Abstract
Background
Genome wide association studies (GWAS) have revealed significant association of caveolin-1 (Cav1) gene variants with increased risk of cardiac arrhythmias. Nevertheless, the mechanism for this linkage is unclear.
Methods and Results
Using adult Cav1−/− mice, we revealed a marked reduction in the left ventricular (LV) conduction velocity in the absence of myocardial Cav1, which is accompanied with increased inducibility of ventricular arrhythmias. Further studies demonstrated that loss of Cav1 leads to the activation of cSrc tyrosine kinase, resulting in the downregulation of connexin 43 (Cx43) and subsequent electrical abnormalities. Pharmacological inhibition of cSrc mitigates Cx43 downregulation, slow conduction and arrhythmia inducibility in Cav1−/− animals. Using a transgenic mouse model with cardiac-specific overexpression of angiotensin converting enzyme (ACE8/8), we demonstrated that, upon enhanced cardiac RAS activity, Cav1 dissociated from cSrc because of increased Cav1 S-nitrosation (SNO) at Cys156, leading to c-Src activation, Cx43 reduction, impaired gap junction function, and subsequent increase in the propensity for ventricular arrhythmias and sudden cardiac death. RAS-induced Cav1 SNO was associated with increased Cav1-eNOS binding in response to increased mitochondrial reactive oxidative species (ROS) generation.
Conclusions
The present studies reveal the critical role of Cav1 in modulating cSrc activation, gap junction remodeling and ventricular arrhythmias. These data provide a mechanistic explanation for the observed genetic link between Cav1 and cardiac arrhythmias in humans and suggest that targeted regulation of Cav1 may reduce arrhythmic risk in cardiac diseases associated with RAS activation.
Keywords: connexin43, arrhythmia (mechanisms), renin angiotensin system, caveolin 1
Introduction
Human genetic studies have revealed an important link between caveolins and cardiac arrhythmias.1–3 Among the genes (CAV1, CAV2, CAV3) encoding the three distinct caveolin isoforms named caveolins 1 to 3, mutations in CAV3 have been shown to lead to congenital long-QT (LQT)1 and sudden infant death4 syndromes, whereas human genome-wide association studies (GWAS) have observed significant association of CAV1 variants with PR intervals2 and increased susceptibility to cardiac arrhythmias.2,3 It is clear now that caveolin-3 (Cav3) interacts with and regulates cardiac sodium channel Nav1.5,1 and that mutations in CAV3 can lead to a 3-to 5-fold increase in late sodium currents, resulting in delayed repolarization, prolonged QT intervals and arrhythmogenic phenotype.1,4 In contrast, albeit caveolin 1 (Cav1) is known to express in cardiomyocytes5,6 and has been implicated in regulating ion channels in in vitro studies,7,8 there is no established mechanism explaining the genetic link between CAV1 and cardiac arrhythmias. We sought to determine the mechanistic link between Cav1 and cardiac arrhythmias.
Methods
Animals were handled in accordance with the NIH Guide for the Care and Use of Laboratory Animals. All protocols involving animals were approved by the Animal Studies Committee at the University of Illinois at Chicago, Lifespan, or the Veterans Administration San Diego Healthcare System.
In vivo electrophysiological studies, including electrocardiogram (ECG) recordings, programmed stimulation, and ventricular conduction velocity were performed on Cav1−/− and ACE8/8 mice (all in C57/Bl6 background) that were derived and maintained as described previously.9–11 Left ventricular (LV) tissue and/or cardiomyocytes isolated from Cav1−/−, Cav3−/− and ACE8/8 mice were used for Western blotting, immunoprecipitation, S-nitrosation assay, NO measurement and transcript analyses.
All measurements were presented in dot plots with means ± SEM. The inducibility of VT was presented as percentage of all tested animals in the same group. The statistical significance of differences between experimental groups was evaluated by the exact version of the Mann-Whitney U test or Fisher’s exact test, followed by Holm test to correct for multiple comparisons; P values <0.05 are considered statistically significant. Detailed methods are available in the Supplemental Material.
Results
Loss of Cav1 results in slowed cardiac conduction and increased risk of ventricular arrhythmia
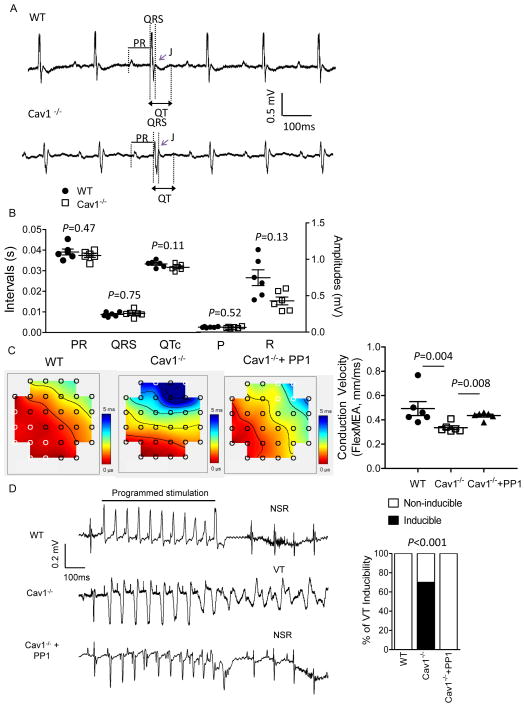
To determine the potential impact of genetic deletion of Cav1 on cardiac electric functioning, adult (2–4 months) WT and Cav1−/− mice were first subjected to surface ECG recordings (Figure 1A). Cav1−/− mice were viable and fertile without evidence of cardiac structural abnormality up to 5 months of age.10 The ECG recordings revealed that the morphologies of the P, J and T waves, as well as the durations of the PR, QRS, and corrected QT (QTc) intervals (Figure 1B) measured in WT and Cav1−/− animals were indistinguishable, although the R wave amplitudes were trending lower in Cav1−/− compared with WT mice (Figure 1A and 1B). Using a 72-electrode Flex-Multi-electrode array (Flex-MEA), the LV epicardial conduction velocity was measured in WT and Cav1−/− mice. As shown in Figure 1C, the LV conduction velocity in Cav1−/− (n=6, 0.35±0.03 mm/ms, median 0.32 mm/ms) was significantly (P=0.004) lower than that in WT (n=6, 0.50±0.09 mm/ms, median 0.44 mm/ms) mice. To test if the reduced LV conduction velocity observed in Cav1−/− mice is associated with increased arrhythmia risk, epicardial programmed electrical stimulation was conducted in WT and Cav1−/− mice. These experiments revealed that none of the WT mice (8 with double and 14 with triple extra-stimuli) was inducible for ventricular tachycardia (VT), whereas 70% (7 out of 10, P<0.001 by Fisher’s exact test) and 79% (11 out of 14, P<0.0001) of the Cav1−/− mice were inducible for VT using double and triple extra-stimuli, respectively (Figure 1D and Supplemental Table). Single extra-stimulus failed to induce arrhythmias in any of the animals studied. Taken together, initial electrophysiological studies demonstrated that loss of Cav1 resulted in slowed LV conduction velocity and increased ventricular arrhythmia inducibility.
Figure 1.
Knockout of Cav1 leads to reduced LV conduction velocity and increased inducibility of ventricular arrhythmias, both of which can be prevented by cSrc inhibition. (A) Representative ECG (lead II) waveforms from anesthetized adult (2–4 months) WT and Cav1−/− mice are illustrated; (B) Mean ± SEM PR, QRS and QTc intervals, as well as P and R wave amplitudes measured in WT (n=6) and Cav1−/− (n=6) mices were not significantly different, albeit R wave amplitudes were trending lower in Cav1−/− compared to WT mice. (C) Representative LV epicardial conduction velocity recordings in WT, Cav1−/−, and Cav1−/− mice treated with 4 weeks of cSrc kinase inhibitor PP1, using a 72-electrode FLEX-MEA, were shown. The epicardial conduction velocity was significantly (P=0.004) reduced in Cav1−/− (n=6) compared with WT (n=6), LV. The LV conduction velocity in Cav1−/− LV can be normalized with 4 weeks of PP1 treatment. (D) Representative surface ECG recordings from WT, Cav1−/− and Cav1−/− treated with PP1 during epicardial programmed electrical stimulation. With the use of double extra-stimuli, none of the WT animals (n=8) were inducible for ventricular arrhythmias, whereas 70% (n=10) of Cav1−/− mice were inducible for ventricular tachycardia (VT) (P<0.001). PP1 treatment in Cav1−/− mice significantly reduced the inducibility of ventricular arrhythmias (0% inducible, n=8) with programmed stimulation.
Electrical abnormalities observed in Cav1−/− mice result from LV Cx43 downregulation by activated cSrc tyrosine kinase
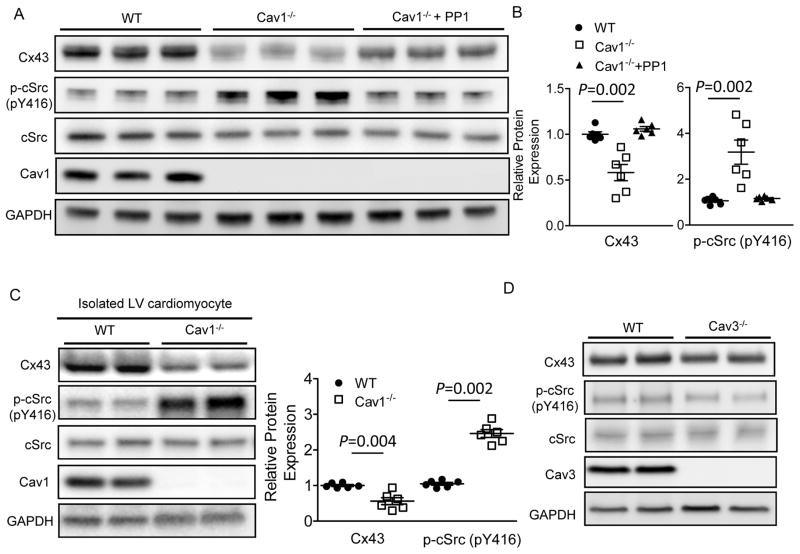
Slow myocardial conduction velocity can result from reduced Na+ current (INa) or from increased cell-cell conduction resistance caused by increased fibrosis or decreased gap junction function.12 Whole-cell voltage clamp experiments in LV cardiomyocytes, as well as Mason-trichrome staining of the LV cross-sections, were conducted in WT and Cav1−/− mice to determine if changes in INa currents or the presence of cardiac fibrosis may contribute to the conduction abnormality and increased arrhythmia inducibility observed in Cav1−/− mice. As shown in Supplemental Figure 1A and 1B, the densities of INa, as well as the steady state inactivation properties of INa, were similar in WT and Cav1−/− LV myocytes. Also similar to WT LV, there was no significant fibrosis detected in Cav1−/− LV (Supplemental Figure 1 C,D). In contrast, Western blot analyses revealed a 42% reduction of the Cx43 expression in Cav1−/−, compared with WT LV (Figure 2A,B). Quantification of Cx43 of different phosphorylation states, P0, P1 and P3, relative to total Cx43 levels, did not reveal significant difference between WT and Cav1−/− LV samples (Supplemental Figure 2), suggesting that genetic deletion of Cav1 does not affect the phosphorylation state of cardiac Cx43. In addition, immunofluorescent staining of Cx43 and N-cadherin, a protein marker of the intercalated discs (Supplemental Figure 3A and 3B), revealed that the percentage of Cx43 colocalized with N-cadherin was similar in WT and Cav1−/− LV, suggesting the proportion of cellular Cx43 incorporated into gap junctions was not affected in the absence of Cav1. Isolated myocytes from WT and Cav1−/− LV were used in additional Western blots to confirm that Cx43 expression levels were indeed markedly reduced in Cav1−/− compared to WT LV cardiomyocytes (52% reduction, P=0.004; Figure 2C). Taken together, these data suggest that the conduction abnormality and increased inducibility for ventricular arrhythmias observed in Cav1−/− mice can be attributed largely to Cx43 downregulation.
Figure 2.
Loss of Cav1 results in cardiac cSrc activation and Cx43 downregulation, which can be reversed by cSrc inhibition. (A) Representative Western blots of the LV protein lysates from WT, Cav1−/− mice and Cav1−/− mice treated with 4 weeks of cSrc inhibitor PP1 (1.5 mg/kg/dose intraperitoneally, 3 times per week). (B) cSrc phosphorylation was significantly (P=0.002) increased in Cav1−/− (n=6) compared with WT (n=6), LV, whereas Cx43 was markedly reduced in Cav1−/− LV. Four weeks of PP1 treatments prevented cSrc phosphorylation/activation and Cx43 downregulation in Cav1−/− LV (n=6). (C) Representative Western blots of the isolated LV cardiomyocytes from WT (n=4) and Cav1−/− (n=4) mice confirmed markedly reduced Cx43 (by 52%, P=0.004) and increased p-cSrc (by 2.6 fold, P=0.002) in cardiomyocytes with genetic deletion of Cav1. (D) The p-cSrc and Cx43 protein levels were not different in WT and Cav3−/− LV.
It is known that Cav1 negatively regulates a redox-sensitive tyrosine kinase cSrc, the activation of which has been shown to cause the downregulation of cardiac Cx43.13 We hypothesized that the observed Cx43 downregulation, slow conduction and increased arrhythmic inducibility in Cav1−/− mice resulted from loss of Cav1 inhibition of cSrc. To test this, we first examined the expression levels of phosphorylated cSrc at Tyr416 (p-cSrc, the active form of cSrc) in the ventricular myocardium and isolated LV cardiomyocytes from WT and Cav1−/− mice. As shown in Figure 2A and 2C, the protein expression level of p-cSrc was significantly upregulated in Cav1−/− LV (by 2.8 fold, P=0.002) and isolated LV cardiomyocytes (by 2.6 fold, P=0.002), compared to WT. In addition, pharmacological inhibition of cSrc activity with 4 weeks of the cSrc inhibitor PP1 (1.5 mg/kg/dose, 3 times per week for 4 weeks, intraperitoneally) in Cav1−/− mice normalized LV p-cSrc and Cx43 expression to levels similar to that in WT (Figure 2A and 2B). Consistent with the notion that cSrc regulates Cx43 post-transcriptionally,13 quantitative RT-PCR did not reveal a significant difference in the LV Cx43 mRNA expression levels in WT, Cav1−/− and PP1-treated Cav1−/− mice (Supplemental Figure 4, see Discussion).
In line with the reversal of Cx43 downregulation with cSrc inhibition, the slow LV conduction and increased ventricular arrhythmia inducibility observed in Cav1−/− mice could be mitigated by 4-week treatment with cSrc inhibitor PP1 (mean LV conduction velocity 0.43±0.01 mm/ms, median 0.45 mm/ms; 0% inducible for VT with double extra-stimuli, n=8, Figure 1C, 1D and Supplemental Table). In contrast to Cav1−/− mice, the LV p-cSrc and Cx43 expression in Cav3−/− LV were similar to that in WT (Figure 2D), suggesting no obvious role of Cav3 in cSrc/Cx43 regulation. Taken together, these results suggest that Cav1, but not Cav3, plays a critical role in maintaining cardiac Cx43 homeostasis through regulating cSrc activity. In the absence of Cav1, cSrc becomes activated, leading to Cx43 downregulation, subsequent conduction abnormality, and increased inducibility for arrhythmias.
Reduced binding between Cav1 and cSrc results in cSrc activation and subsequent Cx43 downregulation upon enhanced cardiac RAS signaling
The electrophysiological abnormalities linked to Cx43 dysregulation observed in Cav1−/− mice were reminiscent of the phenotype of the mouse models with increased cardiac RAS activity.9,14 These animals have a high incidence of conduction block, ventricular arrhythmias and sudden death resulting from reduced cardiac Cx43 and impaired gap junction function. Using a gene-targeted mouse model of cardiac-specific ACE overexpression (ACE8/8),9,15 we have previously demonstrated that enhanced cardiac RAS signaling can lead to cSrc activation, Cx43 degradation, reduce myocyte coupling, increased inducibility of ventricular arrhythmias and sudden cardiac death, all of which can be reversed by pharmacological inhibition of cSrc.9,15 Given the similarity in the electrophysiological phenotypes of Cav1−/− and ACE8/8 mice, we hypothesized that Cav1 was likely involved in RAS-induced cardiac cSrc activation and Cx43 reduction.
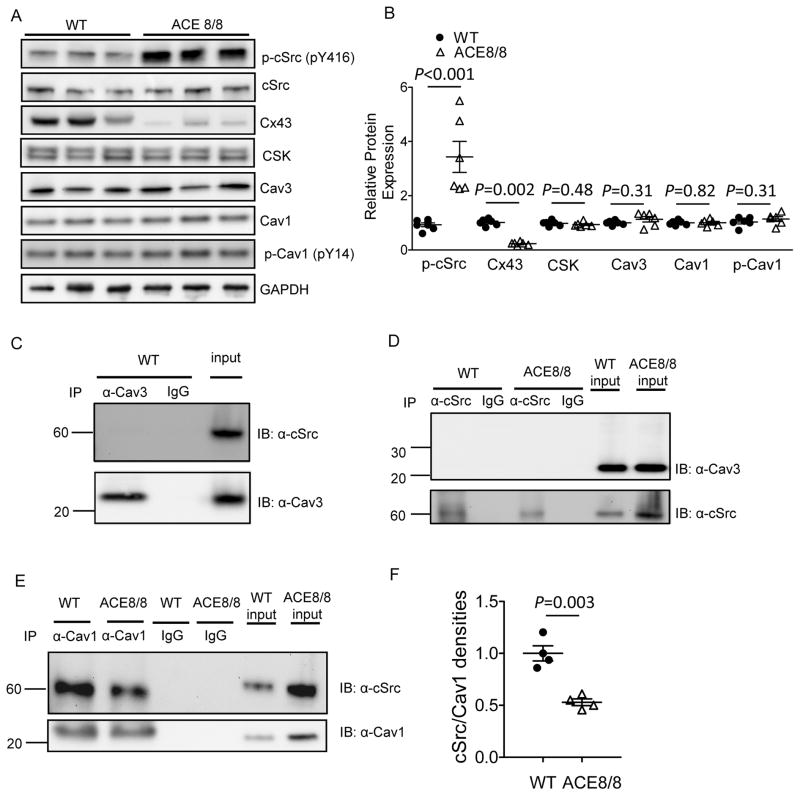
Increased cardiac RAS activity in ACE8/8 mice was accompanied by a 3.4 fold increase (P<0.001) in cSrc activation/phosphorylation and 77% reduction in Cx43 (P=0.002) compared to WT LV (Figure 3A,B). The intrinsic kinase activity of cSrc is controlled by autophosphorylation of Tyr416 located within the kinase domain that results in cSrc activation and by phosphorylation at Tyr527 that results in cSrc inactivation.16 Phosphorylation of Tyr527 is mediated by the C-terminal Src kinase (CSK),17,18 whereas cSrc Tyr416 autophosphorylation can be suppressed by the direct binding with the scaffolding proteins Cav1 and Cav3.19 Cav1 is also necessary for CSK recruitment to cSrc.5 We hypothesized that enhanced RAS signaling activated cSrc either through decreasing the availability of the negative regulator(s) or through abrogating the interaction between cSrc and its negative regulator(s). To test this, the protein expression levels of CSK, Cav3, Cav1, as well as phosphorylated Cav1 (at Tyr14), the active form of Cav1 shown to inhibit cSrc activity,18 were examined and compared in WT and ACE8/8 LV samples. As shown in Figure 3A and 3B, the protein expression of cSrc negative regulators, CSK, Cav3 and Cav1/p-Cav1, were not significantly different in WT and ACE8/8 LV. Next, we assessed the interaction between cSrc and its negative regulators in the mouse LV. Interestingly, cSrc failed to co-immunoprecipitate with CSK (Supplemental Figure 5) or Cav3 (Figure 3C,D), whereas cSrc co-immunoprecipitated with Cav1 in mouse LV (Figure 3E). In addition, the interaction between cSrc and Cav1 was markedly reduced (by 50%, P=0.003) in ACE8/8 compared with WT LV (Figure 3E,F). Taken together, these results suggest that reduced interaction between Cav1 and cSrc abrogates the inhibitory effects of Cav1 on cSrc, thereby contributing to cSrc activation upon enhanced RAS signaling in mouse ventricular myocardium.
Figure 3.
Cardiac RAS-induced cSrc activation and Cx43 downregulation were accompanied by decreased Cav1-cSrc binding. Western blots (A, B) revealed significantly increased cSrc activation (phosphorylation at pY416) and Cx43 downregulation in ACE8/8 (n=6) compared with WT (n=6) LV (P<0.001). The protein expression levels of CSK, Cav1, Cav3, and p-Cav1 (pY14) were not different in ACE8/8 and WT LV. Immunoprecipitation with either Cav3 (C) or cSrc (D) antibody did not show an interaction between Cav3 and cSrc in mouse LV. By contrast, cSrc co-immunoprecipitated with Cav1 in mouse LV (E,F), and the interaction between cSrc and Cav1 was significantly reduced (P=0.003, by ~50%) in ACE8/8 (n=4), compared with WT (n=4) LV.
Enhanced RAS signaling increases S-nitrosation of Cav1, resulting in reduced Cav1-cSrc interaction in LV cardiomyocytes
It is known that the interaction between Cav1 and cSrc at the cell membrane depends on the coupling between the N-terminal myristoyl moiety of cSrc and the palmitoylated Cys156 of Cav1.20 Protein palmitoylation can be disrupted by nitrosation of cysteine residues (S-nitrosation, SNO) by direct competition for cysteine or by the displacement of palmitate;21 SNO cysteine modification is known to modulate the activity of various signaling molecules including PSD-95,22 β-adrenergic receptor23 and Cav1.24 We hypothesized that increased SNO of Cav1 may contribute to the observed uncoupling of cardiac Cav1 and cSrc upon enhanced RAS signaling.
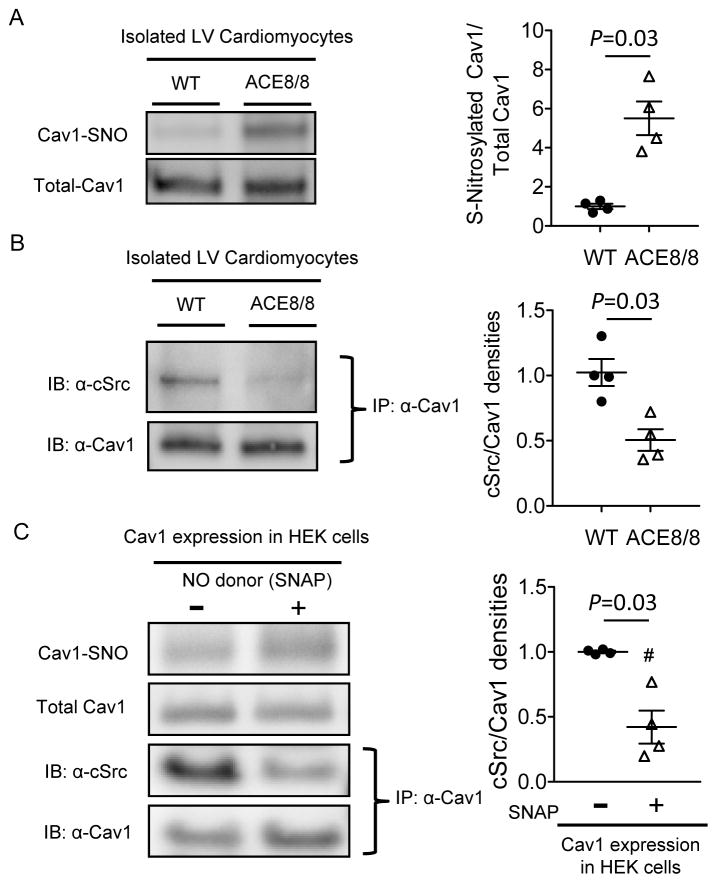
To test this hypothesis directly, a biotin-switch assay to detect protein SNO was conducted using isolated cardiomyocytes from WT and ACE8/8 LV. As shown in Figure 4A, there was a 5.5 fold increase (P=0.03) of Cav1 SNO in isolated myocytes from ACE8/8, compared to WT LV. The increased Cav1 SNO with increased RAS activity was accompanied by a 50% reduction (P=0.03) in Cav1-cSrc interaction in ACE8/8 compared with WT LV myocytes (Figure 4B). To test if increased Cav1 SNO could result in Cav1-cSrc dissociation, human embryonic kidney (HEK) cells transfected with mouse Cav1 and cSrc were treated with 20 μM nitric oxide (NO) donor S-nitroso-N-acetyl-DL-penicillamine (SNAP) or vehicle for 10 min. Increased Cav1 SNO induced by SNAP treatment resulted in decreased (by 58% compared to control, P=0.03) Cav1-cSrc binding (Figure 4C), suggesting that increased Cav1 SNO directly disrupted the Cav1-cSrc interaction.
Figure 4.
RAS activation induces Cav1 S-nitrosation, resulting in Cav1-cSrc dissociation. (A) Cav1 SNO was assessed using biotin-switch assay in the cardiomyocytes isolated from WT (n=4) and ACE8/8 (n=4) LV, which showed the level of Cav1 SNO was significantly (P=0.03) higher in ACE8/8 than in WT LV myocytes. (B) Co-immunoprecipitation experiments revealed that the interaction between cSrc and Cav1 was reduced in ACE8/8 (n=4), compared with WT (n=4), LV myocytes. (F) HEK cells co-transfected with mouse cSrc and Cav1 cDNA were subjected to NO donor (SNAP, 20 μM, 10 min) treatment, where Cav1 SNO was increased, resulting in reduced interaction between cSrc and Cav1 (P=0.03, n=4 in each group).
Cys156, but not Cys133 or Cys143, is critical for Cav1 S-nitrosation
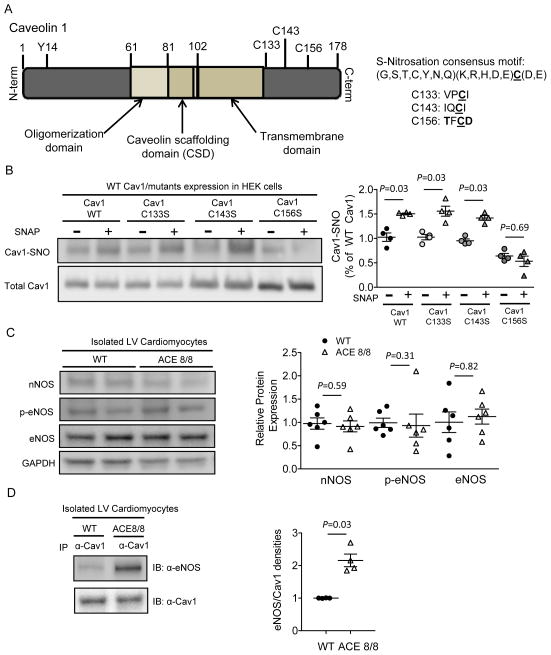
Cav1 contains three cysteines (C133, C143 and C156) that can be palmitoylated, tethering Cav1 to the plasma membrane (Figure 5A). Because protein S-nitrosation, like phosphorylation, usually occurs in the presence of conserved motifs in the primary amino acid sequence,25 we examined the amino acid sequences surrounding the cysteine residues of Cav1 to identify potential sites for S-nitrosation. Of the three cysteines present in Cav1, only Cys156 resides within a consensus motif (G,S,T,C,Y,N,Q)(K,R,H,D,E)C(D,E) for S-nitrosation (Figure 5A),25 predicting Cys156 as the Cav1 SNO site. To test this prediction, HEK cells transfected either with WT Cav1 or one of the nitrosation-resistant Cys-to-Ser (C133S, C143S or C156S) Cav1 mutants were treated with SNAP (20 μM, 10 min) and assayed for Cav1 SNO by biotin-switch assay. As shown in Figure 5B, SNAP treatment increased S-nitrosation in WT, C133S- and C143S-Cav1, but not in C156S-Cav1, suggesting Cys156 was the critical cysteine residue required for Cav1 SNO. Consistent with this result, a recent study also reported that human Cav1 SNO at Cys156 is critical for cSrc activation in response to chronic pulmonary vascular inflammation.26
Figure 5.
Cav1 is nitrosated at Cys156 and Cav1 SNO upon RAS activation is associated with increased eNOS-Cav1 binding. (A) Schematic illustration of mouse Cav1, containing three cysteine residues (C133, C143 and C156) close to the C-terminus, among which only C156 is predicted to be nitrosated. (B) HEK cells transfected with mouse cSrc, as well as with either WT mouse Cav1 cDNA or Cav1 containing Cys133, Cys143 or Cys156 to Ser (nitrosation-resistant) single amino acid mutation, were subjected to SNAP treatment. SNAP treatment significantly increased SNO in WT, C133S and C143S, but not in C156S, Cav1 molecule (P=0.03, n=4 in each pair), suggesting C156 is the only cysteine residue in Cav1 that can be nitrosated. (C) Western blot did not reveal significant differences in the protein expression levels of nNOS, eNOS or p-eNOS in the isolated LV myocytes from WT (n=6) and ACE8/8 (n=6) mice. (D) Co-immunoprecipitation experiments demonstrated significantly (P=0.03) increased eNOS-Cav1 binding in ACE8/8 (n=4), compared with WT (n=4), isolated LV cardiomyocytes.
Cardiac Cav1 S-nitrosation upon enhanced RAS signaling is facilitated by increased eNOS-Cav1 association
Physiologically, the chemical reaction of protein S-nitrosation is favored upon increased availability of NO, either through increased NO production27 or by close proximity to the enzymes that synthesize NO, NO synthase (NOS).27,28 To test if the increased Cav1 SNO upon enhanced cardiac RAS signaling was the result of elevated NO production, we examined the protein expression levels of NOS in isolated LV cardiomyocytes from WT and ACE8/8 animals. As shown in Figure 5C, the protein expression levels of neuronal (nNOS) and endothelial (eNOS) NOS, as well as phospho-eNOS, the active form of eNOS, were not significantly different in WT and ACE8/8 cardiomyocytes. In addition, a direct quantification of NO concentration did not reveal a measurable difference in NO production from isolated WT and ACE8/8 ventricular cardiomyocytes (Supplemental Figure 6). To test if enhanced RAS signaling makes NO available to Cav1 by bringing NOS in proximity to Cav1, we examined the amount of NOS that could be co-immunoprecipitated with Cav1 in WT and ACE8/8 LV myocytes. As shown in Figure 5D, Western blots of the Cav1-pull down lysates revealed a 2.2-fold increase (P=0.03) in the binding between eNOS and Cav1 in ACE8/8, compared with WT isolated LV myocytes. nNOS, however, did not co-immunoprecipitate with Cav1 in either WT or ACE8/8 LV myocytes (data not shown). Taken together, these data suggest that increased Cav1 SNO with enhanced cardiac RAS signaling is related to increased Cav1-eNOS binding.
Cardiac RAS-induced eNOS-Cav1 association is dependent on increased mitochondrial ROS
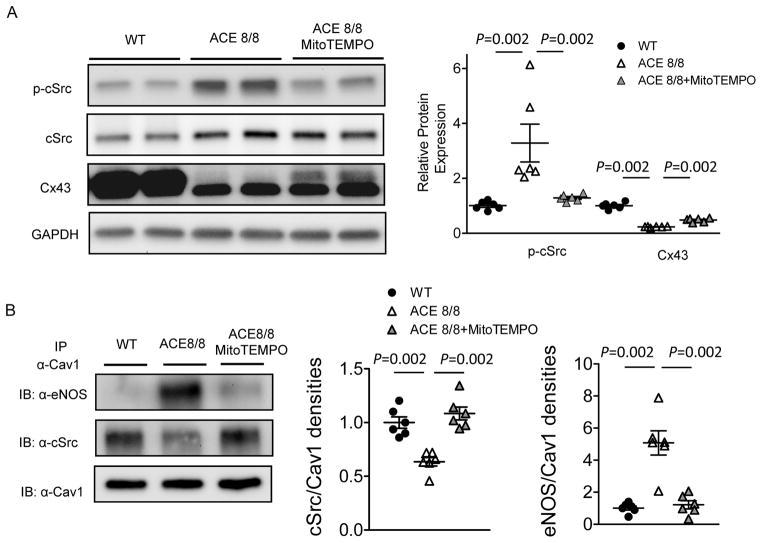
Using the same ACE8/8 mouse model, we have recently demonstrated that cardiac ROS, specifically mitochondrial ROS (mitoROS), is markedly increased with enhanced RAS signaling.15,29 Treatment with mitochondria-targeted antioxidant MitoTEMPO, but not the other types of antioxidants, restores the Cx43 expression, normalizes gap junction conduction, as well as ameliorates ventricular arrhythmias and sudden cardiac death in ACE8/8 mice.29 We hypothesized that increased mitoROS upon enhanced RAS signaling mediated Cx43 degradation through modulating the Cav1-cSrc interaction and cSrc activity. To test this, 4 week ACE8/8 animals were treated with MitoTEMPO (0.7 mg/kg/day, intraperitoneally) for 2 weeks, a regimen that has been demonstrated to normalize elevated mitoROS in ACE8/8 hearts to the levels similar to WT controls.29 As shown in Figure 6A and consistent with previous results,29 MitoTEMPO treatment in ACE8/8 mice resulted in reduced cardiac cSrc phosphorylation (by 65 %, P=0.002) and increased Cx43 expression (by 1.9 fold, P=0.002) compared to untreated ACE8/8 animals. Importantly, co-immunoprecipitation experiments revealed that the increased Cav1-eNOS binding and decreased Cav1-cSrc interaction observed in ACE8/8 LV were both reversed with the treatment of MitoTEMPO (Figure 6B), suggesting that the increased Cav1-eNOS binding and subsequent Cav1-cSrc dissociation upon enhanced RAS signaling were dependent on mitochondrial ROS.
Figure 6.
Mitochondria-targeted antioxidant MitoTEMPO ameliorates cardiac RAS activation-induced cSrc activation and Cx43 downregulation through reducing Cav1-eNOS interaction and restoring Cav1-cSrc binding. (A) Two weeks of MitoTEMPO (0.7 mg/kg/day, intraperitoneally) treatment significantly attenuated cSrc activation/phosphorylation (P=0.002) and Cx43 downregulation (P=0.002) in ACE8/8 LV (n=6 in each group). (B) MitoTEMPO treatment significantly reduced Cav1-eNOS interaction (P=0.002) and restored Cav1-cSrc binding (P=0.002) in ACE8/8 LV (n=6 in each group).
Discussion
Accumulating evidence has suggested that Cav1 is involved in the regulation of cardiac electrical functioning. For example, Cav1 binds to the human ether-a-go-go related gene (hERG) K+ channel and regulates its function7 and degradation.30 L-type Ca2+ channels,8 as well as Cx43,31 have been shown to be targeted to lipid rafts/caveolae and directly interact with Cav1. Importantly, human genome-wide association studies have revealed significant association of Cav1 variants with increased risk of cardiac arrhythmias.2,3 Using two different mouse models (Cav1−/− and ACE8/8) in the present study, we have demonstrated the essential role of Cav1 in maintaining the homeostasis of cardiac Cx43 by modulating cSrc activity. With the abrogation of Cav1-mediated cSrc inhibition, either through genetic deletion of Cav1 or via Cav1 SNO induced by enhanced RAS signaling, cSrc became activated, leading to downregulation of Cx43, reduced ventricular conduction velocity, and increased propensity for ventricular arrhythmias.
The renin-angiotensin system (RAS) is a critical component of the physiological and pathological responses of the cardiovascular system. Angiotensin II (AngII), the central signaling effector of RAS, binds to AngII type 1 receptor (AT1R) and activates NAD(P)H oxidases leading to increased production of cytosolic as well as mitochondrial ROS. It has been demonstrated that mitochondrial, but not cytosolic, ROS plays a critical role in RAS-mediated connexon remodeling and ventricular arrhythmias.29 The present study provides a mechanistic link between RAS-induced oxidative stress and ventricular arrhythmias, where RAS-induced mitochondrial ROS triggers increased eNOS-Cav1 association and Cav1-S-nitrosation, resulting in cSrc activation, Cx43 downregulation and subsequent electrical abnormalities.
It has been demonstrated previously that increased cardiac p-cSrc can compete with Cx43 for the binding with ZO-1 protein at the intercalated disc, promoting Cx43 internalization and degradation.13 With the robust increases in p-cSrc, it is likely that Cx43 downregulation observed in Cav1−/− LV can be attributed to p-cSrc-mediated Cx43 depletion. Indeed, Cx43 mRNA expression levels were not different in the LV from WT, Cav1−/− and Cav1−/−+PP1 mice (Supplemental Figure 4), suggesting that the production of Cx43, at least on the transcriptional level, is not affected in Cav1−/− LV. Our experiments, however, could not exclude the possibility that the efficiency of ventricular Cx43 protein translation or trafficking could be impaired in the absence of Cav1.
Intriguingly, Cav3, the muscle-specific caveolin isoform that is essential for caveolae formation in cardiomyocytes,32 was not involved in the regulation of cSrc and Cx43, since Cav3 did not interact with cSrc (Figure 3C,D) and knockout of Cav3 did not alter cardiac cSrc activity or Cx43 expression levels (Figure 2D). The observation that cSrc is not activated in Cav3−/− LV suggests that Cav1-mediated cSrc inhibition is unaffected in Cav3−/− hearts. Because caveolae are completely absent in Cav3−/− cardiomyocytes,32 the preserved Cav1-cSrc interaction in Cav3−/− hearts suggests that Cav1 interacts with and regulates cSrc outside of caveolae in cardiomyocytes. Indeed, recent studies indicate that caveolin can regulate cellular functions in non-caveolar regions. Examples include cell adhesion,33 reactive neuronal plasticity34 and oxidative stress-induced responses.35 Taken together, the data presented here provide evidence suggesting the non-caveolar role of Cav1-mediated cSrc and Cx43 regulation in cardiomyocytes.
Cav1 is known to negatively regulate eNOS activity in endothelial cells in a caveolae-dependent manner.36 In cells where Cav1 does not drive caveolae assembly, however, the ability of Cav1 to inhibit eNOS activity is diminished, albeit the Cav1-eNOS interaction remains.36 The observation that Cav1-eNOS binding increased without altering eNOS activity (levels of p-eNOS) in ACE8/8 cardiomyocytes (Figure 5C,D) suggests that the Cav1-eNOS interaction in cardiomyocytes is non-caveolar. Therefore, upon enhanced RAS activity and increased mitoROS, eNOS actively redistributes to non-caveolar compartments, allowing spatially confined NO release to targets such as Cav1. This observation highlights the importance of the spatial coupling and direct interaction between eNOS and its targets in NO-mediated signaling pathways.37 In addition, the paradox that binding between eNOS and its negative regulator Cav1 in ACE8/8 mouse hearts allows nitrosation of Cav1 suggests that Cav1 may cease to inhibit eNOS if an appropriate signal is given. It is possible that upon an enhanced RAS state, the non-caveolar interaction between eNOS and Cav1 is increased, and this leads to potential increased local activity of eNOS to facilitate Cav1 SNO. The differential eNOS activities in caveolar and non-caveolar compartments also suggest that the lipid environment may contribute to the negative regulation of eNOS,38 where eNOS targeted to non-caveolar regions can be activated even in the presence of Cav1.
The present study also revealed that increased eNOS-Cav1 binding upon RAS activation in cardiomyocytes was dependent on mitoROS. In line with the recent evidence showing that mitochondrial-targeted, but not general, antioxidants, can ameliorate RAS activation-induced Cx43 downregulation and ventricular arrhythmias,29 these findings reflect the critical role of mitoROS in cardiac cSrc and Cx43 regulation. These data are also consistent with the emerging role of mitoROS as signaling molecules in regulating physiological functions. It is intriguing to understand how mitoROS signals the redistribution of eNOS to non-caveolar Cav1, causes Cav1 SNO, and contributes to subsequent cSrc and Cx43 dysregulation. It has been reported that a subpopulation of eNOS is “docked” to the mitochondrial outer membrane both in endothelial cells39 and neurons.40 It is possible that this subpopulation of eNOS senses the increased mitoROS upon RAS activation, resulting in its displacement from the mitochondria outer membrane and redistribution to non-caveolar compartments where eNOS-Cav1-cSrc interaction occurs. Further experiments are required to test this hypothesis directly.
The observation that the LV conduction velocity is reduced by 30% in Cav1−/− mice, with a ~50% reduction in Cx43 comparing to the WT, is intriguing. Based on the observation in connexin knockout mice, it is generally considered that there exists a significant redundancy of myocardial gap junctions, and significant myocardial conduction slowing occurs only with near-complete connexin depletion.41,42 Several studies in human and animal myocardium, however, reported significant changes in ventricular conduction velocity with relatively small changes in Cx43 levels.43–45 In a recent study by Dhillon et al., a continuous relationship between gap-junction conductance and ventricular conduction velocity was observed in human and guinea-pig myocardium.46 These findings, along with the data presented here, suggest that significant conduction slowing can occur with modest decrease in gap-junction conduction in mammalian myocardium.
Cav1 is abundantly expressed in fibroblasts and endothelial cells. Although the effects of Cav1 deletion on cSrc and Cx43 regulation were observed in isolated cardiomyocytes, we could not completely exclude the possibility that Cav1 deletion might exert non-cell-autonomous effects on cardiomyocytes indirectly through fibroblasts or endothelial cells in the mouse heart. A cardiac-specific Cav1 knockout mouse line, which is not available so far, would be a desirable tool to demonstrate cell autonomous effects of Cav1 deletion in cardiomyocytes.
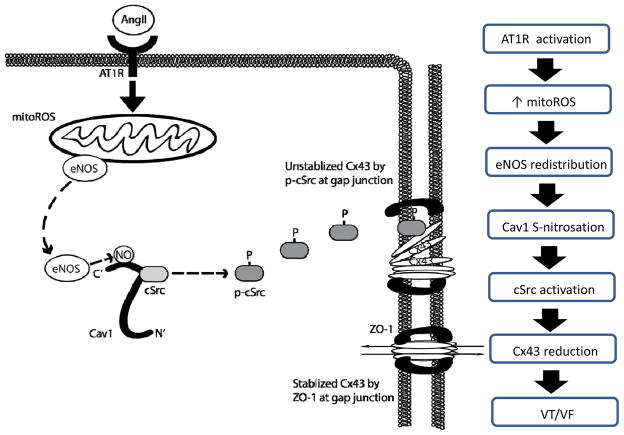
In summary, the present study, for the first time, demonstrates the critical role of Cav1 in maintaining the homeostasis of cardiac Cx43 by interacting with and inhibiting cSrc tyrosine kinase. The disrupted Cav1-cSrc interaction upon pathological conditions such as enhanced RAS signaling resulted in the activation of cSrc, Cx43 reduction, slow conduction and increased risk for ventricular arrhythmias. As summarized in the schematic illustration (Figure 7), our data suggest that mitoROS production increases upon RAS activation, which triggers the redistribution of eNOS and increased Cav1-eNOS interaction, resulting in Cav1 SNO, Cav1-cSrc dissociation, cSrc activation, Cx43 downregulation and subsequently, slow cardiac conduction and increased propensity for arrhythmias. Our findings provide a potential explanation for the genetic association of Cav1 and human arrhythmias, as well as the insights into the mechanistic link between RAS-induced mitochondrial ROS and Cx43 hemichannel regulation. These results suggest the potential therapeutic approach of targeting the regulation of Cav1 or mitochondrial ROS to ameliorate arrhythmic risk caused by RAS activation in various cardiac diseases.
Figure 7.
Schematics illustrating molecular mechanisms linking RAS activation to gap junction remodeling and ventricular arrhythmias. Upon RAS activation, AngII binds to the AT1 receptor, which elevates the level of mitochondrial ROS (mitoROS). Increased mitoROS triggers the redistribution of eNOS and increases the binding between eNOS and Cav1, resulting in increased Cav1 SNO at C156. Increased Cav1 SNO reduces the interaction between Cav1 and cSrc, resulting in Cav1-cSrc dissociation and subsequent phosphorylation/activation of cSrc. Phosphorylated cSrc then competes with and displaces Cx43 from ZO-1 at the intercalated disc, leading to degradation of Cx43, conduction block, and increased propensity of ventricular arrhythmias.
Supplementary Material
Acknowledgments
Funding Sources: This work was funded by National Institutes of Health (NIH) Grants RO1 HL104025 (SCD), HL106592 (SCD), HL091071 (HHP), HL107200 (HHP), HL060678 (RDM), HL071626 (RDM), Veterans Affairs MERIT grants BX000859 (SCD) and BX001963 (HHP), and American Heart Association Midwest Affiliation Postdoctoral Fellowship AHA13POST14380029 (KCY).
Footnotes
Conflict of Interest Disclosure: Dr. Dudley is an inventor of 13/551,790 A Method for Ameliorating or Preventing Arrhythmic Risk Associated with Cardiomyopathy by Improving Conduction Velocity and 13/507,319 A Method for Modulating or Controlling Connexin43 (Cx43) Level of a Cell and Reducing Arrhythmic Risk
References
- 1.Vatta M, Ackerman MJ, Ye B, Makielski JC, Ughanze EE, Taylor EW, Tester DJ, Balijepalli RC, Foell JD, Li Z, Kamp TJ, Towbin JA. Mutant caveolin-3 induces persistent late sodium current and is associated with long-QT syndrome. Circulation. 2006;114:2104–2112. doi: 10.1161/CIRCULATIONAHA.106.635268. [DOI] [PubMed] [Google Scholar]
- 2.Holm H, Gudbjartsson DF, Arnar DO, Thorleifsson G, Thorgeirsson G, Stefansdottir H, Gudjonsson SA, Jonasdottir A, Mathiesen EB, Njolstad I, Nyrnes A, Wilsgaard T, Hald EM, Hveem K, Stoltenberg C, Lochen ML, Kong A, Thorsteinsdottir U, Stefansson K. Several common variants modulate heart rate, PR interval and QRS duration. Nat Genet. 2010;42:117–122. doi: 10.1038/ng.511. [DOI] [PubMed] [Google Scholar]
- 3.Ellinor PT, Lunetta KL, Albert CM, Glazer NL, Ritchie MD, Smith AV, Arking DE, Muller-Nurasyid M, Krijthe BP, Lubitz SA, Bis JC, Chung MK, Dorr M, Ozaki K, Roberts JD, Smith JG, Pfeufer A, Sinner MF, Lohman K, Ding J, Smith NL, Smith JD, Rienstra M, Rice KM, Van Wagoner DR, Magnani JW, Wakili R, Clauss S, Rotter JI, Steinbeck G, Launer LJ, Davies RW, Borkovich M, Harris TB, Lin H, Volker U, Volzke H, Milan DJ, Hofman A, Boerwinkle E, Chen LY, Soliman EZ, Voight BF, Li G, Chakravarti A, Kubo M, Tedrow UB, Rose LM, Ridker PM, Conen D, Tsunoda T, Furukawa T, Sotoodehnia N, Xu S, Kamatani N, Levy D, Nakamura Y, Parvez B, Mahida S, Furie KL, Rosand J, Muhammad R, Psaty BM, Meitinger T, Perz S, Wichmann HE, Witteman JC, Kao WH, Kathiresan S, Roden DM, Uitterlinden AG, Rivadeneira F, McKnight B, Sjogren M, Newman AB, Liu Y, Gollob MH, Melander O, Tanaka T, Stricker BH, Felix SB, Alonso A, Darbar D, Barnard J, Chasman DI, Heckbert SR, Benjamin EJ, Gudnason V, Kaab S. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet. 2012;44:670–675. doi: 10.1038/ng.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cronk LB, Ye B, Kaku T, Tester DJ, Vatta M, Makielski JC, Ackerman MJ. Novel mechanism for sudden infant death syndrome: persistent late sodium current secondary to mutations in caveolin-3. Heart Rhythm. 2007;4:161–166. doi: 10.1016/j.hrthm.2006.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel HH, Tsutsumi YM, Head BP, Niesman IR, Jennings M, Horikawa Y, Huang D, Moreno AL, Patel PM, Insel PA, Roth DM. Mechanisms of cardiac protection from ischemia/reperfusion injury: a role for caveolae and caveolin-1. FASEB J. 2007;21:1565–1574. doi: 10.1096/fj.06-7719com. [DOI] [PubMed] [Google Scholar]
- 6.Robenek H, Weissen-Plenz G, Severs NJ. Freeze-fracture replica immunolabelling reveals caveolin-1 in the human cardiomyocyte plasma membrane. J Cell Mol Med. 2008;12:2519–2521. doi: 10.1111/j.1582-4934.2008.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin J, Lin S, Choy PC, Shen X, Deng C, Kuang S, Wu J, Xu W. The regulation of the cardiac potassium channel (HERG) by caveolin-1. Biochem Cell Biol. 2008;86:405–415. doi: 10.1139/o08-118. [DOI] [PubMed] [Google Scholar]
- 8.Darby PJ, Kwan CY, Daniel EE. Caveolae from canine airway smooth muscle contain the necessary components for a role in Ca2+ handling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1226–1235. doi: 10.1152/ajplung.2000.279.6.L1226. [DOI] [PubMed] [Google Scholar]
- 9.Xiao HD, Fuchs S, Campbell DJ, Lewis W, Dudley SC, Jr, Kasi VS, Hoit BD, Keshelava G, Zhao H, Capecchi MR, Bernstein KE. Mice with cardiac-restricted angiotensin-converting enzyme (ACE) have atrial enlargement, cardiac arrhythmia, and sudden death. Am J Pathol. 2004;165:1019–1032. doi: 10.1016/S0002-9440(10)63363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H, Jr, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 11.Hagiwara Y, Sasaoka T, Araishi K, Imamura M, Yorifuji H, Nonaka I, Ozawa E, Kikuchi T. Caveolin-3 deficiency causes muscle degeneration in mice. Hum Mol Genet. 2000;9:3047–3054. doi: 10.1093/hmg/9.20.3047. [DOI] [PubMed] [Google Scholar]
- 12.King JH, Huang CL, Fraser JA. Determinants of myocardial conduction velocity: implications for arrhythmogenesis. Front Physiol. 2013;4:154. doi: 10.3389/fphys.2013.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kieken F, Mutsaers N, Dolmatova E, Virgil K, Wit AL, Kellezi A, Hirst-Jensen BJ, Duffy HS, Sorgen PL. Structural and molecular mechanisms of gap junction remodeling in epicardial border zone myocytes following myocardial infarction. Circ Res. 2009;104:1103–1112. doi: 10.1161/CIRCRESAHA.108.190454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donoghue M, Wakimoto H, Maguire CT, Acton S, Hales P, Stagliano N, Fairchild-Huntress V, Xu J, Lorenz JN, Kadambi V, Berul CI, Breitbart RE. Heart block, ventricular tachycardia, and sudden death in ACE2 transgenic mice with downregulated connexins. J Mol Cell Cardiol. 2003;35:1043–1053. doi: 10.1016/s0022-2828(03)00177-9. [DOI] [PubMed] [Google Scholar]
- 15.Sovari AA, Iravanian S, Dolmatova E, Jiao Z, Liu H, Zandieh S, Kumar V, Wang K, Bernstein KE, Bonini MG, Duffy HS, Dudley SC. Inhibition of c-Src tyrosine kinase prevents angiotensin II-mediated connexin-43 remodeling and sudden cardiac death. J Am Coll Cardiol. 2011;58:2332–2339. doi: 10.1016/j.jacc.2011.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown MT, Cooper JA. Regulation, substrates and functions of src. Biochim Biophys Acta. 1996;1287:121–149. doi: 10.1016/0304-419x(96)00003-0. [DOI] [PubMed] [Google Scholar]
- 17.Okada M, Nada S, Yamanashi Y, Yamamoto T, Nakagawa H. CSK: a protein-tyrosine kinase involved in regulation of Src family kinases. J Biol Chem. 1991;266:24249–24252. [PubMed] [Google Scholar]
- 18.Place AT, Chen Z, Bakhshi FR, Liu G, O’Bryan JP, Minshall RD. Cooperative role of caveolin-1 and C-terminal Src kinase binding protein in C-terminal Src kinase-mediated negative regulation of c-Src. Mol Pharmacol. 2011;80:665–672. doi: 10.1124/mol.111.073957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li S, Couet J, Lisanti MP. Src tyrosine kinases, Galpha subunits, and H-Ras share a common membrane-anchored scaffolding protein, caveolin. Caveolin binding negatively regulates the auto-activation of Src tyrosine kinases. J Biol Chem. 1996;271:29182–29190. doi: 10.1074/jbc.271.46.29182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee H, Woodman SE, Engelman JA, Volonte D, Galbiati F, Kaufman HL, Lublin DM, Lisanti MP. Palmitoylation of caveolin-1 at a single site (Cys-156) controls its coupling to the c-Src tyrosine kinase: targeting of dually acylated molecules (GPI-linked, transmembrane, or cytoplasmic) to caveolae effectively uncouples c-Src and caveolin-1 (TYR-14) J Biol Chem. 2001;276:35150–35158. doi: 10.1074/jbc.M104530200. [DOI] [PubMed] [Google Scholar]
- 21.Salaun C, Greaves J, Chamberlain LH. The intracellular dynamic of protein palmitoylation. J Cell Biol. 2010;191:1229–1238. doi: 10.1083/jcb.201008160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho GP, Selvakumar B, Mukai J, Hester LD, Wang Y, Gogos JA, Snyder SH. S-nitrosylation and S-palmitoylation reciprocally regulate synaptic targeting of PSD-95. Neuron. 2011;71:131–141. doi: 10.1016/j.neuron.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adam L, Bouvier M, Jones TL. Nitric oxide modulates β2-adrenergic receptor palmitoylation and signaling. J Biol Chem. 1999;274:26337–26343. doi: 10.1074/jbc.274.37.26337. [DOI] [PubMed] [Google Scholar]
- 24.Baker TL, Booden MA, Buss JE. S-Nitrosocysteine increases palmitate turnover on Ha-Ras in NIH 3T3 cells. J Biol Chem. 2000;275:22037–22047. doi: 10.1074/jbc.M001813200. [DOI] [PubMed] [Google Scholar]
- 25.Stamler JS, Toone EJ, Lipton SA, Sucher NJ. (S)NO signals: translocation, regulation, and a consensus motif. Neuron. 1997;18:691–696. doi: 10.1016/s0896-6273(00)80310-4. [DOI] [PubMed] [Google Scholar]
- 26.Bakhshi FR, Mao M, Shajahan AN, Piegeler T, Chen Z, Chernaya O, Sharma T, Elliott WM, Szulcek R, Bogaard HJ, Comhair S, Erzurum S, van Nieuw Amerongen GP, Bonini MG, Minshall RD. Nitrosation-dependent caveolin 1 phosphorylation, ubiquitination, and degradation and its association with idiopathic pulmonary arterial hypertension. Pulmonary Circulation. 2013;3:816–830. doi: 10.1086/674753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 28.Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and α1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- 29.Sovari AA, Rutledge CA, Jeong EM, Dolmatova E, Arasu D, Liu H, Vahdani N, Gu L, Zandieh S, Xiao L, Bonini MG, Duffy HS, Dudley SC., Jr Mitochondria oxidative stress, connexin43 remodeling, and sudden arrhythmic death. Circ Arrhythm Electrophysiol. 2013;6:623–631. doi: 10.1161/CIRCEP.112.976787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Massaeli H, Sun T, Li X, Shallow H, Wu J, Xu J, Li W, Hanson C, Guo J, Zhang S. Involvement of caveolin in low K+-induced endocytic degradation of cell-surface human ether-a-go-go-related gene (hERG) channels. J Biol Chem. 2010;285:27259–27264. doi: 10.1074/jbc.M110.124909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schubert AL, Schubert W, Spray DC, Lisanti MP. Connexin family members target to lipid raft domains and interact with caveolin-1. Biochemistry. 2002;41:5754–5764. doi: 10.1021/bi0121656. [DOI] [PubMed] [Google Scholar]
- 32.Woodman SE, Park DS, Cohen AW, Cheung MW, Chandra M, Shirani J, Tang B, Jelicks LA, Kitsis RN, Christ GJ, Factor SM, Tanowitz HB, Lisanti MP. Caveolin-3 knock-out mice develop a progressive cardiomyopathy and show hyperactivation of the p42/44 MAPK cascade. J Biol Chem. 2002;277:38988–38997. doi: 10.1074/jbc.M205511200. [DOI] [PubMed] [Google Scholar]
- 33.del Pozo MA, Balasubramanian N, Alderson NB, Kiosses WB, Grande-Garcia A, Anderson RG, Schwartz MA. Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat Cell Biol. 2005;7:901–908. doi: 10.1038/ncb1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaudreault SB, Blain JF, Gratton JP, Poirier J. A role for caveolin-1 in post-injury reactive neuronal plasticity. J Neurochem. 2005;92:831–839. doi: 10.1111/j.1471-4159.2004.02917.x. [DOI] [PubMed] [Google Scholar]
- 35.Khan EM, Heidinger JM, Levy M, Lisanti MP, Ravid T, Goldkorn T. Epidermal growth factor receptor exposed to oxidative stress undergoes Src- and caveolin-1-dependent perinuclear trafficking. J Biol Chem. 2006;281:14486–14493. doi: 10.1074/jbc.M509332200. [DOI] [PubMed] [Google Scholar]
- 36.Sowa G, Pypaert M, Sessa WC. Distinction between signaling mechanisms in lipid rafts vs. caveolae. Proc Natl Acad Sci U S A. 2001;98:14072–14077. doi: 10.1073/pnas.241409998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nedvetsky PI, Sessa WC, Schmidt HH. There’s NO binding like NOS binding: protein-protein interactions in NO/cGMP signaling. Proc Natl Acad Sci U S A. 2002;99:16510–16512. doi: 10.1073/pnas.262701999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michel T, Feron O. Nitric oxide synthases: which, where, how, and why? J Clin Invest. 1997;100:2146–2152. doi: 10.1172/JCI119750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao S, Chen J, Brodsky SV, Huang H, Adler S, Lee JH, Dhadwal N, Cohen-Gould L, Gross SS, Goligorsky MS. Docking of endothelial nitric oxide synthase (eNOS) to the mitochondrial outer membrane: a pentabasic amino acid sequence in the autoinhibitory domain of eNOS targets a proteinase K-cleavable peptide on the cytoplasmic face of mitochondria. J Biol Chem. 2004;279:15968–15974. doi: 10.1074/jbc.M308504200. [DOI] [PubMed] [Google Scholar]
- 40.Henrich M, Hoffmann K, Konig P, Gruss M, Fischbach T, Godecke A, Hempelmann G, Kummer W. Sensory neurons respond to hypoxia with NO production associated with mitochondria. Mol Cell Neurosci. 2002;20:307–322. doi: 10.1006/mcne.2002.1111. [DOI] [PubMed] [Google Scholar]
- 41.Gutstein DE, Morley GE, Tamaddon H, Vaidya D, Schneider MD, Chen J, Chien KR, Stuhlmann H, Fishman GI. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ Res. 2001;88:333–339. doi: 10.1161/01.res.88.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaidya D, Tamaddon HS, Lo CW, Taffet SM, Delmar M, Morley GE, Jalife J. Null mutation of connexin43 causes slow propagation of ventricular activation in the late stages of mouse embryonic development. Circ Res. 2001;88:1196–1202. doi: 10.1161/hh1101.091107. [DOI] [PubMed] [Google Scholar]
- 43.Kostin S, Rieger M, Dammer S, Hein S, Richter M, Klovekorn WP, Bauer EP, Schaper J. Gap junction remodeling and altered connexin43 expression in the failing human heart. Mol Cell Biochem. 2003;242:135–144. [PubMed] [Google Scholar]
- 44.Glukhov AV, Fedorov VV, Kalish PW, Ravikumar VK, Lou Q, Janks D, Schuessler RB, Moazami N, Efimov IR. Conduction remodeling in human end-stage nonischemic left ventricular cardiomyopathy. Circulation. 2012;125:1835–1847. doi: 10.1161/CIRCULATIONAHA.111.047274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guerrero PA, Schuessler RB, Davis LM, Beyer EC, Johnson CM, Yamada KA, Saffitz JE. Slow ventricular conduction in mice heterozygous for a connexin43 null mutation. J Clin Invest. 1997;99:1991–1998. doi: 10.1172/JCI119367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dhillon PS, Gray R, Kojodjojo P, Jabr R, Chowdhury R, Fry CH, Peters NS. Relationship between gap-junctional conductance and conduction velocity in Mammalian myocardium. Circ Arrhythm Electrophysiol. 2013;6:1208–1214. doi: 10.1161/CIRCEP.113.000848. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.