Abstract
From the results of deletion analyses, the FERM domain of FAK has been proposed to inhibit enzymatic activity and repress FAK signaling. We have identified a sequence in the FERM domain that is important for FAK signaling in vivo. Point mutations in this sequence had little effect upon catalytic activity in vitro. However, the mutant exhibits reduced tyrosine phosphorylation and dramatically reduced Src family kinase binding. Further, the abilities of the mutant to transduce biochemical signals and to promote cell migration were severely impaired. The results implicate a FERM domain interaction in cell adhesion-dependent activation of FAK and downstream signaling. We also show that the purified FERM domain of FAK interacts with full-length FAK in vitro, and mutation of this sequence disrupts the interaction. These findings are discussed in the context of models of FAK regulation by its FERM domain.
The focal adhesion kinase, FAK, plays a major role in transducing signals downstream of integrins (40, 47). Upon integrin-mediated adhesion, FAK becomes tyrosine phosphorylated and activated. Additional signaling molecules, e.g., Src and phosphatidylinositol 3-kinase, are recruited into complexes with FAK, leading to the transduction of biochemical signals that control important biological processes. Integrin signaling via FAK regulates cell migration, proliferation, and survival. FAK-dependent regulation of one or more of these processes is essential, since fak−/− mice exhibit embryonic lethality (27). Conversely, enhanced FAK signaling may lead to aberrant cell proliferation, survival, or migration, which may have pathological consequences in humans. For example, aberrant FAK signaling may contribute to cancer development and progression to metastatic disease (40).
FAK contains three major domains, an N-terminal domain, a central catalytic domain, and a C-terminal domain (40, 47). The C-terminal domain can be further subdivided into the focal adhesion targeting (FAT) sequence, comprising the C-terminal 140 amino acids of the protein, and the region between the catalytic domain and the FAT sequence. Focal adhesion-associated FAT sequence binding partners have been identified, and insight into the molecular basis of FAT sequence function was recently obtained from crystal and nuclear magnetic resonance structure analyses (3, 14, 23, 26, 31). The sequence between the catalytic domain and the FAT sequence contains docking sites for SH3 domain-containing proteins and thus serves as a scaffold for the recruitment of signaling proteins. Several sites of tyrosine phosphorylation play important regulatory roles in FAK. Within the catalytic domain, two tyrosine residues in the activation loop, tyrosines 576 and 577, regulate catalytic activity. The major site of autophosphorylation, tyrosine 397, lies just N terminal to the catalytic domain and serves as a binding site for Src family kinases. While details regarding the function of the catalytic and C-terminal domains have been elucidated, fewer studies have examined the function of the N-terminal domain of FAK.
The N-terminal domain of FAK exhibits homology with FERM domains, which are structurally conserved domains found in many proteins (18). FERM domains are present in structural proteins such as talin and the ezrin-radixin-moesin (ERM) family of proteins (18; A. H. Chishti, A. C. Kim, S. M. Marfatia, M. Lutchman, M. Hanspal, H. Jindal, S. C. Liu, P. S. Low, G. A. Rouleau, N. Mohandas, J. A. Chasis, J. G. Conboy, P. Gascard, Y. Takakuwa, S. C. Huang, E. J. Benz, Jr., A. Bretscher, R. G. Fehon, J. F. Gusella, V. Ramesh, F. Solomon, V. T. Marchesi, S. Tsukita, S. Tsukita, and K. B. Hoover, Letter, Trends Biochem. Sci. 23:281-282, 1998) as well as in signaling proteins such as the JAK family of tyrosine kinases and several tyrosine phosphatases (18; Chishti et al., letter). FERM domains mediate protein-protein interactions, and two different paradigms of interaction have been described. First, FERM domains can mediate intermolecular interactions, usually allowing docking with the cytoplasmic tails of transmembrane proteins. Second, FERM domains function in either intramolecular or homophilic intermolecular interactions. The best-characterized FERM domain-mediated interactions are those of the ERM family of proteins. The ERM proteins bind via their FERM domains to the cytoplasmic domains of transmembrane proteins, e.g., CD44 (32). Other examples of FERM domain-mediated intermolecular interactions include binding of the talin FERM domain to the β subunit of integrins and the interaction of the FERM domains of JAKs with the γc and gp130 subunits of cytokine receptors (4, 25, 60). The FERM domains of the ERM proteins also bind intramolecularly to a site within the C terminus (32). This interaction obscures the CD44 binding site within the FERM domain and an actin binding site in the C-terminal tail. Regulation of this intramolecular interaction modulates ERM protein-mediated intermolecular interactions. The FERM domain of JAK3 interacts with the C-terminal catalytic domain, and this interaction is essential for catalytic activity (60).
Like other FERM domains, the FAK FERM domain also mediates protein-protein interactions. Several binding partners have been identified, including the cytoplasmic tails of the β1 integrin subunit and growth factor receptors (19, 44, 53). The FERM domain of FAK also mediates an interaction with the cytoplasmic tyrosine kinase Etk/Bmx, and interacts weakly with the FERM domain of ezrin (7, 37). Disruption of the FAK-growth factor receptor interaction impairs chemotaxis, and mutants of Etk defective for FAK binding fail to promote cell motility in CHO cells (7, 53). Thus, several of these FERM domain-mediated interactions may function in the control of cell motility.
Several reports have demonstrated that deletion of the N-terminal domain of FAK can elevate catalytic activity in vitro (6, 9, 57). A mutant with deletion of part of the N-terminal domain (residues 1 to 100) exhibited elevated tyrosine phosphorylation and binding to Src and Grb2 under serum starvation conditions, whereas wild-type FAK was poorly tyrosine phosphorylated and did not associate with these binding partners (48). In contrast, this mutant and other mutants with large N-terminal deletions exhibited a very modest increase in tyrosine phosphorylation in adherent cells grown in the presence of serum (6, 24, 48). A recent report describing another series of deletion mutants demonstrates that removal of the FERM domain results in elevated phosphotyrosine levels of FAK in cells in suspension and enhanced phosphorylation of paxillin (9). From these observations, it was proposed that the N-terminal domain of FAK plays an inhibitory role under conditions where FAK activity is suppressed. Further, it was speculated that this might occur via an intramolecular interaction (17, 49). Indeed, an interaction between the FERM domain and the catalytic domain was recently demonstrated (9). Exogenous FERM domain was also shown to impair catalytic activity of full-length FAK in vitro and FAK signaling in vivo (9), providing compelling evidence in support of the hypothesis.
We were also interested in examining whether the FAK FERM domain could mediate intra- or intermolecular interactions. Recombinant FERM domain constructs were utilized and shown to associate with full-length FAK in vitro. However, the interaction that we observed appears distinct from the previously described interaction (9), since both the N-terminal and catalytic domains of full-length FAK are required for its interaction with the FERM domain in vitro. A highly conserved, basic patch on the surface of the FAK FERM domain was identified by molecular modeling. Mutation of residues in this basic patch disrupted the ability of the FERM domain to associate with full-length FAK in vitro. To assess the role of the basic patch of the FERM domain in the biochemical and biological functions of FAK, substitutions were engineered in full-length FAK. The mutant exhibited near-wild-type catalytic activity in vitro but was defective for transducing biochemical and biological signals in vivo. These observations suggest that the basic patch of the FERM domain is required for the efficient activation of FAK and transmission of downstream signals.
MATERIALS AND METHODS
Cells.
Chicken embryo (CE) cells were prepared and maintained as described previously (38). The RCAS A avian replication-competent retroviral vector encoding wild-type FAK and CAKβ (Pyk2/CadTK/RAFTK), FAK mutants, and FAK/CAKβ chimeras have been described previously (10, 13, 43, 46). These constructs contain a C-terminal KT3 tag, which is an 11-amino-acid epitope derived from simian virus 40 large T antigen (33). Rat-1 cells were cultured in Dulbecco's modified Eagle's medium plus 10% fetal bovine serum. HEK 293 cells were maintained in Dulbecco's modified Eagle's medium-F12 containing 10% fetal bovine serum. T47D cells were maintained in RPMI 1640 containing 10% fetal bovine serum and 0.2 U of insulin per ml. Cells were transfected by using Lipofectamine PLUS (Life Technologies, Gaithersburg, Md.). For adhesion experiments, cells were trypsinized, held in suspension, and plated onto dishes coated with extracellular matrix proteins. CE cells were plated on dishes coated with fibronectin (50 μg/ml) (13, 50). T47D cells were plated on dishes coated with collagen I (5 μg/ml). In some experiments, cells were treated with 50 μM vanadate for 16 h prior to lysis (46). Motility assays were performed as described previously, using 40 μg of collagen I per ml as the haptotactic stimulus (13, 28). A mixed-model test as well as paired and unpaired Student t tests were performed with the SAS (Cary, N.C.) software to identify statistically significant differences in the average fold change in motility.
Protein analysis.
Cells were washed twice with phosphate-buffered saline and lysed in Triton X-100 lysis buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 1% Triton X-100, 10% glycerol) or Tx-RIPA (50 mM Tris [pH 7.3], 150 mM NaCl, 1% Triton X-100, 0.5% deoxycholate) containing protease and phosphatase inhibitors as described previously (51, 55). Protein concentrations were determined with the bicinchoninic acid assay (Pierce, Rockford, Ill.). The KT3 monoclonal antibody (Covance, Princeton, N.J.), FAK phosphorylation site-specific antibodies (Biosource International, Camarillo, Calif.), a Pyk2/CAKβ monoclonal antibody and the 4G10 phosphotyrosine antibody (Upstate USA Inc., Lake Placid, N.Y.), and the RC20 phosphotyrosine antibody (BD Biosciences, San Diego, Calif.) were purchased commercially. The BC4 polyclonal antiserum has been described previously (41, 50). The Fyn polyclonal antiserum was a generous gift from André Veillette (Institut de Recherches, Clinique de Montreal), and the FAK monoclonal antibodies, 2A7 and 4.47, were generous gifts from Tom Parsons (University of Virginia) and Bill Cance (University of Florida), respectively. Immunoprecipitations and Western blotting were performed as previously described (13). For coimmunoprecipitations, the immune complexes were washed with IPW buffer (20 mM Tris [pH 7.4], 10% glycerol, 50 mM NaCl, 0.2% Triton X-100). For in vitro kinase assays, immune complexes were washed with IPW buffer, phosphate-buffered saline, and kinase reaction buffer {20 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)] [pH 7.2], 7.5 μM MnCl2, 2.5 μM MgCl2}. The immune complexes were incubated in kinase reaction buffer containing 10 μCi of [γ-32P]ATP, 5 μM cold ATP, and 50 μg of poly(Glu,Tyr) for the indicated times at room temperature. Kinase reactions were terminated with the addition of sample buffer and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography. Quantitative analysis was performed with a Storm phosphorimager and ImageQuant software.
Homology modeling of the FERM domain of FAK.
Homology modeling was performed in the Structural Bioinformatics Core facility at University of North Carolina, Chapel Hill. The crystal structure of the FERM domain of moesin (PDB identification, 1E5W) (11) was used as the template for homology modeling of the FERM domain of FAK. The initial alignment of the sequence of human FAK with the sequence of moesin was made by using the 3D-PSSM fold recognition program and modified by using the Clustal X multiple-sequence alignment program (29, 56). The Modeler module of the InsightII molecular modeling system (Accelrys Inc.) was used to create the model of the N-terminal domain of FAK. The sequence-structure compatibility of the model was evaluated by using the Verify function of the Profiles-3D module of InsightII. The sequence alignment of FAK and moesin was modified in regions of the model with low Profiles-3D/Verify scores. A new model was generated by using the new alignment, and the sequence-structure compatibility of the new model was evaluated. This process was repeated until the sequence-structure compatibility score could not be improved. The first two strands of the FAK FERM domain repeatedly received a low self-compatibility score and were deleted from the final model. The final model, which contains FAK residues 60 to 349, received a self-compatibility score of 119.9 out of a maximum expected score of 131.9. A score below 59 would indicate an incorrect structure. For comparison, the X-ray crystal structure of the FERM domain of moesin was analyzed by using the Verify function of Profiles-3D. This structure received a self-compatibility score of 153 out of a maximum expected score of 158. This analysis suggests that this structure is a reasonably good homology model.
Recombinant proteins.
The avian FAK or rat CAKβ sequences encoding residues 1 to 405 were amplified by PCR and subcloned into pGEX-KG in frame with the glutathione S-transferase (GST) coding sequences (20). All amplified sequences were analyzed by nucleotide sequencing at the University of North Carolina, Chapel Hill, Automated DNA Sequencing Facility on a model 377 DNA sequencer (Perkin-Elmer, Applied Biosystems Division) with the ABI PRISM dye terminator cycle sequencing ready reaction kit with AmpliTaq DNA polymerase FS (Perkin-Elmer, Applied Biosystems Division). The GST fusion proteins were induced as previously described (55). For binding assays, cell lysates (1 to 2 mg) were precleared with GST (25 μg) immobilized on glutathione-agarose beads (Sigma) for 1 h at 4°C. Precleared lysates were incubated with GST fusion proteins (25 μg) immobilized on glutathione-agarose beads for 1 h at 4°C with constant rocking and then washed three times in IPW buffer. Bound proteins were eluted with Laemmli sample buffer and analyzed by Western blotting.
To explore direct binding of the recombinant FERM domain to the recombinant catalytic domain, the catalytic domain of FAK (residues 390 to 696) was amplified by PCR and ligated into a modified pET vector containing an amino-terminal His6 tag with a TEV cleavage site. The expression of recombinant protein in Escherichia coli BL21 cells was induced at an optical density at 600 nm of 0.6 to 0.7 by the addition of 0.2 mM IPTG (isopropyl-β-d-thiogalactopyranoside), and cells were grown for an additional 12 to 16 h at 18°C. The cells were harvested and frozen at −70°C. Cell pellets were thawed in buffer A (20 mM Tris [pH 8.0], 200 mM NaCl, 5 mM imidazole, 5 mM β-mercaptoethanol) with the addition of 0.5% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamadine, 10 μg of leupeptin per ml, 10 mg of lysozyme per ml, and 1 mg of DNase per ml and lysed by sonication on ice. After clarification, supernatants were loaded onto an Ni-chelating column. Following extensive washing, protein was eluted with buffer A containing 300 mM imidazole. FAK kinase domain-containing fractions were pooled, and the His6 tag was cleaved by incubation with 1 μg of TEV protease per mg of protein overnight at 4°C. The digested protein was diluted with buffer B (20 mM Tris [pH 8.0], 2 mM NaCl, 5 mM β-mercaptoethanol) to a final salt concentration of 50 mM NaCl. Protein was loaded onto a 5 ml Hi-Trap Q column equilibrated in buffer A containing 50 mM NaCl and eluted with buffer A containing 500 mM NaCl. The kinase domain was concentrated and further purified on an S75 size exclusion column previously equilibrated in buffer C (20 mM Tris [pH 8.0], 100 mM NaCl, 5 mM β-mercaptoethanol). GST-FERM proteins on glutathione beads were incubated with purified FAK kinase domain in buffer D (20 mM Tris [pH 8.0], 50 mM NaCl, 5 mM β-mercaptoethanol, 5 mM MgCl2, 1 mM ATP) for 20 min at 25°C. The beads were washed three times with buffer D (with no MgCl2 or ATP), and protein was eluted with buffer D containing 25 mM glutathione. Duplicate samples were analyzed by Western blotting with 4G10 and SDS-PAGE followed by Coomassie blue staining.
Molecular biology.
FAK mutants were engineered by using pBluescript-FAK as a template (41). Oligonucleotides were designed to substitute alanine residues for the amino acids targeted for mutagenesis, and the mutations were created by using the QuikChange mutagenesis kit (Stratagene, La Jolla Calif.). Mutants were analyzed by sequence analysis to verify the intended point mutations and that no unintended mutations were present. The N-terminal domains of the mutants (residues 1 to 405) were amplified and subcloned into pGEX-KG as described above. For transient expression of the N-terminal domain of FAK in mammalian cells, residues 1 to 405 were amplified and subcloned into pcDNA3. For expression in CE cells, the mutant full-length FAK cDNAs were subcloned into RCAS A.
Immunofluorescence.
Cells were fixed in 3% formaldehyde and permeabilized with 0.4% Triton X-100. FAK was detected by using BC4 and a rhodamine-conjugated anti-rabbit antibody as previously described (8, 42). Cells were visualized by using a Leitz Orthoplan fluorescence microscope, and images captured with a Hamamatsu digital camera and Metamorph imaging software (Universal Imaging Corporation, West Chester, Pa.). Images were taken with identical exposure times.
RESULTS
The FAK FERM domain binds FAK in vitro.
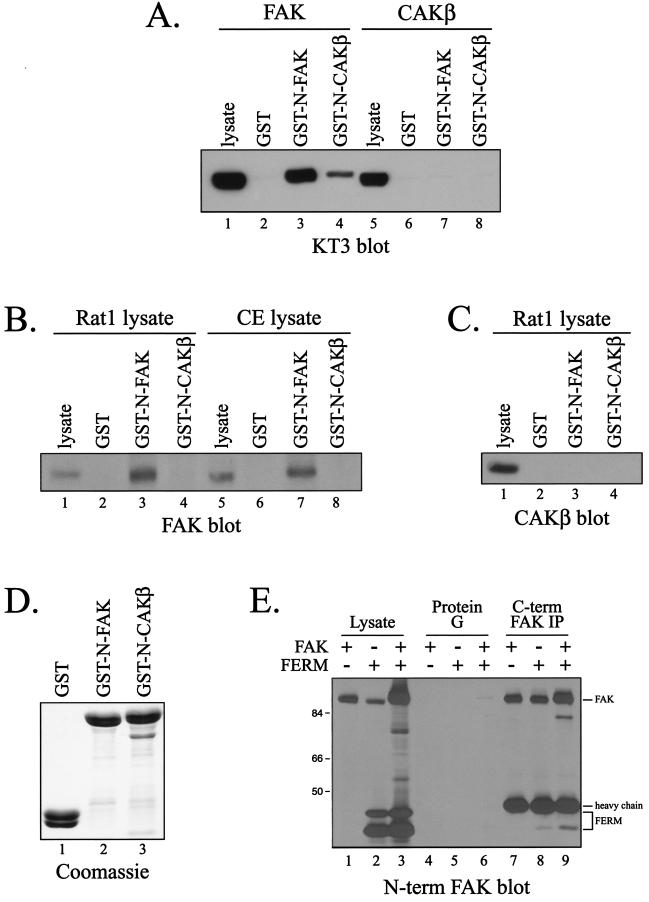
Since FERM domains of other proteins mediate intramolecular interactions, the possibility that the FERM domain of FAK exhibited FAK binding activity was explored. A GST fusion protein containing the N-terminal domain of FAK (including the entire FERM domain), immobilized to glutathione-agarose beads, was incubated with lysates of CE cells overexpressing wild-type, epitope-tagged FAK. Bound proteins were analyzed by Western blotting with the KT3 monoclonal antibody, which recognizes the tag. FAK bound to the GST-FAK N-terminal domain fusion protein but not to GST alone (Fig. 1A). Since CAKβ/Pyk2/CadTK/RAFTK exhibits extensive sequence similarity to FAK, the N-terminal domain of CAKβ was also examined for its ability to bind FAK. The CAKβ N-terminal domain also associated with FAK in vitro, although less FAK bound to the N terminus of CAKβ than to the N terminus of FAK (Fig. 1A). The ability of the N termini of FAK and CAKβ to bind to CAKβ was also examined by incubating GST-FAK N terminus and GST-CAKβ N terminus fusion proteins, immobilized on glutathione-agarose beads, with lysate from CE cells expressing exogenous, epitope-tagged CAKβ. Although CAKβ was efficiently expressed in CE cells, neither the N-terminal domain of FAK nor that of CAKβ bound to CAKβ (Fig. 1A). Thus, the N terminus of FAK and, to a lesser extent the N terminus of CAKβ, associate with exogenously expressed FAK in vitro but not with CAKβ.
FIG. 1.
The N-terminal domain of FAK associates with FAK in vitro. (A) Epitope-tagged FAK (lanes 1 to 4) or CAKβ (lanes 5 to 8) was expressed in CE cells. Cell lysates (1 mg) were precleared with GST bound to glutathione beads and then incubated with 25 μg of GST, GST-N-FAK, or GST-N-CAKβ. Bound proteins were detected by Western blotting with KT3. Cell lysate (25 μg) was analyzed directly by Western blotting as a control (lanes 1 and 5). (B) Rat-1 cells (lanes 1 to 4) or CE cells (lanes 5 to 8) were lysed, precleared with GST, and then incubated with GST, GST-N-FAK, or GST-N-CAKβ. Endogenous FAK bound to the beads was detected by Western blotting with BC4. Lysate (25 μg) was directly blotted as a control (lanes 1 and 5). (C) The blot in panel B was stripped and reprobed with a CAKβ monoclonal antibody to examine endogenous CAKβ binding to the GST fusion proteins. Note that CE cells express no endogenous CAKβ, and these samples were excluded from the analysis. (D) A fraction of the products from the GST pulldowns performed on Rat-1 cell lysates (B and C) were analyzed by SDS-PAGE andCoomassie blue staining to demonstrate that equal amounts of each fusion protein was used in the analysis. (E) The FAK N-terminal domain was transiently coexpressed with full-length FAK in 293 cells. Expression of each was determined by Western blotting of 25 μg of lysate with monoclonal antibody 4.47 (lanes 1 to 3). Full-length FAK was immunoprecipitated (IP) with monoclonal antibody 2A7, and the immunoprecipitated FAK and coimmunoprecipitating N-terminal domain were detected by Western blotting with monoclonal antibody 4.47 (lanes 7 to 9). As a specificity control for coimmunoprecipitation, mock precipitations with protein G alone were also performed (lanes 4 to 6). Numbers on the left are molecular weights in thousands.
To further corroborate these findings, the ability of GST fusion proteins containing the N terminus of FAK or CAKβ to associate with endogenous FAK and CAKβ was examined. Fusion proteins immobilized on glutathione-agarose beads were incubated with lysates of CE cells or Rat-1 cells, and bound FAK was detected by Western blotting. The N-terminal domain of FAK associated with endogenous FAK in vitro (Fig. 1B). The N-terminal domain of CAKβ bound very weakly to endogenous FAK and was not detectable in the exposure shown. No binding to GST alone was detected. Further, equal amounts of each GST fusion protein were used in the analysis (Fig. 1D). CAKβ is not expressed endogenously in CE cells, and thus the interaction of the N-terminal domains of FAK and CAKβ with endogenous CAKβ was examined by using lysates of Rat-1 cells. Similar to the results with exogenously expressed CAKβ, endogenous CAKβ failed to associate with the N-terminal domain of either FAK or CAKβ in vitro (Fig. 1C). These findings demonstrate that the N terminus of FAK, and to a lesser extent the N terminus of CAKβ, is capable of associating directly or indirectly with FAK in vitro but that these N-terminal domains are not capable of associating with CAKβ.
To further validate the observed interaction of the FERM domain with full-length FAK, the FERM domain was subcloned into pcDNA3 for transient expression in mammalian cells. Full-length FAK and the FERM domain were coexpressed in HEK 293 cells, and FAK was immunoprecipitated with monoclonal antibody 2A7, which recognizes the C-terminal domain of FAK. Coimmunoprecipitating FERM domain was detected by using monoclonal antibody 4.47, which recognizes the N-terminal domain. The results show coimmunoprecipitation of the FERM domain with full-length FAK, although the stoichiometry of association was quite low (Fig. 1E, lane 9). A reduced amount of the FERM domain was coimmunoprecipitated with 4.47 in the absence of exogenously expressed FAK, reflecting association of the FERM domain with endogenous FAK (Fig. 1E, lane 8). Control immunoprecipitations nonspecifically trapped very small amounts of the FERM domain (Fig. 1E, lanes 4 to 6). These results demonstrate that the FERM domain of FAK can associate with full-length FAK in vivo, albeit with low stoichiometry.
The central region of FAK containing the catalytic domain is required for N-terminal domain binding.
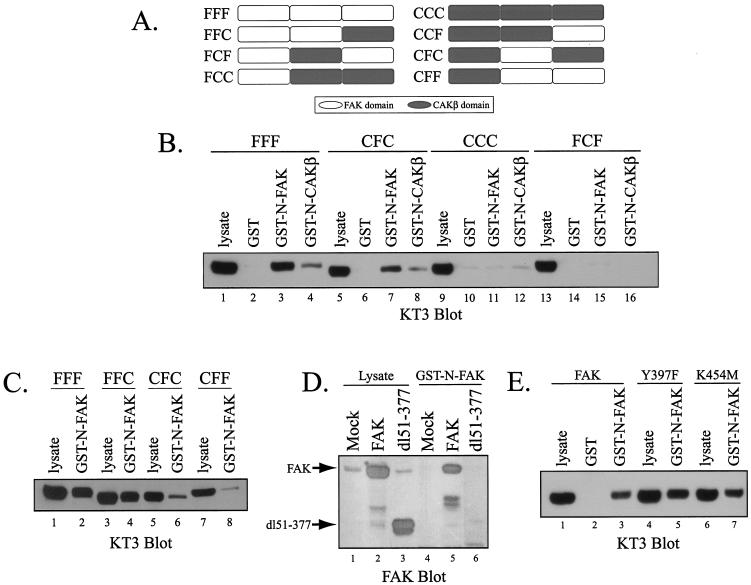
The observation that the N termini of FAK and CAKβ can associate with FAK, but not with CAKβ, allowed the use of chimeric FAK/CAKβ molecules to map the region of FAK that interacts with the N-terminal domain (Fig. 2A) (10). GST-FAK N terminus fusion protein immobilized on glutathione-agarose beads was incubated with lysates of CE cells expressing various FAK/CAKβ chimeric proteins. The association of the chimeras with the N terminus of FAK was determined by Western blotting with KT3 (Fig. 2B and C). The central region of FAK, which contains the catalytic domain, was the principal determinant of the ability to bind the N-terminal domain of FAK. Whereas chimeras containing the FAK catalytic domain, FFF (Fig. 2B, lanes 3 and 4), and CFC (Fig. 2B, lanes 7 and 8), associated with the N termini of FAK and CAKβ in vitro, chimeras containing the catalytic domain of CAKβ, CCC (Fig. 2B, lanes 11 and 12) and FCF (Fig. 2B, lanes 15 and 16) did not bind to the N-terminal domain of FAK or CAKβ. These data implicate the central region of FAK in the interaction with the FAK FERM domain. As the central regions swapped between chimeras are comprised of the catalytic domain with small N- and C-terminal extensions, the most likely site of interaction is within the catalytic domain. However, additional analysis suggested that the N-terminal domain of FAK may modulate this interaction, since chimeras containing both the FAK N-terminal domain and the FAK catalytic domain bound more efficiently to the GST-FAK N terminus fusion protein than chimeras containing the N-terminal domain of CAKβ (Fig. 2C). CFC (Fig. 2C, lane 6) and CFF (Fig. 2C, lane 8) exhibited weaker binding to the FAK FERM domain than the analogous chimeras containing the N terminus of FAK, FFC (Fig. 2C, lane 4) and FFF (Fig. 2C, lane 2). Further, a mutant with a large N-terminal deletion, dl51-377, associated very poorly with the FERM domain of FAK (Fig. 2D). Thus, both the catalytic and N-terminal domains of FAK are required to associate with the FAK FERM domain in vitro. This observation is distinct from a previous report of an interaction between the FERM domain and catalytic domain of FAK (9).
FIG. 2.
Association of the FERM domain with FAK/CAKβ chimeras and FAK mutants. (A) Schematic illustration of FAK/CAKβ chimeras utilized in this analysis. (B) Lysates of cells expressing the chimeras FFF (lanes 1 to 4), CFC (lanes 5 to 8), CCC (lanes 9 to 12), and FCF (lanes 13 to 16) were incubated with GST (lanes 2, 6, 10, and 14), GST-N-FAK (lanes 3, 7, 11, and 15), or GST-N-CAKβ (lanes 4, 8, 12, and 16) immobilized to glutathione-agarose beads. Chimeric proteins that bound to the GST fusion proteins were detected by Western blotting with KT3. Lysate was directly analyzed as a control (lanes 1, 5, 9, and 13). (C) Lysates of cells expressing the chimeras FFF (lanes 1 and 2), FFC (lanes 3 and 4) CFC (lanes 5 and 6), and CFF (lanes 7 and 8) were incubated with GST-N-FAK (lanes 2, 4, 6, and 8) immobilized to glutathione-agarose beads, and bound protein was detected by Western blotting with KT3. Lysate was directly analyzed as a control (lanes 1, 3, 5, and 7). (D) Lysates of untransfected CE cells (lane 4) or cells expressing wild-type FAK (lanes 5) or the FAK mutant dl51-377 (lane 6) were incubated with GST-N-FAK, and bound FAK was detected by Western blotting with the BC4 polyclonal antiserum. Lysates were directly analyzed as a control (lanes 1 to 3). (E) Lysates of CE cells expressing FAK (lanes 1 to 3), FAKY397F (lanes 4 and 5), or FAKK454M (lanes 6 and 7) were incubated with GST-N-FAK, and bound FAK was detected by Western blotting with KT3. Lysates were directly analyzed by Western blotting as a control (lanes 1, 4, and 6). As a negative control for FAK binding, GST was used.
A number of FAK mutants were expressed in CE cells and characterized for their ability to associate with the N-terminal domain of FAK in vitro. The major autophosphorylation site of FAK, tyrosine 397, is dispensable for the interaction with the N-terminal domain of FAK, since the 397F FAK mutant bound to the N-terminal domain (Fig. 2E). Catalytic activity of FAK was also not required for association with the FAK N-terminal domain in vitro, since two catalytically defective mutants of FAK, K454 M and D564A, associated with the N-terminal domain (Fig. 2E and data not shown). Thus, neither catalytic activity nor phosphorylation of FAK at its major autophosphorylation site was required for the interaction with the FAK N terminus in vitro.
Homology modeling of the FERM domain of FAK.
Based upon sequence analysis, a FERM domain was identified within the N-terminal domain of FAK (18). To gain insight into the function of the N-terminal domain, a molecular model of the FERM domain was created by using the InsightII homology modeling program. The structures of several FERM domains were used as templates for homology modeling, and the best model was produced by using the crystal structure of moesin as a template (11). Residues flanking the FERM domain could not be modeled, since the secondary structures of these regions were predicted to be neither α-helices nor β-strands.
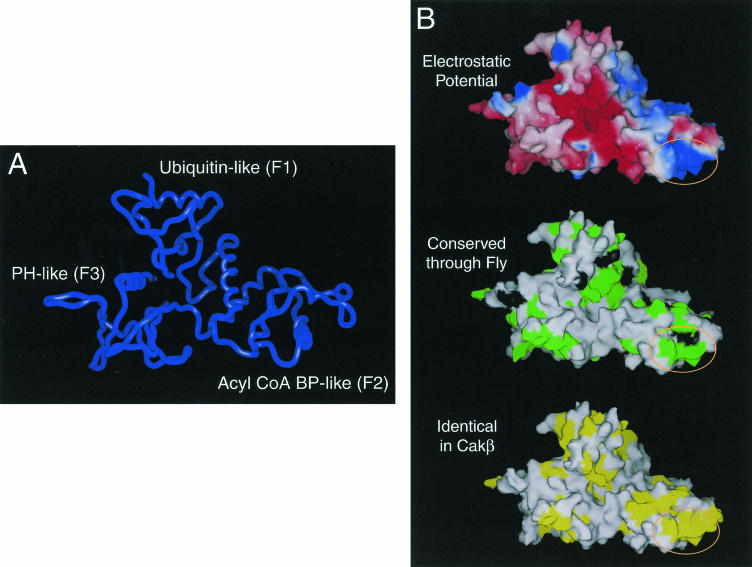
FERM domains contain three lobes, F1, F2, and F3 (also referred to as the A, B, and C subdomains) (21, 36). The F1 subdomain resembles ubiquitin in its structure, the F2 subdomain has a protein fold similar to that of acyl coenzyme A (acyl-CoA) binding protein, and the F3 subdomain is similar in structure to PH/PTB/EVH1 domains (11, 21, 36). The first two strands of the ubiquitin-like domain of the FAK FERM domain could not be accurately modeled and were therefore omitted from the final structure (Fig. 3A).
FIG. 3.
Model of the FERM domain of FAK. (A) The backbone of the model of the FERM domain of FAK is shown. This model contains FAK residues 60 to 349. The ubiquitin-like subdomain (F1), acyl-CoA binding protein (BP)-like subdomain (F2) and PH/PTB/EVH-like subdomain (F3) are indicated. (B) The surface of the model of the FERM domain is shown. In the top panel, electrostatic potential is indicated colorimetrically, with red indicating negative potential and blue indicating positive potential. In the middle panel, the surface of the FERM domain is shown with residues that are identical from Drosophila to human (green) and highly conserved residues (black) indicated. In the bottom panel, the surface of the FERM domain is shown with residues that are identical between FAK and CAKβ in yellow. The circled region indicates a highly conserved basic patch at the tip of the F2 subdomain.
Identification of N-terminal domain residues critical for FAK binding.
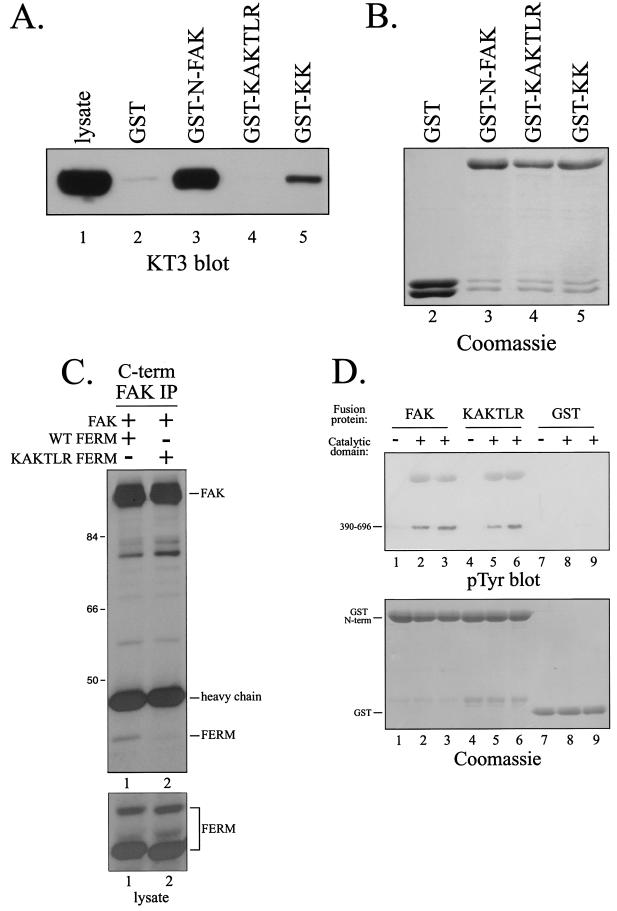
One of the striking features of the FAK FERM model was a large patch of basic residues on the surface at the tip of the F2 subdomain (Fig. 3B, top). Analysis of the X-ray crystal structure of the FAK FERM domain (D. F. J. Ceccarelli, H. K. Song, F. Poy, M. D. Schaller, and M. J. Eck, unpublished data) verified that this basic patch was solvent exposed. These residues are highly conserved in FAK (Fig. 3B, middle) and are also conserved between FAK and CAKβ (Fig. 3B, bottom). As CAKβ exhibits partial binding activity, such a conserved region may form part of the interaction site. Therefore, this region was considered a candidate for the FAK binding site within the FERM domain of FAK. To explore the role of these residues in FAK binding, alanine substitutions were made for several basic residues. Two mutants, one with alanine substitutions for K190 and K191 (KK) and one with alanine substitutions for K216, K218, and R221 (KAKTLR), were created. These mutants were engineered into the GST-FAK N-terminal domain fusion protein construct, expressed, and tested for their ability to associate with FAK in vitro. Wild-type FAK and several other FERM domain mutants efficiently associated with FAK in vitro (Fig. 4A and data not shown). In contrast, KK exhibited reduced binding relative to the wild-type FERM domain, and KAKTLR was devoid of binding activity (Fig. 4A). Comparable amounts of fusion proteins were used in these experiments (Fig. 4B). To further explore this interaction, the KAKTLR FERM domain variant was coexpressed with wild-type FAK in HEK 293 cells. The wild-type FERM domain coimmunoprecipitated with full-length FAK (Fig. 4C, lane 1). In contrast, the interaction of the FERM domain containing the KAKTLR mutation with full-length FAK was dramatically reduced (Fig. 4C, lane 2). These findings suggest that conserved basic residues on the surface of the F2 subdomain of the FAK FERM domain are important for the interaction with full-length FAK.
FIG. 4.
Point mutations in the FERM domain disrupt the interaction with FAK. (A) Lysates of CE cells overexpressing epitope-tagged FAK were precleared with GST and then incubated with GST (lane 2), GST-N-FAK (lane 3), GST-KAKTLR (lane 4), or GST-KK (lane 5). Bound FAK was detected by Western blotting with KT3. Lysate (25 μg) was analyzed as a control (lane 1). (B) As a loading control, a fraction of product from each pulldown from panel A was analyzed by SDS-PAGE and Coomassie blue staining. (C) The wild-type (WT) FERM domain (lane 1) or FERM domain with the KAKTLR mutation (lane 2) was transiently coexpressed with full-length, wild-type FAK in HEK 293 cells. FAK was immunoprecipitated with 2A7, and the immune complexes were analyzed by Western blotting with 4.47 (top panel). Lysates were blotted with 4.47 to verify equal expression of the FERM domains (bottom panel). Numbers on the left are molecular weights in thousands. (D) Purified recombinant catalytic domain of FAK was incubated with GST-N-FAK (lanes 2 and 3), GST-KAKTLR (lanes 5 and 6), or GST (lanes 8 and 9) immobilized on glutathione beads in the presence of Mg2+ and ATP. After washing and elution with free glutathione, samples were analyzed by Western blotting for phosphotyrosine (pTyr) (top panel) or by SDS-PAGE and Coomassie blue staining (bottom panel). As controls, GST fusion proteins were also incubated in the absence of the purified recombinant catalytic domain (lanes 1, 4, and 7).
To further explore the interaction between the FERM and catalytic domains, each was expressed as recombinant protein in E. coli. The purified catalytic domain was incubated with GST fusion proteins immobilized on beads in the presence of Mg2+ and ATP, allowing autophosphorylation of the catalytic domain and transphosphorylation of the FERM domain. After washing, proteins were eluted with free glutathione, and the presence of the catalytic domain was detected by blotting with a phosphotyrosine antibody (Fig. 4D, top panel). Although this interaction was very weak (i.e., not detected in the Coomassie blue-stained gel), the catalytic domain associated directly with the FERM domain of FAK in vitro (Fig. 4D, lanes 2 and 3). The catalytic domain failed to associate with the GST alone (Fig. 4D, lanes 8 and 9) or with other control GST fusion proteins (data not shown). Surprisingly, the catalytic domain associated with the KAKTLR mutant of FAK (Fig. 4D, lanes 5 and 6). Similar amounts of GST-N-FAK (lanes 1 to 3), GST-KAKTLR (lanes 4 to 6), and GST (lanes 7 to 9) were used in this analysis (Fig. 4D, bottom). These findings demonstrate the direct interaction of the FAK FERM domain with the FAK catalytic domain in vitro, presumably reflecting the recently described FERM-catalytic domain interaction (9). However, the basic patch at the tip of the F2 subdomain is not required for this direct interaction.
The FERM domain-mediated interaction modulates FAK phosphorylation.
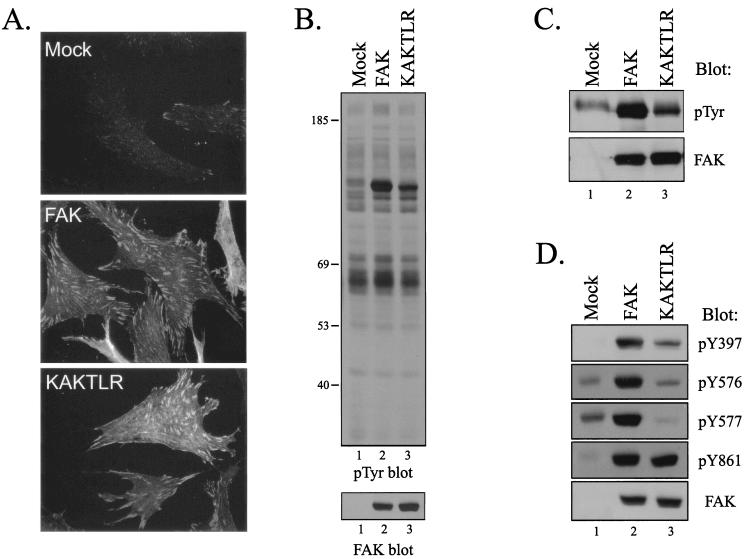
In order to assess the consequences of disrupting the interaction mediated by the basic patch of the FERM domain of FAK, the KAKTLR mutant was selected for further characterization. The mutant was subcloned into the RCAS A retroviral vector and expressed in CE cells. KAKTLR could be expressed in CE cells to similar levels as wild-type FAK and was correctly localized to focal adhesions (Fig. 5A and B). Overexpression of FAK had little effect on the levels of cellular tyrosine phosphorylation, except that the exogenously expressed FAK protein now appeared as the major tyrosine-phosphorylated protein in the cell (Fig. 5B, top panel) (45, 46). Similarly, overexpression of KAKTLR had no effect on the phosphotyrosine levels of cellular proteins; however, tyrosine phosphorylation of KAKTLR was apparently reduced relative to that of wild-type FAK (Fig. 5B). To verify this observation, exogenously expressed wild-type FAK and KAKTLR were immunoprecipitated and Western blotted with a phosphotyrosine antibody. KAKTLR exhibited reduced levels of phosphotyrosine relative to wild-type FAK (Fig. 5C). The blot was stripped and reprobed to verify that equal levels of exogenously expressed FAK proteins were recovered. To determine whether KAKTLR was defective for phosphorylation at specific tyrosine residues, cell lysates were analyzed by Western blotting with phospho-specific antibodies recognizing phosphorylated Y397 (PY397), Y576 (PY576), and Y577 (PY577). Phosphorylation of KAKTLR at each of these sites was reduced relative to that of wild-type FAK (Fig. 5D, lanes 2 and 3). In contrast to these results, similar levels of phosphorylation of tyrosine residue 861 were seen in the wild-type and mutant FAK proteins. A FAK Western blot verified that equal amounts of wild-type FAK and KAKTLR were present in the lysates (Fig. 5D, bottom). These results demonstrate that the mutation disrupting the FERM domain-mediated interaction leads to reduced tyrosine phosphorylation of FAK, including key regulatory sites of phosphorylation at tyrosines 397, 576, and 577.
FIG. 5.
KAKTLR is defective for tyrosine phosphorylation in vivo. (A) CE cells (top panel) and CE cells overexpressing wild-type FAK (middle panel) or the KAKTLR mutant (bottom panel) were fixed and stained with the BC4 polyclonal FAK antibody. (B) Upper panel, 25 μg of cell lysate from control transfected CE cells (lane 1) or FAK (lane 2)- or KAKTLR (lane 3)-expressing CE cells was analyzed by Western blotting for phosphotyrosine (pTyr). The positions of molecular weight markers (in thousands) are indicated on the left. Lower panel, the blot was stripped and reprobed for FAK. (C) FAK was immunoprecipitated from lysates of CE cells containing empty vector (lane 1) or expressing FAK (lane 2) or KAKTLR (lane 3). Immune complexes were Western blotted for phosphotyrosine (top panel) and then stripped and reprobed for FAK (bottom panel). (D) Twenty-five micrograms of cell lysate from control transfected CE cells (lane 1) or FAK (lane 2)- or KAKTLR (lane 3)-expressing CE cells was analyzed by Western blotting with phospho-specific antibodies recognizing FAK when phosphorylated on Y397, Y576, Y577, or Y861, as indicated. Lysate was also blotted for FAK to demonstrate equal expression of protein (bottom panel).
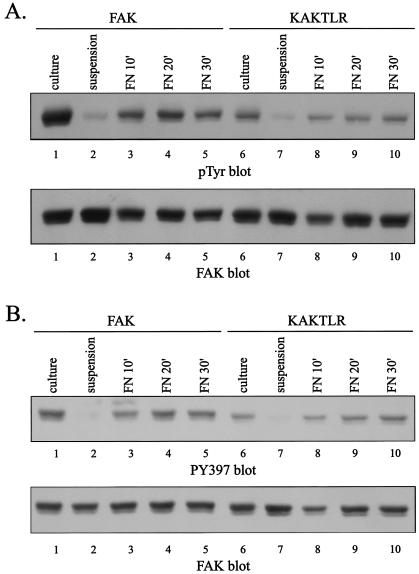
To further characterize KAKTLR, tyrosine phosphorylation in response to cell adhesion to fibronectin was examined. CE cells expressing wild-type FAK or the KAKTLR mutant were trypsinized, held in suspension for 45 min, and then plated on fibronectin for the various times. FAK and KAKTLR were immunoprecipitated and analyzed by Western blotting for phosphotyrosine. The phosphotyrosine content of both wild-type FAK and KAKTLR was reduced when cells were held in suspension and increased upon cell adhesion to fibronectin (Fig. 6A). However, the level of tyrosine phosphorylation of KAKTLR was reduced relative to that of wild-type FAK. Tyrosine phosphorylation was further analyzed by using the PY397 phospho-specific antibody. When the cells were held in suspension, phosphorylation of tyrosine 397 in FAK and KAKTLR was dramatically reduced (Fig. 6B). Upon adhesion to fibronectin, both become phosphorylated at tyrosine 397. However, the level of phosphorylation at tyrosine 397 in KAKTLR was reduced relative to that in wild-type FAK following adhesion to fibronectin. Differences in phosphotyrosine levels were not due to differences in expression or recovery of wild-type and mutant FAK by immunoprecipitation (Fig. 6). These results demonstrate that the KAKTLR mutation, which inhibits the ability of the FERM domain to interact with FAK in vitro, also impairs tyrosine phosphorylation of FAK in vivo in response to a physiological stimulus.
FIG. 6.
KAKTLR is defective for adhesion dependent tyrosine phosphorylation. CE cells expressing wild-type FAK (lanes 1 to 5) or KAKTLR (lanes 6 to 10) were held in suspension or plated onto fibronectin for the indicated times. Tyrosine phosphorylation in subconfluent cells in culture was also examined (lanes 1 and 6). FAK or KAKTLR was immunoprecipitated and analyzed by Western blotting for phosphotyrosine (pTyr) (panel A, top panel). Tyrosine phosphorylation at position 397 was examined by Western blotting of whole cell lysates with PY397 (panel B, top panel). As a loading control, these blots were stripped and reprobed with BC4 (bottom panels).
The FERM domain-mediated interaction is dispensable for catalytic activity.
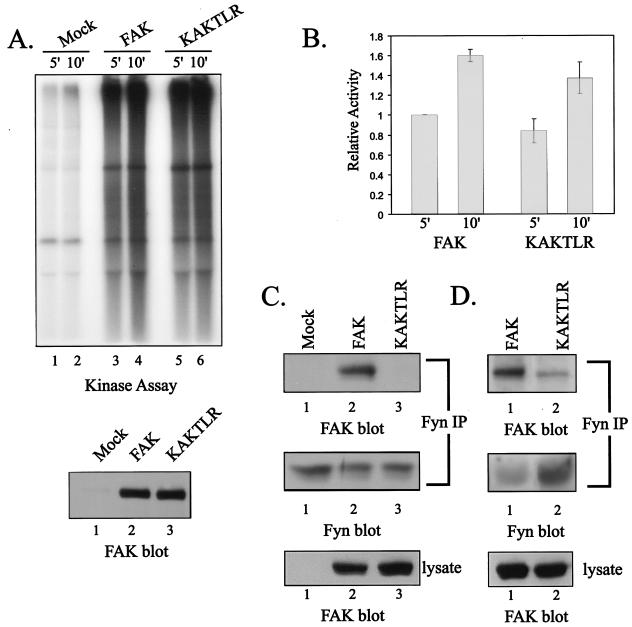
Since KAKTLR exhibits reduced tyrosine phosphorylation in vivo, and given the precedent that the FERM domain-catalytic domain interaction is critical for catalytic activity of JAK3 (60), it seemed possible that the KAKTLR mutant would exhibit a catalytic defect. This possibility was examined by incubating FAK immune complexes in an in vitro kinase assay with poly(Glu,Tyr) as an exogenous substrate. Under these conditions, wild-type FAK and the KAKTLR mutant catalyzed phosphorylation of the exogenous substrate. There was comparable catalytic activity of the KAKTLR mutant relative to the wild type as measured in this assay (Fig. 7A). Quantification of five independent experiments by phosphorimager analysis revealed an approximately 20% reduction in the catalytic activity of the mutant (Fig. 7B). Western blotting of the immune complexes revealed that equal amounts of wild-type FAK and KAKTLR were immunoprecipitated (Fig. 7A, bottom). Therefore, the mutation disrupting the FERM domain-mediated interaction had very little effect upon kinase activity in vitro.
FIG. 7.
KAKTLR is catalytically active but is defective for Fyn binding. (A) Endogenous FAK (lanes 1 and 2) and exogenously expressed wild-type FAK (lanes 3 and 4) or KAKTLR (lanes 5 and 6) were immunoprecipitated from CE cell lysates, and the immune complexes were incubated in an in vitro kinase assay with poly(Glu,Tyr) for 5 or 10 min (top panel). A fraction of each immune complex was also blotted for FAK to verify equal recovery of wild-type FAK (lane 2) and KAKTLR (lane 3) (bottom panel). (B) The results from five experiments were quantified by phosphorimager analysis. Phosphorylation of substrate following 5 min incubation with FAK was arbitrarily set as 1. The error bars indicate standard errors. (C) CE lysates containing empty vector (lanes 1) or expressing FAK (lanes 2) or KAKTLR (lanes 3) were used for immunoprecipitations (IP) with a Fyn polyclonal antiserum. The immune complexes were blotted with BC4 to detect coimmunoprecipitated FAK (top panel), and then the blot was stripped and reprobed for Fyn (middle panel) to demonstrate equal recovery of Fyn in the immunoprecipitations. Expression of wild-type and mutant FAK was compared by blotting 25 μg of cell lysate with BC4 (bottom panel). (D) Fyn was immunoprecipitated from lysates of FAK (lanes 1)- or KAKTLR (lanes 2)-overexpressing cells following adhesion to fibronectin-coated plates for 20 min. Immune complexes were analyzed by Western blotting with BC4 (top panel), and the blots were stripped and reprobed for Fyn (middle panel). Expression of wild-type and mutant FAK was compared by blotting 25 μg of cell lysate with BC4 (bottom panel).
Perturbation of the FERM domain-mediated interaction disrupts binding of Src family kinases.
The KAKTLR mutant exhibits a defect in in vivo phosphorylation of tyrosine 397, a phosphorylation site that regulates binding to several SH2 domain-containing proteins, including Src family kinases. To explore the effect of the KAKTLR mutation upon association with Src kinases, wild-type or mutant FAK proteins were expressed in CE cells and association with Fyn was examined. Endogenous Fyn was immunoprecipitated from cell lysates, and coimmunoprecipitated FAK was examined by Western blotting. Wild-type FAK was readily coimmunoprecipitated with Fyn (Fig. 7C, top panel, lane 2). In contrast, KAKTLR exhibited very weak binding to Fyn (Fig. 7C, top panel, lane 3). Equal amounts of Fyn were immunoprecipitated from each of the lysates (Fig. 7C, middle panel), and comparable levels of wild-type and mutant FAK were expressed (Fig. 7C, bottom panel). Reduced association of the KAKTLR mutation with Fyn was also observed in cells that adhered to fibronectin for 20 min prior to lysis (Fig. 7D). These results demonstrate that the interaction mediated by the basic patch of the F2 subdomain of the FERM domain is required for the efficient phosphorylation of FAK at tyrosine 397 and the subsequent binding of Src family kinases.
Disruption of the FERM domain interaction impairs signaling in vivo.
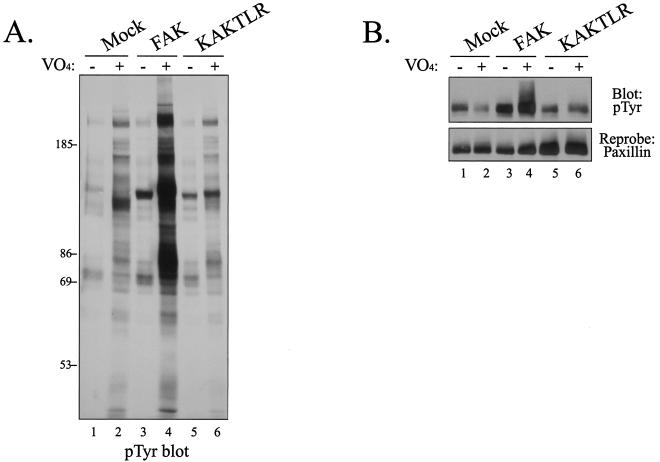
Since KAKTLR exhibited reduced tyrosine phosphorylation in vivo and tyrosine phosphorylation plays an important regulatory role in FAK signaling, the ability of the mutant to transmit biochemical signals in vivo was explored. Whereas overexpression of FAK in CE cells has little effect upon tyrosine phosphorylation of focal adhesion-associated proteins, inhibition of cellular phosphatases by treatment with vanadate induces a dramatic increase in tyrosine phosphorylation of focal adhesion-associated proteins in FAK-overexpressing cells (45, 46). To explore transmission of downstream signals in this system, CE cells overexpressing wild-type FAK or KAKTLR were treated with vanadate, and cellular phosphotyrosine levels were examined by Western blotting. Vanadate treatment had little effect on phosphotyrosine levels in mock-transfected cells but induced a dramatic increase in tyrosine phosphorylation in FAK-overexpressing cells (Fig. 8A, lanes 3 and 4). In contrast, vanadate treatment of KAKTLR-overexpressing cells had little effect on cellular phosphotyrosine levels (lanes 5 and 6), similar to the effect observed in mock-transfected cells. To examine tyrosine phosphorylation of a specific substrate, paxillin was immunoprecipitated from these lysates, and tyrosine phosphorylation was examined by Western blotting. Whereas vanadate treatment of FAK-overexpressing cells dramatically increased tyrosine phosphorylation of paxillin (Fig. 8B, lane 4), vanadate treatment of KAKTLR-expressing cells failed to induce an increase in tyrosine phosphorylation of paxillin (Fig. 8B, lane 6). Equal amounts of paxillin were immunoprecipitated for this analysis (Fig. 8B, bottom). These results demonstrate that disruption of the FERM domain interaction impairs the ability of FAK to transmit biochemical signals in vivo.
FIG. 8.
KAKTLR is defective for signaling in vivo. (A) Lysates of CE cells expressing empty RCAS A (lanes 1 and 2), FAK (lanes 3 and 4), or KAKTLR (lanes 5 and 6) were analyzed by Western blotting for phosphotyrosine (pTyr). Prior to lysis, cells were either left untreated or treated with 50 μM vanadate (VO4) for 16 h. (B) Endogenous paxillin was immunoprecipitated from lysates of control cells (lanes 1 and 2) or cells expressing FAK (lanes 3 and 4) or KAKTLR (lanes 5 and 6) treated as for panel A. Tyrosine phosphorylation was examined by Western blotting (top). The blot was stripped and reprobed with a monoclonal antibody recognizing paxillin (bottom).
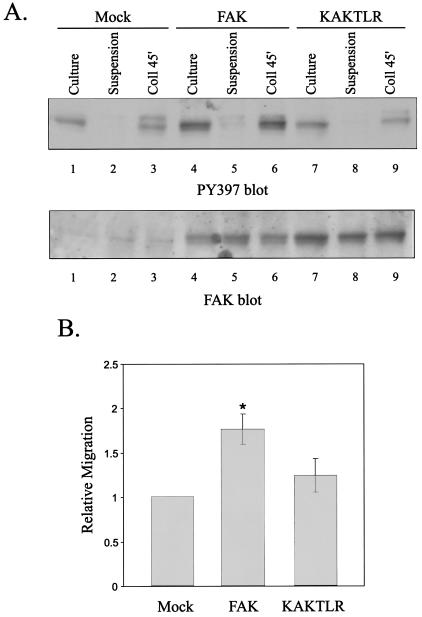
To examine the importance of the FERM domain-mediated interaction in mediating biological functions controlled by FAK, the ability of KAKTLR to promote cell motility was examined. T47D/tva cells, a derivative of the T47D breast cancer cell line that is susceptible to infection with avian retroviral vectors, were used for this analysis (13). T47D/tva cells were infected with the empty RCAS A vector or with RCAS A containing the wild-type FAK or KAKTLR cDNA. Expression levels of FAK and KAKTLR were comparable, as determined by Western blotting (Fig. 9A). Regulation of tyrosine phosphorylation of FAK in T47D cells following cell adhesion to collagen was examined. Cells were held in suspension or plated onto collagen-coated plates for 45 min prior to lysis. Tyrosine phosphorylation at the major autophosphorylation site was measured by Western blotting with PY397, and the amount of FAK in each lysate was determined by stripping and reprobing with a FAK polyclonal antiserum. Phosphorylation of endogenous FAK precipitously declined upon detachment and incubation in suspension. Replating on collagen restored phosphorylation at tyrosine 397 (Fig. 9A, top panel). In cells expressing exogenous FAK, the level of FAK was increased and, concomitantly, phosphorylation of tyrosine 397 was increased (Fig. 9A, lanes 4 to 6). As in control cells, phosphorylation declined when FAK-overexpressing cells were held in suspension, and tyrosine 397 became phosphorylated upon adhesion to collagen. Despite similar expression levels of wild-type FAK and KAKTLR, the level of phosphorylation at tyrosine 397 on KAKTLR was similar to the amount of phosphotyrosine detected on endogenous FAK (Fig. 9A, lanes 7 to 9). These results demonstrate that the KAKTLR mutation produces defects in adhesion-dependent phosphorylation in human epithelial cells, in addition to avian fibroblasts. To measure motility, cells were suspended in serum-free medium and placed in the upper chamber of a transwell. Cell migration in response to a haptotactic stimulus, i.e., collagen applied to the underside of the transwell membrane, was examined (Fig. 9B). Overexpression of FAK resulted in a statistically significant 1.8-fold increase in the number of migrating cells compared to control cells. Overexpression of KAKTLR also increased the motility of T47D/tva cells. However, the mutant was defective for promoting cell motility, increasing migration by only 1.2-fold relative to the motility of control cells, which was not statistically significant. This result demonstrates that the interaction mediated by the FERM domain of FAK is not only required for biochemical signaling but is also required for the control of biological responses by FAK.
FIG. 9.
KAKTLR is defective for promoting motility in T47D cells. T47D/tva cells were infected with empty RCAS A (mock) or RCAS A vectors engineered to express wild-type FAK or KAKTLR. (A) Control cells (lanes 1 to 3) or FAK (lanes 4 to 6)- or KAKTLR (lanes 7 to 9)-expressing cells growing in culture (lanes 1, 4, and 7), held in suspension (lanes 2, 5, and 8) or plated onto collagen for 45 min (lanes 3, 6, and 9) were lysed, and phosphorylation of FAK at tyrosine 397 was examined by Western blotting with PY397 (top). The blot was stripped and reprobed with BC4 as a loading control (bottom). (B) T47D/tva cells infected with empty RCAS A or expressing FAK or KAKTLR were assessed for haptotactic motility in response to collagen I. The average fold change in motility for nine experiments, each performed in triplicate, is shown. Error bars denote standard errors. *, the difference in motility between FAK- and mock-expressing cells was statistically significant (P < 0.05). The difference in motility between KAKTLR- and mock-expressing cells was not statistically significant.
DISCUSSION
The N-terminal region of FAK contains a FERM domain, a module that mediates protein-protein interactions. Like other FERM domains, the FAK FERM domain mediates interactions with several proteins, including transmembrane proteins (e.g., the platelet-derived growth factor receptor) and cytosolic proteins (e.g., Etk) (7, 53). An intramolecular FERM domain-mediated interaction has also been proposed as a mechanism for regulation of FAK activity (9, 17, 49). We have observed that the FAK FERM domain can associate with full-length FAK in vitro. Point mutations within the FERM domain that disrupt this interaction have been identified and shown to impair FAK signaling in vivo. This contrasts with previous findings that suggest a negative regulatory role for the FERM domain. Whereas studies to date have relied upon FERM domain deletion mutants, this is the first study using FERM domain point mutations. One caveat of mutational analysis is loss of function due to inadvertent indirect effects such as misfolding. Several observations demonstrate that this is not the case in this study. Analysis of the recombinant wild-type and mutant FERM domains by circular dichroism suggests the mutant domain is folded similarly to the wild-type domain (data not shown). Further, similar catalytic activities and localization to focal adhesions demonstrate that the catalytic and FAT domains of the mutant and wild-type proteins are correctly folded and function comparably. The results of this study reveal a potentially important role of the FERM domain in cell adhesion-dependent activation of FAK signaling, a function that was not readily apparent from the analysis of N-terminal deletion mutants.
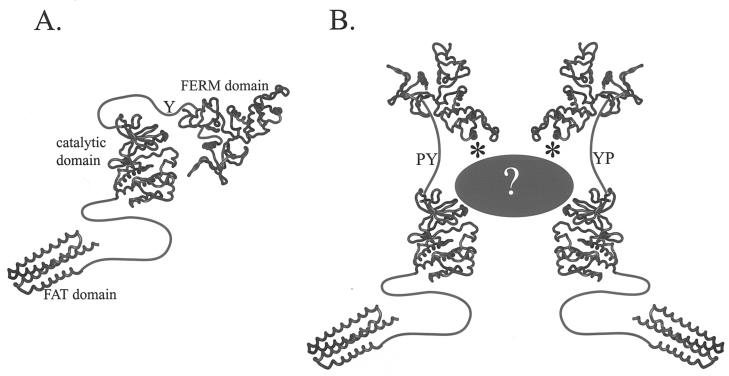
The observed interaction between the FERM domain and full-length FAK appears to be distinct from another reported interaction between the FERM domain and FAK (9). In that published report, the FERM domain was found to be able to interact with the catalytic domain of FAK in vitro. This contrasts with the interaction that we have observed, in which both the FERM domain and catalytic domain of full-length FAK are required to associate with the recombinant FAK FERM domain in vitro. The interaction of the FAK FERM and catalytic domains is presumed to be direct (9). We have partially addressed whether our observed interaction is direct or indirect by using recombinant fragments of FAK. A direct interaction between the recombinant FERM domain and recombinant catalytic domain of FAK was observed, although the interaction was weak. Interestingly, the mutations that abolish the interaction of recombinant FERM domain with full-length FAK in vitro (the KAKTLR mutant) have no effect on the interaction of the purified FERM domain with purified catalytic domain. It therefore appears that multiple FERM domain-mediated interactions may be involved in FAK regulation. The first is the direct FERM domain-catalytic domain interaction described by Cooper et al. (9), and the second is the intermolecular FERM domain-full-length FAK interaction, which we speculate is indirect. The former interaction inhibits FAK activity and signaling (9). The latter interaction may be required for FAK activation, since mutation of the basic patch that mediates the interaction impairs phosphorylation of FAK at tyrosine 397, recruitment of Src family kinases, tyrosine phosphorylation of downstream substrates, and stimulation of cell migration. One model consistent with these data is that the FERM domain negatively regulates FAK via interaction with the catalytic domain in the absence of stimulus (Fig. 10A). Interactions mediated by the basic patch of the FERM domain might alleviate the inhibitory interaction of the FERM domain with the catalytic domain, resulting in FAK activation following cell adhesion (Fig. 10B). We have considered the possibility that the KAKTLR mutant might have dominant negative properties. This is unlikely, as the mutant does not drastically impair tyrosine phosphorylation of paxillin, nor does it inhibit motility. This contrasts with results observed with FRNK, a potent dominant negative mutant of FAK (16, 39).
FIG. 10.
Model of FAK Regulation. (A) In its inactive state, the FERM domain is believed to interact with the catalytic domain to repress activity. (B) Upon activation, this interaction may be disrupted. The basic patch in the F2 subdomain of the FERM domain (asterisk) is required for optimal activation and is perhaps involved in relieving FERM domain-mediated repression of kinase activity.
One intriguing but surprising observation was that the N-terminal domains of FAK and CAKβ could interact with full-length FAK but not with full-length CAKβ. This is presumably due to differences in sequence between FAK and CAKβ. There are precedents for differential interactions of proteins with FAK and CAKβ. For example, FIP200 binds both FAK and CAKβ, but the molecular mechanisms of interaction with each are different. The C-terminal domain of FIP200 mediates binding to the catalytic domain of CAKβ (1, 58). The interaction with FAK is more complex, as there are multiple sites of interaction. The C-terminal domain of FIP200 binds to the N-terminal domain of FAK, not the catalytic domain (1). In addition, both the N-terminal and middle domains of FIP200 mediate the interaction with the catalytic domain of FAK (1). The significance of differential FAK and CAKβ FERM domain interactions is not yet clear, but they could be relevant to differential responses of the kinases to different stimuli.
To gain insight into the function of the FERM domain of FAK, a molecular model was generated. The molecular model has been useful in identifying highly conserved residues on the surface of the FAK FERM domain that may participate in protein-protein interactions. A basic patch at the tip of the acyl-CoA binding protein-like subdomain (F2) of the FAK FERM domain mediates an interaction with full-length FAK in vitro, as demonstrated by the KAKTLR mutant. Interestingly, two diverse lines of investigation suggest that a similar region of the merlin FERM domain may also mediate protein-protein interactions. First, a point mutation in merlin associated with the development of neurofibromatosis type 2 resides at the tip of the F2 subdomain (52). Second, mutation of the highly conserved “blue box” in Drosophila merlin, which is located at the tip of the F2 subdomain, abolishes function of the protein (30). These mutations are predicted to have no consequence for structure but rather to disrupt a protein-protein interaction(s) important for merlin function. These observations suggest that the tip of the F2 subdomain mediates protein-protein interactions in multiple FERM domains, although the molecular bases for these interactions may not be conserved. This finding may be significant, since other interactions with FERM domains have been localized to other regions of the domain, either to the convex surface of the domain across multiple subdomains or to the interface of the F1 and F3 domains (15, 22, 36).
Another interesting observation was that the KAKTLR mutant exhibited a defect in phosphorylation of tyrosine residues 397, 576, and 577 (Fig. 5) yet demonstrated levels of phosphorylation at tyrosine 861 that were equivalent to those for wild-type FAK. Other studies have also shown that phosphorylations of different tyrosine residues of FAK are differentially regulated. For example, stimulation of MCF-7 cells with heregulin (59), of HUVEC cells with vascular endothelial growth factor (2), and of NMuMG cells with transforming growth factor β (35) promotes phosphorylation of tyrosine 861 in FAK with little effect on the phosphorylation of other tyrosine residues. Further, phosphorylation of tyrosine 861 was dramatically reduced in confluent NMuMG cells, whereas phosphorylation of tyrosine 397 was unaffected by cell density (35). Conversely, in several prostate cancer cell lines, phosphorylation of tyrosine 397 was cell adhesion dependent, whereas phosphorylation of tyrosine 861 was independent of cell adhesion (54). Thus, there are a number of precedents in which tyrosine 861 phosphorylation is regulated differently than other FAK tyrosine residues. Despite differential regulation, tyrosine phosphorylation of 861 in response to all of these stimuli is Src dependent (2, 12, 54, 59). It is perhaps surprising that the KAKTLR mutant is defective for Src kinase binding and phosphorylation of some Src-dependent phosphorylation sites (Y576 and Y577) but is fully competent for phosphorylation of another Src-dependent phosphorylation site (Y861). While recruitment of Src into a complex with FAK can promote phosphorylation of tyrosine residues within FAK (42, 48), several reports have demonstrated that Src-dependent phosphorylation of FAK can occur without assembly of the FAK-Src complex (5, 34). The tyrosine phosphorylation pattern of the KAKTLR mutant could indicate that phosphorylation of certain sites on FAK by Src may be more dependent upon formation of the FAK-Src complex than phosphorylation of others, a hypothesis that has not been completely tested.
The properties of the KAKTLR mutant are different from the reported properties of other N-terminal FAK mutants, which to date have been those with large deletions. The results of these previous studies suggest that the N-terminal FERM domain may inhibit the catalytic activity and signaling capacity of FAK. Three mutants, dlN82 (with a deletion extending to serine 333), dl1-386, and Δ375, exhibit dramatically elevated catalytic activity in vitro (6, 9, 57). A fourth mutant, dl51-377, exhibited only a modest increase in kinase activity in vitro (24). A number of mutants have also been characterized for their signaling abilities in vivo. One mutant, Δ1-100, was heavily phosphorylated on tyrosine and associated with Src and Grb2 in adherent, serum-starved cells, in contrast to wild-type FAK, which was modestly phosphorylated and did not associate with Src or Grb2 under these conditions (48). A second mutant, Δ375, exhibited elevated phosphotyrosine levels in cells in suspension (9). In contrast, two other mutants, dl51-377 and dl1-361, exhibited cell adhesion-dependent tyrosine phosphorylation similar to that of wild-type FAK (50). The Δ1-100 mutant exhibited an elevation in levels of phosphotyrosine following adhesion to fibronectin but associated with similar amounts of Src and Grb2 as wild-type FAK (48). In serum-containing medium or following adhesion to fibronectin, dlN82, dl51-377, and dl1-361 exhibited phosphotyrosine levels similar to those of wild-type FAK (6, 50), whereas Δ375 exhibited some increase in tyrosine phosphorylation under the same conditions (9). Furthermore, in vanadate-treated fibroblasts, dl51-377 and dl1-361 promoted tyrosine phosphorylation of paxillin to the same level as wild-type FAK (50). In contrast, transient expression of Δ375 in 293 cells induces tyrosine phosphorylation of paxillin to higher levels than wild-type FAK (9). Clearly, the role of the FAK N terminus in the regulation of signaling is complex and remains to be fully elucidated. Some results suggest that the N terminus impairs catalytic activity in vitro and is necessary for suppression of FAK phosphorylation and signaling under some conditions. However, under conditions where FAK is active, removal of the N-terminal domain does not have as dramatic effect upon FAK phosphorylation or signaling. The KAKTLR mutant differs from these deletion mutants in several ways. First, it exhibits wild-type levels of catalytic activity in vitro. Thus, the inhibitory function of the N terminus, which is apparently relieved by deletion of N-terminal fragments, appears to be intact in this mutant. Second, this mutant is defective for adhesion-dependent tyrosine phosphorylation and downstream signaling, demonstrating a role for the F2 subdomain in activation of the full-length FAK protein in vivo.
Previous hypotheses have speculated that the FERM domain of FAK may regulate signaling via several distinct mechanisms. One proposed mechanism involves an intramolecular inhibitory interaction involving the FERM domain of FAK (9, 17, 49, 57). A second mechanism involves the interaction of inhibitory molecules, e.g., FIP200 (58). The interaction of FIP200 with FAK is particularly complex, with three different binding sites on FIP200 interacting with at least two distinct binding sites on FAK (1). At least in part, the ability of FIP200 to repress the catalytic activity of FAK may depend upon the interaction of its C-terminal domain with the N-terminal domain of FAK; however, the major mode of inhibition is apparently mediated via interaction of the middle and N-terminal domains of FIP200 with the catalytic domain of FAK (1). Our present results suggest that a FERM domain-mediated interaction is also required for the activation of FAK signaling following cell adhesion. These findings suggest that certain FERM domain-mediated interactions function in inhibition of FAK signaling, whereas another FERM domain-mediated interaction is required for activation. One of the most intriguing aspects of the interaction mediated via the basic patch of the F2 subdomain of the FERM domain is the attenuation of signaling upon disruption of the interaction. This observation suggests that the F2 subdomain of FAK may be an effective target for therapeutic intervention in FAK signaling. Pharmacological disruption of this interaction might reduce phosphorylation of FAK and Src binding in vivo and impair aberrant cell motility or survival induced by FAK under pathological conditions.
Acknowledgments
We thank Joan Taylor, Shelley Earp, Lee Graves, Vidhya Iyer, and Pat Lyons for helpful comments on the manuscript. Thanks also go to Brenda Temple for help with homology modeling and to Vita Golubovskaya for providing purified FAK. Thanks go to Alison Worsham for exceptional technical assistance and to Keith Burridge for the use of his microscope. Special thanks go to Danielle Scheswohl and Martin Playford for their assistance during revision of the manuscript. We also thank Eric Schaefer for helpful discussions during the course of this study.
This project is supported by NIH grant CA90901 (to M.D.S.). V.G.-N. is supported by a predoctoral assistantship from the Department of Defense (DAMD 17-00-1-0377).
REFERENCES
- 1.Abbi, S., H. Ueda, C. Zheng, L. A. Cooper, J. Zhao, R. Christopher, and J. L. Guan. 2002. Regulation of focal adhesion kinase by a novel protein inhibitor FIP200. Mol. Biol. Cell 13:3178-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu-Ghazaleh, R., J. Kabir, H. Jia, M. Lobo, and I. Zachary. 2001. Src mediates stimulation by vascular endothelial growth factor of the phosphorylation of focal adhesion kinase at tyrosine 861, and migration and anti-apoptosis in endothelial cells. Biochem. J. 360:255-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arold, S. T., M. K. Hoellerer, and M. E. M. Noble. 2002. The structural basis of localization and signaling by the focal adhesion targeting domain. Structure 10:319-327. [DOI] [PubMed] [Google Scholar]
- 4.Calderwood, D. A., B. Yan, J. M. de Pereda, B. G. Alvarez, Y. Fujioka, R. C. Liddington, and M. H. Ginsberg. 2002. The phosphotyrosine binding-like domain of talin activates integrins. J. Biol. Chem. 277:21749-21758. [DOI] [PubMed] [Google Scholar]
- 5.Cary, L. A., R. A. Klinghoffer, C. Sachsenmaier, and J. A. Cooper. 2002. SRC catalytic but not scaffolding function is needed for integrin-regulated tyrosine phosphorylation, cell migration, and cell spreading. Mol. Cell. Biol. 22:2427-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan, P. Y., S. B. Kanner, G. Whitney, and A. Aruffo. 1994. A transmembrane-anchored chimeric focal adhesion kinase is constitutively activated and phosphorylated at tyrosine residues identical to pp125FAK. J. Biol. Chem. 269:20567-20574. [PubMed] [Google Scholar]
- 7.Chen, R., O. Kim, M. Li, X. Xiong, J. L. Guan, H. J. Kung, H. Chen, Y. Shimizu, and Y. Qiu. 2001. Regulation of the PH-domain-containing tyrosine kinase Etk by focal adhesion kinase through the FERM domain. Nat. Cell Biol. 3:439-444. [DOI] [PubMed] [Google Scholar]
- 8.Cooley, M. A., J. M. Broome, C. Ohngemach, L. H. Romer, and M. D. Schaller. 2000. Paxillin binding is not the sole determinant of focal adhesion localization or dominant-negative activity of focal adhesion kinase/focal adhesion kinase-related nonkinase. Mol. Biol. Cell 11:3247-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper, L. A., T. L. Shen, and J. L. Guan. 2003. Regulation of focal adhesion kinase by its amino-terminal domain through an autoinhibitory interaction. Mol. Cell. Biol. 23:8030-8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunty, J. M., and M. D. Schaller. 2002. The amino termini of FAK family kinases regulate substrate phosphorylation, localization, and cell morphology. J. Biol. Chem. 277:45644-45654. [DOI] [PubMed] [Google Scholar]
- 11.Edwards, S. D., and N. H. Keep. 2001. The 2.7 A crystal structure of the activated FERM domain of moesin: an analysis of structural changes on activation. Biochemistry 40:7061-7068. [DOI] [PubMed] [Google Scholar]
- 12.Eliceiri, B. P., X. S. Puente, J. D. Hood, D. G. Stupack, D. D. Schlaepfer, X. Z. Huang, D. Sheppard, and D. A. Cheresh. 2002. Src-mediated coupling of focal adhesion kinase to integrin alpha(v)beta5 in vascular endothelial growth factor signaling. J. Cell Biol. 157:149-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabarra-Niecko, V., P. J. Keely, and M. D. Schaller. 2002. Characterization of an activated mutant of focal adhesion kinase: SuperFAK. Biochem. J. 365:591-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao, G., K. C. Prutzman, M. L. King, D. M. Scheswohl, E. F. DeRose, R. E. London, M. D. Schaller, and S. L. Campbell. 2004. NMR solution structure of the focal adhesion targeting domain of focal adhesion kinase in complex with a paxillin LD peptide: evidence for a two-site binding model. J. Biol. Chem. 279:8441-8451. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Alvarez, B., J. M. de Pereda, D. A. Calderwood, T. S. Ulmer, D. Critchley, I. D. Campbell, M. H. Ginsberg, and R. C. Liddington. 2003. Structural determinants of integrin recognition by Talin. Mol. Cell 11:49-58. [DOI] [PubMed] [Google Scholar]
- 16.Gilmore, A. P., and L. H. Romer. 1996. Inhibition of focal adhesion kinase (FAK) signaling in focal adhesions decreases cell motility and proliferation. Mol. Biol. Cell 7:1209-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girault, J. A., A. Costa, P. Derkinderen, J. M. Studler, and M. Toutant. 1999. FAK and PYK2/CAKbeta in the nervous system: a link between neuronal activity, plasticity and survival? Trends Neurosci. 22:257-263. [DOI] [PubMed] [Google Scholar]
- 18.Girault, J. A., G. Labesse, J. P. Mornon, and I. Callebaut. 1999. The N-termini of FAK and JAKs contain divergent band 4.1 domains. Trends Biochem. Sci. 24:54-57. [DOI] [PubMed] [Google Scholar]
- 19.Golubovskaya, V., L. Beviglia, L. H. Xu, H. S. Earp III, R. Craven, and W. Cance. 2002. Dual inhibition of focal adhesion kinase and epidermal growth factor receptor pathways cooperatively induces death receptor-mediated apoptosis in human breast cancer cells. J. Biol. Chem. 277:38978-38987. [DOI] [PubMed] [Google Scholar]
- 20.Guan, K. L., and J. E. Dixon. 1991. Eukaryotic proteins expressed in Escherichia coli and improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal. Biochem. 192:262-267. [DOI] [PubMed] [Google Scholar]
- 21.Hamada, K., T. Shimizu, T. Matsui, S. Tsukita, and T. Hakoshima. 2000. Structural basis of the membrane-targeting and unmasking mechanisms of the radixin FERM domain. EMBO J. 19:4449-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamada, K., T. Shimizu, S. Yonemura, S. Tsukita, S. Tsukita, and T. Hakoshima. 2003. Structural basis of adhesion-molecule recognition by ERM proteins revealed by the crystal structure of the radixin-ICAM-2 complex. EMBO J. 22:502-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayashi, I., K. Vuori, and R. C. Liddington. 2002. The focal adhesion targeting (FAT) region of focal adhesion kinase is a four-helix bundle that binds paxillin. Nat. Struct. Biol. 9:101-106. [DOI] [PubMed] [Google Scholar]
- 24.Hildebrand, J. D., M. D. Schaller, and J. T. Parsons. 1993. Identification of sequences required for the efficient localization of the focal adhesion kinase, pp125FAK, to cellular focal adhesions. J. Cell Biol. 123:993-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hilkens, C. M., H. Is'harc, B. F. Lillemeier, B. Strobl, P. A. Bates, I. Behrmann, and I. M. Kerr. 2001. A region encompassing the FERM domain of Jak1 is necessary for binding to the cytokine receptor gp130. FEBS Lett. 505:87-91. [DOI] [PubMed] [Google Scholar]
- 26.Hoellerer, M. K., M. E. M. Noble, G. Labesse, I. D. Campbell, J. M. Werner, and S. T. Arold. 2003. Molecular recognition of paxillin LD motifs by the focal adhesion targeting domain. Structure 11:1207-1217. [DOI] [PubMed] [Google Scholar]
- 27.Ilic, D., Y. Furuta, S. Kanazawa, N. Takeda, K. Sobue, N. Nakatsuji, S. Nomura, J. Fujimoto, M. Okada, and T. Yamamoto. 1995. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 377:539-544. [DOI] [PubMed] [Google Scholar]
- 28.Keely, P. J., A. M. Fong, M. M. Zutter, and S. A. Santoro. 1995. Alteration of collagen-dependent adhesion, motility, and morphogenesis by the expression of antisense alpha 2 integrin mRNA in mammary cells. J. Cell Sci. 108:595-607. [DOI] [PubMed] [Google Scholar]
- 29.Kelley, L. A., R. M. MacCallum, and M. J. Sternberg. 2000. Enhanced genome annotation using structural profiles in the program 3D-PSSM. J. Mol. Biol. 299:499-520. [DOI] [PubMed] [Google Scholar]
- 30.LaJeunesse, D. R., B. M. McCartney, and R. G. Fehon. 1998. Structural analysis of Drosophila merlin reveals functional domains important for growth control and subcellular localization. J. Cell Biol. 141:1589-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu, G., C. D. Guibao, and J. Zheng. 2002. Structural insight into the mechanisms of targeting and signaling of focal adhesion kinase. Mol. Cell. Biol. 22:2751-2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Louvet-Vallee, S. 2000. ERM proteins: from cellular architecture to cell signaling. Biol. Cell 92:305-316. [DOI] [PubMed] [Google Scholar]
- 33.MacArthur, H., and G. Walter. 1984. Monoclonal antibodies specific for the carboxy terminus of simian virus 40 large T antigen. J. Virol. 52:483-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McLean, G. W., V. J. Fincham, and M. C. Frame. 2000. v-Src induces tyrosine phosphorylation of focal adhesion kinase independently of tyrosine 397 and formation of a complex with Src. J. Biol. Chem. 275:23333-23339. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura, K., H. Yano, E. Schaefer, and H. A. Sabe. 2001. Different modes and qualities of tyrosine phosphorylation of Fak and Pyk2 during epithelial-mesenchymal transdifferentiation and cell migration: analysis of specific phosphorylation events using site-directed antibodies. Oncogene 20:2626-2635. [DOI] [PubMed] [Google Scholar]
- 36.Pearson, M. A., D. Reczek, A. Bretscher, and P. A. Karplus. 2000. Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell 101:259-270. [DOI] [PubMed] [Google Scholar]
- 37.Poullet, P., A. Gautreau, G. Kadare, J. A. Girault, D. Louvard, and M. Arpin. 2001. Ezrin interacts with focal adhesion kinase and induces its activation independently of cell-matrix adhesion. J. Biol. Chem. 276:37686-37691. [DOI] [PubMed] [Google Scholar]
- 38.Reynolds, A. B., D. J. Roesel, S. B. Kanner, and J. T. Parsons. 1989. Transformation-specific tyrosine phosphorylation of a novel cellular protein in chicken cells expressing oncogenic variants of the avian cellular src gene. Mol. Cell. Biol. 9:629-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richardson, A., and T. Parsons. 1996. A mechanism for regulation of the adhesion-associated proteintyrosine kinase pp125FAK. Nature 380:538-540. (Erratum, 381:810.) [DOI] [PubMed]
- 40.Schaller, M. D. 2001. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim. Biophys. Acta 1540:1-21. [DOI] [PubMed] [Google Scholar]
- 41.Schaller, M. D., C. A. Borgman, B. S. Cobb, R. R. Vines, A. B. Reynolds, and J. T. Parsons. 1992. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc. Natl. Acad. Sci. USA 89:5192-5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schaller, M. D., J. D. Hildebrand, and J. T. Parsons. 1999. Complex formation with focal adhesion kinase: a mechanism to regulate activity and subcellular localization of Src kinases. Mol. Biol. Cell 10:3489-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schaller, M. D., J. D. Hildebrand, J. D. Shannon, J. W. Fox, R. R. Vines, and J. T. Parsons. 1994. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol. Cell. Biol. 14:1680-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schaller, M. D., C. A. Otey, J. D. Hildebrand, and J. T. Parsons. 1995. Focal adhesion kinase and paxillin bind to peptides mimicking beta integrin cytoplasmic domains. J. Cell Biol. 130:1181-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schaller, M. D., and J. T. Parsons. 1995. pp125FAK-dependent tyrosine phosphorylation of paxillin creates a high-affinity binding site for Crk. Mol. Cell. Biol. 15:2635-2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schaller, M. D., and T. Sasaki. 1997. Differential signaling by the focal adhesion kinase and cell adhesion kinase beta. J. Biol. Chem. 272:25319-25325. [DOI] [PubMed] [Google Scholar]
- 47.Schlaepfer, D. D., C. R. Hauck, and D. J. Sieg. 1999. Signaling through focal adhesion kinase. Prog. Biophys. Mol. Biol. 71:435-478. [DOI] [PubMed] [Google Scholar]
- 48.Schlaepfer, D. D., and T. Hunter. 1996. Evidence for in vivo phosphorylation of the Grb2 SH2-domain binding site on focal adhesion kinase by Src-family protein-tyrosine kinases. Mol. Cell Biol. 16:5623-5633. (Erratum, 16:7182-7184.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schlaepfer, D. D., and T. Hunter. 1998. Integrin signalling and tyrosine phosphorylation: just the FAKs? Trends Cell Biol. 8:151-157. [DOI] [PubMed] [Google Scholar]
- 50.Shen, Y., and M. D. Schaller. 1999. Focal adhesion targeting: the critical determinant of FAK regulation and substrate phosphorylation. Mol. Biol. Cell 10:2507-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen, Y., G. Schneider, J. F. Cloutier, A. Veillette, and M. D. Schaller. 1998. Direct association of protein-tyrosine phosphatase PTP-PEST with paxillin. J. Biol. Chem. 273:6474-6481. [DOI] [PubMed] [Google Scholar]
- 52.Shimizu, T., A. Seto, N. Maita, K. Hamada, S. Tsukita, S. Tsukita, and T. Hakoshima. 2002. Structural basis for neurofibromatosis type 2. Crystal structure of the merlin FERM domain. J. Biol. Chem. 277:10332-10336. [DOI] [PubMed] [Google Scholar]
- 53.Sieg, D. J., C. R. Hauck, D. Ilic, C. K. Klingbeil, E. Schaefer, C. H. Damsky, and D. D. Schlaepfer. 2000. FAK integrates growth-factor and integrin signals to promote cell migration. Nat. Cell Biol. 2:249-256. [DOI] [PubMed] [Google Scholar]
- 54.Slack, J. K., R. B. Adams, J. D. Rovin, E. A. Bissonette, C. E. Stoker, and J. T. Parsons. 2001. Alterations in the focal adhesion kinase/Src signal transduction pathway correlate with increased migratory capacity of prostate carcinoma cells. Oncogene 20:1152-1163. [DOI] [PubMed] [Google Scholar]
- 55.Thomas, J. W., M. A. Cooley, J. M. Broome, R. Salgia, J. D. Griffin, C. R. Lombardo, and M. D. Schaller. 1999. The role of focal adhesion kinase binding in the regulation of tyrosine phosphorylation of paxillin. J. Biol. Chem. 274:36684-36692. [DOI] [PubMed] [Google Scholar]
- 56.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toutant, M., A. Costa, J. M. Studler, G. Kadare, M. Carnaud, and J. A. Girault. 2002. Alternative splicing controls the mechanisms of FAK autophosphorylation. Mol. Cell. Biol. 22:7731-7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ueda, H., S. Abbi, C. Zheng, and J. L. Guan. 2000. Suppression of Pyk2 kinase and cellular activities by FIP200. J. Cell Biol. 149:423-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vadlamudi, R. K., A. A. Sahin, L. Adam, R. A. Wang, and R. Kumar. 2003. Heregulin and HER2 signaling selectively activates c-Src phosphorylation at tyrosine 215. FEBS Lett. 543:76-80. [DOI] [PubMed] [Google Scholar]
- 60.Zhou, Y. J., M. Chen, N. A. Cusack, L. H. Kimmel, K. S. Magnuson, J. G. Boyd, W. Lin, J. L. Roberts, A. Lengi, R. H. Buckley, R. L. Geahlen, F. Candotti, M. Gadina, P. S. Changelian, and J. J. O'Shea. 2001. Unexpected effects of FERM domain mutations on catalytic activity of Jak3: structural implication for Janus kinases. Mol. Cell 8:959-969. [DOI] [PubMed] [Google Scholar]