Abstract
Background
Findings from previous studies linking brain-derived neurotrophic factor (BDNF) and schizophrenia are inconsistent and few studies have assessed the relationship between BDNF C270T gene polymorphisms and the clinical and cognitive symptoms of schizophrenia.
Aim
Compare the prevalence of the BDNF C270T gene polymorphisms between patients with schizophrenia and controls and, in the patients, assess the relationship of genotypes to the severity of symptoms.
Methods
BDNF C270T genotype and allele frequency were measured using Polymerase Chain Reaction methods in 224 drug-free patients with schizophrenia and 220 controls. Psychotic symptoms were assessed using the Positive and Negative Syndrome Scale (PANSS), and cognitive functioning was assessed using the Wisconsin Card Sorting Test (WCST) and the Trail Making Test (TMT). In the patient group, differences in severity of symptoms across the three genotypes (i.e., C/C, C/T, and T/T) of C270T were assessed using one-way analysis of variance.
Results
The frequency of the T allele was much higher in patients than in controls (15.6% vs. 4.3%, χ2=31.47, p<0.001) and the C/T genotype was more common among patients than controls (27.7% vs. 7.7%, χ2=34.93, p<0.001). Compared to controls, patients performed poorly on all the cognitive tests, but there were no significant differences in the cognitive measures between patients with the three different genotypes. The total PANSS score, the PANSS negative symptoms subscale score, and the PANSS general psychopathology subscale score were not significantly different between the three groups of patients. However, the PANSS positive symptoms subscale score showed a small, statistically significant elevation in the severity of positive symptoms in the C/T genotype compared to the C/C genotype.
Conclusion
We confirm previous findings about differences in the prevalence of the BDNF C270T gene polymorphisms in schizophrenia, but do not find strong evidence of a relationship between different genotypes and the severity of the clinical or cognitive symptoms of schizophrenia. Clinical and cognitive symptoms in schizophrenia fluctuate over the course of the illness and with treatment, so stable, individual-specific measures of these parameters (that is, traits) need to be identified before it will be possible to definitively assess their relationship to different genotypes.
Abstract
背景
关于脑源性神经营养因子(brain-derived neurotrophic factor, BDNF) 与精神分裂症的关系研究结果不尽一致,BDNF C270T基因多态性与精神分裂症精神病理症状和认知功能的关系研究较少。
目的
比较精神分裂症患者与对照的BDNF C270T基因多态性,并评估精神分裂症患者的基因型与症状严重程度之间的关系。
方法
采用聚合酶链反应技术检测224例未服药精神分裂症患者和220名正常人的BDNF C270T基因型和等位基因分布频率,采用阳性与阴性症状量表(Positive and Negative Syndrome Scale, PANSS)评定精神分裂症的精神病理症状,采用威斯康星卡片分类测验(Wisconsin Card Sorting Test, WCST)和连线测验(Trail Making Test, TMT)评定认知功能。采用单因素方差分析评估C270T三种不同基因型(C/C、C/T和T/T)患者症状严重程度的差异。
结果
患者的T等位基因频率高于对照组(15.6% 比 4.3%, χ2=31.47, p<0.001),C/T基因型更常见于患者组(27.7% 比7.7%, χ2=34.93, p<0.001)。与对照相比,患者组所有的认知测验表现均较差,但三种不同基因型患者的认知评定结果无统计学差异。三组患者的PANSS总分、PANSS阴性症状分和PANSS一般精神病理量表评分无统计学差异。但是,C/T基因型患者的PANSS阳性症状分略高于C/C基因型患者,差异具有统计学意义。
结论
我们证实了既往报道中精神分裂症患者BDNF C270T基因多态性不一致的结果,但未发现不同基因型与精神分裂症的临床或认知症状严重程度相关的强有力证据。精神分裂症患者的临床和认知症状随病程和治疗而波动,因此需要确定稳定的、个体特异性(即特质性)的评定上述参数的指标,才有可能明确它们与不同基因型间的关系。
1. Background
It has been widely documented that multiple genes are involved in the development of schizophrenia. Previous studies have estimated the heritability of schizophrenia to be 80 to 85%.[1]–[3] Recently, there has been increased interest in the relationship between brain-derived neurotrophic factor (BDNF) and schizophrenia. The BDNF gene is expressed in numerous brain regions including the neocortex, hippocampus and amygdale. The BDNF gene is located on chromosome 11p13 and the C270T single nucleotide polymorphism (SNP) is in the 5′-noncoding promoter region of the gene. The C-T exchange in the C270T SNP may influence transcription of the BDNF gene and subsequently impact the expression of the protein.[4] The biological plausibility of this potentially causative mechanism spurred interest in the relationship between BDNF C270T polymorphism and schizophrenia. Previous studies on this issue from China and other countries have yielded inconsistent results.[5]–[7] Moreover, there have been no studies in China about the relationship between BDNF C270T polymorphism and the psychotic symptoms (including positive and negative symptoms) or cognitive functioning of patients with schizophrenia.
The current study uses a case-control design to assess the relationship between BDNF C270T polymorphism and schizophrenia using a sample of 224 patients with schizophrenia and 220 controls without schizophrenia. Within the patient group we also assess the relationship between symptoms, cognitive functioning, and BDNF C270T polymorphisms.
2. Methods
2.1. Sample
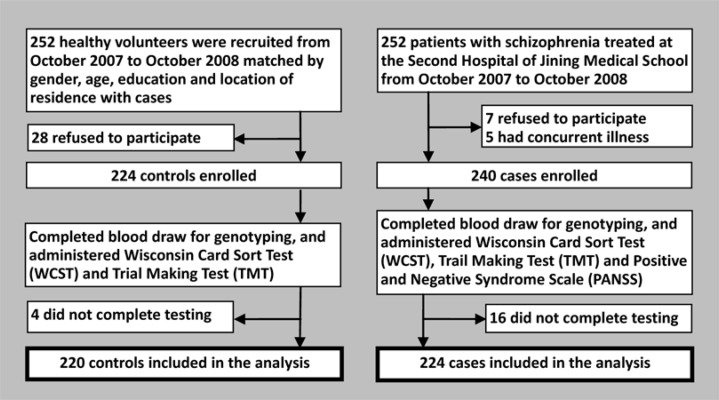
The identification of cases and controls is shown in Figure 1. Patients with schizophrenia were recruited from the Second Hospital of Jining Medical University from October 2007 to October 2008. A total of 252 patients with schizophrenia were identified and 224 completed the assessments. The diagnosis of schizophrenia was based on criteria specified in the American Psychiatric Association's Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV).[8] Exclusion criteria included the following: blood relative of another participant, neurological illnesses, other mental disorders, substance use disorders, family history of neurological or mental disorders, secondary cognitive impairment from brain trauma, severe physical illnesses, or an IQ of less than 70. Patients who had ever received electroconvulsive therapy or who were currently using antipsychotic, antidepressant or antimanic medications were also excluded.
Figure 1. Enrollment of cases and controls.
All cases were of Han ethnicity from Shandong province. There were 148 inpatients and 76 outpatients including 118 males and 106 females. Patients were 16 to 60 years old with a mean (sd) age of 31.5 (12.2) years. Age of onset was 12 to 50 years of age with a mean (sd) of 23.4 (6.6) years. The mean (sd) duration of illness was 42.3 (9.8) months. Mean years of education was 11.4 (2.4) years. Among these cases, 144 were drug-naïve (i.e., had never used antipsychotic medication) and the remaining 80 had been drug-free for a minimum of two months prior to the assessment.
Controls consisted of healthy volunteers (using the same exclusion criteria as those for cases) matched with cases on gender, age, educational level and location of residence (urban or rural resident). A total of 252 potential controls were identified and 220 completed the assessments; they included 115 males and 105 females, were 17 to 60 years of old, and had a mean age of 32.4 (11.6) years.
2.2. Clinical assessments
The Positive and Negative Syndrome Scale (PANSS) was used to assess clinical symptoms among the patients.[9],[10] This clinician-administered scale generates a total score and three subscale scores: a positive symptom score, a negative symptom score and a general psychopathology score. The Chinese translation of this scale has good test-retest reliability for the total score and the three subscale scores (ICC, 0.77 to 0.89) and good internal consistency for the total score (alpha=0.87) and the three subscale scores (alpha, 0.74 to 0.90). Two trained psychiatrists, neither of whom participated in the genotyping, administered the PANSS.
The Wisconsin Card Sorting Test (WCST-64)[11] and Trail Making Tests (TMT)[12] were used to assess cognitive functioning. The total number of completed categories and the number of perseverative errors were used as the outcome measures for the WCST and the time to completion of Trail Making Test A and Trail Making Test B were used as the outcome measures for the TMT.
2.3. DNA extraction and genotyping
All participants provided 5ml of blood in the morning. Edetic acid (12g/L) was used to prevent coagulation; mononuclear cells were separated with a lymph cell separation buffer; and the routine phenol/chloroform method was used to extract DNA. DNA was stored at -70°C.
Polymerase Chain Reaction (PCR) was used to analyze BDNF C270T polymorphism with the upstream primer 5′-CAGAGGAGCCAGCCCGGTGCG-3′ and downstream primer 5′-CTCCTGCACCAAGCCCCATTC-3′ (provided by Shenzhen JingmeiCo.). 100ng of DNA was dissolved in a 25µl system that included 1.1µmol/LMgCl2, 200µmol/LdNTP (dATP, dCTP, dGTP, dTTP), 1µmol/L primers, and 1.5uTaq enzyme.
Amplification of DNA was conducted by Polymerase Chain Reaction (PCR). First, DNA was predenatured at 94°C for 5 minutes. Next, PCR was performed by 30 cycles of DNA denaturation at 94°C for 30s, primer annealing at 60°C for 30s, and DNA elongation at 72°C for 30s. Subsequently, DNA was further elongated at 72°C for 30s. Amplified DNA was cut by the Hinfl enzyme and then separated via polyacrylamide gel electrophoresis (PAGE, 60g/L). Silver dye was used to visualize the band and molecular standardswere used to compare the size of pieces in order to read the genotype. A single section of 213bp is T/T genotype; two sections of 87bp and 126bp each are C/C genotype; three sections of 213bp, 126bp, and 87bp are each C/T genotype.
2.4. Statistical analysis
Data were analysed using SPSS16.0 statistical software. Hardy-Weinberg equilibrium was tested using Pearson's χ2 test for the case group and, because of the low prevalence of the T allele in the control group (4.3%), by an exact method[13] in the control group. Variations in BDNF C270T polymorphisms and allele frequencies between cases and controls were analysed using χ2 tests. A Tukey-type multiple comparison method based on the arcsin transformation of the original proportions was used to compare the proportion of cases and controls in the three genotypes.[14] In the patient group, one-way analysis of variance (ANOVA) was used to compare measures of the severity of symptoms (PANSS) and of cognitive functioning (WCST and TMT) between the three C270T genotypes. If the F-test for continuous variables was statistically significant, the Student-Newman-Keuls (SNK-q) test was used for post-hoc multiple comparisons between groups.
3. Results
3.1. Hardy-Weinberg equilibrium
Results from the χ2 test for the case group found no significant deviation in the observed proportions of BDNF genotypes compared to the expected proportions based on the Hardy-Weinberg equilibrium (χ2=0.55, p=0.758). Results of using the exact method to assess Hardy-Weinberg equilibrium[13] in the control group found a nonsignificant excess in the proportion of controls with the T/T genotype (exact p=0.051).
3.2. Differences in BDNF C270T polymorphisms and allele frequencies between cases and controls
As shown in Table 1, the T allele frequency and the proportion of individuals with the C/T genotype were both significantly higher among patients with schizophrenia than in controls. This was true for both males and females. There were, however, no significant differences in the prevalence of the two alleles in male versus female patients (χ2=0.56, p=0.453) or in the proportions of the three genotypes in male versus female patients (χ2=0.66, p=0.717).
Table 1. BDNF C270T polymorphisms and allele frequencies in cases and controls (n, %).
| Genotype |
Allele frequencies |
||||||
| C/C | C/T | T/T | C | T | |||
| ALL SUBJECTS | |||||||
| Cases (n=224) | 158 (70.5) | 62 (27.7) | 4 (1.7) | 378 (84.4) | 70 (15.6) | ||
| Controls (n=220) | 203 (92.3) | 15 (7.7) | 2 (0.9) | 421 (95.7) | 19 (4.3) | ||
| χ2 | 34.93a | --- | --- | 31.47 | --- | ||
| p | <0.001 | --- | --- | <0.001 | --- | ||
| MALE SUBJECTS | |||||||
| Cases (n=118) | 86 (72.9) | 30 (25.4) | 2 (1.7) | 202 (85.6) | 34 (14.4) | ||
| Controls (n=115) | 104 (90.4) | 9 (7.8) | 2 (1.7) | 217 (94.3) | 13 (5.7) | ||
| χ2 | 12.98a | --- | --- | 9.84 | --- | ||
| p | 0.002 | --- | --- | 0.002 | --- | ||
| FEMALE SUBJECTS | |||||||
| Cases (n=106) | 72 (67.9) | 32 (30.2) | 2 (1.8) | 176 (83.0) | 36 (17.0) | ||
| Controls (n=105) | 99 (94.3) | 6 (5.7) | 0 (0) | 204 (97.1) | 6 (2.9) | ||
| χ2 | 24.05a | --- | --- | 23.48 | --- | ||
| p | <0.001 | --- | --- | <0.001 | --- | ||
aMultiple-comparison testing based on arsine transformation of original proportions[14] finds that the proportion of C/T genotype in the case group is significantly greater than in the control group (p<0.001).
3.3. Relationship of C270T polymorphism and schizophrenia symptoms
As shown in Table 2, there were no statistically significant differences between the three genotypes in the total PANSS score or in the PANSS negative symptoms subscale score or the PANSS general psychopathology subscale score. However there was a small but statistically significant higher PANSS positive symptoms subscale score in patients with the C/T genotype than in patients with the C/C genotype
Table 2. PANSS scores in patients with schizophrenia who have C/C, C/T, and T/T BDNF C270T genotypes (mean [sd]).
| Genotype | Total score | Positive symptoms score | Negative symptoms score | General psychopathology score |
| C/C (n=158) | 78.6 (9.9) | 22.3 (4.4) | 18.6 (5.5) | 38.0 (5.4) |
| C/T (n=62) | 78.4 (9.8) | 22.8 (4.4) | 18.3 (5.8) | 38.5 (5.9) |
| T/T (n=4) | 77.5 (9.3) | 22.7 (4.5) | 17.4 (3.9) | 37.7 (5.8) |
| F | 2.87 | 4.37 | 2.24 | 2.93 |
| p | 0.086 | 0.034a | 0.095 | 0.074 |
PANSS, Positive and Negative Symptom Scale
a. Post-hoc SNK tests: C/C vs. C/T, q=3.38, p=0.032; C/C vs. T/T, q=0.89, p=0.465; C/T vs. T/T, q=1.02, p=0.088.
3.4. Relationship of C270T polymorphism and cognitive functioning
As assessed by the number of categories completed and by number of perseverative errors on the WCST and by the completion time of the TMT-A and TMT-B, the cognitive functioning of patients is significantly worse than that of controls (Table 3). However, there were no significant differences in these measures of cognitive functioning between patients with the three different BDNF C270T polymorphisms.
Table 3. Cognitive functioning measures from the Wisconsin Card Sorting Test (WCST) and the Trail Making Test (TMT) in controls and in patients with schizophrenia who have C/C, C/T, and T/T BDNF C270T genotypes (mean [sd]).
| Genotype | WCST number of categories achieved | WCST perseverative errors | TMT-A (seconds) | TMT-B (seconds) |
| Patients | ||||
| C/C (n=158) | 1.4 (2.9) | 16.3 (7.7) | 46.6 (17.9) | 115.8 (47.8) |
| C/T (n=62) | 1.4 (2.8) | 15.9 (7.7) | 46.3 (18.5) | 116.7 (48.8) |
| T/T (n=4) | 1.4 (2.9) | 16.4 (7.7) | 46.6 (18.0) | 117.1 (49.2) |
| Controls (n=220) | 2.2 (2.4) | 10.2 (5.3) | 32.6 (12.2) | 56.6 (33.8) |
| F | 10.36a | 13.68a | 26.62a | 16.56a |
| p | 0.038 | 0.026 | 0.006 | 0.002 |
a Post-hoc SNK tests for all variables found that the three patient groups were all significantly different from controls but there were no significant differences between the three patient groups.
4. Discussion
4.1. Main findings
As a member in the family of neurotrophic factors, BDNF can promote the proliferation, migration, and differentiation of neural stem cells into various types of neurons. It can boost the plasticity of synapses, change the shape of neurons and, thus, increase the density of synapses and the growth of dendrites and axons. There is also evidence that BDNF plays a role in learning and long-term memory.[15] During brain development BDNF influences the growth and connections of neurons, directs neuronal survival and apoptosis, and participates in neuronal responses to stress. Importantly, BDNF may influence the differentiation of serotonin and dopamine neurons, which are related to schizophrenia.[4]
Thus, it is possible that the expression of the BDNF gene is related to the occurrence of schizophrenia and to the cognitive functioning and symptoms in patients with schizophrenia.[16]–[18] Our study confirmed differences in the distribution of BDNF C270T genotypes in schizophrenia. Compared to the control group, patients in the case group had a significantly higher prevalence of the T-allele and of the C/T genotype. This result parallels results from other studies in China[19] and elsewhere.[20]
However, our study found no association between BDNF C270T polymorphisms and cognitive functioning as assessed by the WCST and TMT. Nor did we find any relationship between BDNF C270T polymorphisms and the negative symptoms of schizophrenia or the level of general psychopathology in schizophrenia as assessed by PANSS. The one, small (though statistically significant) difference that we identified was slightly more severe positive psychotic symptoms (as measured by PANSS) in patients with schizophrenia who have the BDNF C270T C/T genotype than in those with the C/C genotype. This result is different from that reported by He and colleagues[18] who found that the C/T genotype was associated with more severe deficit symptoms (i.e., negative symptoms). He and colleagues[18] and other researchers[20] also found that the BDNF C270T C/T genotype was more strongly associated with schizophrenia in females than in males, but our results did not confirm this difference by gender.
4.2. Limitations
The clinical symptoms and cognitive functioning of patients with schizophrenia fluctuate during different phases of the illness and can be affected by pharmacological treatments, cognitive-based treatments[21] and, possibly, diet.[22] Thus correlating genotypes with symptoms and cognitive measures is fraught with problems, particularly in cross-sectional studies. It is, moreover, unclear which measures of symptoms and cognition would be most closely related to genotype; is it the most severe level, the premedication level, the level in the residual phase, or some combination of the three? We tried to limit the variability by restricting subjects to those who were drug-free at the time of the assessment, but this only controlled one factor (current medication use). Our sample included patients with different durations of illness and it included those who were drug-naïve and those who had previously used antipsychotic medication; these factors may have influenced the results. Longitudinal studies that assess symptoms and cognitive functioning at different stages of illness are needed to identify the appropriate target measures (individual-specific traits that are stable over time) for assessing the effect (if any) of genotype on the symptoms and cognitive functioning in schizophrenia.
Our measures of cognitive functioning were restricted to two measures from the WCST and two from the TMT; a more comprehensive assessment of cognitive functioning may have identified cognitive differences by genotype.
Schizophrenia is a complex multi-gene disorder with high genetic heterogeneity as a result of numerous genetic and environmental influences. The relatively small sample size and the very low prevalence of the T/T genotype in the current study made it impossible to adjust the results for the many potential confounders (age, duration of illness, gender, etc.) so some of the negative results may be due to insufficient sample size. The small sample size and low prevalence of the T allele was also the probable cause for the nonsignificant deviation of the control group from Hardy-Weinberg equilibrium, a problem that may have introduced biases in the comparison of cases and controls. Moreover, given the presumed polygenetic cause of schizophrenia, a single gene like BDNF may only make a trivial contribution to the phenotype or it may require interaction with other genetic or environmental factors to manifest a phenotypic effect. Thus the failure to identify significant differences in the cognitive and clinical symptoms of patients with different BDNF C270T genotypes is not sufficient to rule out this gene as a possible biomarker for the occurrence, clinical responsiveness and prognosis of schizophrenia.
There are two additional limitations to the study. We did not collect detailed information on the characteristics of individuals with schizophrenia who were excluded from participation in the study, so it is difficult to be certain of how representative the subjects in the study are of all treated patients with schizophrenia. And the study was originally organized as a matched study so we should have used paired statistical tests, but to avoid discarding the valuable genetic data we collected (for cases or controls that did not have matching data because of dropouts) we analysed the cases and controls as independent samples; this approach may under-estimate variance in the estimated parameters because of artificial homogeneity in the data.
4.3. Significance
Recent advances in molecular biology and imaging techniques have accelerated research about neurotransmitters in mental disorders including schizophrenia. This has increased interest in the neurodevelopmental and cell apoptosis hypotheses about the causes of schizophrenia and resulted in an explosion of studies about the role of BDNF in the development and course of the disorder.[6] Our study adds to this growing corpus: it supports the hypothesis about the association of specific BDNF polymorphisms to schizophrenia but fails to find clear evidence about a relationship of BDNF polymorphisms to the severity of clinical or cognitive symptoms.
Biography

Professor JinguoZhai graduated from Jining Medical College, Shandong Province in 1989 and received a Master's of Medicine from Central South University in Changsha, Hunan Province in 2006. He is currently Director of the Clinical Psychiatry Research Center in the Department of Psychiatry at the Jining Medical University. His research interests are clinical psychopharmacology and the molecular biology of psychiatric disorders.
Footnotes
Conflict of interest: The authors report no conflict of interest related to this manuscript.
Funding: The current study was supported by the Ministry of Health Public Welfare Research Project (201002003); the Natural Science Foundation of Shandong Province (ZR2012HM065); and the Plan of Development in Medical Science and Technology of Shandong Province (2007HW037; 2009HZ012).
References
- 1.Doherty J, O'Donovan M, Owen M. Recent genomic advances in schizophrenia. Clin Genet. 2012;81(2):103–109. doi: 10.1111/j.1399-0004.2011.01773.x. [DOI] [PubMed] [Google Scholar]
- 2.Kaymaz N, van Os J. Heritability of structural brain traits an endophenotype approach to deconstruct schizophrenia. Int Rev Neurobiol. 2009;89:85–130. doi: 10.1016/S0074-7742(09)89005-3. [DOI] [PubMed] [Google Scholar]
- 3.Peper JS, Schnack HG, Brouwer RM, Van Baal GC, Pjetri E, Székely E, et al. Heritability of regional and global brain structure at the onset of puberty: a magnetic resonance imaging study in 9-year-old twin pairs. Hum Brain Mapp. 2009;30(7):2184–2196. doi: 10.1002/hbm.20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo XJ, Hang WH, Guo LY. A family based assiociation study of BDNF C270T polymorphism with schizophrenia. Chin J Nerv Ment Dis. 2011;37(9):568–570. (in Chinese) [Google Scholar]
- 5.Sun RF, Zhu YS, Kuang WJ, Liu Y, Li SB. The G-712A polymorphism of brain-derived neurotrophic factor is associated with major depression but not schizophrenia. NeurosciLett. 2011;489(1):34–37. doi: 10.1016/j.neulet.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 6.Zintzaras E. Brain-derived neurotrophic factor gene polymorphisms and schizophrenia: a meta-analysis. Psychiatr Genet. 2007;17(2):69–75. doi: 10.1097/YPG.0b013e32801119da. [DOI] [PubMed] [Google Scholar]
- 7.Mirowska-Guzel D, Mach A, Gromadzka G, Czlonkowski A, Czlonkowska A. BDNF A196G and C270T gene polymorphisms and susceptibility to multiple sclerosis in the Polish population. Gender differences. J Neuroimmunol. 2008;193(1–2):170–172. doi: 10.1016/j.jneuroim.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 8.American Psychological Association . Diagnostic and Statistical Manual of Mental Disorders. Fourth Ed. Washington: American Psychiatric Association; 1994. [Google Scholar]
- 9.Si TM, Yang JZ, Su L. Reliability,validity of PANSS and its implication. Chinese Mental Health Journal. 2004;18(1):45–47. (in Chinese) [Google Scholar]
- 10.Editorial Committee of Chinese Journal of Behavioral Medical Science . Handbook of Behavioral Medical ScienceRating Scales. Beijing: Chinese Medical Electronic AudioVedio Press; 2005. (in Chinese) [Google Scholar]
- 11.Tan YL, Zou YZ, Qu Y, Guo XF. Stability of commonly used measures in the Wisconsin Card Sorting Test. Chinese Mental Health Journal. 2002;16(12):831–833. (in Chinese) [Google Scholar]
- 12.Guo XF. One-year outcomes of schizophrenia: evaluating effectiveness of psychosocial intervention and comparative effectiveness of antipsychotics. Central South University; 2007. (in Chinese) [Google Scholar]
- 13.Wigginton JE, Cutler DJ, Abecsis GR. A note on exact tests of Hardy-Weinberg Equilibrium. Am J Hum Genet. 2005;76(5):887–893. doi: 10.1086/429864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zar HG. Biostatistical Analysis. (4th edition) Prentice Hall: New Jersey; 1999. pp. 563–565. [Google Scholar]
- 15.Liu P, Liu Z. Effect of BDNF on study and memory in the hippocampus. Journal of Changchun University of Traditional Chinese Medicine. 2009;25(2):195–197. (in Chinese) [Google Scholar]
- 16.Li W, Wei J, Zhou DF, Tan YL, Cao YL, Zhang XY, et al. Lack of association between the BDNF C270T polymorphism and schizophrenia in a Chinese Han population. Schizophr Res. 2007;97(1–3):297–298. doi: 10.1016/j.schres.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe Y, Nunokawa A, Kaneko N, Someya T. Meta-analysis of case-control association studies between the C270T polymorphism of the brain-derived neurotrophic factor gene and schizophrenia. Schizophr Res. 2007;95(1–3):250–252. doi: 10.1016/j.schres.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 18.He XL, Zhao JP, Liu T. Relationship between polymorphism in the brain-derived neurotrophic factor gene and qualitative or quantitative characters of schizophrenic phenotype. Journal of Clinical Psychosomatic Diseases. 2005;11(3):197–199. (in Chinese) [Google Scholar]
- 19.He XL, Zhao JP, Liu TQ, Liu T, Zhang XH. Association of schizophrenia and C270T polymorphism of brain-derived neurotrophic factor gene. Chinese Journal of Clinical Rehabilitation. 2005;9(28):245–247. (in Chinese) [Google Scholar]
- 20.Szekeres G, Juhász A, Rimanóczy A, Kéri S, Janka Z. The C270T polymorphism of the brain-derived neurotrophic factor gene is associated with schizophrenia. Schizophr Res. 2003;65(1):15–18. doi: 10.1016/s0920-9964(02)00505-4. [DOI] [PubMed] [Google Scholar]
- 21.Vinogradov S, Fisher M, Holland C, Shelly W, Wolkowitz O, Mellon SH. Is serum brain-derived neurotrophic factor a biomarker for cognitive enhancement in schizophrenia? Biol Psychiatry. 2009;66(6):549–553. doi: 10.1016/j.biopsych.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung YC, Park CH, Kwon HK, Park YM, Kim YS, Doo JK, et al. Improved cognitive performance following supplementation with a mixed-grain diet in high school students: a randomized controlled trial. Nutrition. 2012;28(2):165–72. doi: 10.1016/j.nut.2011.05.017. [DOI] [PubMed] [Google Scholar]