SUMMARY
Intestinal stem cells in the adult Drosophila midgut are regulated by growth factors produced from the surrounding niche cells including enterocytes and visceral muscle. The role of the other major cell type, the secretory enteroendocrine cells, in regulating intestinal stem cells remains unclear. We show here that newly eclosed scute loss of function mutant flies are completely devoid of enteroendocrine cells. These enteroendocrine cell-less flies have normal ingestion and fecundity but shorter life span. Moreover, in these newly eclosed mutant flies, the diet-stimulated midgut growth that depends on the insulin-like peptide 3 expression in the surrounding muscle is defective. The depletion of Tachykinin producing enteroendocrine cells or knockdown of Tachykinin leads to a similar although less severe phenotype. These results together establish that enteroendocrine cells serve as an important link between diet and visceral muscle expression of an insulin-like growth factor to stimulate intestinal stem cell proliferation and tissue growth.
Keywords: Drosophila, DILP3, enteroendocrine cells, homeostasis, intestine, scute, stem cells, Tachykinin
INTRODUCTION
The gastrointestinal (GI) tract is a complex organ essential for nutrient absorption and whole body metabolism (Miguel-Aliaga, 2012). The Drosophila midgut is an equivalent of the mammalian stomach and small intestine. The midgut epithelium has no crypt-villus structure but instead is a monolayer of absorptive enterocytes (ECs), with interspersed intestinal stem cells (ISCs), enteroblasts (EBs) and enteroendocrine cells (EEs) located closer to the basement membrane (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006).
All cells in the midgut likely constitute together the niche that regulates ISC proliferation and EB differentiation for tissue homeostasis. The visceral muscle secretes Wingless, insulin-like peptides, epidermal growth factor receptor (EGFR) ligands and Decapentaplegic (Dpp)/BMP (Guo et al., 2013; Jiang et al., 2010; Lin et al., 2008; O’Brien et al., 2011). The mature ECs are a major source of stress induced Dpp, EGFR ligands, and the JAK-STAT pathway ligands Unpaired (Upd) 1–3 (Biteau and Jasper, 2011; Buchon et al., 2010; Guo et al., 2013; Jiang et al., 2010; Jiang et al., 2009; Li et al., 2013a; Osman et al., 2012; Tian and Jiang, 2014; Xu et al., 2011). The differentiating EBs also produce Upds, Wingless and EGFR ligands (Cordero et al., 2012; Jiang et al., 2010; Zhou et al., 2013). The surrounding trachea secretes Dpp while the innervating neurons can also regulate intestinal physiology (Cognigni et al., 2011; Li et al., 2013b).
EEs constitute a major cell type in the Drosophila midgut epithelium. While the mammalian secretory lineage is differentiated into Paneth cells, goblet cells, enteroendocrine cells and tuft cells (Gerbe et al., 2012), the entire population of secretory cells in the Drosophila midgut is collectively called EEs and marked by the homeodomain protein Prospero (Pros) (Micchelli and Perrimon, 2006). Nonetheless, different subsets of hormones are produced from different subtypes of midgut EEs (Ohlstein and Spradling, 2006). In the mouse intestine, the Lgr5+ ISCs directly contact Paneth cells and isolated ISC-Paneth cell doublets have higher efficiency to form organoids (Sato et al., 2011). However, mouse genetic knockout that has Paneth cells removed did not result in the loss of Lgr5+ ISCs (Durand et al., 2012). Only recently Drosophila midgut EEs have been shown to negatively regulate ISC proliferation via EGFR ligand production and to regulate ISC differentiation via the Slit/Robo pathway (Biteau and Jasper, 2014; Scopelliti et al., 2014). Therefore, the function of EEs in regulating stem cell activity largely remains to be investigated. Here we show that Drosophila midgut EEs serve a niche function by producing hormones such as Tachykinin (Tk) to regulate insulin peptide expression in the surrounding muscle that in turn affects intestinal homeostasis.
Results and Discussion
scute (sc) RNAi and deletion result in EE-less flies
Previous evidence shows that adult midgut mutant clones that have all the AS-C genes deleted are defective in EE formation while overexpression of sc or asense (ase) is sufficient to increase EE formation (Bardin et al., 2010). Moreover, the Notch pathway with a downstream requirement of ase also regulate EE differentiation (Micchelli and Perrimon, 2006; Takashima et al., 2011; Zeng et al., 2013). To study the requirement of EEs in midgut homeostasis, we first attempted to delete all EEs by knocking down each of the AS-C transcripts using the ISC/EB driver esg-Gal4. The results show that sc RNAi was the only one that caused the loss of all EEs in the adult midgut (Fig. 1A, Fig. S1A–F). The esg-Gal4 driver is expressed in both larval and adult midguts, but the esg>sc RNAi larvae were normal while the newly eclosed adults had no EEs. Therefore, sc is likely required for all EE formation during metamorphosis when the adult midgut epithelium is reformed from precursors/stem cells (Jiang and Edgar, 2009; Micchelli et al., 2011).
Figure 1. EE-less fly guts after loss of sc function have growth defects.
(A) The number of Pros+ nuclei was counted within 0.08 mm2 surface area of a microscopic image from a similar region of each posterior midgut. The scRNAi midguts were completely devoid of EEs. For this and all other figures, data are presented as mean +/− SEM (error bar). (B) EE quantification in the midguts of flies with the genotypes indicated. Control was w-, the deficiency for sc was Df(1)sc10-1 and for ato was Df(3R)p13. Young flies were 7 days old and aged flies were 21 days old. NS is non-significant with P>0.05, and all P values are from Student’s t test. (C, D) Light microscope images of control and esg>scRNAi fly midguts. The arrow and hair line point to the posterior midgut region where images were taken to measure the diameter. (E) Quantification of the diameter of the midguts after starving (1% sucrose) or normal feeding condition. (F) The cross-section area of each enterocyte based on confocal images of Armadillo staining that outlined the cell shape was measured and the average is plotted as shown. (G, H) Midguts from fertilized females (7–10 days old) were homogenized and used for enzymatic assay. Each genotype corresponded to 5–6 samples of 10 midguts each. The sample with the highest activity in each enzyme assay was set as 100% and all others were calculated as a fraction.
The sc6/sc10-1 hemizygous mutant adults were also completely devoid of midgut EEs (Fig. 1B, Fig. S1G–H), while other hemizygous combinations including sc1, sc3B, and sc5 were normal in terms of EE number. Df(1)sc10-1 is a small deficiency that has both ac and sc uncovered. sc1 and sc3B each contains a gypsy insertion in far upstream regions of sc, while sc5 and sc6 are 1.3 and 17.4 kb deletions, respectively, in the sc 3′ regulatory region (Garcia-Bellido and de Celis, 2009). The sc6/sc10-1 combination may affect sc expression during midgut metamorphosis and thus the formation of all adult EEs.
The atonal homolog 1 (Atoh1) is required for all secretory cell differentiation in mouse (Durand et al., 2012; VanDussen and Samuelson, 2010). However, esg-Gal4 driven atonal (ato) RNAi and the amorphic combination ato1/Df(3R)p13 showed normal EE formation (Fig. 1A, B, Fig. S1F, I, J). Nonetheless, we found that older ato1/Df(3R)p13 flies exhibited a significantly lower increase of EE number (Fig. 1B), suggesting a role of ato in EE differentiation in adult flies.
Changing the number of EEs alters life span
In sc RNAi guts the mRNA expression of allatostatin (Ast), allatostatin C (AstC), tachykinin (Tk), diuretic hormone (DH31), and neuropeptide F (NPF) was almost abolished (Fig. S1K), consistent with the absence of all EEs. On the other hand, the mRNA expression of the same peptide genes in heads showed no significant change (Fig. S1L). Even though the EEs and regulatory peptides were absent from the midgut, the flies were viable and showed no apparent morphological defects. There was no significant difference in the number of eggs laid and the number of pupae formed from control and sc RNAi flies (Fig. S1M), suggesting that the flies probably have sufficient nutrient uptake to support the major physiological task of reproduction. However, when we examined the longevity of these animals, the EE-less flies after sc RNAi showed significantly shorter life span (Fig. S1N). In addition, when the number of EEs was increased in adult flies by esgGal4;tubGal80ts (esgts>) driven sc overexpression (Bardin et al. 2010 and Fig. S3), an even shorter life span was observed. These results suggest that a balanced number of EEs is essential for the long-term health of the animal. Moreover, there may be important physiological changes in these EE-less flies that are yet to be uncovered, such as reduced intestinal growth described in detail below.
EE-less flies have reduced intestinal growth as observed under starvation conditions
One of the phenotypic changes we found for the sc RNAi/EE-less flies was that under normal feeding condition their midguts had a significantly narrower diameter than that of control midguts (Fig. 1C, D). When reared in poor nutrition of 1% sucrose, both WT and EE-less flies had thinner midguts. When reared in normal food, WT flies had substantially bigger midgut diameter while EE-less flies had grown significantly less (Fig. 1E). The cross-section area of enterocytes in the EE-less midguts was smaller (Fig. 1F), suggesting that there is also a growth defect at the individual cell level.
A series of experiments showed that ingestion of food dye by the sc RNAi/EE-less flies was not lower than control flies (Fig. S2C). The measurement of food intake by OD of gut dye contents also showed similar ingestion (Fig. S2D). The measurement of excretion by counting colored deposits and visual examination of dye clearing from guts showed that there was no significant change in food passage (Fig. S2E, F). The normal fecundity shown in Fig. S1M also suggested that the mutant flies likely had absorbed sufficient nutrient for reproduction. Nonetheless, another phenotype we could detect was a substantial reduction of intestinal digestive enzyme activities including trypsin, chymotrypsin, aminopeptidase and acetate esterase (Fig. 1G, H and Fig. S2A, B). These enzyme activities exhibit strong reduction after starvation of WT flies. The EE-less flies therefore have a physiological response as if they experience starvation although they are provided with a normal diet.
EE-less midguts have reduced ISC division and Dilp3 expression
A previous report has established that newly eclosed flies respond to nutrient availability by increasing ISC division that leads to a jump start of intestinal growth (O’Brien et al., 2011). When we fed newly eclosed flies on the poor diet of 1% sucrose, both wild type and sc RNAi/EE-less guts had very low number of p-H3-positive cells (Fig. 2A), which represent mitotic ISCs because ISCs are the only dividing cells in the adult midgut. When fed on normal diet the wild type guts had significantly higher p-H3 counts, but the sc RNAi/EE-less guts were consistently lower at all the time points. The sc6/sc10-1 hemizygous mutant combination exhibited a similarly lower mitotic activity on normal diet (Fig. 2B).
Figure 2. EE-less guts have ISC proliferation and Dilp3 expression defects.
(A–B) Newly hatched flies (day 1) were collected and kept in normal food vials or plastic vials with filter paper soaked with 1% sucrose (starved). Each day after, midguts were dissected from flies of the indicated genotypes and stained for p-H3 to detect mitotic cells. Average number of p-H3+ cells is plotted as shown. The esg>GFP in panel A or sc/+ in panel B served as controls. The deficiency is Df(1)sc10-1. (C) Dilp3 mRNA expression assayed by qPCR. Newly hatched esg>GFP (control) and esg>GFP, scRNAi flies were kept in normal food vials for 1 to 5 days as indicated. At each indicated day, 10 flies from each sample were used for gut dissection, RNA isolation and qPCR. Each qPCR cycle number of Dilp3 was normalized with that of rp49 in a parallel reaction of the same RNA sample. The lowest Dilp3 expressing sample esg>scRNAi at day 1 was set as 1 (first black bar) and all other samples were calculated as relative level and plotted as shown. (D–E) Dilp3 promoter-Gal4 driven UAS-GFP expression (Dilp3>GFP) illuminates the smooth muscle surrounding the adult midgut epithelium. This expression of muscle Dilp3>GFP is not altered when the UAS-scRNAi construct is also driven by this Dilp3 promoter. (F–K) Confocal images of midgut at an outer focal plane showing the visceral muscle staining, an inner focal plane showing the epithelium staining and 3D reconstruction of multiple focal planes. The control flies contained the combination of esg-Gal4 and Dilp3-Gal4 together driving UAS-GFP expression. The bottom panels I–K were from a fly strain that also contained the scRNAi construct.
When we investigated possible signaling defects in the EE-less flies, we found that in addition to other gut peptide mRNAs, the level of Dilp3 mRNA was also highly decreased in these guts while the head Dilp3 was normal (Fig. 2C, Fig. S1L). This is somewhat surprising because Dilp3 is expressed not in the epithelium or EEs but in the surrounding muscle (O’Brien et al., 2011; Veenstra et al., 2008). We used the Dilp3 promoter-Gal4 driven UAS-GFP expression (Dilp3>GFP) to visualize the expression in muscle (Fig. 2D). Both control and sc RNAi under this driver showed normal muscle GFP expression (Fig. 2E), demonstrating that sc does not function within the smooth muscle to regulate Dilp3 expression. We then combined the esg-Gal4 and Dilp3-Gal4, and the control UAS-GFP samples showed the expected expression in both midgut precursors and surrounding muscles (Fig. 2F–H). When these combined Gal4 drivers were used to drive sc RNAi, the smooth muscle GFP signal was clearly reduced (Fig. 2I–K). These guts also exhibited no Prospero staining and overall fewer cells with small sizes as expected from esg>sc RNAi (Fig. 2I–K).
The report by O’Brien (2011) showed an increase of Dilp3 expression from the surrounding muscle in newly eclosed flies under well-fed diet (see also Fig. 2C). This muscle Dilp3 expression precedes brain expression and is essential for the initial nutrient stimulated intestinal growth. Our EE-less flies show similar growth and Dilp3 expression defects, suggesting that EE is a link between nutrient sensing and Dilp3 expression during this early growth phase.
Increasing the number of EEs promotes ISC division partly via Dilp3 expression
Wild type and AS-C deletion (scB57) mutant clones in adult midguts did not exhibit a difference in their cell numbers (Bardin et al., 2010). Moreover, we performed esgts>sc RNAi in adult flies for 3 days but did not observe a decrease of mitotic count or EE number. Together these results suggest that sc is not required directly in ISC for proliferation, and imply that the ISC division defects observed in the sc mutant/EE-less flies is likely due to the loss of EEs. To investigate this idea further, we used the esgts> system to up and down shift the expression of sc at various time points and measure the correlation of sc expression, EE number and ISC mitotic activity. The overexpression of sc after shifting to 29°C for a few days correlated with increased EE number, expression of gut peptides and increased ISC activity (Fig. S3A–I). Then we downshifted back to room temperature (23°C) to allow the Gal80ts repressor to function again. The sc mRNA expression was quickly reduced within 2 days and remained low for 4 days (Fig. 3A). Although we did not have a working antibody to check the Sc protein stability, the expression of a probable downstream gene phyllopod (Reeves and Posakony, 2005) showed the same up and down regulation (Fig. 3B), revealing that Sc function returned to normal after the temperature downshift. Meanwhile, the number of Pros+ cells and p-H3 count remained higher after the downshift (Fig. 3C, D). Therefore, the number of EEs, but not sc mRNA or function, correlates with ISC mitotic activity.
Figure 3. Increasing the number of EEs promotes ISC division partly via Dilp3.
(A–D) Down-shift experiments were performed by placing the esgGal4-tubulinGal80ts (esgts>) flies first at 29°C for 3 days and then back to room temperature (approximately 23°C). On the indicated days, fly guts were dissected to use for sc mRNA and phyllopod mRNA PCR assays. Each sc and phyllopod PCR cycle number was normalized with that of rp49, and the normalized control sample at each time point was set as 1 and the normalized UAS-sc sample of the same time point was calculated as fold change over the control. Equal numbers of flies from the same vials were used separately for Pros and p-H3 staining and quantification. (E) 3 days old flies kept at room temperature with the genotype esgts>GFP (control) and esgts>GFP, UAS-sc were shifted to 29°C for 3 days and then shifted back to room temperature for additional 3 days. The normalized Dilp3 mRNA of control at each time point was set as 1 and that of the UAS-sc of the same time point was calculated as fold change. (F) The tubulin-Gal4 driver with the temperature sensitive repressor tubulinGal80ts (tubulin, ts>) were crossed together with the transgenic constructs UAS-GFP as control, UAS-sc to increase the EE formation, and UAS-Dilp3RNAi to deplete the Dilp3 RNA. Flies approximately 7 days old were transferred to 29°C for 4 days to allow the expression of the transgenes. The flies were then used for gut dissection and p-H3 staining.
We performed another experiment that was independent of sc expression or expression in ISCs. The anti-apoptotic protein p35 was driven by the pros-Gal4 driver, which is expressed in a subset of EEs in the middle and posterior midgut (Fig. S4B–E). This resulted in a significant albeit smaller increase in EE number, and a concomitant increase in mitotic activity (Fig. S3J, K), which was counted only in the middle and posterior midgut due to some EC expression of this driver in the anterior region (Fig. S4C). Therefore, the different approaches show consistent correlation between EE number and ISC division.
Dilp3 expression was significantly although modestly increased in flies that had increased EE number after sc overexpression (Fig. 3E), similar to that observed in fed versus fasted flies (O’Brien et al., 2011). We tested whether Dilp3 was functionally important in this EE-driven mitotic activity. Due to the lethality, we could not obtain a fly strain that had esg-Gal4, Dilp3-Gal4, UAS-Dilp3RNAi, tub-Gal80ts and UAS-sc to perform a comparable experiment as shown in Fig. 2. So instead, we generated flies that contained a ubiquitous driver with temperature controlled expression, i.e. tub-Gal80ts/UAS-sc; tub-Gal4/UAS-Dilp3RNAi. These fly guts showed a significantly lower number of p-H3+ cells than that in the tub-Gal80ts/UAS-sc; tub-Gal4/+ control flies (Fig. 3F). These results demonstrate that the EE-regulated ISC division is partly dependent on Dilp3. The expression of an activated insulin receptor by esg-Gal4 could highly increase midgut proliferation and this effect was dominant over the loss of EEs after scRNAi (Fig. S4A), which is consistent with an important function of insulin signaling in the midgut.
Tk secreting EEs have a role in regulating Dilp3 and ISC proliferation
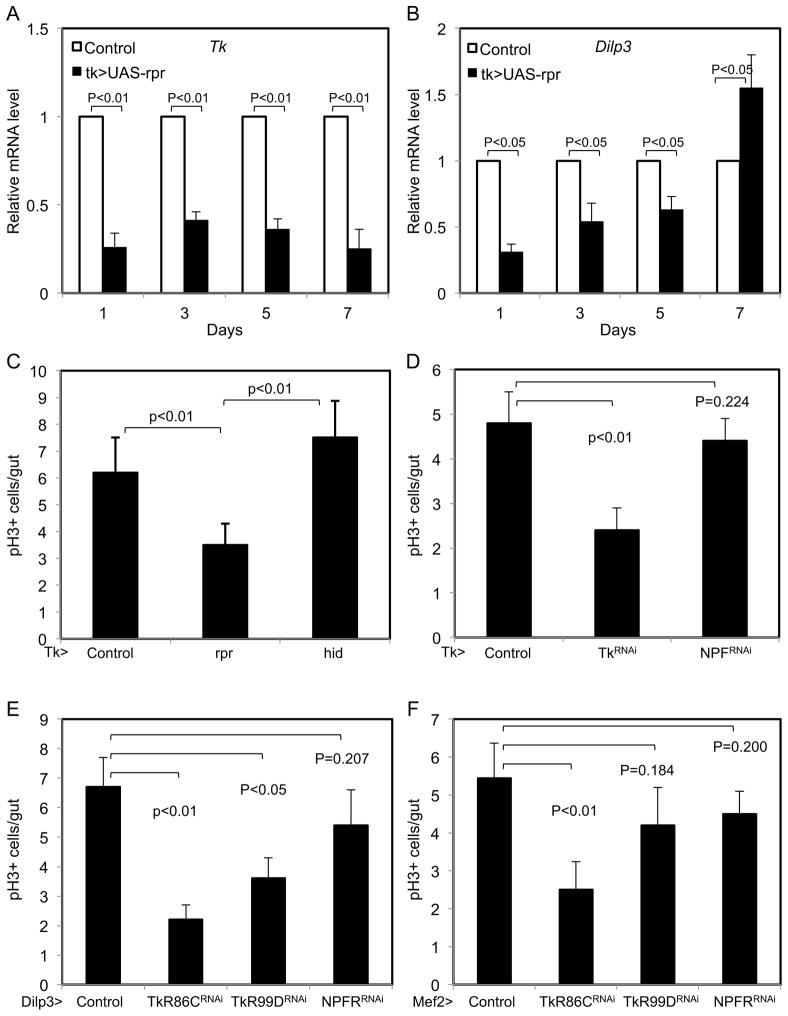
As stated above, normally hatched flies did not lower their EE number after esgts>sc RNAi, perhaps due to redundant function with other bHLH proteins in adults. The expression of proapoptotic proteins by the prosts-Gal4 also could not reduce the EE number. We thus screened other drivers and identified a Tk promoter Gal4 (Tk-Gal4) that had expression recapitulating the Tk staining pattern representing a subset of EEs (Fig. S4B, F–I″). More importantly, this driver when used to express the proapoptotic protein Reaper (Rpr) caused a significant reduction of EE number (Fig. S4J), of Tk and Dilp3 mRNA (Fig. 4A, B), and of mitotic count (Fig. 4C). The Tk-Gal4 driven expression of another proapoptotic protein Hid caused a less efficient killing of EEs (Fig. S4J) and subsequently no reduction of p-H3 count (Fig. 4C). The knockdown of Tk itself by Tk-Gal4 also caused significant reduction of p-H3 count (Fig. 4D). A previous report revealed the expression by antibody staining of a Tk receptor (TkR86C) in visceral muscles (Poels et al., 2009), and our knockdown of TkR86C in smooth muscle by Dilp3-Gal4 or Mef2-Gal4 showed a modest but significant decrease in ISC proliferation (Fig. 4E, F). There was a concomitant reduction of Dilp3 mRNA in guts of all these experiments (Fig. S4K–M), while the head Dilp3 mRNA had no significant change in all these experiments. As a comparison, TkR99D or NPFR RNAi did not show the same consistent defect.
Figure 4. Tk secreting EEs have a role in regulating Dilp3 and ISC proliferation.
(A–B) Tk-Gal4 flies were crossed with UAS-rpr to induce killing of a sub-population of EE cells. Tk>GFP was the control. Flies at the indicated days at room temperature after hatch were used for gut dissection and PCR assay. Each PCR cycle number of Tk and Dilp3 was normalized with the cycle number of rp49 in a parallel reaction of the same RNA sample. The control sample at each time point was set as 1 and UAS-rpr samples were plotted as a fraction of the control. (C) The same flies at 3 days as above and together with Tk>hid were used to quantify the number p-H3+ mitotic ISCs. (D) The flies containing the Tk-Gal4 driver were crossed with UAS-RNAi strains for Tk and NPF. The control was UAS-GFP. 3 days old progeny flies were dissected for p-H3 staining and quantification. (E–F) The flies containing the Dilp3-Gal4 or Mef2-Gal4 expressing in visceral muscle were crossed with UAS-RNAi strains for the receptors TkR86C, TkR99D and NPFR. The control was UAS-GFP. 3 days old progeny flies were dissected for p-H3 staining and quantification.
In conclusion, we show that among the AS-C genes sc is the one essential for the formation of all adult midgut EEs, and is probably required during metamorphosis when the midgut is re-formed. In newly eclosed flies, EEs serve as a link between diet-stimulated Dilp3 expression in the visceral muscle and ISC proliferation (see graphic highlight). Depletion of Tk expressing EEs caused similar Dilp3 expression and ISC proliferation defects, although the defects appeared to be less severe than that in the sc RNAi/EE-less guts. The results together suggest that Tk expressing EEs are part of the EE population required for this regulatory circuit. The approach we report here has established the Drosophila midgut as a model to dissect the function of EEs in intestinal homeostasis and whole animal physiology.
Experimental Procedures
Drosophila stocks and tissue staining
All Drosophila stocks were maintained at room temperature in yeast extract/cornmeal/molasses/agar food medium. UAS-mCD8GFP and w1118 were used for crossing with Gal4 and mutant lines as control. The fly stocks acRNAi (29586), aseRNAi (31895), lscRNAi (27058), scRNAi (26206), atoRNAi (26316), TkRNAi (25800), NPFRNAi (27237), sc1, sc3B, sc5, sc6, ato1, Df(1)sc10-1, Df(3R)p13 and UAS-sc were obtained from Bloomington stock center. TkR86CRNAi (13392), TkR99DRNAi (43329) and NPFRRNAi (107663) were obtained from VDRC. esg-Gal4, Dilp3RNAi (33681), Dilp3-Gal4, Mef2-Gal4 and pros-Gal4 have been described (Micchelli and Perrimon, 2006; O’Brien et al., 2011; Sen et al., 2004). The Tk-Gal4 line was among a set of Tk promoter Gal4 lines screened for expression in the adult midgut, and contains an approximately 1 kb fragment 2.5 kb upstream of the Tk transcription start (Song and Perrimon, Cell Reports, 2014, in press). Female flies were used for routine gut dissection, because of the bigger size. Immunofluorescence staining, antibodies used, microscope image acquisition and processing were as described (Amcheslavsky et al., 2011; Amcheslavsky et al., 2009).
Feeding, fecundity, enzyme assays
For feeding experiments, newly hatched or appropriately aged flies were kept in regular food vials or in plastic vials with a filter paper soaked with 1% sucrose in water, and transferred to fresh vials every day. For dye ingestion experiments, 20 flies were transferred to a plastic vial with a filter paper soaked with 5% sucrose and 0.5% bromophenol blue sodium salt (B5525, Sigma). At the indicated time flies that showed visible blue abdomen were counted, or used for gut extract preparation and OD measurement. For defecation experiments, flies were placed in new vials with sucrose/bromophenol blue and colored excreta on the vial wall were counted at 4- and 24-hour time points. For gut clearance assays, flies were first fed with bromophenol blue and ten flies that had blue abdomen were transferred to a new vial containing 5% sucrose only. At 2 and 24 hours after, flies were counted based on whether they still had blue abdomen or not. For fecundity assays, newly hatched male and virgin female flies were aged for 5 days on normal diet. A group of 10 females and 5 males were put together in a new food vial and transferred to fresh food vial every day. The number of eggs was counted in each vial for 10 days. Vials were kept to allow larvae and pupae to develop and the number of pupae was counted for every vial. For digestive enzyme assays, midguts from fertilized females (7–10 days old) were homogenized in 50 μL PBS at 5000 rpm 15 seconds (Precellys 24, Bertin Technologies) and centrifuged (10.000x g 10 min). Substrates for trypsin enzymatic assay (C8022) was purchased from Sigma-Aldrich and the reaction was set up following the manufacturer’s instructions. Increase in absorbance (405 nm) or fluorescence (355 nm/460 nm) after substrate cleavage was monitored by a microplate reader (Mithras LB 940, Berthold Technologies). Each genotype corresponded to 5–6 samples of 10 midguts each.
Real-time qPCR
Total RNA was isolated from 10 dissected female guts and used to prepare cDNA for qPCR using a Bio-Rad iQ5 System (Amcheslavsky et al., 2011). The qPCR was performed in duplicate from each of at least 3 independent biological samples. The ribosomal protein 49 (rp49) gene expression was used as the internal control for normalization of cycle number. The primer sequences are listed in Supplement.
All error bars represent standard error of the mean and P value is from Student’s t test.
Supplementary Material
Figure S1. EE number, hormone gene expression and life span after loss of sc function. (A–F) Confocal images of adult midgut surface views. esg-Gal4 flies were crossed with w- or the indicated UAS-RNAi constructs. 7 days old flies were used for dissection and staining. For all images in this figure, blue is DAPI for DNA, red membrane is Armadillo (β-catenin) and red nuclear is Prospero. The arrows point to examples of EEs. Scale bar is 20 μm. (G–J) Confocal images from the midguts of flies with the indicated genotypes. The deficiency for sc was Df(1)sc10–1 and for ato was Df(3R)p13. (K, L) Peptide hormone gene expression in midgut and head. 5 days old flies were used for dissection and RNA isolation from heads and midguts. The cycle number of each gene was normalized with that of rp49 in a parallel reaction of the same RNA sample. The level of expression relative to rp49 in control sample is shown at the top of each white bar, and this normalized expression was set as 1 for each gene. The expression of that gene in the scRNAi guts was calculated as the ratio to that in control. (M) For fecundity assay, males and females were put together and transferred to new vials every day. Eggs were counted in each vial for 10 days. Four independent experiments were performed and the average cumulative number of eggs laid per female fly was plotted. After flies were transferred out, the vials were kept and the embryos were let grown and the pupae were counted. Four independent experiments were performed and the average pupae number per female fly was plotted. (N) 100 flies of each genotype were kept at 29°C in normal food vials and transferred to fresh vials every other day. The percent of flies survived at each time was recorded. The experiment was performed 3 times, and the average at each time point was plotted. The P value was calculated by comparing the survival of control sample on the same day as 50% survival of the sc samples.
Figure S2. Digestive enzyme activities, food intake and food passage assays in EEless flies. (A–B) For digestive enzyme assay, midguts from 7 days old females were homogenized and mixed with the corresponding substrate for the indicated enzymatic assay. Each genotype analyzed had 5–6 samples of 10 midguts each. (C) 20 control and esg>scRNAi flies were transferred to plastic vials containing a filter paper soaked with 5% sucrose in water and 0.5% bromophenol blue (BPB) sodium salt. Every 30 minutes flies that had visible ingested dye in the abdomen were counted. Six independent experiments were performed and the average accumulative at each time point was plotted. (D) 10 midguts from 7 days old females of each genotype were pooled at 3 and 6 h after transferring the flies to a plastic vial containing a solution of sucrose 50 mM (1.71%) and 2.5% (w/v) blue dye (food colorant E131). Food intake was monitored by the increase in absorbance at 630 nm (Mithras LB 940, Berthold Technologies) of the supernatant after midgut homogenization and centrifugation (10.000x g 10 min). Average of 3 experiments was plotted. (E–F) The fly strains were kept in 5% sucrose and 0.5% BPB. At the indicated times, blue deposits on the plastic vials were counted. Similarly, 10 flies were well fed to have visible blue abdomens and then transferred to a new vial containing 5% sucrose solution. Flies that had clear abdomens were counted after the time as indicated. Three independent experiments were performed and the average was plotted.
Figure S3. EE number correlates with ISC proliferation. (A) The flies approximately 7 days old were shifted to 29°C for 2 to 6 days to inactivate Gal80ts and allow Gal4 to activate UAS-sc expression. Quantification of Prospero+ nuclei were performed from midguts of esgts>GFP control and esgts>GFP, UAS-sc flies. (B–G) Representative confocal images of midgut surface views from control and sc over-expression flies. Blue is DAPI for DNA, red nuclear is Prospero and green is mCD8GFP. The scale bar is 20 μm. (H) The esgts>GFP control and the esgts>GFP, UAS-sc flies were shifted to 29°C for 4 days and used for gut dissection and RNA isolation. The primer sets for the indicated genes were used for qPCR. The cycle number of each gene was normalized with that of rp49 in a parallel reaction of the same RNA sample. The normalized expression of each gene in the control sample was set as 1 and the expression of that gene in the UAS-sc background was calculated as fold change. (I) Average number of p-H3+ cells in whole midguts of esgts>GFP control and esgts>GFP, UAS-sc flies. 7 days old flies were shifted to 29°C to initiate sc overexpression. At the days indicated, a portion of the flies was used for midgut dissection and p-H3 staining. (J–K) The pros promoter-Gal4 and tubulinGal80ts (prosts>)were crossed with UAS-GFP control and UAS-p35 transgenic flies. 5 days old flies were shifted to 29°C for 4 days and then used for gut dissection, Pros and p-H3 staining, and quantification.
Figure S4. Midgut expression of pros-Gal4 and Tk-Gal4 and functional assays of TK signaling. (A) The esg-Gal4 was used to express an activated insulin receptor (InRAct), together with the scRNAi. Flies 3 days after eclosion were used for gut dissection and p-H3 quantification. (B) Quantification of Pros+ staining overlapping with the Gal4 driven GFP+ cells. The ratios are calculated to show the percentage of GFP+ cells that should be EEs, or to show the percentage of EEs that have the Gal4>GFP expression. (C–I″) UAS-GFP were crossed with the Gal4 drivers as indicated and midguts of 5–7 days old flies were dissected and stained using antibodies for Pros (C–H) or Tk (I–I″) proteins. The arrows indicate examples of Pros+ nuclear staining, and the wide green arrows indicate overlapping GFP+ staining in some EEs. The GFP signal in panel C reveals pros-Gal4 expression in the ECs of a small anterior midgut region. Although we did not observe obvious increase of p-H3+ cells in this anterior region from the pros-Gal4/UAS-p35 experiments, we counted p-H3 staining from middle and posterior midguts only for these experiments. (J) The same experiments as in Fig. 4C, and the guts were stained and quantified for Pros+ EEs. (K) The same experiments as in Fig. 4D, and the guts were used for PCR quantification of Dilp3 mRNA. (L, M) The same experiments as in Fig. 4E and F, and the guts were used for PCR quantification of Dilp3 mRNA.
Acknowledgments
We acknowledge the Vienna Drosophila RNAi Center and the Bloomington Drosophila Stock Center for providing fly stocks. We thank Lucy O’Brien for the kind provision of fly stocks and reagents. YTI is supported by an NIH grant (DK83450), is a member of the UMass DERC (DK32520), a member of the UMass Center for Clinical and Translational Science (UL1TR000161) and a member of the Guangdong Innovative Research Team Program (No. 201001Y0104789252). Work in the Perrimon laboratory is supported by HHMI and NIH. Work in DF’s laboratory was funded by CNRS and ANR Drosogut. IB was supported by the Sao Paulo regional government (Brazil).
Footnotes
Author Contributions
Alla Amcheslavsky, Qi Li, Yingchao Nie, and Tony Ip designed, carried out and analysed the experiments. Wei Roc Song and Norbert Perrimon performed the experiments that identified the TK-Gal4 gut driver, expression pattern and cell killing conditions. Ivan Bragatto and Dominique Ferrandon designed and performed the gut digestive enzyme and feeding assays. Alla Amcheslavsky and Tony Ip wrote the manuscript and all authors amended the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amcheslavsky A, Ito N, Jiang J, Ip YT. Tuberous sclerosis complex and Myc coordinate the growth and division of Drosophila intestinal stem cells. J Cell Biol. 2011;193:695–710. doi: 10.1083/jcb.201103018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009;4:49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin AJ, Perdigoto CN, Southall TD, Brand AH, Schweisguth F. Transcriptional control of stem cell maintenance in the Drosophila intestine. Development. 2010;137:705–714. doi: 10.1242/dev.039404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B, Jasper H. EGF signaling regulates the proliferation of intestinal stem cells in Drosophila. Development. 2011;138:1045–1055. doi: 10.1242/dev.056671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B, Jasper H. Slit/Robo signaling regulates cell fate decisions in the intestinal stem cell lineage of Drosophila. Cell Rep. 2014;7:1867–1875. doi: 10.1016/j.celrep.2014.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Kuraishi T, Lemaitre B. Drosophila EGFR pathway coordinates stem cell proliferation and gut remodeling following infection. BMC Biol. 2010;8:152. doi: 10.1186/1741-7007-8-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cognigni P, Bailey AP, Miguel-Aliaga I. Enteric neurons and systemic signals couple nutritional and reproductive status with intestinal homeostasis. Cell Metab. 2011;13:92–104. doi: 10.1016/j.cmet.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero JB, Stefanatos RK, Scopelliti A, Vidal M, Sansom OJ. Inducible progenitor-derived Wingless regulates adult midgut regeneration in Drosophila. EMBO J. 2012;31:3901–3917. doi: 10.1038/emboj.2012.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand A, Donahue B, Peignon G, Letourneur F, Cagnard N, Slomianny C, Perret C, Shroyer NF, Romagnolo B. Functional intestinal stem cells after Paneth cell ablation induced by the loss of transcription factor Math1 (Atoh1) Proc Natl Acad Sci U S A. 2012;109:8965–8970. doi: 10.1073/pnas.1201652109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bellido A, de Celis JF. The complex tale of the achaete-scute complex: a paradigmatic case in the analysis of gene organization and function during development. Genetics. 2009;182:631–639. doi: 10.1534/genetics.109.104083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbe F, Legraverend C, Jay P. The intestinal epithelium tuft cells: specification and function. Cell Mol Life Sci. 2012;69:2907–2917. doi: 10.1007/s00018-012-0984-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Driver I, Ohlstein B. Injury-induced BMP signaling negatively regulates Drosophila midgut homeostasis. J Cell Biol. 2013;201:945–961. doi: 10.1083/jcb.201302049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Edgar BA. EGFR signaling regulates the proliferation of Drosophila adult midgut progenitors. Development. 2009;136:483–493. doi: 10.1242/dev.026955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Grenley MO, Bravo MJ, Blumhagen RZ, Edgar BA. EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell Stem Cell. 2010;8:84–95. doi: 10.1016/j.stem.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Qi Y, Jasper H. Dpp Signaling Determines Regional Stem Cell Identity in the Regenerating Adult Drosophila Gastrointestinal Tract. Cell Reports. 2013a;4:10–18. doi: 10.1016/j.celrep.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zhang Y, Han L, Shi L, Lin X. Trachea-derived dpp controls adult midgut homeostasis in Drosophila. Dev Cell. 2013b;24:133–143. doi: 10.1016/j.devcel.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Lin G, Xu N, Xi R. Paracrine Wingless signalling controls self-renewal of Drosophila intestinal stem cells. Nature. 2008;455:1119–1123. doi: 10.1038/nature07329. [DOI] [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- Micchelli CA, Sudmeier L, Perrimon N, Tang S, Beehler-Evans R. Identification of adult midgut precursors in Drosophila. Gene Expr Patterns. 2011;11:12–21. doi: 10.1016/j.gep.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Miguel-Aliaga I. Nerveless and gutsy: intestinal nutrient sensing from invertebrates to humans. Semin Cell Dev Biol. 2012;23:614–620. doi: 10.1016/j.semcdb.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien LE, Soliman SS, Li X, Bilder D. Altered modes of stem cell division drive adaptive intestinal growth. Cell. 2011;147:603–614. doi: 10.1016/j.cell.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Osman D, Buchon N, Chakrabarti S, Huang YT, Su WC, Poidevin M, Tsai YC, Lemaitre B. Autocrine and paracrine unpaired signaling regulate intestinal stem cell maintenance and division. J Cell Sci. 2012;125:5944–5949. doi: 10.1242/jcs.113100. [DOI] [PubMed] [Google Scholar]
- Poels J, Birse RT, Nachman RJ, Fichna J, Janecka A, Vanden Broeck J, Nassel DR. Characterization and distribution of NKD, a receptor for Drosophila tachykinin-related peptide 6. Peptides. 2009;30:545–556. doi: 10.1016/j.peptides.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Reeves N, Posakony JW. Genetic programs activated by proneural proteins in the developing Drosophila PNS. Dev Cell. 2005;8:413–425. doi: 10.1016/j.devcel.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scopelliti A, Cordero JB, Diao F, Strathdee K, White BH, Sansom OJ, Vidal M. Local control of intestinal stem cell homeostasis by enteroendocrine cells in the adult Drosophila midgut. Curr Biol. 2014;24:1199–1211. doi: 10.1016/j.cub.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A, Kuruvilla D, Pinto L, Sarin A, Rodrigues V. Programmed cell death and context dependent activation of the EGF pathway regulate gliogenesis in the Drosophila olfactory system. Mech Dev. 2004;121:65–78. doi: 10.1016/j.mod.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Song W, Perrimon N. Cell Reports. 2014 in press. [Google Scholar]
- Takashima S, Adams KL, Ortiz PA, Ying CT, Moridzadeh R, Younossi-Hartenstein A, Hartenstein V. Development of the Drosophila entero-endocrine lineage and its specification by the Notch signaling pathway. Dev Biol. 2011;353:161–172. doi: 10.1016/j.ydbio.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian A, Jiang J. Intestinal epithelium-drived BMP controls stem cell self-renewal in Drosophila adult midgut. Elife. 2014;3:e01857. doi: 10.7554/eLife.01857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDussen KL, Samuelson LC. Mouse atonal homolog 1 directs intestinal progenitors to secretory cell rather than absorptive cell fate. Dev Biol. 2010;346:215–223. doi: 10.1016/j.ydbio.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra JA, Agricola HJ, Sellami A. Regulatory peptides in fruit fly midgut. Cell Tissue Res. 2008;334:499–516. doi: 10.1007/s00441-008-0708-3. [DOI] [PubMed] [Google Scholar]
- Xu N, Wang SQ, Tan D, Gao Y, Lin G, Xi R. EGFR, Wingless and JAK/STAT signaling cooperatively maintain Drosophila intestinal stem cells. Dev Biol. 2011;354:31–43. doi: 10.1016/j.ydbio.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Zeng X, Lin X, Hou SX. The Osa-containing SWI/SNF chromatin-remodeling complex regulates stem cell commitment in the adult Drosophila intestine. Development. 2013;140:3532–3540. doi: 10.1242/dev.096891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Rasmussen A, Lee S, Agaisse H. The UPD3 cytokine couples environmental challenge and intestinal stem cell division through modulation of JAK/STAT signaling in the stem cell microenvironment. Dev Biol. 2013;373:389–393. doi: 10.1016/j.ydbio.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. EE number, hormone gene expression and life span after loss of sc function. (A–F) Confocal images of adult midgut surface views. esg-Gal4 flies were crossed with w- or the indicated UAS-RNAi constructs. 7 days old flies were used for dissection and staining. For all images in this figure, blue is DAPI for DNA, red membrane is Armadillo (β-catenin) and red nuclear is Prospero. The arrows point to examples of EEs. Scale bar is 20 μm. (G–J) Confocal images from the midguts of flies with the indicated genotypes. The deficiency for sc was Df(1)sc10–1 and for ato was Df(3R)p13. (K, L) Peptide hormone gene expression in midgut and head. 5 days old flies were used for dissection and RNA isolation from heads and midguts. The cycle number of each gene was normalized with that of rp49 in a parallel reaction of the same RNA sample. The level of expression relative to rp49 in control sample is shown at the top of each white bar, and this normalized expression was set as 1 for each gene. The expression of that gene in the scRNAi guts was calculated as the ratio to that in control. (M) For fecundity assay, males and females were put together and transferred to new vials every day. Eggs were counted in each vial for 10 days. Four independent experiments were performed and the average cumulative number of eggs laid per female fly was plotted. After flies were transferred out, the vials were kept and the embryos were let grown and the pupae were counted. Four independent experiments were performed and the average pupae number per female fly was plotted. (N) 100 flies of each genotype were kept at 29°C in normal food vials and transferred to fresh vials every other day. The percent of flies survived at each time was recorded. The experiment was performed 3 times, and the average at each time point was plotted. The P value was calculated by comparing the survival of control sample on the same day as 50% survival of the sc samples.
Figure S2. Digestive enzyme activities, food intake and food passage assays in EEless flies. (A–B) For digestive enzyme assay, midguts from 7 days old females were homogenized and mixed with the corresponding substrate for the indicated enzymatic assay. Each genotype analyzed had 5–6 samples of 10 midguts each. (C) 20 control and esg>scRNAi flies were transferred to plastic vials containing a filter paper soaked with 5% sucrose in water and 0.5% bromophenol blue (BPB) sodium salt. Every 30 minutes flies that had visible ingested dye in the abdomen were counted. Six independent experiments were performed and the average accumulative at each time point was plotted. (D) 10 midguts from 7 days old females of each genotype were pooled at 3 and 6 h after transferring the flies to a plastic vial containing a solution of sucrose 50 mM (1.71%) and 2.5% (w/v) blue dye (food colorant E131). Food intake was monitored by the increase in absorbance at 630 nm (Mithras LB 940, Berthold Technologies) of the supernatant after midgut homogenization and centrifugation (10.000x g 10 min). Average of 3 experiments was plotted. (E–F) The fly strains were kept in 5% sucrose and 0.5% BPB. At the indicated times, blue deposits on the plastic vials were counted. Similarly, 10 flies were well fed to have visible blue abdomens and then transferred to a new vial containing 5% sucrose solution. Flies that had clear abdomens were counted after the time as indicated. Three independent experiments were performed and the average was plotted.
Figure S3. EE number correlates with ISC proliferation. (A) The flies approximately 7 days old were shifted to 29°C for 2 to 6 days to inactivate Gal80ts and allow Gal4 to activate UAS-sc expression. Quantification of Prospero+ nuclei were performed from midguts of esgts>GFP control and esgts>GFP, UAS-sc flies. (B–G) Representative confocal images of midgut surface views from control and sc over-expression flies. Blue is DAPI for DNA, red nuclear is Prospero and green is mCD8GFP. The scale bar is 20 μm. (H) The esgts>GFP control and the esgts>GFP, UAS-sc flies were shifted to 29°C for 4 days and used for gut dissection and RNA isolation. The primer sets for the indicated genes were used for qPCR. The cycle number of each gene was normalized with that of rp49 in a parallel reaction of the same RNA sample. The normalized expression of each gene in the control sample was set as 1 and the expression of that gene in the UAS-sc background was calculated as fold change. (I) Average number of p-H3+ cells in whole midguts of esgts>GFP control and esgts>GFP, UAS-sc flies. 7 days old flies were shifted to 29°C to initiate sc overexpression. At the days indicated, a portion of the flies was used for midgut dissection and p-H3 staining. (J–K) The pros promoter-Gal4 and tubulinGal80ts (prosts>)were crossed with UAS-GFP control and UAS-p35 transgenic flies. 5 days old flies were shifted to 29°C for 4 days and then used for gut dissection, Pros and p-H3 staining, and quantification.
Figure S4. Midgut expression of pros-Gal4 and Tk-Gal4 and functional assays of TK signaling. (A) The esg-Gal4 was used to express an activated insulin receptor (InRAct), together with the scRNAi. Flies 3 days after eclosion were used for gut dissection and p-H3 quantification. (B) Quantification of Pros+ staining overlapping with the Gal4 driven GFP+ cells. The ratios are calculated to show the percentage of GFP+ cells that should be EEs, or to show the percentage of EEs that have the Gal4>GFP expression. (C–I″) UAS-GFP were crossed with the Gal4 drivers as indicated and midguts of 5–7 days old flies were dissected and stained using antibodies for Pros (C–H) or Tk (I–I″) proteins. The arrows indicate examples of Pros+ nuclear staining, and the wide green arrows indicate overlapping GFP+ staining in some EEs. The GFP signal in panel C reveals pros-Gal4 expression in the ECs of a small anterior midgut region. Although we did not observe obvious increase of p-H3+ cells in this anterior region from the pros-Gal4/UAS-p35 experiments, we counted p-H3 staining from middle and posterior midguts only for these experiments. (J) The same experiments as in Fig. 4C, and the guts were stained and quantified for Pros+ EEs. (K) The same experiments as in Fig. 4D, and the guts were used for PCR quantification of Dilp3 mRNA. (L, M) The same experiments as in Fig. 4E and F, and the guts were used for PCR quantification of Dilp3 mRNA.