Abstract
The plant hormone ethylene plays a role in various growth related processes. In this detailed study of the vegetative growth of Arabidopsis, Nicotiana tabacum, and Petunia x hybrida plants, we show that ethylene insensitivity does not result in an increased total leaf area or relative growth rate (RGR) under optimal growth conditions. When grown in semiclosed containers, leaf area of ethylene-insensitive plants was larger compared to the wild type. This effect was caused by a buildup of ethylene inside these containers, which inhibited the growth of wild-type plants. Ethylene-insensitive Arabidopsis and N. tabacum plants had a lower biomass, which was mainly the result of a smaller seed mass. RGR of vegetative plants was not affected by ethylene insensitivity, but the underlying components of RGR differed; specific leaf area (leaf area per unit leaf mass) was higher, and unit leaf rate (growth rate per unit leaf area) was lower. The latter was a result of a slower rate of photosynthesis per unit leaf area in the ethylene-insensitive plants.
Auxins, cytokinins, gibberellic acid, and abscisic acid play a role in the regulation of growth and can affect the rate of photosynthesis (Mansfield and McAinsh, 1995; Brenner and Cheikh, 1995) as well as plant morphology (Reid and Howel, 1995). The gas ethylene is also highly active morphogenetically, and its effects have been extensively studied in a range of species, including Arabidopsis seedlings (e.g. Bleecker et al., 1988; Guzman and Ecker, 1990; Smalle et al., 1997; Hall et al., 1999). Thickening of the hypocotyl, reduction in hypocotyl and root elongation, and a diageotropic orientation are typical effects of exogenously applied ethylene (Abeles et al., 1992). Although in many (semi)aquatic plants ethylene stimulates leaf and petiole extension (Voesenek et al., 2003), in terrestrial plants ethylene generally inhibits leaf expansion (Abeles et al., 1992). In support of this view are observations of larger rosette leaves in ethylene-insensitive Arabidopsis mutants (Guzman and Ecker, 1990; Ecker, 1995). More specifically, total leaf area of rosette leaves of ethylene-insensitive Arabidopsis genotypes (etr1-1 and ers1) was reported to be 25% to 50% larger than that of wild-type plants (Bleecker et al., 1988; Hua et al., 1995). The increase in leaf area was attributed to an increased cell expansion in ethylene-insensitive plants (Hua et al., 1995). A greater leaf area in ethylene-insensitive plants suggests that the low endogenous ethylene concentrations in the wild type have an inhibiting effect on leaf expansion. However, it could be that leaf expansion of wild-type plants was inhibited because ethylene accumulated in semiclosed tissue-culture containers used in some of this work (Kieber et al., 1993).
Leaf area is not necessarily a good descriptor of growth. A way to achieve a better understanding of the underlying growth processes is a growth analysis as described in, for example, Poorter (2002). In such an analysis, one of the parameters used is the leaf area per unit leaf dry mass or specific leaf area (SLA). Leaves vary in SLA, which affects the amount of photosynthetically active radiation captured per unit of mass. For example, a leaf with a high SLA will capture more light per unit mass than a leaf with a low SLA. Variation in SLA is of major importance when explaining differences between species in the increase of biomass per unit mass per day (relative growth rate [RGR]; Poorter and Remkes, 1990; Garnier, 1992; Atkin et al., 1996). A second factor affecting RGR is the allocation of mass to leaves. More allocation of mass to leaves, compared to stems and roots, results in a larger leaf area and, thus, more light capture per unit plant mass. The third factor is the increase in biomass per unit leaf area per day and is called unit leaf rate (ULR). ULR is driven by the carbon fixation in the process of photosynthesis. A part of the carbon fixated is respired by shoots and roots, providing energy for biosynthesis and maintenance; the remaining carbon being incorporated into the biomass of the plant. In contrast with SLA, variation in ULR appears to be of minor importance for explaining differences in RGR between species (Poorter and Van der Werf, 1998). It has been shown previously that a decrease of the ULR due to environmental factors such as low light or CO2 levels can be compensated by an increase in SLA (Poorter and Nagel, 2000).
To our knowledge, no growth analysis has ever been made of ethylene-insensitive plants, but a small number of previous studies describe the effects of ethylene insensitivity on photosynthetic processes. Zhou et al. (1998) observed that the greening of cotyledons in the presence of high glucose levels is more strongly inhibited in Arabidopsis etr1-1 mutants compared to wild-type plants. This suggests a possible effect of ethylene insensitivity on the sugar-dependent repression of photosynthetic gene expression (Krapp et al., 1993). Furthermore, Grbić and Bleecker (1995) showed that, in nonsenescing leaves, ethylene-insensitive etr1-1 mutants have less chlorophyll and a lower amount of active Rubisco per area than wild-type Arabidopsis leaves. It is likely that less Rubisco is linked to a lower organic nitrogen content and a lower rate of photosynthesis per unit leaf area (Evans and Seemann, 1989).
Given that the current knowledge about the role of ethylene in vegetative plant growth is largely incomplete, we aim to improve the understanding of whole-plant growth and allocation in ethylene-insensitive genotypes. To evaluate possible species-specific effects of ethylene insensitivity on plant growth parameters, three different species were examined. A growth analysis was performed on hydroponics as well as on soil to make the results more broadly applicable. First, we tested the sensitivity of ethylene-sensitive and -insensitive Arabidopsis, Nicotiana tabacum, and Petunia x hybrida genotypes to ethylene. Second, we determined the total leaf area of Arabidopsis grown on petri dishes with or without an ethylene absorbing compound. These results were compared with the total leaf area of plants growing in a well-ventilated climate room. Third, we studied the RGR and its components in ethylene-sensitive and -insensitive genotypes of the three species. Last, we analyzed whether the lower amount of active Rubisco per area reported for Arabidopsis etr1-1 mutants is reflected in a lower nitrogen content and photosynthesis per unit leaf area in all three ethylene-insensitive genotypes.
We conclude that leaf area of ethylene-sensitive Arabidopsis was reduced when the plants were grown for 2 weeks in semiclosed containers. There was no difference in total leaf area or RGR between ethylene-sensitive and -insensitive plants under optimal conditions. However, the components underlying RGR were different: The ethylene-insensitive plants were found to have a higher SLA but a lower ULR than the ethylene-sensitive controls. The lower ULR was shown to be the outcome of a lower rate of photosynthesis in ethylene-insensitive plants.
RESULTS
Ethylene Sensitivity
To confirm the ethylene insensitivity of the mutant and transgenic lines, their response capacity to 10 μL L−1 applied ethylene was assessed in terms of extension growth of seedling roots, seedling hypocotyls, adult roots, and chlorophyll content of full-grown leaves (Table I). All mutant and transgenic lines showed an impaired response to the applied ethylene. Overall, P. hybrida Atetr1-1 showed the most residual responsiveness and N. tabacum Atetr1-1 the least.
Table I.
Effects of applying 10 μL L−1 ethylene on the length of seedling roots and hypocotyls (Arabidopsis, 3 d old; N. tabacum and P. hybrida, 7 d old), root length of 3-week-old plants, and chlorophyll content per unit area in full-grown leaves of 3-week-old plants
| Arabidopsis (%)
|
N. tabacum (%)
|
P. hybrida (%)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| WT | etr1-1 | P | NT | Atetr1-1 | P | NT | Atetr1-1 | P | |
| Seedling root length | 72 ± 1 | 5 ± 3 | *** | 79 ± 1 | 2 ± 1 | *** | 68 ± 1 | 30 ± 4 | *** |
| Seedling hypocotyl length | 56 ± 2 | 12 ± 6 | *** | 39 ± 11 | −1 ± 1 | *** | 31 ± 1 | 30 ± 1 | NS |
| Adult root length | 23 ± 2 | 8 ± 6 | * | 13 ± 2 | 4 ± 5 | * | 15 ± 1 | 5 ± 5 | * |
| Chlorophyll content per unit area | 36 ± 2 | 14 ± 5 | ** | 39 ± 3 | 2 ± 1 | *** | 55 ± 4 | 40 ± 2 | * |
The etr1-1 mutant and transgenic plants are compared to the wild type (WT) or nontransformed (NT) controls. The ethylene response capacity is expressed as the reduction in length (or chlorophyll content per unit area) relative to air-grown plants. Mean values ± se (n = 6). ***, P < 0.001; **, P < 0.01; *, P < 0.05; NS, Not significant.
Ethylene Accumulation and Total Leaf Area
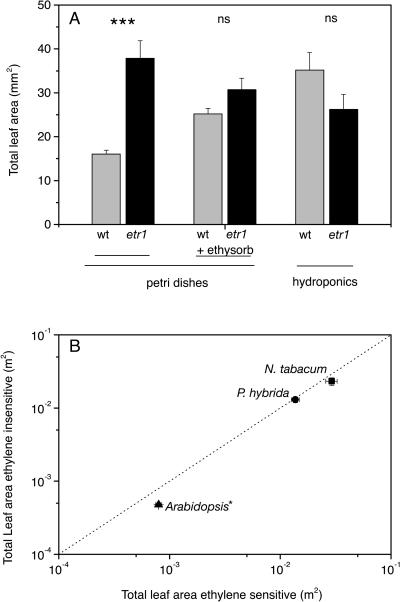
After 2 weeks of growth in petri dishes, wild-type Arabidopsis plants enriched the enclosed gas space with more than 0.08 μL L−1 ethylene. This concentration was 8 times larger than the ambient concentration in the growth room. The concentration of ethylene in petri dishes in which the etr1-1 mutants were growing was almost an order of magnitude greater (0.70 μL L−1), a likely outcome of larger plant size and a constitutive higher ethylene production rate (Guzman and Ecker, 1990). Total leaf area of the wild type was 57% (P < 0.001) smaller than ethylene-insensitive etr1-1 (Fig. 1A). However, adding an ethylene absorbent (Ethysorb) to the petri dishes almost completely rescued the growth reduction of the wild type, giving a leaf area indistinguishable from that of ethylene-insensitive mutants. Furthermore, when germinating and growing the Arabidopsis seedlings in a well-ventilated climate room that avoided ethylene accumulation, total leaf area of 14-d-old etr1-1 mutants was not larger than that of the wild type (Fig. 1A). Similar results were obtained with Arabidopsis, N. tabacum, and P. hybrida grown on hydroponics for a longer time period. In this case, total leaf area of ethylene-insensitive Arabidopsis plants was even slightly smaller compared to ethylene-sensitive controls (Fig. 1B). Further confirmation was sought using plants grown in well-ventilated conditions using soil as the growing medium. Again, no stimulating effect of ethylene insensitivity was observed on total leaf area (Fig. 2). However, ethylene insensitivity was not without some influence on plant growth since the total plant dry mass of all ethylene-insensitive genotypes was found to be significantly smaller than that of ethylene-sensitive controls (Table II).
Figure 1.
Comparison of total leaf areas of ethylene-sensitive and -insensitive genotypes. A, Arabidopsis wild-type and ethylene-insensitive etr1-1 mutants grown for 2 weeks in sealed petri dishes (n = 40), in petri dishes containing Ethysorb (n = 40), and on hydroponics in the open (n = 6). Mean values ± se. ***, significant difference between ethylene-sensitive and -insensitive plants (P < 0.001). Ethylene concentrations inside the petri dishes were: wild type, 0.08 μL L−1; etr1-1, 0.70 μL L−1; wild type + Ethysorb, 0.02 μL L−1; and etr1-1 + Ethysorb, 0.03 μL L−1. B, Total leaf area after 2 weeks (Arabidopsis, triangles) or 4 weeks (N. tabacum, squares; P. hybrida, circles) of growth on hydroponics. The dotted line represents the 1:1 ratio. *, significant difference between ethylene-sensitive and -insensitive plants (P < 0.05). Mean values ± se (n = 18).
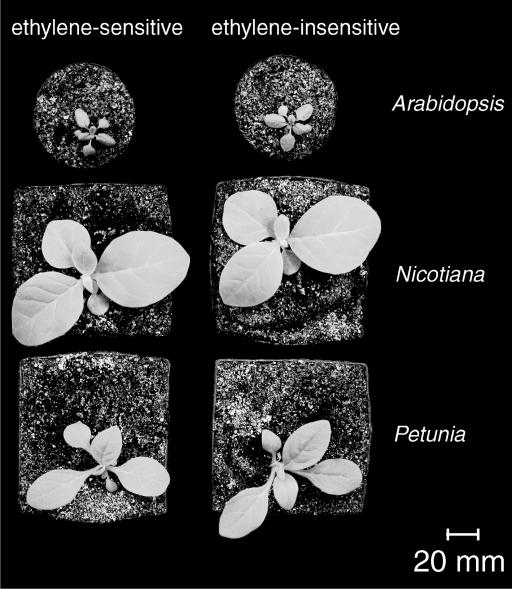
Figure 2.
Ethylene-sensitive (left) and -insensitive (right) Arabidopsis (top), N. tabacum (middle), and P. hybrida (bottom) plants after 14 d of growth on soil.
Table II.
Growth parameters of plants grown on soil
| Arabidopsis
|
N. tabacum
|
P. hybrida
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| WT | etr1-1 | P | NT | Atetr1-1 | P | NT | Atetr1-1 | P | |
| Total dry mass (g) | 0.035 ± 0.003 | 0.014 ± 0.001 | *** | 3.0 ± 0.1 | 2.0 ± 0.1 | *** | 1.5 ± 0.1 | 1.1 ± 0.1 | ** |
| RGR (mg g−1 d−1) | 260 ± 14 | 268 ± 13 | NS | 230 ± 7 | 219 ± 9 | NS | 220 ± 4 | 205 ± 12 | NS |
| SLA (m2 kg−1) | 47 ± 2 | 57 ± 2 | *** | 31 ± 2 | 46 ± 2 | *** | 42 ± 2 | 45.8 ± 0.9 | * |
| LMF (g g−1) | 0.71 ± 0.02 | 0.71 ± 0.02 | NS | 0.65 ± 0.01 | 0.68 ± 0.01 | * | 0.57 ± 0.01 | 0.61 ± 0.01 | ** |
| ULR (g m−2 d−1) | 8.0 ± 0.4 | 6.5 ± 0.4 | * | 12.1 ± 0.7 | 7.3 ± 0.5 | *** | 9.7 ± 0.6 | 7.3 ± 0.5 | ** |
The etr1-1 mutant and transgenic plants are compared to the wild type (WT) or nontransformed (NT) controls. Mean values ± se (n = 7). ***, P < 0.001. **, P < 0.01. *, P < 0.05. NS, Not significant.
Growth Analysis
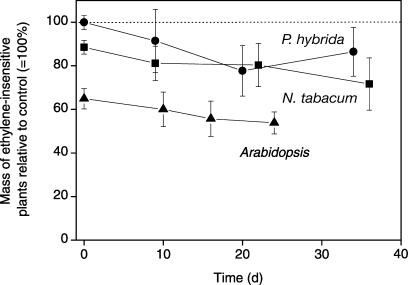
There are several factors that determine total plant biomass at any given time. First, differences in seed mass can have a large effect on the size of the adult plants to which they give rise (Leishman et al., 2000). Second, a slower germination can delay development and thus result in smaller plants. Third, the plants can differ in their relative growth rate. To separate out these possibilities, we studied the contribution of seed mass, timing of germination, and growth rate of the adult plant to the lower biomass of the ethylene-insensitive genotypes. Surprisingly, seed mass of ethylene-insensitive N. tabacum and Arabidopsis was 15% and 35% smaller compared to ethylene-sensitive seeds. The relative biomass of ethylene-insensitive plants did not decrease significantly between the seed and seedling stage (Fig. 3), a result of a similar growth rate and the fact that in all examined species, there was less than a day difference in the timing of germination (data not shown). In addition, there was no further significant decrease in relative biomass of the ethylene-insensitive genotypes after transplantation of the seedlings to hydroponics, signifying no differences in growth rate during growth on hydroponics. In conclusion, the lower biomass of ethylene-insensitive genotypes was found to arise mainly from a lighter seed mass.
Figure 3.
Total dry mass of ethylene-insensitive Arabidopsis, N. tabacum, and P. hybrida relative to the ethylene-sensitive controls (represented by the dotted line). The first time point is at the seed stage, the second time point is a seedling with two leaves, and the third and fourth time points correspond with the two harvests taken for the growth analysis. In Arabidopsis and N. tabacum, the differences in mass in adult plants are predominantly caused by a difference in seed mass.
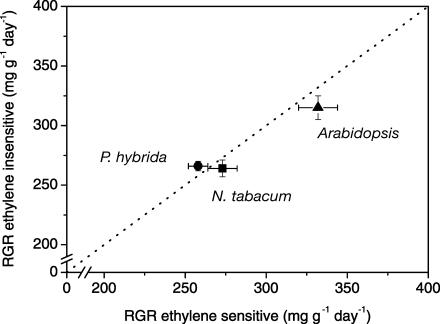
Growth in ethylene-sensitive and -insensitive plants was examined in greater detail, through analysis of RGR and its underlying components (as described in “Materials and Methods”). On hydroponics, all three species had high growth rates (above 250 mg g−1 d−1), and the RGR of ethylene-sensitive genotypes was statistically indistinguishable from that of the corresponding ethylene-sensitive plants of the same species (Fig. 4). For plants grown on soil, the growth rates were lower compared to hydroponics culture, but again no differences between ethylene-sensitive and -insensitive plants were observed (Table II).
Figure 4.
Effect of ethylene insensitivity on the RGR of plants growing on hydroponics for 2 weeks (Arabidopsis, triangles) or 4 weeks (N. tabacum, squares; P. hybrida, circles). The dotted line represents the 1:1 ratio. Mean values ± se (n = 18).
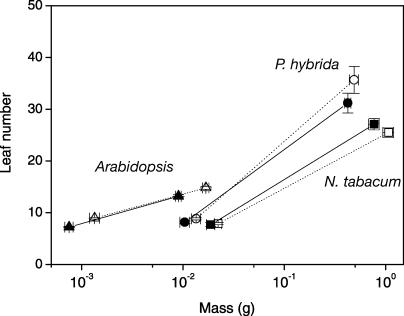
Growth parameters determining RGR are known to vary depending on the species and the developmental stage of the plants. To overcome this complication when making comparisons between different lines, all growth parameters were plotted against total plant dry mass. Since leaf number is a common measure for the developmental stage of Arabidopsis, we also examined the relationship between the total dry mass and leaf number for all three species. Figure 5 shows that there were no significant differences in leaf number between ethylene-sensitive and -insensitive plants of the same mass over the time period of our experiments.
Figure 5.
The relationship between leaf number and total plant dry mass of Arabidopsis (triangles), N. tabacum (squares), and P. hybrida (circles). Closed symbols and continuous lines represent the ethylene-insensitive genotypes, whereas open symbols and dotted lines represent the ethylene-sensitive plants. Mean values ± se (n = 18) are shown.
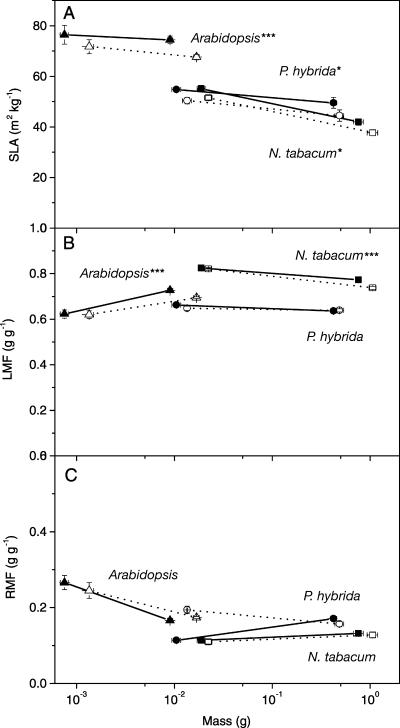
When examining components that determine relative growth rate, it was found that the leaf area per leaf mass (SLA) was significantly greater in all three ethylene-insensitive genotypes (Fig. 6A; Table II). A higher SLA can be the outcome of thinner leaves or leaves with a lower density. Ethylene-insensitive leaves indeed had a lower density than ethylene-sensitive plants, but this was not due to an increase in leaf porosity (25% air volume per total volume for N. tabacum and P. hybrida, 19% for Arabidopsis). The biomass allocation to the leaves, expressed as leaf mass fraction (LMF), was somewhat higher (5%, P < 0.05) in both ethylene-insensitive Arabidopsis and N. tabacum as compared to ethylene-sensitive controls (Fig. 6B). In ethylene-insensitive P. hybrida and N. tabacum, a significantly higher LMF was observed when the plants were grown on soil (Table II). Allocation of biomass to the roots, expressed as root mass fraction (RMF), was lower for ethylene-insensitive P. hybrida plants during the early stages of growth on hydroponics; in all other cases, we found no significant differences (Fig. 6C). The growth rate per unit leaf area (ULR) of ethylene-insensitive Arabidopsis and N. tabacum was almost 15% lower compared to controls, but in P. hybrida the effect was not statistically significant (Fig. 7A) unless the plants were grown on soil (Table II). We conclude that, in ethylene-insensitive genotypes, a lower ULR (−15%) antagonizes the effect of a slightly higher SLA (10%) and LMF (5%), leading to a RGR similar to that of ethylene-sensitive plants.
Figure 6.
Effect of ethylene insensitivity on leaf area per unit leaf mass (SLA; A), leaf mass per plant mass (LMF; B), and root mass per plant mass (RMF; C) of Arabidopsis (triangles), N. tabacum (squares), and P. hybrida (circles). Values are plotted against whole-plant dry mass so that plants of similar size and stage of development can be compared. Closed symbols and continuous lines represent the ethylene-insensitive genotypes, and open symbols and dotted lines represent the ethylene-sensitive plants. ***, significant difference in the last harvest with P < 0.001; *, significant difference with P < 0.05. Mean values ± se (n = 18) are shown.
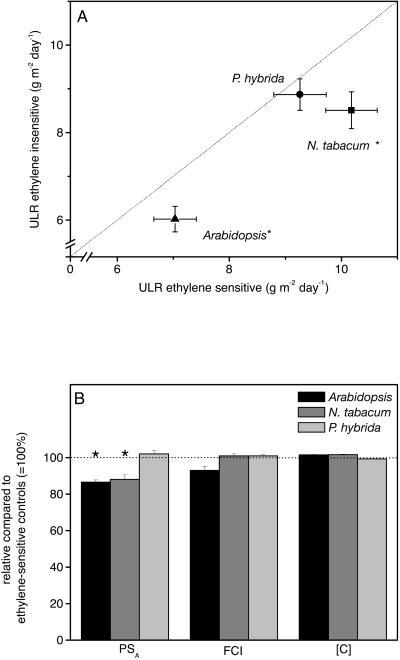
Figure 7.
Effect of ethylene insensitivity on growth rate per unit area (ULR; A). Symbols and lines are explained in Figure 4. * represents a significant difference with P < 0.05. Mean values ± se are given, n = 18. B, Components of the ULR expressed as percentage from ethylene-sensitive plants (100%): photosynthesis (PSA), fraction of daily fixed carbon that is incorporated (FCI), and carbon concentration ([C]). n = 8.
Photosynthesis, Respiration, and Nitrogen Content
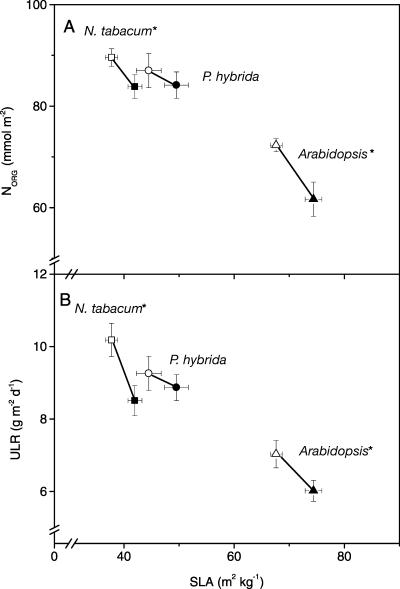
The growth parameter ULR can also be calculated from physiological parameters using Equation 5 (see “Materials and Methods”). The resulting differences in ULR between ethylene-sensitive and -insensitive plants were found to be similar to the differences in ULR calculated from biomass and area measurements (Eq. 4) as described above (Fig. 7A). Figure 7B shows that the lower ULR in ethylene-insensitive plants is predominantly due to a slower rate of photosynthesis per unit area (PSA; Table III) and not a consequence of the fraction of photosynthetically fixed carbon that is incorporated into biomass instead of respired (FCI) or of differences in the carbon content ([C]). Only in ethylene-insensitive P. hybrida plants we found no significant decrease in net PSA (Fig. 7B). In the ethylene-insensitive genotypes with a slower rate of photosynthesis per area, we also found a lower organic nitrogen content per unit leaf area (Fig. 8A). There was no significant difference in photosynthetic nitrogen use efficiency (rate of photosynthesis per mol of nitrogen) between ethylene-sensitive and -insensitive plants (data not shown).
Table III.
Rate of photosynthesis per area of the ethylene-sensitive and -insensitive genotypes
| Net Photosynthesis (μmol CO2 m−2 s−1)
|
||
|---|---|---|
| Ethylene Sensitive | Ethylene Insensitive | |
| Arabidopsis | 6.6 ± 0.3 | 5.8 ± 0.3* |
| N. tabacum | 10.5 ± 0.5 | 9.2 ± 0.4* |
| P. hybrida | 11.8 ± 0.2 | 11.6 ± 0.3 |
Mean values ± se (n = 8).
The ethylene-insensitive plants were significantly different from the sensitive plants (P < 0.05).
Figure 8.
Effect of ethylene insensitivity on organic nitrogen content (NORG; A) and the relationship between ULR and SLA (B). The relationship between SLA and ULR or NORG suggests a trade-off resulting in a constant RGR. Symbols are explained in Figure 4. Mean values ± se are given (*, P < 0.05).
DISCUSSION
The impact of insensitivity to ethylene on growth was assessed using plants containing the mutated ethylene receptor gene etr1-1 from Arabidopsis. No stimulating effect of ethylene insensitivity on total leaf area was found when using plants growing in well-ventilated conditions using soil or hydroponics. In separate experiments, similar results were obtained with ethylene-insensitive Arabidopsis ein2 and ein4 mutants (data not shown). When growing plants in (semi)closed tissue-culture containers, leaf growth of wild-type plants was inhibited. We have shown that this was a result of ethylene accumulation inside the containers (Fig. 1), as was suggested previously by Kieber et al. (1993). Similar effects of enclosure have been reported before in studies of leaf abscission and ethylene-mediated auxin effects (Chadwick and Burg, 1970) and can be overcome by ethylene absorption (Jackson and Osborne, 1970) or ventilation of the containers (Zobayed et al., 2001). Accordingly, in our work a leaf growth inhibiting effect of accumulated ethylene was revealed by the promotive influence of an ethylene absorbent.
The absence of a stimulating effect of ethylene insensitivity on total leaf area seems to contrast with previously reported results (Bleecker et al., 1988; Guzman and Ecker, 1990; Grbić and Bleecker, 1995; Hua et al., 1995). In those studies, ethylene-insensitive plants show an increased leaf area, even when grown on soil and in well-ventilated conditions (J. Hua, personal communication; A. Bleecker, personal communication). Although the light levels typically used in those studies were lower than 200 μmol m−2 s−1, we found no increase in the leaf area of etr1-1 mutants relative to the wild type when the plants were exposed to only 100 μmol m−2 s−1 (data not shown). We think the discrepancy between previous findings and our results can be explained by a difference in the moment of measuring the leaves. Our results were obtained by studying plants during the vegetative growth stage, in contrast with the other work mentioned in which a larger total leaf area was found during or after bolting, measured when the leaves of etr1-1 had (nearly) fully expanded (Guzman and Ecker, 1990; J. Hua, personal communication; A. Bleecker, personal communication). Leaf longevity in etr1-1 is increased by 30% as a result of the later onset of senescence (Grbić and Bleecker, 1995). This longer growth period of etr1-1 leaves is a likely explanation for the larger full-grown leaves previously reported for ethylene-insensitive mutants.
Taken together, our data show that ethylene-insensitive plants do not have a larger leaf area compared to ethylene-sensitive plants during their vegetative growth stage. In this view, the low, endogenous ethylene concentrations normally present in the wild type have no negative impact on vegetative growth. In fact, it could well be that very low concentrations (<0.05 μL L−1) of ethylene can stimulate leaf expansion, both in dicots (Lee and Reid, 1997) and monocots (Fiorani et al., 2002).
Previous work on ethylene perception mutants focused on the differences in leaf area as a parameter of plant growth. In this work the differences in total plant dry mass were also examined. We observed a reduced biomass in the ethylene-insensitive Arabidopsis and N. tabacum. This could be caused by a smaller seed mass, delayed germination, or a reduced RGR. It has been reported that the ethylene-insensitive Arabidopsis mutant displays enhanced seed dormancy (Bleecker et al., 1988; Beaudoin et al., 2000). However, our results indicate that the reduced biomass of ethylene-insensitive Arabidopsis and N. tabacum was mainly caused by a lower seed mass (Fig. 3), that is carried through into a smaller plant size for at least 5 weeks. An equal RGR between ethylene-sensitive and -insensitive genotypes does not automatically imply that the underlying growth components are not affected. RGR is determined by leaf area per unit leaf mass (SLA), the relative amount of leaf biomass (LMF), and the growth rate per unit leaf area (ULR). In our experiments, we found that ethylene insensitivity conferred a 10% increase in SLA and a 5% increase in LMF in Arabidopsis and N. tabacum. The latter is a result of a lower stem mass per plant mass, as we observed a later onset of stem development of the insensitive genotypes (data not shown; Pierik et al., 2003).
Defects in ethylene perception can result in abnormal development of tomato (Lycopersicon esculentum) and P. hybrida roots, especially in response to mechanical impedance (Clark et al., 1999). In this work, we found no differences in total biomass allocation to the roots (RMF) at the last harvest. A lower RMF was observed in the first harvest of ethylene-insensitive P. hybrida, just after transplanting the plants from sand to hydroponics (Fig. 6C).
A lower chlorophyll and active Rubisco content per unit leaf area was shown by Grbić and Bleecker (1995) in Arabidopsis etr1-1 mutants, but no explanation was given for this fact, and the actual rate of photosynthesis in ethylene-insensitive plants was not measured. The lower organic nitrogen content per area, found in our study, suggests that there is a dilution of the photosynthetic machinery over the leaf area, and this may be the cause of slower photosynthesis per unit leaf area in ethylene-insensitive plants. We found a negative relationship between ULR and SLA for all species (Fig. 8B). Konings (1989) and Poorter (1989) already suggested that an increased SLA may decrease nitrogen content and photosynthesis per unit area. Indeed, measurements of the organic nitrogen content per unit leaf area show this expected negative correlation with SLA (Fig. 8A).
Interestingly, there are some similarities between ethylene-insensitive plants and plants grown at low irradiances. We observed a higher SLA and lower nitrogen content per area in the ethylene-insensitive plants. Growing plants at low light also results in a higher SLA and a lower nitrogen content per unit leaf area (Evans and Poorter, 2001). Up to 75% of the leaf organic nitrogen is present in the chloroplasts, most of it in the photosynthetic machinery (Evans and Seemann, 1989). In a study comparing plants with different amounts of organic nitrogen content per area, Konings (1989) suggested that a higher SLA resulted in fewer photosynthetically active mesophyll cells per area. If the larger cell size previously reported for the epidermis of ethylene-insensitive Arabidopsis (Hua et al., 1995) also occurs in the mesophyll, this may provide an explanation for the differences in leaf area per leaf mass and photosynthesis we observed. However, cross-sections of full-grown leaves did not show clearly visible differences in the sizes of mesophyll cells or of intracellular air spaces (data not shown). An alternative explanation for the observed differences in SLA is a difference in the amount or composition of cell wall material. Less deposition of secondary cell wall thickenings would lead to a lower leaf density.
Ethylene can alter cell wall synthesis and composition (Abeles et al., 1992), and it has recently been suggested that ethylene-insensitive N. tabacum and Arabidopsis plants may be more susceptible to Pythium spp. due to an altered cell wall composition (Geraats et al., 2002). However, this last explanation does not account for the observed lower rate of photosynthesis.
In contrast to Arabidopsis and N. tabacum, SLA was the only growth parameter of P. hybrida grown on hydroponics that was significantly affected by ethylene insensitivity, although ULR and LMF differed in soil-grown P. hybrida plants. A possible explanation is that P. hybrida Atetr1-1 plants are less insensitive to ethylene compared to the other ethylene-insensitive genotypes (Table I), possibly reducing the differences in growth parameters between P. hybrida Atetr1-1 and the nontransformed controls.
For at least the three species examined in this work, ethylene insensitivity had little effect on vegetative growth and development under optimal conditions. These results make the view that ethylene, in contrast to other hormones, is not essential for normal development more broadly applicable. This is also clearly apparent in the aquatic monocot Potamogeton pectinatus, a species that lacks ethylene production and yet develops completely and reproduces both sexually and asexually (Summers et al., 1996). Ethylene is better seen as playing a more subtle role in tuning development in response to environmental stimuli or to innate circumstances (such as ripening or senescence) that induce abnormally fast endogenous production of the gas.
CONCLUSIONS
When plants are grown in semiclosed containers, accumulation of ethylene results in a smaller leaf area of ethylene-sensitive Arabidopsis compared to ethylene-insensitive plants. However, when grown in well-ventilated conditions, no increased total leaf area was found during the vegetative growth phase in the ethylene-insensitive genotypes of three different species. Growth rates were similar for ethylene-sensitive and -insensitive plants, but a lower seed mass in Arabidopsis etr1-1 and N. tabacum Atetr1-1 resulted in smaller adult plants. Ethylene-insensitive plants had a larger leaf area per leaf mass, which generally promotes growth. However, this effect was counteracted by a slower rate of photosynthesis per unit leaf area. Overall, these findings indicate little impact of endogenous levels of ethylene on the growth of nonstressed plants.
MATERIALS AND METHODS
Plant Material
Arabidopsis Columbia wild-type and etr1-1 (Bleecker et al., 1988) seeds were kindly provided by Prof. M. Koornneef (Department of Genetics, Wageningen University, The Netherlands). Seeds of Nicotiana tabacum cv Samsun NN Atetr1::Atetr1-1 and nontransformed control lines (Knoester et al., 1998) were provided by Prof. L.C. van Loon (Department of Phytopathology, Utrecht University, The Netherlands). Petunia x hybrida (W115, nontransformed, and 35S::Atetr1-1 lines) seeds were provided by Prof. G. Angenent (Plant Research International, Wageningen, The Netherlands). At least three different independent transformation lines of N. tabacum and P. hybrida were used in a triple-response assay (data not shown); further experiments were done with the most ethylene-insensitive lines.
Ethylene Response Capacity
Seedlings of all six genotypes where grown in open petri dishes placed in closed 11-L dessicators in the absence or presence of a saturating ethylene concentration (10 μL L−1; Hoek Loos, Amsterdam). The glass dessicators contained a butyl-rubber septum for taking gas samples with a 1-mL syringe. The petri dishes contained 20 wild-type or etr1-1 Arabidopsis seeds on a half-strength Murashige and Skoog medium (Murashige and Skoog, 1962) containing 0.7% (w/v) plant agar. All components of the medium were obtained from Duchefa Biochemie BV (Haarlem, The Netherlands). The pH of the medium was 5.8. The ethylene concentration was measured with a gas chromatograph (Syntech Spectras GC 955, 100 Series; Groningen, The Netherlands) equipped with a Haye Sep 80/100 column and photo-ionization detector (oven T = 105°C; minimum detectable concentration, 0.01 μL L−1).The hypocotyl and root length of eight seedlings per treatment was measured after 3 d (Arabidopsis) or 7 d (N. tabacum and P. hybrida) growth in the dark at 20°C.
The ethylene response capacity of the roots of 3-week-old plants was measured by growing six plants of each genotype on 6-L buckets filled with a modified Hoagland solution, described in Poorter and Remkes (1990). The buckets were in a growth room with an average temperature of 20 ± 0.5°C, a relative humidity of 65%, and 230 ± 20 μmol m−2 s−1 photosynthetically active radiation during a 16-h photoperiod. The nutrient solution was continuously aerated with an air flow of 0.5 L min−1. In half of the buckets, the air was mixed with 0.05 L min−1, 100 μL L−1 ethylene gas. For ethylene measurement in the nutrient solution, gas samples were taken with a syringe from a small air bubble captured for several minutes under a petri dish at the bottom of the buckets. The ethylene concentrations in all buckets varied within 10% of the applied concentration of 10 μL L−1. The growth room was well ventilated, and no increase in ethylene levels could be detected in the air of the climate room. After 1 week of growth on the buckets, the plants were harvested, and the length of the longest roots were measured.
The ethylene response capacity of full-grown leaves of 3-week-old plants was measured in terms of change in chlorophyll concentration. Parts of full-grown leaves were placed in a small volume of tap water inside 22-L dessicators in the absence or presence of 10 μL L−1 ethylene gas. For 5 d, the leaves were incubated and then removed from the containers, after which small discs were cut from the tissue. The leaf discs were quickly transferred to a vial containing N,N-dimethylformamide and stored for 5 d at 4°C in darkness. After 1 week of incubation in N,N-dimethylformamide, chlorophyll absorption was measured spectrophotometrically (Inskeep and Bloom, 1985; Porra et al., 1989).
The ethylene response capacity (ERC) of the genotypes was calculated as follows:
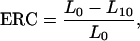 |
(Eq. 1) |
where L0 and L10 are the response values (length and chlorophyll concentration) at 0 μL L−1 and 10 μL L−1 exogenously applied ethylene.
Ethylene Accumulation
To test the effect of ethylene accumulation, 40 wild-type or etr1-1 Arabidopsis seeds were germinated in 32 glass petri dishes (ø = 10 cm) on the previously described Murashige and Skoog medium with 1% (w/v) sucrose. The petri dishes contained a butyl-rubber septum for taking gas samples and were sealed with one layer of parafilm. Note that the parafilm is gas permeable and allows ethylene to slowly diffuse out of the petri dishes. For each of the two genotypes, one gram Ethysorb, an ethylene absorbing compound (Stay Fresh, London), was placed in a small plastic container inside half of the petri dishes. The petri dishes were kept in a growth room under the same conditions as described above. After 14 d, gas samples were taken from the petri dishes with a syringe, and ethylene concentrations were determined. Thereafter, five seedlings per petri dish were harvested and total leaf area per seedling was determined.
Growth Experiments
Prior to each experiment, seed mass (dried at 70°C for 48 h) was determined. A separate batch of seeds was germinated on sand in trays covered with a glass plate and watered with a modified Hoagland solution with a nitrate concentration of 2 mm (Poorter and Remkes, 1990). The trays were kept in a growth room with an average temperature of 20 ± 0.5°C, a relative humidity of 65%, and 230 ± 20 μmol m−2 s−1 photosynthetically active radiation during a 16-h photoperiod. At 10 d (Arabidopsis) or 9 d (P. hybrida and N. tabacum) after emergence, seedling mass was determined and the plants transferred to 32-L containers of aerated nutrient solution (Poorter and Remkes, 1990). The pH was adjusted regularly to 5.8 with 1 n KOH. Eighteen plants per genotype were harvested at two time points (Fig. 3) during the growth period. The results were verified with a separate time course experiment, in which six plants per genotype were harvested at six time points over 7 d (Arabidopsis) or 14 d (N. tabacum and P. hybrida; data not shown). Fresh mass of leaves, stems, and roots and total leaf area (LI-COR LI-3100 leaf area meter; LI-COR, Lincoln, NE) were determined at each harvest. Dry masses were determined after the material had been oven-dried for at least 48 h at 70°C.
The leaf porosity (% air spaces) of full-grown leaves was measured by determining leaf buoyancy before and after vacuum infiltration of the gas spaces (Raskin, 1983; Colmer et al., 1998) with a 0.1% Tween 20 (v/v) solution (Duchefa Biochemie BV).
The growth analysis was repeated by growing the plants in soil, under the same light levels and daylength as previously described. N. tabacum and P. hybrida were grown in 500-mL pots, each containing a mixture of potting soil and perlite (1/1, v/v) including 3 g L−1 osmocote, 2 g L−1 MgO/CaO, and 2.5 mm KH2PO4. For Arabidopsis, 70-mL pots were used containing a mixture of potting soil and perlite (1/2, v/v) including 2 g L−1 osmocote and 2 g L−1 MgO/CaO. At the start, Arabidopsis plants were watered with a half-strength Hoagland nutrient solution. The pots were then transferred to irrigation mats (Maasmond-Westland, Utrecht, The Netherlands), which were automatically watered twice a day to saturation with tap water and the excess water drained.
Photosynthesis, Respiration, and Chemical Analysis
After 7 (Arabidopsis) or 14 d (N. tabacum and P. hybrida) of growth on hydroponics, eight plants were used to measure whole-plant gas exchange (Poorter et al., 1990) at the growth conditions described before. Leaf area was measured with the LI-COR LI-3100 leaf area meter, and the material was freeze-dried (VirTis, Gardiner, NY) for 48 h. Carbon and nitrogen concentrations of the freeze-dried material were determined with a C-H-N analyzer (model 1106; Carlo Erba, Milan). Nitrate content was determined from the same plant material using a colorimetrical assay as described by Cataldo et al. (1975). Organic nitrogen content was calculated as the total nitrogen content minus the nitrate content.
Growth Analysis
On two time points, leaf number, leaf area, and the dry and fresh mass of roots, stem and leaves of each plant were measured. From these measurements, the following growth parameters can be calculated. The net dry biomass increase per unit dry mass per day is the RGR (mg g−1 d−1). RGR was calculated using the classical approach (Hunt, 1982):
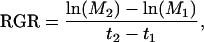 |
(Eq. 2) |
where M1 and M2 is the plant dry mass at time t1 and t2, respectively. RGR can be factorized into three components (Evans, 1972). The first component is the leaf area per leaf dry mass or SLA (m2 kg−1). The second is the fraction of total biomass allocated to the leaves or LMF (g g−1). The third is the increase of biomass per unit leaf area per day or ULR (g m−2 d−1). The formula for RGR then becomes:
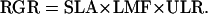 |
(Eq. 3) |
The SLA is calculated as the leaf area divided by the leaf mass. The SLA is also the reciprocal of the product of leaf thickness (m) and leaf density (kg m−3). Leaf density is dependent on the amount of air space inside the leaf tissue (leaf porosity) and the amount of water per dry mass (leaf water content). LMF is calculated as the leaf mass divided by the plant mass. ULR can be calculated from plant mass and leaf area on two time points:
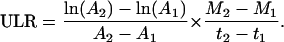 |
(Eq. 4) |
ULR can also be calculated from measurements of gas exchange and carbon concentration. The ULR depends on (1) photosynthesis per unit leaf area (PSA; mol C fixed m−2 leaf area d−1); (2) fraction of daily fixed carbon that is not respired but incorporated into the biomass of a plant (FCI; mol C incorporated mol−1 C fixed); and (3) the amount of biomass that can be formed with 1 mol carbon, referred to by the carbon concentration ([C]; mol C g−1 dry mass). This can be represented as (Poorter, 2002):
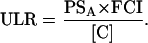 |
(Eq. 5) |
The results were analyzed using the SPSS statistical package (release 8.0; SPSS, Chicago). Differences between growth parameters were tested using a Student's t test on the normalized averages. Differences in leaf area of seedlings grown in Petri dishes were tested using an ANOVA.
Acknowledgments
We thank Rob Welschen, Ankie Ammerlaan, Yvonne de Jong-van Berkel, and Petra Burger for technical assistance. Prof. M.B. Jackson and Fabio Fiorani made many helpful comments on the manuscript. We thank Prof. M. Koornneef and Prof. G. Angenent for providing the tobacco and petunia seeds used in this study. In addition, we thank Prof. L.C. van Loon for his advice and support as coordinator of the research program of the Earth and Life Sciences Foundation.
This work was supported by the Earth and Life Sciences Foundation, which is subsidized by the Netherlands Organization for Scientific Research (NWO; grant no. 805.33.463) and by the NWO (PIONIER grant no. 800.84.470 to L.A.C.J.V.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.034389.
References
- Abeles FB, Morgan PW, Saltveit ME (1992) Ethylene in Plant Biology. Academic Press, New York
- Atkin OK, Botman B, Lambers H (1996) The causes of inherently slow growth in alpine plants: an analysis based on the underlying carbon economies of alpine and lowland Poa species. Funct Ecol 10: 698–707 [Google Scholar]
- Beaudoin N, Serizet C, Gosti F, Giraudat J (2000) Interactions between abscisic acid and ethylene signaling cascades. Plant Cell 12: 1103–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker AB, Estelle MA, Somerville C, Kende H (1988) Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science 141: 1086–1087 [DOI] [PubMed] [Google Scholar]
- Brenner ML, Cheikh N (1995) Hormones in photosynthate partitioning and seed filling. In PJ Davies, ed, Plant Hormones. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 649–670
- Cataldo DA, Haroon M, Schrader LF, Youngs VL (1975) Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun Soil Sci Plant Anal 6: 71–80 [Google Scholar]
- Chadwick AV, Burg SP (1970) Regulation of root growth by auxin-ethylene interaction. Plant Physiol 54: 192–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DG, Gubrium EK, Barrett JE, Nell TA, Klee HJ (1999) Root formation in ethylene-insensitive plants. Plant Physiol 121: 53–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmer TD, Gibberd MR, Wiengweera A, Tinh TK (1998) The barrier to radial oxygen loss from roots of rice (Oryza sativa L.) is induced by growth in stagnant solution. J Exp Bot 49: 1431–1436 [Google Scholar]
- Ecker J (1995) The ethylene signal transduction pathway in plants. Science 268: 667–675 [DOI] [PubMed] [Google Scholar]
- Evans GC (1972) The Quantitative Analysis of Plant Growth. Blackwell Scientific Publications, Oxford
- Evans JR, Poorter H (2001) Photosynthetic acclimation of plants to growth irradiance: the relative importance of SLA and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ 24: 755–767 [Google Scholar]
- Evans JR, Seemann JR (1989) The allocation of protein nitrogen in the photosynthetic apparatus: costs, consequences, and control. In WR Briggs, ed, Photosynthesis. Alan R. Liss, New York, pp 183–205
- Fiorani F, Bögemann GM, Visser EJW, Lambers H, Voesenek LACJ (2002) Ethylene emission and responsiveness to applied ethylene vary among Poa species that inherently differ in leaf elongation rates. Plant Physiol 129: 1382–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier E (1992) Growth analysis of congeneric annual and perennial grass species. J Ecol 80: 665–675 [Google Scholar]
- Geraats BPJ, Bakker AHM, van Loon LC (2002) Ethylene insensitivity impairs resistance to soilborne pathogens in tobacco and Arabidopsis thaliana. Mol Plant Microbe Interact 15: 1078–1085 [DOI] [PubMed] [Google Scholar]
- Grbić V, Bleecker AB (1995) Ethylene regulates the timing of leaf senescence in Arabidopsis. Plant J 8: 595–602 [Google Scholar]
- Guzman P, Ecker J (1990) Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2: 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A, Chen Q, Findell J, Schaller G, Bleecker A (1999) The relationship between ethylene binding and dominant insensitivity conferred by mutant forms of the ETR1 ethylene receptor. Plant Physiol 121: 291–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, Chang C, Sun Q, Meyerowitz EM (1995) Ethylene insensitivity conferred by Arabidopsis ERS gene. Science 269: 1712–1714 [DOI] [PubMed] [Google Scholar]
- Hunt R (1982) Plant Growth Curves. E. Arnold Publishers, London
- Inskeep WP, Bloom PR (1985) Extinction coefficients of chlorophyll a and b in N,N-dimethylformamide and 80% acetone. Plant Physiol 77: 483–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB, Osborne DJ (1970) Ethylene, the natural regulator of leaf abscission. Nature 225: 1019. [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR (1993) CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell 72: 427–441 [DOI] [PubMed] [Google Scholar]
- Knoester M, van Loon L, van den Heuvel J, Hennig J, Bol J, Linthorst H (1998) Ethylene-insensitive tobacco lacks nonhost resistance against soil-borne fungi. Proc Natl Acad Sci USA 95: 1933–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings H (1989) Physiological and morphological differences between plants with a high NAR or a high LAR as related to environmental conditions. In H Lambers, ML Cambridge, H Konings, and TL Pons, eds, Causes and Consequences of Variation in Growth Rate and Productivity in Plants. SPB Academic Publishing, The Hague, The Netherlands, pp 101–123
- Krapp A, Hofmann B, Schafer C, Stitt M (1993) Regulation of the expression of rbcS and other photosynthetic genes by carbohydrates: a mechanism for the sink regulation of photosynthesis? Plant J 3: 817–828 [Google Scholar]
- Lee S, Reid D (1997) The role of endogenous ethylene in the expansion of Helianthus annuus leaves. Can J Bot 75: 501–509 [DOI] [PubMed] [Google Scholar]
- Leishman M, Wright IJ, Moles AT, Westoby M (2000) The evolutionary ecology of seed size. In M Fenner, ed, Seeds - The Ecology of Regeneration in Plant Communities, Ed 2. CAB International, Wallingford, UK, pp 31–57
- Mansfield TA, McAinsh MR (1995) Hormones as regulators of water balance. In PJ Davies, ed, Plant Hormones. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 598–616
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Pierik R, Visser EJW, de Kroon H, Voesenek LACJ (2003) Ethylene is required in tobacco to successfully compete with proximate neighbours. Plant Cell Environ 26: 1229–1234 [Google Scholar]
- Poorter H (1989) Interspecific variation in relative growth rate: on ecological causes and physiological consequences. In H Lambers, ML Cambridge, H Konings, and TL Pons, eds, Causes and Consequences of Variation in Growth Rate and Productivity in Plants. SPB Academic Publishing, The Hague, The Netherlands, pp 101–123
- Poorter H (2002) Plant growth and carbon economy. In Encyclopedia of Life Sciences, www.els.net. Macmillan Publishers, Nature Publishing Group, London
- Poorter H, Nagel O (2000) The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review. Aust J Plant Physiol 27: 595–607 [Google Scholar]
- Poorter H, Remkes C (1990) Leaf area ratio and net assimilation rate of 24 wild species differing in relative growth rate. Oecologia 83: 553–559 [DOI] [PubMed] [Google Scholar]
- Poorter H, Remkes C, Lambers H (1990) Carbon and nitrogen economy of 24 wild species differing in relative growth rate. Plant Physiol 94: 621–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorter H, Van der Werf A (1998) Is inherent variation in RGR determined by LAR at low irradiance and by NAR at high irradiance? A review of herbaceous species. In H Lambers, H Poorter, and MMI Van Vuuren, eds, Inherent Variation in Plant Growth. Physiological Mechanisms and Ecological Consequences. Backhuys Publishers, Leiden, The Netherlands, pp 309–336
- Porra RJ, Thompson WA, Kreidemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975: 348–394 [Google Scholar]
- Raskin I (1983) A method for measuring leaf volume, density, thickness, and internal gas volume. Hortscience 18: 698–699 [Google Scholar]
- Reid JB, Howel H (1995) Hormone mutants and plant development. In PJ Davies, ed, Plant Hormones. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 598–616
- Smalle J, Haegman M, Kurepa J, Van Montagu M, Straeten D (1997) Ethylene can stimulate Arabidopsis hypocotyl elongation in the light. Proc Natl Acad Sci USA 94: 2756–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers JE, Voesenek LACJ, Blom CWPM, Lewis MJ, Jackson MB (1996) Potamogeton pectinatus is constitutively incapable of synthesizing ethylene and lacks 1-aminocyclopropane-1-carboxylic acid oxidase. Plant Physiol 111: 901–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voesenek LACJ, Benschop JJ, Bou J, Cox MCH, Groeneveld HW, Millenaar FF, Vreeburg RAM, Peeters AJM (2003) Interaction between plant hormones regulates submergence-induced shoot elongation in the flooding-tolerant dicot Rumex palustris. Ann Bot 91: 205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Jang J, Jones T, Sheen J (1998) Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsis glucose-insensitive mutant. Proc Natl Acad Sci USA 95: 10294–10299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobayed SMA, Armstrong J, Armstrong W (2001) Micropropagation of potato: evaluation of closed, diffusive and forced ventilation on growth and tuberization. Ann Bot 87: 53–59 [Google Scholar]