Abstract
Impaired cognitive function, along with positive and negative symptoms, is a core clinical feature of schizophrenia. Earlier studies suggest that impaired cognitive functioning should be assessed from the perspective of brain networks. The recently developed brainnetome approach to evaluating brain networks—an approach that was initially developed by Chinese scientists—provides a new methodology for studying this issue. In this paper we first introduce the concept of brainnetome. We then review recent progress in developing a brainnetome of impaired cognitive function in people with schizophrenia. The models of the relevant brain networks considered were created using data obtained from functional and anatomical brain imaging technologies at different levels of analysis: networks centered on regions of interest, networks related to specific cognitive functions, whole brain networks, and the attributes of brain networks. Finally, we discuss the current challenges and potential new directions for research about brainnetome.
Keywords: Brainnetome, Magnetic resonance imaging, Schizophrenia, Cognitive function
Schizophrenia is a severely disabling disease with a lifetime prevalence of 1% that mainly occurs in young and middle-aged people. The clinical symptoms are variable and range from hallucinations and delusions (positive symptoms) to flat or blunted affect and emotions (negative symptoms). Although these positive and negative symptoms are generally considered to be the characteristic features of schizophrenia, studies conducted in the last two decades have shown that impaired cognition should also be considered a core clinical symptom[1]–[4]. There is still disagreement about whether impaired cognition should be included in the diagnostic criteria for schizophrenia, but researchers agree that the extent of impaired cognitive function reflects the severity of the disease. For example, the American Psychiatric Association's upcoming fifth edition of the Diagnostic and Statistical Manual for mental disorders is planning to list impaired cognition as an important dimension for evaluating the severity of schizophrenia[5]. Working memory dysfunction is the most common and important type of impaired cognition in people with schizophrenia [6]–[10] so an understanding of the neural basis behind impaired working memory is considered essential to understanding the pathophysiology of schizophrenia [10]. The present manuscript discusses the contribution of a construct called the brainnetome in understanding impaired working memory and cognition in people with schizophrenia.
1. Brainnetome
The underlying neural mechanisms of impaired cognition, including the working memory dysfunction in people with schizophrenia, have been studied since the late 20th century. Goldman-Rakic and colleagues[10] suggested that the working memory dysfunction in individuals with schizophrenia was the result of abnormal brain networks. Based on electrophysiology results from non-human primates, he proposed that the neural activity underlying working memory is not limited to the frontal cortex but also requires interactions between the frontal cortex and other remote regions including the posterior parietal cortex, inferior temporal cortex, cingulate cortex and the hippocampus. These brain regions form fiber connections with the dorsolateral prefrontal cortex and interact via these connections. Since working memory plays a critical role in the integrity of various thought processes, abnormalities in this working memory circuit may be the neural basis of impaired cognition and, thus, be related to the psychotic symptoms observed in schizophrenia. Goldman-Rakic and colleagues proposed that impaired cognition in people with schizophrenia be studied in terms of brain networks but they provided no specific hypothesis about the neural mechanisms underlying this impaired cognition, possibly because of a shortage of technical strategies for studying neural connections.
The creation and development of the brainnetome provides a new opportunity for studying the neural mechanisms of impaired cognition in people with schizophrenia. Brain network research is on the frontier of international science and is one of the seven important areas of research identified by the journal Science in 2007. The National Institute of Health in the United States launched the Human Connectome Project [11],[12] that focuses on brain networks. During the past decade, a team led by the first author of this review proposed the development of an international project on the brainnetome[13], organized the 393rd Xiang-shan scientific symposium focusing on the brainnetome, and launched the brainnetome project with the support of China's National Program on Key Basic Research (the ‘973 program’)[14].
While different brain connectomes address the structural connections between different neurons (nuclei) or brain regions, brainnetome not only focuses on structural connections between neurons and brain regions but also emphasizes the dynamic characteristics of fully constructed brain networks and the relationship between brain network changes and neuropsychological disease. Specifically, the Brainnetome research project [13] focuses on the study of the topological structure, dynamics, and functions of brain networks as well as the superficial characteristics and genetic contents of dysfunctional brain networks from different temporospatial scales. By combining imaging techniques that have high temporospatial resolution with modern computational techniques, these programs can be used to develop models of the interactions between different brain regions and the dynamic evolution of brain networks. These models will facilitate systematic study of the structure and function of the brain on different temporospatial dimensions, help in the analysis of transmission mechanisms of internal signals using different brain indices, identity pathways for studying complicated information interactions and high-efficacy tissue structures within the brain, and explore new pathways for understanding information processing and the higher functions of the human brain. The study of brain networks has become a key research focus for information science, neuroscience, clinical medicine and other fields. The investigation of abnormal models and the specificity of these abnormal models in disease with respect to brain networks is not only helpful for promoting the understanding of normal and abnormal brain functions, but can also aid in the identification of specific imaging characteristics of diseases at the brain network level. This will be helpful in improving current diagnostic standards and, thus, is important both for theory and in the clinic. Currently, the brainnetome represents a new direction for studying the pathophysiological mechanisms of impaired cognition in schizophrenia.
2. Brain network study of impaired cognition in schizophrenia
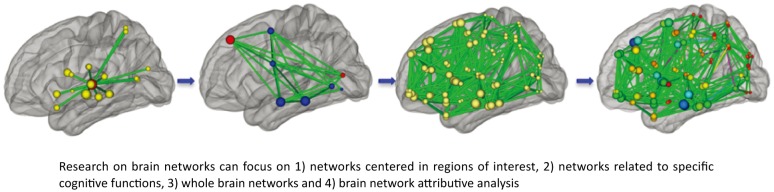
The human brain integrates and merges information from multiple regions to perform high-level cognitive functions. The human brain is a system with multiple levels and therefore needs to be studied at different levels. Brain network research can be divided into functional or anatomic approaches based on modes of imaging. Alternatively, this research can be divided into brain network analysis based on single region-of-interest, specific brain network analysis, whole brain network analysis, and brain network attributive analysis (Figure 1). By choosing investigative strategies based on different levels of analysis and imaging modes, we have generated some primary information about the mechanisms of impaired cognition and abnormal working memory in people with schizophrenia.
Figure 1. Schematic of brain networks.
2.1. Functional brain network during task
Single-unit recordings from non-human primates and imaging studies of the normal human brain have contributed to the recognition of the dorsolateral prefrontal cortex (DLPFC) as a core region of the working memory network. The majority of fMRI studies on working memory in patient with schizophrenia are focused on the DLPFC [15]–[19]. Using region-of-interest functional connectivity analyses, it was found that the task load of working memory can modulate the functional connectivity between the hippocampus and DLPFC in normal humans. However, in patients with schizophrenia, the correlation between the left hippocampus and the right DLPFC was not modulated by the task load [20]. By analyzing subcomponents of working memory, it was found that in patients with schizophrenia the connectivities between the prefrontal cortex and the hippocampus and intraparietal cortex were reduced during the non-articulatory maintenance of phonological information[21]. Moreover, connectivity between the DLPFC and the cerebellum was increased during the high-load state, and this was correlated with improvement of working memory in patients with schizophrenia[22].
The lateral frontoparietal network is considered the core network underlying adaptive cognition control[23],[24] so this was also a focus of study for the brainnetome investigation of impaired cognition in people with schizophrenia. By using independent components analyses methods, it was found that the connectivity in the bilateral DLPFC and the left inferior parietal lobe was increased during a verbal working memory task[25]. Furthermore, accuracy during the working memory task was positively correlated with the strength of functional connectivity in the left DLPFC [25]. The abnormal enhancement of fronto-parietal connections suggested the presence of a compensatory process in patients, that is, the DLPFC needs more involvement and interaction with the parietal cortex to satisfy the task requirement. However, when separating the encoding stage from the recognition stage during working memory, it was found that during the encoding stage the integration function of the bilateral fronto-parietal network in patient with schizophrenia was reduced and that the left fronto-parietal network displayed abnormal activity with a load-dependent functional response[26].
Although these studies found abnormal activity in the brain networks of patients while performing working memory tasks, these abnormal patterns were not consistent. This may be explained by the complexity of working memory tasks. Working memory tasks involve several different cognitive processes, including the initial information encoding stage, the subsequent maintenance stage (which includes updating and managing information) and the final selecting and responding stage. Each stage has a different neurobiological basis[27]. Additional study is necessary to further investigate the characteristics of brain networks of patients with schizophrenia by distinguishing the different cognitive stages or processes of working memory.
2.2. Functional brain network during rest
Resting fMRI studies have recently become popular because of various advantages, including the ease of working with subjects with neuropsychiatric disorders (without task design or subject training) and their comparability across different studies. At rest, blood-oxygen-level-dependent (BOLD) signals show spontaneous low-frequency fluctuations (SLFF) (<0.1 Hz), which have been verified as physiologically meaningful signals[28]. Through studies of the temporal correlation of the SLFF between different brain regions (via functional connectivities), it was found that functional connectivities during the resting state were not derived from respiration or heartbeats. In addition, the functional connectivities had anatomical foundations and existed at different resting conditions (e.g., eye-opening, eye-closing, visual fixation, and anesthesia), suggesting that resting-state functional connectivities reflect intrinsic brain activities [28]. The default-mode network is always the focus of studies of brain function in resting conditions[29],[30]. The default-mode network refers to the brain regions that are negatively activated in goal-directed tasks but are active in resting conditions. Regions involved in this network include the posterior cingulate cortex, the medial frontal cortex, and the lateral parietal cortex, among others. These brain regions have significant functional connectivities in resting conditions, and may reflect the active state of brain function at the “baseline” stage. Therefore, these were defined as the default-mode network[31],[32].
The functional activities of the brain default-mode network are modulated by working memory tasks and can predict individual variations in cognitive capability. Previous studies in normal humans investigated the functional connectivity between the core node in the default-mode network (i.e., the posterior cingulate cortex) and the core node in the medial prefrontal cortex [33], and assessed functional connectivities between the posterior cingulate cortex and other brain regions[34] during working memory tasks and during the resting state. The results indicated that during working memory tasks the functional connectivities between the two default-mode brain regions were weaker than during the resting state. However, the functional connectivities between the default-mode brain network and the working memory-related brain regions were enhanced, including those between the posterior cingulate cortex and the bilateral inferior parietal gyri, the medial prefrontal cortex, the left inferior frontal gyrus, and the superior parietal lobule [33],[34]. Using functional connectivity/network analysis methods based on graph theory, it was determined that the topological structure of core brain regions in the default-mode network during a working memory task was similar to that during rest, but the degree of connectivity between the brain regions related to task execution increased. This supports the notion of the increased importance of these brain regions during task performance[35]. Although the topological structure of networks in normal humans does not change, tasks do have modulating effects on the default-mode network. The scores on the working memory task are not only positively correlated with the functional connectivity strength of the default-mode network under different task conditions, but also positively correlated with the connectivity strength under the resting condition. This suggests that the strength of functional connectivities of the default-mode network during rest can predict individual differences in the cognitive ability required to complete the working memory task[33].
Researchers currently attribute abnormal cognitive function and impaired working memory in patients with schizophrenia to interference of the default-mode network[36], that is, the patient cannot transfer the necessary attention from intrinsic thoughts and emotions to the exterior stimulus, resulting in low cognitive scores. Studies have repeatedly found that the extent of deactivation of default-mode networks during working memory tasks is reduced in schizophrenia patients[37]–[40]. Previous investigations found that the functional connectivities between the bilateral DLPFC and the posterior cingulate gyrus in patients with first-episodic schizophrenia are reduced during rest[41]. However, the resting-state functional connectivities in the regions constituting the default-mode brain network, the connectivities in the regions constituting the task-positive brain network, and the connectivities between these two brain networks are increased in patients with paranoid schizophrenia[42]. Additionally, the abnormally enhanced functional connectivities in the default-mode brain network are also found in healthy siblings of people with schizophrenia, suggesting that the abnormal functional connectivities of the default-mode brain network may be important characteristics of the disease [43]. An investigation of the modulating effect of working memory task state on functional connectivities of the default-mode network in patients with schizophrenia revealed that the functional connectivities of the default-mode network are found both while performing working memory tasks and during rest, similar to findings in normal controls. However, these connectivities were significantly stronger in people with schizophrenia than in normal individuals and were correlated with the severity of positive symptoms[39].
3. Anatomical brain network
Diffusion Tensor Imaging (DTI) is a recent advance in MRI technology that can provide diffusion information about hydrogen molecules in brain tissue and thus indirectly reflect the physical and functional characteristics of white matter in the brain[44]. DTI represents a departure from the traditional in vitro fiber tracing methods of white matter and makes it possible to study white matter fibers in vivo. Based on anatomical knowledge, all white matter fibers originate from gray matter structures and end in other gray matter structures. Therefore, DTI is a powerful technique for directly describing the anatomical networks of brain.
Previous studies have indicated that the superior frontal-intraparietal network, which comprises the superior longitudinal fasciculus connecting the frontal and parietal cortices and the adjacent gray matter, is an important component of the working memory brain network [45]. The integrity of the superior longitudinal fasciculus is decreased in people with recent-onset schizophrenia, and performance on a linguistic working memory task is correlated with disrupted fiber integrity. This structural change may be one reason for the difficulty in recovery of cognitive abilities in people with schizophrenia [46]. It has also been demonstrated that the anatomical connectivity between the DLPFC and the anterior cingulate cortex is reduced in patients with schizophrenia and that this structural abnormality is related to working memory task performance [47],[48]. By combining DTI and fMRI findings on working memory tasks, Schlosser and colleagues[49] found that the fractional anisotropy (FA) in the right medial parietal and frontal lobes was reduced in people with schizophrenia, and that the activation of prefrontal, superior parietal, and occipital lobes during a working memory task was weakened. More importantly, the abnormal white matter in the prefrontal lobes of patients with schizophrenia was directly correlated with the extent of activation of the prefrontal and occipital lobes. These results suggest that the integrity of the anatomical connectivities underlying the working memory network are related to behavioral test scores, and that abnormal anatomical connectivities can result in changes of brain activation during working memory tasks. However, these studies are only the starting point—the etiological role of abnormal anatomical connectivities between the core brain regions in the working memory network as a cause of the impaired working memory in people with schizophrenia needs to be verified in future studies.
4. Topological properties of the brain network
Brain network analysis based on graph theory is a new method for studying the pathophysiological mechanisms of neuropsychological diseases. A variety of imaging studies have indicated that the human brain is a complicated network with small-world topology [50],[51]. This efficient topological structure changes with aging[52],[53] and state[54], and has a genetic basis[55]. Using this analysis method, we found that abnormal resting-state functional connectivities were widely distributed within the entire brain in people with schizophrenia [56]. Furthermore, we found that the topological properties of the brain functional network are changed in people with schizophrenia. These differences are demonstrated by a decreased cluster coefficient, prolonged minimal path length, and reduced information transmission efficiency, all of which suggest abnormal information exchange of the brain functional network[57]. Additionally, the topological structural characteristics of the brain functional network were significantly correlated with clinical indices; the longer the duration of illness the lower the efficiency of information transmission[57] and the extent of the abnormal brain anatomical characteristics was correlated with clinical severity [58]. Similar results were obtained by constructing brain networks using EEG[59],[60] and structural MRI[61]. All of these studies of brain networks revealed abnormal connectivity patterns in people with schizophrenia. The integrated information obtained by brain network analysis provides a new strategy for understanding the pathophysiological mechanisms of schizophrenia, potentially aiding early diagnosis and improving the efficacy of clinical evaluations.
Cognitive functions like working memory cannot be independently executed by one neuron or one single brain region but are accomplished by interactions between multiple brain regions. The study of local brain regions and the interactions among these brain regions is the core focus of brainnetome studies. The dynamic interaction mode between brain regions is the basis of human cognitive processes and operations[62]. Understanding how the human brain generates cognition depends on knowledge of large-scale brain organization [63]. It is known that the characteristics of the brain structural network are significantly correlated with intelligence[64]. A team lead by Professor Bullmore at the University of Cambridge[65] found that behavioral performance on a verbal fluency task was positively correlated with functional connectivities and with the topological characteristics of the functional network during rest. Specifically, better behavioral performance was positively correlated with greater functional connectivity strength and integration, higher small-world-like characteristics and clustering coefficiency, and a more hub-dominated degree of distribution during rest. The team found that this relationship also existed in patients with schizophrenia, among whom there was reduced clustering and small-world-like characteristics, a reduced probability of high-degree hubs, and increased robustness. Relatively intact global properties of network topology in the brain functional network were found in people with first-episode schizophrenia while performing a cognitive control task, but there were widespread abnormalities in the connectivities, particularly in cognitive control-related brain regions [66]. Using magnetoencephalography, this team also compared brain networks in people with schizophrenia and normal controls while performing a working memory task. The results indicated that working memory performance was positively correlated with the global cost efficiency of the beta-band network and with the cost efficiency of nodes in the left lateral, parietal and frontal areas in both groups[67] .
5. Current situation of brainnetome study in China
Brainnetome is a field of study that was originally proposed by Chinese scientists and is gradually being accepted by more and more researchers. Chinese researchers have made important contributions in this area. Through several years of investigations, the team led by the first author of this review (Professor Jiang) progressed from study of the functional connection of areas of interest, to the study of functional connection analysis of brain sub-systems, and finally to the study of the functional connection modes of the whole brain and the characteristic analysis of brain networks. Through these studies they systemically established a new framework for data analysis of the brain functional network in the resting state and applied it to the study of multiple brain diseases, including schizophrenia [41]–[43],[56],[57]. Their models of the functioning of brain networks in various diseases established a methodological basis for completely and correctly understanding the mechanisms of information management in the brain and the changes in brain function that accompany disease conditions. There method of studying the brain structural network of normal subjects and neuropsychological patients by measuring cortical thickness using structural MRI and cortical reflection techniques resulted in the generation of important structural evidence for understanding the pathophysiological mechanisms of “connection loss” seen in people with Alzheimer's disease [68]. Extension of this method for constructing brain anatomic networks using fiber tracing, successfully identified the weighted and non-weighted whole-brain anatomic network of young participants and verified the significant correlation between individual intelligence and brain network characteristics [64]. This research group also used these methods to study brain anatomic networks based on diffused MRI to compare the normal population to patient populations and found that the information transmission efficacy of the brain network in people with schizophrenia was significantly decreased and, moreover, was significantly correlated with clinical scores reflecting disease severity (that is, greater disease severity was related to lower information transmission efficacy) which provided new imaging evidence for schizophrenia research [58].
Domestic researchers in China have their own areas of specialization. For example, Yong He and colleagues in Beijing Normal University have a unique contribution to the study of brain structural networks based on morphological data from structural MRI. For the first time, this team established the brain structural network in a normal population using morphological data about brain white matter from structural MRI images and applied these to the study of Alzheimer's disease[69] and multiple sclerosis[70]. The team led by De-zhong Yao from the institute of Electronic Science and Technology established a brain network using EEG techniques[71] and proposed the spatial fusion method of EEG and fMRI for researching brain networks[72]–[74].
Compared to foreign research teams, Chinese researchers take advantage of the large number of available subjects with different diseases. Combining the large samples makes it possible to adjust for the variability in findings caused by patient heterogeneity and long-term medication use, thus providing a clearer picture of the pathophysiological mechanisms of schizophrenia. Currently, with the support of China's 973 National Natural Science Foundation Key program, a consortium of 10 specialized centers in China is establishing a multiple-center imaging database of people with schizophrenia. On the basis of this collaboration, new methods and techniques of brainnetome research are being developed that will generate an improved brain map with clearer functional significance and more detailed anatomical classifications. It is hoped that this work will lead to important breakthroughs in the study of cognitive disorders in people with schizophrenia.
6. Prospects
The study of schizophrenia from the perspective of the brainnetome is just beginning. Despite exciting new findings in the brainnetome study of cognitive dysfunction in people with schizophrenia, there are many issues that remain unresolved. There is no clear answer to core scientific questions regarding which core brain regions and key abnormal connectivities are related to abnormal cognition in people with schizophrenia. Currently, knowledge about the brain mechanisms of cognitive disorders is mainly derived from functional imaging studies; there is very little understanding of the related anatomical networks. Brain anatomical networks are the basis of functional networks and provide anatomical constructs for functional interactions so it is essential to combine studies of anatomical and functional networks to fully explain the brain network mechanisms of cognitive disorders in schizophrenia. In summary, the brainnetome brings a new and exciting perspective to the study of psychological diseases that has the potential of increasing our understanding of the basic mechanisms that result in schizophrenia.
Footnotes
Funding: Supported by the National Key Basic Research Program of China (2011CB707800), the Young Program from the National Natural Science Foundation of China (30900487) and the Open Project of National Laboratory of Pattern Recognition.
References
- 1.Andreasen NC. Linking mind and brain in the study of mental illnesses: a project for a scientific psychopathology. Science. 1997;275(5306):1586–1593. doi: 10.1126/science.275.5306.1586. [DOI] [PubMed] [Google Scholar]
- 2.Elvevag B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol. 2000;14(1):1–21. [PubMed] [Google Scholar]
- 3.Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6(4):348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- 4.Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, "just the facts" 4. Clinical features and conceptualization. Schizophr Res. 2009;110(1–3):1–23. doi: 10.1016/j.schres.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 5.American Psychiatric Association DSM-5 development: B 00 Schizophrenia. [2011-12-6] http://www.dsm5.org/ProposedRevision/Pages/proposedrevision.aspx?rid=411#.
- 6.Goldman-Rakic PS. Regional and cellular fractionation of working memory. Proc Natl Acad Sci USA. 1996;93(24):13473–13480. doi: 10.1073/pnas.93.24.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baddeley A. The fractionation of working memory. Proc Natl Acad Sci USA. 1996;93(24):13458–13472. doi: 10.1073/pnas.93.24.13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piskulic D, Olver JS, Norman TR, Maruff P. Behavioural studies of spatial working memory dysfunction in schizophrenia: a quantitative literature review. Psychiatry Res. 2007;150(2):111–121. doi: 10.1016/j.psychres.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 9.Silver H, Feldman P, Bilker W, Gur RC. Working memory deficit as a core neuropsychological dysfunction in schizophrenia. Am J Psychiatry. 2003;160(10):1809–1816. doi: 10.1176/appi.ajp.160.10.1809. [DOI] [PubMed] [Google Scholar]
- 10.Goldman-Rakic PS. The physiological approach: functional architecture of working memory and disordered cognition in schizophrenia. Biol Psychiatry. 1999;46(5):650–661. doi: 10.1016/s0006-3223(99)00130-4. [DOI] [PubMed] [Google Scholar]
- 11. http://www.humanconnectomeproject.org/.
- 12. http://humanconnectome.org/.
- 13.Yao Z, Zhang Y, Lin L, Zhou Y, Xu C, Jiang T. Alzheimer's disease neuroimaging initiative. Abnormal cortical networks in mild cognitive impairment and Alzheimer's disease. PLoS Comput Biol. 2010;6(11):e1001006. doi: 10.1371/journal.pcbi.1001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. http://www.brainnetome.org/.
- 15.Callicott JH, Mattay VS, Verchinski BA, Marenco S, Eqan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry. 2003;160(12):2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- 16.Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, et al. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Human Brain Mapp. 2005;25(1):60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karlsgodt KH, Glahn DC, van Erp TG, Therman S, Huttunen M, Manninen M, et al. The relationship between performance and fMRI signal during working memory in patients with schizophrenia, unaffected co-twins, and control subjects. Schizophr Res. 2007;89(1–3):191–197. doi: 10.1016/j.schres.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Metzak PD, Riley JD, Wang L, Whitman JC, Ngan ET, Woodward TS. Decreased efficiency of task-positive and task-negative networks during working memory in schizophrenia. Schizophr Bull. 2011 doi: 10.1093/schbul/sbq154. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Potkin SG, Turner JA, Brown GG, McCarthy G, Greve DN, Glover GH, et al. Working memory and DLPFC inefficiency in schizophrenia: the FBIRN study. Schizophr Bull. 2009;35(1):19–31. doi: 10.1093/schbul/sbn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer-Lindenberg AS, Olsen RK, Kohn RD, Brown T, Egan MF, Weinberger DR, et al. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry. 2005;62(4):379–386. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- 21.Henseler I, Falkai P, Gruber O. Disturbed functional connectivity within brain networks subserving domain-specific subcomponents of working memory in schizophrenia: relation to performance and clinical symptoms. J Psychiatr Res. 2010;44(6):364–372. doi: 10.1016/j.jpsychires.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Kumari V, Peters ER, Fannon D, Antonova E, Premkumar P, Anilkumar AP, et al. Dorsolateral prefrontal cortex activity predicts responsiveness to cognitive-behavioral therapy in schizophrenia. Biol Psychiatry. 2009;66(6):594–602. doi: 10.1016/j.biopsych.2009.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Desenbach RA, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolf RC, Vasic N, Sambataro F, Höse A, Frasch K, Schmid M, et al. Temporally anticorrelated brain networks during working memory performance reveal aberrant prefrontal and hippocampal connectivity in patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(8):1464–1473. doi: 10.1016/j.pnpbp.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 26.Meda SA, Stevens MC, Folley BS, Calhoun VD, Pearlson GD. Evidence for anomalous network connectivity during working memory encoding in schizophrenia: an ICA based analysis. PLoS One. 2009;4(11):e7911. doi: 10.1371/journal.pone.0007911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Driesen NR, Leung HC, Calhoun VD, Constable RT, Gueorguieva R, Hoffman R, et al. Impairment of working memory maintenance and response in schizophrenia: functional magnetic resonance imaging evidence. Biol Psychiatry. 2008;64(12):1026–1034. doi: 10.1016/j.biopsych.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 29.Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33(3):279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 31.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26(51):13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bluhm RL, Clark CR, McFarlane AC, Moores KA, Shaw ME, Lanius RA. Default network connectivity during a working memory task. Human Brain Mapp. 2011;32(7):1029–1035. doi: 10.1002/hbm.21090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci. 2009;29(6):1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sonuga-Barke EJ, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci Biobehav Rev. 2007;31(7):977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Guerrero-Pedraza A, McKenna PJ, Gomar JJ, Sarró S, Salvador R, Amann B, et al. First-episode psychosis is characterized by failure of deactivation but not by hypo- or hyperfrontality. Psychol Med. 2012;42(1):73–84. doi: 10.1017/S0033291711001073. [DOI] [PubMed] [Google Scholar]
- 38.Pomarol-Clotet E, Canales-Rodriques EJ, Salvador R, Sarró S, Gomar JJ, Vila F, et al. Medial prefrontal cortex pathology in schizophrenia as revealed by convergent findings from multimodal imaging. Mol Psychiatry. 2010;15(8):823–830. doi: 10.1038/mp.2009.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA. 2009;106(4):1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salvador R, Martinez A, Pomarol-Clotet E, Gomar J, Vila F, Sarró S, et al. A simple view of the brain through a frequency-specific functional connectivity measure. Neuroimage. 2008;39(1):279–289. doi: 10.1016/j.neuroimage.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 41.Zhou Y, Liang M, Jiang T, Tian L, Liu Y, Liu Z, et al. Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neurosci Lett. 2007;417(3):297–302. doi: 10.1016/j.neulet.2007.02.081. [DOI] [PubMed] [Google Scholar]
- 42.Zhou Y, Liang M, Tian L, Wang K, Hao Y, Liu H, et al. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res. 2007;97(1–3):194–205. doi: 10.1016/j.schres.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 43.Liu H, Kaneko Y, Ouyang X, Li L, Hao Y, Chen EY, et al. Schizophrenic patients and their unaffected siblings share increased resting-state connectivity in the task-negative network but not its anticorrelated task-positive network. Schizophr Bull. 2010 doi: 10.1093/schbul/sbq074. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 45.Klingberg T. Development of a superior frontal-intraparietal network for visuo-spatial working memory. Neuropsychologia. 2006;44(11):2171–2177. doi: 10.1016/j.neuropsychologia.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 46.Karlsgodt KH, van Erp TG, Poldrack RA, Bearden CE, Nuechterlein KH, Cannon TD. Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent-onset schizophrenia. Biol Psychiatry. 2008;63(5):512–518. doi: 10.1016/j.biopsych.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 47.Kubicki M, Westin CF, Nestor PG, Wible CG, Frumin M, Maier SE, et al. Cingulate fasciculus integrity disruption in schizophrenia: a magnetic resonance diffusion tensor imaging study. Biol Psychiatry. 2003;54(11):1171–1180. doi: 10.1016/s0006-3223(03)00419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meyer-Lindenberg A, Poline JB, Kohn PD, Holt JL, Egan MF, Weinberger DR, et al. Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry. 2001;158(11):1809–1817. doi: 10.1176/appi.ajp.158.11.1809. [DOI] [PubMed] [Google Scholar]
- 49.Schlösser RG, Nenadic I, Wagner G, Güllmar D, von Consbruch K, Köhler S, et al. White matter abnormalities and brain activation in schizophrenia: a combined DTI and fMRI study. Schizophr Res. 2007;89(1–3):1–11. doi: 10.1016/j.schres.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 50.Bullmore ET, Bassett DS. Brain graphs: graphical models of the human brain connectome. Annu Rev Clin Psychol. 2011;7:113–140. doi: 10.1146/annurev-clinpsy-040510-143934. [DOI] [PubMed] [Google Scholar]
- 51.Stam CJ, Reijneveld JC. Graph theoretical analysis of complex networks in the brain. Nonlinear Biomed Physics. 2007;1(1):3. doi: 10.1186/1753-4631-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Achard S, Bullmore E. Efficiency and cost of economical brain functional networks. PLoS Comput Biol. 2007;3(2):e17. doi: 10.1371/journal.pcbi.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Micheloyannis S, Vourkas M, Tsirka V, Karakonstantaki E, Kanatsouli K, Stam CJ. The influence of ageing on complex brain networks: a graph theoretical analysis. Human Brain Mapp. 2009;30(1):200–208. doi: 10.1002/hbm.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferri R, Rundo F, Bruni O, Terzano MG, Stam CJ. Small-world network organization of functional connectivity of EEG slow-wave activity during sleep. Clin Neurophysiol. 2007;118(2):449–456. doi: 10.1016/j.clinph.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 55.Posthuma D, de Geus EJ, Mulder EJ, Smit DJ, Boomsma DI, Stam CJ. Genetic components of functional connectivity in the brain: the heritability of synchronization likelihood. Human Brain Mapp. 2005;26(3):191–198. doi: 10.1002/hbm.20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liang M, Zhou Y, Jiang T, Liu Z, Tian L, Liu H, et al. Widespread functional disconnectivity in schizophrenia with resting-state functional magnetic resonance imaging. Neuroreport. 2006;17(2):209–213. doi: 10.1097/01.wnr.0000198434.06518.b8. [DOI] [PubMed] [Google Scholar]
- 57.Liu Y, Liang M, Zhou Y, He Y, Hao Y, Song M, et al. Disrupted small-world networks in schizophrenia. Brain. 2008;131(4):945–961. doi: 10.1093/brain/awn018. [DOI] [PubMed] [Google Scholar]
- 58.Wang Q, Su TP, Zhou Y, Chou KH, Chen IY, Jiang T, et al. Anatomical insights into disrupted small-world networks in schizophrenia. NeuroImage. 2012;59(2):1085–1093. doi: 10.1016/j.neuroimage.2011.09.035. [DOI] [PubMed] [Google Scholar]
- 59.Micheloyannis S, Pachou E, Stam CJ, Breakspear M, Bitsios P, Vourkas M, et al. Small-world networks and disturbed functional connectivity in schizophrenia. Schizophr Res. 2006;87(1–3):60–66. doi: 10.1016/j.schres.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 60.Rubinov M, Knock SA, Stam CJ, Micheloyannis S, Harris AW, Williams LM, et al. Small-world properties of nonlinear brain activity in schizophrenia. Hum Brain Mapp. 2009;30(2):403–416. doi: 10.1002/hbm.20517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci. 2008;28(37):9239–9248. doi: 10.1523/JNEUROSCI.1929-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deco G, Buehlmann A, Masquelier T, Hugues E. The role of rhythmic neural synchronization in rest and task conditions. Front Hum Neurosci. 2011;5:4. doi: 10.3389/fnhum.2011.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci. 2010;14(6):277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 64.Li Y, Liu Y, Li J, Qin W, Li K, Yu C, et al. Brain anatomical network and intelligence. PLoS Comput Biol. 2009;5(5):e1000395. doi: 10.1371/journal.pcbi.1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lynall ME, Bassett DS, Kerwin R, McKenna PJ, Kitzbichler M, Muller U, et al. Functional connectivity and brain networks in schizophrenia. J Neurosci. 2010;30(28):9477–9487. doi: 10.1523/JNEUROSCI.0333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fornito A, Yoon J, Zalesky A, Bullmore ET, Carter CS. General and specific functional connectivity disturbances in first-episode schizophrenia during cognitive control performance. Biol Psychiatry. 2011;70(1):64–72. doi: 10.1016/j.biopsych.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bassett DS, Bullmore ET, Meyer-Lindenberg A, Apud JA, Weinberger DR, Coppola R. Cognitive fitness of cost-efficient brain functional networks. Proc Natl Acad Sci USA. 2009;106(28):11747–11752. doi: 10.1073/pnas.0903641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yao Z, Zhang Y, Lin L, Zhou Y, Xu C, Jiang T. Abnormal cortical networks in mild cognitive impairment and Alzheimer's disease. PLoS Comput Biol. 2010;6(11):e1001006. doi: 10.1371/journal.pcbi.1001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.He Y, Chen Z, Evans A. Structural insights into aberrant topological patterns of large-scale cortical networks in Alzheimer's disease. J Neurosci. 2008;28(18):4756–4766. doi: 10.1523/JNEUROSCI.0141-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He Y, Dagher A, Chen Z, Charil A, Zijdenbos A, Worsley K, et al. Impaired small-world efficiency in structural cortical networks in multiple sclerosis associated with white matter lesion load. Brain. 2009;132(12):3366–3379. doi: 10.1093/brain/awp089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qin Y, Xu P, Yao D. A comparative study of different references for EEG default mode network: the use of the infinity reference. Clin Neurophysiol. 2010;121(12):1981–1991. doi: 10.1016/j.clinph.2010.03.056. [DOI] [PubMed] [Google Scholar]
- 72.Lei X, Hu J, Yao D. Incorporating fMRI functional networks in EEG source imaging: a Bayesian Model comparison approach. Brain Topogr. 2012;25(1):27–38. doi: 10.1007/s10548-011-0187-9. [DOI] [PubMed] [Google Scholar]
- 73.Lei X, Ostwald D, Hu J, Qiu C, Porcaro C, Baqshaw AP, et al. Multimodal functional network connectivity: an EEG-fMRI fusion in network space. PLoS ONE. 2011;6(9):e24642. doi: 10.1371/journal.pone.0024642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lei X, Xu P, Luo C, Zhao J, Zhou D, Yao D. fMRI functional networks for EEG source imaging. Hum Brain Mapp. 2011;32(7):1141–1160. doi: 10.1002/hbm.21098. [DOI] [PMC free article] [PubMed] [Google Scholar]


