Abstract
Neural culture of human pluripotent stem cells is useful for neuroscience research, but the optimal feeding schedule for these in vitro systems is unclear. We evaluated the survival and neural differentiation profiles of human embryonic and induced pluripotent stem cells cultured with medium exchange schedules of five, six, or seven days weekly through two months of differentiation. No significant differences were seen in cell numbers or neural differentiation markers through this culture interval with either human pluripotent cell type. We conclude that there is unlikely to be an advantage of feeding more than five days weekly for this culture system.
Keywords: feeding, medium exchange, cell culture, survival, neural, differentiation, human, embryonic stem cells, induced pluripotent stem cells
Introduction
Many cell-level human neuroscience questions can be approached through in vitro model systems involving neural differentiation of cultured human embryonic stem cells and/or human induced pluripotent stem cells.(6) The optimal feeding (medium exchange) schedule for the generation and maintenance of these cultured human neural cell types is unclear. There are obvious practical advantages to schedules involving fewer feedings per unit of time. Feeding cells less frequently could decrease demands on investigator time, which is often a major issue in cell culture labs,(5) but particularly so for smaller laboratory groups with less personnel. Less frequent medium exchange decreases material costs, as some components of the required culture reagents and supplies are expensive and can make up significant portions of ever-tightening research budgets.(7) Less frequent handling of the cells also decreased the risk of culture contamination, as each interaction carries the potential to introduce pathogens into the medium.(1) For these reasons, we asked if the survival of human embryonic stem cells and human induced pluripotent stem cells could be maintained with less frequent feedings, and if there would be negative effects on their neural differentiation profiles in vitro.
Methods
A single group of human embryonic stem cells (H9) were divided into three groups fed 5, 6, or 7 days a week (groups E5, E6, and E7), and a single group of human induced pluripotent stem cells (iPS-DF6-9-9T) were also divided into three groups fed 5, 6, or 7 days a week (groups I5, I6, and I7, figure 1). Each group had 3 replicates. The cells of all groups were expanded as pluripotent cells and differentiated to neural lineages at 37C and 5% CO2 in a Heracell 240 incubator (Heraeus) as previously described with the following modifications.(3,4)
Figure 1.
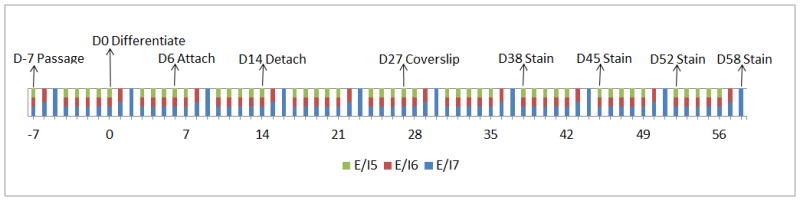
Timeline over differentiation days (D) −7 through 58 of feeding schedules for the human embryonic or induced pluripotent stem cell lines fed 5 (groups E/I5, green bars), 6 (groups E/I6, red bars), or 7 (groups E/I7, blue bars) days a week.
All groups were expanded in the pluripotent state in 6-well plates (Nunc) on a feeder layer of irradiated mouse embryonic fibroblasts in 3 ml per well of proliferation medium with FGF2 (PM+FGF2) that consisted of Dulbecco’s modified Eagle medium: nutrient mixture F-12 (DMEM/F12) with 2.5mM L-Glutamine and 15mM HEPES Buffer (Fisher), 20% serum replacement (Gibco), 1% minimum essential medium Eagle: nonessential amino acids (MEM-NEAA; Gibco), 0.1 mM beta-mercaptoethanol (Sigma), and 4 ng/mL fibroblast growth factor 2 (FGF2; R&D Systems). All groups were passaged to new plates every 7 days with 0.1 mg/mL dispase (Gibco) at 37C for 5 minutes and mechanical dissociation. With each passage 5/6 of the cell suspension from each plate was started in the neural differentiation protocol described below (passage day was differentiation day 0 [D0]), and 1/6 of the cell suspension from each plate was passaged to new plates for continued proliferation of the pluripotent cells. The proliferating pluripotent cells were fed with exchange of 2 of the 3 ml of PM+FGF2 daily except for E6 and I6, which were not fed the day after passage, and E5 and I5, which were not fed for the 2 days after passage.
On D0, cell colonies starting the neural differentiation protocol were suspended in 15 ml of PM (without FGF2) in 25 ml flasks (Fisher). The cell colonies grew as floating clusters, while any remaining feeder cells adhered to the flask and were removed by transferring the cell cluster suspension into new flasks. The cells were fed with exchange of 10 of the 15 ml of PM on D1 for E7 and I7; D2 for E6, I6, E7, and I7; and D3 for all the groups. On D4, all the medium was changed for all the groups to neural medium (NM) that consisted of DMEM/F12 with 2.5mM L-Glutamine and 15mM HEPES Buffer, 1% MEM-NEAA, 1% N2 supplement (Gibco), and 2 mg/mL heparin (Sigma). On D5, 10 of 15 ml of NM was exchanged for all groups. On D6, the clusters of all groups were attached to 6-well plates with 3 ml per well of NM with 10% fetal bovine serum (Gibco) for 18 hours. On D7, all the groups had exchange of all the medium for 3 ml of NM. The cells were fed with exchange of 2 of the 3 ml of NM on D8 for E7 and I7; D9 for E6, I6, E7, and I7; and D10-13 for all the groups. On D14, rosettes in the adherent colonies were separated from the surrounding flat cells with 0.1 mg/mL of dispase at 37C for 1 minute and mechanical dissociation, and again grown as floating clusters in 15 ml of NIM in 25 ml flasks. Cells were fed by exchange of 10 of the 15 ml of NM on D15-26 for E7 and I7; D16-21 and 23-26 for E6 and I6; and D17-21 and 24-26 for E5 and I5.
Cell numbers were quantified on D-7 (7 days prior to D0), and on D0, 6, 14, 21, and 27. On these days the cluster solution was well mixed and a small portion was immediately removed for counting. The clusters in that portion were dissociated to a single-cell suspension with accutase (Fisher), and the numbers of live cells were determined using trypan blue staining and a hemocytometer.
On D27, equal numbers of cells were attached to glass coverslips coated with polyornithine (Sigma) and laminin (Invitrogen) in 24-well plates (Fisher). On D28, 300 ul of NM was added to each well. Cells were fed by exchange of 200 of the 300 ul of NM on D29-57 for E7 and I7; D30-35, 37-41, 43-47, 49-54, and 56-57 for E6 and I6; and D31-35, 38-41, 44-47, 50-54, and 57 for E5 and I5.
On D38, 45, 52, and 58 cells were immunostained with primary antibodies for the following differentiation markers, with 2 markers assessed per coverslip: PAX6 for neural progenitors (1:500, DSHB), SOX1 for neural progenitors (1:1000, R&D Systems), KI67 for dividing cells (1:200, Millipore), NESTIN for neural progenitors (1:1000, Fisher), HOXB4 for caudal neural identity (1:50, DSHB), OTX2 for rostral neural identity (1:1000, R&D Systems), βIIItubulin for neurons (1:1000, Sigma), GFAP for astrocytes (1:1000, DAKO), MAP2 for neurons (1:1000, Sigma), and SYNAPSIN for synapses (1:250, Millipore). Species-matched secondary antibodies conjugated to Cy3 (1:1000, Fisher) or AF488 (1:1000, Invitrogen) were used on these coverslips, as well as coverslips for which primary antibodies had not been used to monitor for nonspecific staining. All nuclei were stained with Hoechst (1:1000, Invitrogen). The coverslips were mounted on slides with fluoromount G (Southern Biotech) and 4 consecutive 10X images were systematically captured through the center of the coverslip under identical light conditions with a Nikon E600 fluorescence microscope, Photometrics CoolSnap HQ2 camera, and NIS-Elements software. All labeled nuclei were manually counted on the images, and the percentage of the first 100 cells that labeled for each differentiation marker was manually counted. ImageJ (NIH) was used to measure the Integrated Density (ID) of each of the fluorescence channels on the images: red for the marker paired with Cy3, green for the marker paired with AF488, and blue for nuclei stained with Hoechst.
Data are presented as mean +/− standard deviation (SD), and groups were compared with repeated measures analysis of variance.
Results
Mean cell numbers from D-7 through D27, prior to attachment to coverslips, did not differ significantly between the different feeding schedules for either the embryonic or induced pluripotent stem cell lines (p>0.05, figure 2). Neither mean cell numbers nor Hoechst ID values after attachment to coverslips from D38 through D58 differed significantly between the different feeding schedules for either the embryonic or induced pluripotent stem cell lines (p>0.05, figure 3). There were no significant differences for the mean percentage or ID values of the tested differentiation markers from D38 through D58 between the different feeding schedules for either the embryonic or induced pluripotent stem cell lines (p>0.05, data not shown).
Figure 2.
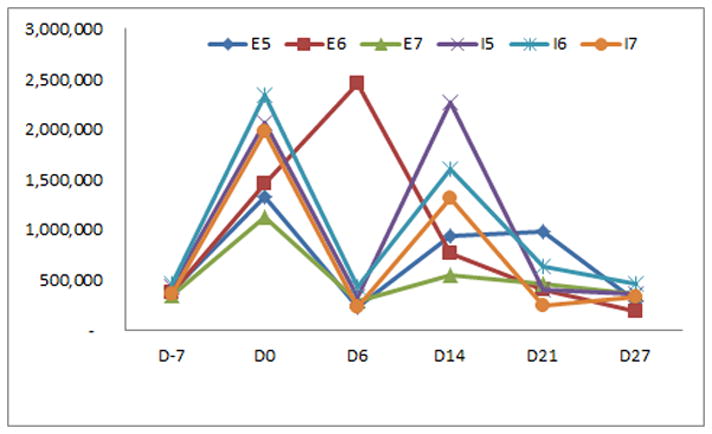
Mean cell numbers over differentiation days (D) −7, 0, 6, 14, 21, and 27 did not differ significantly between the different feeding schedules for the human embryonic or induced pluripotent stem cell lines fed 5, 6, or 7 days a week (groups E5-7 and I5-7).
Figure 3.
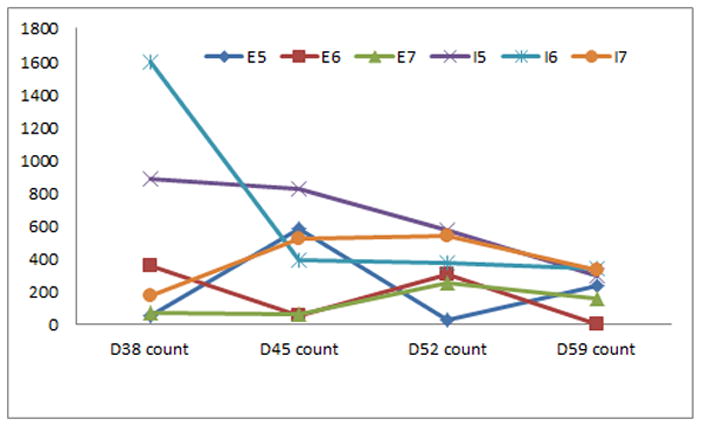
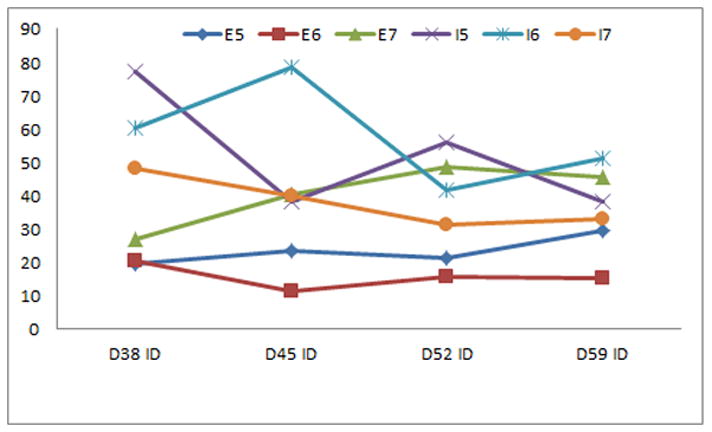
Neither mean cell numbers nor ID values for the nuclear stain Hoechst over D38, 45, 52, and 58 differed significantly between the different feeding schedules for the human embryonic or induced pluripotent stem cell lines fed 5, 6, or 7 days a week.
Conclusions
We did not find significant differences in assessments of survival or neural differentiation through two months for the tested human embryonic stem cell and human induced pluripotent stem cell lines fed for five, six, or seven days a week. We had expected to see a decrease in the total number of cells when they were fed less often, on the assumption that more frequent addition of nutrients and removal of waste products would lead to greater proliferation and/or survival of the cells. A likely explanation for the lack of difference in cell numbers could be that feeding more than five days per week provides more nutrients and waste removal than the cells can use, at least with the volumes and medium recipes that we used.
The expected neural differentiation progression of cells in our culture system, from dividing neural progenitors expressing KI67, PAX6, SOX1, or NESTIN, to more mature neural cell types expressing βIIItubulin, MAP2, SYNAPSIN, or GFAP,(2) showed no difference between the different feeding schedules. This could be because the relative amounts of nutrient supply and waste removal have no effect on neural differentiation of these cell types, or because the maximum necessary amounts are less than that supplied by the minimum feeding schedule we tested.
Strengths of our study include systematic assessments of survival and neural differentiation of multiple replicates of both human embryonic and induced pluripotent stem cells through 58 days of differentiation in vitro. Our study also has weaknesses. Our results may not be applicable to other cell lines, other in vitro differentiation systems, or longer time frames. Cell survival and differentiation may be maintained with even less frequent feeding schedules beyond those we tested. More involved assessments of neural differentiation, such as quantification of protein and/or gene expression over time of the markers we examined as well as additional neural factors might have detected more subtle differences between the groups.
In conclusion, our study suggests that feeding human embryonic or human induced pluripotent stem cells more than five days a week does not improve cell survival or neural differentiation.
Acknowledgments
Funding: Supported by NIH grants NINDS 1K08NS079622-01A1 and P30 HD03352
References
- 1.Davis J. Animal cell culture: essential methods. Hoboken, NJ: John Wiley & Sons Inc; 2011. [Google Scholar]
- 2.Hu BY, Weick JP, Yu J, et al. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci U S A. 2010;107(9):4335–4340. doi: 10.1073/pnas.0910012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jensen MB, Krishnaney-Davison R, Cohen LK, et al. Injected Versus Oral Cyclosporine for Human Neural Progenitor Grafting in Rats. Journal of Stem Cell Research & Therapy. 2012 doi: 10.4172/2157-7633.S10-003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jensen MB, Yan H, Krishnaney-Davison R, et al. Survival and Differentiation of Transplanted Neural Stem Cells Derived from Human Induced Pluripotent Stem Cells in a Rat Stroke Model. J Stroke Cerebrovasc Dis. 2011 doi: 10.1016/j.jstrokecerebrovasdis.2011.09.008. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ledford H. Work ethic: The 24/7 lab. Nature. 2011;477(7362):20–22. doi: 10.1038/477020a. [DOI] [PubMed] [Google Scholar]
- 6.Liu H, Zhang SC. Specification of neuronal and glial subtypes from human pluripotent stem cells. Cell Mol Life Sci. 2011;68(24):3995–4008. doi: 10.1007/s00018-011-0770-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Research, Transparency Market. Cell Culture Market is Expected to Reach USD 16.85 Billion Globally in 2018. 2013. [Google Scholar]


