Abstract
Purpose
The introduction of the mumps vaccine has dramatically reduced the number of mumps cases, but outbreaks have recently occurred among highly vaccinated populations in developed countries. Epidemiological and clinical characteristics of patients with mumps admitted between 1989 and 2012 in a single hospital in Korea are described in the present study.
Methods
We retrospectively evaluated inpatients with mumps between 1989 and 2012 and outpatients and inpatients with mumps in 2011-2012.
Results
A total of 152 patients with mumps were admitted between 1989 and 2012, and 163 patients were recorded in 2011-2012. The highest number of admitted cases occurred in 1998 and 2012 (35 and 34 cases, respectively). Among the patients admitted in 2011-2012, the highest frequency was observed among people aged 15-19 years, and low frequency was observed in those aged <4 years and >20 years, compatible to the city data and national data. In patients admitted to our department in 1998 (35 cases) and in 2010-2012 (27 cases), there were significant differences in the mean age and the rate of secondary measles-mumps-rubella (MMR) vaccination, but had similar clinical features, including complications, except aseptic meningitis. Antimumps immunoglobulin (Ig) G was positive in 83% and 100%, and IgM was positive in 67% and 41%, respectively, in the two periods.
Conclusion
In Korea, recent mumps outbreaks have occurred mainly among secondary school students who received two doses of the MMR vaccine. The vaccinees might have a modified immune reaction to viral insults, manifesting modified epidemiological and clinical features.
Keywords: Mumps, Disease outbreaks, Measles-mumps-rubella vaccine, Antibodies, Immnoglobulin G, Immunoglobulin M
Introduction
Mumps is an acute systemic self-limited illness caused by a single-stranded RNA virus belonging to the Paramyxoviridae family. Its clinical manifestations are characterized by parotid gland swelling with preceding prodromal symptoms of several days, including fever, headache, malaise and myalgia, although the disease can pass unnoticed, particularly in children. The majority of affected patients recover from the disease uneventfully, but some severely affected patients complain of complications, including meningitis, orchitis, oophoritis, pancreatitis and rarely encephalitis, deafness, carditis and nephritis1,2).
After introduction of the mumps vaccine and the measles-mumps-rubella (MMR) vaccine, the mumps incidence declined dramatically. However, multiple outbreaks of mumps were recently reported among highly vaccinated populations in many countries3,4,5,6,7,8). In Korea, the MMR vaccination program was started in 1983, and two-dose MMR vaccination has been recommended since 1994 after the 1993 measles epidemic by Korean Pediatric Society. After the 2000-2001 measles epidemic, children who entered primary school were required to present a certificate of the two-dose MMR vaccination9,10). With these programs, mumps cases were markedly reduced, but sporadic outbreaks have occurred since early 1990s, with a nationwide epidemic occurring in 1998 including in Daejeon11). Since 2007, nationwide local outbreaks have occurred mainly in secondary school students with large outbreaks occurring in Daejeon in 201212,13,14,15).
In this study, we evaluated the epidemiological and clinical data from our institution during the period from 1989 to 2012, and presented the national epidemiological data published by the Korea Centers for Disease Control and Prevention (KCDC) during study period14,15). We compared the clinical and laboratory characteristics of the patients admitted in 1998 and those in 2010-2012. We also focused on discussion regarding the immunopathogenesis of mumps including the reason for high seopositivity of anti-immunoglobulin G (IgG) antibodies and low seropositivity of anti-immunoglobulin M (IgM) antibodies in mumps patients in recent outbreaks.
Materials and methods
We retrospectively analyzed the medical records of 152 inpatients with mumps, who were diagnosed from January 1989 through December 2012, and 163 mumps patients (116 outpatients and 47 inpatients) diagnosed from January 2011 to December 2012 at The Catholic University of Korea, Daejeon St. Mary's Hospital. All 152 admitted patients with mumps satisfied the clinical diagnostic criteria. The patients had parotid gland swelling and increased amylase with/without complications such as orchitis and meningitis, and they received antimumps IgM and IgG test (Platelia Mumps IgM; immunoenzymatic capture method and Platelia Mumps IgG, enzyme-linked immunosorbent assay [ELISA]; Bio-Rad, Marnes-la-Coquette, France) during hospitalization. However, no patients were tested for mumps virus culture and for other possible viruses of parotitis.
We analyzed demographic characteristics including admission time, age and sex in the inpatients (1989-2012) and the outpatients and inpatients from 2011 to 2012. KCDC has been published annual cases of various infectious diseases including mumps with regional frequencies. We reviewed the data published by the KCDC during the study period (1989-2012)14,15). We also evaluated and compared the clinical and laboratory characteristics of the inpatients admitted to Department of Pediatrics according to the time of admission (the patient group admitted in 1998, 35 cases; the patient group admitted from 2010 to 2012, 27 cases). MMR vaccination history was obtained from parents or guardian statements and partly based on vaccination records. Some data compiled in this study regarding the patient group admitted in 1998 was previously published11). The study was approved by the Institutional Review Board of The Catholic University of Korea, Daejeon St. Mary's Hospital.
Statistical analyses were performed using the SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). Categorical variables were assessed using the chi-square test and Fisher exact test, and continuous variables were assessed using the independent t-test. A P value of <0.05 was considered significant for the statistical tests.
Results
1. Annual inpatient cases during the study period
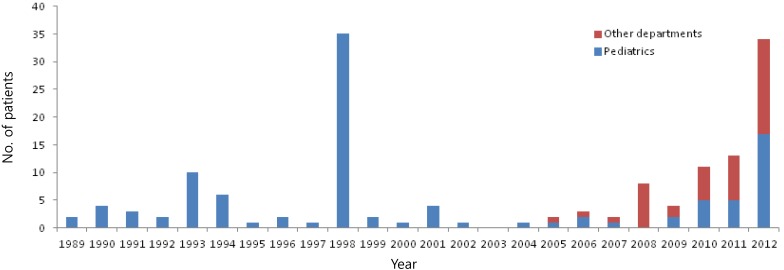
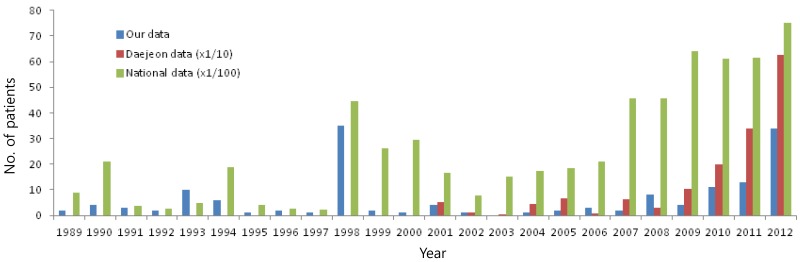
During the study period (1989-2012), a total of 152 patients were admitted to our hospital, and the annual number of patients is shown in Fig. 1. Before 2004, there were few patients who were admitted to other departments except the Department of Pediatrics; however, since 2005, there were 44 patients who were admitted to other departments; 17 cases in Department of Urology, 14 cases in Department of Internal Medicine, 10 cases in Departments of Ear, nose, and throat (ENT), and 3 cases in other departments; red bars in Fig. 1. The median age of the inpatients (152 cases) was 12 years (range, 2-52 years of age) and the male-to-female ratio was 3.2:1 (116/36). There were two peaks of number of the patients during the study period occurring in 1998 (35 cases) and 2012 (34 cases), and a range of 0-13 sporadic cases in other years. According to the nationwide data including Daejeon (our city, 2001-2012), there might be nationwide local outbreaks since 2007, and in Daejeon, there were outbreaks since 2010 with the highest number of cases occurring in 2012, which is in agreement with our data (Fig. 2).
Fig. 1.
Annual inpatient cases during the study period (1989-2012). Other departments: Department of Urology, Department of Internal Medicine, Department of Ear, Nose, and Throat, etc.
Fig. 2.
Annual cases in this study, in Daejeon and in nationwide data during the study period. The data of Daejeon are expressed since 200114,15).
2. Age distribution of mumps patients from 2011-2012
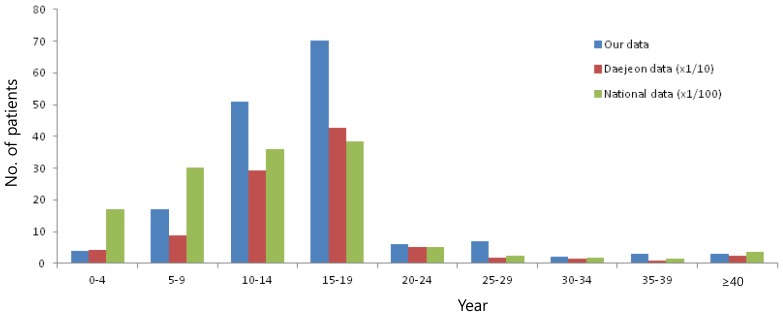
From 2011-2012, there were 163 patients (116 outpatients and 47 inpatients) in our institution. The age distribution of mumps patients is shown in Fig. 2. The age of patients ranged from 2 to 50 years, and the peak age group was 15-19 years and followed the 10-14 years group with rare occurring in patients >20 years and <4 years. This age distribution pattern was similar to the Daejeon data and the national data during 2011-2012, both were published by KCDC14,15) (Fig. 3).
Fig. 3.
Age distribution of mumps patients from 2011-2012 in our institution, in Daejeon and in nationwide data.
3. Comparison of demographic and clinical findings in two outbreaks
For evaluation of clinical or laboratory differences between the one-dose MMR vaccinees and the two-dose MMR vaccinees, we evaluated the patients admitted in 1998 (35 cases) and the patients admitted from 2010 to 2012 (27 cases) in our department. The male patients were predominant in both groups without a significance, but the mean age were significantly different between the groups (P<0.01). In clinical features, there was no difference in parotid gland involvement, fever and headache, but in complications, fewer patients with meningitis were observed in recent years (P<0.01). The positive rates of antimumps IgG and IgM were 83.3% (15 of 18) and 66.6% (12 of 18) in the 1998 group, and 100% (27 of 27) and 40.7% (10 of 27) in the 2010-2012 group, respectively. In MMR vaccination history, 33 of 35 in the 1998 group received the one-dose MMR vaccine and no patients received the second MMR vaccine, while 23 of 27 patients in the 2010-2012 group received the two-dose MMR vaccine (Table 1).
Table 1.
Clinical and laboratory characteristics between the patients admitted in 1998 and in 2010-2012
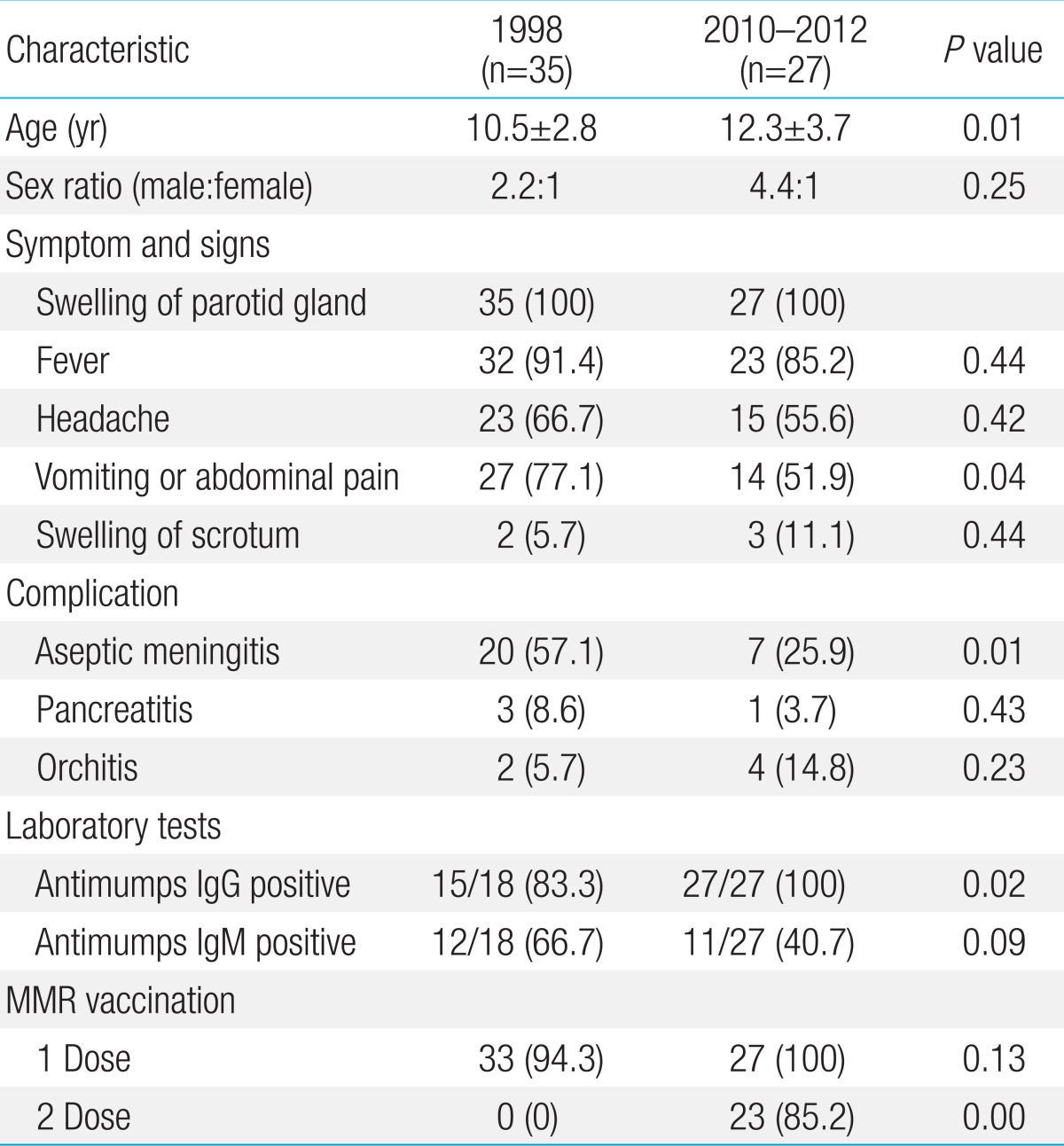
Values are presented as mean±standard deviation or number (%).
IgG, immunoglobulin G; IgM, immunoglobulin M; MMR, measles-mumps-rubella.
Discussion
Although MMR vaccination has greatly reduced the number of mumps and measles cases worldwide, a large number of mumps cases in two-dose MMR vaccinees have recently occurred globally3,4,5,6,7,8). In the United States, after the introduction of the mumps vaccine in 1967, reported cases fell dramatically by 98% from 1968 to 1985. In the late 1980s, outbreaks occurred in both unvaccinated and vaccinated adolescents and young adults, and wide use of the two-dose MMR vaccine for measles control was followed by low cases during 2000-2005. However since 2006, sporadic outbreaks have occurred mainly in the 18-24 years of age group, with the majority having received the two-dose MMR vaccine3,4). In Korea, a nationwide MMR vaccination program was started in 1983, and a two-dose MMR vaccination has been recommended at 15 months and at 4-6 years of age since 1995 after the 1993-1994 measles epidemic. The Jeryl-Lynn mumps virus strain contained MMR vaccine has only been used since 2002. At the time of the 2000-2001 measles epidemic, the one-dose MMR vaccination coverage was reported in approximately 90% of children born after 1987. Since 2001, children who entered primary school have been required to present a certificate of the two-dose MMR vaccination and 95%-99% of the population born after 1995 was covered by the two-dose MMR vaccination9,10). Therefore, more than 95% of the population under 16 years of age in 2012 in Korea may be two-dose vaccinees, and the patients admitted to our department in 2010-2012 were all <16 years of age. After one-dose MMR vaccine introduction, mumps cases were markedly reduced, but sporadic outbreaks occurred from early 1990s and a nationwide epidemic occurred in 1998 as well as in Daejeon11,15). During the two-dose MMR vaccination period, nationwide outbreaks occurred after 2007, with large outbreak in Daejeon in 2012. Although we evaluated only the admitted patients with mumps, the epidemiological pattern in this study was compatible with data from the Korea government14,15). Similar phenomena are observed in other populations where the two-dose MMR vaccination schedule is well-established4,7,8). Considering the national and international data, more nationwide local outbreaks in Korea are possible over time.
Regarding the reason for outbreaks in two-dose vaccinated subjects, the decreased vaccine effectiveness over time after vaccination (waning immunity or secondary vaccine failure) may play a major role3), although other factors may have contributed, including high population density with close contact in schools or parties4,6,8), incomplete vaccine-induced immunity to wild virus (primary vaccine failure), antigenic variation of wild-type strains16), a limited effectiveness of virus strains used in vaccines17) and less than optimum herd immunity in a subpopulation5,18).
In primary mumps infection, antimumps IgMs were detected in the majority of mumps patients first at the parotitis stage, and then IgG production followed, as well as any acute systemic infections1,19). Although the level of vaccine induced antimumps IgG can decrease with time after vaccination16,20), IgG antibodies are detected in the majority of mumps patients that received MMR vaccine at presentation7,19). This finding was also observed in this study; the patients in 1998 (one-dose MMR vaccinees) showed 83.3% IgG seropositivity and the patients in 2010-2012 (two-dose MMR vaccinees) showed 100%, when using an enzyme immunoassay, while IgM antibodies were detected in 66.6% and 40.7% of the patients, respectively. After a viral infection, a variety of specific antibodies against viral particle are thought to be produced by immune cells (plasma cells). A kind of plasma cell clone has B-cell receptors for certain-sized protein as an antigen and produce specific antibodies against the protein. Thus, the antigens that induce specific IgG antibodies are not a whole virion (large complex protein), but the fragments of structural virus proteins and/or the proteins from viral gene products, although some specific IgG antibodies against these antigens can bind to the virions in vitro and in vivo. Several serologic methods for mumps diagnosis have been developed, including serum neutralization, complement fixation, hemagglutination-inhibition, immunofluorescence and enzyme immunoassay including ELISA21,22). The antigens used in each diagnostic tool may differ slightly, and accordingly, specific antibodies are different as well, showing of somewhat different positivity of the antibodies23). Therefore in mumps virus infection, waning immunity is not reflected by the vaccine-induced IgG antibodies, and other undetermined immune functions may fade over time after vaccination. Similar finding can be observed with other viral infections. For example, in the 2009 H1N1 pandemic influenza in Korea, the prepandemic seroprevalence of neutralizing IgG antibodies that were cross-reactive to the H1N1 influenza viruses were not different among all adult group (20.0% in the 19-59 years of age group and 27.3% in the >60 years group), but the adults >40 years of age consisted of only 6% of all affected patients24). Thus, older people could possibly have pre-existing immunity not detectable by cross-reactive IgG antibodies.
The immunopathogenesis of mumps parotitis and other complications such as orchitis and meningoencephalitis is not clearly understood. It has been believed that mumps viruses themselves are involved in parotid gland inflammation and other tissue inflammations (meningitis, orchitis, pancreatitis, etc.). However in general, an infectious disease has a primary infection site (focus) where pathogens are multiplied. The focus of a virus infection produces many substances, including whole virions, byproducts of the virus replication process including viral structural fragments, materials from virus-infected injured cells, and materials from activated immune cells including proinflammatory cytokines (tumor necrosis factor-α, interleukin-6, etc.), which may consist of various sized proteins. Therefore, the beginning of systemic prodromal symptoms and signs, including fever, malaise and viremia, may coincide with the release of viruses and the substances that formed during incubation period from the focus (possibly upper respiratory track cells and nearby secondary immune organs) into the systemic circulation. We previously hypothesized that circulating immune cells may have their own functions against the size and characteristics of these substances (proteins) within the "protein homeostasis system" of the host25,26,27). Various immune cells and immune proteins (immunoglobulins) in vivo are only effectors for control of the toxic substances against the host cells, thus disease progression is dependent on the action of corresponding immune cells.
Considering many enigmas in mumps as followed; each virus in various viral diseases has their own host tissue cells having receptors for virus entry and replication with variable incubation period, but the receptors on host cells in mumps are not clearly defined1); mumps viruses have no cytopathic-effect on some kind of human cell lines in vitro28); clinical course of mumps is self-limited with variable phenotypes including mumps meningtits without parotitis29); viruses or polymerase chain reaction products are detected on upper respiratory tract (around the parotid glands) only at the beginning of the illness and only a part of the patients30,31); specific antibodies (IgM and IgG) against mumps viruses are not detected in the incubation period and the early stage of primary infection; and other viruses including Epstein-Barr virus and influenza viruses can induce parotitis32), the immunopathogenesis of mumps may not the virus-induced cytopathy, but hypothetically the immunological reaction of host immune cells against the substances that have affinity to the host target cells (parotid gland cells, testicle cells, central nervous system cells, and other tissue cells), as well as the majority of other viral and bacterial infections including influenza and mycoplasma infections25,26,27).
The positive rate of antimumps IgM antibodies in vaccinees is well-known to be lower compared with nonvaccinees ranged from 19% to 50%4,7,8,23), but the reason of this finding needs further investigation. In general, IgM antibodies in any systemic viral infections do not appear at the beginning of the illness such as fever onset (prodromal stage), but 3-4 days after the illness onset at the earliest. The host's immune system, including IgM antibodies, controls the pathogens and other inflammatory substances from the initial infection sites and subsequent materials produced during inflammations in an infection, and complete removal of these substances results in the host's full recovery from the disease. IgM antibodies may control the virions that are exposed into systemic circulation. The exposed virions may not induce a cytopathy of parotid gland cells by intracellular replication, but the smaller toxic substances from the virus-infected injured host cells, including virus-associated byproducts, in the focus and corresponding immune cells may induce the parotid gland and other tissue inflammations. Thus, it is possible that in the vaccinees, small amounts of virions are produced at the primary focus and/or virus particles are released late from the focus into the systemic circulation (early examination of IgM antibodies), or pre-existing IgG antibodies may interfere with the exposed viruses and production of IgM antibodies. Since the production of small amount of viruses in the infected person may need close personal contact to transmit the disease and may have a limitation to widespread, this assumption could explain the epidemiologic characteristics in recent local outbreaks; the outbreaks occurred in mainly school students, and the onset of outbreak was sudden increased number of cases within a month period and followed by a sudden decrease with subsiding within several months in the highly vaccinated subpopulations3,18).
Regarding epidemiological data between the patients in 1998 (32 of 35 were one-dose MMR vaccinees) and the patients in 2010-2012 (23 of 27 were two-dose MMR vaccinees), the age distribution was somewhat different, although the number of cases was small. The peak age group in the 1998 outbreak was 10-11 years (vaccination at 15 months of age) and in recent outbreaks was 13-14 years (booster vaccination at 4-6 years of age). The difference of age distribution was observed in the United States between the patients in late 1980s outbreaks and the patients in recent outbreaks3,33). Therefore, the undetermined protective immune function from a vaccine may wane within 10 years after last vaccination. On the other hand, in the age distribution of mumps patients in this study and in the national data from 2011 to 2012, the 15-19 years group of high school students was predominant and the persons of >20-30 years were relatively sparse. This age pattern is somewhat different to the data from other countries. In the United States, college students of aged 18-24 years mainly affected with mumps in recent local outbreaks with some variations according to different districts3,4,8).
After use of MMR vaccine, the most susceptible group moved from the 5-9 years to adolescent and young adults1). Thus, male patients had a higher rate of complications (orchitis) than did female patients in recent outbreaks3,4). Clinical manifestations in vaccinees are considered to be less severe than in the unvaccinated patients, due to the vaccine's effects on the host's immune function1). The fever duration of the patients with orchitis was shorter in vaccinees than in nonvaccinees34). In this study, the rate of meningitis was significantly lower in patient in the recent outbreak. It may be explained that two-dose vaccinees have milder clinical course than one-dose vaccinees, however the sample size is very small.
The long-term incubation period (2-3 weeks), the high rate of subclinical infection (~40%), the transmission from patients with mild vaccine-modified disease and the time-limited effectiveness of a vaccine may cause persistent outbreaks and difficulty of eradicating mumps. Recently, third mumps vaccine trials in mumps outbreaks were reported to be effective in halting the spread of mumps in a subpopulation8,35). However, further studies are needed to apply this policy to all susceptible populations.
This retrospective observational study has some limitations. Although our epidemiological data appeared to be similar to nationwide data from the Korean government, our data resulted from a small number of admitted patients. We did not perform extensive diagnostic viral studies for parotitis, and cannot rule out the possibility of other pathogens.
In conclusion, the majority of mumps patients in recent outbreaks in Korea were two-dose MMR vaccinees, and the results were compatible with the data from other countries. Almost all the patients had anti-mumps IgG with approximately 40% IgM positivity, and they had a timegap of approximately one decade from last MMR vaccination to be infected. Although clinical manifestations in recent outbreaks seem to be milder compared to the prevaccine era, new preventive policies may be needed to reduce future outbreaks.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Plotkin SA, Rubin SA. Mumps vaccine. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 5th ed. Philadelphia: Elsevier; 2008. pp. 435–465. [Google Scholar]
- 2.Hviid A, Rubin S, Muhlemann K. Mumps. Lancet. 2008;371:932–944. doi: 10.1016/S0140-6736(08)60419-5. [DOI] [PubMed] [Google Scholar]
- 3.Dayan GH, Quinlisk MP, Parker AA, Barskey AE, Harris ML, Schwartz JM, et al. Recent resurgence of mumps in the United States. N Engl J Med. 2008;358:1580–1589. doi: 10.1056/NEJMoa0706589. [DOI] [PubMed] [Google Scholar]
- 4.Barskey AE, Schulte C, Rosen JB, Handschur EF, Rausch-Phung E, Doll MK, et al. Mumps outbreak in Orthodox Jewish communities in the United States. N Engl J Med. 2012;367:1704–1713. doi: 10.1056/NEJMoa1202865. [DOI] [PubMed] [Google Scholar]
- 5.Kay D, Roche M, Atkinson J, Lamden K, Vivancos R. Mumps outbreaks in four universities in the North West of England: prevention, detection and response. Vaccine. 2011;29:3883–3887. doi: 10.1016/j.vaccine.2011.03.037. [DOI] [PubMed] [Google Scholar]
- 6.Greenland K, Whelan J, Fanoy E, Borgert M, Hulshof K, Yap KB, et al. Mumps outbreak among vaccinated university students associated with a large party, the Netherlands, 2010. Vaccine. 2012;30:4676–4680. doi: 10.1016/j.vaccine.2012.04.083. [DOI] [PubMed] [Google Scholar]
- 7.Bangor-Jones RD, Dowse GK, Giele CM, van Buynder PG, Hodge MM, Whitty MM. A prolonged mumps outbreak among highly vaccinated Aboriginal people in the Kimberley region of Western Australia. Med J Aust. 2009;191:398–401. doi: 10.5694/j.1326-5377.2009.tb02850.x. [DOI] [PubMed] [Google Scholar]
- 8.Nelson GE, Aguon A, Valencia E, Oliva R, Guerrero ML, Reyes R, et al. Epidemiology of a mumps outbreak in a highly vaccinated island population and use of a third dose of measles-mumps-rubella vaccine for outbreak control: Guam 2009 to 2010. Pediatr Infect Dis J. 2013;32:374–380. doi: 10.1097/INF.0b013e318279f593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee KY, Lee HS, Hur JK, Kang JH, Lee BC. Clinical features of measles according to age in a measles epidemic. Scand J Infect Dis. 2005;37:471–475. doi: 10.1080/00365540510037803. [DOI] [PubMed] [Google Scholar]
- 10.Lee KY, Lee HS, Hur JK, Kang JH, Lee BC. The changing epidemiology of hospitalized pediatric patients in three measles outbreaks. J Infect. 2007;54:167–172. doi: 10.1016/j.jinf.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Kang HD, Lee KY, Cha SW, Yoon KY, Lee DJ, Han JW, et al. An outbreak of mumps in Taejon, Korea, 1998. Korean J Pediatr Infect Dis. 1999;6:239–244. [Google Scholar]
- 12.Choi KM. Reemergence of mumps. Korean J Pediatr. 2010;53:623–628. doi: 10.3345/kjp.2010.53.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho SY, Lee SY, Kang JH, Hwang HS. Mumps outbreak in Incheon, Korea, 2009. Korean J Pediatr. 2010;53:67–71. [Google Scholar]
- 14.Korea Centers for Disease Control and Prevention. Disease web statistics system [Internet] Cheongju: Korea Centers for Disease Control and Prevention; c2012. [cited 2013 Aug 10]. Available from: http://is.cdc.go.kr/nstat/index.jsp. [Google Scholar]
- 15.Korea Centers for Disease Control and Prevention. Infectious diseases surveillance yearbook, 2011. Cheongju: Korea Centers for Disease Control and Prevention; 2012. pp. 67pp. 82pp. 324–557. [Google Scholar]
- 16.Rubin SA, Qi L, Audet SA, Sullivan B, Carbone KM, Bellini WJ, et al. Antibody induced by immunization with the Jeryl Lynn mumps vaccine strain effectively neutralizes a heterologous wild-type mumps virus associated with a large outbreak. J Infect Dis. 2008;198:508–515. doi: 10.1086/590115. [DOI] [PubMed] [Google Scholar]
- 17.Peltola H, Kulkarni PS, Kapre SV, Paunio M, Jadhav SS, Dhere RM. Mumps outbreaks in Canada and the United States: time for new thinking on mumps vaccines. Clin Infect Dis. 2007;45:459–466. doi: 10.1086/520028. [DOI] [PubMed] [Google Scholar]
- 18.Barskey AE, Glasser JW, LeBaron CW. Mumps resurgences in the United States: a historical perspective on unexpected elements. Vaccine. 2009;27:6186–6195. doi: 10.1016/j.vaccine.2009.06.109. [DOI] [PubMed] [Google Scholar]
- 19.Sanz-Moreno JC, Limia-Sanchez A, Garcia-Comas L, Mosquera-Gutierrez MM, Echevarria-Mayo JE, Castellanos-Nadal A, et al. Detection of secondary mumps vaccine failure by means of avidity testing for specific immunoglobulin G. Vaccine. 2005;23:4921–4925. doi: 10.1016/j.vaccine.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Davidkin I, Valle M, Julkunen I. Persistence of anti-mumps virus antibodies after a two-dose MMR vaccination: a nine-year follow-up. Vaccine. 1995;13:1617–1622. doi: 10.1016/0264-410x(95)00064-8. [DOI] [PubMed] [Google Scholar]
- 21.Brown GC, Baublis JV, O'Leary TP. Development and duration of mumps fluorescent antibodies in various immunoglobulin fractions of human serum. J Immunol. 1970;104:86–94. [PubMed] [Google Scholar]
- 22.Gut JP, Spiess C, Schmitt S, Kirn A. Rapid diagnosis of acute mumps infection by a direct immunoglobulin M antibody capture enzyme immunoassay with labeled antigen. J Clin Microbiol. 1985;21:346–352. doi: 10.1128/jcm.21.3.346-352.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krause CH, Molyneaux PJ, Ho-Yen DO, McIntyre P, Carman WF, Templeton KE. Comparison of mumps-IgM ELISAs in acute infection. J Clin Virol. 2007;38:153–156. doi: 10.1016/j.jcv.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Rhim JW, Go EJ, Lee KY, Youn YS, Kim MS, Park SH, et al. Pandemic 2009 H1N1 virus infection in children and adults: a cohort study at a single hospital throughout the epidemic. Int Arch Med. 2012;5:13. doi: 10.1186/1755-7682-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee KY, Rhim JW, Kang JH. Hyperactive immune cells (T cells) may be responsible for acute lung injury in influenza virus infections: a need for early immune-modulators for severe cases. Med Hypotheses. 2011;76:64–69. doi: 10.1016/j.mehy.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Youn YS, Lee KY. Mycoplasma pneumoniae pneumonia in children. Korean J Pediatr. 2012;55:42–47. doi: 10.3345/kjp.2012.55.2.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee KY, Rhim JW, Kang JH. Kawasaki disease: laboratory findings and an immunopathogenesis on the premise of a "protein homeostasis system". Yonsei Med J. 2012;53:262–275. doi: 10.3349/ymj.2012.53.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Afzal MA, Dussupt V, Minor PD, Pipkin PA, Fleck R, Hockley DJ, et al. Assessment of mumps virus growth on various continuous cell lines by virological, immunological, molecular and morphological investigations. J Virol Methods. 2005;126:149–156. doi: 10.1016/j.jviromet.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 29.Vandana KE, Arunkumar G, Bairy I. Role of laboratory in rapid diagnosis of atypical mumps. Braz J Infect Dis. 2010;14:201–202. doi: 10.1590/s1413-86702010000200018. [DOI] [PubMed] [Google Scholar]
- 30.Bitsko RH, Cortese MM, Dayan GH, Rota PA, Lowe L, Iversen SC, et al. Detection of RNA of mumps virus during an outbreak in a population with a high level of measles, mumps, and rubella vaccine coverage. J Clin Microbiol. 2008;46:1101–1103. doi: 10.1128/JCM.01803-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hatchette T, Davidson R, Clay S, Pettipas J, Leblanc J, Sarwal S, et al. Laboratory diagnosis of mumps in a partially immunized population: The Nova Scotia experience. Can J Infect Dis Med Microbiol. 2009;20:e157–e162. doi: 10.1155/2009/493275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davidkin I, Jokinen S, Paananen A, Leinikki P, Peltola H. Etiology of mumps-like illnesses in children and adolescents vaccinated for measles, mumps, and rubella. J Infect Dis. 2005;191:719–723. doi: 10.1086/427338. [DOI] [PubMed] [Google Scholar]
- 33.Hersh BS, Fine PE, Kent WK, Cochi SL, Kahn LH, Zell ER, et al. Mumps outbreak in a highly vaccinated population. J Pediatr. 1991;119:187–193. doi: 10.1016/s0022-3476(05)80726-7. [DOI] [PubMed] [Google Scholar]
- 34.Tae BS, Ham BK, Kim JH, Park JY, Bae JH. Clinical features of mumps orchitis in vaccinated postpubertal males: a single-center series of 62 patients. Korean J Urol. 2012;53:865–869. doi: 10.4111/kju.2012.53.12.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogbuanu IU, Kutty PK, Hudson JM, Blog D, Abedi GR, Goodell S, et al. Impact of a third dose of measles-mumps-rubella vaccine on a mumps outbreak. Pediatrics. 2012;130:e1567–e1574. doi: 10.1542/peds.2012-0177. [DOI] [PubMed] [Google Scholar]