Abstract
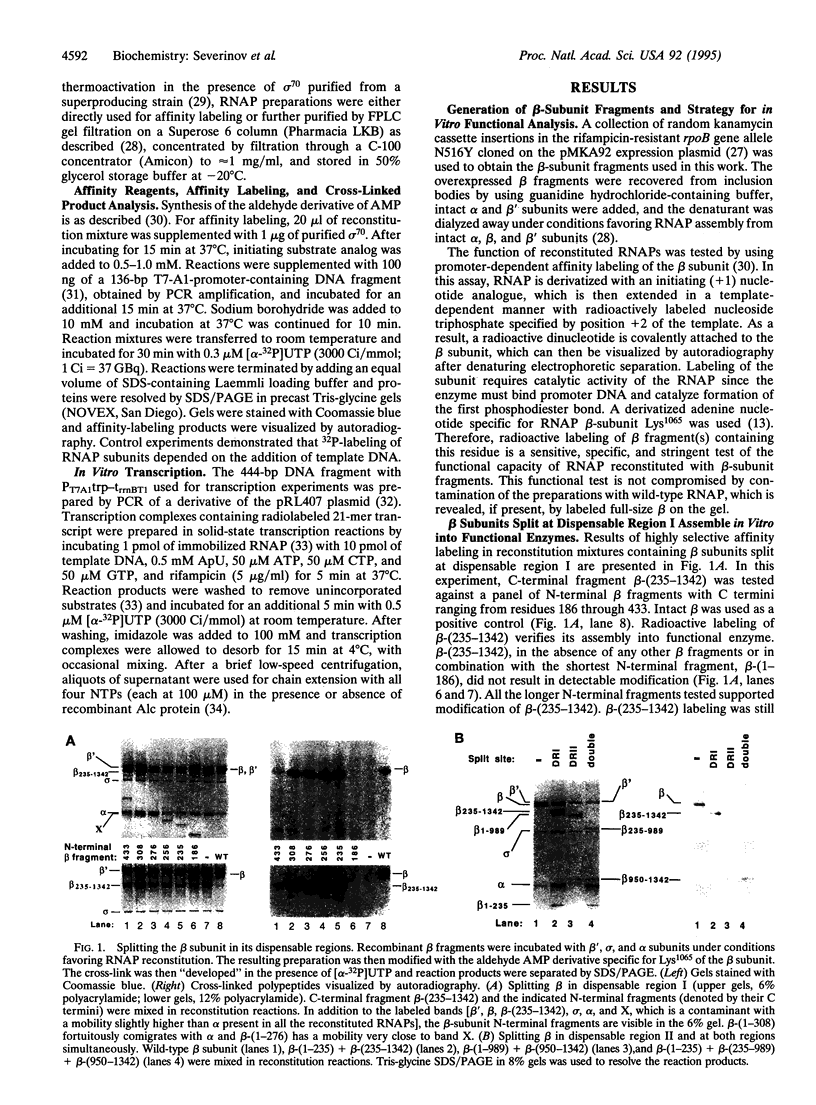
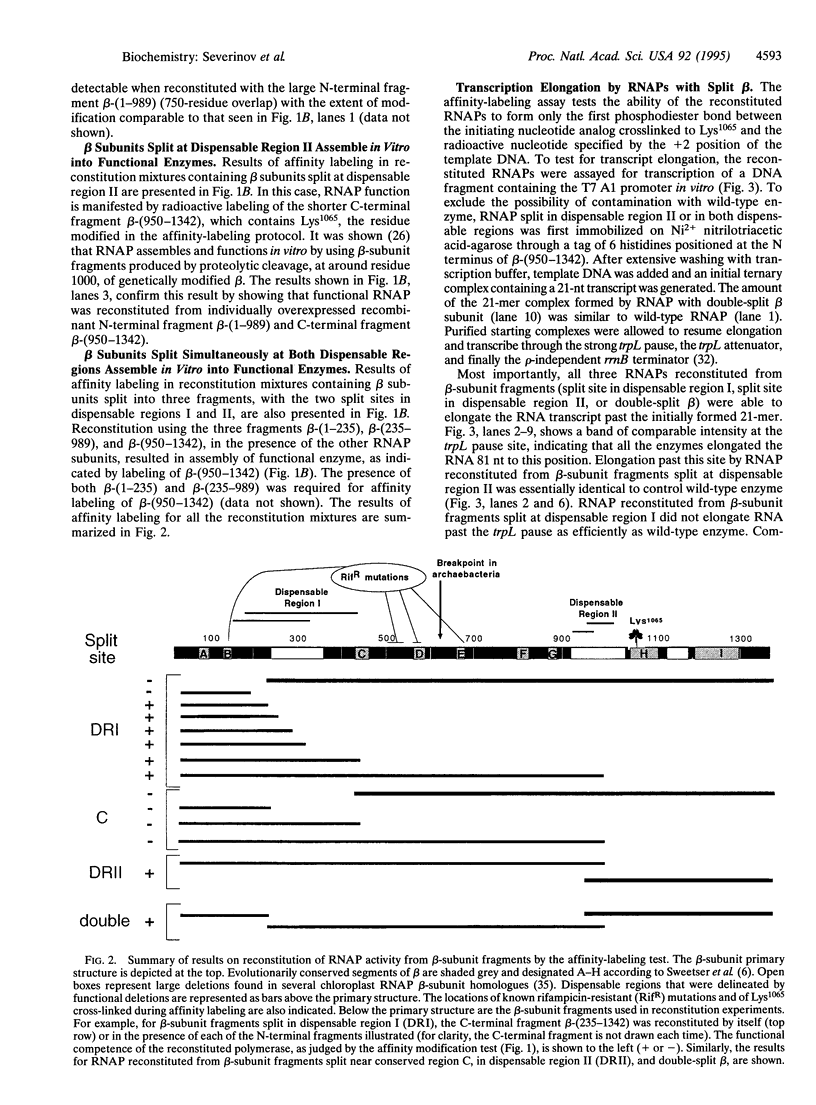
The Escherichia coli rpoB gene, which codes for the 1342-residue beta subunit of RNA polymerase (RNAP), contains two dispensable regions centered around codons 300 and 1000. To test whether these regions demarcate domains of the RNAP beta subunit, fragments encoded by segments of rpoB flanking the dispensable regions were individually overexpressed and purified. We show that these beta-subunit polypeptide fragments, when added with purified recombinant beta', sigma, and alpha subunits of RNAP, reconstitute a functional enzyme in vitro. These results demonstrate that the beta subunit is composed of at least three distinct domains and open another avenue for in vitro studies of RNAP assembly and structure.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahearn J. M., Jr, Bartolomei M. S., West M. L., Cisek L. J., Corden J. L. Cloning and sequence analysis of the mouse genomic locus encoding the largest subunit of RNA polymerase II. J Biol Chem. 1987 Aug 5;262(22):10695–10705. [PubMed] [Google Scholar]
- Allison L. A., Moyle M., Shales M., Ingles C. J. Extensive homology among the largest subunits of eukaryotic and prokaryotic RNA polymerases. Cell. 1985 Sep;42(2):599–610. doi: 10.1016/0092-8674(85)90117-5. [DOI] [PubMed] [Google Scholar]
- Amann E., Brosius J., Ptashne M. Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene. 1983 Nov;25(2-3):167–178. doi: 10.1016/0378-1119(83)90222-6. [DOI] [PubMed] [Google Scholar]
- Berghöfer B., Kröckel L., Körtner C., Truss M., Schallenberg J., Klein A. Relatedness of archaebacterial RNA polymerase core subunits to their eubacterial and eukaryotic equivalents. Nucleic Acids Res. 1988 Aug 25;16(16):8113–8128. doi: 10.1093/nar/16.16.8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsland K. J., Haselkorn R. Evolutionary relationships among eubacteria, cyanobacteria, and chloroplasts: evidence from the rpoC1 gene of Anabaena sp. strain PCC 7120. J Bacteriol. 1991 Jun;173(11):3446–3455. doi: 10.1128/jb.173.11.3446-3455.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibi E., Kaback H. R. In vivo expression of the lacY gene in two segments leads to functional lac permease. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4325–4329. doi: 10.1073/pnas.87.11.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs J., Searles L. L., Greenleaf A. L. Structure of the eukaryotic transcription apparatus: features of the gene for the largest subunit of Drosophila RNA polymerase II. Cell. 1985 Sep;42(2):611–621. doi: 10.1016/0092-8674(85)90118-7. [DOI] [PubMed] [Google Scholar]
- Borukhov S., Severinov K., Kashlev M., Lebedev A., Bass I., Rowland G. C., Lim P. P., Glass R. E., Nikiforov V., Goldfarb A. Mapping of trypsin cleavage and antibody-binding sites and delineation of a dispensable domain in the beta subunit of Escherichia coli RNA polymerase. J Biol Chem. 1991 Dec 15;266(35):23921–23926. [PubMed] [Google Scholar]
- Burbaum J. J., Schimmel P. Assembly of a class I tRNA synthetase from products of an artificially split gene. Biochemistry. 1991 Jan 15;30(2):319–324. doi: 10.1021/bi00216a002. [DOI] [PubMed] [Google Scholar]
- Corradin G., Harbury H. A. Reconstitution of horse heart cytochrome c: interaction of the components obtained upon cleavage of the peptide bond following methionine residue 65. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3036–3039. doi: 10.1073/pnas.68.12.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik C. S., Buchman S. R., Beychok S. Characterization of globin domains: heme binding to the central exon product. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1384–1388. doi: 10.1073/pnas.77.3.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darst S. A., Edwards A. M., Kubalek E. W., Kornberg R. D. Three-dimensional structure of yeast RNA polymerase II at 16 A resolution. Cell. 1991 Jul 12;66(1):121–128. doi: 10.1016/0092-8674(91)90144-n. [DOI] [PubMed] [Google Scholar]
- Darst S. A., Kubalek E. W., Kornberg R. D. Three-dimensional structure of Escherichia coli RNA polymerase holoenzyme determined by electron crystallography. Nature. 1989 Aug 31;340(6236):730–732. doi: 10.1038/340730a0. [DOI] [PubMed] [Google Scholar]
- Falkenburg D., Dworniczak B., Faust D. M., Bautz E. K. RNA polymerase II of Drosophila. Relation of its 140,000 Mr subunit to the beta subunit of Escherichia coli RNA polymerase. J Mol Biol. 1987 Jun 20;195(4):929–937. doi: 10.1016/0022-2836(87)90496-7. [DOI] [PubMed] [Google Scholar]
- Galakatos N. G., Walsh C. T. Specific proteolysis of native alanine racemases from Salmonella typhimurium: identification of the cleavage site and characterization of the clipped two-domain proteins. Biochemistry. 1987 Dec 15;26(25):8475–8480. doi: 10.1021/bi00399a066. [DOI] [PubMed] [Google Scholar]
- Grachev M. A., Kolocheva T. I., Lukhtanov E. A., Mustaev A. A. Studies on the functional topography of Escherichia coli RNA polymerase. Highly selective affinity labelling by analogues of initiating substrates. Eur J Biochem. 1987 Feb 16;163(1):113–121. doi: 10.1111/j.1432-1033.1987.tb10743.x. [DOI] [PubMed] [Google Scholar]
- Heisler L. M., Suzuki H., Landick R., Gross C. A. Four contiguous amino acids define the target for streptolydigin resistance in the beta subunit of Escherichia coli RNA polymerase. J Biol Chem. 1993 Dec 5;268(34):25369–25375. [PubMed] [Google Scholar]
- Heumann H., Lederer H., Kammerer W., Palm P., Metzger W., Baer G. Large-scale preparation of a DNA fragment containing the strong promoter A1 of the phage T7. Biochim Biophys Acta. 1987 Jul 14;909(2):126–132. doi: 10.1016/0167-4781(87)90034-0. [DOI] [PubMed] [Google Scholar]
- Holmgren A., Slabý I. Thioredoxin-C': mechanism of noncovalent complementation and reactions of the refolded complex and the active site containing fragment with thioredoxin reductase. Biochemistry. 1979 Dec 11;18(25):5591–5599. doi: 10.1021/bi00592a011. [DOI] [PubMed] [Google Scholar]
- Honoré N., Bergh S., Chanteau S., Doucet-Populaire F., Eiglmeier K., Garnier T., Georges C., Launois P., Limpaiboon T., Newton S. Nucleotide sequence of the first cosmid from the Mycobacterium leprae genome project: structure and function of the Rif-Str regions. Mol Microbiol. 1993 Jan;7(2):207–214. doi: 10.1111/j.1365-2958.1993.tb01112.x. [DOI] [PubMed] [Google Scholar]
- Hudson G. S., Holton T. A., Whitfield P. R., Bottomley W. Spinach chloroplast rpoBC genes encode three subunits of the chloroplast RNA polymerase. J Mol Biol. 1988 Apr 20;200(4):639–654. doi: 10.1016/0022-2836(88)90477-9. [DOI] [PubMed] [Google Scholar]
- Iwabe N., Kuma K., Kishino H., Hasegawa M., Miyata T. Evolution of RNA polymerases and branching patterns of the three major groups of Archaebacteria. J Mol Evol. 1991 Jan;32(1):70–78. doi: 10.1007/BF02099931. [DOI] [PubMed] [Google Scholar]
- Jin D. J., Gross C. A. Mapping and sequencing of mutations in the Escherichia coli rpoB gene that lead to rifampicin resistance. J Mol Biol. 1988 Jul 5;202(1):45–58. doi: 10.1016/0022-2836(88)90517-7. [DOI] [PubMed] [Google Scholar]
- Kashlev M., Lee J., Zalenskaya K., Nikiforov V., Goldfarb A. Blocking of the initiation-to-elongation transition by a transdominant RNA polymerase mutation. Science. 1990 May 25;248(4958):1006–1009. doi: 10.1126/science.1693014. [DOI] [PubMed] [Google Scholar]
- Kashlev M., Martin E., Polyakov A., Severinov K., Nikiforov V., Goldfarb A. Histidine-tagged RNA polymerase: dissection of the transcription cycle using immobilized enzyme. Gene. 1993 Aug 16;130(1):9–14. doi: 10.1016/0378-1119(93)90340-9. [DOI] [PubMed] [Google Scholar]
- Kashlev M., Nudler E., Goldfarb A., White T., Kutter E. Bacteriophage T4 Alc protein: a transcription termination factor sensing local modification of DNA. Cell. 1993 Oct 8;75(1):147–154. [PubMed] [Google Scholar]
- Kato I., Anfinsen C. B. On the stabilization of ribonuclease S-protein by ribonuclease S-peptide. J Biol Chem. 1969 Feb 10;244(3):1004–1007. [PubMed] [Google Scholar]
- Krummel B., Chamberlin M. J. Structural analysis of ternary complexes of Escherichia coli RNA polymerase. Deoxyribonuclease I footprinting of defined complexes. J Mol Biol. 1992 May 20;225(2):239–250. doi: 10.1016/0022-2836(92)90918-a. [DOI] [PubMed] [Google Scholar]
- Landick R., Stewart J., Lee D. N. Amino acid changes in conserved regions of the beta-subunit of Escherichia coli RNA polymerase alter transcription pausing and termination. Genes Dev. 1990 Sep;4(9):1623–1636. doi: 10.1101/gad.4.9.1623. [DOI] [PubMed] [Google Scholar]
- Li C. H., Bewley T. A. Human pituitary growth hormone: restoration of full biological activity by noncovalent interaction of two fragments of the hormone. Proc Natl Acad Sci U S A. 1976 May;73(5):1476–1479. doi: 10.1073/pnas.73.5.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisitsyn N. A., Sverdlov E. D., Moiseyeva E. P., Danilevskaya O. N., Nikiforov V. G. Mutation to rifampicin resistance at the beginning of the RNA polymerase beta subunit gene in Escherichia coli. Mol Gen Genet. 1984;196(1):173–174. doi: 10.1007/BF00334112. [DOI] [PubMed] [Google Scholar]
- Mustaev A., Kashlev M., Lee J. Y., Polyakov A., Lebedev A., Zalenskaya K., Grachev M., Goldfarb A., Nikiforov V. Mapping of the priming substrate contacts in the active center of Escherichia coli RNA polymerase. J Biol Chem. 1991 Dec 15;266(35):23927–23931. [PubMed] [Google Scholar]
- Mustaev A., Kashlev M., Zaychikov E., Grachev M., Goldfarb A. Active center rearrangement in RNA polymerase initiation complex. J Biol Chem. 1993 Sep 15;268(26):19185–19187. [PubMed] [Google Scholar]
- Nudler E., Goldfarb A., Kashlev M. Discontinuous mechanism of transcription elongation. Science. 1994 Aug 5;265(5173):793–796. doi: 10.1126/science.8047884. [DOI] [PubMed] [Google Scholar]
- Ogryz'ko E. P., Nikiforov V. G., Borodin A. M., Danilkovich A. V., Monastyrskaia G. S. Aminokislotnye zameny v beta-sub''edinitse RNK-polimerazy E. coli, kompensiruiushchie mutatsionnye povrezhdeniia faktora terminatsii ro. Bioorg Khim. 1988 Jul;14(7):963–964. [PubMed] [Google Scholar]
- Ovchinnikov YuA, Monastyrskaya G. S., Gubanov V. V., Lipkin V. M., Sverdlov E. D., Kiver I. F., Bass I. A., Mindlin S. Z., Danilevskaya O. N., Khesin R. B. Primary structure of Escherichia coli RNA polymerase nucleotide substitution in the beta subunit gene of the rifampicin resistant rpoB255 mutant. Mol Gen Genet. 1981;184(3):536–538. doi: 10.1007/BF00352535. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov Y. A., Monastyrskaya G. S., Gubanov V. V., Guryev S. O., Chertov OYu, Modyanov N. N., Grinkevich V. A., Makarova I. A., Marchenko T. V., Polovnikova I. N. The primary structure of Escherichia coli RNA polymerase. Nucleotide sequence of the rpoB gene and amino-acid sequence of the beta-subunit. Eur J Biochem. 1981 Jun 1;116(3):621–629. doi: 10.1111/j.1432-1033.1981.tb05381.x. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov Y. A., Monastyrskaya G. S., Guriev S. O., Kalinina N. F., Sverdlov E. D., Gragerov A. I., Bass I. A., Kiver I. F., Moiseyeva E. P., Igumnov V. N. RNA polymerase rifampicin resistance mutations in Escherichia coli: sequence changes and dominance. Mol Gen Genet. 1983;190(2):344–348. doi: 10.1007/BF00330662. [DOI] [PubMed] [Google Scholar]
- Pühler G., Leffers H., Gropp F., Palm P., Klenk H. P., Lottspeich F., Garrett R. A., Zillig W. Archaebacterial DNA-dependent RNA polymerases testify to the evolution of the eukaryotic nuclear genome. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4569–4573. doi: 10.1073/pnas.86.12.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint Girons I., Gilles A. M., Margarita D., Michelson S., Monnot M., Fermandjian S., Danchin A., Bârzu O. Structural and catalytic characteristics of Escherichia coli adenylate kinase. J Biol Chem. 1987 Jan 15;262(2):622–629. [PubMed] [Google Scholar]
- Schultz P., Célia H., Riva M., Sentenac A., Oudet P. Three-dimensional model of yeast RNA polymerase I determined by electron microscopy of two-dimensional crystals. EMBO J. 1993 Jul;12(7):2601–2607. doi: 10.1002/j.1460-2075.1993.tb05920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severinov K., Kashlev M., Severinova E., Bass I., McWilliams K., Kutter E., Nikiforov V., Snyder L., Goldfarb A. A non-essential domain of Escherichia coli RNA polymerase required for the action of the termination factor Alc. J Biol Chem. 1994 May 13;269(19):14254–14259. [PubMed] [Google Scholar]
- Severinov K., Mustaev A., Kashlev M., Borukhov S., Nikiforov V., Goldfarb A. Dissection of the beta subunit in the Escherichia coli RNA polymerase into domains by proteolytic cleavage. J Biol Chem. 1992 Jun 25;267(18):12813–12819. [PubMed] [Google Scholar]
- Severinov K., Soushko M., Goldfarb A., Nikiforov V. RifR mutations in the beginning of the Escherichia coli rpoB gene. Mol Gen Genet. 1994 Jul 25;244(2):120–126. doi: 10.1007/BF00283512. [DOI] [PubMed] [Google Scholar]
- Shiba K., Schimmel P. Functional assembly of a randomly cleaved protein. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1880–1884. doi: 10.1073/pnas.89.5.1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba K., Schimmel P. Tripartite functional assembly of a large class I aminoacyl tRNA synthetase. J Biol Chem. 1992 Nov 15;267(32):22703–22706. [PubMed] [Google Scholar]
- Strittmatter P., Barry R. E., Corcoran D. Tryptic conversion of cytochrome b 5 reductase to an active derivative containing two peptide chains. J Biol Chem. 1972 May 10;247(9):2768–2775. [PubMed] [Google Scholar]
- Sweetser D., Nonet M., Young R. A. Prokaryotic and eukaryotic RNA polymerases have homologous core subunits. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1192–1196. doi: 10.1073/pnas.84.5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrubel W., Stochaj U., Sonnewald U., Theres C., Ehring R. Reconstitution of an active lactose carrier in vivo by simultaneous synthesis of two complementary protein fragments. J Bacteriol. 1990 Sep;172(9):5374–5381. doi: 10.1128/jb.172.9.5374-5381.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalenskaya K., Lee J., Gujuluva C. N., Shin Y. K., Slutsky M., Goldfarb A. Recombinant RNA polymerase: inducible overexpression, purification and assembly of Escherichia coli rpo gene products. Gene. 1990 Apr 30;89(1):7–12. doi: 10.1016/0378-1119(90)90199-2. [DOI] [PubMed] [Google Scholar]