Abstract
BACKGROUND/OBJECTIVES
Acanthopanax sessiliflorus is a native Korean plant and used as traditional medicine or an ingredient in many Korean foods. The free radical theory of aging suggests that cellular oxidative stress caused by free radicals is the main cause of aging. Free radicals can be removed by cellular anti-oxidants.
MATERIALS/METHODS
Here, we examined the anti-oxidant activity of Acanthopanax sessiliflorus extract both in vitro and in vivo. Survival of nematode C. elegans under stress conditions was also compared between control and Acanthopanax sessiliflorus extract-treated groups. Then, anti-aging effect of Acanthopanax sessiliflorus extract was monitored in C. elegans.
RESULTS
Stem extract significantly reduced oxidative DNA damage in lymphocyte, which was not observed by leaves or root extract. Survival of C. elegans under oxidative-stress conditions was significantly enhanced by Acanthopanax sessiliflorus stem extract. In addition, Acanthopanax sessiliflorus stem increased resistance to other environmental stresses, including heat shock and ultraviolet irradiation. Treatment with Acanthopanax sessiliflorus stem extract significantly extended both mean and maximum lifespan in C. elegans. However, fertility was not affected by Acanthopanax sessiliflorus stem.
CONCLUSION
Different parts of Acanthopanax sessiliflorus have different bioactivities and stem extract have strong anti-oxidant activity in both rat lymphocytes and C. elegans, and conferred a longevity phenotype without reduced reproduction in C. elegans, which provides conclusive evidence to support the free radical theory of aging.
Keywords: Acanthopanax sessiliflorus, Caenorhabditis elegans, lifespan, stress response, fertility
INTRODUCTION
Many studies have focused on elucidating the mechanisms of aging and discovering possible lifespan-extending interventions. However, the causes and mechanisms of aging are not clearly known until now. Among theories of aging suggested so far, the most widely accepted is the free radical theory [1]. Free radicals are byproducts of cellular metabolism that cause oxidative damages to cellular macromolecules. Accumulated oxidative damage by free radicals with aging can lead to a functional decline in cells and tissues and eventually to death [2,3]. Reactive oxygen species (ROS), byproducts of mitochondrial respiration, are major free radicals in cells. Cellular ROS can be removed by both anti-oxidant enzymes, such as catalase and superoxide dismutase, and anti-oxidants, including glutathione, vitamin C, and vitamin E. The effect of dietary supplementation with anti-oxidants on aging has been widely studied in various organisms. Diallyl trisulfide, one of the pharmacologically active compounds contained in garlic, increases lifespan of Caenorhabditis elegans [4]. Green tea polyphenols increase lifespan and reduce the incidence rate of aging-related disease [5]. Baraquillo obtained from cocoa showed protective effects against oxidative stress and β-amyloid peptide toxicity [6]. In mice, middle-age onset dietary supplementation with vitamin E partially restores age-related alterations in gene expression profiling [7]. The expression of aging biomarkers, identified through genome-wide transcriptional profiling, is significantly affected by supplementation with anti-oxidants in tissues-pecific ways [8]. A recent study showed that electrolyzed-reduced water has strong anti-oxidant activity in vivo and can extend both mean and maximum lifespan of C. elegans [9,10].
Acanthopanax species are plants that inhabit in Korea, Japan, and China. Acanthopanax species have been used as a traditional treatment for various diseases including diabetes, tumors, and rheumatoid arthritis [11,12]. Chiisanoside is a major constituent of Acanthopanax species and has anti-inflammatory, anti-hepatotoxic, anti-diabetic, and anti-viral activities [13,14]. Mitogen-induced lymphocyte proliferation is inhibited by chiisanoside [13,14,15]. Extract of Acanthopanax species shows immune-stimulating activity and reduces body weight gain in high-fat diet mice [16,17,18]. Acanthopanax species functions as a strong anti-oxidant in vivo. Cellular DNA damage caused by oxidative stress and protein glycation are significantly reduced by Acanthopanax species [19,20]. Recent studies also suggest that Acanthopanax species can extend lifespan and delay onset of age-related diseases. The root of Acanthopanax senicosus reduces susceptibility to oxidative stress and confers a longevity phenotype in C. elegans [21]. Extract from Acanthopanax sessiliflorus (A. sessiliflorus) leaves significantly increases both mean and maximum lifespan without accompanying reduced reproduction [22]. Dopaminergic neurons in Parkinson's disease model mice are protected by the root and rhizome of Acanthopanax senicosus [23].
Here, we studied the effect of A. sessiliflorus stem extract on resistance to various environmental stresses and aging. Susceptibility to oxidative stress, heat stress, and ultraviolet irradiation was monitored in vivo using C. elegans as the model system. In addition, the lifespan-extending effect of A. sessiliflorus stem and the change in reproduction by administering A. sessiliflorus stem extract were examined.
MATERIALS AND METHODS
Oxidative DNA damage: Comet assay
Lymphocytes were isolated from male rats using Histopaque 1077 (Sigma-Aldrich, St. Louis, USA). The isolated lymphocytes were pre-treated with A. sessiliflorus stem extract for 30 min at 37℃ and then treated with 400 µM dieldrin for 1 h on ice. After treatment, the lymphocytes were mixed with 75 µL 0.7% low-melting-point agarose and added to slides pre-coated with 1% normal-melting-point agarose. After immersing in lysis solution (2.5 M NaCl, 100 mM EDTA, 10 mM Tris, 1% Triton X-100 and 10% DMSO) for 1 h at 4℃ in the dark, the slides were placed in an electrophoresis tank containing 300 mM NaOH and 10 mM Na2EDTA (pH 13.0) for 20 min. Electrophoresis was performed at 25 V/300 mA for 20 min at 4℃. The slides were washed with neutralizing buffer (0.4 M Tris·HCl, pH 7.5) three times and treated with ethanol for 5 min. The slides were stained with 10 µL 50 µM ethidium bromide. Fluorescence intensity was measured using a fluorescence microscope (Leica, Wetzlar, Germany) and Komet 5.5 software (Kinetic Imaging, UK). The olive tail moment was calculated as (tail.mean-head. mean) × tail% DNA/100. In total, 100 cells were randomly captured in each group. This protocol was approved by the Institutional Animal Care and Use Committee of Soonchunhyang University (SCH10_03_01).
Worm and sample preparation
The C. elegans wild type N2 strain was purchased from the C. elegans Genome Center (CGC, Minneapolis, USA). N2 worms were cultured on NGM (1.7% agar, 2.5 mg/mL peptone, 25 mM NaCl, 50 mM KH2PO4 (pH 6.0), 5 µg/mL, cholesterol, 1 mM CaCl2, and 1 mM MgSO4) plates containing E. coli OP50 as a food. Extract of A. sessiliflorus stem was provided by Sushin Ogapy Co., Ltd (Cheonan, Chungnam, Korea). A 200 g of A. sessiliflorus stem were extracted using hot water extraction with 1.5 L distilled water for 16 hs. Then, the extract was filtered through filter paper and concentrated using vacuum evaporation. The extract was dissolved in distilled water and sterilized using 0.2 µm cellulose acetate hydrophilic filters (Advantec, Tokyo, Japan).
Resistance to oxidative stress
Five 3-day-old N2 worms were placed on small NGM plates and allowed to lay eggs for 5 hs at 20℃. After eliminating all five adult worms from the plates, newly-laid eggs were grown for 3 days at 20℃. Sixty age-synchronized adult worms were transferred to fresh NGM plates containing five different concentrations (0, 50, 100, 500, and 1000 mg/L) of A. sessiliflorus stem extract. The next day, the worms were transferred to fresh NGM plates containing both A. sessiliflorus stem extract and 20 mM paraquat (Sigma-Aldrich, St. Louis, USA), which induces oxidative stress in the worms. We counted living and dead worms three times per day until all worms were dead. Worms not responding to mechanical stimulation were scored as dead. We performed two independent experiment.
Thermotolerance
Sixty age-synchronized worms (3-day-old) were transferred to NGM plates containing 500 mg/L of A. sessiliflorus extract and incubated at 20℃ for 24 hs. Then, the worms were shifted to 35℃ for 10 hs. After the heat stress, the worms were shifted back to 20℃. We monitored survival of the worms after a 24 hs of incubation at 20℃. The experiment was repeated three times independently. A P-value was calculated using the standard two-tailed Student's t-test.
Resistance to ultraviolet irradiation
Sixty age-synchronized worms were cultured in NGM plates containing 500 mg/L A. sessiliflorus extract for 24 hs. Then, the plates were incubated in a 254 nm-ultraviolet crosslinker (BLX-254, VILBER Lourmat Co., Torcy, France) for 1 min at 20 J/cm2/min. After ultraviolet irradiation, the plates were transferred back to the 20℃ incubator. The next day, living and dead worms were scored every day until all worms were dead. The experiment was repeated twice to confirm the results.
Lifespan assay
Sixty age-synchronized 3-day-old worms were transferred to fresh NGM plates containing the A. sessiliflorus extract and 12.5 µg/mL 5-fluoro-2'-deoxyruridine (FuDR) (Sigma-Aldrich, St. Louis, MO, USA), which prevents eggs from hatching. Thereafter, worms were transferred to fresh NGM plates with the A. sessiliflorus extract and FuDR every other day. The number of living and dead worms was recorded every day. Two independent experiments were performed.
Fertility assay
Five young-adult worms were allowed to lay eggs on NGM plates containing the A. sessiliflorus extract for 5 hs at 20℃. After a 2-day 20℃ incubation, a single adult worm was transferred to a fresh NGM plate containing the A. sessiliflorus extract. Ten worms were transferred individually to 10 fresh NGM plates containing the A. sessiliflorus extract every day until they stopped laying eggs. The plates including new eggs laid by each worm for 24 hs was incubated at 20℃ for 48 hs and the hatched worms were counted.
Statistical analysis
Comet data were analyzed using the SPSS package for Windows ver. 13 (SPSS Inc., Chicago, USA). The mean DNA damage values (olive tail moment) for each treatment were compared using one-way analysis of variance followed by Duncan's multiple range test [24]. We used the log-rank test to analyze resistance to oxidative stress, ultraviolet irradiation, and for the lifespan assay [25]. The log-rank test is a non-parametric Mantel-Cox test and widely used to compare two time-course survival curves. Statistical significance in the other experiments was assessed with the standard two-tailed Student's t-test. A P-value lower than 0.05 was regarded as significant.
RESULTS
Suppressive effects of A. sessiliflorus stem on oxidative DNA damage in lymphocyte
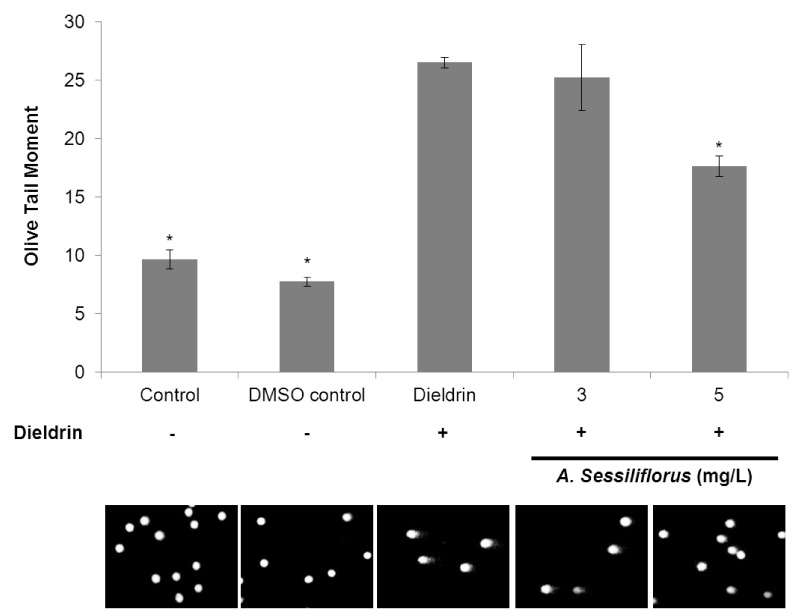
The anti-oxidant activity of A. sessiliflorus stem extract was tested in vitro using lymphocytes from rats. The olive tail moments resulting from damaged DNA increased by 2.7-fold under oxidative-stress conditions (9.7 ± 0.83 (mean ± SE) in the control and 26.5 ± 0.43 in the dieldrin-treated group). This finding indicates the oxidative DNA damage in lymphocytes increased significantly by the oxidative-stress inducer dieldrin (Fig. 1). The olive tail moments in the 3 and 5 µg/mL A. sessiliflorus stem extract-treated groups were 25.2 ± 0.87 and 17.6 ± 0.87, respectively. The 3 µg/mL A. sessiliflours stem extract treatment failed to significantly reduce the level of oxidative DNA damage in lymphocytes. However, 5 µg/mL of A. sessiliflours stem extract effectively suppressed DNA damage caused by oxidative stress (P < 0.05). These results suggest that A. sessiliflours stem extract acts as a strong anti-oxidant and decreases oxidative stress in cells.
Fig. 1.
Suppressive effect of A. sessiliflorus stem on oxidative DNA damage in lymphocytes. The inhibitory effect of in vitro supplementation of different concentrations of A. sessiliflorus stem extract on oxidative DNA damage in lymphocytes was determined by alkaline single-cell gel electrophoresis (comet assay). Pre-treatment with A. sessiliflorus stem extract significantly reduced oxidative DNA damage in lymphocytes. Bottom figures show that dieldrin treatment induced DNA tailing and 5 mg/L of A. sessiliflorus stem extract significantly reduced tailing of DNA. *significantly different from dieldrin-alone treated lymphocytes at P < 0.05 by Duncan's multiple range test.
A. sessiliflorus stem increases resistance to oxidative stress in C. elegans
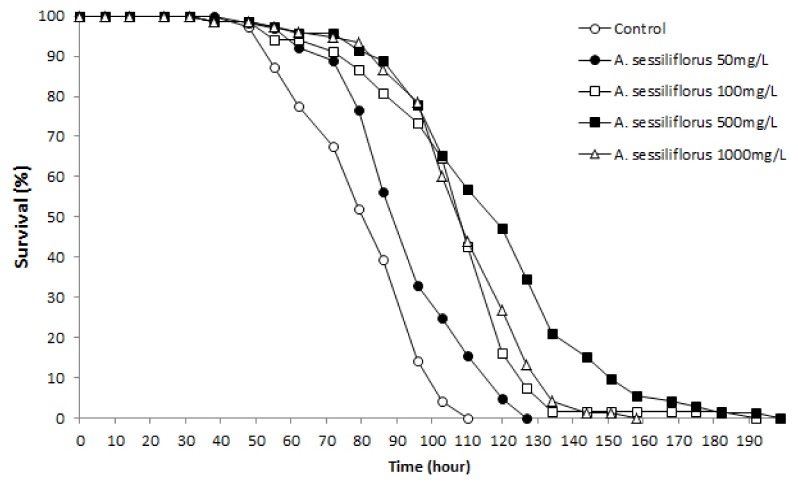
To test the anti-oxidant effect of A. sessiliflorus stem in vivo, we monitored time-course survival in C. elegans under oxidative stress. Resistance to oxidative stress increased significantly in response to A. sessiliflorus stem extract. Mean survival time was extended by all concentrations of A. sessiliflorus stem extract (Fig. 2). Mean survival time of the control was 74.1 h and that of the A. sessiliflorus stem extract treated groups extended to 85.9 h at 50 mg/L (P = 0.003), 99.6 h at 100 mg/L (P < 0.001), 111.9 h by 500 mg/L (P < 0.001), and 102.6 h by 1000 mg/L (P < 0.001). In the replicative experiment, only 500 mg/L of A. sessiliflorus stem extract showed significant extension in resistance to oxidative stress. Mean survival time increased from 55.5 to 72.1 h at 500 mg/L of A. sessiliflorus stem extract (P < 0.001). In both experiments, the most effective concentration of A. sessiliflorus stem extract was 500 mg/L (50.9% and 29.9% increase in the first and second experiment, respectively) and 1000 mg/L of A. sessiliflorus rather diminished the anti-oxidant activity of A. sessiliflorus stem extract (Table 1).
Fig. 2.
Effect of A. sessiliflorus stem extract on resistance to oxidative stress in C. elegans. Paraquat was used as the oxidative-stress inducer. Viability under the oxidative-stress condition increased significantly after treatment with different concentrations of A. sessiliflorus stem extract (P < 0.05). X-axis indicates the time exposed to paraquat.
Table 1.
The effect of A. sessiliflorus on resistance to oxidative stress
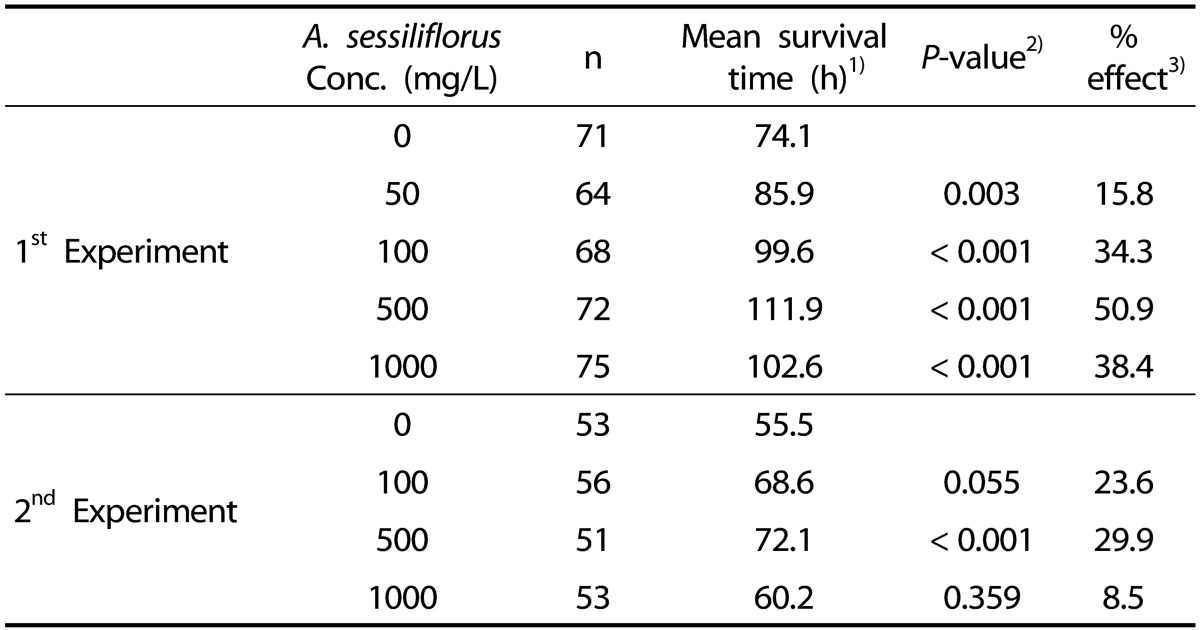
1)Data expressed as mean survival time after treating worms with 20 mM paraquat. Mean survival time is the time when 50% of worms are survived.
2)P-value was calculated using the long-rank test by comparing each concentration of A. sessiliflorus stem extract with control (0 mg/L of A. sessiliflorus stem extract).
3)% effect was calculated by (A-C)/C*100, where A is the mean survival time of C. elegans treated with each concentration of A. sessiliflorus stem extract, and C is the mean survival time of control.
Increased thermotolerance of C. elegans by A. sessiliflorus stem
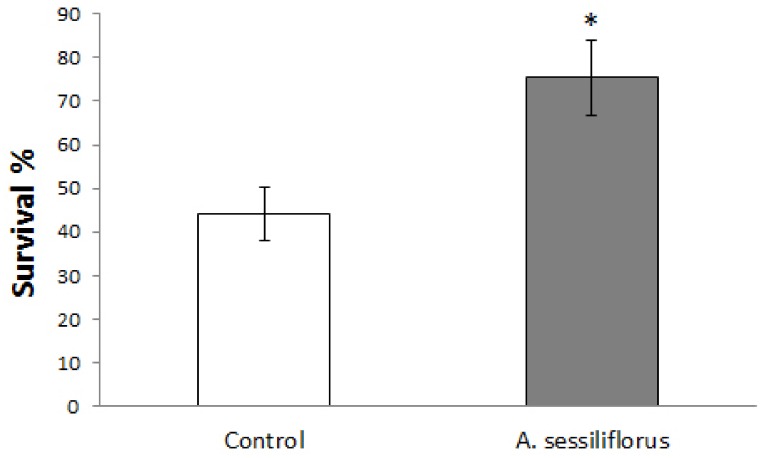
Next, we examined the effect of A. sessiliflorus stem extract on susceptibility to heat stress. C. elegans can usually be grown at room temperature (16 to 25℃), but temperatures higher than 25℃ confer heat stress to worms and causes premature death [26]. In this study, we applied a 35℃ heat stress to young adult worms for 10 hs and monitored the change in survival rate caused by A. sessiliflorus stem extract. Susceptibility to heat stress was decreased significantly following treatment with 500 mg/L A. sessiliflorus stem extract (Fig. 3). After 10 hs of heat stress, 44.2 ± 6.29% (mean ± SE) of the worms survived in the control. However, pre-treatment with A. sessiliflorus stem extract augmented survival rate up to 75.5 ± 8.70% (P = 0.043).
Fig. 3.
A. sessiliflorus stem extract increased thermotolerance in C. elegans. Y axis indicates the survival rate of each group after 10 hs of 35℃ heat stress. The 500 mg/L treatment of A. sessiliflorus stem extract was used in this test. Values are mean ± SE of three independent experiments (n = 60). *P < 0.05, significantly different from control.
Resistance to ultraviolet irradiation is extended by A. sessiliflorus stem
Resistance to ultraviolet irradiation increased significantly following treatment with 500 mg/L A. sessiliflorus stem extract (Fig. 4). Mean survival time of control worms was 5.98 days and that of worms pre-treated with A. sessiliflorus stem extract was 6.78 days (P = 0.022). In the replicative experiment, mean survival time increased from 3.57 to 4.20 days after treatment with A. sessliflorus stem extract (P = 0.054). Mean survival time increased by 13.3 and 16.7% in the first and second experiments, respectively, following pre-treatment with A. sessiliflorus stem extract.
Fig. 4.
Resistance to ultraviolet irradiation increased following treatment with A. sessiliflorus stem extract. Age-synchronized young adult worms were irradiated with 20 J/cm2/min ultraviolet for 1 min to determine the effect of A. sessiliflorus stem extract on resistance to ultraviolet irradiation. Survival after ultraviolet irradiation increased following treatment with A. sessiliflorus stem extract (P < 0.05). X-axis indicates days after UV irradiation.
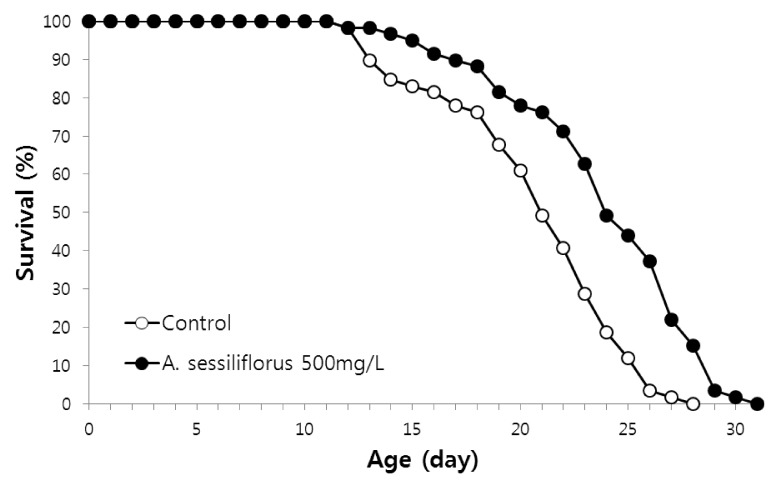
A. sessiliflorus stem extends lifespan in C. elegans
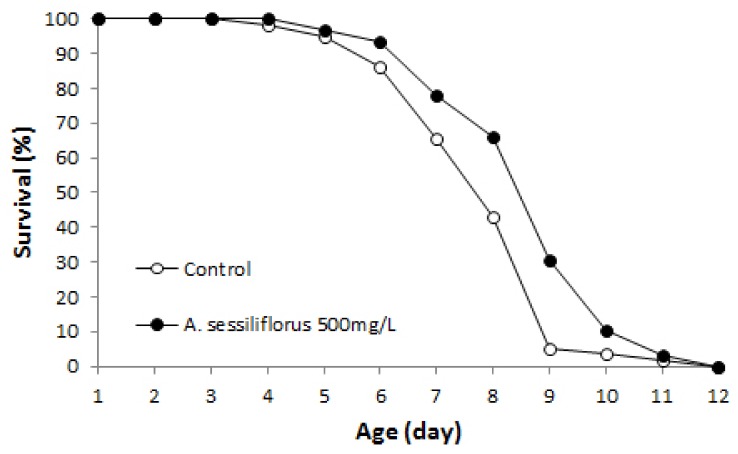
The free radical theory of aging suggests that cellular oxidative damage, mainly caused by ROS, plays an important role in normal aging and determines the lifespan of an organism [3,27]. Having observed increased resistance to oxidative stress by A. sessiliflorus stem extract, we next examined the effect of A. sessiliflorus stem extract (500 mg/L) on lifespan. The mean and maximum lifespans of the control was 19.7 and 27 days, whereas mean and maximum lifespans of worms treated with A. sessiliflorus stem extract were 20.6 and 30 days, respectively (Fig. 5 and Table 2). Mean lifespan increased 16.8% (P < 0.001). A replicative experiment also resulted in a significantly extended lifespan in the A. sessiliflorus stem extract-treated group. Mean lifespan was 20.6 days in the control and that of the A. sessiliflorus stem extract-treated group was 24.3 days (18% increase, P < 0.001). Thus, maximum lifespan was extended up to 5 days by A. sessiliflorus stem extract (Table 2).
Fig. 5.
Lifespan extension by A. sessiliflorus stem extract in C. elegans. The lifespans of C. elegans grown in normal NGM plate and an NGM plate containing 500 mg/L A. sessiliflorus stem extract was compared. Both mean and maximum lifespan increased significantly by A. sessiliflorus stem extract. Mean lifespans of animals grown in the control and A. sessiliflorus stem extract-treated NGM were 18.3 and 21.5 days, respectively. Mean lifespan of worms increased up to 18.8% following A. sessiliflorus stem extract treatment (P < 0.001). The log-rank test was employed for the statistical analysis of the survival curve.
Table 2.
Longevity effect of A. sessiliflorus in C. elegans
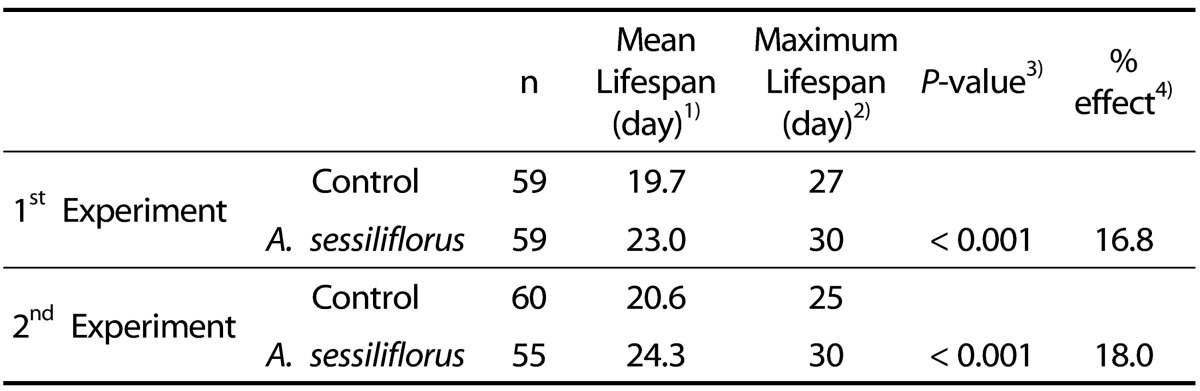
1)Mean lifespan was the day when 50% of worms used in the assay alive.
2)Maximum lifespan was the oldest age reached by the last surviving worm in each group.
3)P-value was calculated using the log-rank test by comparing the control and A. sessiliflorus stem extract-treated groups.
4)% effect was calculated by (A-C)/C*100, where A is the mean lifespan of C. elegans treated with A. sessiliflorus stem extract and C is the mean lifespan of control.
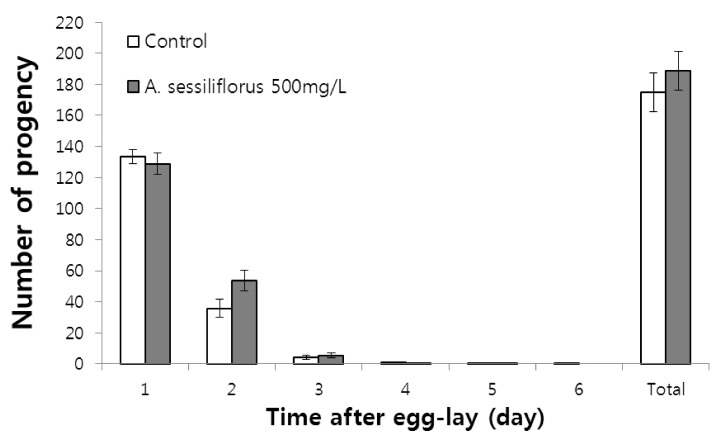
No reduction in fertility by A. sessiliflorus stem
Most of the lifespan-extension phenotypes found in C. elegans accompany reduced reproduction or delayed progeny production possibly due to allocating cellular resources to maintenance rather than to reproduction [28,29,30]. Thus, we examined fertility of worms treated with 500 mg/L A. sessiliflorus stem extract. As shown in Fig. 6, the total number of progeny produced throughout the gravid period was not different between the control and A. sessiliflorus stem extract-treated groups (175.0 ± 12.56 (mean ± SE) and 188.5 ± 12.56 in control and A. sessiliflorus stem extract-treated group, respectively). We also counted the number of progeny each day during the gravid period to determine the effect of A. sessiliflorus stem extract on reproductive duration. No difference in time-course distribution of progeny production was observed between the control and A. sessiliflorus stem extract-treated groups (Fig. 6).
Fig. 6.
Effect of A. sessiliflorus stem extract on fertility of C. elegans. Time-course distribution of fertility and total number of progeny produced by control and A. sessiliflorus stem extract-treated worms is shown. Total number of progeny produced was 175.0 ± 12.56 in the control and 188.5 ± 12.56 in the A. sessiliflorus stem extract-treated group (P = 0.412). Values are mean ± SE (P = 10).
DISCUSSION
A. sessiliflorus has been widely consumed as a functional food in Korea because of its various biological activities [11,31,32]. A. sessiliflorus juice has been commercialized for its strong anti-diabetic, liver protecting, and blood-pressure lowering effects [33]. In this study, we examined the effect of A. sessiliflorus stem extract on response to various environmental stresses. Resistance to oxidative stress increased significantly following pre-treatment with A. sessiliflorus stem extract both in vitro and in vivo. Oxidative DNA damage reduced markedly in rat lymphocytes. In C. elegans, survival under oxidative-stress conditions increased up to 50% following treatment of worms with A. sessiliflorus stem extract. These findings indicate that A. sessiliflorus acts as a strong ROS scavenger in cells. Oxidative stress results from accumulated ROS with aging and is one of the major causal factors of many age-related diseases, such as Alzheimer's disease, Parkinson's disease, and heart failure [34,35]. Our data support the possibility that A. sessiliflorus stem could be used as a preventive natural compound for those age-related diseases.
Changes in response to other environmental stresses were also examined. Thermotolerance of C. elegans increased significantly by A. sessiliflorus stem extract. In addition, worms pre-treated with A. sessiliflorus stem extract survived longer after ultraviolet irradiation compared to controls. A recent study showed that anti-oxidant electrolyzed-reduced water also confers increased resistance to heat shock and ultraviolet irradiation [9]. Further studies are needed to determine the underlying mechanisms involved in increased resistance to environmental stresses by A. sessiliflorus stem.
Importantly, only stem extract significantly reduced oxidative DNA damage in rat lymphocytes: treatment of leaves or root extract has no effect on DNA damage caused by oxidative stress in lymphocytes (unpublished data). The effect on resistance to UV irradiation was also different among leaves, stem, and root extract: the effect of stem extract is lower than that of leaves or root extract (unpublished data). These findings suggest that the different parts of A. sessiliflorus have different bioactivities and it is worth to study the effect of different parts separately. Further studies regarding the effect of combinations of extract from different parts of A. sessiliflorus on stress response and lifespan seem to be necessary to figure out whether there is any additional or synergistic effect by different parts of the plant.
The role of ROS in normal aging and the effect of antioxidants on lifespan have been reported in various model organisms [36,37,39]. However, whether ROS is the determining factor in aging and lifespan remains controversial. Cognitive impairment observed in aged rats is prevented by vitamin E supplementation, and centenarians are characterized as having the highest levels of vitamin A and E in plasma [40]. In contrast, disease incidence and lifespan do not change following vitamin E supplementation in mice [41]. However, a high dose of vitamin E supplementation (5000 mg/kg) improves brain mitochondrial function and leads to lifespan extension in mice [42]. Resveratrol, a polyphenol compound found in red wine, is a strong anti-oxidant and increases lifespan in rotifers, C. elegans, and Drosophila [43]. However, the effect of resveratrol on lifespan in mammals is still elusive [44]. In the present study, we observed a significant lifespan-extending effect of A. sessiliflorus stem extract in C. elegans. However, lifespan assay alone was not enough to support anti-aging effect of stem extract. Additional data, such as effects on biomarkers of aging and changes in lifespan of long-lived mutants, are necessary to prove anti-aging effects of A. sessiliflorus stem extract. We suggest that the longevity phenotype conferred by A. sessiliflorus stem extract could be due to its anti-oxidant activity. Thus, the lifespan-extending effects of A. sessiliflours stem in mammals needs to be elucidated to support the free radical theory of aging.
The disposable soma theory of aging focuses on the importance of the distribution of limited cellular resources between reproduction and maintenance of somatic cells with aging [45]. Many lifespan-extending mutations in C. elegans also induce reduced or delayed progeny production [28,29,30]. The long-lived age-1 mutant has an extended reproductive period, and complete knockout of germ cells results in a longer lifespan [30,46]. These long-lived strains seem to re-locate their cellular resources from reproduction to somatic maintenance. To our surprise, the total number of progeny and the gravid period were not altered by A. sessiliflorus stem extract in this study. This finding indicates that the lifespan-extending effect of A. sessiliflorus is not accompanied by reduced reproduction unlike other long-lived mutants in C. elegans. We have provided convincing evidence that A. sessiliflorus, a native Korean plant, has strong anti-oxidant and stress-resisting activities and can extend lifespan without a reduced reproduction in C. elegans.
Footnotes
This research was supported by the Soonchunhyang University Research Fund and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0022429).
References
- 1.Harman D. The free radical theory of aging. Antioxid Redox Signal. 2003;5:557–561. doi: 10.1089/152308603770310202. [DOI] [PubMed] [Google Scholar]
- 2.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 3.Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 4.Powolny AA, Singh SV, Melov S, Hubbard A, Fisher AL. The garlic constituent diallyl trisulfide increases the lifespan of C. elegans via skn-1 activation. Exp Gerontol. 2011;46:441–452. doi: 10.1016/j.exger.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitani K, Yokozawa T, Osawa T. Interventions in aging and age-associated pathologies by means of nutritional approaches. Ann N Y Acad Sci. 2004;1019:424–426. doi: 10.1196/annals.1297.075. [DOI] [PubMed] [Google Scholar]
- 6.Martorell P, Bataller E, Llopis S, Gonzalez N, Alvarez B, Montón F, Ortiz P, Ramón D, Genovés S. A cocoa peptide protects Caenorhabditis elegans from oxidative stress and β-amyloid peptide toxicity. PLoS One. 2013;8:e63283. doi: 10.1371/journal.pone.0063283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park SK, Page GP, Kim K, Allison DB, Meydani M, Weindruch R, Prolla TA. alpha- and gamma-Tocopherol prevent age-related transcriptional alterations in the heart and brain of mice. J Nutr. 2008;138:1010–1018. doi: 10.1093/jn/138.6.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park SK, Kim K, Page GP, Allison DB, Weindruch R, Prolla TA. Gene expression profiling of aging in multiple mouse strains: identification of aging biomarkers and impact of dietary antioxidants. Aging Cell. 2009;8:484–495. doi: 10.1111/j.1474-9726.2009.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park SK, Kim JJ, Yu AR, Lee MY, Park SK. Electrolyzed-reduced water confers increased resistance to environmental stresses. Mol Cell Toxicol. 2012;8:241–247. [Google Scholar]
- 10.Park SK, Park SK. Electrolyzed-reduced water increases resistance to oxidative stress, fertility, and lifespan via insulin/IGF-1-like signal in C. elegans. Biol Res. 2013;46:147–152. doi: 10.4067/S0716-97602013000200005. [DOI] [PubMed] [Google Scholar]
- 11.Jung BS, Shin MK. Hyang Yak Dae Sa Jeon. 3rd ed. Seoul: Young Lim Sa Publisher; 2003. [Google Scholar]
- 12.Fujikawa T, Yamaguchi A, Morita I, Takeda H, Nishibe S. Protective effects of Acanthopanax senticosus Harms from Hokkaido and its components on gastric ulcer in restrained cold water stressed rats. Biol Pharm Bull. 1996;19:1227–1230. doi: 10.1248/bpb.19.1227. [DOI] [PubMed] [Google Scholar]
- 13.Bae EA, Yook CS, Oh OJ, Chang SY, Nohara T, Kim DH. Metabolism of chiisanoside from Acanthopanax divaricatus var. albeofructus by human intestinal bacteria and its relation to some biological activities. Biol Pharm Bull. 2001;24:582–585. doi: 10.1248/bpb.24.582. [DOI] [PubMed] [Google Scholar]
- 14.Jung HJ, Nam JH, Choi J, Lee KT, Park HJ. Antiinflammatory effects of chiisanoside and chiisanogenin obtained from the leaves of Acanthopanax chiisanensis in the carrageenan- and Freund's complete adjuvant-induced rats. J Ethnopharmacol. 2005;97:359–367. doi: 10.1016/j.jep.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 15.Kim YO, Cho DH, Chung HJ, Kim JH, Chang SY, Yook CS, Yang KS, Oh OJ. Effects of lupane-triterpenoids on mitogen-induced proliferation of lymphocytes. Yakhak Hoeji. 1999;43:208–213. [Google Scholar]
- 16.Jeong SC, Jeong YT, Yang BK, Song CH. Chemical characteristics and immuno-stimulating properties of biopolymers extracted from Acanthopanax sessiliflorus. J Biochem Mol Biol. 2006;39:84–90. doi: 10.5483/bmbrep.2006.39.1.084. [DOI] [PubMed] [Google Scholar]
- 17.Jeong SC, Yang BK, Jeong YT, Rao KS, Song CH. Isolation and characterization of biopolymers extracted from the bark of Acanthopanax sessiliflorus and their anticomplement activity. J Microbiol Biotechnol. 2007;17:21–28. [PubMed] [Google Scholar]
- 18.Yoshizumi K, Hirano K, Ando H, Hirai Y, Ida Y, Tsuji T, Tanaka T, Satouchi K, Terao J. Lupane-type saponins from leaves of Acanthopanax sessiliflorus and their inhibitory activity on pancreatic lipase. J Agric Food Chem. 2006;54:335–341. doi: 10.1021/jf052047f. [DOI] [PubMed] [Google Scholar]
- 19.Ryu AR, Kim JH, Lee MY. Suppressive effect of Acanthopanax sessiliflorus extract on the DNA and cell damage by Dieldrin. Korean J Med Crop Sci. 2012;20:245–250. [Google Scholar]
- 20.Kim HY, Kim K. Protein glycation inhibitory and antioxidative activities of some plant extracts in vitro. J Agric Food Chem. 2003;51:1586–1591. doi: 10.1021/jf020850t. [DOI] [PubMed] [Google Scholar]
- 21.Wiegant FA, Surinova S, Ytsma E, Langelaar-Makkinje M, Wikman G, Post JA. Plant adaptogens increase lifespan and stress resistance in C. elegans. Biogerontology. 2009;10:27–42. doi: 10.1007/s10522-008-9151-9. [DOI] [PubMed] [Google Scholar]
- 22.Kim CK, Park SK. Effect of Acanthopanax sessiliflorus extracts on stress response and aging in Caenorhabditis elegans. Food Sci Technol Res. 2013;19:439–444. [Google Scholar]
- 23.Liu SM, Li XZ, Huo Y, Lu F. Protective effect of extract of Acanthopanax senticosus Harms on dopaminergic neurons in Parkinson's disease mice. Phytomedicine. 2012;19:631–638. doi: 10.1016/j.phymed.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Park SK, Lee MY. Suppressive effects of various antioxidants on melamine-induced oxidative DNA damage in human lymphocytes. Mol Cell Toxicol. 2009;5:243–249. [Google Scholar]
- 25.Peto R, Peto J. Asymptotically efficient rank invarient test procedures. J R Stat Soc Ser A Stat Soc. 1972;135:185–207. [Google Scholar]
- 26.Treinin M, Shliar J, Jiang H, Powell-Coffman JA, Bromberg Z, Horowitz M. HIF-1 is required for heat acclimation in the nematode Caenorhabditis elegans. Physiol Genomics. 2003;14:17–24. doi: 10.1152/physiolgenomics.00179.2002. [DOI] [PubMed] [Google Scholar]
- 27.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hughes SE, Evason K, Xiong C, Kornfeld K. Genetic and pharmacological factors that influence reproductive aging in nematodes. PLoS Genet. 2007;3:e25. doi: 10.1371/journal.pgen.0030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larsen PL, Albert PS, Riddle DL. Genes that regulate both development and longevity in Caenorhabditis elegans. Genetics. 1995;139:1567–1583. doi: 10.1093/genetics/139.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gems D, Sutton AJ, Sundermeyer ML, Albert PS, King KV, Edgley ML, Larsen PL, Riddle DL. Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics. 1998;150:129–155. doi: 10.1093/genetics/150.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yook CS, Rho YS, Seo SH, Leem JY, Han DR. Chemical components of Acanthopanax divaricatus and anticancer effect in leaves. Yakhak Hoeji. 1996;40:251–261. [Google Scholar]
- 32.Hahn DR, Kim CJ, Kim JH. A study on the chemical constituents of Acanthopanax koreanum Nakai and its pharmaco-biological activities. Yakhak Hoeji. 1985;29:357–361. [Google Scholar]
- 33.Fu J, Fu J, Yuan J, Zhang N, Gao B, Fu G, Tu Y, Zhang Y. Anti-diabetic activities of Acanthopanax senticosus polysaccharide (ASP) in combination with metformin. Int J Biol Macromol. 2012;50:619–623. doi: 10.1016/j.ijbiomac.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 34.Liu XZ, Yan D. Ageing and hearing loss. J Pathol. 2007;211:188–197. doi: 10.1002/path.2102. [DOI] [PubMed] [Google Scholar]
- 35.Lee CK, Weindruch R, Prolla TA. Gene-expression profile of the ageing brain in mice. Nat Genet. 2000;25:294–297. doi: 10.1038/77046. [DOI] [PubMed] [Google Scholar]
- 36.Suthammarak W, Somerlot BH, Opheim E, Sedensky M, Morgan PG. Novel interactions between mitochondrial superoxide dismutases and the electron transport chain. Aging Cell. 2013;12:1132–1140. doi: 10.1111/acel.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lagouge M, Larsson NG. The role of mitochondrial DNA mutations and free radicals in disease and ageing. J Intern Med. 2013;273:529–543. doi: 10.1111/joim.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ivanova DG, Yankova TM. The free radical theory of aging in search of a strategy for increasing life span. Folia Med (Plovdiv) 2013;55:33–41. doi: 10.2478/folmed-2013-0003. [DOI] [PubMed] [Google Scholar]
- 39.Goto S. Evidence for and against anti-aging effects based on model animal studies. Nihon Rinsho. 2009;67:1337–1340. [PubMed] [Google Scholar]
- 40.Mecocci P, Polidori MC, Troiano L, Cherubini A, Cecchetti R, Pini G, Straatman M, Monti D, Stahl W, Sies H, Franceschi C, Senin U. Plasma antioxidants and longevity: a study on healthy centenarians. Free Radic Biol Med. 2000;28:1243–1248. doi: 10.1016/s0891-5849(00)00246-x. [DOI] [PubMed] [Google Scholar]
- 41.Lipman RD, Bronson RT, Wu D, Smith DE, Prior R, Cao G, Han SN, Martin KR, Meydani SN, Meydani M. Disease incidence and longevity are unaltered by dietary antioxidant supplementation initiated during middle age in C57BL/6 mice. Mech Ageing Dev. 1998;103:269–284. doi: 10.1016/s0047-6374(98)00048-7. [DOI] [PubMed] [Google Scholar]
- 42.Navarro A, Gómez C, Sánchez-Pino MJ, González H, Bández MJ, Boveris AD, Boveris A. Vitamin E at high doses improves survival, neurological performance, and brain mitochondrial function in aging male mice. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1392–R1399. doi: 10.1152/ajpregu.00834.2004. [DOI] [PubMed] [Google Scholar]
- 43.Bass TM, Weinkove D, Houthoofd K, Gems D, Partridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech Ageing Dev. 2007;128:546–552. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 44.Agarwal B, Baur JA. Resveratrol and life extension. Ann N Y Acad Sci. 2011;1215:138–143. doi: 10.1111/j.1749-6632.2010.05850.x. [DOI] [PubMed] [Google Scholar]
- 45.Kirkwood TB. Evolution of ageing. Nature. 1977;270:301–304. doi: 10.1038/270301a0. [DOI] [PubMed] [Google Scholar]
- 46.Hsin H, Kenyon C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature. 1999;399:362–366. doi: 10.1038/20694. [DOI] [PubMed] [Google Scholar]