Abstract
Jak2 is a hormone-receptor-coupled kinase that mediates the tyrosine phosphorylation and activation of signal transducers and activators of transcription (Stat). The biological relevance of Jak2-Stat signaling in hormone-responsive adult tissues is difficult to investigate since Jak2 deficiency leads to embryonic lethality. We generated Jak2 conditional knockout mice to study essential functions of Jak2 during mammary gland development. The mouse mammary tumor virus-Cre-mediated excision of the first coding exon resulted in a Jak2 null mutation that uncouples signaling from the prolactin receptor (PRL-R) to its downstream mediator Stat5 in the presence of normal and supraphysiological levels of PRL. Jak2-deficient females were unable to lactate as a result of impaired alveologenesis. Unlike Stat5a knockouts, multiple gestation cycles could not reverse the Jak2-deficient phenotype, suggesting that neither other components of the PRL-R signaling cascade nor other growth factors and their signal transducers were able to compensate for the loss of Jak2 function to activate Stat5 in vivo. A comparative analysis of Jak2-deficient mammary glands with transplants from Stat5a/b knockouts revealed that Jak2 deficiency also impairs the pregnancy-induced branching morphogenesis. Jak2 conditional mutants therefore resemble PRL-R knockouts more closely, which suggested that Jak2 deficiency might affect additional PRL-R downstream mediators other than Stat5a and Stat5b. To address whether Jak2 is required for the maintenance of PRL-responsive, differentiating alveolar cells, we utilized a transgenic strain that expresses Cre recombinase under regulatory elements of the whey acidic protein gene (Wap). The Wap-Cre-mediated excision of Jak2 resulted in a negative selection of differentiated alveolar cells, suggesting that Jak2 is required not only for the proliferation and differentiation of alveolar cells but also for their maintenance during lactation.
Prolactin (PRL), in synergy with other peptide and steroid hormones as well as local growth factors, controls the proliferation and differentiation of mammary epithelial cells (12, 13, 38). PRL seems to play a central role in this process, as it acts as a mitogen and a morphogen (15, 32). Upon dimerization of its receptor, PRL is suggested to signal through Janus kinases (Jak) and signal transducers and activators of transcription (Stat), in particular Jak2, Stat5a, and Stat5b (14, 34), as well as Ras/mitogen-activated protein kinase (6, 8), phosphatidylinositol 3-kinase-AKT, and the phospholipase C-protein kinase C pathway (10, 26). Loss-of-function studies with mouse models provide new insights into biologically relevant functions of individual components of the PRL receptor (PRL-R) signaling cascade. Surprisingly, the lack of PRL (15), the PRL-R (32), and Stat5a (25, 37) exhibited an unexpected level of specificity during mammogenesis. Indispensable functions of these proteins are restricted to pregnancy-associated changes in the mammary gland, and all three knockout models (PRL−/−, PRL-R−/−, and Stat5a−/− mice) lack alveolar proliferation and differentiation. Based on this line of evidence, it is apparent that Stat5a mediates essential, biologically relevant functions of PRL signaling in normal mammary epithelial cells. It is known from studies using in vitro model systems that upon ligand-induced aggregation of the receptor, Jak2 is stably recruited to the receptor complex. Jak2 then undergoes autophosphorylation and phosphorylates the PRL-R on various tyrosine residues, thus generating binding sites for several SH2 domain-bearing signaling molecules including Stat5a and Stat5b (reviewed in reference 14). The subsequent phosphorylation of Stat5a and Stat5b by Jak2 triggers their homo- and heterodimerization and translocation into the nucleus, where they bind to specific DNA sequence motifs and activate transcription of target genes such as β-casein and the whey acidic protein gene (Wap).
Although the biological relevance of Stat5 activation in response to PRL signaling has been demonstrated with knockout mice, the enzymatic coupling between the PRL-R and Stat5 in vivo and biologically relevant functions of Jak2 are difficult to elucidate due to the prenatal lethality of Jak2 conventional knockouts (29, 33). Pivotal studies with conventional knockout mice revealed that the biological functions of Jak2 are pleiotropic, which is consistent with its suggested coupling to multiple cytokine receptors in specific target cells (reviewed in reference 20). Specifically, Jak2 is suggested to mediate signals through a variety of single-chain receptors for ligands such as PRL, growth hormone (GH), erythropoietin (EPO), and thrombopoietin as well as to the multichain interleukin 3 receptor family (e.g., interleukin 3 receptor and granulocyte-macrophage colony-stimulating factor receptor) and members of the gp130 receptor family. To define a role for Jak2 during mammary development, Shillingford and coworkers (35) recently reported the first successful attempt to transplant mammary gland anlagen from Jak2-deficient embryos into wild-type recipients. These initial studies revealed that Jak2-deficient mammary epithelial cells showed a reduced proliferation index in animals treated with estrogen and progesterone, and the lack of Jak2 impaired the specification of secretory epithelial cells. Since alveolar precursors do not proliferate, it is evident that this transplantation model lacks the entire alveolar lineage. Consequently, transplantation models do not allow the examination of essential functions of Jak2 in differentiating epithelial subtypes during pregnancy and lactation. Moreover, in transplanted epithelia of postpartum females, differentiated cells immediately inactivate Stat5, upregulate Stat3, and initiate proapoptotic signaling pathways (e.g., expression of Bax and Bcl-xs) due to the lack of a nipple and, consequently, milk stasis (22).
To identify essential functions of Jak2 at defined stages of mammary development, we have generated two types of mamma-specific knockout mice on the basis of the Cre-lox system. The mouse mammary tumor virus (MMTV)-Cre-mediated excision of the first coding exon resulted in a Jak2 null mutation, which is incapable of activating Stat5 in mammary epithelial cells in response to PRL signaling. Like in the transplant model, the MMTV-Cre-mediated deletion of Jak2 from ductal progenitors had no effect on ductal elongation and branching morphogenesis in nulliparous females. Mutants were, however, unable to lactate as a result of impaired alveolar specification during early gestation. Unlike the Stat5a knockout (23), multiple gestation cycles did not reverse this abnormal phenotype, suggesting that neither other components of the PRL-R signaling cascade nor other growth factors and their signal transducers, such as Src and ErbB family members (18, 31), were able to compensate for the loss of Jak2 function to activate Stat5 in vivo. To study the function of Jak2 specifically in the alveolar lineage, we utilized Wap-Cre transgenic mice to delete the Jak2 gene specifically from PRL-responsive, differentiating alveolar cells during the last phase of pregnancy and lactation. This approach revealed a strong negative selection of differentiated alveolar cells, suggesting that Jak2 is required not only for the proliferation and initiation of differentiation of immature alveolar cells but also for their maintenance at the differentiated stage during lactation.
MATERIALS AND METHODS
Mouse models and genotyping protocols.
Technical details about the cloning and sequencing of the Jak2 genomic locus (GenBank accession number AY157991), the construction of the targeting construct, and the production of genetically engineered mice with two Jak2 conditional knockout alleles (Jak2fl/fl or Jak2tm1Kuw) or the derived null alleles will be described elsewhere (A. Krempler, submitted for publication). The PCR protocols for genotyping Wap-Cre, MMTV-Cre, and Rosa-lacZ mice have been described previously (36, 39, 41). The Stat5a/b double knockout mice (37) were a kind gift from James N. Ihle (St. Jude Children's Research Hospital, Memphis, Tenn.) to K.U.W. Athymic nude mice (NCr strain, National Cancer Institute) were used for transplantation studies. All animals used in the studies were treated humanely and in accordance with federal guidelines and institutional policies.
Transplantation method.
The surgical procedures for clearing the fat pad of 3-week-old female mice and the method of implanting tissue fragments and cell suspensions have been described recently in great detail (43). Briefly, random fragments (∼1.0 mm3) of mammary epithelium were taken from nulliparous Stat5a/b−/− females and implanted into the cleared fat pad of 3-week-old recipients (athymic NCr-nu mice). The recipients were kept as nulliparous virgins for 12 weeks to provide sufficient time for the transplanted epithelium to form a ductal tree. Subsequently, the transplant-carrying females were bred with a heterozygous athymic NCr-nu male, and mammary glands were taken from the recipients immediately after delivering the young. The mammary glands were prepared as whole mounts and stained as described below.
PRL injections into mice.
Ovine PRL (NIDDK-oPRL-21, AFP-10692C) was kindly provided by A. F. Parlow under the sponsorship of the National Hormone and Pituitary Program, National Institute of Diabetes and Digestive and Kidney Diseases (National Institutes of Health, Bethesda, Md.). Nulliparous Jak2-deficient mice and their wild-type controls were injected intraperitoneally with approximately 100 μg of PRL (5 μg per g of body weight) or plain saline solution. Mammary glands were taken 30 min later and fixed overnight at 4°C in 10% buffered formalin. The next day, the tissues were dehydrated, sectioned, and stained with an anti-phospho-STAT5a/b antibody.
Carmine alum and X-Gal staining of mammary whole mounts.
Whole mounts were prepared and stained in carmine alum as described previously (42). A standard X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining procedure (39) was performed to detect β-galactosidase activity in mammary gland whole mounts. Tissues were incubated overnight at 30°C in X-Gal staining buffer containing a final concentration of 1 mg of X-Gal/ml. X-Gal-stained mammary whole mounts were postfixed in 10% buffered formalin (Fisher Scientific Company), dehydrated to 100% ethanol, and placed overnight in HistoClear (National Diagnostics, Atlanta, Ga.) before microscopic analysis. For the examination of tissue sections, mammary glands were dehydrated following the X-Gal procedure, embedded in paraffin, and sectioned.
Immunohistochemistry.
For bromodeoxyuridine labeling, TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) assay, or general immunohistochemistry, mammary glands were fixed overnight at 4°C in 10% buffered formalin (Fisher Scientific Company). Paraffin sections were processed and stained with anti-Stat5 and anti-phospho-Stat5a/b (Tyr694/Tyr699) antibodies as described previously (30). The anti-Stat5a-specific antibody was a kind gift from Lothar Hennighausen (National Institutes of Health). The antibromodeoxyuridine mouse monoclonal antibody was a component of the cell proliferation kit from Amersham Pharmacia Biotech. Biotinylated or Alexa Fluor 488-conjugated secondary antibodies were used to detect the phosphorylated and/or inactive form of Stat5. The labeling of apoptotic nuclei with ChromaTide Alexa Fluor 488-5-dUTP was performed as described previously (19). Slides were counterstained briefly with DAPI (4′,6′-diamidino-2-phenylindole) or hematoxylin to visualize nuclei. Bright-field and fluorescence images of histological slides were taken on a Nikon Labophot microscope equipped with a Nikon Coolpix 990 camera as well as fluorescein isothiocyanate and DAPI filter sets.
Southern blot of recombined tissues.
Genomic DNA from tissues was prepared by using standard phenol-chloroform extraction. Fifteen micrograms was digested with EcoRI at 37°C overnight and separated on a 0.8% agarose gel. The DNA was denatured and blotted onto a nylon membrane (Genescreen plus; NEN) and hybridized with a 32P-labeled probe. The 3′ external probe, approximately 643 bp in size, was generated by PCR using the 1724-1725 primer pair (5′-CCA GGT TCA TAC ATC TCA AAA CC-3′ and 5′-GTC ACA GTA GTC CTT TGT CAG G-3′). Membranes were washed in 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) buffer containing sodium dodecyl sulfate and exposed for 16 h to a Kodak XOMAT-AR film.
Cell culture.
Mouse embryonic fibroblasts (MEFs) were derived from 12.5-day-old embryos and grown at 37°C with 5% CO2 until confluence in 75-cm2 culture flasks in Dulbecco's modified Eagle's medium (Biofluids, Rockville, Md.) containing 10% fetal calf serum (Atlanta Biologicals, Norcross, Ga.), 2 mM l-glutamine, 0.1 mM nonessential amino acids, 10 μg of gentamicin/ml, and penicillin-streptomycin (50 IU/ml and 50 μg/ml, respectively). Cells were starved in serum-free Dulbecco's modified Eagle's medium for 16 h and then incubated with or without ovine 20 nM GH for 15 min at 37°C. Cells were harvested, frozen on dry ice, and stored at −80°C before being used in protein immunoblotting experiments.
Protein analysis.
Embryos (12.5 days old) were pooled into groups of three and homogenized on ice in a Triton X-100-based lysis buffer with protease inhibitors and processed as described previously (30). Frozen cell pellets from MEFs were lysed directly in the same buffer. Clarified tissue lysates corresponding to 2.0 mg of total protein were immunoprecipitated with 4 μg of a Jak2 polyclonal rabbit antibody (Upstate Biotechnology, Lake Placid, N.Y.) per ml. Clarified cell lysates were immunoprecipitated with 4 μg of either the Jak2 antibody or a panStat5a/b antibody (AX55; Advantex Bioreagents, Conroe, Tex.) per ml. Immunoprecipitates or whole-cell extracts were resolved by sodium dodecyl sulfate-7.5% polyacrylamide gel electrophoresis and immunoblotted with either phosphoStat5a/b (Y694/9) antibody (0.5 μg/ml; AX1; Advantex Bioreagents), panStat5a/b antibody (2 μg/ml), phosphotyrosine antibody (1 μg/ml; 4G10; Upstate Biotechnology), or Jak2 antibody (1:3,000). Horseradish peroxidase-coupled secondary antibodies and enhanced chemiluminescence (Amersham, Piscataway, N.J.) were used for detection.
RESULTS
The conditional deletion of Jak2 results in a true null mutation.
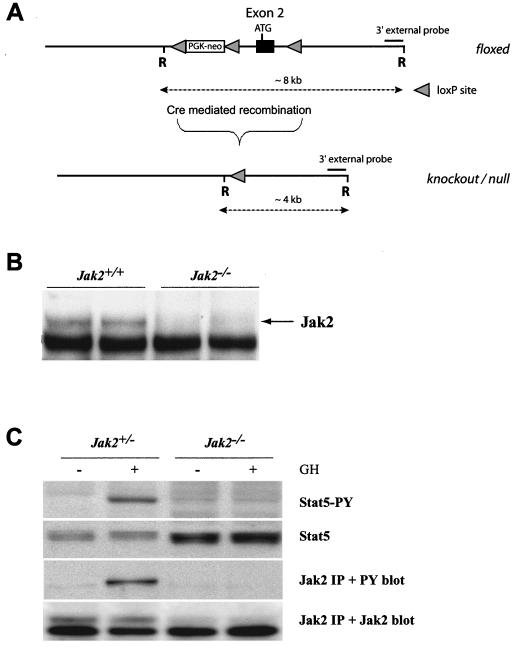
We generated conditional knockout mice in which the first coding exon of Jak2 can be deleted through Cre-mediated recombination (Fig. 1A). A detailed description of the construction of the targeting vector and phenotypic consequences of the deletion of Jak2 in the germ line will be described elsewhere (Krempler et al., submitted). In brief, the Jak2 conditional knockout allele contains a floxed PGK-neo selectable marker approximately 600 bp upstream of the first coding exon (exon 2). A third loxP site was placed 500 bp downstream of this exon. Homozygous mutant mice that carried two floxed Jak2 alleles developed normally until adulthood. Both males and females were fertile, and they exhibited no phenotypic abnormalities, suggesting that the insertion of the selectable marker upstream of the first coding exon did not affect the transcriptional regulation of Jak2. Next, we generated knockout mice that lack Jak2 completely in all organs (Jak2−/−) to assess phenotypic abnormalities that were reported previously in Jak2 conventional knockout mice with targeted deletions of exons 2 or 3 (29, 33). The Cre-mediated deletion of Jak2 in the germ line of mutant mice resulted in embryonic lethality at midgestation due to a lack of EPO signaling and defective definitive erythropoiesis (Krempler et al., submitted). These phenotypic data suggested that the conditional deletion of the first coding exon results in a true null mutation of Jak2 in cells expressing Cre recombinase.
FIG. 1.
Conditional knockout of the Jak2 gene. (A) The targeted insertion of loxP sites around the first coding exon (exon 2) created a conditional knockout allele of Jak2 that can be excised upon Cre-mediated recombination. The deletion of Jak2 can be monitored by using Southern blot analysis with a 3′ external probe following an EcoRI (R) restriction digest. Alternatively, a PCR assay with the 1786-1787 primer set can be employed to verify the presence of a Jak2 null allele in cells expressing Cre. (B) Immunoprecipitation and Western blot analysis of Jak2 derived from embryos carrying two wild-type or two knockout alleles. (C) Immunoprecipitation (IP) and Western blot analysis to monitor the activation of Stat5 and autophosphorylation of Jak2 in GH-treated MEFs that are heterozygous or homozygous knockouts. Note that Jak2 deficiency (Jak2−/−) prevents the GH-mediated tyrosine phosphorylation (PY) of Stat5.
To verify the absence of the Jak2 protein in cells with two null alleles, we established primary MEF cultures from anemic embryos at day 12.5 of gestation and their normal-looking wild-type or heterozygous littermates. The presence of two Jak2 null alleles (Jak2−/−) in retarded embryos and derived fibroblasts was verified by PCR and Southern blot analysis (data not shown). The immunoprecipitation and Western blot analysis demonstrated that the Jak2 protein was not expressed in Jak2−/− MEFs (Fig. 1B). We also did not detect any smaller protein variants of Jak2 in these knockout cells (data not shown), suggesting that other downstream ATG codons were insufficient to initiate translation and that the Jak2 null allele does not produce a residual protein with limited functionality. The phenotypic resemblance of the mutant mice with other targeted Jak2 mutations (29, 33) supported this conclusion.
Jak2-deficient (Jak2−/−) fibroblasts multiplied normally in vitro, suggesting that MEFs do not depend on GH-mediated activation of Stat5. Heterozygous control cells (Jak2+/−) do not have active Stat5 (Fig. 1C), but GH-receptor-mediated autophosphorylation of Jak2 and activation of Stat5 can be induced in control cells immediately after adding GH into the growth medium. In contrast, Jak2-deficient MEFs lack the GH-induced activation of Stat5, suggesting that the functional inhibition of Jak2 is sufficient to decouple GH from its downstream mediators Stat5a and Stat5b. In summary, the phenotypic examination of Jak2-deficient embryos and the biochemical analysis of derived MEFs clearly demonstrate that a knockout of Jak2 is sufficient to suppress EPO and GH signaling through their corresponding single-chain receptors.
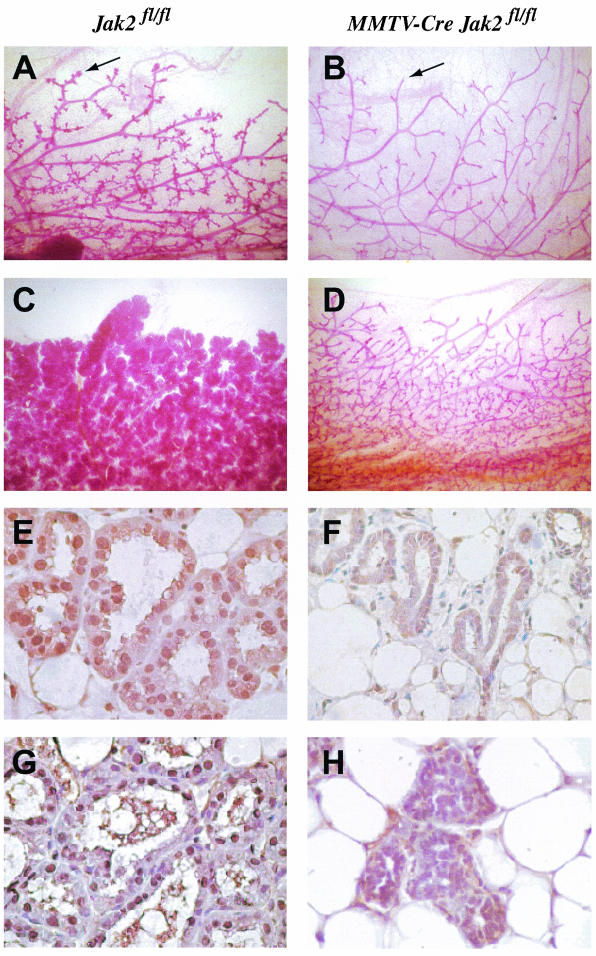
The MMTV-Cre-mediated deletion of Jak2 results in impaired mammary gland development in virgin, pregnant, and postpartum females.
We generated conditional knockout mice that carry an MMTV-Cre transgene in the Jak2 homozygous floxed background (MMTV-Cre Jak2fl/fl) to address important biological functions of Jak2 during mammary gland development. The MMTV-Cre transgene is expressed in all major epithelial subtypes (i.e., luminal and myoepithelial cells) of the prepubescent mammary gland (40). Mutant mice lacking Jak2 somatically in MMTV-Cre target tissues develop normally and are fertile. Jak2-deficient dams were, however, unable to lactate (n = 17). Similar to the Jak2−/− transplantation model by Shillingford et al. (35), the MMTV-Cre-mediated deletion of Jak2 in nulliparous females does not cause phenotypic abnormalities during ductal elongation and the formation of secondary side branches (Fig. 2A and B). A careful analysis of the conditional knockouts, however, revealed an important difference in the architecture of virgin mammary glands that was not reported previously. Lobular units that usually reside on terminal ducts of tertiary side branches of mature virgins (Fig. 2A, arrow) were absent in nulliparous Jak2 conditional knockouts (Fig. 2B). This finding suggests that Jak2-mediated signal transduction plays an important role in the origination of alveolar precursors in response to the concerted action of hormones during the normal estrus cycle. Due to the lack of alveolar precursors, the development of secretory lobules is severely impaired in pregnant and postpartum MMTV-Cre Jak2fl/fl females (Fig. 2C and D). During early pregnancy (day 7.5 of gestation), the proliferation index is significantly decreased in Jak2 conditional knockouts compared to in their littermate wild-type controls (4 versus 11%; n > 3,000). Shillingford et al. demonstrated previously that after the administration of estrogen and progesterone into nulliparous females, the multiplication of epithelial cells is affected in Jak2-deficient transplants. Based on our observations of the conditional knockout, the difference in the proliferation index is likely a consequence of the lack of the entire alveolar lineage since progesterone is a mitogen for this specific epithelial subtype. To verify the lack of alveolar specification in the Jak2 conditional knockout, we analyzed the expression of ductal versus alveolar markers, in particular Nkcc1 (Slc12a2) and Npt2b (Slc34a2), in Jak2-deficient mammary glands. Indeed, Jak2−/− epithelia did not undergo alveolar specification during pregnancy (data not shown). In addition, we examined the functional differentiation and expression of late milk protein gene Wap, which is known to be regulated by PRL signaling and Stat5a activation (25, 27). While the Wap protein was highly abundant within the lumen and secretory alveolar cells in postpartum wild-type controls, Jak2-deficient females failed to synthesize this milk protein, suggesting that in addition to alveolar specification, mammary epithelial cells do not undergo terminal differentiation in the absence of Jak2 (data not shown).
FIG. 2.
Whole-mount analysis (A to D) and examination of Stat5 activity (E to H) of mammary glands from tissue-specific Jak2 knockout mice (MMTV-Cre Jak2fl/fl [B, D, F, and H]) and their littermate controls (Jak2fl/fl [A, C, E, and G]). (A and B) Whole mounts of the number 4 inguinal glands of nulliparous females 5 months of age (magnification, ×10). Note the absence of alveolar buds at the end of terminal ducts in the mutants (B, arrow). The arrow in panel A indicates lobular units that usually reside on terminal ducts of tertiary side branches of mature virgins. (C and D) Whole mounts of inguinal mammary glands of females shortly after parturition (magnification, ×10). (E and F) Immunohistochemistryof the total (inactive and activated) Stat5a protein expression. Note that the majority of Stat5a is confined to the nucleus in normal secretory alveolar cells (E), whereas Jak2-deficient cells express lower levels of Stat5a localized in cytoplasm (F) (magnification, ×400). Slides were counterstained with hematoxylin. (G and H) Immunohistochemistry of activated (tyrosine-phosphorylated [PY]) Stat5. Active Stat5 is exclusively nuclear in the controls (G), whereas lobular units in the conditional knockouts do not contain activated Stat5 (H) (magnification, ×400).
It is a common feature of many genetically engineered mouse strains that lactation can be restored after multiple pregnancies. For instance, PRL-R heterozygous mutants and Stat5a knockout mice develop normal secretory alveoli after multiple pregnancy cycles (23, 32). We recently proposed a universal mechanism for the selective amplification of alveolar cells expressing differentiation markers while retaining characteristics of stem-progenitor cells in the remodeled mammary gland (39). In analogy to these studies, we monitored Jak2-deficient dams during multiple pregnancy cycles for their ability to produce milk to determine whether Jak2 deficiency can be compensated by accompanying signaling pathways that have been implicated in Jak2-independent activation of Stat5 (18, 31). Mice that exhibited a lack of lactogenesis as single-parous dams did not resume milk production after four to seven pregnancies (n = 6), suggesting that mammary epithelial stem cells or specific precursors of the alveolar lineage were unable to activate alternative signal transduction cascades to bypass the lack of Jak2.
Jak2-deficient mammary epithelial cells fail to activate Stat5.
The Jak2-mediated phosphorylation of Stat5a and Stat5b triggers their dimerization and translocation into the nucleus, where they activate the transcription of target genes such as Csnb (β-casein) and Wap (9, 24). We could demonstrate that in cultured fibroblasts, a null mutation of Jak2 was able to abolish the GH-induced phosphorylation of Stat5 (see above). To verify that Jak2 deficiency results in a lack of Stat5 activation in vivo, we examined the localization and phosphorylation of Stat5 using immunohistochemistry on histological sections of mammary tissues derived from conditional knockouts and their littermate controls. At parturition, the vast majority of the Stat5a protein is localized in the nucleus of luminal epithelial cells in the wild-type controls (Fig. 2E). In contrast, Stat5a was confined to the cytoplasm in mammary epithelia of Jak2 knockout mice (Fig. 2F). Using a phosphorylation-specific antibody against Stat5, we observed that the nuclear protein was phosphorylated in the controls but not in Jak2-deficient dams (Fig. 2G and H). In conclusion, these observations suggest that other receptor tyrosine kinases are unable to activate Stat5 under normal physiological conditions in mammary epithelia lacking Jak2.
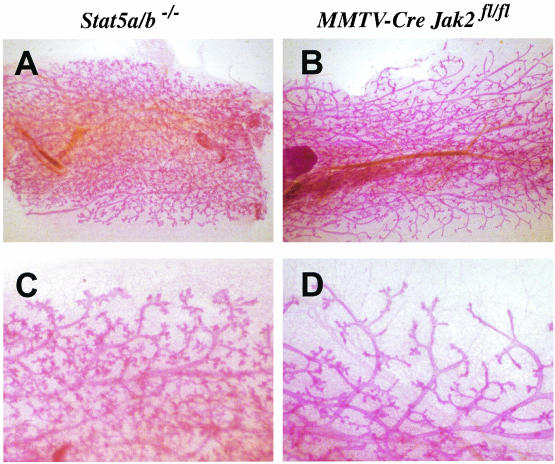
Our findings that Jak2-deficient mammary epithelial cells lack active Stat5 in virgin, pregnant, and postpartum animals is supported by the fact that Jak2 mammary-specific knockout mice largely resemble the phenotype observed for Stat5a-deficient females (25). Since Jak2 is able to activate both Stat5a and Stat5b in the murine mammary gland (24), we performed a comparative analysis of mammary epithelia that lack Jak2 or both Stat5 proteins (Fig. 3). Female Stat5a/b double knockout mice are infertile (37). We therefore transplanted mutant mammary epithelia into wild-type recipients to generate a mamma-specific knockout to study alveologenesis in the absence of both Stat5 proteins. As expected, Stat5a/b mutant epithelia lack alveoli in postpartum females (Fig. 3A and C). The formation of pregnancy-induced side branches on the terminal end of the ducts was, however, not affected by the absence of Stat5. In contrast, mammary glands of Jak2-deficient females appeared to be more severely impaired at parturition (Fig. 3B and D). Notably, Jak2 deficiency does not just lead to impaired alveolar proliferation and differentiation. In addition, the pregnancy-mediated branching morphogenesis at the terminal ducts seemed to be inhibited by the loss of Jak2. A similar observation was recently described for PRL-R−/− epithelia compared to Stat5a/b−/− knockouts both transplanted into collateral glands of the same wild-type recipient (27). In conclusion, Jak2 deficiency seems to more closely resemble the PRL-R-deficient phenotype than abnormalities observed in Stat5a mutants or Stat5a/b double knockout transplants. This suggests that Jak2 deficiency might affect additional downstream mediators other than Stat5a and Stat5b.
FIG. 3.
Comparative whole-mount analysis of mammary epithelia lacking Jak2 or both Stat5 proteins in postpartum females. (A and C) Whole-mount analysis of Stat5a/b mutant epithelia transplanted into wild-type recipients. Magnifications, ×4 (A) and ×20 (C). (B and D) MMTV-Cre Jak2fl/fl females several hours after delivering the young. Magnifications, ×4 (B) and ×20 (D). Note the lack of parity-induced side branches in Jak2-deficient dams (D) in contrast to in the transplant model lacking Stat5 (C).
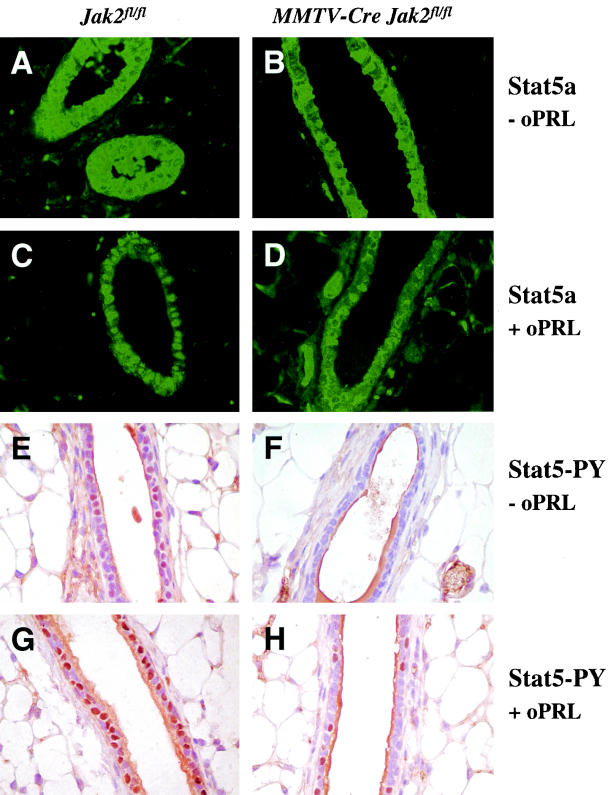
Jak2 deficiency uncouples PRL signaling from its downstream mediator Stat5.
It was previously demonstrated that Stat5 is continuously phosphorylated in a number of mammary epithelial cells in the nonpregnant murine mammary gland and the human breast (30). This basal activation of Stat5 seems to be controlled by hormones of the pituitary gland, in particular, by PRL. Phosphorylated Stat5 was not observed in hypophysectomized females and in nulliparous PRL-R knockouts. Although a large amount of the Stat5a protein is generally confined to the cytoplasm (Fig. 4A), phosphorylated Stat5 can be clearly detected in a significant number of nuclei in luminal epithelial cells of nonpregnant wild-type females (Fig. 4E). Consistent with our previous studies of hypophysectomized virgins and PRL-R mutants, the deletion of Jak2 results in a significant inhibition of the basal Stat5 activation. Nuclear Stat5 is virtually absent in MMTV-Cre Jak2fl/fl animals (Fig. 4B and F). The administration of supraphysiological levels of ovine PRL induced a nearly uniform phosphorylation and nuclear translocation of the bulk of the Stat5 protein in nonpregnant wild-type controls (Fig. 4C and G). In contrast, Stat5 remained cytoplasmic or perinuclear in the majority of luminal epithelial cells in the Jak2 mutants injected with the same amount of PRL (Fig. 4D and H). In conclusion, the results of the comparative analysis and the PRL injection study confirm that (i) the basal activation of Stat5 in nulliparous females is mediated by PRL via Jak2 and (ii) Jak2 deficiency disrupts PRL-R signaling in luminal epithelial cells. Based on the severity of the phenotype observed in the Jak2 mutants compared to that of the Stat5a/b null transplants, a knockout of Jak2 might curtail PRL-R downstream targets other than Stat5.
FIG. 4.
Lack of PRL-induced phosphorylation and nuclear translocation of Stat5 in mammary epithelial cells of nulliparous Jak2 conditional knockouts (MMTV-Cre Jak2fl/fl [B, D, F, and H]) compared to nonpregnant controls (Jak2fl/fl [A, C, E, and G]). (A to D) Immunofluorescence staining of the total (inactive and activated) Stat5a protein in nonpregnant females injected with ovine PRL (oPRL) (+ oPRL [C and D]) or saline (− oPRL [A and B]). Note that the majority of the Stat5a protein is confined to the nucleus in wild-type cells after administration of supraphysiological levels of oPRL (C), whereas Jak2-deficient mice exhibit no major changes in the nuclear transport of Stat5 (D). Magnification, ×400. (E to H) Immunohistochemistry of activated (tyrosine-phosphorylated [PY]) Stat5 in nonpregnant females injected with oPRL (G and H) or saline (E and F). Active Stat5 can be detected in a number of nuclei in luminal epithelial cells of nonpregnant wild-type females (E), whereas Jak2-deficient mice have a significantly reduced basal activation of Stat5 (F). A virtually uniform activation of Stat5 is evident in nulliparous females after administration of supraphysiological levels of oPRL (G). In contrast, Jak2-deficient mice have a significantly reduced PRL-induced phosphorylation and nuclear translocation of Stat5 after administration of oPRL (H). Magnification, ×400.
Jak2 is required for PRL-mediated maintenance of functionally differentiated alveolar cells.
The MMTV-Cre-mediated mamma-specific knockout of Jak2 in nulliparous, pregnant, and postpartum females revealed that alveolar proliferation, specification, and differentiation are critically dependent on Jak2. We then wanted to determine whether Jak2 is also required for the maintenance and survival of differentiated, secretory alveolar cells. Declining circulating levels of PRL in combination with an activation of proapoptotic factors induced by milk stasis are responsible for the collapse of alveolar structures during the first phase of involution at the end of lactation (22, 42). The dephosphorylation of Stat5 and activation of Stat3 are two molecular events that seem to stimulate the programmed cell death of terminally differentiated cells (reviewed in reference 13). To examine whether Jak2-Stat5 signaling is required for the PRL-mediated survival and maintenance of differentiated mammary epithelial cells, we specifically deleted Jak2 from this secretory epithelial subpopulation during late pregnancy and lactation.
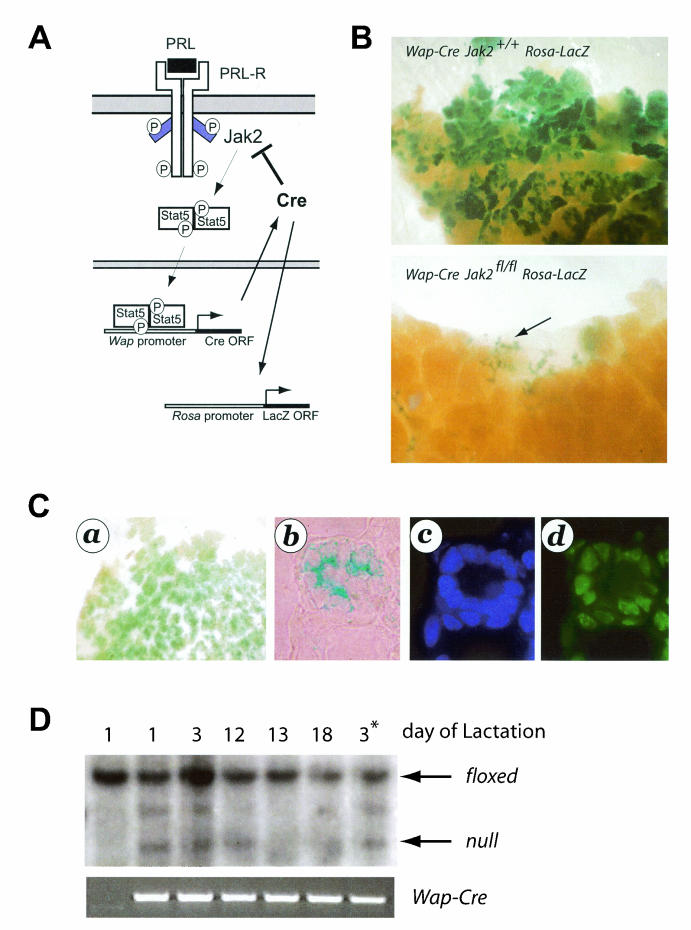
The experimental design for this study is illustrated in Fig. 5A. As discussed earlier, Wap is a PRL-induced milk protein gene whose expression is largely confined to secretory alveolar cells. Since Wap is highly upregulated during the last phase of pregnancy, the expression of this gene is generally applied as a terminal differentiation marker for mammary epithelial cells. We developed transgenic mice that express Cre under the control of the Wap gene promoter (Wap-Cre) to target this recombinase specifically to PRL-responsive, differentiating mammary epithelial cells (41). A careful examination of the Wap-Cre expression profile by using a Cre-lox reporter strain, Rosa-lox-Stop-lox-LacZ (36), revealed that Cre expression and activation of the reporter coincide with the upregulation of the endogenous Wap locus during the second half of pregnancy (39). In this context, it is important that adequate levels of Wap (and therefore Wap-Cre expression) require the formation of a proper three-dimensional structure of an alveolus during early pregnancy (4). Primary ducts and large side branches do not express Wap-Cre (39). According to our experimental design, the Wap-Cre-mediated excision of Jak2 (Wap-Cre Jak2fl/fl) should disrupt the enduring Jak2-Stat5 signaling cascade in differentiated alveolar cells, i.e., an epithelial cell population that is highly dependent on the presence of Jak2 from the earliest stages of pregnancy, when these cells emerge from undifferentiated alveolar progenitors (see phenotypic abnormalities in the MMTV-Cre Jak2fl/fl model). This strategy permitted us to evaluate the role of Jak2 in the maintenance and survival of cells in this epithelial compartment. Because we hypothesized that a loss of Jak2 in differentiated alveolar cells would lead to cell death and a progressive negative selection of Jak2-deficient cells, we bred the Rosa-lox-Stop-lox-LacZ reporter (herein called Rosa-LacZ) into the Jak2 conditional knockout mice (Wap-Cre Jak2fl/fl Rosa-LacZ). The X-Gal staining technique of mammary gland whole mounts allowed us to readily monitor Cre expression and thereby the loss of Jak2, as well as any cell selection that might occur as a consequence of Jak2 deficiency.
FIG. 5.
Wap-Cre-mediated deletion of Jak2 in differentiating alveolar cells during pregnancy and lactation. (A) Experimental design. Cre recombinase is under the control of the promoter of the Wap gene, which is a PRL target gene that is predominantly expressed in secretory alveolar cells during late pregnancy and lactation. Therefore, Jak2 is deleted in these cells after they initiate the differentiation program. P, (tyrosine) phosphorylation; ORF, open reading frame. (B) The inclusion of a Rosa-lox-Stop-lox-LacZ (i.e., Rosa-LacZ) reporter transgene in this experimental design permits the detection of Cre-expressing (i.e., Jak2-deficient) cells in mammary whole mounts stained with X-Gal. Note that many alveolar cells express β-galactosidase in lactating dams that are the wild type for Jak2 (B, upper panel), whereas these cells are absent in postpartum Wap-Cre Jak2fl/fl females (B, lower panel). Magnification, ×20. (C) X-Gal staining of a whole mount (a) or a histological section of differentiating alveolar cells (b) derived from a Wap-Cre Jak2fl/fl Rosa-LacZ female at day 16.5 of pregnancy. X-Gal-positive (i.e., Jak2−/−) cells were stained with DAPI (4′,6′-diamidino-2-phenylindole) (c) and labeled with ChromaTide Alexa Fluor 488-5-dUTP (d) to detect apoptotic cells. Magnification, ×400. (D) Southern blot on genomic DNA derived from mammary tissues of lactating Wap-Cre Jak2fl/fl dams (lanes 2 to 6) and a Jak2fl/fl control (lane 1) to monitor the negative selection of differentiated cells lacking Jak2. The schematic of the Southern blot strategy to distinguish between the floxed allele (∼8 kb) and the null allele (∼4 kb) is illustrated in Fig. 1. An intermediate band of ∼5.5 kb represents a partial recombined allele lacking the coding exon. Note that Jak2-deficient cells (complete or partially deleted alleles of Jak2) are negatively selected during the lactation period. *, second lactation period.
Indeed, the prediction that Jak2 is critical for the survival and maintenance of differentiated alveolar cells was supported by our findings in the Wap-Cre Jak2fl/fl Rosa-LacZ model. While numerous alveolar cells expressed β-galactosidase in the Wap-Cre Jak2+/+ Rosa-LacZ controls (Fig. 5B, upper panel), the number of X-Gal-positive cells significantly declined when Jak2 was excised from differentiating cells (Fig. 5B, lower panel, arrow). These observations suggest that continuous PRL signaling through Jak2-Stat5 is required for the maintenance of secretory alveolar cells. To verify this assumption, we monitored the induction of apoptosis in cells activating Wap-Cre and Rosa-LacZ (i.e., cells lacking Jak2) during early stages of alveolar differentiation (Fig. 5C). Unlike in postpartum females (Fig. 5B), Wap-Cre Jak2fl/fl Rosa-LacZ mice possess numerous X-Gal-positive cells at day 16.5 of pregnancy (Fig. 5C, panel a). X-Gal-positive cells that are confined to differentiating alveoli (Fig. 5C, panel b) undergo apoptosis as determined by a TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) assay (Fig. 5C, panels c and d). The negative selection against differentiated cells lacking Jak2 was further verified by using a Southern blot assay to determine the amount of recombined cells during an entire lactation period (Fig. 5D). While mammary tissues contained some Jak2-deficient cells during the first few days of lactation (Fig. 5D, lanes 2 to 4), these cells were completely absent during the remainder of the lactation period (Fig. 5D, lanes 5 and 6). The negative selection of Jak2-deficient, differentiating cells seemed to not affect undifferentiated alveolar progenitors that do not express Wap-Cre in this model. A new generation of Jak2 knockout cells was generated with each subsequent pregnancy (Fig. 5D, lane 7). In summary, this set of experiments with Wap-Cre-mediated conditional knockout mice conclusively determined that Jak2 is critical for the PRL-mediated survival and maintenance of differentiated alveolar cells in the mammary glands of pregnant and lactating females.
DISCUSSION
Jak2 is indispensable for the PRL-R-mediated activation of Stat5.
We generated the first conditional knockout mouse model that allows the targeted disruption of Jak2-Stat5 signaling from any given cell type at virtually any stage of development. The Cre-mediated excision of the first coding exon of Jak2 from the germ line resulted in mutant embryos with identical phenotypes described previously (29, 33), suggesting that the conditional knockout approach creates a true null allele of Jak2 in cells where Cre recombinase is active. Using an MMTV-Cre-mediated inactivation of Jak2 in the mammary gland, we determined nonredundant functions of this gene for alveolar proliferation and differentiation in virgin, pregnant, and lactating females. The exogenous administration of PRL into the conditional knockouts and their controls verifies the pivotal role of Jak2 for the enzymatic coupling of PRL-R signaling and Stat5 activation in vivo. The results of our studies of the Jak2 conditional knockout model imply that growth factor receptors and their signal transducers, in particular Src and ErbB family members, do not activate Stat5 in a Jak2-independent manner in normal mammary epithelial cells as was previously proposed (18, 31).
While some phenotypic consequences of Jak2 deficiency during pregnancy have been observed previously in a mammary anlagen transplant model (35), there are several new observations and consequential implications that we obtained by analyzing Jak2 conditional knockout mice. First, a deficiency of Jak2 resulted in blunted tertiary ducts lacking lobular units that are typically found in wild-type, mature virgins. A very similar phenotype was observed in PRL-R knockout mice (2). Since MMTV-Cre Jak2fl/fl females are fertile, it is unlikely that the lack of lobular units in nulliparous mutants can be attributed simply to impaired ovarian function. Second, the comparative analysis of postpartum mammary glands from Jak2 mutants and epithelial transplants lacking Stat5a and Stat5b also revealed a difference in the pregnancy-induced ductal morphogenesis. The phenotype in the Jak2 conditional knockout appears, therefore, to be more similar to abnormalities observed in PRL-R−/− epithelial transplants engrafted into wild-type mice (27), suggesting that Jak2-coupled PRL signaling targets other downstream mediators in addition to Stat5. Jak2 phosphorylates the receptor, thereby creating docking sites for other SH2-domain proteins such as Src, Fyn, and Tec that might signal in a Stat5-independent manner (1, 5, 21). The defect in the formation of terminal side branches during pregnancy is likely to be intrinsic to Jak2−/− mammary epithelia since the conditional knockouts have normal levels of PRL, normal ovarian function (i.e., full-term pregnancy), and, consequently, adequate steroid hormone synthesis. PRL, on the other hand, has been demonstrated to increase estrogen receptor expression in mammary epithelial cells in vitro (7). The estrogen receptor is known to increase both progesterone receptor (PR) and PRL-R expression (11, 28). PR signaling is indispensable for the formation of small side branches in terminal duct lobular units and alveologenesis (3, 16). The disruption of PRL-R signaling in the Jak2 conditional knockouts might therefore affect its own feedback mechanism and lead to reduced PRL-R expression and reduced PR signaling and therefore impaired ductal branching morphogenesis during pregnancy, when levels of progesterone in serum are normally elevated. The Jak2 conditional knockout will be a very valuable tool to address this aspect of a synergistic function of PR and PRL-R signaling in much greater detail.
PRL-R-Jak2 signaling supports the survival of secretory epithelial cells.
Unlike transplantation models, Cre-lox-based conditional knockouts permit an inhibition of a target gene at defined stages of development. We utilized this distinct feature to delete Jak2 specifically from differentiating alveolar cells that initiated a differentiation program in response to pregnancy hormones, in particular PRL, to examine the importance of PRL-R-Jak2 signaling for cell survival. The Wap-Cre-mediated excision of Jak2 from PRL-responsive, differentiating alveolar cells had a profound negative impact on their survival. The vast majority of Jak2-deficient cells died during late pregnancy and the first week of lactation. The Jak2-deficient cells were progressively replaced by alveolar cells that did not express the Wap-Cre transgene (i.e., cells with functional, unrecombined Jak2 alleles). There is evidence from previously published observations to suggest that lactogenic hormones and Stat5 activation are important for the survival of secretory epithelial cells during lactation. While lactogenic hormone levels, including PRL levels, decline after the weaning of the young, cell-autonomous, proapoptotic pathways become active in response to milk stasis (22, 42). Shortly after the weaning of the litter, Stat5 is readily dephosphorylated. The deactivation of Stat5 is, however, not just the result of reduced levels of PRL in circulation. Three different experimental designs that induce milk stasis in the presence of a normal suckling stimulus and therefore high levels of lactogenic hormones have demonstrated that cell-autonomous pathways are able to modulate PRL-R signaling and Stat5 activation, (i) the sealed nipple experiment, (ii) the transplantation method, and (iii) oxytocin knockouts with an impaired milk let-down reflex (22, 42). Is the activation of Stat5, however, truly a prerequisite for the survival of PRL-responsive, secretory epithelial cells? The results obtained from the Jak2 conditional knockout and recently published data on a Stat5 overexpression model show that this might indeed be the case. Mice expressing a dominant-active form of Stat5 exhibited a higher proliferation index, delayed involution, and a higher susceptibility to mammary tumorigenesis (17). Clearly, the Wap-Cre-mediated deletion model of Jak2 supports the hypothesis of Stat5 as a survival factor for differentiated alveolar cells. More specifically, the deletion of Jak2 disrupts the enzymatic coupling between the PRL-R and Stat5 in the presence of elevated circulating levels of PRL during pregnancy and lactation. This experimental design might therefore recapitulate the decoupling of extracellular peptide hormones, in particular PRL, from intracellular processes, which is an essential step during the first phase of involution.
Acknowledgments
This work was supported in part by the Public Health Service grants CA93797 (to K.U.W.) and CA101841 (to H.R. and K.U.W.) from the National Cancer Institute. H.R. receives a Public Health Service grant from the National Institutes of Health (DK052013). A.K. received a stipend from the Deutsche Forschungsgemeinschaft (DFG, KR 2107/1-1). Support provided to K.U.W. by the Nebraska Cancer and Smoking Disease Research Program (NE DHHS LB595) and Cattlemen's Ball of Nebraska, Inc., was imperative to finance the generation of the Jak2-deficient animal model.
We thank James Ihle (St. Jude Children's Research Hospital) for providing Stat5a/b double knockout mice. We also thank Lothar Hennighausen and Jim Turner (National Institutes of Health) as well as Jurg Biber (University of Zurich, Zurich, Switzerland) for contributing various antibodies (Nkcc1 and Npt2b) to this study.
REFERENCES
- 1.Berlanga, J. J., J. A. F. Vara, J. Martin-Perez, and J. P. Garcia-Ruiz. 1995. Prolactin receptor is associated with c-src kinase in rat liver. Mol. Endocrinol. 9:1461-1467. [DOI] [PubMed] [Google Scholar]
- 2.Brisken, C., S. Kaur, T. E. Chavarria, N. Binart, R. L. Sutherland, R. A. Weinberg, P. A. Kelly, and C. J. Ormandy. 1999. Prolactin controls mammary gland development via direct and indirect mechanisms. Dev. Biol. 210:96-106. [DOI] [PubMed] [Google Scholar]
- 3.Brisken, C., S. Park, T. Vass, J. P. Lydon, B. W. O'Malley, and R. A. Weinberg. 1998. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc. Natl. Acad. Sci. USA 95:5076-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, L. H., and M. J. Bissell. 1989. A novel regulatory mechanism for whey acidic protein gene expression. Cell Regul. 1:45-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clevenger, C. V., and M. V. Medaglia. 1994. The protein tyrosine kinase P59fyn is associated with prolactin (PRL) receptor and is activated by PRL stimulation of T-lymphocytes. Mol. Endocrinol. 8:674-681. [DOI] [PubMed] [Google Scholar]
- 6.Das, R., and B. K. Vonderhaar. 1997. Prolactin as a mitogen in mammary cells. J. Mammary Gland Biol. Neoplasia 2:29-39. [DOI] [PubMed] [Google Scholar]
- 7.Edery, M., W. Imagawa, L. Larson, and S. Nandi. 1985. Regulation of estrogen and progesterone receptor levels in mouse mammary epithelial cells grown in serum-free collagen gel cultures. Endocrinology 116:105-112. [DOI] [PubMed] [Google Scholar]
- 8.Erwin, R. A., R. A. Kirken, M. G. Malabarba, W. L. Farrar, and H. Rui. 1995. Prolactin activates Ras via signaling proteins SHC, growth factor receptor bound 2, and son of sevenless. Endocrinology 136:3512-3518. [DOI] [PubMed] [Google Scholar]
- 9.Gouilleux, F., H. Wakao, M. Mundt, and B. Groner. 1994. Prolactin induces phosphorylation of Tyr694 of Stat5 (MGF), a prerequisite for DNA binding and induction of transcription. EMBO J. 13:4361-4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grimley, P. M., F. Dong, and H. Rui. 1999. Stat5a and Stat5b: fraternal twins of signal transduction and transcriptional activation. Cytokine Growth Factor Rev. 10:131-157. [DOI] [PubMed] [Google Scholar]
- 11.Haslam, S. Z., and G. Shyamala. 1979. Effect of oestradiol on progesterone receptors in normal mammary glands and its relationship with lactation. Biochem. J. 182:127-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hennighausen, L., and G. W. Robinson. 1998. Think globally, act locally: the making of a mouse mammary gland. Genes Dev. 12:449-455. [DOI] [PubMed] [Google Scholar]
- 13.Hennighausen, L., and G. W. Robinson. 2001. Signaling pathways in mammary gland development. Dev. Cell 1:467-475. [DOI] [PubMed] [Google Scholar]
- 14.Hennighausen, L., G. W. Robinson, K. U. Wagner, and W. Liu. 1997. Prolactin signaling in mammary gland development. J. Biol. Chem. 272:7567-7569. [DOI] [PubMed] [Google Scholar]
- 15.Horseman, N. D., W. Zhao, E. Montecino-Rodriguez, M. Tanaka, K. Nakashima, S. J. Engle, F. Smith, E. Markoff, and K. Dorshkind. 1997. Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J. 16:6926-6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Humphreys, R. C., J. Lydon, B. W. O'Malley, and J. M. Rosen. 1997. Mammary gland development is mediated by both stromal and epithelial progesterone receptors. Mol. Endocrinol. 11:801-811. [DOI] [PubMed] [Google Scholar]
- 17.Iavnilovitch, E., B. Groner, and I. Barash. 2002. Overexpression and forced activation of stat5 in mammary gland of transgenic mice promotes cellular proliferation, enhances differentiation, and delays postlactational apoptosis. Mol. Cancer Res. 1:32-47. [PubMed] [Google Scholar]
- 18.Jones, F. E., T. Welte, X. Y. Fu, and D. F. Stern. 1999. ErbB4 signaling in the mammary gland is required for lobuloalveolar development and Stat5 activation during lactation. J. Cell Biol. 147:77-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kingsley-Kallesen, M., S. S. Mukhopadhyay, S. L. Wyszomierski, S. Schanler, G. Schutz, and J. M. Rosen. 2002. The mineralocorticoid receptor may compensate for the loss of the glucocorticoid receptor at specific stages of mammary gland development. Mol. Endocrinol. 16:2008-2018. [DOI] [PubMed] [Google Scholar]
- 20.Kisseleva, T., S. Bhattacharya, J. Braunstein, and C. W. Schindler. 2002. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene 285:1-24. [DOI] [PubMed] [Google Scholar]
- 21.Kline, J. B., D. J. Moore, and C. V. Clevenger. 2001. Activation and association of the Tec tyrosine kinase with the human prolactin receptor: mapping of a Tec/Vav1-receptor binding site. Mol. Endocrinol. 15:832-841. [DOI] [PubMed] [Google Scholar]
- 22.Li, M., X. Liu, G. Robinson, U. Bar-Peled, K. U. Wagner, W. S. Young, L. Hennighausen, and P. A. Furth. 1997. Mammary-derived signals activate programmed cell death during the first stage of mammary gland involution. Proc. Natl. Acad. Sci. USA 94:3425-3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu, X., M. I. Gallego, G. H. Smith, G. W. Robinson, and L. Hennighausen. 1998. Functional release of Stat5a-null mammary tissue through the activation of compensating signals including Stat5b. Cell Growth Differ. 9:795-803. [PubMed] [Google Scholar]
- 24.Liu, X., G. W. Robinson, and L. Hennighausen. 1996. Activation of Stat5a and Stat5b by tyrosine phosphorylation is tightly linked to mammary gland differentiation. Mol. Endocrinol. 10:1496-1506. [DOI] [PubMed] [Google Scholar]
- 25.Liu, X., G. W. Robinson, K. U. Wagner, L. Garrett, A. Wynshaw-Boris, and L. Hennighausen. 1997. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 11:179-186. [DOI] [PubMed] [Google Scholar]
- 26.Llovera, M., P. Touraine, P. A. Kelly, and V. Goffin. 2000. Involvement of prolactin in breast cancer: redefining the molecular targets. Exp. Gerontol. 35:41-51. [DOI] [PubMed] [Google Scholar]
- 27.Miyoshi, K., J. M. Shillingford, G. H. Smith, S. L. Grimm, K. U. Wagner, T. Oka, J. M. Rosen, G. W. Robinson, and L. Hennighausen. 2001. Signal transducer and activator of transcription (Stat) 5 controls the proliferation and differentiation of mammary alveolar epithelium. J. Cell Biol. 155:531-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mizoguchi, Y., J. Y. Kim, J. Enami, and S. Sakai. 1997. The regulation of the prolactin receptor gene expression in the mammary gland of early pregnant mouse. Endocr. J. 44:53-58. [DOI] [PubMed] [Google Scholar]
- 29.Neubauer, H., A. Cumano, M. Muller, H. Wu, U. Huffstadt, and K. Pfeffer. 1998. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell 93:397-409. [DOI] [PubMed] [Google Scholar]
- 30.Nevalainen, M. T., J. Xie, L. Bubendorf, K. U. Wagner, and H. Rui. 2002. Basal activation of transcription factor signal transducer and activator of transcription (stat5) in nonpregnant mouse and human breast epithelium. Mol. Endocrinol. 16:1108-1124. [DOI] [PubMed] [Google Scholar]
- 31.Olayioye, M. A., I. Beuvink, K. Horsch, J. M. Daly, and N. E. Hynes. 1999. ErbB receptor-induced activation of stat transcription factors is mediated by Src tyrosine kinases. J. Biol. Chem. 274:17209-17218. [DOI] [PubMed] [Google Scholar]
- 32.Ormandy, C. J., A. Camus, J. Barra, D. Damotte, B. Lucas, H. Buteau, M. Edery, N. Brousse, C. Babinet, N. Binart, and P. A. Kelly. 1997. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 11:167-178. [DOI] [PubMed] [Google Scholar]
- 33.Parganas, E., D. Wang, D. Stravopodis, D. J. Topham, J. C. Marine, S. Teglund, E. F. Vanin, S. Bodner, O. R. Colamonici, J. M. van Deursen, G. Grosveld, and J. N. Ihle. 1998. Jak2 is essential for signaling through a variety of cytokine receptors. Cell 93:385-395. [DOI] [PubMed] [Google Scholar]
- 34.Rui, H., R. A. Kirken, and W. L. Farrar. 1994. Activation of receptor-associated tyrosine kinase JAK2 by prolactin. J. Biol. Chem. 269:5364-5368. [PubMed] [Google Scholar]
- 35.Shillingford, J. M., K. Miyoshi, G. W. Robinson, S. L. Grimm, J. M. Rosen, H. Neubauer, K. Pfeffer, and L. Hennighausen. 2002. Jak2 is an essential tyrosine kinase involved in pregnancy-mediated development of mammary secretory epithelium. Mol. Endocrinol. 16:563-570. [DOI] [PubMed] [Google Scholar]
- 36.Soriano, P. 1999. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21:70-71. [DOI] [PubMed] [Google Scholar]
- 37.Teglund, S., C. McKay, E. Schuetz, J. M. van Deursen, D. Stravopodis, D. Wang, M. Brown, S. Bodner, G. Grosveld, and J. N. Ihle. 1998. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell 93:841-850. [DOI] [PubMed] [Google Scholar]
- 38.Topper, Y. J., and C. S. Freeman. 1980. Multiple hormone interactions in the developmental biology of the mammary gland. Physiol. Rev. 60:1049-1106. [DOI] [PubMed] [Google Scholar]
- 39.Wagner, K. U., C. A. Boulanger, M. D. Henry, M. Sgagias, L. Hennighausen, and G. H. Smith. 2002. An adjunct mammary epithelial cell population in parous females: its role in functional adaptation and tissue renewal. Development 129:1377-1386. [DOI] [PubMed] [Google Scholar]
- 40.Wagner, K. U., K. McAllister, T. Ward, B. Davis, R. Wiseman, and L. Hennighausen. 2001. Spatial and temporal expression of the Cre gene under the control of the MMTV-LTR in different lines of transgenic mice. Transgenic Res. 10:545-553. [DOI] [PubMed] [Google Scholar]
- 41.Wagner, K. U., R. J. Wall, L. St. Onge, P. Gruss, A. Wynshaw-Boris, L. Garrett, M. Li, P. A. Furth, and L. Hennighausen. 1997. Cre-mediated gene deletion in the mammary gland. Nucleic Acids Res. 25:4323-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagner, K. U., W. S. Young, X. Liu, E. I. Ginns, M. Li, P. A. Furth, and L. Hennighausen. 1997. Oxytocin and milk removal are required for post-partum mammary-gland development. Genes Funct. 1:233-244. [DOI] [PubMed] [Google Scholar]
- 43.Young, L. J. T. 2000. The cleared mammary fat pad and the transplantation of mammary gland morphological structures and cells, p. 67-74. In M. M. Ip and B. B. Ash, Methods in mammary gland biology and breast cancer. Kluwer Academic/Plenum Publishers, New York, N.Y.