Abstract
Human paraoxonase 1 (PON-1) is a serum high-density lipoprotein-associated enzyme mainly secreted by the liver. It has endogenous and exogenous substrates and displays protective properties with respect to cardiovascular disease and organophosphate intoxication. In the HuH7 human hepatoma cell line, PON-1 activity and mRNA levels were increased by dietary polyphenolic compounds such as quercetin but also by toxic ligands of the aryl hydrocarbon receptor (AhR) such as 3-methylcholanthrene (3-MC). However, the 2,3,7,8-tetrachlorobenzo(p)dioxin (TCDD) was a poor inducer. Transient and stable transfection assays indicated that these compounds increased the PON-1 gene promoter activity in an AhR-dependent manner, since their effect was inhibited by 7-keto-cholesterol and AhR-directed short interfering RNA. Deletions and mutations studies showed that a xenobiotic responsive element (XRE)-like sequence within the PON-1 promoter mediated the effect of 3-MC and quercetin. In contrast with consensus XREs from the cytochrome P450 1A1 gene, the PON-1 XRE-like element mediated preferentially the effect of quercetin compared to the results seen with TCDD. Furthermore, AhR binding to this element was preferentially activated by quercetin. These observations provide a molecular mechanism for the regulation of the cardioprotective enzyme PON-1 by polyphenols. They suggest also that AhR ligands may differentially regulate gene expression depending on the DNA target sequence.
Paraoxonase 1 (PON-1) is a high-density lipoprotein (HDL)-associated serum enzyme mainly secreted by the liver (13). It was initially characterized by its ability to hydrolyze organophosphates (OPs) such as paraoxon but was later shown to hydrolyze a number of exogenous and endogenous compounds. This enzyme is generally believed to display protective properties with respect to OP intoxication and cardiovascular disease (CVD).
OPs are commonly used insecticides (parathion, chlopyrifos) and are also chemical warfare agents (sarin, soman). Because of its ability to hydrolyze and hence to detoxify a large number of OPs (12), PON-1 seems to play an important role in OP intoxication sensitivity (8, 25). PON-1-deficient mice have an increased sensitivity to OPs, whereas serum enzyme injection or exogenous overexpression of PON-1 by an adenoviral vector reduces OP toxicity in mice (11, 40).
PON-1 has also been shown to protect against CVD: (i) it prevents the formation of oxidized HDLs and low-density lipoproteins (LDLs) (6); (ii) it hydrolyses the thiolactone form of homocysteine, which alters proteins in the arterial wall (22); and (iii) it hydrolyses platelet-activating factor, a bioactive phospholipid which is involved in vascular disease development (36). Several studies suggest that a low-level plasma PON-1 activity is associated with increased prevalence of atherosclerosis and could be an independent risk factor for coronary events (24). PON-1-deficient mice are more susceptible to lipoprotein oxidation and atherosclerosis (40), while transgenic mice overexpressing PON-1 display decreased atherosclerotic lesions (42).
The PON-1 gene displays polymorphisms in the coding sequence which affect the catalytic activity of PON-1 and in the promoter region which affect the levels of the PON-1 gene expression (10). The PON-1 status is determined by the association of these polymorphisms and the factors modulating serum PON-1 activity. This status has been linked to individual sensitivity to OP toxicity and to susceptibility to CVD (10). Since nutritional and environmental factors likely explain some of the individual variation in serum PON-1 activity levels, this enzyme can be considered a promising target for pharmaceutical drugs (10, 15). Therefore, pharmacological modulation of PON-1 activity or PON-1 gene expression could constitute a useful approach for the prevention of CVD and OP intoxication.
Currently, little is know about PON-1 gene regulation. Regarding pharmacological regulation, the PON-1 gene was recently shown to be regulated in vitro and in vivo by fenofibrate and statins (14, 19, 26). Inflammatory conditions appear to decrease PON-1 gene expression in vitro (16, 43). Serum PON-1 activity also depends on a number of physiological and pathological conditions: low levels of serum activity have been found in cases of renal disease, diabetes mellitus, HDL deficiencies, and liver cirrhosis (10). High-fat diets were also shown to decrease PON-1 activity, while daily moderate alcohol consumption increased it (10). Among dietary or lifestyle factors, wine consumption and some polyphenols present in wine or fruit juice increase serum PON-1 activity in humans and mice (15, 18).
Increased PON-1 activity following consumption of polyphenol-rich diets is consistent with the beneficial cardiovascular effects of such diets. It was suggested that polyphenols could act through their antioxidant properties (PON-1 activity is known to be inhibited by oxidative stress) (5). However, it is unclear whether other antioxidants (vitamins) display similar effects on PON-1 activity (26). Therefore, polyphenols could also act through other mechanisms that were not elucidated.
In addition to their antioxidant properties, polyphenols have been shown to modulate gene expression (9, 23). They have been found to interact with nuclear receptors; owing to their chemical structure, several of these molecules, for example, naringenin, flavone, catechin, and quercetin, display in vitro agonist properties on the aryl hydrocarbon receptor (AhR) (2, 3, 9). The AhR is a ligand-activated transcription factor belonging to the basic helix-loop-helix/per-ARNT-Sim family of proteins. It is classically activated by synthetic xenobiotics including dioxins such as TCDD [2,3,7,8-tetrachlorobenzo(p)dioxin] or polycyclic aromatic hydrocarbons (PAHs) such as benzo(a)pyrene [B(a)P] and 3-methylcholanthrene (3-MC). Following ligand binding, AhR translocates to the nucleus and forms a heterodimer with the AhR nuclear translocator (ARNT). The AhR/ARNT heterodimer binds to xenobiotic responsive elements (XREs) (minimal consensus core sequence, GCGTG) in promoter regions of target genes to regulate their transcription. Induction of cytochrome P4501A1 (CYP1A1) has been studied extensively as a model of AhR-activated transcription (41).
Recently, 7-ketocholesterol (7-KC) has been identified as a physiological negative modulator of AhR (37) but the existence of relevant endogenous ligand(s) is still unclear. Apart from its role in xenobiotics metabolism, the physiological role of AhR remains to be investigated. Several studies have suggested its implication in cell-cycle regulation, triglyceride synthesis, and adipocyte differentiation (1, 37).
The biological relevance of the AhR activation by dietary compounds such as polyphenols remains to be elucidated. An interesting issue is how toxic compounds such as dioxins and protective dietary polyphenols could act through the same nuclear receptor. The present study addressed this question.
We hypothesized that a molecular mechanism modulating PON-1 gene expression could itself be involved in the potential benefits of polyphenols. We further hypothesized that the AhR could be involved in such regulation. In addition to the AhR-activating properties of some polyphenols, this hypothesis was formulated on the basis of recent observation showing that wine polyphenols but also 3-MC, a potent AhR agonist devoid of antioxidant properties (and, rather, a pro-oxidant), were able to increase serum PON-1 activity in rats and mice (18, 35). We investigated the effect of polyphenols and toxic AhR ligands on the PON-1 gene regulation. We also established the contribution of AhR and showed that this receptor could differentially activate target DNA sequences, depending on its ligand. The study provides additional insights into the protective effects of polyphenols and suggests a mechanism for AhR-dependent ligand-specific gene regulation.
MATERIALS AND METHODS
Chemicals.
TCDD (0.155 mM stock) was obtained from Promochem (Molsheim, France). Other chemicals [3-MC, B(a)P, naringenin, flavone, catechin, quercetin, and 7-KC] were obtained from Sigma (Saint-Quentin Fallavier, France). Fenofibric acid (FA) was a kind gift of A. Edgar (Laboratoires Fournier, Daix, France). AhR monoclonal antibody (clone RPT9) was obtained from Affinity Bioreagents (Golden, Colo.).
Cell culture.
The human hepatoma cell line HuH7 was maintained at 37°C in an atmosphere containing 5% CO2. The Dulbecco's modified Eagle's medium was supplemented with 10% fetal calf serum (Invitrogen, Cergy-Pontoise, France), 100 U of penicillin (Diamant, Puteaux, France)/ml, 100 U of streptomycin (Invitrogen)/ml, and 0.5 mg of amphotericin B (Bristol Myers Squibb, Princeton, N.J.)/ml.
Animals.
Adult male BALB/c mice weighing 18 g at the time of sacrifice were used in this study. Two mice were treated intraperitoneally for three consecutive days with 3-MC (40 mg in corn oil/kg of body weight/day). Two mice were treated with the solvent vehicle alone (corn oil). Mice were sacrificed 24 h after the last dose. This treatment has been previously standardized in terms of dose and duration (35).
PON-1 enzymatic activity.
The cell-associated PON-1 arylesterase activity was measured following a method adapted from the work of Deakin et al. (13). The tested compounds were incubated with the cells (5 × 105 cells/6-well dish) for 48 h in medium containing standard fetal calf serum to allow full cell growth. After treatment, cells were washed with phosphate-buffered saline (PBS) (Invitrogen) to eliminate serum traces that might have contained PON-1 and incubated for 10 min in their culture dishes with the appropriate buffer (50 mM Tris-HCl [pH 8.0], 1 mM CaCl2) and 5 mM phenylacetate as a substrate. The optical density at 270 nm of an aliquot of the supernatant was then measured to determine the extent of phenylacetate hydrolysis. These values were normalized to the protein content of the cells lysates and expressed as (Δoptical density at 270 nm − blank)/protein content.
Northern blots.
Mouse liver total RNAs were prepared with TriPure isolation reagent (Roche, Meylan, France). HuH7 human hepatoma cell line total RNAs were prepared using an RNA Easy Midi kit (QIAGEN, Les Ulis, France). HuH7 polyA+ mRNAs were purified with an Oligotex mRNA purification kit (QIAGEN). Northern blots were performed as already described (19), using 10 μg of mice liver total RNA or 3 μg of polyA+ HuH7 mRNA. The probe used to detect the human PON-1 mRNA is a 283-bp fragment of the 3′ untranslated region (3′-UTR) of the PON-1 mRNA starting immediately after the stop codon. The probe used to detect the murine PON-1 mRNA was a kind gift of N. Janel (Paris, France) (34). The probe used to detect CYP1A1 corresponds to the complete human CYP1A1 cDNA. PON-1, CYP1A1, and actin probes were labeled with a Megaprime DNA labeling kit (Amersham Biosciences, Orsay, France) according to the manufacturer's instructions. Hybridization was performed overnight using Rapid-hybrid buffer (Amersham Biosciences). The membrane was washed 30 min at 65°C with 2× SSC (1×SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.1% sodium dodecyl sulfate and 30 min at 65°C with 0.5× SSC-0.1% sodium dodecyl sulfate. After exposure and revelation with a PhosphorImager, quantifications were performed with ImageQuant software (Molecular Dynamics, Inc.).
Plasmids.
The sequence of the PON-1 gene is accessible in GenBank under accession number AC004022 (BAC clone GS1-155M11). Several plasmids were constructed that respectively contained 1,009 bp (from −1013 to −4), 190 bp (−194 to −4), 142 bp (−146 to −4), 122 bp (−126 to −4), 102 bp (−106 to −4), and 67 bp (−71 to −4) of the PON-1 gene 5′ region cloned into the firefly luciferase reporter vector pGL3 basic (Promega, Charbonnières, France) (19). Since the PON-1 gene promoter displays several transcription starts (19), the +1 position corresponds to the A of the translation start codon.
The pPON1000mut-FL plasmid corresponds to the pPON1000-FL plasmid, with two mutations located at positions −112 and −111 (Table 1). The p(XRE-PON)3-FL and p(mutXRE-PON)3-FL vectors contain, respectively, three copies of the wild-type or mutated fragment of the PON-1 gene promoter corresponding to positions −-126 to −106 and a minimal TATA element (from the adenovirus E1B gene) cloned into pGL3 basic reporter vector. The p(XRE-1A1)-FL contains three functional XREs of the human CYP1A1 gene cloned in front of this TATA minimal element (30). The pTATA-FL contains only this minimal TATA element. The pSG5-AhR, expressing the human AhR, was a generous gift from J.F. Savouret (INSERM U530, Paris, France). The empty expression vector pSG5 was purchased from Stratagene (Amsterdam, The Netherlands).
TABLE 1.
XRE-like sequences in the PON-1 gene promotera
| Sequence and type | Position | Consensus fit |
|---|---|---|
| Consensus core XRE | ||
| GCGTG | 5/5 | |
| Identified responsive element | ||
| GCGGGb | −112 to −108 | 4/5 |
| TAGGGc | −112 to −108 | 2/5 |
| Core XRE like | ||
| GAGTG | −544 to −540 | 4/5 |
| GCCTG | −467 to −463 | 4/5 |
| CAAGC | −388 to −384 | 4/5 |
| GACGC | −367 to −363 | 4/5 |
| CGCGC | −154 to −150 | 4/5 |
| GCGGG | −97 to −93 | 4/5 |
Positions in the promoter sequence and their fitting with the consensus sequence are indicated (the +1 position corresponds to the A of the translation start codon).
Wild type.
The mutation is underlined.
Transfection experiments.
Transient transfection experiments were performed with HuH7 cells by the calcium phosphate coprecipitation technique, as previously described (19). Briefly, at 1 day prior to the transfection, cells (1.5 × 105 cells/12-well dish) were seeded into the usual culture medium. Firefly luciferase reporter vectors (2 μg) (except for short interfering RNA [siRNA] cotransfection experiments, in which 0.5 μg was used), the human AhR-expressing vector (0 to 500 ng), and the control or AhR-targeted siRNA (0.5 μg) were introduced into the cells. Eighteen hours later, cells were exposed to chemicals which were added to the culture medium, with dimethyl sulfoxide (DMSO) (0.1% [vol/vol]) as the solvent vehicle. After 48 h of incubation, cells were lysed for luciferase assays. In siRNA cotransfection experiments, chemicals were exposed only for 24 h, as gene silencing was most efficient at around 40 h after transfection. A firefly luciferase assay was performed with a Promega kit according to the manufacturer's instructions and with a Bio-Orbit 1253 luminometer (Bio-Orbit, Turku, Finland). Results are expressed in arbitrary light units (ALU). For each transfection experiment, the means ± standard errors of the means (SEM) of these values are indicated in the figure legends. Blanks were obtained by assaying luciferase activity in mock-transfected cells; in all transfection experiments, this value was <0.01 ALU.
HuH7 stably transfected clones (expressing the luciferase under the control of the 1,009-bp promoter of the PON-1 gene) were obtained as previously described (19). Cells (0.3 × 106 cells/6-well dish) were seeded into culture medium. At 24 h later, cells were exposed to chemicals, which were added to the culture medium. After 48 h of incubation, cells were lysed for enzymatic assays. A firefly luciferase assay was performed as described above, and results were normalized to the protein content of cell lysates.
Design of AhR-targeted specific siRNA.
An AhR-specific siRNA duplex sequence was designed according to instructions available at the QIAGEN web site. A region corresponding to positions +1024 to + 1044 relative to the translation start site of human AhR cDNA (5′-AAGACTGGAGAAAGTGGCATG-3′) was identified. We used a National Center for Biotechnology Information standard nucleotide-nucleotide BLAST program to verify that this sequence did not match with that of any other human gene. The control siRNA used in this study is a nonsilencing siRNA designed by QIAGEN (5-AATTCTCCGAACGTGTCACGT-3′). SiRNAs were synthesized by the use of oligoribonucleotides from Xeragon, a QIAGEN company. Lyophilized oligoribonucleotides were resuspended in an RNase-free annealing buffer (100 mM potassium acetate, 30 mM HEPES-KOH, 2 mM magnesium acetate, pH 7.4) to obtain a 20 μM solution (0.3 μg/μl), heated at 90°C for 1 min, incubated 1 h at 37°C to allow duplex formation and to disrupt larger aggregates, and stored at −20°C.
Electrophoretic mobility shift assay (EMSA).
For nuclear extract preparation, HuH7 cells (3 × 106 cells/10-cm-diameter dish) were seeded into the usual culture medium. At 40 h later, cells were treated for 90 min with 10 nM TCDD-5 μM 3-MC-50 μM quercetin or the solvent vehicle alone (0.1% DMSO). Cells were then washed, scraped in 1 ml of PBS, and centrifuged at 230 × g. Cells were then resuspended in 1 ml of PBS and centrifuged at 825 × g. Cells were resuspended in 900 μl of an reticulocyte standard buffer containing 10 mM Tris, 10 mM NaCl, 3 mM MgCl2, 0.5 mM dithiothreitol (DTT), 0.1 EGTA, and antiprotease tablets (Roche) and incubated for 5 min with 0.5% NP-40. Cells were centrifuged at 3,300 × g, resuspended in 1 ml of reticulocyte standard buffer, and centrifuged at 6,000 × g. Cells were resuspended in 100 μl of a solution containing 20 mM HEPES (pH 7.4), 0.4 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, 20% glycerol, and antiprotease tablets, incubated for 30 min on ice, and centrifuged at 100,000 × g for 15 min. Supernatant was collected, and 100 μl of a solution containing 20 mM HEPES, 0.2 mM EDTA, 0.5 mM DTT, 50 mM KCl, and antiprotease tablets was added. Nuclear extracts were then frozen in liquid nitrogen.
Synthetic double-stranded DNA probes (4 pg) were labeled with [α-32P]dCTP (Amersham Biosciences) and the large Klenow fragment of DNA polymerase I (Ozyme, Saint Quentin en Yvelines, France). Nuclear extracts (5 μg) were incubated for 5 min with a 10-fold excess of nonlabeled probes or antibodies (5 μg) at room temperature, and the labeled probe (50 fmol [corresponding to 200,000 cpm]) was added. The binding reactions were performed for 15 min at room temperature in a final volume of 18 μl of a binding buffer containing 50 mM HEPES (pH 7.9), 5 mM EDTA, 300 mM KCl, 50% glycerol, 10 mM DTT, 1 μg of polydeoxyinosinic-deoxycytidylic acid (Amersham Biosciences), and 500 ng of salmon sperm DNA. DNA-protein complexes were separated under nondenaturing conditions on a 6% (wt/vol) polyacrylamide gel containing 2.5% glycerol, with 1× Tris-borate-EDTA (45 mM Tris borate, 45 mM boric acid, 2 mM EDTA) as a running buffer. The gels were dried, and the DNA-protein complexes were detected and quantified with a PhosphorImager and ImageQuant software (Molecular Dynamics, Inc).
Several double-stranded DNA sequences were used in this study: the wild-type PON-1 gene (−126 to −106) region (GGAGGCTGCGGACCCGGCGGGGAGGGGT) (underlined characters represent the core XREs), the mutated PON-1 gene (−126 to −106) region (GGAGGCTGCGGACCCCTAGGGGAGGGGT), and a consensus XRE from the CYP1A1 gene (GGAGGTAGGCGTTGCGTGAGAAGGACCG).
Statistics.
Student's two-tailed t tests were performed using Statview software (Abacus Concepts, Inc.).
RESULTS
Effect of synthetic AhR ligands and dietary polyphenols on PON-1 mRNA levels.
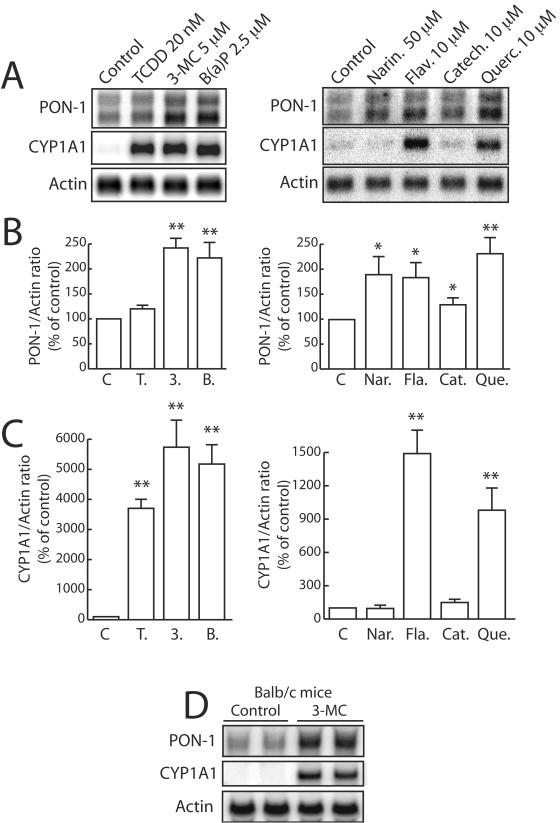
We examined the effect of two types of AhR ligands, classical “synthetic” molecules and natural dietary compounds, on PON-1 gene mRNA levels. As shown in Fig. 1A, Northern blot analysis revealed that treatment of HuH7 cells for 48 h with different AhR ligands resulted in a twofold increase in mRNA levels with classical PAH [3-MC and B(a)P]. Treatment with the dioxin TCDD elicited a small increase (Fig. 1A and B). All these compounds potently induced mRNA levels of CYP1A1, a typical gene activated by AhR ligands (Fig. 1A and C).
FIG. 1.
Synthetic AhR ligands and natural dietary compounds increase PON-1 mRNA levels. (A to C) HuH7 cells were treated for 48 h with the synthetic compounds TCDD, 3-MC, and B(a)P or with the dietary polyphenols naringenin (Narin.), flavone (Flav.), catechin (Catech.), and quercetin (Querc.) at the indicated concentrations or with the solvent vehicle alone (Control [0.1% DMSO]). mRNAs were extracted, and Northern-blot analysis was performed as described in Materials and Methods. (A) Representative Northern blot hybridization as revealed with a PhosphorImager. The actin gene mRNA was used as a normalizing control. The CYP1A1 gene (typical target of AhR) mRNA was used as a positive control. (B) The histogram shows the means ± SEM (n = 4) of the quantification ratio of the PON-1 and actin gene mRNAs. A level of 100% corresponds to the ratio in cells treated with DMSO alone. Statistically significant (P < 0.01) differences in results compared to those obtained with this control are marked with double asterisks. C, control; T., TCDD; 3., 3-MC; B., B(a)P; Nar., naringenin; Fla., flavone; Cat., catechin; Que., quercetin. (C) The histogram shows the means ± SEM (n = 4) of the quantification ratio of the CYP1A1 and actin gene mRNAs. A level of 100% corresponds to the ratio in cells treated with DMSO alone (control condition). Statistically significant (P < 0.01) differences in results compared to those obtained with this control are marked with double asterisks. (D) Adult BALB/c mice were treated for three consecutive days with 3-MC (40 mg/kg of body weight/day) or with the solvent vehicle (corn oil) alone and were sacrificed 24 h after the last dose was administered. In vivo experiments, liver total RNAs extraction, and Northern blot analysis were performed as described in Materials and Methods.
Among dietary polyphenol compounds, naringenin, flavone, and quercetin increased PON-1 mRNA about twofold but catechin was a poor inducer (Fig. 1A and B). Under the same conditions, only flavone and quercetin induced CYP1A1 mRNA expression but at far lesser levels than those seen with the classical synthetic AhR ligands (Fig. 1A and C). It thus appears that the PON-1 and CYP1A1 genes display different induction patterns with natural dietary polyphenols and that some compounds, like naringenin, can increase the PON-1 gene expression without affecting that of CYP1A1. It is also noticeable that quercetin was able to induce the PON-1 gene similarly to 3-MC and more potently than TCDD. Under the same conditions, TCDD and PAH displayed a much stronger effect on the CYP1A1 gene expression than dietary polyphenols.
We also examined the effects of TCDD, 3-MC, and quercetin on cell-associated PON-1 arylesterase activity (data not shown). In consistency with Northern blot experiment results, treatment of HuH7 cells for 48 h with the same concentrations of 3-MC, quercetin, and TCDD as described above resulted in a significant increase of cell-associated PON-1 activity (+49% ± 6%, +50% ± 2%, and +18% ± 4%, respectively; P < 0.01, n = 9). In these experiments, the protein content of HuH7 cells was not significantly affected by the treatments, suggesting that these drugs were not cytotoxic at the doses used in this study (data not shown).
To confirm these results, the effect of a typical AhR ligand on the PON-1 gene expression was investigated in vivo. Adult BALB/c mice were treated for 72 h with 3-MC or left untreated. Figure 1D shows that in consistency with the results of in vitro experiments, 3-MC treatment markedly increased the liver PON-1 mRNA levels of these mice (mean increase, 2.6-fold).
Effect of synthetic AhR ligands and dietary polyphenols on PON-1 promoter activity.
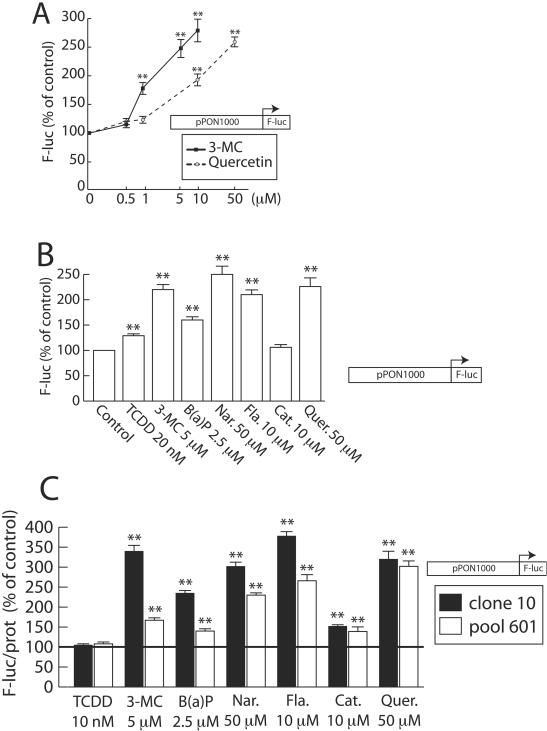
Dietary polyphenols and synthetic ligands were tested using transient transfection assays with the pPON1000-FL reporter vector in HuH7 cells. This plasmid carries the luciferase reporter gene driven by the 1-kb promoter of the PON-1 gene (see Materials and Methods). As shown in Fig. 2A, both 3-MC and quercetin increased the PON-1 gene promoter activity in a dose-dependent manner. The induction reached about 2.5-fold for 10 μM 3-MC and 50 μM quercetin, without any toxicity. Figure 2B shows the effect on the PON-1 gene promoter activity of all the molecules used previously in mRNA analysis. Significant induction levels (from 1.5- to 2.3-fold) were observed with 3-MC, B(a)P, naringenin, flavone, and quercetin and to a lesser extent with TCDD. No induction was observed with catechin. These results were consistent with Northern blot experiment data (particularly for the magnitude of induction and the relative potencies of the different molecules).
FIG. 2.
Synthetic AhR ligands and natural dietary compounds increase the activity of the PON-1 gene promoter. (A) HuH7 cells were transiently transfected with the pPON1000-FL plasmid and treated for 48 h with the indicated concentration of 3-MC or quercetin (solvent vehicle, 0.1% DMSO). Firefly luciferase was assayed as described in Materials and Methods. Results are means ± SEM (n = 12). A level of 100% corresponds to the luciferase value in cells treated with DMSO alone (ALU, 4.24 ± 1.14; n = 12). Statistically significant differences with this control are marked with double asterisks (P < 0.01). (B) HuH7 cells were transiently transfected with the pPON1000-FL plasmid and treated for 48 h with the different compounds (Nar., naringenin; Fla., flavone; Cat., catechin; Quer., quercetin) at the indicated concentrations or with the solvent vehicle alone (Control [0.1% DMSO]). Firefly luciferase was assayed as described in Materials and Methods. Results are means ± SEM (n = 12). A level of 100% corresponds to the luciferase value in cells treated with DMSO alone (ALU, 3.14 ± 0.50; n = 12). Statistically significant differences with this control are marked with double asterisks (P < 0.01). (C) An HuH7 clone (clone 10) and a pool of clones (pool 601), expressing the firefly luciferase reporter gene as a result of the stable transfection of pPON1000-FL (19), were treated with the same compounds as described for panel B at the indicated concentrations or with the solvent vehicle alone (0.1% DMSO) for 48 h. Firefly luciferase and protein content were assayed as described in Materials and Methods. Results were expressed as ratios of firefly luciferase activity/protein content (mean, ± SEM, n = 9). For each group (the clone and the pool), 100% corresponds to the luciferase value in cells treated with DMSO alone (ALU for the clone, 0.78 ± 0.11; ALU for the pool, 0.178 ± 0.02 [n = 9]; represented by a horizontal line). In each group, statistically significant (P < 0.01) differences from control results are marked with double asterisks.
The effect of the different molecules tested on the PON-1 gene promoter activity was also assessed in engineered cell lines. Several stably transfected HuH7 clones expressing luciferase under the control of the 1-kb gene promoter were tested. Figure 2C shows the results of these experiments, which are consistent with that of transient transfection. TCDD displayed no effect in these experiments. Again, the protein content of HuH7 cells was not significantly affected by the treatments used in these experiments (data not shown).
Mapping of responsive elements within the promoter sequence.
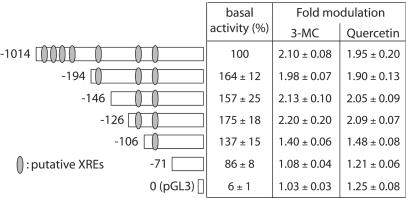
Various deletions of the PON-1 gene promoter were constructed, and reporter vectors containing these deletions were used in transient transfection assays to define the location of putative responsive elements involved in the regulation of the PON-1 gene by synthetic and dietary AhR ligands (Fig. 3). The basal activity of the smallest fragment (−71, containing the main transcription start sites) (19) was somewhat decreased but remained far above the background level of the pGL3 empty vector. These deleted promoters allowed us to identify a 20-bp sequence possibly involved in the regulation by 3-MC and quercetin. Indeed, promoter activation by both compounds was significantly lower (P < 0.01) with the two shorter deleted promoters (−106 and −71) than with the four larger ones (−1014, −194, −146, and −126). No significant induction was observed with the smallest promoter (−71) and the control pGL3 vector. Deletion of the promoter up to position −126 did not significantly alter the response to 3-MC and quercetin. In conclusion, a regulatory element mediating, at least partially, the 3-MC and quercetin effects is located between positions −126 and −106. A weaker regulatory element could also be located between the −106 and −71 positions.
FIG. 3.
Mapping of the PON-1 gene promoter by the use of deleted fragments. HuH7 cells were transiently transfected with the reporter vector driven by deleted promoters containing fragments (the 3′ end of each fragment is −4, and the 5′ end is indicated). The localizations of XRE-like sequences within the PON-1 gene promoter are indicated (see Table 1). Cells were treated for 48 h with 5 μM 3-MC or 50 μM quercetin (solvent vehicle, 0.1% DMSO). Firefly luciferase was assayed as described in Materials and Methods. Results are means ± SEM (n = 15). For the basal activities of the different promoter fragments, results are expressed as the percentages of the luciferase activity in cells transfected with the full-length pPON1000-FL (ALU, 4.18 ± 0.80; n = 15). Regarding the effects of both compounds, results are expressed as severalfold modulation relative to the basal activities for each construction.
AhR-dependent gene regulation is known to be mediated by XREs (core consensus sequence, GCGTG) in target gene promoters (41). The human PON-1 gene promoter does not contain any consensus XRE. However, we have identified motifs (which are listed as core XRE-like sequences in Table 1) similar to the consensus core sequence. The (−126 to −106) fragment contains one of these core XRE-like sequences located between positions −112 and −108 (see Table 1) and only matching four-fifths of the core consensus sequence.
Characterization of the target DNA sequence mediating the PON-1 gene induction.
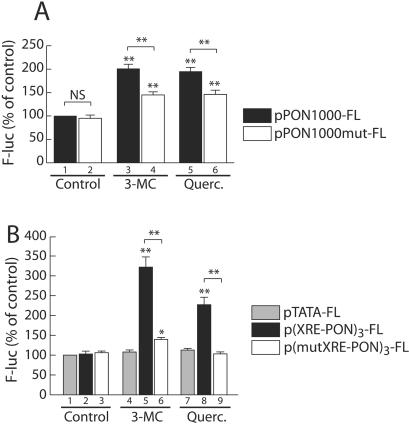
To assess whether the (−126 to −106) region was necessary for mediation of the observed regulation, a mutation was introduced within the putative XRE in the 1-kb promoter fragment (see Table 1). The effect of quercetin and 3-MC on this mutated PON-1 gene promoter was investigated by transient transfection experiments (Fig. 4A). The basal activity was not significantly affected (compare bars 1 and 2). The induction achieved by 3-MC and quercetin was significantly lower with the mutated promoter than with the wild-type promoter (1.4- versus 2-fold with 3-MC and 1.4- versus 1.9-fold for quercetin). These results suggest that this putative XRE-like sequence is the main target mediating the regulation of the PON-1 gene by synthetic and dietary AhR ligands. However, 3-MC and quercetin moderately but still significantly induced the −106 deleted promoter (Fig. 4) as well as the full-length mutated promoter. Thus, the identified (−126 to −106) sequence alone does not account for the entire effect of the tested molecules.
FIG. 4.
Characterization of the target DNA sequence mediating the PON-1 gene induction. (A) HuH7 cells were transiently transfected with either the pPON-1000-FL (filled bars) or pPON1000mut-FL (open bars) plasmids. The mutations are located within the 20 bp identified as critical in Fig. 4 (for sequences, see Table 1). Cells were treated for 48 h with 5 μM 3-MC, 50 μM quercetin (Querc.), or the solvent vehicle alone (0.1% DMSO). Firefly luciferase was assayed as described in Materials and Methods. Results are means ± SEM (n = 15). A level of 100% corresponds to the luciferase value in cells transfected with pPON1000-FL (wild-type promoter) and treated with DMSO alone (ALU, 3.89 ± 0.85; n = 15). For each plasmid, statistically significant differences between treated and untreated conditions are marked with double asterisks (P < 0.01). For each treatment, statistically significant differences between wild-type and mutated promoter activities are marked with double asterisks (P < 0.01). (B) Cells were transiently transfected with the p(XRE-PON)3-FL (black-shaded bars), p(mutXRE-PON)3-FL (open bars), or the pTATA-FL (grey-shaded bars) plasmids and treated for 48 h with 5 μM 3-MC, 50 μM quercetin, or the solvent vehicle alone (0.1% DMSO). Firefly luciferase was assayed as described in Materials and Methods. Results are means ± SEM (n = 15). The mutation present in p(mutXRE-PON)3-FL is the same as that shown in Fig. 5 (see Table 1). A level of 100% corresponds to the luciferase value in cells transfected with pTATA-FL and treated with DMSO alone (ALU, 0.30 ± 0.02; n = 15). For each plasmid, statistically significant (P < 0.01) differences between treated and untreated conditions are marked with double asterisks. For each treatment, statistically significant (P < 0.01) differences between wild-type and mutated promoter activities are marked with double asterisks.
To functionally characterize the critical sequence and to assess whether it was sufficient to mediate PON-1 regulation, three copies of the wild-type or mutated (−126 to −106) region of the PON-1 gene promoter were introduced upstream of a classical minimal TATA element driving the luciferase reporter gene. As shown in Fig. 4B, the activity of the promoter containing the wild-type XRE-like element was significantly induced by quercetin and 3-MC (while that of the TATA minimal element alone was not significantly modified). Under the same conditions, the presence of the mutation within the XRE-like element abolished the effect of quercetin (compare bars 9 and 8) and dramatically decreased the effect of 3-MC (compare bars 6 and 5). These results confirm the implication of the PON-1 gene promoter region (−126 to −106), in particular, the noncanonical XRE located between positions −112 and −108, in the positive regulation of the PON-1 gene by quercetin and 3-MC.
Effect of overexpression of the AhR on the regulation of the PON-1 gene promoter.
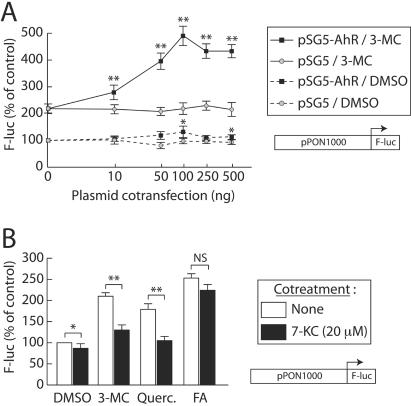
Since the various polyphenols were previously described as putative AhR ligands (2, 3, 9) and since an XRE-like element mediates their effect on the PON-1 gene (as described above), the involvement of the AhR was investigated. The effect of AhR overexpression was studied using transient transfection experiments and the HuH7 cell line. Figure 5A shows the effect of the cotransfection of increasing amounts of an AhR-expressing vector (or its parent empty vector pSG5) on the induction elicited by 3-MC. pSG5 cotransfection did not affect the basal activity of the promoter or the 3-MC-induced activity. Cotransfection of the AhR-expressing vector in the absence of ligand slightly increased the basal activity of the promoter (22% with 100 ng of the AhR-expressing vector). The cotransfection clearly enhanced the 3-MC induction; this effect was dose dependent and reached a plateau above 100 ng of cotransfected expression vector. The increases in the inducing effects of 3-MC in the absence or presence of 100 ng of the AhR-expressing vector were two- and fivefold, respectively.
FIG. 5.
AhR overexpression enhances the inducing effect of 3-MC on the PON-1 gene promoter activity, and cotreatment with the AhR antagonist 7-KC partially abolishes the effect of synthetic and natural dietary compounds. (A) HuH7 cells were transiently transfected with the pPON1000-FL plasmid and cotransfected with the indicated amount of the AhR-expressing plasmid pSG5-AhR (squares) or its corresponding empty vector pSG5 (circles). Cells were treated for 48 h with 5 μM 3-MC (solid lines) or the solvent vehicle alone (0.1% DMSO; dashed lines). Firefly luciferase was assayed as described in Materials and Methods. Results are means ± SEM (n = 12). A level of 100% corresponds to the luciferase value in cells without cotransfection and treated with DMSO alone (ALU, 3.15 ± 0.79; n = 12). For each group (DMSO or 3-MC treatment; identical amounts of cotransfected plasmid were used), statistically significant differences between pSG5 and pAhR cotransfected conditions are marked with a single asterisk (P < 0.05) or a double asterisk (P < 0.01). (B) HuH7 cells were transiently transfected with the pPON1000-FL plasmid and treated for 48 h with 5 μM 3-MC, 10 μM quercetin (Querc.), 250 μM FA, or the solvent vehicle alone (0.1% DMSO). In addition, cells were treated with the AhR antagonist 7-KC (37) (20 μM) or left untreated. Firefly luciferase was assayed as described in Materials and Methods. Results are means ± SEM (n = 10). A level of 100% corresponds to the luciferase value in cells treated with DMSO alone (ALU, 2.89 ± 0.92; n = 10). For each treatment, statistically significant differences between untreated and 7-KC cotreatment conditions are marked with a single asterisk (P < 0.05) or a double asterisk (P < 0.01).
Similar cotransfection assays were carried out with all the synthetic and dietary AhR ligands used in this study (Table 2). In these experiments AhR overexpression weakly increased the basal activity of the promoter (1.22-fold [consistent with data presented in Fig. 5]). It also specifically enhanced the effect of 3-MC, B(a)P, flavone, and quercetin (1.5- to almost 2-fold; P < 0.01, n = 12). The effects of TCDD and catechin, the compounds that displayed a very weak activation of the PON-1 gene expression in previous experiments (Northern blot and transfection experiments) were also slightly improved. Under the same conditions, AhR overexpression had no significant effect on the induction of the PON-1 gene promoter activity elicited by FA, a previously identified PON-1 gene inducer (19). This highlights the specificity of the effect observed for the other compounds.
TABLE 2.
Effect of AhR overexpression on the regulation of the PON-1 gene promotera
| Control | Luciferase value (ALU) for:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Synthetic compound
|
Natural dietary compound
|
Control FA; (250 μM) | |||||||
| TCDD (20 nM) | 3-MC (5 μM) | B(a)P (2.5 μM) | Naringenin (50 μM) | Flavone (10 μM) | Catechin (10 μM) | Quercetin (50 μM) | |||
| pSG5 | 100b | 130 ± 7 | 237 ± 15 | 144 ± 6 | 251 ± 9 | 205 ± 11 | 107 ± 5 | 228 ± 17 | 250 ± 12 |
| pSG5-AhR | 100c | 175 ± 7 (1.40 ± 0.08d) | 439 ± 16 (1.85 ± 0.12d) | 216 ± 8 (1.60 ± 0.07d) | 282 ± 9 (1.19 ± 0.16e) | 325 ± 16 (1.63 ± 0.06d) | 140 ± 6 (1.35 ± 0.09d) | 362 ± 23 (1.59 ± 0.15d) | 228 ± 9 (0.95 ± 0.09e) |
HuH7 cells were transfected with pPON1000-FL and 100 ng of the AhR-expressing vector (pSG5-AhR) or the empty vector (pSG5) and treated for 48 h with the indicated compounds or with the solvent vehicle alone (0.1% DMSO). Firefly luciferase was assayed as described in Materials and Methods. Results represent means ± SEM (n = 12). For each experiment, ratios of luciferase values in pSG5-AhR and pSG5-cotransfected conditions were calculated. Means ± SEM. (n = 12) of these ratios are indicated in parentheses. They represent the relative enhancement of the inducing molecule effect owing to AhR overexpression.
A level of 100% corresponds to the luciferase value in cells treated with the solvent alone and cotransfected with the empty pSG5 vector (ALU, 2.10 ± 0.39; n = 12).
A level of 100% corresponds to the luciferase value in cells treated with the solvent alone and cotransfected with the pSG5-AhR vector (this value was 22% higher than the control value in cells transfected with pSG5).
Significant enhancement (P < 0.01).
NS, not significant.
Effect of the cotreatment with an AhR antagonist.
We next tested an antagonist of the AhR receptor; 7-KC has recently been shown to be an endogenous AhR modulator, inhibiting AhR-mediated transactivation through competitive binding towards xenobiotic ligands. It has been shown to prevent the CYP1A1 induction elicited by 10 nM TCDD with an IC50 of 5 μM in the HepG2 hepatoma cell line (37). Using transient transfection experiments in the HuH7 cell line, we assessed the effect of 20 μM 7-KC on the PON-1 gene induction elicited by quercetin and 3-MC. As shown in Fig. 5B, treatment of HuH7 cells with 7-KC alone slightly decreased the basal activity of the promoter. Cotreatment with 7-KC strongly (P < 0.01; n = 12) decreased the induction of the promoter activity elicited by the indicated molecules. In the same experiments, the induction ratio elicited by FA was not significantly affected by the cotreatment with 7-KC, suggesting that the effect of 7-KC was specific. These results show that the inhibition of the AhR antagonizes at least partially the inducing effect of 3-MC and quercetin on the PON-1 gene promoter activity.
Effect of the siRNA-mediated AhR gene silencing.
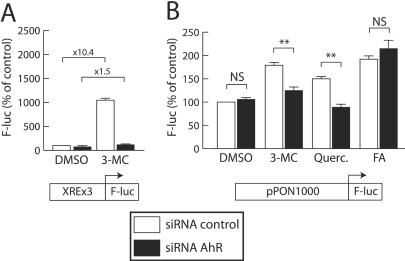
Because pharmacological inhibition of AhR may not be sufficiently specific, we used targeted gene silencing. Endogenous AhR expression was inhibited with a specific AhR-targeted siRNA. As shown in Fig. 6A, the validity and efficiency of this approach was established using a typical AhR-regulated promoter containing three consensus XREs. The effect of 3-MC on this promoter decreased from a 10-fold induction in the presence of a control (nonsilencing) siRNA to a 1.5-fold induction in the presence of the AhR-targeted siRNA.
FIG. 6.
siRNA-mediated AhR gene silencing antagonizes the inducing effect of quercetin and 3-MC on the PON-1 gene promoter activity. (A) As a control experiment for siRNA efficiency, HuH7 cells were transiently transfected with 500 ng of an AhR-inducible luciferase reporter vector (pXRE3-G5-FL) and cotransfected with 500 ng of the AhR-targeted siRNA (filled bars) or the nonsilencing control siRNA (open bars) as described in Materials and Methods. Cells were treated for 24 h with 5 μM 3-MC or the solvent vehicle alone (0.1% DMSO). Firefly luciferase was assayed as described in Materials and Methods. Results are means ± SEM (n = 15). For each plasmid, 100% corresponds to the luciferase value in cells cotransfected with the control siRNA and treated with DMSO alone (ALU, 1.01 ± 0.07; n = 15). Severalfold inductions elicited by 3-MC in each cotransfection conditions are indicated above the bars. (B) HuH7 cells were transiently transfected with 500 ng of the pPON1000-FL plasmid and cotransfected with 500 ng of the AhR-targeted siRNA (filled bars) or the nonsilencing control siRNA (open bars). Cells were treated for 24 h with 5 μM 3-MC, 50 μM quercetin (Querc.), 250 μM FA as a negative control, or the solvent vehicle alone (0.1% DMSO). Firefly luciferase was assayed as described in Materials and Methods. Results are means ± SEM (n = 15). For each plasmid, 100% corresponds to the luciferase value in cells cotransfected with the control siRNA and treated with DMSO alone (ALU, 0.32 ± 0.02; n = 15). For each treatment, statistically significant (P < 0.01) differences between control and AhR-targeted siRNA cotransfected conditions are marked with double asterisks.
Figure 6B shows the effect of AhR gene silencing on the induction of the PON-1 gene promoter elicited by quercetin and 3-MC. The basal activity of the promoter was not affected by AhR gene silencing. In these experiments, cells were treated for only 24 h, since preliminary experiments showed that siRNA-mediated gene silencing was most efficient about 40 h after their transfection. The inducing effects of quercetin and 3-MC were therefore more limited than those previously shown (Fig. 2). These effects were almost completely abolished when the AhR-targeted siRNA was cotransfected, interestingly, thus showing the involvement of AhR in the regulation of the PON-1 gene by quercetin and 3-MC. AhR gene silencing had no effect on the induction of the promoter activity by FA, suggesting that the effect observed with quercetin and 3-MC was specific.
DNA-protein interaction at the regulatory sequence.
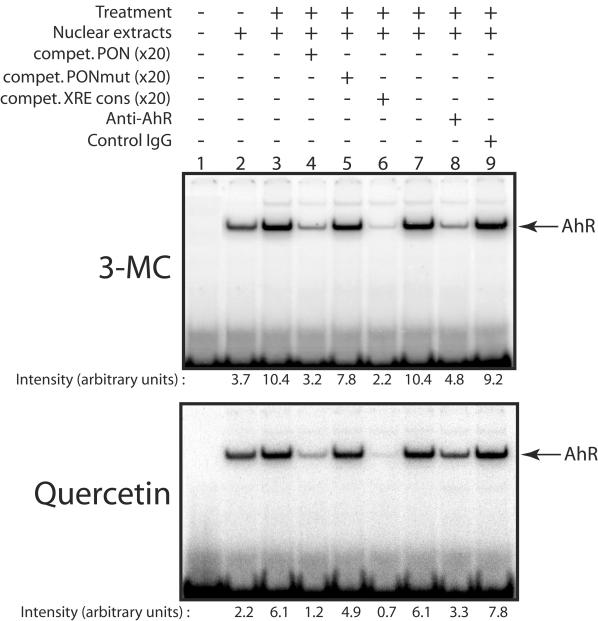
The effect of several inducers of the PON-1 gene on the binding of transcription factors to the XRE-like sequence was investigated by EMSA with the 20-bp (−126 to −106) PON-1 promoter sequence as a probe. As shown in Fig. 7, a complex was formed when nuclear extracts from untreated cells were added to this probe (see below). The intensity of this complex was increased when nuclear extracts from quercetin or 3-MC treated cells were used (compare lanes 3 and 2). The formation of the DNA-protein complex was competed by unlabeled PON-1 DNA sequence and by a 20-bp DNA sequence from the CYP1A1 gene containing a consensus XRE (lanes 4 and 6, respectively). Under the same conditions, the mutated PON-1 DNA sequence (containing the same mutations within the XRE-like motif as described above for transfection experiments; see Table 1) did not prevent the formation of the protein-DNA complex (lane 5). The identified XRE-like sequence is also reminiscent of an Sp1 binding site, but competition by a sequence containing such a site did not prevent the complex formation (data not shown). In addition, anti-AhR monoclonal antibodies decreased the intensity of the DNA-protein complex (compare lanes 8 and 7) whereas control immunoglobulin G had no effect (compare lanes 9 and 7). These observations suggest that 3-MC and quercetin treatments increase the binding of AhR to the (−126 to −106) region of the PON-1 gene promoter. Moreover, this binding appears to depend on the integrity of the core XRE-like element.
FIG. 7.
Effect of 3-MC and quercetin on the binding of AhR to the PON-1 gene promoter. HuH7 cells were treated for 90 min with 5 μM 3-MC, 50 μM quercetin, or the solvent vehicle alone (0.1% DMSO). Nuclear extracts were prepared as described in Materials and Methods and were incubated with the 32P-labeled PON-1 gene (−126 to −106) region and subjected to EMSA. Competition was performed with an excess (×20) of wild-type PON-1 (compet. PON) (lane 4), mutated PON-1 (compet. PONmut) (lane 5), or consensus XRE (compet. XRE cons) (lane 6) unlabeled double-stranded oligonucleotides. Nuclear extracts were also incubated with 4 μg of an anti-AhR monoclonal antibody (lane 8) or the same amount of control immunoglobulin G (IgG) (raised against PON-1; lane 9). PhosphorImager pictures show representative experiments (the same results were observed with several different preparations of nuclear extracts). Means (n = 5) of the PhosphorImager quantifications of the observed DNA-protein complexes are indicated under the lanes.
It is noticeable that a significant amount of AhR/DNA complex can be observed by the use of extracts from untreated cells. This basal complex also contains the AhR, since its formation is inhibited by an anti-AhR antibody and by competition with an unlabeled oligonucleotide containing a consensus XRE but not by competition with an Sp1 DNA binding site (data not shown).
Induction patterns of different AhR ligands with the PON-1 and CYP1A1 promoters.
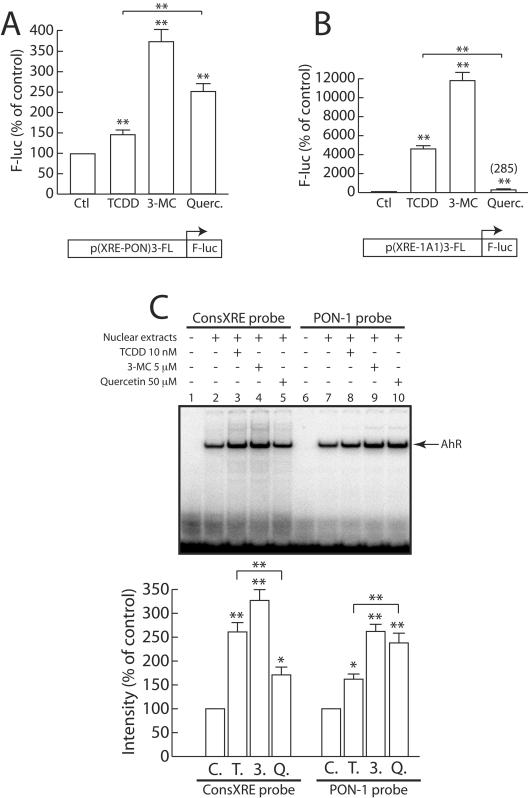
We tested the effects of TCDD, 3-MC, and quercetin (AhR ligands from three different classes) on the activity of a promoter containing either three copies of the (−126 to −106) critical region of the PON-1 gene promoter (XRE-PON) or three consensus XREs from the human CYP1A1 gene (XRE-1A1) upstream of a TATA minimal element. The basal activity of the CYP1A1 sequence was eightfold higher than that of the PON-1 gene-regulating element (data not shown). As shown in Fig. 8A, the promoter activity of the (XRE-PON)3 was significantly induced by quercetin and 3-MC and to a lesser extent by TCDD. In comparison, TCDD and 3-MC strongly activated the promoter containing the (XRE-1A1)3 promoter whereas quercetin displayed a much weaker inducing effect (Fig. 8B). The inducing effects of TCDD and quercetin are statistically different (P < 0.01; n = 15) for both the PON-1 (quercetin > TCDD) and the CYP1A1 (TCDD ≫ quercetin) promoter elements. Similar observations were made respecting the activities of both the full-length PON-1 and CYP1A1 gene promoters (data not shown).
FIG. 8.
Induction patterns of different AhR ligands for the PON-1 and CYP1A1 promoters. (A) HuH7 cells were transfected with the p(XRE-PON)3-FL plasmid and treated for 48 h with 20 nM TCDD, 5 μM 3-MC, 50 μM quercetin (Querc.), or the solvent vehicle alone (0.1% DMSO) (Ctl). Firefly luciferase was assayed as described in Materials and Methods. Results are means ± SEM (n = 15). A level of 100% corresponds to the luciferase value in cells treated with DMSO alone (ALU, 6.05 ± 0.68; n = 15). Statistically significant (P < 0.01) differences between treated and untreated conditions are marked with double asterisks. (B) Cells were transfected with the p(XRE-1A1)-FL plasmid and treated for 48 h with 20 nM TCDD, 5 μM 3-MC, 50 μM quercetin, or the solvent vehicle alone (0.1% DMSO). Firefly luciferase was assayed as described in Materials and Methods. Results are means ± SEM (n = 15). A level of 100% corresponds to the luciferase value in cells treated with DMSO alone (ALU, 0.37 ± 0.02; n = 15). Statistically significant differences between treated and untreated conditions are marked with double asterisks (P < 0.01). (C) HuH7 cells were treated for 90 min with 20 nM TCDD (lanes 3 and 8) (T.), 5 μM 3-MC (lanes 4 and 9) (3.), 50 μM quercetin (lanes 5 and 10) (Q.), or the solvent vehicle alone (0.1% DMSO; lanes 2 and 7) (C.). Nuclear extracts were prepared as described in Materials and Methods and were incubated with the 32P-labeled PON-1 gene (−126 to −106) region or the 32P-labeled consensus XRE probe and subjected to EMSA. A PhosphorImager picture shows a representative experiment. The histogram shows the means ± SEM (n = 4) of the PhosphorImager quantification of the observed DNA-protein complexes. For each probe (consXRE and PON-1), 100% corresponds to the cells treated with DMSO alone; statistically significant differences from results obtained with this control are marked with a single asterisk (P < 0.05) or a double asterisk (P < 0.01).
We also compared the effects of TCDD, 3-MC, and quercetin on the binding of AhR to both the (−126 to −106) region of the PON-1 gene promoter and a consensus XRE element from the CYP1A1 gene promoter (see Materials and Methods). As shown in Fig. 8C, 3-MC and quercetin and, to a lesser extent, TCDD treatments caused an increase in the intensity of the complex formed by nuclear proteins and the PON-1 gene probe (compare lanes 8, 9, and 10). The effect of quercetin was significantly higher than that of TCDD (P < 0.01, n = 4). Interestingly, the intensity profile of the binding to the CYP1A1 (consensus XRE) probe was different. Indeed, in the same conditions TCDD elicited a significantly (P < 0.01, n = 4) stronger DNA-protein complex intensity than quercetin (compare lanes 3 and 5).
These result are consistent with those observed in Northern blot experiments (Fig. 1), thus suggesting that the different induction patterns observed for the PON-1 and CYP1A1 genes are due, at least partially, to the sequence of the responsive element mediating these effects.
DISCUSSION
In this study, we demonstrated that the PON-1 gene expression can be increased by synthetic and natural dietary AhR ligands. In the HuH7 hepatoma cell line, treatment with these compounds increased PON-1 arylesterase activity and mRNA levels. Further investigations showed that these compounds stimulate the PON-1 gene promoter activity. These effects were observed at concentrations consistent with those measured in vivo after dietary exposure to these compounds (3, 39). Recent in vivo studies have revealed an increase of PON-1 serum activity following treatment with flavonoids (4, 18) or 3-MC (35). Some polyphenols are thought to exert a direct antioxidant effect in serum, which could be beneficial for PON-1 activity (which is inactivated by oxidative stress and oxidized LDL in the serum) (5). We show here that polyphenols could also modulate the expression level of the PON-1 gene itself. Our results provide a molecular mechanism involving a transcriptional activation. The PON-1 gene activation by synthetic pollutants (TCDD or PAH) and natural dietary polyphenols appears to be mediated by AhR, since (i) overexpression of the AhR in our cellular model specifically enhanced the observed inductions; (ii) cotreatment with 7-KC, an endogenous inhibitor of AhR, totally or partially abolished these effects; (iii) AhR-targeted gene silencing by siRNA resulted in a specific loss of inductions; and (iv) AhR specifically bound the identified critical DNA regulatory sequence within the PON-1 gene promoter and this binding was increased by 3-MC and quercetin treatments. Some AhR ligands have been shown to activate gene transcription through an indirect mechanism involving oxidative stress (owing to their metabolism). We tested this hypothesis but found that oxidative stress did not activate the PON-1 gene expression (mRNA levels and promoter activity) (data not shown). In addition, antioxidants did not prevent the inducing effect of 3-MC (data not shown). Altogether, our data support the hypothesis of a direct involvement of AhR in the observed effects.
AhR trans-activating function was first thought to require consensus XRE sequences and a TATA-based transcription initiation mechanism (41). Regarding the TATA motif, recent studies have shown this may not be always the case: the AhR repressor (AhRR) gene is regulated by AhR in spite of the absence of a TATA box (7). The human PON-1 gene promoter does not contain a classical XRE or a TATA box. However, a 20-bp regulatory region involved in the effect of quercetin and 3-MC was identified and was shown to bind the activated AhR. This region contains a nonclassical core XRE-like motif (GCGGG) matching four-fifths of the pentameric core XRE consensus sequence (GCGTG). The results of EMSA studies suggested that this motif, though reminiscent of an Sp1-binding site, does not bind the Sp1 transcription factor (data not shown). The observation of such a noncanonical functional AhR-responsive element is unusual. Indeed, the four core nucleotides CGTG were shown to define the minimal and necessary sequence that is recognized by the TCDD-induced AhR/ARNT heterodimer because their substitution decreases AhR binding affinity by 100- to 800-fold (41, 44). Nucleotides that flank this core site are also involved in dictating the efficiency of AhR binding but are not conserved (41). Our data show that the PON-1 gene is activated in vivo by the AhR ligand 3-MC. The scanning of the murine PON-1 gene promoter did not reveal strong homologies with the human sequence. Yet two pentanucleotidic motifs matching four-fifths of the consensus XRE core sequence are present in the proximal region of the promoter.
TCDD and PAH are well-known AhR activators, and several polyphenols were described in vitro as putative AhR ligands (2, 3, 9). However, the AhR-mediated biological functions of dietary compounds remained elusive. Dietary polyphenols are thought to have agonist or antagonist properties on the AhR, depending on the molecule and its concentration. Studies using CYP1A1 as a paradigm of AhR-dependent transcription showed that quercetin was an activator (9) and that other compounds could antagonize TCDD-induced transcription (through an inhibition of AhR nuclear translocation and DNA binding [20] or possibly by a perturbation of the transcriptional machinery recruitment [21]).
Polyphenols are found in different amounts in various edible sources like fruits, vegetables, or wine. They are absorbed following consumption and are found in plasma and tissues. The concentrations used in this study are consistent with those observed in vivo after ingestion of a polyphenol-enriched diet (3, 9, 39). Consumption of such a polyphenol-enriched diet was shown to be inversely associated with morbidity and mortality from CVD (the so-called “French paradox”) (5). The mechanisms involved still need to be elucidated, however, though some studies supported a chemical hypothesis (dietary polyphenols have been shown to chemically protect LDL from oxidation) (5). Wine polyphenols have also been shown to enhance NO synthase expression as well as NO release from endothelial cells (23). We show here that the up-regulation of the PON-1 gene could likely contribute to the cardiovascular protective effects attributed to wine and dietary polyphenols, which offers a new molecular explanation for the clinical observations.
In addition to its atheroprotective role, PON-1 is also a xenobiotic metabolizing enzyme (XME) that detoxifies OPs and arylesters. AhR is known to control the expression of a battery of target XME genes (such as CYP1A1), which are often autoregulated by their own substrates (for example, CYP1A1 metabolizes and is regulated by PAH). Polyphenols were recently shown to be extensively metabolized into phenolic acids (32). Since such acids are metabolized by PON-1 (phenylacetate is used to assay PON-1 activity), the up-regulation of the PON-1 gene by quercetin could constitute an adaptive mechanism.
The endogenous AhR ligand(s) and the AhR main physiological function, apart from xenobiotic metabolism, have not been yet clearly identified. In AhR knockout mice the predominant phenotype was delayed hepatic development and bile duct fibrosis, suggesting a role of AhR in cell cycle regulation and differentiation. Moreover, some studies suggest that AhR could be involved in the regulation of lipid metabolism, especially in cholesterol metabolism, triglyceride synthesis, and adipocyte differentiation (1, 37). Induction of PON-1 (which hydrolyses oxidized lipids) by AhR can contribute to this effect. Furthermore, LXRα and SREBP-2, other nuclear receptors involved in lipid metabolism, appear to control the PON-1 gene regulation (14, 19). The PON-1 gene may thus be regulated by AhR ligands (exogenous or endogenous) as a part of the complex regulation of lipid metabolism. Yet AhR is thought to play a deleterious role in the development of atherosclerosis, since some epidemiological studies linked exposure to its synthetic ligands (TCDD and PAH) with increased serum triglycerides and CVD. No clear specific molecular mechanisms linking AhR activation and CVD were identified (38), however, though it is well established that PAH can induce genetic lesions and oxidative stress, largely contributing to their general toxicity. The study of the transcriptional signature of TCDD in human HepG2 hepatoma cells (33) provided contradictory results: TCDD induced “proatherogenic” genes (like endothelin) but also induced antiatherogenic genes (like NO synthases) and repressed atherogenic genes (like the hypertension-mediating vasopressin and neuropeptide Y receptors). Another recent study demonstrated that AhR represses T-cadherin expression in vascular smooth-muscle cells (31). T-cadherin has been previously shown to be markedly up-regulated in atherosclerotic lesions (28). Therefore, whether there is a direct implication of AhR in atherosclerosis development remains unclear.
It seems paradoxical that both cardioprotective agents such as polyphenols and toxic agents such as PAH are ligands of the AhR. However, we have to take into consideration the structure, bioavailability, and metabolism of the different compounds as well as a differential targeting of gene promoters. The uncoupling of PAH metabolism by XME (such as CYP1A1) results in the formation of reactive oxygen species, which are known to promote the development of atherosclerosis. In contrast, polyphenols display antioxidant properties known to be atheroprotective in vivo (5). Although we have shown here that some PAH induce the PON-1 gene expression, they may also inhibit PON-1 activity because of the oxidative stress they induce. Moreover, PAHs are metabolized into highly mutagenic compounds which clearly account for their proatherogenic effect (29). TCDD is unique because of its extremely high-level affinity for AhR and its very poor metabolism (TCDD displays a half-life in the range of several years). The potential proatherogenic effect of TCDD could possibly stem from sustained long-time exposure and subsequent activation of “toxic” genes (like CYP1A1, which generates H2O2). Other AhR ligands displaying a much shorter half-life (like natural polyphenols) may have different properties. Quercetin, which seems to have properties protective against atherosclerosis development (5), has a half-life of about 25 h in the body (3), and our data support the hypothesis that this protective role is related, at least partially, to AhR activation.
Another yet-unexplored explanation of the putative difference between toxic and beneficial AhR ligands could be a differential targeting of AhR-responsive genes. We show here that under the same conditions, quercetin is a better activator of the PON-1 gene than TCDD whereas TCDD is a better inducer of CYP1A1 than quercetin. Moreover, naringenin increases PON-1 mRNA levels and has no effect on CYP1A1 mRNA levels. Several molecular mechanisms may account for these observations. Our data support the hypothesis that the DNA target of AhR is involved, since (i) the induction profiles of the various AhR ligands are not the same with respect to the CYP1A1 and the PON-1 gene mRNA levels; (ii) the same observation applies for gene promoter activity (data not shown); (iii) the PON-1 gene only displays XRE-like core sequences, whereas the CYP1A1 gene contains several consensus XREs; (iv) synthetic promoters containing either a consensus sequence or the PON-1 XRE-like core sequence display differences in their induction profiles; and (v) the intensity profiles of DNA-protein complexes following treatment with the various AhR ligands for a consensus XRE and for the identified PON-1 gene DNA target are different.
A recent study showed differential transcriptional and physiological effects of two AhR ligands (TCDD and dimethyl-benzanthracene) due to differences in the targeted nucleotide sequences (27). Our data are consistent with the hypothesis that TCDD and quercetin-liganded AhR bind with different levels of efficiency to different DNA target sequences. However, the global impact on transcription efficiency may also involve a differential recruitment of coactivators and/or a modification of the DNA superstructure. It may depend on the liganded AhR conformation itself and/or on the particular DNA sequence of the target (or on the larger promoter context). Further studies are required to investigate these molecular mechanisms. The transcriptional machinery recruitment could be one of these molecular mechanisms. Indeed, the recruitment of transcriptional coactivators (histone acetyltransferases) and RNA polymerase II was recently shown to depend on the nature of the AhR ligand (21).
Different approaches led to the discovery of new putative AhR target genes. Savouret et al. searched sequence databases for human genes containing consensus XRE sequence(s) (38), while others used microarrays to characterize the transcriptional signature of TCDD in the HepG2 hepatoma cell line (17, 33). Microarray experiments allowed the identification of more target genes than the in silico study. One of the explanations could be that some of the AhR target genes do not contain a consensus XRE sequence (as is the case for PON-1). Moreover, large-scale gene expression studies should also be undertaken using AhR ligands other than TCDD, since different genes may be regulated. The identification of new AhR target genes may shed light on nonclassical AhR-dependent gene regulation mechanisms and gene induction profiles. It would likely allow the identification of genes and DNA motifs preferentially targeted by AhR following activation by natural (and maybe endogenous) ligands.
In conclusion, this study reports the induction of the PON-1 gene expression by dietary polyphenols and provides a molecular mechanism which may account for the observed protective effects of these compounds with respect to atherosclerosis. Since PON-1 is presented as a potential therapeutic target for CVD prevention (10, 15) and low-dose chronic OP toxicity (8, 25) and since the level of the PON-1 gene expression has a significant impact on serum PON-1 activity, the efficiency of dietary polyphenols in both applications may be studied. Furthermore, the synthesis of modified molecules by combinatorial chemistry may lead to the discovery of more active and nontoxic AhR ligands for these indications, possibly allowing a somewhat specific gene targeting.
Acknowledgments
We are grateful to J. F. Savouret (INSERM U530, Paris, France) for discussions and for providing us with plasmids. We also thank L. Taysse (CEB, Vert Le Petit, France) for the treatment of mice and N. Janel (EA3508, Paris, France) for providing us with murine PON-1 Northern-blot probe.
This work was supported by the Délégation Générale pour l'Armement (DGA/STTC contract 99 CO 099), Université René Descartes, Région Ile de France, Fondation pour la Recherche Médicale, Société Française de Toxicologie, and Ligue Nationale contre le Cancer. C.G. was supported by a Délégation Générale pour l'Armement—Centre National de la Recherche Scientifique grant and Société Française de Toxicologie and Fondation pour la Recherche Médicale grants.
REFERENCES
- 1.Alexander, D. L., L. G. Ganem, P. Fernandez-Salguero, F. Gonzalez, and C. R. Jefcoate. 1998. Aryl-hydrocarbon receptor is an inhibitory regulator of lipid synthesis and of commitment to adipogenesis. J. Cell Sci. 111(Pt. 22):3311-3322. [DOI] [PubMed] [Google Scholar]
- 2.Amakura, Y., T. Tsutsumi, M. Nakamura, H. Kitagawa, J. Fujino, K. Sasaki, M. Toyoda, T. Yoshida, and T. Maitani. 2003. Activation of the aryl hydrocarbon receptor by some vegetable constituents determined using in vitro reporter gene assay. Biol. Pharm. Bull. 26:532-539. [DOI] [PubMed] [Google Scholar]
- 3.Ashida, H., I. Fukuda, T. Yamashita, and K. Kanazawa. 2000. Flavones and flavonols at dietary levels inhibit a transformation of aryl hydrocarbon receptor induced by dioxin. FEBS Lett. 476:213-217. [DOI] [PubMed] [Google Scholar]
- 4.Aviram, M., L. Dornfeld, M. Rosenblat, N. Volkova, M. Kaplan, R. Coleman, T. Hayek, D. Presser, and B. Fuhrman. 2000. Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: studies in humans and in atherosclerotic apolipoprotein E-deficient mice. Am. J. Clin. Nutr. 71:1062-1076. [DOI] [PubMed] [Google Scholar]
- 5.Aviram, M., and B. Fuhrman. 2002. Wine flavonoids protect against LDL oxidation and atherosclerosis. Ann. N. Y. Acad. Sci. 957:146-161. [DOI] [PubMed] [Google Scholar]
- 6.Aviram, M., M. Rosenblat, C. L. Bisgaier, R. S. Newton, S. L. Primo-Parmo, and B. N. La Du. 1998. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J. Clin. Investig. 101:1581-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baba, T., J. Mimura, K. Gradin, A. Kuroiwa, T. Watanabe, Y. Matsuda, J. Inazawa, K. Sogawa, and Y. Fujii-Kuriyama. 2001. Structure and expression of the Ah receptor repressor gene. J. Biol. Chem. 276:33101-33110. [DOI] [PubMed] [Google Scholar]
- 8.Cherry, N., M. Mackness, P. Durrington, A. Povey, M. Dippnall, T. Smith, and B. Mackness. 2002. Paraoxonase (PON1) polymorphisms in farmers attributing ill health to sheep dip. Lancet 359:763-764. [DOI] [PubMed] [Google Scholar]
- 9.Ciolino, H. P., P. J. Daschner, and G. C. Yeh. 1999. Dietary flavonols quercetin and kaempferol are ligands of the aryl hydrocarbon receptor that affect CYP1A1 transcription differentially. Biochem. J. 340(Pt. 3):715-722. [PMC free article] [PubMed] [Google Scholar]
- 10.Costa, L. G., T. B. Cole, G. P. Jarvik, and C. E. Furlong. 2003. Functional genomics of the paraoxonase (PON1) polymorphisms: effects on pesticide sensitivity, cardiovascular disease, and drug metabolism. Annu. Rev. Med. 54:371-392. [DOI] [PubMed] [Google Scholar]
- 11.Cowan, J., C. M. Sinton, A. W. Varley, F. H. Wians, R. W. Haley, and R. S. Munford. 2001. Gene therapy to prevent organophosphate intoxication. Toxicol. Appl. Pharmacol. 173:1-6. [DOI] [PubMed] [Google Scholar]
- 12.Davies, H. G., R. J. Richter, M. Keifer, C. A. Broomfield, J. Sowalla, and C. E. Furlong. 1996. The effect of the human serum paraoxonase polymorphism is reversed with diazoxon, soman and sarin. Nat. Genet. 14:334-336. [DOI] [PubMed] [Google Scholar]
- 13.Deakin, S., I. Leviev, M. Gomaraschi, L. Calabresi, G. Franceschini, and R. W. James. 2001. Enzymatically active paraoxonase-1 is located at the external membrane of producing cells and released by a high-affinity, saturable, desorption mechanism. J. Biol. Chem. 277:4301-4308. [DOI] [PubMed] [Google Scholar]
- 14.Deakin, S., I. Leviev, S. Guernier, and R. W. James. 2003. Statin modulates expression of the PON1 gene and increases serum paraoxonase. A role for sterol regulatory element-binding protein-2. Arterioscler. Thromb. Vasc. Biol. [DOI] [PubMed]
- 15.Durrington, P. N., B. Mackness, and M. I. Mackness. 2002. The hunt for nutritional and pharmacological modulators of paraoxonase. Arterioscler. Thromb. Vasc. Biol. 22:1248-1250. [DOI] [PubMed] [Google Scholar]
- 16.Feingold, K. R., R. A. Memon, A. H. Moser, and C. Grunfeld. 1998. Paraoxonase activity in the serum and hepatic mRNA levels decrease during the acute phase response. Atherosclerosis 139:307-315. [DOI] [PubMed] [Google Scholar]
- 17.Frueh, F. W., K. C. Hayashibara, P. O. Brown, and J. P. Whitlock, Jr. 2001. Use of cDNA microarrays to analyze dioxin-induced changes in human liver gene expression. Toxicol. Lett. 122:189-203. [DOI] [PubMed] [Google Scholar]
- 18.Fuhrman, B., and M. Aviram. 2002. Preservation of paraoxonase activity by wine flavonoids: possible role in protection of LDL from lipid peroxidation. Ann. N. Y. Acad. Sci. 957:321-324. [DOI] [PubMed] [Google Scholar]
- 19.Gouedard, C., N. Koum-Besson, R. Barouki, and Y. Morel. 2003. Opposite regulation of the human paraoxonase-1 Gene PON-1 by fenofibrate and statins. Mol. Pharmacol. 63:945-956. [DOI] [PubMed] [Google Scholar]
- 20.Henry, E. C., A. S. Kende, G. Rucci, M. J. Totleben, J. J. Willey, S. D. Dertinger, R. S. Pollenz, J. P. Jones, and T. A. Gasiewicz. 1999. Flavone antagonists bind competitively with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) to the aryl hydrocarbon receptor but inhibit nuclear uptake and transformation. Mol. Pharmacol. 55:716-725. [PubMed] [Google Scholar]
- 21.Hestermann, E. V., and M. Brown. 2003. Agonist and chemopreventative ligands induce differential transcriptional cofactor recruitment by aryl hydrocarbon receptor. Mol. Cell. Biol. 23:7920-7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jakubowski, H. 2000. Calcium-dependent human serum homocysteine thiolactone hydrolase. A protective mechanism against protein N-homocysteinylation. J. Biol. Chem. 275:3957-3962. [DOI] [PubMed] [Google Scholar]
- 23.Leikert, J. F., T. R. Rathel, P. Wohlfart, V. Cheynier, A. M. Vollmar, and V. M. Dirsch. 2002. Red wine polyphenols enhance endothelial nitric oxide synthase expression and subsequent nitric oxide release from endothelial cells. Circulation 106:1614-1617. [DOI] [PubMed] [Google Scholar]
- 24.Mackness, B., P. Durrington, P. McElduff, J. Yarnell, N. Azam, M. Watt, and M. Mackness. 2003. Low paraoxonase activity predicts coronary events in the Caerphilly Prospective Study. Circulation 107:2775-2779. [DOI] [PubMed] [Google Scholar]
- 25.Mackness, B., P. Durrington, A. Povey, S. Thomson, M. Dippnall, M. Mackness, T. Smith, and N. Cherry. 2003. Paraoxonase and susceptibility to organophosphorus poisoning in farmers dipping sheep. Pharmacogenetics 13:81-88. [DOI] [PubMed] [Google Scholar]
- 26.Mackness, M. I., B. Mackness, and P. N. Durrington. 2002. Paraoxonase and coronary heart disease. Atheroscler Suppl. 3:49-55. [DOI] [PubMed] [Google Scholar]
- 27.Matikainen, T., G. I. Perez, A. Jurisicova, J. K. Pru, J. J. Schlezinger, H. Y. Ryu, J. Laine, T. Sakai, S. J. Korsmeyer, R. F. Casper, D. H. Sherr, and J. L. Tilly. 2001. Aromatic hydrocarbon receptor-driven Bax gene expression is required for premature ovarian failure caused by biohazardous environmental chemicals. Nat. Genet. 28:355-360. [DOI] [PubMed] [Google Scholar]
- 28.Moiseeva, E. P. 2001. Adhesion receptors of vascular smooth muscle cells and their functions. Cardiovasc. Res. 52:372-386. [DOI] [PubMed] [Google Scholar]
- 29.Moorthy, B., K. P. Miller, W. Jiang, and K. S. Ramos. 2002. The atherogen 3-methylcholanthrene induces multiple DNA adducts in mouse aortic smooth muscle cells: role of cytochrome P4501B1. Cardiovasc. Res. 53:1002-1009. [DOI] [PubMed] [Google Scholar]
- 30.Morel, Y., N. Mermod, and R. Barouki. 1999. An autoregulatory loop controlling CYP1A1 gene expression: role of H2O2 and NFI. Mol. Cell. Biol. 19:6825-6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niermann, T., S. Schmutz, P. Erne, and T. Resink. 2003. Aryl hydrocarbon receptor ligands repress T-cadherin expression in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 300:943-949. [DOI] [PubMed] [Google Scholar]
- 32.Olthof, M. R., P. C. Hollman, M. N. Buijsman, J. M. van Amelsvoort, and M. B. Katan. 2003. Chlorogenic acid, quercetin-3-rutinoside and black tea phenols are extensively metabolized in humans. J. Nutr. 133:1806-1814. [DOI] [PubMed] [Google Scholar]
- 33.Puga, A., A. Maier, and M. Medvedovic. 2000. The transcriptional signature of dioxin in human hepatoma HepG2 cells. Biochem. Pharmacol. 60:1129-1142. [DOI] [PubMed] [Google Scholar]
- 34.Robert, K., J. F. Chasse, D. Santiard-Baron, C. Vayssettes, A. Chabli, J. Aupetit, N. Maeda, P. Kamoun, J. London, and N. Janel. 2003. Altered gene expression in liver from a murine model of hyperhomocysteinemia. J. Biol. Chem. 278:31504-31511. [DOI] [PubMed] [Google Scholar]
- 35.Rodrigo, L., A. F. Hernandez, J. J. Lopez-Caballero, F. Gil, and A. Pla. 2001. Immunohistochemical evidence for the expression and induction of paraoxonase in rat liver, kidney, lung and brain tissue. Implications for its physiological role. Chem.-Biol. Interact. 137:123-137. [DOI] [PubMed] [Google Scholar]
- 36.Rodrigo, L., B. Mackness, P. N. Durrington, A. Hernandez, and M. I. Mackness. 2001. Hydrolysis of platelet-activating factor by human serum paraoxonase. Biochem. J. 354:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savouret, J. F., M. Antenos, M. Quesne, J. Xu, E. Milgrom, and R. F. Casper. 2001. 7-Ketocholesterol is an endogenous modulator for the arylhydrocarbon receptor. J. Biol. Chem. 276:3054-3059. [DOI] [PubMed] [Google Scholar]
- 38.Savouret, J. F., A. Berdeaux, and R. F. Casper. 2003. The aryl hydrocarbon receptor and its xenobiotic ligands: a fundamental trigger for cardiovascular diseases. Nutr. Metab. Cardiovasc. Dis. 13:104-113. [DOI] [PubMed] [Google Scholar]
- 39.Scalbert, A., and G. Williamson. 2000. Dietary intake and bioavailability of polyphenols. J. Nutr. 130:2073S-2085S. [DOI] [PubMed] [Google Scholar]
- 40.Shih, D. M., L. Gu, Y. R. Xia, M. Navab, W. F. Li, S. Hama, L. W. Castellani, C. E. Furlong, L. G. Costa, A. M. Fogelman, and A. J. Lusis. 1998. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature 394:284-287. [DOI] [PubMed] [Google Scholar]
- 41.Swanson, H. I. 2002. DNA binding and protein interactions of the AHR/ARNT heterodimer that facilitate gene activation. Chem.-Biol. Interact. 141:63-76. [DOI] [PubMed] [Google Scholar]
- 42.Tward, A., Y. R. Xia, X. P. Wang, Y. S. Shi, C. Park, L. W. Castellani, A. J. Lusis, and D. M. Shih. 2002. Decreased atherosclerotic lesion formation in human serum paraoxonase transgenic mice. Circulation 106:484-490. [DOI] [PubMed] [Google Scholar]
- 43.Van Lenten, B. J., A. C. Wagner, M. Navab, and A. M. Fogelman. 2001. Oxidized phospholipids induce changes in hepatic paraoxonase and ApoJ but not monocyte chemoattractant protein-1 via interleukin-6. J. Biol. Chem. 276:1923-1929. [DOI] [PubMed] [Google Scholar]
- 44.Yao, E. F., and M. S. Denison. 1992. DNA sequence determinants for binding of transformed Ah receptor to a dioxin-responsive enhancer. Biochemistry 31:5060-5067. [DOI] [PubMed] [Google Scholar]