Depending on one’s perspective, αIIbβ3 antagonists can be viewed as a great success story or as an exasperating disappointment1. Certainly, millions of patients have been treated with the three FDA approved αIIbβ3 antagonists, abcixibmab, epitibitide, tirofiban; based on their reduction in mortality in clinical trials, one can calculate that many lives have been saved by these drugs2; and they continue to be administered to prevent thrombotic events, primarily in the setting of percutaneous coronary interventions3. On the other hand, the vision that αIIbβ3 antagonists would be broadly administered as safe, orally active agents to patients at risk for acute coronary syndromes and other cardiovascular diseases not only did come to fruition materialize but were abandoned as being unsafe4;5. Indeed, the perceived side effects of existing αIIbβ3 antagonists, bleeding6;7 and thrombocytopenia8–10, in combination with the emergence of alternative and inexpensive anti-platelet and anti-thrombotic drugs, has led to waning use of αIIbβ3 antagonists over the past decade. Thus, the story of αIIbβ3 antagonists appears to be heading towards its closing chapter. To rewrite or extend the ending of this story would require development of a new class of αIIbβ3 antagonists, one with a distinct mechanism of action that would distinguish it from the existing αIIbβ3 antagonists and their associated complications, bleeding and thrombocytopenia, and, above all, be targeted to a new and broader therapeutic indication. The article by Li et al appearing in this issue11 of ATVB describes the properties and early preclinical testing of RUC-4 as a new αIIbβ3 antagonist.
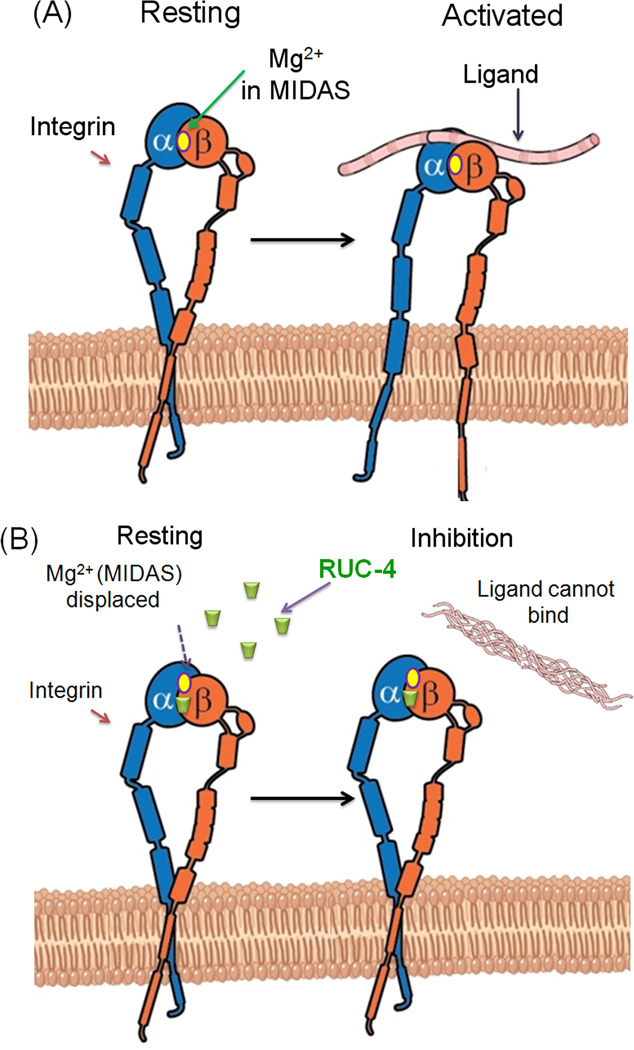
RUC-4 (mol wt = 386) is closely related to its predecessors RUC-110;12 and RUC-210, which were identified through high throughput screens for small molecule inhibitors of fibrinogen binding to αIIbβ3. Like RUC-2, RUC-4 is a potent inhibitor of platelet aggregation; it is specific for αIIbβ3 and does not react with αVβ3. The solubility properties of RUC-4 in physiologically compatible solvent are superior to that RUC-2. Both compounds “work” by competing with Mg2+ bound to the Metal Ion Dependent Adhesion Site in the integrin β I domain for a key coordinating site in the β3 subunit (see Figure). This displacement locks the receptor in a resting state so that it can not bind ligand with high affinity and does not undergo the conformational changes associated with ligand binding. Hence, αIIbβ3 does not become activated upon binding of RUC-4 and does not express neoepitopes induced by ligand binding (LIBS)13 that may become the targets for naturally occurring antibodies that may lead to the thrombocytopenia observed in some patients treated with αIIbβ3 antagonists9;14–17. The manuscript presents detailed molecular dynamic simulations to explain and compare the binding mechanisms of RUC-4 and RUC-2 to the αIIbβ3 at a structural level.
Figure 1.
Mechanism of action of RUC-4. (A) Ligands bind near MIDAS in the β integrin subunit leading to activation of resting integrins. (B) Unlike conventional αIIbβ3 integrin antagonists, RUC-4 displaces Mg2+ to bind at MIDAS. As no conformational change ensues, integrins cannot bind ligands and thus remain inhibited.
The remainder of the manuscript deals with an in vivo analysis of RUC-4 in comparison to RUC-2. Since neither RUC-4 nor RUC-2 react with mouse αIIbβ3, mice developed by Blue et al12 which express human αIIb complexed to murine β3, were used as an initial test of the anti-platelet activity of the two agents in vivo. Doses of RUC-2 administered by intraperitoneal (IP) injection were found that completely inhibited platelet aggregation induced by high dose ADP within 15 min of injection with a return towards normalization within 45 min to 4hr. Even lower dosed of RUC-4, administered by intramuscular (IM) injection, also led to complete inhibition of platelet aggregation within 5 minutes with partial return of aggregation by 4 hours. Indeed, the plasma absorption of RUC-4 through the IM route was more rapid than that of RUC-2 through the IP route. With these encouraging results, RUC-4 was moved into test into cynomolgus monkeys. The animals were given IM injections of ~4, 2 and 1 mg/kg of RUC-4. The extent and duration of inhibition of platelet aggregation ranged from complete to partial inhibition of platelet aggregation within 15 minutes and paralleled the dose of administered from RUC-4 as did the recovery of normal platelet function. None of the animals developed thrombocytopenia, major bleeds or other overt health problems. In the final set of analyses, the authors returned to murine models and examined the effects of RUC-2 and RUC-4 in two models of thrombosis. In a ferric chloric carotid injury model and in a vWF mutant mouse model, RUC-4 protected the mouse against development of thrombosis by IM administration in the former model and IV injection in the latter model.
The study presented by Li et al ( ) identifies RUC-4 as having a favorable preclinical safety and efficacy profile and properties clearly justifying further exploration. Particularly intriguing is the route of its administration, intramuscular, and the rapidity with which full inhibition of platelet aggregation, as rapidly as 15 minutes in subhuman primates, can be achieved. These characteristic open the possibility that a drug with the profile of RUC-4 could be administered by emergency medical personnel to patients with myocardial infarctions where rapid intervention is not only life saving but impacts on subsequent complications18. The currently approved FDA approved αIIbβ3 antagonists requiring IV injection and prolonged administration are not amenable to fulfilling this role. Obviously, the present study is only the initial step in a long road to the development of RUC-4 or its derivatives as a therapeutic drug. Even at the preclinical level, it remains to be shown that the drug does not cause clinically significant bleeds or does not lead to thrombotic episodes as the drug dissipates or has other adverse effects. Moreover, the design of appropriate of clinical trials and the cost of such trials represents as major hurdles to drug development in the cardiovascular arena. Nevertheless, an important step has been come to realization- the possibility of a new αIIbβ3 antagonist may have risen.
Acknowledgement
We thank David Schumick for his assistance in the development of the figure. This work was supported by National Institutes of Health grant P01 HL073311.
Footnotes
Disclosures
The authors report no conflicts.
Reference List
- 1.Bledzka K, Smyth SS, Plow EF. Integrin aIIbB3: From Discovery to Efficacious Therapeutic Target. Circ.Res. 2013;112:1189–1200. doi: 10.1161/CIRCRESAHA.112.300570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coller BS. Translating from the rivers of Babylon to the coronary bloodstream. J.Clin.Invest. 2012;122:4293–4299. doi: 10.1172/JCI66867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosch X, Marrugat J, Sanchis J. Platelet glycoprotein IIb/IIIa blockers during percutaneous coronary intervention and as the initial medical treatment of non-ST segment elevation acute coronary syndromes. Cochrane.Database.Syst.Rev. 2010 doi: 10.1002/14651858.CD002130.pub2. CD002130. [DOI] [PubMed] [Google Scholar]
- 4.Cox D. Oral GPIIb/IIIa antagonists: what went wrong? Curr.Pharm.Des. 2004;10:1587–1596. doi: 10.2174/1381612043384673. [DOI] [PubMed] [Google Scholar]
- 5.Chew DP, Bhatt DL, Sapp S, Topol EJ. Increased mortality with oral platelet glycoprotein IIb/IIIa antagonists - A meta-analysis of phase III multicenter randomized trials. Circulation. 2001;103:201–206. doi: 10.1161/01.cir.103.2.201. [DOI] [PubMed] [Google Scholar]
- 6.Ndrepepa G, Guerra E, Schulz S, et al. Weight of the bleeding impact on early and late mortality after percutaneous coronary intervention. J.Thromb.Thrombolysis. 2014 doi: 10.1007/s11239-014-1084-3. [DOI] [PubMed] [Google Scholar]
- 7.Ndrepepa G, Kastrati A, Neumann FJ, et al. Five-year outcome of patients with acute myocardial infarction enrolled in a randomised trial assessing the value of abciximab during coronary artery stenting. Eur.Heart J. 2004;25:1635–1640. doi: 10.1016/j.ehj.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 8.Tcheng JE. Clinical challenges of platelet glycoprotein IIb/IIIa receptor inhibitor therapy: bleeding, reversal, thrombocytopenia, and retreatment. Am.Heart J. 2000;139:S38–S45. doi: 10.1067/mhj.2000.103742. [DOI] [PubMed] [Google Scholar]
- 9.Tcheng JE, Kereiakes DJ, Lincoff AM, et al. Abciximab readministration: results of the ReoPro Readministration Registry. Circulation. 2001;104:870–875. doi: 10.1161/hc3301.094533. [DOI] [PubMed] [Google Scholar]
- 10.Zhu J, Zhu J, Negri A, et al. Closed headpiece of integrin alphaIIbbeta3 and its complex with an alphaIIbbeta3-specific antagonist that does not induce opening. Blood. 2010;116:5050–5059. doi: 10.1182/blood-2010-04-281154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. This article from ATVB. [Google Scholar]
- 12.Blue R, Kowalska MA, Hirsch J. Structural and therapeutic insights from the species specificity and in vivo antithrombotic activity of a novel alphaIIb-specific alphaIIbbeta3 antagonist. Blood. 2009;114:195–201. doi: 10.1182/blood-2008-08-169243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frelinger AL, III, Du X, Plow EF, Ginsberg MH. Monoclonal antibodies to ligand-occupied conformers of integrin αIIbβ3 (glycoprotein IIb-IIIa) alter receptor affinity, specificity and function. J.Biol.Chem. 1991;266:17106–17111. [PubMed] [Google Scholar]
- 14.Bougie DW, Wilker PR, Wuitschick ED, et al. Acute thrombocytopenia after treatment with tirofiban or eptifibatide is associated with antibodies specific for ligand-occupied GPIIb/IIIa. Blood. 2002;100:2071–2076. [PubMed] [Google Scholar]
- 15.Weitz JI, Bates SM. Beyond heparin and aspirin: new treatments for unstable angina and non-Q-wave myocardial infarction. Arch.Intern.Med. 2000;160:749–758. doi: 10.1001/archinte.160.6.749. [DOI] [PubMed] [Google Scholar]
- 16.Madan M, Berkowitz SD. Understanding thrombocytopenia and antigenicity with glycoprotein IIb-IIIa inhibitors. Am.Heart J. 1999;138:317–326. doi: 10.1053/hj.1999.v138.a100465. [DOI] [PubMed] [Google Scholar]
- 17.Bednar B, Cook JJ, Holahan MA, et al. Fibrinogen receptor antagonist-induced thrombocytopenia in chimpanzee and rhesus monkey associated with preexisting drug-dependent antibodies to platelet glycoprotein IIb/IIIa. Blood. 1999;94:587–599. [PubMed] [Google Scholar]
- 18.Goel K, Pinto DS, Gibson CM. Association of time to reperfusion with left ventricular function and heart failure in patients with acute myocardial infarction treated with primary percutaneous coronary intervention: a systematic review. Am.Heart J. 2013;165:451–467. doi: 10.1016/j.ahj.2012.11.014. [DOI] [PubMed] [Google Scholar]