Abstract
Glucose homeostasis is controlled in part by regulation of glucose uptake into muscle and adipose tissue. Intracellular membrane vesicles containing the GLUT4 glucose transporter move towards the cell cortex in response to insulin and then fuse with the plasma membrane. Here we show that the fusion step is retarded by the inhibition of phosphatidylinositol (PI) 3-kinase. Treatment of insulin-stimulated 3T3-L1 adipocytes with the PI 3-kinase inhibitor LY294002 causes the accumulation of GLUT4-containing vesicles just beneath the cell surface. This accumulation of GLUT4-containing vesicles near the plasma membrane prior to fusion requires an intact cytoskeletal network and the unconventional myosin motor Myo1c. Remarkably, enhanced Myo1c expression under these conditions causes extensive membrane ruffling and overrides the block in membrane fusion caused by LY294002, restoring the display of GLUT4 on the cell exterior. Ultrafast microscopic analysis revealed that insulin treatment leads to the mobilization of GLUT4-containing vesicles to these regions of Myo1c-induced membrane ruffles. Thus, localized membrane remodeling driven by the Myo1c motor appears to facilitate the fusion of exocytic GLUT4-containing vesicles with the adipocyte plasma membrane.
The plasma membranes of living cells undergo constant recycling through the dynamic processes of membrane retrieval and membrane insertion. Through these endocytic and exocytic events, which include intermediate membrane transport pathways, the relative abundance of specific plasma membrane components and the secretion of molecules can be regulated. Complex mechanisms involving protein-protein interactions have evolved to coordinate such membrane trafficking processes to ensure that transported membranes are appropriately targeted to their specific destinations (36). These mechanisms include directed movements on cytoskeletal tracks (39) and the concerted actions of tethering proteins that anchor transport vesicles to cognate target membranes (27). The membrane fusion step in exocytosis is highly dependent on proteins that join membranes in close proximity such that two separate lipid bilayers merge into one (16). One class of proteins that function in this fusion step are the SNAREs (35), of which the synaptic vesicle proteins synaptobrevin/VAMP (15) and syntaxin 1 (1), as well as SNAP-25 (13) on the plasma membrane, are best characterized. Although SNAREs are clearly important for fusion, they may not be involved in directly executing the fusion reaction. The hypothesis that several other cofactors are necessary to complete fusion is supported by the observation that at least under some specialized conditions the deficiencies of certain SNAREs do not prevent fusion (5, 9, 32, 42). Furthermore, specialized exocytic systems such as those in neuronal synapses contain unique proteins that facilitate targeting or regulation of membrane fusion (28). Thus, the molecular details of membrane fusion processes remain an active area of investigation.
Among a number of newly discovered proteins that have been implicated in the membrane targeting and fusion processes are the SM family of proteins (38), the septins (12), and RIM (40) and associated proteins. A recent report has also suggested the involvement of a class V myosin, myo52, in fission yeast in mediating vacuole fusion under osmotic stress, providing the first link between an actin-based motor and homotypic membrane fusion (23). This suggestion is particularly interesting in light of findings in our laboratory that a myosin I family member (Myo1c) is required for optimal insulin-stimulated translocation of intracellular membranes containing GLUT4 glucose transporters to the plasma membrane (3). In this membrane trafficking system, GLUT4 recycles between intracellular and plasma membrane compartments and insulin acutely stimulates GLUT4 exocytosis through a phosphatidylinositol (PI) 3-kinase-dependent pathway (6, 19, 21, 24, 34). The detailed mechanism by which GLUT4-containing membranes fuse with the plasma membrane requires interaction between syntaxin 4 (t-SNARE) (41) and VAMP-2 (v-SNARE) (7). However, it is not known which components or processes that function in the GLUT4 recycling pathway are directly downstream of PI 3-kinase signaling or require the myosin Myo1c.
The aim of the present studies was to characterize the role of Myo1c in the trafficking pathway of GLUT4-containing membranes and its relationship to PI 3-kinase-sensitive steps. Previous work had shown that the expression of high levels of Myo1c in cultured adipocytes enhances the extent to which GLUT4 is translocated to the plasma membrane in response to insulin (3). In other recent studies, PI 3-kinase signaling was implicated in the fusion step of exocytosis of GLUT4-containing membranes (25). Consistent with this hypothesis, we report here that the blockade of PI 3-kinase inhibits the fusion of GLUT4-containing membrane vesicles with the plasma membrane and causes the accumulation of these vesicles just beneath the cell surface. Remarkably, high expression of Myo1c could override the block in membrane fusion caused by PI 3-kinase inhibition when insulin is also present. These data suggest that Myo1c drives a process that promotes the fusion of GLUT4-containing vesicles with the plasma membrane.
MATERIALS AND METHODS
Materials and chemicals.
LY294002 was purchased from BIOMOL. Mouse anti-Myc (clone 9E10) monoclonal antibody was purchased from Neomarkers Inc. Rhodamine-labeled goat anti-mouse antibodies and BODIPY 581/591 were purchased from Molecular Probes.
DNA constructs.
The construction of Myc-GLUT4-GFP, Myc-GLUT4-CFP, and YFP-Myo1c has been described previously (3, 17). The YFP-Myo1c(T) plasmid was constructed by subcloning a Myo1c coding sequence encompassing residues 767 to 1028, amplified by PCR, into the BamHI and XhoI restriction sites of pEYFPC1 in frame with yellow fluorescent protein (YFP). The construct was sequenced, and its expression was verified in COS-1 cells prior to experiments with 3T3-L1 adipocytes.
Cell culture, cell treatments, and transfection of differentiated 3T3-L1 adipocytes.
3T3-L1 fibroblasts were grown to confluence and differentiated as described previously (3). Differentiated adipocytes were transfected on the fifth day postdifferentiation by electroporation as described previously. The cells were then allowed to recover for 24 h before serum starvation in Dulbecco modified Eagle medium plus 0.5% bovine serum albumin for 5 h and then treated with the reagents described in the figure legends. Cells were pretreated with either 5 μM latrunculin and 50 μM colchicine for 1 h or 100 μM LY294002 for 15 min prior to stimulation with 100 nM insulin for 30 min.
Myc-GLUT4-CFP internalization assay.
Differentiated 3T3-L1 adipocytes were electroporated either with Myc-GLUT4-CFP alone or with YFP-Myo1c, as indicated. After 24 h, cells were stimulated with insulin for 1 h to allow the Myc-GLUT4-CFP to translocate to the cell surface. Cells were then washed twice with ice-cold phosphate-buffered saline, incubated at 4°C with monoclonal anti-Myc antibodies for 1 h, washed twice with ice-cold phosphate-buffered saline, and warmed to 37°C for the indicated times in order to allow the Myc-GLUT4-CFP that had translocated to the cell surface in presence of insulin to internalize. Cells were then fixed and permeabilized, and the Myc-GLUT4-CFP-expressing cells were detected by staining with rhodamine-conjugated anti-mouse secondary antibodies.
Live-cell imaging.
In live-cell imaging experiments, adipocytes were seeded in 35-mm-diameter plastic tissue culture dishes with glass coverslip bottoms (MatTek). Images of fluorescently labeled live cells were obtained with an IX 70 inverted microscope (Olympus) with a ×100 NA 1.4 objective lens, a Coolsnap HQ (Roper Scientific) digital camera, and an excitation filter wheel and shutter (Sutter) in the epifluorescence light path and an emission filter wheel (Sutter) in the imaging light path. Metamorph Image acquisition and analysis software (Universal Imaging) controlled the hardware and acquired the data. Time lapse images were taken every 5 s for 10 to 15 min by using rhodamine and YFP filters (Chroma). For Fig. 5, the images were deblurred by using the Metamorph no-neighbors algorithm, in which images are reblurred by convolution with the microscope point spread function and a fraction of the reblurred image is subtracted from the original blurred image. The microscope point spread function was calculated from the values of the numerical aperture of the objective lens, the wavelength of the fluorescence, and the size of the image pixels.
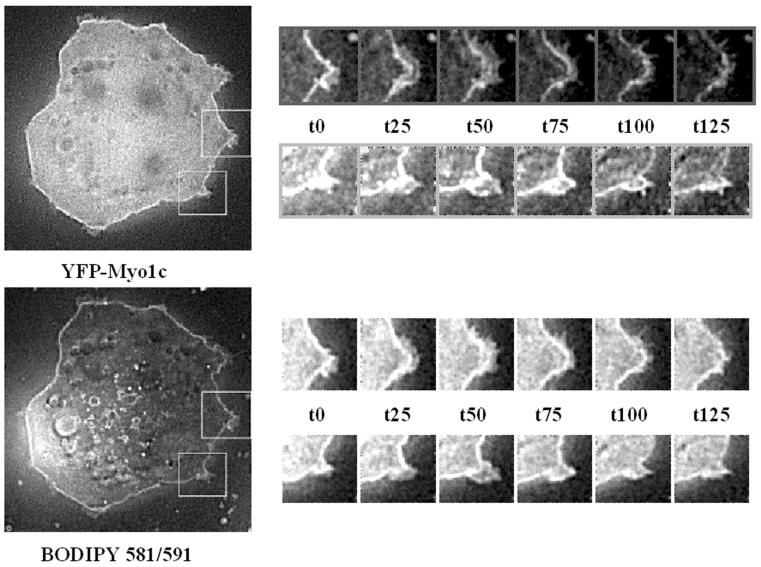
FIG. 5.
Myo1c expression in 3T3-L1 adipocytes causes insulin-independent membrane ruffles. Differentiated 3T3-L1 adipocytes expressing YFP-Myo1c were serum starved. BODIPY 581/591 was added to the media at a final concentration of 1 μM, and the cells were incubated for 20 min at 37°C. Membrane ruffling was observed by monitoring the cells live for 10 min, imaging them at 5-s intervals. Shown are six frames, each 25 s apart, for both BODIPY 581/591 stain and YFP-Myo1c. The images are all single optical sections from three-dimensional images following image restoration.
Ultrafast microscopy.
Differentiated 3T3-L1 adipocytes expressing YFP-Myo1c and Myc-GLUT4-CFP and were imaged 24 h after transfection by using high-speed, three-dimensional microscopy (8, 26, 30, 43). Cyan fluorescent protein (CFP) and YFP donor-acceptor pairs were imaged by using a Coherent Argon laser tuned to 458 nm, a wavelength at which both fluorophores were simultaneously excited. The 128- by 128-pixel camera field was split into two subfields of 128 by 60 pixels by using a dual-view module (Optical Insights, Santa Fe, N.M.). The donor and acceptor fields were filtered by 480/30 and 535/40 emission filters, respectively. This optical configuration insured true simultaneity of donor-acceptor excitation and emission. Two-dimensional images of both CFP and YFP were acquired by using exposure times of 5 ms. Each three-dimensional image set consisted of 21 optical sections spaced 250 nm apart, with an additional 20 ms being allowed for changing focus to the next optical section. A three-dimensional image set was acquired in less than 600 ms. In this manner, three-dimensional images were acquired every 10 s for 1,000 continuous seconds (16.7 min).
The images were first corrected for camera dark current. Next, since both fluorophores were simultaneously excited by the 458-nm laser line, cells expressing CFP only and cells expressing only YFP were imaged to determine both the relative alignment of the CFP and YFP subfields and the percentages of YFP fluorescence in the CFP channel and of CFP fluorescence in the YFP channel (data not shown). YFP bleed-through into the CFP image was negligible. CFP bleed-through into the spatially corresponding YFP pixel (after realignment) was determined as follows: YFP = YFP − (1.09 × CFP). Finally, the haze originating from light sources outside the in-focus plane of the cell was reduced by image restoration (7). The microscope point spread function was empirically determined by imaging 190-nm fluorescent beads with the exact same optical configuration used for the CFP-YFP data.
For analysis of the concentration of plasma membrane-associated GLUT4 in ruffling versus nonruffling areas shown in Fig. 6, a three-dimensional time series image set of Myc-GLUT4-CFP and YFP-Myo1c was first subjected to intensity thresholding. Separate thresholds for the Myc-GLUT4-CFP and YFP-Myo1c images were chosen such that 95% of the cytosolic pixels were excluded in both cases. Then, the average fluorescence intensity of the above-threshold pixels in the GLUT4 image series in regions with and without apparent ruffling was computed for each time point. The selected intensity threshold value was halved and doubled to recalculate the GLUT4-CFP fluorescence in order to investigate the sensitivity of the analysis (data not shown). The results were essentially the same.
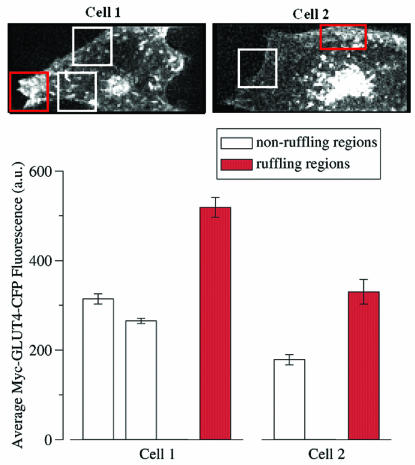
FIG. 6.
Ultrafast microscopy reveals enrichment of GLUT4 and Myo1c in ruffling areas compared to nonruffling areas. (Top) Three-dimensional projection images of two different cells, showing Myc-GLUT4-CFP distribution. The images shown were selected from a sequence of 100 such three-dimensional images of each cell spanning 16.7 min (see Materials and Methods). The three-dimensional images were first subjected to image restoration to remove out-of-focus light, and then maximum-intensity projections were made by retaining the maximum (brightest) intensity at each pixel position of the 21 optical sections. Regions of active membrane ruffling (red boxes) were identified visually and compared with regions not showing significant ruffling (white boxes). See the supplemental material for complete movie sequences. (Bottom) In order to separate cytosolic from membrane-associated Myc-GLUT4-CFP fluorescence, an intensity threshold was chosen for each cell such that 95% of the Myc-GLUT4-CFP pixels in the cytosol were below the threshold. Then, for each region of each cell, the average Myc-GLUT4-CFP fluorescence of just the pixels whose intensities were above the threshold and within the indicated region was computed for each time point. The mean and standard deviation of the average Myc-GLUT4-CFP concentration for all 100 time points were calculated and plotted as shown.
RESULTS AND DISCUSSION
PI 3-kinase is required for fusion of GLUT4-containing vesicles with the plasma membrane.
To differentiate between GLUT4 vesicles that translocate to the cell periphery in response to insulin and those that actually fuse with the plasma membrane, we employed a GLUT4 construct (Myc-GLUT4-EGFP) containing a Myc epitope in an exofacial loop of the transporter and a green fluorescent protein (GFP) fusion at its cytoplasmic COOH terminus (17). The fusion of intracellular vesicles containing this construct with the plasma membrane exposes the Myc epitope to reaction with Myc antibody added to intact adipocytes. As shown in Fig. 1, little or no Myc antibody was detected on the cell surfaces of unstimulated 3T3-L1 adipocytes expressing this Myc-GLUT4-EGFP protein. Insulin treatment of these cells acutely translocates intracellular Myc-GLUT4-EGFP to the cell surface, as evidenced by the rim of GFP signal around the cell periphery and the striking anti-Myc staining of the cell surface. Consistent with previous studies (33), the anti-Myc signal is markedly reduced when 3T3-L1 adipocytes are treated with the PI 3-kinase inhibitor LY294002 prior to stimulation with insulin, indicating that PI 3-kinase is required for this insulin effect. However, upon careful examination, we noted that in many of these cells treated with LY294002 and insulin, a significant rim of GFP signal could be observed in spite of the absence of anti-Myc staining (Fig. 1A and B) (33). This rim was not observed in cells treated with the inhibitor alone. These results are consistent with previous data suggesting that GLUT4-containing vesicles can translocate to the cell periphery in response to insulin through movements on cytoskeletal tracks even in the absence of PI 3-kinase activity (33). Thus, fusion of the vesicles with the plasma membrane is apparently inhibited by LY294002.
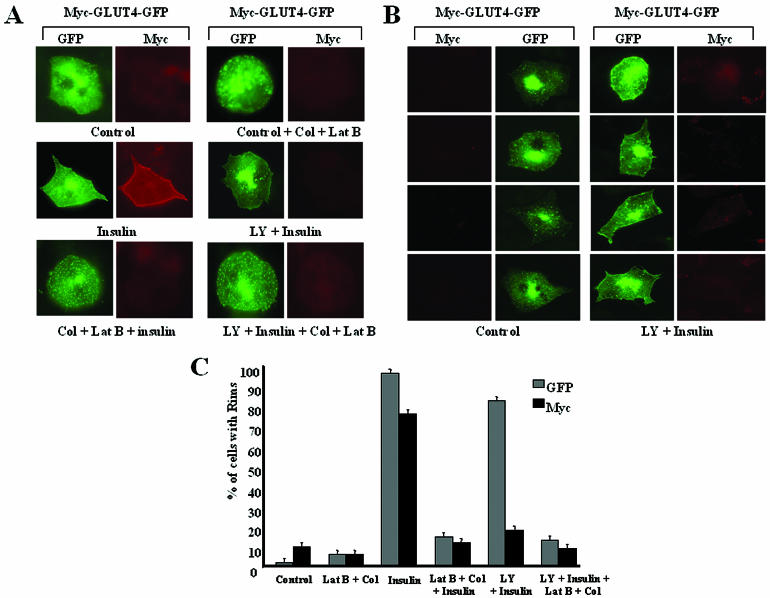
FIG. 1.
PI 3-kinase inhibitor LY294002 blocks insulin-stimulated fusion of GLUT4-containing vesicles with the plasma membrane. (A) Differentiated 3T3-L1 adipocytes expressing Myc-GLUT4-GFP were serum starved and were then either left untreated (Control), treated with colchicine and latrunculin B (Control + Col + Lat B), stimulated with insulin and treated with LY294002 followed by insulin (LY + Insulin), treated with colchicine and latrunculin B followed by insulin (Col + Lat B + insulin), or treated with LY 294002 followed by insulin for 30 min and then with colchicine and latrunculin B for 1 h (LY + Insulin + Col + Lat B). The cells were then fixed and stained with anti-Myc followed by rhodamine-labeled secondary antibody. (B) Four fields of differentiated 3T3-L1 adipocytes expressing Myc-GLUT4-GFP under serum starvation or after treatment with LY294002 (LY) and insulin from the experiment presented in panel A are shown. (C) Cells treated with the reagents mentioned above for panel A were counted for both GFP and Myc rims. At least 200 cells for each condition were counted and scored blindly for GFP and Myc rims. The images were all taken at the same exposure. The percentages of cells with GFP and Myc rims are shown. The results reflect averages for three identical experiments.
Based on these data, we directly tested whether the appearance of GLUT4-containing vesicles at the cell periphery was influenced by the disassembly of actin filaments and microtubules by using the inhibitors latrunculin B and colchicine, respectively. Figure 1A shows marked dispersion of GLUT4 from the perinuclear region of a typical adipocyte in response to these inhibitors, as well as their ability to decrease the amount of GLUT4 just beneath the plasma membrane in cells treated with LY294002 and insulin. As previously reported (33), latrunculin B plus colchicine also dramatically inhibited the display of the Myc epitope (from Myc-GLUT4-EGFP) on the cell surface in response to insulin (Fig. 1A). These data are quantified in Fig. 1C and show that over 80% of cells exhibit GFP rims in the presence of the PI 3-kinase inhibitor plus insulin, whereas less than 20% display such rims when the cytoskeleton is disrupted. Taken together, these data suggest that insulin treatment of differentiated 3T3-L1 adipocytes leads to the recruitment of GLUT4-containing vesicles from the intracellular storage compartment to the cell periphery. This translocation is microtubule and F-actin dependent but is independent of PI 3-kinase activity. Once these vesicles reach the cortical region of the cell, they apparently undergo fusion by a process that requires PI 3-kinase activity.
Myo1c is required for accumulation of GLUT4-containing vesicles at the cell cortex.
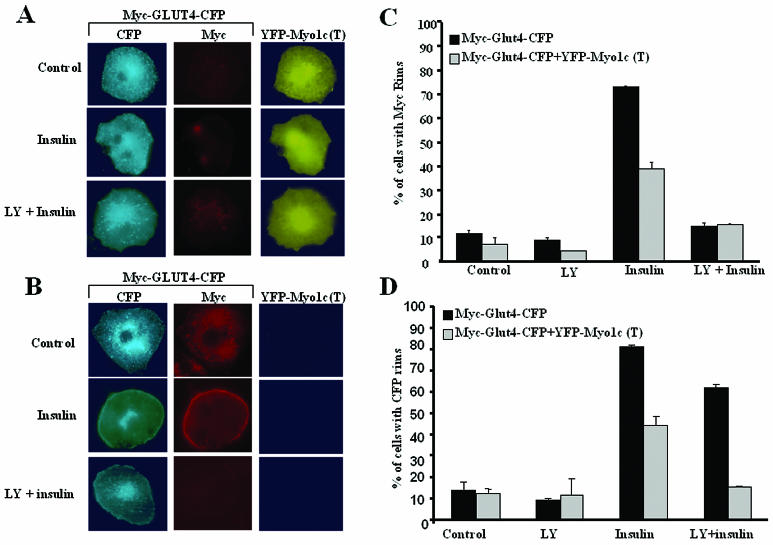
Since pretreatment with LY294002 prior to insulin stimulation results in the accumulation of GLUT4-containing vesicles, presumably anchored to actin filaments, near the cortical region of the cell, we sought to test whether Myo1c is required for this accumulation. A dominant-negative Myo1c(T) construct was expressed in cells that were also expressing Myc-GLUT4-CFP. This Myo1c(T) truncated mutant has the ability to bind cargo but is missing the actin binding region and the motor domain (29). As expected, the expression of Myo1c(T) significantly inhibited insulin-stimulated GLUT4 translocation to the cell surface, as assayed by counting cells with anti-Myc rims both in the presence and in the absence of PI 3-kinase activity (Fig. 2A and C). We did not observe complete inhibition of insulin-stimulated GLUT4 translocation in all of the cells expressing Myo1c(T), presumably because of the inherent variability in the expression of the dominant-negative Myo1c. As observed before, cells expressing only Myc-GLUT4-CFP displayed CFP signal around the rims of the cells when pretreated with the PI 3-kinase inhibitor LY294002 in the presence of insulin (Fig. 2B and C). However, this cortical accumulation of GLUT4 was significantly inhibited in cells expressing the dominant-negative isoform of Myo1c (Fig. 2A and D). These results suggest that upon insulin stimulation, the movement of GLUT4-containing vesicles to the cortical region of the cell or their anchoring to the actin cytoskeleton prior to fusion requires Myo1c.
FIG. 2.
Myo1c is required to localize insulin-stimulated GLUT4-containing vesicles close to the plasma membrane. (A) Differentiated 3T3-L1 adipocytes expressing Myc-GLUT4-CFP and YFP-Myo1c(T) were serum starved and either left untreated (Control), treated with insulin (Insulin), or treated with LY294002 followed by insulin (LY + Insulin). The cells were then fixed and stained with anti-Myc followed by rhodamine-labeled secondary antibody. Cells expressing both YFP-Myo1c(T) and Myc-GLUT4-CFP are shown. (B) Cells expressing only Myc-GLUT4-CFP. (C) Cells from panels A and B were counted for Myc rims at the cell surfaces. (D) Cells from panels A and B were counted for CFP signals at the cell surfaces. More than 100 cells were scored blindly for Myc and CFP rims. The data represent averages for three similar experiments.
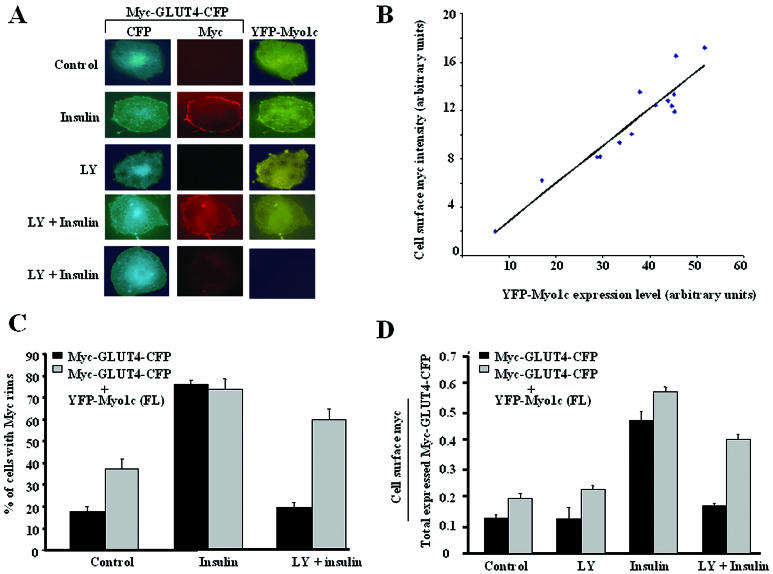
Myo1c expression overrides the LY294002-mediated block in fusion of GLUT4-containing vesicles with the plasma membrane.
We have recently reported that high expression of Myo1c augments the insulin-stimulated appearance of GLUT4 on the plasma membrane of differentiated 3T3-L1 adipocytes (3). Data presented here (Fig. 1) indicate that PI 3-kinase regulates the fusion of GLUT4-containing vesicles with the plasma membrane. To test whether Myo1c function can influence the block in fusion caused by the PI 3-kinase inhibitor LY294002, we coexpressed YFP-tagged Myo1c with Myc-GLUT4-CFP in cultured adipocytes. In the absence of insulin, very little GLUT4 was displayed on the cell surface in the presence or absence of LY294002, as assayed by cell surface anti-Myc signal. After insulin stimulation, about 80% of these intact cells displayed anti-Myc binding at the cell surface, signifying that much of the GLUT4 at the cell surface was in the plasma membrane. Interestingly, the amount of Myc displayed at the cell surface correlated directly with the amount of YFP-Myo1c expression in these cells (Fig. 3B). When these cells were treated with LY294002 prior to insulin stimulation, most of the cells expressing YFP-Myo1c displayed cell surface anti-Myc staining, compared to few of the cells expressing Myc-GLUT4-CFP but not YFP-Myo1c (Fig. 3A and C and Fig. SA3 in the supplemental material). Quantification of anti-Myc binding at the cell surface normalized to the expression of Myc-GLUT4-CFP in these YFP-Myo1c-expressing cells revealed about 60% of cells having anti-Myc rims, compared to about 20% in cells not expressing YFP-Myo1c (Fig. 3D). These data indicate that Myo1c can function to reverse the block in membrane fusion caused by the blockade of PI 3-kinase activity (Fig. 3C and D).
FIG. 3.
Myo1c expression partially overcomes the LY294002-induced block in fusion of GLUT4-containing vesicles. (A) Differentiated 3T3-L1 adipocytes expressing Myc-GLUT4-CFP and YFP-Myo1c were serum starved and then either left untreated (Control), treated with insulin (Insulin), or treated with LY294002 followed by insulin (LY + Insulin). The top four rows of panels show cells expressing both Myc-GLUT4-CFP and YFP-Myo1c, and the fifth row of panels show a cell expressing only Myc-GLUT4-CFP. (B) Differentiated 3T3-L1 adipocytes expressing Myc-GLUT4-CFP and YFP-Myo1c were stimulated with insulin. The cell surface Myc signal (rhodamine) intensity in these cells was quantitated and compared with the YFP-Myo1c signal intensity in these cells. (C) Cells shown in panel A were counted for anti-Myc rims at the cell surfaces. More than 100 cells for each condition were scored blindly for Myc rims. (D) Quantification of cell surface anti-Myc signal (rhodamine) intensity in the cells shown in panel A. The arbitrary unit represents the ratio of the cell surface anti-Myc signal to the total CFP signal in each cell. The data represent averages for five similar experiments.
One interpretation of these findings is that Myo1c potentiates fusion by delivering an increased number of exocytic GLUT4-containing vesicles to fusion sites, thus increasing the number of possible fusions per unit time. This would tend to increase the number of fusion events even when fusion is partially inhibited by LY294002. This explanation is supported by the fact that there was a small but significant increase in the number of fused GLUT4-containing vesicles under basal conditions when these cells expressed Myo1c. According to this model, in the presence of insulin there are more vesicles present at the cell periphery and hence the effect of the expression of Myo1c under these conditions is more pronounced. Another possibility is that Myo1c actually functions in the molecular mechanism of membrane fusion. Opposing these interpretations is a potential negative effect Myo1c expression could have in reducing the endocytosis of GLUT4 containing vesicles, thereby indirectly increasing the number of these vesicles at the cell surface.
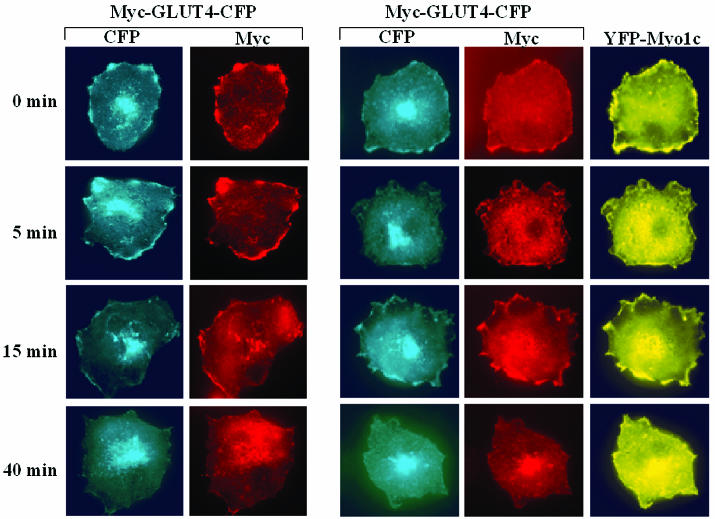
In order to distinguish between the effects of Myo1c on exocytosis and those on endocytosis, we monitored Myc-GLUT4-CFP internalization from the cell surface in cultured adipocytes expressing high levels of YFP-Myo1c and in those with only endogenous Myo1c. In the experiment depicted in Fig. 4, adipocytes were initially stimulated with insulin for 30 min to allow Myc-GLUT4-CFP to translocate to the cell surfaces. The cells were then washed to remove insulin, labeled with anti-Myc antibody at 4°C, warmed at 37°C to allow Myc-GLUT4-CFP internalization, and then fixed at various time points. To assess Myc-GLUT4-CFP endocytosis, the cells were then permeabilized and stained with secondary antibody labeled with rhodamine. After 5 min of incubation at 37°C, very little anti-Myc signal was detected in the cytoplasm of cells expressing Myc-GLUT4-CFP with or without YFP-Myo1c, with most of the anti-Myc being displayed at the cell surface. Anti-Myc could be detected within these cells with increasing perinuclear localization after 15- and 40-min incubations, however, and a marked decrease of anti-Myc signal at the cell surface was observed at these times. However, the amounts of internalized anti-Myc in cells expressing both Myc-GLUT4-CFP and YFP-Myo1c and in cells expressing only Myc-GLUT4-CFP were similar (Fig. 4). Although the experiment shown in Fig. 4 is an accepted method for analyzing GLUT4 endocytosis, the result obtained 5 min after the removal of insulin represents the best estimate of endocytosis, while results obtained after longer times reflect the net results of both endocytosis and exocytosis. In absence of insulin, the exocytic rate is rather low and is presumed not to influence this assay. These results, combined with the data presented in Fig. 3, are consistent with the hypothesis that Myo1c potentiates insulin-stimulated Myc-GLUT4-CFP displayed on the cell surface by increasing the exocytic process rather than inhibiting endocytosis. Although these experiments indicate that Myo1c affects the exocytosis of GLUT4-containing vesicles, it is not yet clear whether these effects of Myo1c are specific for GLUT4-containing vesicles.
FIG. 4.
Myo1c expression does not disrupt Myc-GLUT4-CFP internalization to the perinuclear regions of 3T3-L1 adipocytes. Differentiated 3T3-L1 adipocytes either transfected with Myc-GLUT4-CFP alone or cotransfected with Myc-GLUT4-CFP and YFP-Myo1c were stimulated with insulin for 30 min. The cells were then labeled with anti-Myc antibodies and then warmed to 37°C for the indicated times to allow the Myc-GLUT4-CFP to undergo endocytosis. The cells were then permeabilized and then stained with rhodamine-conjugated secondary antibody to detect total Myc-GLUT4-CFP. The data are representative of three similar experiments.
Myo1c expression in differentiated 3T3-L1 adipocytes induces active membrane ruffling.
An interesting observation made in the experiments depicted in Fig. 3 and 4 was that the expression of YFP-Myo1c plus Myc-GLUT4-CFP in differentiated 3T3-L1 adipocytes induced dramatic membrane ruffling. Insulin is also known to stimulate actin reorganization and the formation of lamellipodia or membrane ruffles in several cell types, including 3T3-L1 adipocytes (22), rat1 fibroblasts (10), CHO-T cells (20), and L6 myotubes (37). Actin polymerization has been postulated to be a plausible mechanism for driving lamellipodia and membrane protrusions (2). An alternative mechanism whereby unconventional myosin is responsible for driving protrusions, with polymerized actin providing the substrate on which force is exerted, has been proposed (2, 11). In order to better assess Myo1c function in membrane ruffling, we expressed YFP-Myo1c in differentiated 3T3-L1 adipocytes and observed these cells by using live-cell fluorescence microscopy. Cultured adipocytes expressing YFP-Myo1c showed dramatic insulin-independent membrane ruffling (Fig. 5 and accompanying movies in Fig. SA1 in the supplemental material), compared to little or no ruffling in untransfected control adipocytes, as has been observed previously by other groups (18). Cells with high YFP-Myo1c expression did not exhibit increased cortical F-actin, as detected by phalloidin staining (data not shown). Insulin also causes membrane ruffling in untransfected cells but not to the extent seen in YFP-Myo1c-expressing adipocytes (data not shown). Adipocytes expressing YFP-Myo1c were also stained with the intensely fluorescent β-BODIPY 581/591 C5-HPC [4,4-difluoro-5-(4-phenyl-1,3-buta-dienyl)-4-bora-3a,4a-diaza-s-indacene-3-pentanoyl)-1-hexa-decanoyl-sn-glycero-3-phosphocholine], which is commonly used as a plasma membrane probe. Several areas of these cells displayed continuous ruffles, while the other regions of the cell surface remained unruffled. Interestingly, the YFP-Myo1c concentrations in active regions of ruffling membranes were significantly higher than those in neighboring regions which displayed little or no ruffling (Fig. 5). In a few cases, we observed areas of the plasma membrane with significant Myo1c concentrations but which were not ruffling during the period of observation. However, we never observed a membrane ruffle in a region without significant Myo1c concentrated in that region. These data indicate that in differentiated 3T3-L1 adipocytes, high levels of Myo1c are sufficient to cause extensive membrane ruffling without evidence of increased actin polymerization.
GLUT4-containing vesicles concentrate in areas of extensive membrane ruffling prior to fusion.
Taken together, the data described above indicate that Myo1c functions to promote membrane ruffling as well as the fusion of GLUT4-containing vesicles with the plasma membrane in the presence of insulin (Fig. 1 to 5). One interpretation of these findings is that membrane ruffles and the F-actin that is beneath these ruffles may play a role in concentrating GLUT4-containing vesicles prior to their fusion with the plasma membrane. This hypothesis has previously been suggested based on experiments with cultured L6 muscle cells (31, 37). In order to test whether GLUT4-containing vesicles are recruited to the regions of membrane ruffling upon insulin stimulation, we monitored the movement of Myc-GLUT4-CFP in cells also expressing YFP-Myo1c by using ultrafast microscopy. Three-dimensional images of these cells were acquired every 10 s for 1,000 continuous seconds, and each set of three-dimensional images consisted of 21 optical sections spaced 250 nm apart, thereby accounting for the entire volume of the cell. Figure 6 and the accompanying movie in Fig. SA2 in the supplemental material show two differentiated 3T3-L1 adipocytes expressing both Myc-GLUT4-CFP and YFP-Myo1c 10 min after insulin stimulation. Figure 6 (top panel) shows the three-dimensional projections encompassing the 21 optical sections of these cells following image restoration to remove out-of-focus light at one given time point in the movie. The average Myc-CFP-GLUT4 fluorescence intensity within regions of the cell adjacent to membrane ruffles was then computed. This computation was done by first visually identifying regions in these cells which were ruffling and neighboring nonruffling regions in the same cells. The average Myc-GLUT4-CFP intensities in the three-dimensional volume near the ruffling and nonruffling areas of the same cell at each time point were then measured over the entire length of the movie (1,000 s). The mean and standard deviation of this Myc-GLUT4-CFP fluorescence intensity in each of these three-dimensional regions of the cell were then plotted.
Based on the data presented in Fig. 6 and in Fig. SA2 in the supplemental material, we can draw two conclusions. First, Myo1c was consistently enriched in regions of active membrane ruffles compared to Myo1c in neighboring nonruffling domains. Second, Myc-GLUT4-CFP vesicles were also predominantly recruited into these cytoplasmic regions of active membrane reorganizations. As is evident from Fig. 6, the regions of the cell near membrane ruffles were highly enriched in both Myc-GLUT4-CFP and YFP-Myo1c (also see the accompanying movies Fig. SA2 in the supplemental material). This aggregation of Myc-GLUT4-CFP vesicles near membrane ruffles was not observed in the absence of insulin, consistent with the hypothesis that these ruffles are enriched in exocytic Myc-GLUT4-CFP vesicles. As shown in Fig. 5, YFP-Myo1c was also predominantly concentrated in these ruffles. These data are consistent with the hypothesis that insulin stimulation of cultured adipocytes results in the recruitment of exocytic GLUT4-containing vesicles to regions of Myo1c-driven membrane ruffling, where they undergo fusion.
Like other regulated exocytic pathways, insulin-stimulated GLUT4 vesicle trafficking involves budding from an internal storage compartment and translocation to the cell periphery, followed by fusion. Insulin-stimulated GLUT4 translocation to the cell surface probably involves microtubules aided by kinesin motors (14, 33). Results presented here confirm that the cytoskeleton is necessary for the translocation and that PI 3-kinase activity is not needed for this process. Our present and previous results indicate that, once GLUT4 reaches the cell periphery, the GLUT4-containing vesicles associate with actin filaments. A novel finding presented here is the requirement of Myo1c in this step, which apparently involves GLUT4 mobilization and anchoring to the cell cortex (Fig. 2). Although direct evidence is lacking, it is possible that the Myo1c cargo domain binds to a putative receptor on the GLUT4-containing vesicles, thereby tethering the vesicle to actin filaments. This process is apparently followed by the fusion of these vesicles with the plasma membrane, which, as we have shown, requires PI 3-kinase activity (Fig. 1). Importantly, the expression of Myo1c can partially overcome the block in fusion in the absence of PI 3-kinase activity (Fig. 3). Coupled with the previously reported observation that the expression of Myo1c potentiates GLUT4 translocation and fusion, these data suggest that Myo1c may be involved in the fusion process.
The hypothesis that Myo1c may directly participate in the mechanism of membrane fusion is further highlighted by the dramatic membrane ruffling induced by Myo1c in cultured adipocytes (Fig. 5). Insulin treatment of these cells results in the recruitment of exocytic GLUT4-containing vesicles to these sites of membrane ruffles (Fig. 6). A recent report suggests that exocytic vesicles are directed to membrane ruffles prior to fusion with the plasma membrane (4). Our data thus suggest that, in cultured adipocytes, Myo1c is responsible for localized membrane remodeling, which facilitates the fusion of GLUT4-containing vesicles with the plasma membrane in the presence of insulin. Future experiments are needed to directly test this hypothesis.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants DK063023 to M.P.C. and DK58133 to S.C.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Bennett, M. K., J. E. Garcia-Arraras, L. A. Elferink, K. Peterson, A. M. Fleming, C. D. Hazuka, and R. H. Scheller. 1993. The syntaxin family of vesicular transport receptors. Cell 74:863-873. [DOI] [PubMed] [Google Scholar]
- 2.Borisy, G. G., and T. M. Svitkina. 2000. Actin machinery: pushing the envelope. Curr. Opin. Cell Biol. 12:104-112. [DOI] [PubMed] [Google Scholar]
- 3.Bose, A., A. Guilherme, S. I. Robida, S. M. C. Nicoloro, Q. L. Zhou, Z. Y. Jiang, D. P. Pomerleau, and M. P. Czech. 2002. Glucose transporter recycling in response to insulin is facilitated by myosin Myo1c. Nature 420:821-824. [DOI] [PubMed] [Google Scholar]
- 4.Bretscher, M. S., and C. Aguado-Velasco. 1998. EGF induces recycling membrane to form ruffles. Curr. Biol. 8:721-724. [DOI] [PubMed] [Google Scholar]
- 5.Broadie, K., A. Prokop, H. J. Bellen, C. J. O'Kane, K. L. Schulze, and S. T. Sweeney. 1995. Syntaxin and synaptobrevin function downstream of vesicle docking in Drosophila. Neuron 15:663-673. [DOI] [PubMed] [Google Scholar]
- 6.Bryant, N. J., R. Govers, and D. E. James. 2002. Regulated transport of the glucose transporter GLUT4. Nat. Rev. Mol. Cell Biol. 3:267-277. [DOI] [PubMed] [Google Scholar]
- 7.Cain, C. C., W. S. Trimble, and G. E. Lienhard. 1992. Members of the VAMP family of synaptic vesicle proteins are components of glucose transporter-containing vesicles from rat adipocytes. J. Biol. Chem. 267:11681-11684. [PubMed] [Google Scholar]
- 8.Carrington, W. A., R. M. Lynch, E. D. Moore, G. Isenberg, K. E. Fogarty, and F. S. Fay. 1995. Superresolution three-dimensional images of fluorescence in cells with minimal light exposure. Science 268:1483-1487. [DOI] [PubMed] [Google Scholar]
- 9.Deitcher, D. L., A. Ueda, B. A. Stewart, R. W. Burgess, Y. Kidokoro, and T. L. Schwarz. 1998. Distinct requirements for evoked and spontaneous release of neurotransmitter are revealed by mutations in the Drosophila gene neuronal-synaptobrevin. J. Neurosci. 18:2028-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong, L. Q., L. R. Landa, M. J. Wick, L. Zhu, H. Mukai, Y. Ono, and F. Liu. 2000. Phosphorylation of protein kinase N by phosphoinositide-dependent protein kinase-1 mediates insulin signals to the actin cytoskeleton. Proc. Natl. Acad. Sci. USA 97:5089-5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evangelista, M., B. M. Klebl, A. H. Tong, B. A. Webb, T. Leeuw, E. Leberer, M. Whiteway, D. Y. Thomas, and C. Boone. 2000. A role for myosin-I in actin assembly through interactions with Vrp1p, Bee1p, and the Arp2/3 complex. J. Cell Biol. 148:353-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faty, M., M. Fink, and Y. Barral. 2002. Septins: a ring to part mother and daughter. Curr. Genet. 41:123-131. [DOI] [PubMed] [Google Scholar]
- 13.Gerst, J. E. 1999. SNAREs and SNARE regulators in membrane fusion and exocytosis. Cell. Mol. Life Sci. 55:707-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imamura, T., J. Huang, I. Usui, H. Satoh, J. Bever, and J. M. Olefsky. 2003. Insulin-induced GLUT4 translocation involves protein kinase C-lambda-mediated functional coupling between Rab4 and the motor protein kinesin. Mol. Cell. Biol. 23:4892-4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jahn, R., and T. C. Sudhof. 1999. Membrane fusion and exocytosis. Annu. Rev. Biochem. 68:863-911. [DOI] [PubMed] [Google Scholar]
- 16.Jahn, R., T. Lang, and T. C. Sudhof. 2003. Membrane fusion. Cell 112:519-533. [DOI] [PubMed] [Google Scholar]
- 17.Jiang, Z. Y., A. Chawla, A. Bose, M. Way, and M. P. Czech. 2002. A phosphatidylinositol 3-kinase-independent insulin signaling pathway to N-WASP/Arp2/3/F-actin required for GLUT4 glucose transporter recycling. J. Biol. Chem. 277:509-515. [DOI] [PubMed] [Google Scholar]
- 18.Kanzaki, M., and J. E. Pessin. 2001. Insulin-stimulated GLUT4 translocation in adipocytes is dependent upon cortical actin remodeling. J. Biol. Chem. 276:42436-42444. [DOI] [PubMed] [Google Scholar]
- 19.Karylowski, O., A. Zeigerer, A. Cohen, and T. E. McGraw. 2004. GLUT4 is retained by an intracellular cycle of vesicle formation and fusion with endosomes. Mol. Biol. Cell 15:870-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotani, K., K. Hara, K. Kotani, K. Yonezawa, and M. Kasuga. 1995. Phosphoinositide 3-kinase as an upstream regulator of the small GTP-binding protein Rac in the insulin signaling of membrane ruffling. Biochem. Biophys. Res. Commun. 208:985-990. [DOI] [PubMed] [Google Scholar]
- 21.Lampson, M. A., A. Racz, S. W. Cushman, and T. E. McGraw. 2000. Demonstration of insulin-responsive trafficking of GLUT4 and vpTR in fibroblasts. J. Cell Sci. 113:4065-4076. [DOI] [PubMed] [Google Scholar]
- 22.Martin, S. S., T. Haruta, A. J. Morris, A. Klippel, L. T. Williams, and J. M. Olefsky. 1996. Activated phosphatidylinositol 3-kinase is sufficient to mediate actin rearrangement and GLUT4 translocation in 3T3-L1 adipocytes. J. Biol. Chem. 271:17605-17608. [DOI] [PubMed] [Google Scholar]
- 23.Mulvihill, D. P., P. J. Pollard, T. Z. Win, and J. S. Hyams. 2001. Myosin V-mediated vacuole distribution and fusion in fission yeast. Curr. Biol. 11:1124-1127. [DOI] [PubMed] [Google Scholar]
- 24.Okada, T., Y. Kawano, T. Sakakibara, O. Hazeki, and M. Ui. 1994. Essential role of phosphatidylinositol 3-kinase in insulin-induced glucose transport and antilipolysis in rat adipocytes. Studies with a selective inhibitor wortmannin. J. Biol. Chem. 269:3568-3573. [PubMed] [Google Scholar]
- 25.Patel, N., A. Rudich, Z. A. Khayat, R. Garg, and A. Klip. 2003. Intracellular segregation of phosphatidylinositol-3,4,5-trisphosphate by insulin-dependent actin remodeling in L6 skeletal muscle cells. Mol. Cell. Biol. 23:4611-4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patki, V., J. Buxton, A. Chawla, L. Lifshitz, K. Fogarty, W. Carrington, R. Tuft, and S. Corvera. 2001. Insulin action on GLUT4 traffic visualized in single 3T3-l1 adipocytes by using ultra-fast microscopy. Mol. Biol. Cell 12:129-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfeffer, S. 2001. Vesicle tethering factors united. Mol. Cell 8:729-730. [DOI] [PubMed] [Google Scholar]
- 28.Pfeffer, S. R. 1999. Transport-vesicle targeting: tethers before SNAREs. Nat. Cell Biol. 1:E17-E22. [DOI] [PubMed] [Google Scholar]
- 29.Reizes, O., B. Barylko, C. Li, T. C. Sudhof, and J. P. Albanesi. 1994. Domain structure of a mammalian myosin I beta. Proc. Natl. Acad. Sci. USA 91:6349-6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rizzuto, R., W. Carrington, and R. A. Tuft. 1998. Digital imaging microscopy of living cells. Trends Cell Biol. 8:288-292. [DOI] [PubMed] [Google Scholar]
- 31.Rudich, A., and A. Klip. 2003. Push/pull mechanisms of GLUT4 traffic in muscle cells. Acta Physiol. Scand. 178:297-308. [DOI] [PubMed] [Google Scholar]
- 32.Saitoe, M., T. L. Schwarz, J. A. Umbach, C. B. Gundersen, and Y. Kidokoro. 2001. Absence of junctional glutamate receptor clusters in Drosophila mutants lacking spontaneous transmitter release. Science 293:514-517. [DOI] [PubMed] [Google Scholar]
- 33.Semiz, S., J. G. Park, S. M. C. Nicoloro, P. Furcinitti, C. Zhang, A. Chawla, J. Leszyk, and M. P. Czech. 2003. Conventional kinesin KIF5B mediates insulin-stimulated GLUT4 movements on microtubules. EMBO J. 22:2387-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simpson, F., J. P. Whitehead, and D. E. James. 2001. GLUT4—at the cross roads between membrane trafficking and signal transduction. Traffic 2:2-11. [DOI] [PubMed] [Google Scholar]
- 35.Sollner, T. H. 2003. Regulated exocytosis and SNARE function. Mol. Membr. Biol. 20:209-220. [DOI] [PubMed] [Google Scholar]
- 36.Sollner, T. H., and J. E. Rothman. 1996. Molecular machinery mediating vesicle budding, docking and fusion. Experientia 52:1021-1025. [DOI] [PubMed] [Google Scholar]
- 37.Tong, P., Z. A. Khayat, C. Huang, N. Patel, A. Ueyama, and A. Klip. 2001. Insulin-induced cortical actin remodeling promotes GLUT4 insertion at muscle cell membrane ruffles. J. Clin. Investig. 108:371-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toonen, R. F., and M. Verhage. 2003. Vesicle trafficking: pleasure and pain from SM genes. Trends Cell Biol. 13:177-186. [DOI] [PubMed] [Google Scholar]
- 39.Vale, R. D. 2003. The molecular motor toolbox for intracellular transport. Cell 112:467-480. [DOI] [PubMed] [Google Scholar]
- 40.Wang, Y., S. Sugita, and T. C. Sudhof. 2000. The RIM/NIM family of neuronal C2 domain proteins. Interactions with Rab3 and a new class of Src homology 3 domain proteins. J. Biol. Chem. 275:20033-20044. [DOI] [PubMed] [Google Scholar]
- 41.Yang, C., K. J. Coker, J. K. Kim, S. Mora, D. C. Thurmond, A. C. Davis, B. Yang, R. A. Williamson, G. I. Shulman, and J. E. Pessin. 2001. Syntaxin 4 heterozygous knockout mice develop muscle insulin resistance. J. Clin. Investig. 107:1311-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshihara, M., A. Ueda, D. Zhang, D. L. Deitcher, T. L. Schwarz, and Y. Kidokoro. 1999. Selective effects of neuronal-synaptobrevin mutations on transmitter release evoked by sustained versus transient Ca2+ increases and by cAMP. J. Neurosci. 19:2432-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Young, A., R. Tuft, W. Carrington, and S. J. Doxsey. 1999. Centrosome dynamics in living cells. Methods Cell Biol. 58:223-238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.