Abstract
Hypoxia-inducible factor 1 (HIF-1) is a heterodimeric basic helix-loop-helix transcription factor composed of HIF-1α and HIF-1β that is the central regulator of responses to hypoxia. The specific binding of HIF-1 to the hypoxia-responsive element (HRE) induces the transcription of genes that respond to hypoxic conditions, including vascular endothelial growth factor (VEGF). Here we report that expression of HIF-1α is increased in diverse Epstein-Barr virus (EBV)-infected type II and III cell lines, which express EBV latent membrane protein 1 (LMP1), the principal EBV oncoprotein, as well as other latency proteins, but not in the parental EBV-negative cell lines. We show first that transfection of an LMP1 expression plasmid into Ad-AH cells, an EBV-negative nasopharyngeal epithelial cell line, induces synthesis of HIF-1α protein without increasing its stability or mRNA level. The mitogen-activated protein kinase (MAPK) kinase inhibitor PD98059 markedly reduces induction of HIF-1α by LMP1. Catalase, an H2O2 scavenger, strongly suppresses LMP1-induced production of H2O2, which results in a decrease in the expression of HIF-1α induced by LMP1. Inhibition of the NF-κB, c-jun N-terminal kinase, p38 MAPK, and phosphatidylinositol 3-kinase pathways did not affect HIF-1α expression. Moreover, LMP1 induces HIF-1 DNA binding activity and upregulates HRE and VEGF promoter transcriptional activity. Finally, LMP1 increases the appearance of VEGF protein in extracellular fluids; induction of VEGF is suppressed by PD98059 or catalase. These results suggest that LMP1 increases HIF-1 activity through induction of HIF-1α protein expression, which is controlled by p42/p44 MAPK activity and H2O2. The ability of EBV, and specifically its major oncoprotein, LMP1, to induce HIF-1α along with other invasiveness and angiogenic factors reported previously discloses additional oncogenic properties of this tumor virus.
Epstein-Barr virus (EBV) is a human herpesvirus that is associated with several types of malignancies, such as Burkitt's lymphoma, lymphoproliferative disorders, T-cell lymphomas, Hodgkin's disease, invasive breast cancer, and nasopharyngeal carcinoma (NPC) (4, 43). In all of the tumors, EBV infection is predominantly latent. In latent infection, the EBV genes expressed are restricted to six EBV nuclear antigens (EBNA1, -2, −3A, −3B, −3C, and -LP), three latent membrane proteins (LMP1, −2A, and −2B), and two small nonpolyadenylated RNAs (EBER1 and −2). On the basis of the pattern of expression of the genes that encode these proteins, latency is classified into three types. In type I latency, exemplified by Burkitt's lymphoma, only EBNA1, EBERs, and sometimes LMP2A are expressed. In type II latency, exemplified by Hodgkin's disease and NPC, EBNA1, EBERs, and the three LMPs are expressed. In type III latency, exemplified by EBV lymphoproliferative diseases, the full set of EBNAs and LMPs, as well as EBERs, is expressed (43).
LMP1 is considered an oncogenic protein because it has transforming properties in continuous rodent fibroblast cell lines and it is essential for immortalization of B lymphocytes in culture (61). LMP1 is an integral membrane protein consisting of 386 amino acids. Six transmembrane-spanning domains connect a short N-terminal cytoplasmic domain with a long C-terminal cytoplasmic tail (14). The C-terminal region of LMP1 contains two functional domains: C-terminal activation region 1 (CTAR1) and CTAR2. CTAR1, the proximal domain, interacts with tumor necrosis factor-associated factors and activates NF-κB (39). CTAR2, the distal domain, interacts with tumor necrosis factor receptor-associated death domain protein and also activates NF-κB (19). In addition, LMP1 activates the c-jun N-terminal kinase (JNK) cascade through CTAR2 and also stimulates p38 mitogen-activated protein kinase (p38 MAPK) through both CTAR1 and CTAR2 (12, 29). LMP1 also activates the p42/p44 MAPK pathway in Rat-1 fibroblasts (46).
We have shown that LMP1 induces the expression of an array of cellular invasion and metastasis factors, including matrix metalloproteinase 9 (MMP-9), which plays a critical role in tumor invasion (66). In addition, LMP1 induces vascular endothelial growth factor (VEGF) through induction of cyclooxygenase 2 (COX-2) (40). Furthermore, LMP1 induces and causes release of fibroblast growth factor 2 (FGF-2) into extracellular fluid (60). Several of the tumors in which LMP1 is expressed are invasive; NPC, for example, is highly invasive and is characterized by new-vessel formation. These observations suggest that a tumor virus may alter tumor cell phenotype such that invasiveness and angiogenesis are promoted. With EBV, LMP1 plays the central role in this complex process.
Hypoxia-inducible factor 1 (HIF-1) is a heterodimeric basic helix-loop-helix transcription factor composed of HIF-1α and HIF-1β subunits (62). HIF-1α is induced exponentially in response to a decrease in the cellular O2 concentration, but HIF-1β is not regulated by cellular oxygen tension (23). The specific binding of HIF-1 to the hypoxic response element (HRE) activates the transcription of genes whose products are required for tumor progression, including VEGF, glucose transporters, and insulin-like growth factor 2 (48). HIF-1α protein undergoes rapid ubiquitination and degradation by proteasomes under normoxic conditions. The oxygen-dependent turnover of HIF-1α protein is regulated by prolyl 4-hydroxylases that modify HIF-1α at two conserved proline residues (Pro-402 and Pro-564) located in the oxygen-dependent degradation domain of the protein (5, 51). Under normal oxygen conditions, HIF-1α is modified by prolyl hydroxylation, which permits binding of von Hippel-Lindau protein (pVHL), a recognition component of the E3 ligase complex. This binding promotes the ubiquitination and degradation of HIF-1α. Because proline hydroxylases require molecular oxygen and iron for their enzymatic activity, exposure to hypoxia or treatment with iron chelators results in HIF-1α stabilization (20, 67). HIF-1 target genes encode proteins that increase O2 delivery and mediate adaptive responses to O2 deprivation. Thus, a single protein, HIF-1α, appears to determine the responses to hypoxic conditions of at least 40 genes (50, 52). HIF-1 activity is increased not only by intratumoral hypoxia but also by genetic alterations, including PTEN and pVHL, as well as gain-of-function mutations in oncogenes that activate the phosphatidylinositol 3-kinase (PI3K), SRC, and MAPK signal transduction pathways under normoxic conditions (22, 35, 45, 70, 71).
So far, only general stimuli have been identified that affect HIF-1α function. In this report, we show that a specific oncogenic viral protein, LMP1, induces synthesis of HIF-1α and induces HIF-1 activity as a transcriptional factor. VEGF is induced, at least in part, through this pathway.
MATERIALS AND METHODS
Cell cultures.
KR-4 is an EBV-positive type III lymphoblastoid cell line (LCL). The KH-1 and KH-2 lines are EBV-positive type II cell lines derived by fusion of KR-4 and HeLa cells (human cervical carcinoma) (kind gifts of Maria Masucci, Karolinska Institute, Stockholm, Sweden) (69). Ad-AH cells, kindly provided by Erik K. Flemington (Tulane University, New Orleans, La.), are an EBV-negative human nasopharyngeal cell line (58). Human embryonic kidney 293 cells and HeLa cells were obtained from the American Type Culture Collection.
MDA-MB-231 (human breast cancer cell line) and EBV-infected MDA-MB-231 clones (C4A3, C1D12, C2G6, and C3B4) were generous gifts from Irene Joab (INSERM EPI 03-34, IUH, Hospital Saint-Louis, Paris, France). The MDA-MB-231 cells were infected with a recombinant virus provided by Kenzo Takada (18, 53). The parental MDA-MB-231 cells and the C4A3 clone are LMP1 negative. C1D12, C3G6, and C3B4 are LMP1-positive clones. LMP1 expression is strongest in the C3B4 clone and weakest in C4A3. KR-4 cells were maintained in RPMI 1640 medium containing 10% fetal bovine serum (FBS), penicillin, and streptomycin. MDA-MB-231 cells were maintained in RPMI 1640 medium with 10% FBS, 4 μM l-glutamine, penicillin, and streptomycin. EBV-infected MDA-MB-231 clones were maintained in the same medium but with G418 at 700 μg/ml. The other cells were maintained in Dulbecco modified Eagle medium (DMEM) with 10% FBS, penicillin, and streptomycin.
Plasmids.
pcDNA3-based LMP1 has been previously described (66). The FLAG-tagged IκBα (S32A/S36A) expression plasmid (srIκBα) was kindly provided by Albert Baldwin (University of North Carolina). IκBα (S32A/S36A) is not phosphorylated because of the S32 and S36 substitutions, which result in prevention of both degradation of IκBα and subsequent translocation of NF-κB into the nucleus. The FLAG-tagged JNK1 dominant negative (DN) expression plasmid is a generous gift from Roger J. Davis (11). The PGL3-based pGL-HRE and pGL-HRE mut luciferase reporter plasmids, which contain wild-type HRE oligonucleotide or HRE oligonucleotide with a CGT-to-AAA mutation, were kind gifts from J. Silvio Gutkind (National Institutes of Health, Bethesda, Md.) (see Fig. 5B) (54). The human VEGF gene luciferase reporter constructs (−1176/+54 and −27/+54), which were cloned in the pGL2 basic vector, were kindly provided by Jacques Pouyssegur and Gilles Pages (University of Nice, Nice, France) (36).
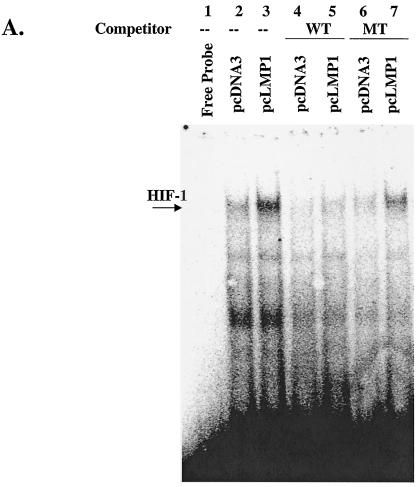
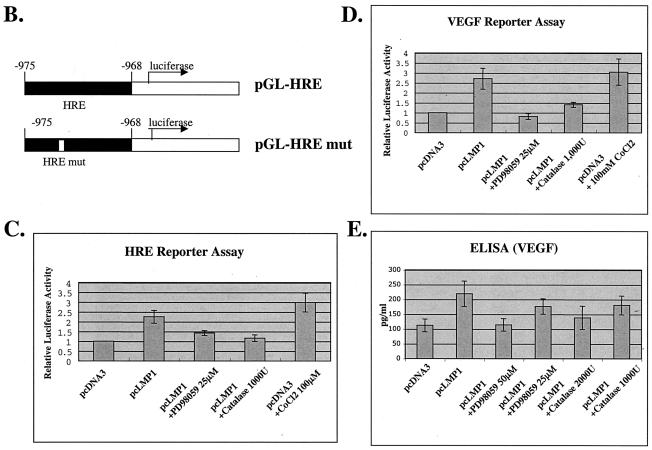
FIG. 5.
(A) LMP-1 induces binding of HIF-1 to the HRE of the VEGF promoter. Nuclear extracts of Ad-AH cells transfected with pcDNA3 or pcLMP1 were incubated with a 32P-labeled probe containing the HIF-1-binding site from the WT VEGF promoter and analyzed by electrophoretic mobility shift assay. Excesses of nonlabeled WT VEGF or MT VEGF were used as competitors. (B) Two promoter constructs, the HRE (pGL-HRE), and a mutated HRE lacking the HIF-1 binding site (pGL-HRE mut), are depicted (54). (C and D) LMP1-induced HRE or VEGF reporter activity was determined by luciferase assay. Relative HRE or VEGF luciferase activity is HRE luciferase activity divided by HRE mut activity or −1176/+54 luciferase activity divided by −27/+54 activity. LMP1 upregulates both relative HRE and VEGF reporter activities more than twofold, and this upregulation is suppressed by PD98059 or catalase. Relative luciferase activity represents the mean and standard deviation of three separate experiments. (E) VEGF protein level in culture medium determined by ELISA. LMP1 increases VEGF protein more than twofold, and this effect is decreased by PD98059 or catalase in a dose-dependent manner. Values represent the mean and standard deviation of the VEGF concentration in the medium in three experiments.
Transient and stable transfection.
Cells were transfected with 2 μg of the appropriate plasmid(s) with the use of the Effectene transfection kit (QIAGEN) in accordance with the manufacturer's instructions. For luciferase reporter assay, cells were transfected with 1 μg of the appropriate plasmids by use of the Superfect transfection kit (QIAGEN) in accordance with the manufacturer's protocol. Stable cell lines were established by cultivating Ad-AH cells in the presence of 800 μg of G418 per ml (Life Technologies).
Western blot analysis.
Whole-cell lysates were extracted in 500 μl of radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% NP-40, 0.50% sodium deoxycholate, 0.10% sodium dodecyl sulfate [SDS], 0.2 mM sodium orthovanadate, 0.1 M NaF, 5 μg of fluoride per ml). Nuclear or cytoplasmic extracts were prepared by the use of NE-Per (Pierce) in accordance with the manufacturer's protocol. Protein concentration was determined by Bio-Rad protein assay. Protein (50 to 100 μg) was boiled in SDS sample buffer for 5 min, electrophoresed on 6 to 10% polyacrylamide gels, and transferred onto a nitrocellulose membrane (Osmonics). Nonspecific reactivity was blocked by incubation for 30 min in a Tris-buffered saline solution containing 0.1% Tween 20 and 10% nonfat dried milk. The membrane was incubated overnight at 4°C with (i) mouse LMP1 monoclonal antibody (DAKO), (ii) mouse HIF-1α monoclonal antibody (Transduction Laboratories), (iii) mouse histone 1 monoclonal antibody (Santa Cruz), (iv) mouse GRP78 monoclonal antibody (Santa Cruz), (v) mouse HIF-1β monoclonal antibody (Transduction Laboratories), (vi) mouse FLAG M2 monoclonal antibody (Sigma), (vii) rabbit phospho-p42/p44 MAPK polyclonal antibody (New England Biolabs), (viii) rabbit phospho-p38 MAPK polyclonal antibody (New England Biolabs), or (ix) rabbit phospho-Akt polyclonal antibody (New England Biolabs). The membrane was washed with Tris-buffered saline solution containing 0.1% Tween 20, incubated with a horseradish peroxidase-conjugated mouse or rabbit secondary antibody (Amersham) at room temperature for 1 h, and washed three times for 15 min with Tris-buffered saline-0.1% Tween 20. Peroxidase activity was detected by enhanced chemiluminescence (Amersham).
RPA.
The human HIF-1α expression vector pCEP4/HIF-1α3.2T7 was a generous gift from Gregg L. Semenza (57). PCR was performed with Hotstar Taq DNA polymerase (QIAGEN) as follows: 35 cycles of 95°C for 30 s, 59°C for 30 s, and 72°C for 30 s. The antisense primer was 5′-CGCGGATCCCAGCCGCTGGAGACACAATC-3′, and the sense primer was 5′-CCGGAATTCCAGCACTACTTCGAAGTGGC-3′. The PCR product was doubly digested with BamHI and EcoRI and subcloned into vector pcDNA3. When linearized with KpnI and transcribed with SP6 RNA polymerase, the [α-32P]UTP-labeled riboprobe protects 197 nucleotides of HIF-1α mRNA. A plasmid to generate a glyceraldehyde-3-phosphate dehydrogenase riboprobe was supplied by United States Biochemicals, Inc. The RNase protection assay (RPA) for detection of HIF-1α mRNA was performed with total RNA and RNase protection kit II (Ambion) in accordance with the manufacturer's instructions and was described previously (60). Signal intensities were calculated with a Molecular Dynamics Phosphorimager (Sunnyvale) and ImageQuant software.
Determination of the half-life of HIF-1α.
Ad-AH cells were transfected with or without pcLMP1 as described above. To determine the half-life of HIF-1α protein, cells were treated with 100 μM cycloheximide to block protein synthesis. Cells were harvested after being incubated with cycloheximide for 0 to 4 min. Expression of HIF-1α was detected at each time point by Western blot analysis.
Metabolic labeling experiments.
Ad-AH cells (10 × 105) were plated in a 100-mm-diameter dish, and 24 h later the cells were transfected with 2 μg of plasmids; 48 h later, the cells were serum starved for 20 h. The cells were washed with phosphate-buffered saline PBS and pulse-labeled for 30 min with 0.3 mCi of [35S]Met-Cys per ml in Met- and Cys-free DMEM. Whole-cell extracts were prepared with RIPA buffer. Extract (500 μg) was precleared with 30 μl of protein A/G beads (Santa Cruz) for 1 h. Ten microliters of HIF-1α antibody and 30 μl of protein A/G beads were added to the supernatant fluid, rotated overnight at 4°C, pelleted, and washed six times with 1 ml of RIPA buffer. An equal volume of 2× SDS loading buffer was added, and the samples were boiled and fractionated by SDS-polyacrylamide gel electrophoresis. The gel was dried, and signal intensities were calculated with a Molecular Dynamics Phosphorimager (Sunnyvale) and ImageQuant software.
Proteasome inhibition assay.
To determine whether LMP1-induced HIF-1α was caused by lowered degradation, we incubated Ad-AH cells with or without LMP1 with 10 μM MG132 (benzyloxycarbonyl-leu-leucinyl-leucinal; Calbiochem), a potent proteasome inhibitor. HIF-1α protein levels were detected by Western blot analysis at different time points.
Luciferase reporter assay.
Reporter assays were performed after transient transfection of each construct as a reporter plasmid with Superfect (QIAGEN) by following the manufacturer's protocol. Luminescence was measured by a luminometer, and the results are expressed as light units per microgram of total protein. Relative luciferase activity was determined as follows. (i) Relative HRE activity was HRE luciferase activity divided by HRE mut activity. (ii) Relative VEGF activity was −1176/+54 luciferase activity divided by −27/+54 activity.
Determination of intracellular ROS generation.
The reactive oxygen species (ROS) measurement was performed in cell suspension by flow cytometry (6). 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) is a lipid-permeable, nonfluorescent compound and is oxidized by intracellular ROS to form the lipid-impermeable and fluorescent compound dichlorodihydrofluorescein (6, 64). The cells were preincubated in DMEM containing 10% FBS with or without catalase. Cells were trypsinized and then resuspended in 1 ml of DMEM without FBS to a final concentration of 106/ml. DCFH-DA (10 μM) was added in cell suspension, followed by incubation at 37°C for 30 min. The cells were then centrifuged and resuspended in PBS for immediate determination of ROS generation by flow cytometry (FACScan; Becton-Dickinson) using 488 nm for excitation and 525 nm for emission.
Electrophoretic mobility shift assay.
Nuclear extracts were prepared from Ad-AH cells as described previously (42). Cells were collected and centrifuged at 4,500 × g for 2 min. The supernatant fluid was discarded, and the cells were resuspended in 500 μl of cell lysis buffer A (10 mM HEPES [pH 8.0], 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol [DTT], 200 mM sucrose, 0.5 mM phenylmethylsulfonyl fluoride, 1 μg of leupeptin per ml, 0.5% NP-40) for 5 min at 4°C. The crude nuclei released by lysis were collected by microcentrifugation. Nuclei were rinsed once in buffer A and resuspended in 100 μl of buffer B (20 mM HEPES [pH 7.9], 1.5 mM MgCl2, 420 mM NaCl, 0.2 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride, 1.0 mM DTT, 1 μg of leupeptin per ml). Nuclei were incubated on a rocking platform at 4°C for 30 min and clarified by centrifugation for 5 min. A 21-bp double-stranded 32P-labeled oligonucleotide probe containing HIF-1 binding site-spanning nucleotides −979 to −959 of the VEGF 5′-flanking sequence (WT VEGF) was synthesized (57). We also prepared unlabeled WT VEGF and MT VEGF, an oligonucleotide containing a 3-bp substitution in the HIF-1 binding site of WT VEGF. For the electrophoretic mobility shift assay, 15 μg of nuclear extract was incubated in DNA-binding buffer (10 mM Tris [pH 7.8], 50 mM NaCl, 1 mM EDTA, 5% glycerol, 1 μl of 100 mM DTT per ml) and poly(dI-dC) (75 ng total) for 20 min at room temperature. The aliquots were then incubated with the WT VEGF probe in the presence or absence of an excess amount of unlabeled oligonucleotide WT VEGF or MT VEGF for 20 min at room temperature. Samples were loaded onto a 5% native polyacrylamide gel in Tris-borate-EDTA buffer. The gel was vacuum dried and exposed for autoradiography.
Determination of VEGF protein levels in cell culture medium.
Subconfluent cells were grown in six-well plates in 2.5 ml of DMEM containing 10% FBS with or without drugs for 2 h. The cells were then incubated in DMEM containing 10% FBS without drugs for 6 h. The medium was collected and clarified by centrifugation at 400 × g for 5 min. The amount of VEGF in the supernatant fluid was determined with an ELISA kit (VEGF-ELISA; R&D Systems) in accordance with the manufacturer's instructions. VEGF was expressed as picograms of VEGF protein per milliliter of medium and per 105 cells.
Injection of cells into athymic mice.
Ad-AH/pcDNA3 or Ad-AH/pcLMP1 (2 × 106) cells, stably transfected with pcDNA3 or pcLMP1, in 0.1 ml of PBS were inoculated subcutaneously into athymic nude mice. About 4 weeks after injection, the mice were sacrificed and equal amounts of each tumor were homogenized in SDS sample buffer for Western blot analysis.
RESULTS
The level of HIF-1α protein is increased in latently infected type II and III cells.
KR-4 is a typical type III LCL. The KH-1 and KH-2 lines were derived by fusion of KR-4 and HeLa cells (69). The level of HIF-1α protein is significantly higher in KR-4, KH-1, and KH-2 cells, which express LMP1, than in HeLa cells. The level of HIF-1α protein appeared to correspond to the level of LMP1 in the different cell lines (Fig. 1A). MDA-MB-231 is an EBV-negative breast cancer cell line. C4A3, C1D12, C2G6, and C3B4 are EBV-infected clones derived from the MDA-MB-231 parental cell line. C1D12, C2G6, and C3B4 cells express LMP1. As shown in Fig. 1B, the HIF-1α protein level is exaggerated in LMP1-positive clones. These results prompted us to examine the role of LMP1 on the induction of HIF-1α protein.
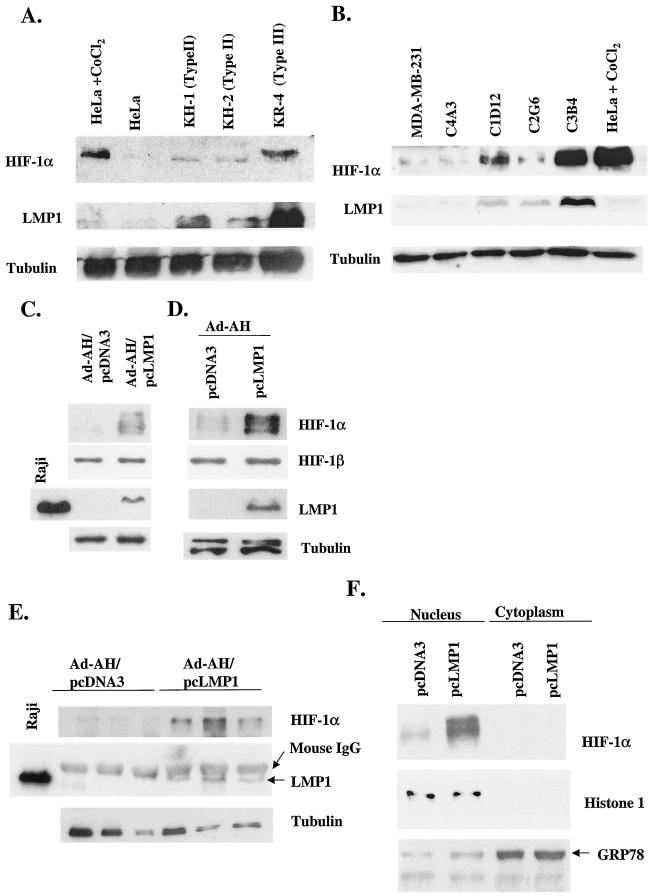
FIG. 1.
(A) Levels of HIF-1α protein are increased in type II and III latently EBV-infected cells. KR-4, type III, is an LCL. The KH-1 and KH-2, type II, cell lines are derived by fusion of KR-4 and HeLa cells (69). Levels of HIF-1α correspond to the level of LMP1 protein. (B) MDA-MB-231 is an EBV-negative breast cancer cell line. C4A3, C1D12, C2G6, and C3B4 are EBV-infected clones derived from the MDA-MB-231 parental cell line. C1D12, C2G6, and C3B4 express LMP1. HIF-1α protein levels are increased depending on LMP1 expression. (C, D, E, and F) LMP1 induces expression of HIF-1α protein in Ad-AH cells, an EBV-negative nasopharyngeal epithelial cell line. Stable (C) or transient (D and F) transfectants of Ad-AH cells, expressing or not expressing LMP1, were incubated under normoxic conditions. (E) HIF-1α levels are increased in LMP1-expressing tumors grown in athymic nude mice. In the whole-cell lysates, the expression of HIF-1α was significantly greater in LMP1-expressing Ad-AH cells detected by Western blotting (C, D, and E). The HIF-1β protein level was not affected by LMP1 (C and D). HIF-1α protein was restricted to the nucleus, and its level was significantly higher in LMP1-expressing cells (F). Histone 1 and GRP78 are nuclear and cytoplasmic markers, respectively.
LMP1 induces expression of HIF-1α protein.
Ad-AH is an EBV-negative nasopharyngeal epithelial cell line. Stable or transient transfectants of Ad-AH cells expressing LMP1 and control Ad-AH cells were incubated under normoxic conditions. In the whole-cell lysates, the expression of HIF-1α protein, but not HIF-1β protein, was significantly higher in LMP1-expressing cells (Fig. 1C and D). Similarly, induction of HIF-1α by LMP1 was detected in human embryonic kidney 293 cells (data not shown).
Since both Ad-AH/pcDNA3 and Ad-AH/pcLMP1 cells are tumorigenic in athymic nude mice, we were able to determine levels of HIF-1α in LMP1-expressing tumors growing in mice. Western blotting showed that there was enhanced expression of HIF-1α in tumors derived from Ad-AH/pcLMP1 cells compared with Ad-AH/pcDNA3-derived tumors (Fig. 1E). These results indicate that LMP1 induces HIF-1α expression in epithelial cells both in vitro and in vivo. In addition to the increase in HIF-1α, HIF-1 needs to be accumulated in the nucleus to act as a transcriptional factor. To determine the localization of HIF-1α, both the nuclear and cytoplasmic fractions were analyzed. As shown in Fig. 1F, HIF-1α protein was restricted to the nucleus, and its level was significantly higher in LMP1-expressing cells than in control cells. This result suggests that induction by LMP1 of HIF-1α may contribute to its activation.
LMP1 does not affect the mRNA level or stability of HIF-1α but stimulates HIF-1α protein synthesis.
To test whether LMP1 can induce the expression of HIF-1α mRNA, Ad-AH cells were transiently transfected with an LMP1 expression plasmid. The level of HIF-1α mRNA detected by RPA was increased by neither LMP1 nor 12,13-phorbol myristate acetate (PMA) compared with that of Ad-AH cells transfected with control vector pcDNA3 (Fig. 2A). Hypoxia increases the level of HIF-1α through an increase in protein stability as a result of decreased ubiquitin-dependent proteasomal degradation (56). In cells exposed to hypoxic conditions, the HIF-1α level remains constant over 60 min despite the lack of ongoing synthesis of the protein in cells exposed to cycloheximide (32). Under normoxic conditions, the half-life of HIF-1α is less than 5 min (30). To investigate whether a similar mechanism is activated by LMP1, the kinetics of HIF-1α decay in Ad-AH cells treated with cycloheximide were determined (Fig. 2B). Regardless of whether cells were transfected with pcDNA3 or pcLMP1, the half-life of HIF-1α protein remained essentially the same at 4 min. These results indicate that LMP1 neither stimulates HIF-1α mRNA expression nor inhibits HIF-1α protein degradation.
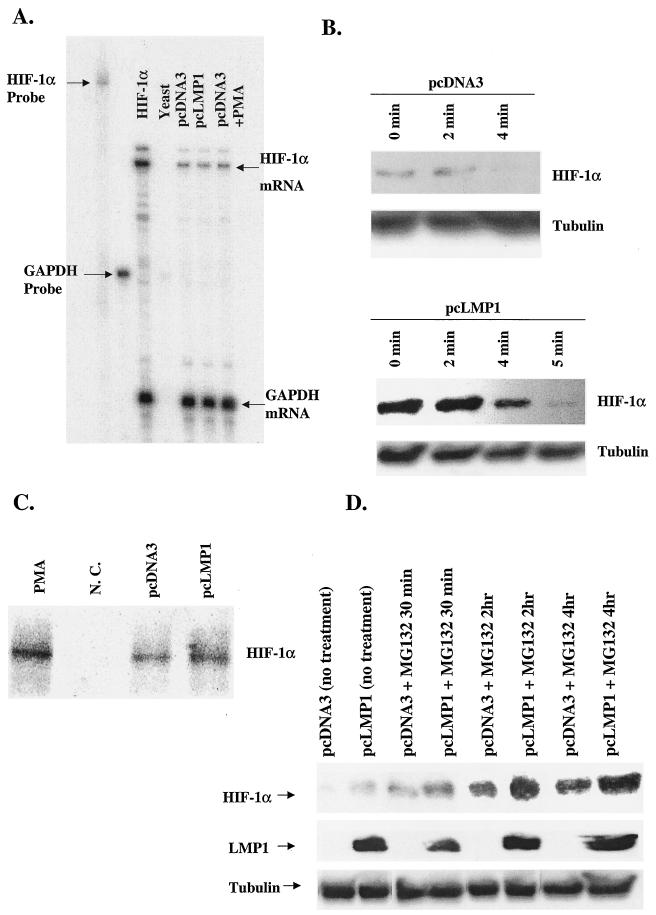
FIG. 2.
LMP1 does not affect the mRNA level or stability of HIF-1α but stimulates HIF-1α protein synthesis. (A) HIF-1α mRNA was detected by RPA. HIF-1α mRNA was induced by neither LMP1 nor PMA. (B) Ad-AH cells were treated with cycloheximide to block protein synthesis. The half-life of HIF-1α protein was constant at 4 min in both LMP1-expressing and control cells. (C) To analyze the rate of HIF-1α protein synthesis, serum-starved Ad-AH cells were pulse-labeled with [35S]Met-Cys for 30 min, followed by immunoprecipitation of HIF-1α protein. 35S-labeled HIF-1α protein was increased in LMP1-expressing cells compared with that in pcDNA3-transfected Ad-AH cells by more than threefold. For the positive control, pcDNA3-transfected Ad-AH cells were treated with PMA and pulse-labeled, followed by immunoprecipitation of HIF-1α protein. For the negative control, LMP1-expressing Ad-AH cells were pulse-labeled, followed by immunoprecipitation by normal mouse immunoglobulin G. N.C., negative control. (D) To determine whether the high level of HIF-1α protein detected in LMP1-positive cells was caused by reduced proteasomal degradation, cells were incubated with MG132 and Western blot assays were performed at the indicated time points. At all time points, levels of HIF-1α protein were significantly higher in LMP1-positive cells than in LMP1-negative cells regardless of the presence of MG132. Data shown are representative of those obtained in three separate experiments.
To analyze the rate of HIF-1α protein synthesis, serum-starved Ad-AH cells were pulse-labeled with [35S]Met-Cys for 30 min, followed by immunoprecipitation of HIF-1α. In contrast to the HIF-1α level in pcDNA3-transfected Ad-AH cells, the 35S-labeled HIF-1α level in the LMP1-expressing cells was increased more than threefold (Fig. 2C). For controls, serum-starved Ad-AH cells were pretreated with PMA, pulse-labeled, and then immunoprecipitated by HIF-1α antibody for the positive control or by normal mouse immunoglobulin G for the negative control. PMA is known to induce synthesis of HIF-1α protein without increasing its stability (44).
To determine whether decreased proteasomal degradation produced the high level of HIF-1α protein detected in LMP1-positive cells, we incubated cells with MG132, a potent proteasome inhibitor, and then performed Western blot analysis at different time points. At all of the time points, levels of HIF-1α protein were significantly higher in LMP1-positive cells than in LMP1-negative cells regardless of the presence of MG132 (Fig. 2D). Therefore, the difference in HIF-1α protein levels between LMP1-positive and LMP1-negative cells is attributable not to differential degradation but to differential expression. Thus, LMP1 increases the level of HIF-1α protein not by induction of its transcription or its stabilization but by increased synthesis of the protein.
The p42/p44 MAPK pathway is involved in LMP1-induced HIF-1α expression.
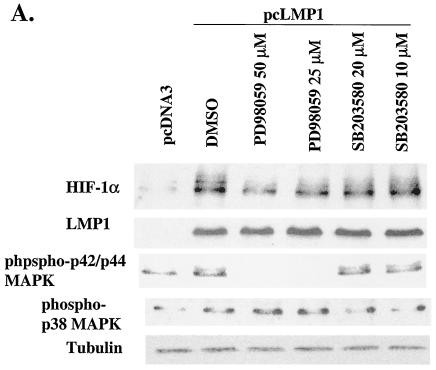
p42/p44 MAPK consists of two serine/threonine protein kinases that modulate the activity of a number of activation factors (10). Richard et al. showed that activation of the p42/p44 MAPK pathway induced the phosphorylation of HIF-1α, which was abolished in the presence of the MAP/ERK (MEK) inhibitor PD98059. They also demonstrated that p42/p44 MAPK activation is sufficient to promote the transcriptional capacity of HIF-1 (45). Hur et al. have also shown that PD98059 changes neither the stabilization nor the DNA-binding ability of HIF-1α, but it inhibits the ability of HIF-1α to transactivate responsive promoters (17a). These data suggest that the p42/p44 MAPK pathway is involved in HIF-1 activity but not in HIF-1α induction. In contrast, Agani and Semenza showed that mersalyl, a thioreactive organomercurial compound, induced the expression of HIF-1α and that PD98059 markedly reduced induction of HIF-1α by mersalyl, but not by hypoxia (1). The role of the p42/p44 MAPK signaling pathway that influences HIF-1α overexpression and HIF-1 transcriptional activity remains somewhat confusing and controversial. Το test whether the p42/p44 MAPK pathway is involved in LMP1-induced HIF-1α expression, we treated LMP1-transfected Ad-AH cells with PD98059. The expression of LMP1-induced HIF-1α protein detected by Western blotting was suppressed by PD98059 in a dose-dependent manner. However, the effect of PD98059 on inhibition of HIF-1α phosphorylation was not clear (Fig. 3A). Although SB203580, a selective p38 MAPK pathway inhibitor, suppressed the LMP1-induced p38 MAPK activity to the basal level (12, 29), the drug did not affect the expression of HIF-1α in our system (Fig. 3A). Thus, p42/p44 MAPK signaling is involved in LMP1-induced HIF-1α expression but the p38 MAPK pathway is not.
FIG. 3.
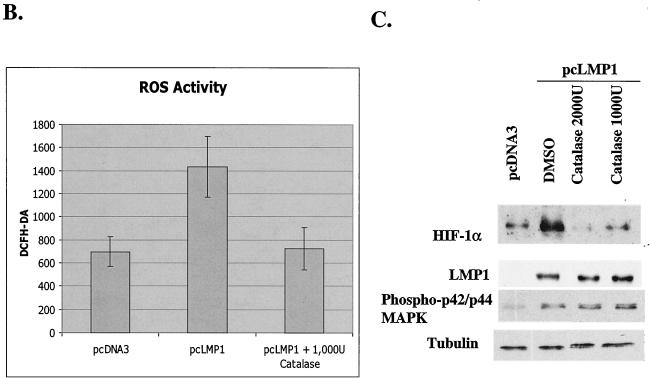
The p42/p44 MAPK pathway and H2O2 are involved in LMP1-induced HIF-1α expression. (A) Western blotting analysis to determine the involvement of the p42/p44 MAPK pathway in LMP1-induced expression of HIF-1α protein. LMP1-induced HIF-1α expression was suppressed in a dose-dependent manner by PD98059 but not by SB203580. (B) LMP1 induces ROS activity, determined by measuring the fluorescence response by flow cytometry and DCFH-DA. Catalase, an H2O2 scavenger, completely suppresses LMP1-induced ROS activity. Relative ROS activity represents the mean and standard deviation of three separate experiments. (C) HIF-1α expression was analyzed by Western blotting to check the effect of catalase. Catalase completely suppresses LMP1-induced HIF-1α expression. DMSO, dimethyl sulfoxide.
LMP1-induced H2O2 stimulates the expression of HIF-1α protein.
ROS have been proposed to participate in the signal transduction process mediating the stabilization and translation of HIF-1α (8, 44). We tested the effect of LMP1 on ROS generation by measuring the fluorescence response by flow cytometry and DCFH-DA and found that LMP1 increased ROS production. Catalase, a hydrogen peroxide (H2O2) scavenger, decreased the generation of ROS to the basal level (Fig. 3B). These results suggest that ROS generated by LMP1 is mostly H2O2. Next, we decided to investigate whether an increase in H2O2 levels was responsible for the induction of HIF-1α mediated by LMP1. When the cells were treated with catalase, HIF-1α induction by LMP1 was completely blocked (Fig. 3C). These results strongly suggest that HIF-1α induction by LMP1 is mediated through H2O2 production.
The PI3K signaling pathway is not involved in LMP1-induced HIF-1α expression.
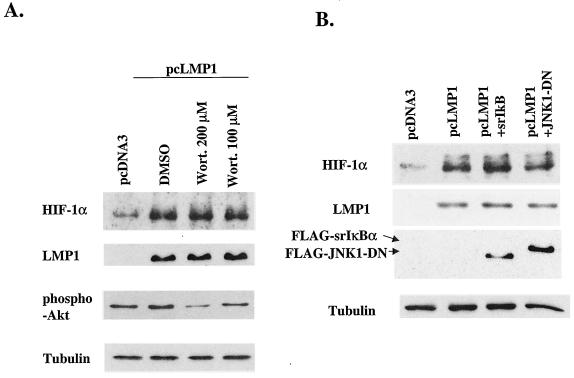
Previous reports suggest that PI3K and Akt are central in the regulation of HIF-1 activity (24, 44, 71). First, we checked whether LMP1 induces PI3K activity by detection of phospho-Akt by Western blotting. Akt is activated by PI3K and lies between PI3K and FKBP-rapamycin-associated protein in this signaling pathway (28). As shown in Fig. 4A, LMP1 does not increase the amount of phospho-Akt, which suggests that LMP1 does not stimulate the PI3K signaling pathway. Next, to determine whether PI3K pathway activity is required for LMP1-induced expression of HIF-1α, cells were exposed to wortmannin, an inhibitor of PI3K. Although wortmannin modestly suppressed phospho-Akt expression in a dose-dependent manner, wortmannin did not affect the level of HIF-1α. Therefore, we conclude that PI3K signaling is not involved in LMP1-induced HIF-1α expression.
FIG. 4.
(A) LMP1 does not increase phospho-Akt. To determine whether the PI3K pathway is involved in LMP1-induced HIF-1α expression, cells were exposed to wortmannin (Wort.), an inhibitor of PI3K. Wortmannin does not suppress LMP1-induced HIF-1α expression. (B) Effect of NF-κB and JNK signaling on LMP1-induced HIF-1α expression. Cotransfection of neither srIκBα nor JNK1-DN suppresses the induction of HIF-1α by LMP1, as shown by Western blotting. DMSO, dimethyl sulfoxide.
NF-κB and JNK signaling are not involved in LMP1-induced HIF-1α expression.
Recently, several reports have indicated a role for NF-κB signaling in HIF-1α induction (15, 26). Jung et al. showed that microtubule-depolymerizing agents induce HIF-1α at the transcriptional level, which depends on activation of NF-κB, in the A549 human lung cancer cell line (26). Figueroa et al. showed that NF-κB plays a key role in HIF-1α protein expression and HIF-1 activation (15). We checked the effect of NF-κB signaling on HIF-1α expression by cotransfecting LMP1 expression plasmid and srIκBα. Despite the importance of NF-κB in LMP1 signaling pathways, its suppression by srIκBα did not affect the LMP1-induced HIF-1α expression (Fig. 4B). Suppression of JNK signaling also did not affect the expression of HIF-1α protein (Fig. 4B).
LMP1-induced HIF-1 has binding activity on the VEGF promoter.
Since LMP1 induces HIF-1α protein expression localized in the nucleus (Fig. 1E), we next checked whether LMP1-induced HIF-1 could bind to the HRE of the VEGF promoter. When a 21-bp oligonucleotide probe containing the HIF-1-binding site from the VEGF promoter, WT VEGF (57), was incubated with nuclear extract from Ad-AH cells with or without LMP1 expression, complexes containing HIF-1 and a constitutively expressed factor(s) were detected in LMP1-expressing cells (Fig. 5A, lane 3). An excess of unlabeled oligonucleotide WT VEGF competed with the probe for binding of HIF-1 (Fig. 5A, lane 5), whereas an oligonucleotide containing a 3-bp substitution in the HIF-1 binding site (MT VEGF) did not compete for binding (Fig. 5A, lane 7). These results indicate that LMP1-induced HIF-1 is active and can bind to the VEGF promoter.
LMP1-induced HIF-1 enhances HRE and VEGF luciferase reporter activity.
As shown in Fig. 5C and D, LMP1 upregulates both HRE and VEGF luciferase reporter activities more than twofold. Low-grade upregulation is a common feature of VEGF luciferase reporter constructs (37). Having shown that the p42/p44 MAPK pathway and H2O2 activity are involved in LMP1-induced HIF-1α expression (Fig. 3A and C), we next treated cells with PD98059, an MEK inhibitor, or catalase, an H2O2 scavenger, to determine the role of these pathways in induction of VEGF by LMP1. PD98059 suppressed LMP1-induced HRE reporter activity by 70% and completely abolished VEGF reporter activity. In addition, catalase suppressed both the HRE and VEGF reporter activities almost to the basal level. These results indicate that LMP1-induced HIF-1α contributes to the transcriptional activation of the gene for VEGF through HIF-1 activation, which depends on p42/p44 MAPK and H2O2 activities. We also checked the secretion of VEGF in the culture medium. As noted before (40), LMP1 increased the level of VEGF protein in extracellular fluid (Fig. 5E). Furthermore, LMP1-induced VEGF was also suppressed both by PD98059 and catalase.
DISCUSSION
In addition to the classical hypoxia-cobalt-desferrioxamine-mediated induction of HIF-1α, a number of agonists such as insulin, insulin-like growth factor 1, angiotensin II, and the organomercurial compound mersalyl also induce the expression of this transcription factor (1, 13, 16, 27, 59). These studies suggest that while hypoxia remains the undisputed ubiquitous inducer of HIF-1, other factors can also modulate increases in HIF-1α protein levels under normoxic conditions. The significant findings from the present work can be briefly summarized as follows: (i) LMP1, the principal EBV oncoprotein, induces HIF-1α protein by increasing its synthesis without increasing its stability or its mRNA level; (ii) activation of the p42/p44 MAPK pathway and synthesis of H2O2 are related to HIF-1α induction by LMP1; and (iii) LMP1 induces expression of VEGF at the transcriptional level, at least in part by activating HIF-1.
Akt, a proto-oncogene, is a major downstream effector of growth factor signaling and has a wide array of progrowth and antiapoptotic effects when activated by growth factors through PI3K (28). Previous reports suggest that the PI3K/Akt pathway is central in the regulation of HIF-1 activity (24, 44, 71). Laughner et al. showed that overexpression of HER2 increases the rate of HIF-1α protein synthesis that is dependent on the activity of PI3K, Akt, and the downstream kinase, FKBP-rapamycin-associated protein, at the posttranscriptional level in mouse 3T3 cells (32). Zundel et al. demonstrated that the stabilization of the HIF-1α protein by hypoxia was prevented by overexpression of PTEN, whereas the activation of Akt was sufficient to promote HIF-1α stabilization under normoxia (71). In contrast, several reports demonstrated that the activation of PI3K/Akt by hypoxia is cell type specific and that the activity of PI3K is not sufficient for the activation of HIF-1, nor is it essential for its induction by hypoxia (2, 3). The role of the PI3K signaling pathway in influencing HIF-1α overexpression remains somewhat confusing and controversial. In our system, LMP1 did not induce PI3K activity. LMP1-induced HIF-1α expression was not affected by wortmannin, a pharmacological inhibitor of PI3K. These results suggest that the PI3K pathway is not involved in LMP1-induced HIF-1α induction.
Recent findings indicate that the p42/p44 MAPK pathway is involved in HIF-1 activation (38, 45). Richard et al. demonstrated that HIF-1α is phosphorylated by p42/p44 MAPK in vitro, not by p38 MAPK or JNK. They also indicated that activation of the p42/p44 MAPK pathway is sufficient to promote the transcriptional activity of HIF-1, which was abolished by the MEK inhibitor PD98059 (45). By use of an in vitro kinase assay, the C-terminal domain of HIF-1α, which contains the transactivation regions of the protein, was demonstrated to be phosphorylated directly by p44 MAPK (38). Moreover, one of the Kaposi's sarcoma-associated herpesvirus (KSHV) genes encoded by open reading frame 74, G protein-coupled receptor, upregulates HIF-1 activity through the p42/p44 and p38 MAPK pathways, which results in VEGF expression in normoxia (54). In these reports, there is no increase in the HIF-1α protein level, which indicates a mechanism different from induction of HIF-1α by LMP1, which induces both expression of HIF-1α protein and HIF-1 activity at least in part in a p42/p44 MAPK pathway-dependent manner. Here we showed that PD98059 only partially suppressed LMP1-induced HRE reporter activity, whereas the drug completely blocked both LMP1-induced VEGF reporter activity and VEGF secretion. These results suggest that p42/p44 MAPK signaling plays a central role in VEGF induction by LMP1 and that LMP1-induced HIF-1 activity is partially involved in VEGF induction by LMP1. Previously, we showed that LMP1 induces VEGF production in part by COX-2 expression, as shown by treatment of cells with NS398, a selective COX-2 inhibitor. However, residual VEGF production was detected despite this treatment (40). Possibly, HIF-1 activity and COX-2 contribute in an additive fashion to induction of VEGF by LMP1.
LMP1 interacts with tumor necrosis factor-associated factors and activates NF-κB signaling (39). Previously, we reported that LMP1 induced COX-2, which resulted in production of prostaglandin E2 (PGE2) via NF-κB signaling (40). Recently, involvement of NF-κB signaling in HIF-1α induction has been proposed (15, 25, 26). Microtubule-depolymerizing agents use an NF-κB-dependent pathway to stabilize HIF-1α protein (26). In contrast, Figueroa et al. proposed a novel role for NF-κB in the induction of HIF-1α at the transcriptional level under hypoxic conditions and reported that the PI3K pathway was involved in NF-κB transactivation (15). Fukuda et al. showed that in HCT116 cells, PGE2 induced HIF-1α protein synthesis dependent on p42/p44 MAPK, PI3K, and C-SRC activity (17). Liu et al. demonstrated that addition of PGE2 to PC-3ML human prostate cancer cells induced HIF-1α stabilization, which results from the promotion of HIF-1α translocation from the cytosol to the nucleus. The effects of PGE2 on HIF-1α were specifically inhibited by PD98059 (34). Jung et al. demonstrated that interleukin-1β (IL-1β)-mediated activation of NF-κB increases COX-2 protein in a lung epithelial cell line and a colon cell line (25). IL-1β-induced NF-κB activation is dependent on the upstream PI3K signaling pathway. PGE2, a major physiological product of COX-2, is also able to increase levels of HIF-1α protein, as a result of its increased stability (25).
It is not clear whether NF-κB and/or PGE2 enhance HIF-1α protein levels by promoting its stabilization or its synthesis. Although NF-κB signaling is involved in LMP1-induced COX-2 expression, the pathway is not involved in HIF-1α induction in our system because cotransfection of srIκΒα did not affect the level of HIF-1α. Although involvement of COX-2 in LMP1-induced HIF-1α expression is not excluded, NF-κB signaling might be only partially involved in LMP1-induced HIF-1α expression in Ad-AH cells. In our system, PI3K signaling was not induced by LMP1. It has been reported that tumor necrosis factor alpha signaling requires phosphorylation events, especially activation of PI3K on HIF-1α stabilization (47). Thus, PI3K signaling activity might be necessary in NF-κB-dependent HIF-1α expression. The influence of cross talk between NF-κB signaling and other signaling pathway needs to be elucidated.
ROS, such as H2O2, O2−, and OH•, are regulated in cells by several pathways. Electron transport through the mitochondrial respiratory chain is extraordinarily efficient, and normally most of the O2 is consumed. However, 1 to 2% of the electrons are leaked to generate O2− in reactions mediated by coenzyme Q and ubiquinone and its complex. Thus, mitochondria are believed to be a major site of ROS production in vivo (21). Another site of electron transport is the endoplasmic reticulum, where O2− is generated by the leakage of electrons from NADPH cytochrome P450 reductase. O2− is also generated by hypoxanthine/xanthine oxidase, lipoxygenase, or COX (21). Superoxide dismutase converts O2− into H2O2, and then the H2O2 generated is degraded to H2O by several cellular enzymes. ROS have also been implicated in HIF-1 activity (7, 49). Chandel et al. showed that hypoxia increases mitochondrial ROS generation at complex III and causes stabilization of HIF-1α protein and that nonmitochondrial ROS generation is involved in the response to CoCl2 (8). Angiotensin II increases HIF-1α translation by ROS-dependent activation of the PI3 kinase pathway that acts on the 5′ untranslated region of HIF-1α mRNA in vascular smooth muscle cells (44). Thioredoxin 1, a redox protein that undergoes reversible NADPH-dependent reduction, increases HIF-1α protein expression (63).
We showed through flow cytometry by the use of DCFH-DA that LMP1 increases ROS production. Also, catalase can completely inhibit the production of ROS by LMP1. Catalase is an antioxidant that acts as an H2O2 scavenger. These results suggest that ROS generated by LMP1 is mostly H2O2. Interestingly, induction of HIF-1α by LMP1 was dependent on H2O2 generation, since catalase completely inhibited induction of HIF-1α by LMP1. Catalase could suppress both HRE and VEGF reporter activities and finally modestly decrease induction of VEGF by LMP1. These results suggest that H2O2 induced by LMP1 plays an important role in LMP1-induced VEGF expression. The mechanism of LMP1-induced H2O2 generation needs investigation.
The mechanism of LMP1-induced HIF-1α expression needs further elucidation. As described above, LMP1-induced HIF-1α expression is p42/p44 MAPK and ROS dependent and PI3K independent. The effects of H2O2 on p42/p44 MAPK are controversial, with some reports showing inhibition and others demonstrating stimulation. Platelet-derived growth factor-induced H2O2 activates the p42/p44 MAPK pathway, which is inhibited by incubation with catalase (55). In neonatal rat ventricular myocytes and pulmonary arterial smooth muscle cells, the p42/p44 MAPK pathway is activated by H2O2 (9, 68). H2O2 activates p42/p44 MAPK in many cell types, although this activation appears to be cell type specific. Catalase almost completely blocked angiotensin II-induced H2O2 generation, which was not accompanied by inhibition of p42/p44 MAPK activation in vascular smooth muscle cells (59). Furthermore, it was also reported that H2O2 downregulated p42/p44 MAPK in human umbilical endothelial cells (33). In our system, LMP1-induced p42/p44 MAPK activation was not H2O2 dependent, because catalase, an H2O2-specific scavenger, did not affect p42/p44 MAPK activity. This result suggests that p42/p44 MAPK and H2O2 stimulate HIF-1α expression through two converging pathways in LMP1-transfected Ad-AH cells.
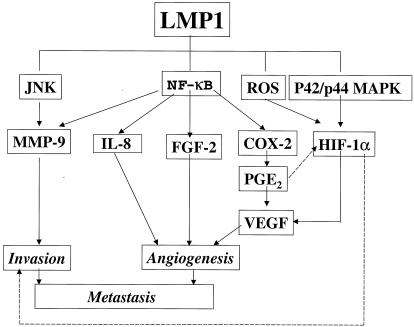
These results add to the emerging picture of the ability of the oncoprotein of a tumor virus to dictate phenotypic changes in tumor cells in addition to transforming cells (Fig. 6). Furthermore, these and other results suggest the biologic significance of our findings. Previously, we have shown that LMP1 induces expression of MMP-9 and in vitro invasiveness, which are suppressed by aspirin, an IκB kinase β inhibitor (41, 66). Furthermore, we have shown that LMP1 induces several angiogenic factors, FGF-2, IL-8, and VEGF (40, 60, 65). NF-κB signaling is involved in the induction of all of these factors by LMP1. Interestingly, we now find that VEGF is also induced by LMP1 through synthesis of HIF-1α, which uses a pathway distinct from NF-κB signaling. Moreover, we have demonstrated a significant correlation between expression of LMP1 and microvessel counts in NPC tissue (65). All of these findings indicate that LMP1 is likely to play a crucial role in tumor invasiveness and angiogenesis and in resultant metastasis. LMP1 may produce these effects on tumor cells in later stages of oncogenesis independently of an etiologic relation between the virus and the malignancy. LMP1 should be a major target for gene therapy designed to control invasion and metastasis, the lethal stage of LMP1-expressing tumors such as NPC.
FIG. 6.
EBV oncoprotein LMP1 induces cellular invasiveness and angiogenic factors. LMP1 induces MMP-9, IL-8, FGF-2, and COX-2 through NF-κB signaling (41, 60, 65, 66). LMP1 induces HIF-1α through the ROS and p42/p44 MAPK pathways. Thus, LMP1 induces invasion and angiogenesis factors and finally may promote tumor metastasis. Although induction of HIF-1α by PGE2 has been proposed, it was not clear in our system (17, 25, 35). Regulation of tumor invasiveness by HIF-1α is a topic of interest for future research (31).
Acknowledgments
We thank Kimryn Rathmell, Kyung-Lib Jang, Luwen Zhang, Leslie Huye, Wei Yue, and Shunbin Ning for critical comments and suggestions. We are also grateful to Albert Baldwin, Roger J. Davis, J. Silvio Gutkind, Jaques Pouyssegur, Gilles Pages, and Gregg L. Semenza for providing srIκBα, the FLAG-tagged JNK1 DN expression plasmid, HRE luciferase reporters, VEGF luciferase reporters, and pCEP4/HIF-1α3.2T7 and to Maria Masucci and Irene Joab for cell lines.
This work was supported by grant P01CA19014 from NCI.
REFERENCES
- 1.Agani, F., and G. L. Semenza. 1998. Mersalyl is a novel inducer of vascular endothelial growth factor gene expression and hypoxia-inducible factor 1 activity. Mol. Pharmacol. 54:749-754. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Tejado, M., A. Alfranca, J. Aragones, A. Vara, M. O. Landazuri, and L. Peso. 2002. Lack of evidence for the involvement of the phosphoinositide 3-kinase/Akt pathway in the activation of hypoxia-inducible factors by low oxygen tension. J. Biol. Chem. 277:13508-13517. [DOI] [PubMed] [Google Scholar]
- 3.Arsham, A. M., D. R. Plas, C. B. Thompson, and M. C. Simon. 2002. Phosphatidylinositol 3-kinase/Akt signaling is neither required for hypoxic stabilization of HIF-1α nor sufficient for HIF-1-dependent target gene transcription. J. Biol. Chem. 277:15162-15170. [DOI] [PubMed] [Google Scholar]
- 4.Bonnet, M., J. M. Guinebretiere, E. Kremmer, V. Grunewald, E. Benhamou, G. Contesso, and I. Joab. 1999. Detection of Epstein-Barr virus in invasive breast cancers. J. Natl. Cancer Inst. 91:1376-1381. [DOI] [PubMed] [Google Scholar]
- 5.Bruick, R. K., and S. L. McKnight. 2001. A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294:1337-1340. [DOI] [PubMed] [Google Scholar]
- 6.Carter, W. O., P. K. Narayanan, and J. P. Robinson. 1994. Intracellular hydrogen peroxide and superoxide anion detection in endothelial cells. J. Leukoc. Biol. 55:253-258. [DOI] [PubMed] [Google Scholar]
- 7.Chandel, N. S., E. Maltepe, E. Goldwasser, C. E. Mathieu, M. C. Simon, and P. T. Schumacker. 1998. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl. Acad. Sci. USA 95:11715-11720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandel, N. S., D. S. McClintock, C. E. Feliciano, T. M. Wood, J. A. Melendez, A. M. Rodriguez, and P. T. Schumacker. 2000. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1α during hypoxia: a mechanism of O2 sensing. J. Biol. Chem. 275:25130-25138. [DOI] [PubMed] [Google Scholar]
- 9.Clerk, A., A. Michael, and P. H. Sugden. 1998. Stimulation of multiple mitogen-activated protein kinase subfamilies by oxidative stress and phosphorylation of the small heat shock protein, HSP25/27, in neonatal ventricular myocytes. Biochem. J. 333:581-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cobb, M. H., and E. J. Goldsmith. 1995. How MAP kinases are regulated. J. Biol. Chem. 270:14843-14846. [DOI] [PubMed] [Google Scholar]
- 11.Dickens, M., J. S. Rogers, J. Cavanagh, A. Ratitano, Z. Xia, J. R. Halpern, M. E. Greenberg, C. L. Sawyers, and R. J. Davis. 1997. A cytoplasmic inhibitor of the JNK signal transduction pathway. Science 277:693-696. [DOI] [PubMed] [Google Scholar]
- 12.Elipoulos, A. G., N. J. Gallgher, S. M. S. Blake, C. W. Dawson, and L. S. Young. 1999. Activation of the p38 mitogen-activated protein kinase pathway by Epstein-Barr virus-encoded latent membrane protein 1 coregulates interleukin-6 and interleukin-8 production. J. Biol. Chem. 274:16085-16096. [DOI] [PubMed] [Google Scholar]
- 13.Feldser, D., F. Agani, N. V. Iyer, B. Pak, B. Ferreira, and G. L. Semenza. 1999. Reciprocal positive regulation of hypoxia-inducible factor 1α and insulin-like growth factor 2. Cancer Res. 59:3915-3918. [PubMed] [Google Scholar]
- 14.Fennewald, S., V. Santen, and E. Kieff. 1984. Nucleotide sequence of an mRNA transcribed in latent growth-transforming virus infection indicates that it may encode a membrane protein. J. Virol. 51:411-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Figueroa, Y. G., A. K. Chan, R. Ibrahim, Y. Tang, M. E. Burrow, J. Alam, and B. S. Scandurro. 2002. NF-κB plays a key role in hypoxia-inducible factor-1-regulated erythropoietin gene expression. Exp. Hematol. 30:1419-1427. [DOI] [PubMed] [Google Scholar]
- 16.Fukuda, R., K. Hirota, F. Fan, Y. D. Jung, L. M. Ellis, and G. L. Semenza. 2002. Insulin-like growth factor 1 induces vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J. Biol. Chem. 277:38205-38211. [DOI] [PubMed] [Google Scholar]
- 17.Fukuda, R., B. Kelly, and G. L. Semenza. 2003. Vascular endothelial growth factor gene expression in colon cancer cells exposed to prostaglandin E2 is mediated by hypoxia-inducible factor 1. Cancer Res. 63:2330-2334. [PubMed] [Google Scholar]
- 17a.Hur, E., K. Y. Chang, S. Lee, and H. Park. 2001. Mitogen-activated protein kinase kinase inhibitor PD98059 blocks the trans-activation but not not the stabilization or DNA binding ability of hypoxia-inducible factor 1α. Mol. Pharmacol. 59:1216-1224. [DOI] [PubMed] [Google Scholar]
- 18.Imai, S., J. Nishikawa, and K. Takada. 1998. Cell to cell contact as efficient mode of Epstein-Barr virus infection of diverse human epithelial cells. J. Virol. 72:4371-4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Izumi, K. M., and E. D. Kieff. 1997. The Epstein-Barr virus oncogene product latent membrane protein 1 engages the tumor necrosis factor receptor-associated death domain protein to mediate B lymphocyte growth transformation and activate NF-κB. Proc. Natl. Acad. Sci. USA 94:12592-12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaakkola, P., D. R. Mole, Y. M. Tian, M. I. Wilson, J. Gielbert, S. J. Gaskell, A. Kriegsheim, H. F. Hebestreit, M. Mukherji, C. J. Schofield, P. H. Maxwell, C. W. Pugh, and P. J. Ratcliffe. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292:468-472. [DOI] [PubMed]
- 21.Jackson, M. J., S. Papa, J. Bolanos, R. Bruckdorfer, H. Carlsen, R. M. Elliott, J. Flier, H. R. Griffiths, S. Heales, B. Holst, M. Lorusso, E. Lund, J. O. Moskaug, U. Moser, M. D. Paola, M. C. Polidori, A. Signorile, W. Stahl, J. Vina-Ribes, and S. B. Astley. 2002. Antioxidants, reactive oxygen and nitrogen species, gene induction and mitochondrial function. Mol. Aspects Med. 23:209-285. [DOI] [PubMed] [Google Scholar]
- 22.Jiang, B. H., F. Agani, A. Passaniti, and G. L. Semenza. 1997. V-SRC induces expression of hypoxia-inducible factor 1 (HIF-1) and transcription of genes encoding vascular endothelial growth factor and enolase 1: involvement of HIF-1 in tumor progression. Cancer Res. 57:5328-5335. [PubMed] [Google Scholar]
- 23.Jiang, B. H., G. L. Semenza, C. Bauer, and H. H. Marti. 1996. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am. J. Physiol. 271:C1172-C1180. [DOI] [PubMed] [Google Scholar]
- 24.Jiang, B. H., J. Z. Zheng, M. Aoki, and P. K. Vogt. 2000. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc. Natl. Acad. Sci. USA 97:1749-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Juang, Y. J., J. S. Isaacs, S. Lee, J. Trepel, and L. Neckers. 2003. IL-1β-mediated up-regulation of HIF-1α via an NFκB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 17:2115-2117. [DOI] [PubMed] [Google Scholar]
- 26.Juang, Y. J., J. S. Isaacs, S. Lee, J. Trepel, and L. Neckers. 2002. Microtubule disruption utilizes an NFκΒ-dependent pathway to stabilize HIF-1α protein. J. Biol. Chem. 278:7445-7452. [DOI] [PubMed] [Google Scholar]
- 27.Kallio, P. J., W. J. Wilson, S. O'Brien, Y. Makino, and L. Poellinger. 1999. Regulation of the hypoxia-inducible transcription factor 1α by the ubiquitin-proteasome pathway. J. Biol. Chem. 274:6519-6525. [DOI] [PubMed] [Google Scholar]
- 28.Kandel, E. S., and N. Hay. 1999. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp. Cell Res. 253:210-229. [DOI] [PubMed] [Google Scholar]
- 29.Kieser, A., E. Kilger, O. Gires, M. Ueffing, W. Kolch, and W. Hammerschmidt. 1997. Epstein-Barr virus latent membrane protein-1 triggers AP-1 activity via the c-Jun N-terminal kinase cascade. EMBO J. 16:6478-6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimura, H., A. Weisz, Y. Kurashima, K. Hashimoto, T. Ogura, F. D'Acquisto, R. Addeo, M. Makuuchi, and H. Esumi. 2000. Hypoxia response element of the human vascular endothelial growth factor gene mediates transcriptional regulation by nitric oxide: control of hypoxia-inducible factor-1 activity by nitric oxide. Blood 95:189-197. [PubMed] [Google Scholar]
- 31.Krishnamachary, B., S. Berg-Dixon, B. Kelly, F. Agani, D. Feldser, G. Ferreira, N. Iyer, J. LaRusch, B. Pak, P. Taghavi, and G. L. Semenza. 2003. Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res. 63:1138-1143. [PubMed] [Google Scholar]
- 32.Laughner, E., P. Taghavi, K. Chiles, P. C. Mahon, and G. L. Semenza. 2001. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1α (HIF-1α) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol. Cell. Biol. 21:3995-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee, Y. J., I. J. Kang, R. Bunger, and Y. H. Kang. 2004. Enhanced survival effect of pyruvate correlates MAPK and NF-κB activation in hydrogen peroxide-treated human endothelial cells. J. Appl. Physiol. 96:793-801. [DOI] [PubMed] [Google Scholar]
- 34.Liu, X. H., A. Kirschenbaum, M. Lu, S. Yao, A. Dosoretz, J. F. Holland, and A. C. Levine. 2002. Prostaglandin E2 induces hypoxia-inducible factor-1α stabilization and nuclear localization in a human prostate cancer cell line. J. Biol. Chem. 277:50081-50086. [DOI] [PubMed] [Google Scholar]
- 35.Maxwell, P. H., M. S. Wiesener, G. W. Chang, S. C. Clifford, E. C. Vaux, M. E. Cockman, C. C. Wykoff, C. W. Pugh, E. R. Maher, and P. J. Ratcliffe. 1999. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399:271-275. [DOI] [PubMed] [Google Scholar]
- 36.Milanini, J., F. Vinals, J. Pouyssegur, and G. Pages. 1998. p42/p44 MAP kinase module plays a key role in the transcriptional regulation of the vascular endothelial growth factor gene in fibroblasts. J. Biol. Chem. 273:18165-18172. [DOI] [PubMed] [Google Scholar]
- 37.Miller, W. E., H. S. Earp, and N. Raab-Traub. 1995. The Epstein-Barr virus latent membrane protein 1 induces expression of the epidermal growth factor receptor. J. Virol. 69:4390-4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minet, E., G. Arnould, I. Michel, D. Roland, M. Mottet, J. Raes, R. Remacle, and C. Michels. 2000. ERK activation upon hypoxia: involvement in HIF-1 activation. FEBS Lett. 468:53-58. [DOI] [PubMed] [Google Scholar]
- 39.Mosialos, G., M. Birkenbach, R. Yalamanchili, T. VanArsdale, C. Ware, and E. Kieff. 1995. The Epstein-Barr virus transforming protein LMP1 engages signaling proteins for the tumor necrosis factor receptor family. Cell 80:389-399. [DOI] [PubMed] [Google Scholar]
- 40.Murono, S., H. Inoue, T. Tanabe, I. Joab, T. Yoshizaki, M. Furukawa, and J. S. Pagano. 2001. Induction of cyclooxygenase-2 by Epstein-Barr virus latent membrane protein 1 is involved in vascular endothelial growth factor production in nasopharyngeal carcinoma cells. Proc. Natl. Acad. Sci. USA 98:6905-6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murono, S., T. Yoshizaki, H. Sato, M. Furukawa, and J. S. Pagano. 2000. Aspirin inhibits tumor cell invasiveness induced by Epstein-Barr virus latent membrane protein 1 through suppression of matrix metalloproteinase-9 expression. Cancer Res. 60:3621-3626. [PubMed] [Google Scholar]
- 42.Narravula, S., and S. P. Colgan. 2001. Hypoxia-inducible factor 1-mediated inhibition of peroxisome proliferator-activated receptor alpha expression during hypoxia. J. Immunol. 166:7543-7548. [DOI] [PubMed] [Google Scholar]
- 43.Pagano, J. S. 1999. Epstein-Barr virus: the first human tumor virus and its role in cancer. Proc. Assoc. Am. Phys. 111:573-580. [DOI] [PubMed] [Google Scholar]
- 44.Page, E. L., G. A. Robitaille, J. Pouyssegur, and D. E. Richard. 2002. Induction of hypoxia-inducible factor-1α by transcriptional and translational mechanisms. J. Biol. Chem. 277:48403-48409. [DOI] [PubMed] [Google Scholar]
- 45.Richard, D. E., E. Berra, E. Gothie, D. Roux, and J. Pouyssegur. 1999. p42/p44 mitogen-activated protein kinases phosphorylate hypoxia-inducible factor 1α (HIF-1α) and enhance the transcriptional activity of HIF-1. J. Biol. Chem. 274:32631-32637. [DOI] [PubMed] [Google Scholar]
- 46.Roberts, M. L., and N. R. Cooper. 1998. Activation of a ras-MAPK-dependent pathway by Epstein-Barr virus latent membrane protein 1 is essential for cellular transformation. Virology 240:93-99. [DOI] [PubMed] [Google Scholar]
- 47.Sandau, K. B., J. Zhou, T. Kietzmann, and B. Brune. 2001. Regulation of hypoxia-inducible factor 1α by the inflammatory mediators nitric oxide and tumor necrosis factor-α in contrast to desferroxamine and phenylarsine oxide. J. Biol. Chem. 276:39805-39811. [DOI] [PubMed] [Google Scholar]
- 48.Semenza, G. L. 1999. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu. Rev. Cell Dev. Biol. 15:551-578. [DOI] [PubMed] [Google Scholar]
- 49.Semenza, G. L. 2000. Expression of hypoxia-inducible factor 1: mechanisms and consequences. Biochem. Pharmacol. 59:47-53. [DOI] [PubMed] [Google Scholar]
- 50.Semenza, G. L. 2000. Hypoxia, clonal selection, and the role of HIF-1 in tumor progression. Crit. Rev. Biochem. Mol. Biol. 35:71-103. [DOI] [PubMed] [Google Scholar]
- 51.Semenza, G. L. 2001. HIF-1, O2, and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell 107:1-3. [DOI] [PubMed] [Google Scholar]
- 52.Semenza, G. L. 2002. Signal transduction to hypoxia-inducible factor 1. Biochem. Pharmacol. 64:993-998. [DOI] [PubMed] [Google Scholar]
- 53.Shimizu, N., H. Yoshiyama, and K. Takada. 1996. Clonal propagation of Epstein-Barr virus (EBV) recombinants in EBV-negative Akata cells. J. Virol. 70:7260-7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sodhi, A., S. Montaner, V. Patel, M. Zohar, C. Bais, E. A. Mesri, and J. S. Gutkind. 2000. The Kaposi's sarcoma-associated herpes virus G protein-coupled receptor up-regulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1α. Cancer Res. 60:4873-4880. [PubMed] [Google Scholar]
- 55.Sundaresan, M., Y. Zu-Xi, V. J. Ferrans, K. Irani, and T. Finkel. 1995. Requirement for generation of HO for platelet-derived growth factor signal transduction. Science 270:296-299. [DOI] [PubMed] [Google Scholar]
- 56.Sutter, C. H., E. Laughner, and G. L. Semenza. 2000. Hypoxia-inducible factor 1α protein expression is controlled by oxygen-regulated ubiquitination that is disrupted by deletions and missense mutations. Proc. Natl. Acad. Sci. USA 97:4748-4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suzuki, Y. J., H. J. Forman, and A. Sevanian. 1997. Oxidants as stimulators of signal transduction. Free Radic. Biol. Med. 22:269-285. [DOI] [PubMed] [Google Scholar]
- 58.Takimoto, T., M. Furukawa, M. Hatano, and R. Umeda. 1984. Epstein-Barr virus nuclear antigen-positive nasopharyngeal hybrid cells. Ann. Otol. Rhinol. Laryngol. 93:166-169. [DOI] [PubMed] [Google Scholar]
- 59.Ushio-Fukai, M., R. W. Alexander, M. Akers, and K. K. Griendling. 1998. p38 MAP kinase is a critical component of the redox-sensitive signaling pathways by angiotensin II: role in vascular smooth muscle cell hypertrophy. J. Biol. Chem. 273:15022-15029. [DOI] [PubMed] [Google Scholar]
- 60.Wakisaka, N., S. Murono, T. Yoshizaki, M. Furukawa, and J. S. Pagano. 2002. Epstein-Barr virus latent membrane protein 1 induces and causes release of fibroblast growth factor-2. Cancer Res. 62:6337-6344. [PubMed] [Google Scholar]
- 61.Wang, D., D. Liebowitz, and E. Kieff. 1985. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell 43:831-840. [DOI] [PubMed] [Google Scholar]
- 62.Wang, G. L., B. H. Jiang, E. A. Rue, and G. L. Semenza. 1995. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 92:5510-5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Welsh, S. J., W. T. Bellamy, M. M. Briehl, and G. Powis. 2002. The redox protein thioredoxin-1 (Trx-1) increases hypoxia-inducible factor 1α protein expression: Trx-1 overexpression results in increased vascular endothelial growth factor production and enhanced tumor angiogenesis. Cancer Res. 62:5089-5095. [PubMed] [Google Scholar]
- 64.Yen, C. H., C. C. Hsieh, S. Y. Chou, and Y. T. Lau. 2001. 17β-Estradiol inhibits oxidized low density lipoprotein-induced generation of reactive oxygen species in endothelial cells. Life Sci. 70:403-413. [DOI] [PubMed] [Google Scholar]
- 65.Yoshizaki, T., T. Horikawa, R. Qing-chun, N. Wakisaka, H. Takeshita, T. S. Sheen, S. Y. Lee, H. Sato, and M. Furukawa. 2001. Induction of interleukin-8 by Epstein-Barr virus latent membrane protein-1 and its correlation to angiogenesis in nasopharyngeal carcinoma. Clin. Cancer Res. 7:1946-1951. [PubMed] [Google Scholar]
- 66.Yoshizaki, T., H. Sato, M. Furukawa, and J. S. Pagano. 1998. The expression of matrix metalloproteinase 9 is enhanced by Epstein-Barr virus latent membrane protein 1. Proc. Natl. Acad. Sci. USA 95:3621-3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu, F., S. B. White, Q. Zhao, and F. S. Lee. 2001. HIF-1α binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc. Natl. Acad. Sci. USA 98:9630-9635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang, J., N. Jin, Y. Liu, and R. A. Rhoades. 1998. Hydrogen peroxide stimulates signal-regulated protein kinases in pulmonary arterial smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 19:324-332. [DOI] [PubMed] [Google Scholar]
- 69.Zhang, L., and J. S. Pagano. 1999. Interferon regulatory factor 2 represses the Epstein-Barr virus BamHI Q latency promoter in type III latency. Mol. Cell. Biol. 19:3216-3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhong, H., D. Chiles, D. Feldser, E. Laughner, C. Hanrahan, M. M. Georgescu, J. W. Simons, and G. L. Semenza. 2000. Modulation of hypoxia-inducible factor 1α expression by the epidermal growth factor/phosphatidylinositol-3 kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 60:1541-1545. [PubMed] [Google Scholar]
- 71.Zundel, W., C. Schindler, D. Haas-Kogan, A. Koong, F. Kaper, E. Chen, A. R. Gottschalk, H. E. Ryan, R. S. Johnson, A. B. Jefferson, D. Stokoe, and A. J. Giaccia. 2000. Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev. 14:391-396. [PMC free article] [PubMed] [Google Scholar]