tumors accounted for 67% of the prior recurrence rate. Single tumors were observed most frequently (62.0%), and a tumor size less than 3 cm (71.4%) was observed more frequently than a tumor size greater than 3 cm. The presence of CIS was observed in a small proportion of patients (6.6%).
TABLE 1.
Patient characteristics and recurrence and progression rates in comparison with the EORTC and CUETO models
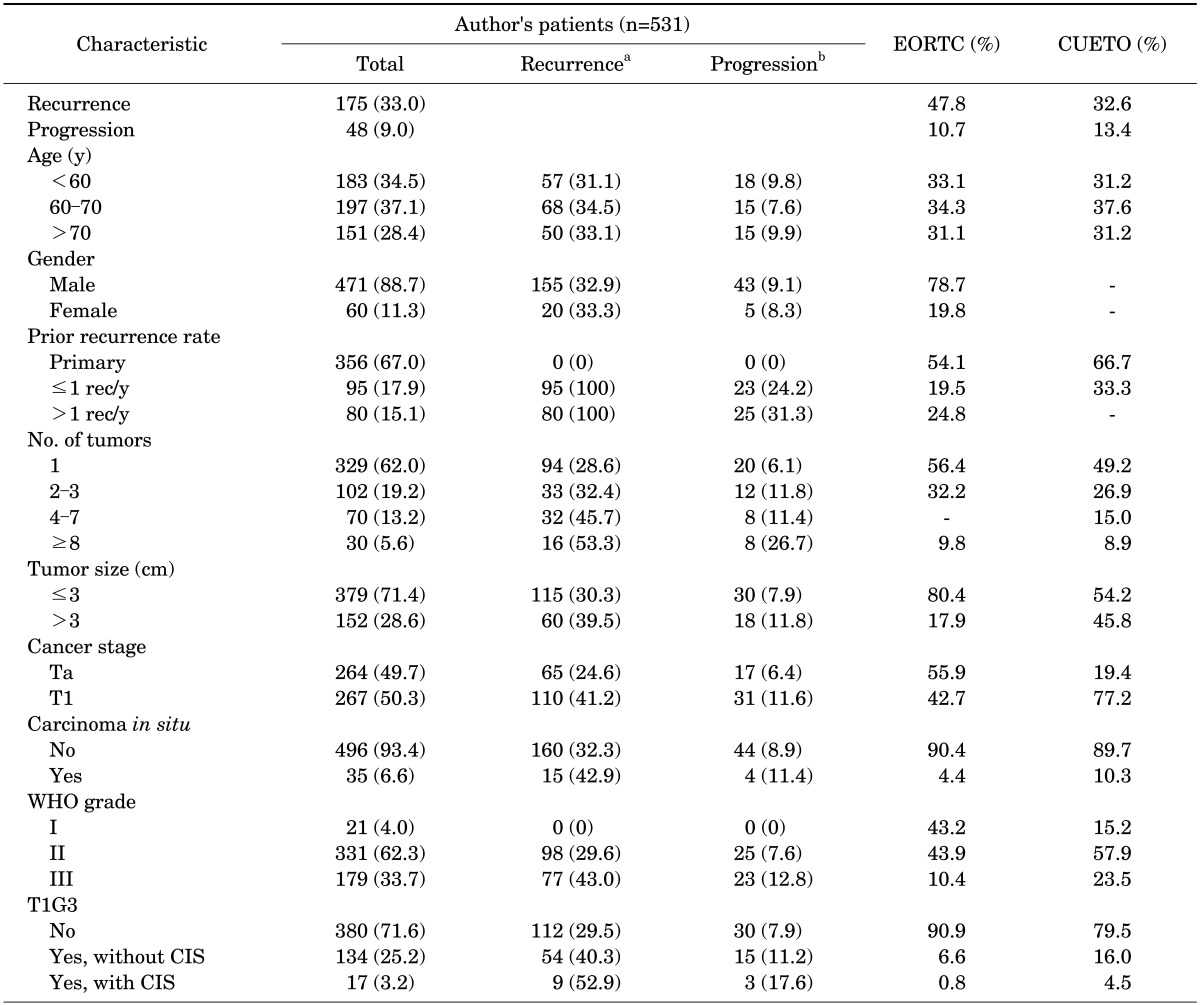
Values are presented as number (%).
EORTC, European Organization for Research and Treatment of Cancer; CUETO, Spanish Urological Club for Oncological Treatment; WHO, World Health Organization; CIS, carcinoma in situ.
a:No. of patients showing recurrence. b:No. of patients showing progression.
In our study, the cancer stages were divided almost equally. By use of the EORTC model, 55.9% of patients had Ta cancer, and by use of the CUETO model, 19.4% of patients did. Also in our study, patients with WHO G1 disease were few (4.0%). With the EORTC model, 43.2% of patients had G1 disease, and with the CUETO model, 15.2% of patients had G1 disease. A total of 282 patients (53.1%) received BCG instillation as the intravesical treatment.
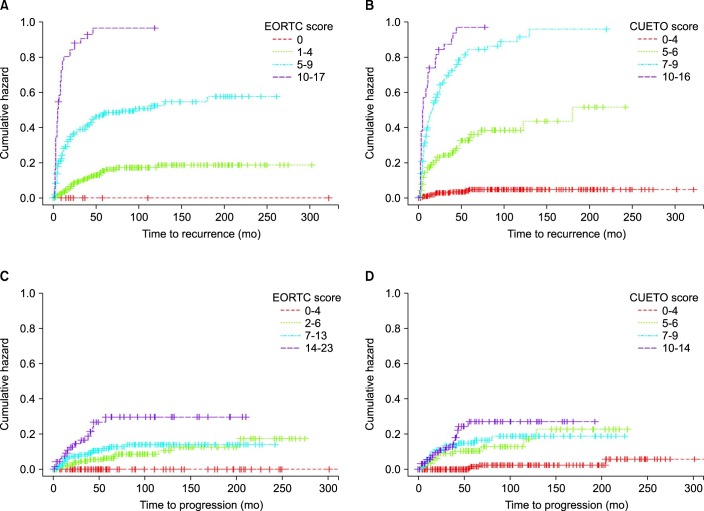
Fig. 1A and B show Kaplan-Meier survival curves of the 4 recurrence risk groups according to each model. By use of the EORTC model, all groups had statistically significant differences except between the group with a score of 0 and the group with a score of 1-4 (p=0.301). By use of the CUETO model, all groups were significantly different without exception. The Harrell's concordance index using the EORTC and CUETO models was 0.759 and 0.836 for recurrence, respectively. Calibration of EORTC and CUETO models showed p-values of 0.856 and 0.688.
FIG. 1.
Kaplan-Meier survival curves of risk of recurrence according to the European Organization for Research and Treatment of Cancer (EORTC) model (A), risk of recurrence according to the Spanish Urological Club for Oncological Treatment (CUETO) model (B), risk of progression according to the EORTC model (C), and risk of progression according to the CUETO model (D).
In Fig. 1C and D, the patients were divided into 4 groups according to model score, and the time to progression is presented for each group. By use of the EORTC model, a significant difference was observed between all groups except between the group with a score of 2-6 and the group with a score of 7-13 (p=0.303). By use of the CUETO model, a significant difference was observed only between the group with a score of 0-4 and the other groups; the other groups did not differ significantly (score 5-6 vs. score 7-9, p=0.616; score 5-6 vs. score 10-14, p=0.121; score 7-9 vs. score 10-14, p=0.307). The Harrell's concordance index using the EORTC and CUETO models was 0.704 and 0.745 for progression, respectively. Calibration of the EORTC and CUETO models showed p-values of 0.974 and 0.994.
Our probabilities of recurrence and progression at 1 and 5 years were compared by use of the EORTC risk table and the CUETO risk table as shown in Tables 2, 3. Our results for recurrence at 1 and 5 years showed larger differences between each score group than the reference probabilities provided by the EORTC and CUETO risk tables. However, our results on comparison with the EORTC system were more distinguishable than our results on comparison with the CUETO system for progression at 1 and 5 years.
TABLE 2.
Comparison of recurrence rate with EORTC and CUETO risk tables according to the total scores in both groups
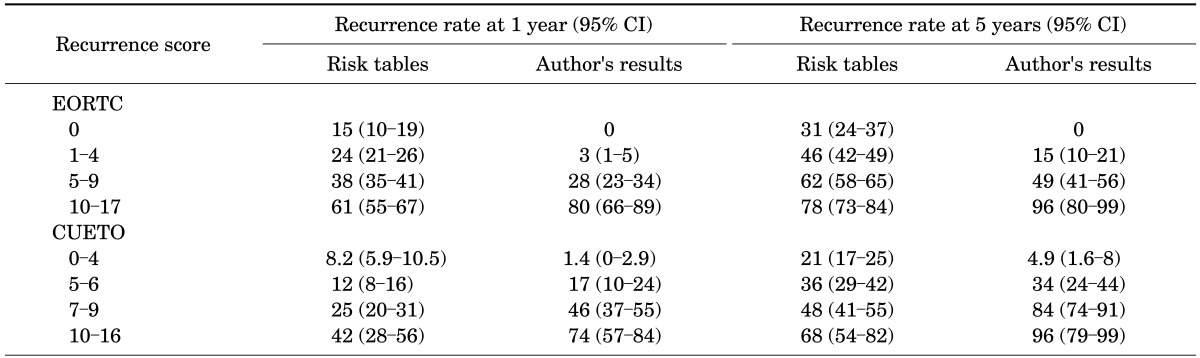
CI, confidence interval; EORTC, European Organization for Research and Treatment of Cancer; CUETO, Spanish Urological Club for Oncological Treatment.
TABLE 3.
Comparison of progression rate with EORTC and CUETO risk tables according to the total scores in both groups
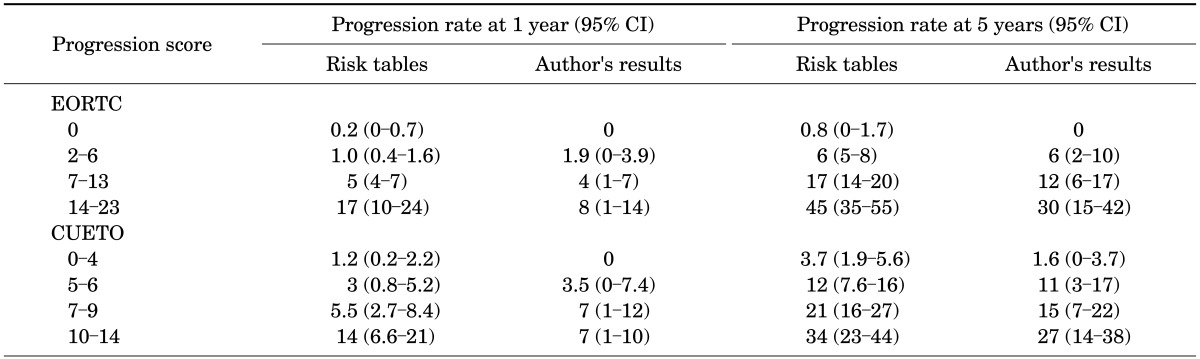
CI, confidence interval; EORTC, European Organization for Research and Treatment of Cancer; CUETO, Spanish Urological Club for Oncological Treatment.
By use of the ROC curve, the EORTC and CUETO scores were found to be useful predictors of recurrence with area under the curve (AUCs) of 0.832 (95% CI, 0.794-0.868) and 0.894 (95% CI, 0.865-0.923), respectively. Comparison of the AUCs of the EORTC and CUETO models showed the AUC of the CUETO model to be significantly higher (p=0.0002). When we applied a cutoff score for recurrence of 5.5, the EORTC system had a sensitivity of 74.3% and specificity of 75.3% in predicting recurrence, and the CUETO system had a sensitivity of 83.4% and a specificity of 82.0% in predicting recurrence.
The EORTC and CUETO scores were not good predictors of progression, with AUCs of 0.722 (95% CI, 0.649-0.779) and 0.724 (95% CI, 0.659-0.795), respectively. They were not significantly different (p=0.932). When we applied a cutoff score of 4.5 for progression, the EORTC system showed a sensitivity of 93.8% and a specificity of 43.9%, and the CUETO system had a sensitivity of 91.7% and a specificity of 46.8%.
In the multivariate analysis of recurrence, prior recurrence rate, CIS, and grade were significant prognostic factors by use of the EORTC model, and recurrent tumor, number of tumors, CIS, and grade were significant prognostic factors by use of the CUETO model. In the multivariate analysis of progression, recurrent tumor, cancer stage, and CIS were significant predictors by use of the EORTC model, and recurrent tumor, cancer stage, CIS, and grade were significant predictors by use of the CUETO model.
DISCUSSION
There are geographical and racial differences in bladder cancer incidence and survival. Environmental triggers may be a cause of some of these differences. Rates of smoking and chemical exposure vary, and both of these are known causes of bladder cancer [5]. Bladder cancer incidence is approximately 3 times higher in white men than in African American men [6]. Furthermore, the survival rate for African Americans is worse than for Asians and whites [7]. In a study predicting the recurrence and progression of bladder cancer by use of the Surveillance, Epidemiology, and End Results Medicare data, stage T1 was associated with a higher rate of recurrence, and female gender, black race, grade, and CIS with T1 were associated with a higher risk of progression [8]. However, a definite method for predicting prognosis has not yet been found, and we should determine risk factors for the Korean population.
Several studies have validated the EORTC model [9,10]. However, only a few studies have made comparisons between models. Xu et al. [11] investigated 363 Chinese patients with NMIBC and found that the EORTC model was more accurate in predicting recurrence and progression than was the CUETO model. They stated that the reason for this occurrence was that most of their patients received intravesical chemotherapy, similar to the patients in the EORTC study. Xylinas et al. [12] explained that both models showed a poor discrimination ability because of overestimation of the risk of recurrence and progression in high-risk patients. These varying results indicate that the basic characteristics of patients could result in differences. The CUETO model showed a higher probability in predicting tumor size, cancer stage, and CIS than did the EORTC model. In the EORTC study, 78% of the patients received intravesical treatment, mostly with chemotherapy, and a few patients were treated with BCG instillation. In the CUETO study, however, 100% of the patients received BCG instillation and 15% of the patients were additionally treated with mitomycin C. Meta-analyses have shown that BCG instillation after TUR-BT reduces the risk of recurrence and progression [13,14]. In our study, 53% of patients received BCG instillation, and tumor size, cancer stage, and CIS were better predictors than in the CUETO study, which resulted in a similar recurrence rate (33%) to that in the CUETO study (32.6%). In addition, in multivariate analyses of data from the CUETO study for recurrence, although age and gender were not significant, other significant variables, especially recurrence, showed a high hazard ratio (4.875). Hence, this may have affected the distinguishable and significant Kaplan-Meier curve of CUETO for recurrence. However, compared with the EORTC study, the variables that were significant in multivariate analyses of data from the CUETO study varied, and the effect of a high hazard ratio was less than in the EORTC study. The CUETO model showed adequate discrimination ability in interpreting our results.
Our results showed a mixed pattern. The Harrell's concordance indexes for recurrence were more distinguishable in the CUETO model than in the EORTC model (p=0.01). Also, the AUC for the cutoff score for recurrence was more significant in the CUETO model than in the EORTC model. However, the EORTC model was found to be a valid tool for assessing the probabilities of recurrence and progression at 1 and 5 years. The weakness of both of these models is the low positive predictive value for progression, especially in patients with high-grade disease. Our results were more distinguishable using not only the EORTC model but also the CUETO model than were the original references provided by the EORTC and CUETO studies. Although their variables were not optimal for our subjects for assessing the results of univariate and multivariate analyses, Sylvester's report showed that the variables in the EORTC and CUETO model were not significant in several studies [15]. The EORTC model was strong for predicting the probabilities of recurrence and progression at 1 and 5 years, and this could help clinicians in individualizing the follow-up schedule according to the patient's risk score. Before performing cystoscopy, assuming an EORTC recurrence score of greater than 5 implies a recurrence rate of greater than 50% at 5 years could help clinicians avoid missing the probability of recurrence. In addition, patients treated with BCG instillation who had a CUETO recurrence score of greater than 5.5 could indicate that there is a high probability of failure of intravesical therapy and the clinician should consider early cystectomy for such patients.
Our study had some limitations. Because of the retrospective analysis and long follow-up period, not all of the patients were treated by the same regimen or the same clinician. This might influence the treatment effect and survival rate. Also, we did not assess intravesical chemotherapy or dosage and discontinuation of BCG instillation owing to incomplete old records. We just checked for a history of BCG instillation. There are recent studies on low-dose BCG and the use of a short period to reduce the side effects of BCG; even reduced BCG instillation has been found to be clinically effective [16,17]. In our hospital, we used the 1973 grading system of the WHO between 1985 and 2006 and the 2004 WHO grading system from 2007 onwards. G1 of the 1973 WHO grading system can correspond to papillary urothelial neoplasm of low malignant potential or low-grade tumor. G2 of the 1973 WHO grading system can correspond to low-grade tumor or high-grade tumor of the 2004 WHO grading system, and G3 of the 1973 WHO grading system can correspond to high-grade tumor of the 2004 WHO grading system [18]. Seventy-eight patients were graded by using the 2004 WHO grading system. Since the EORTC and CUETO models used the 1973 WHO grading system, but the 2004 grading system is used currently in our hospital, our aim was to confirm the applicability of both models to our conditions. Chen et al. [19] found that both the 1973 and the 2004 WHO classifications were effective in predicting progression, whereas the 1973 WHO classification was more suitable for predicting recurrence. According to the European guidelines, both grading systems can be used until clear results are obtained [20].
The other limitation of our study that we could not identify new variables associated with a poor prognosis, such as prostatic urethral involvement or bladder neck involvement, molecular markers, and SNPs [21,22,23,24,25]. However, research on these variables has only recently begun and they may not be recommended for routine examination.
CONCLUSIONS
The EORTC and CUETO scoring systems showed value in predicting recurrence and progression in Korean patients with NMIBC. Especially, the CUETO model showed statistically significant results for recurrence in our study, because of the effect of BCG instillation and the heterogeneous patient characteristics. These models can help clinicians in individualizing the appropriate treatment and follow-up schedule. Prospective, multicenter, and large-scale studies using modified EORTC and CUETO models are needed to predict the accurate recurrence and progression of bladder cancer in the Korean population.
Footnotes
The authors have nothing to disclose.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Donat SM. Evaluation and follow-up strategies for superficial bladder cancer. Urol Clin North Am. 2003;30:765–776. doi: 10.1016/s0094-0143(03)00060-0. [DOI] [PubMed] [Google Scholar]
- 3.Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466–465. doi: 10.1016/j.eururo.2005.12.031. discussion 475-7. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez-Gomez J, Madero R, Solsona E, Unda M, Martinez-Pineiro L, Gonzalez M, et al. Predicting nonmuscle invasive bladder cancer recurrence and progression in patients treated with bacillus Calmette-Guerin: the CUETO scoring model. J Urol. 2009;182:2195–2203. doi: 10.1016/j.juro.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Murta-Nascimento C, Schmitz-Drager BJ, Zeegers MP, Steineck G, Kogevinas M, Real FX, et al. Epidemiology of urinary bladder cancer: from tumor development to patient's death. World J Urol. 2007;25:285–295. doi: 10.1007/s00345-007-0168-5. [DOI] [PubMed] [Google Scholar]
- 6.Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist. 2007;12:20–37. doi: 10.1634/theoncologist.12-1-20. [DOI] [PubMed] [Google Scholar]
- 7.Yee DS, Ishill NM, Lowrance WT, Herr HW, Elkin EB. Ethnic differences in bladder cancer survival. Urology. 2011;78:544–549. doi: 10.1016/j.urology.2011.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chamie K, Litwin MS, Bassett JC, Daskivich TJ, Lai J, Hanley JM, et al. Recurrence of high-risk bladder cancer: a population-based analysis. Cancer. 2013;119:3219–3227. doi: 10.1002/cncr.28147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seo KW, Kim BH, Park CH, Kim CI, Chang HS. The efficacy of the EORTC scoring system and risk tables for the prediction of recurrence and progression of non-muscle-invasive bladder cancer after intravesical bacillus calmette-guerin instillation. Korean J Urol. 2010;51:165–170. doi: 10.4111/kju.2010.51.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hernandez V, De La Pena E, Martin MD, Blazquez C, Diaz FJ, Llorente C. External validation and applicability of the EORTC risk tables for non-muscle-invasive bladder cancer. World J Urol. 2011;29:409–414. doi: 10.1007/s00345-010-0635-2. [DOI] [PubMed] [Google Scholar]
- 11.Xu T, Zhu Z, Zhang X, Wang X, Zhong S, Zhang M, et al. Predicting recurrence and progression in Chinese patients with non-muscle-invasive bladder cancer using EORTC and CUETO scoring models. Urology. 2013;82:387–393. doi: 10.1016/j.urology.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Xylinas E, Kent M, Kluth L, Pycha A, Comploj E, Svatek RS, et al. Accuracy of the EORTC risk tables and of the CUETO scoring model to predict outcomes in non-muscle-invasive urothelial carcinoma of the bladder. Br J Cancer. 2013;109:1460–1466. doi: 10.1038/bjc.2013.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han RF, Pan JG. Can intravesical bacillus Calmette-Guérin reduce recurrence in patients with superficial bladder cancer? A meta-analysis of randomized trials. Urology. 2006;67:1216–1223. doi: 10.1016/j.urology.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Sylvester RJ, van der Meijden AP, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168:1964–1970. doi: 10.1016/S0022-5347(05)64273-5. [DOI] [PubMed] [Google Scholar]
- 15.Sylvester RJ. Natural history, recurrence, and progression in superficial bladder cancer. ScientificWorldJournal. 2006;6:2617–2625. doi: 10.1100/tsw.2006.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ojea A, Nogueira JL, Solsona E, Flores N, Gomez JM, Molina JR, et al. A multicentre, randomised prospective trial comparing three intravesical adjuvant therapies for intermediate-risk superficial bladder cancer: low-dose bacillus Calmette-Guerin (27 mg) versus very low-dose bacillus Calmette-Guerin (13.5 mg) versus mitomycin C. Eur Urol. 2007;52:1398–1406. doi: 10.1016/j.eururo.2007.04.062. [DOI] [PubMed] [Google Scholar]
- 17.Kanagawa Urological Research Group (KURG) A 2-week maintenance regimen of intravesical instillation of bacillus Calmette-Guerin is safe, adherent and effective in patients with non-muscle-invasive bladder cancer: a prospective, multicenter phase II clinical trial. Jpn J Clin Oncol. 2012;42:813–819. doi: 10.1093/jjco/hys097. [DOI] [PubMed] [Google Scholar]
- 18.MacLennan GT, Kirkali Z, Cheng L. Histologic grading of non-invasive papillary urothelial neoplasms. Eur Urol. 2007;51:889–897. doi: 10.1016/j.eururo.2006.10.037. [DOI] [PubMed] [Google Scholar]
- 19.Chen Z, Ding W, Xu K, Tan J, Sun C, Gou Y, et al. The 1973 WHO Classification is more suitable than the 2004 WHO Classification for predicting prognosis in non-muscle-invasive bladder cancer. PLoS One. 2012;7:e47199. doi: 10.1371/journal.pone.0047199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Comperat E, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013;64:639–653. doi: 10.1016/j.eururo.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Oderda M, Ricceri F, Pisano F, Fiorito C, Gurioli A, Casetta G, et al. Prognostic factors including Ki-67 and p53 in Bacillus Calmette-Guérin-treated non-muscle-invasive bladder cancer: a prospective study. Urol Int. 2013;90:184–190. doi: 10.1159/000343431. [DOI] [PubMed] [Google Scholar]
- 22.Shariat SF, Zippe C, Ludecke G, Boman H, Sanchez-Carbayo M, Casella R, et al. Nomograms including nuclear matrix protein 22 for prediction of disease recurrence and progression in patients with Ta, T1 or CIS transitional cell carcinoma of the bladder. J Urol. 2005;173:1518–1525. doi: 10.1097/01.ju.0000154696.48217.75. [DOI] [PubMed] [Google Scholar]
- 23.Ke HL, Chen M, Ye Y, Hildebrandt MA, Wu WJ, Wei H, et al. Genetic variations in micro-RNA biogenesis genes and clinical outcomes in non-muscle-invasive bladder cancer. Carcinogenesis. 2013;34:1006–1011. doi: 10.1093/carcin/bgt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faba OR, Palou J, Breda A, Villavicencio H. High-risk non-muscle-invasive bladder cancer: update for a better identification and treatment. World J Urol. 2012;30:833–840. doi: 10.1007/s00345-012-0967-1. [DOI] [PubMed] [Google Scholar]
- 25.Huguet J, Crego M, Sabate S, Salvador J, Palou J, Villavicencio H. Cystectomy in patients with high risk superficial bladder tumors who fail intravesical BCG therapy: pre-cystectomy prostate involvement as a prognostic factor. Eur Urol. 2005;48:53–59. doi: 10.1016/j.eururo.2005.03.021. [DOI] [PubMed] [Google Scholar]