Abstract
At the onset of neurogenesis in the mammalian central nervous system, neuroepithelial cells switch from symmetric, proliferative to asymmetric, neurogenic divisions. In analogy to the asymmetric division of Drosophila neuroblasts, this switch of mammalian neuroepithelial cells is thought to involve a change in cleavage plane orientation from perpendicular (vertical cleavage) to parallel (horizontal cleavage) relative to the apical surface of the neuroepithelium. Here, we report, using TIS21-GFP knock-in mouse embryos to identify neurogenic neuroepithelial cells, that at the onset as well as advanced stages of neurogenesis the vast majority of neurogenic divisions, like proliferative divisions, show vertical cleavage planes. Remarkably, however, neurogenic divisions of neuroepithelial cells, but not proliferative ones, involve an asymmetric distribution to the daughter cells of the apical plasma membrane, which constitutes only a minute fraction (1–2%) of the entire neuroepithelial cell plasma membrane. Our results support a novel concept for the cell biological basis of asymmetric, neurogenic divisions of neuroepithelial cells in the mammalian central nervous system.
Keywords: asymmetric division, apical plasma membrane, cleavage plane, neurogenesis, TIS21
Introduction
All neurons and macroglial cells of the mammalian central nervous system are derived from neuroepithelial (NE) cells. During development, NE cells initially proliferate to generate more progenitor cells. These proliferative divisions are symmetric in that one NE mother cell gives rise to two NE daughter cells, resulting in an exponential increase in NE cell number. Upon the onset of neurogenesis, an increasing proportion of NE cells switch to differentiating divisions that are thought to be asymmetric in that one NE mother cell generates one NE daughter cell and one post-mitotic neuron (Rakic, 1988; Chenn and McConnell, 1995; Huttner and Brand, 1997). Direct evidence for such asymmetric division of NE cells has come from time-lapse observations of NE cells (Haubensak et al, 2004) and radial glial cells (Miyata et al, 2001; Noctor et al, 2001), which can be regarded as a specialized type of NE cells existing after the onset of neurogenesis (Huttner and Brand, 1997; Kriegstein and Götz, 2003). The cell biological basis and molecular mechanism controlling the switch of mammalian NE cells from symmetric, proliferative divisions to asymmetric, neurogenic divisions are poorly understood.
The division of Drosophila neuroblasts has provided a classical example of how an asymmetric division of a neural progenitor is based on its cell biological organization, specifically the orientation of the cleavage plane in the context of apical–basal polarity (Matsuzaki, 2000; Knoblich, 2001; Wodarz and Huttner, 2003). In contrast to cells in the neuroectodermal epithelium of the Drosophila embryo, whose plane of cleavage is oriented parallel to their apical–basal axis, resulting in symmetric division, Drosophila neuroblasts, while maintaining the axis of apical–basal polarity, re-orient their cleavage plane such that it is positioned perpendicular to this axis. This results in an asymmetric division because cell fate determinants with a polarized intracellular distribution along the apical–basal axis of the neuroblast are differentially distributed to the daughter cells (Matsuzaki, 2000; Knoblich, 2001; Wodarz and Huttner, 2003).
The Drosophila neuroblast has served as a paradigm for the division of vertebrate, mammalian as well non-mammalian, NE cells (Chenn and McConnell, 1995; Cayouette and Raff, 2002; Geldmacher-Voss et al, 2003). Specifically, the switch of mammalian NE cells from symmetric, proliferative to asymmetric, neurogenic divisions has been proposed to involve a change in cleavage plane orientation (Chenn and McConnell, 1995). Symmetric, proliferative divisions of NE cells are thought to require cleavage along their apical–basal axis, that is, perpendicular to the lumenal surface of the neural tube (vertical cleavage). In contrast, asymmetric, neurogenic divisions of NE cells are thought to result from cleavage perpendicular to their apical–basal axis, that is, parallel to the lumenal surface of the neural tube (horizontal cleavage) (Chenn and McConnell, 1995). Consistent with this concept, proteins implicated in cell fate determination of mammalian NE cells, such as Notch (Chenn and McConnell, 1995), Numb (Cayouette et al, 2001; Cayouette and Raff, 2002, 2003) and Minibrain (Hämmerle et al, 2002), have been reported to show a polarized distribution in mitotic NE cells.
However, it has been questioned (Huttner and Brand, 1997) as to whether the proportion of horizontal cleavages that have actually been observed in the mammalian neuroepithelium (Smart, 1973; Landrieu and Goffinet, 1979; Heins et al, 2001) is sufficiently high to explain the large number of neurons generated during the development of mammalian brains. To provide a possible solution to this issue, we have proposed a hypothesis (Huttner and Brand, 1997) that could explain, in cell biological terms, why vertical cleavages may result not only in symmetric, proliferative but also asymmetric, neurogenic divisions. Central to this hypothesis is the distribution of the apical plasma membrane (from now on referred to in short as apical membrane) upon division of mammalian NE cells, rather than the orientation of the cleavage plane relative to the lumenal surface of the neuroepithelium. Specifically, given that NE cells are very elongated, their apical membrane, endowed with a specific protein (Weigmann et al, 1997) (and presumably also lipid (van Meer and Simons, 1988)) composition, should constitute only a minor fraction of their total plasma membrane. Thus, a vertical cleavage could either bisect the apical membrane, leading to its inheritance by both daughter cells and hence a cell biologically symmetric division, or bypass it, leading to its inheritance by only one daughter cell and hence a cell biologically asymmetric division (Huttner and Brand, 1997).
In the present study, we have investigated this hypothesis in the developing mouse embryo. To distinguish between symmetric, proliferative and asymmetric, neurogenic divisions of NE cells, which coexist at the onset of neurogenesis, we exploited our previous observation that the TIS21 gene is selectively expressed in neurogenic, but not proliferating, NE cells (Iacopetti et al, 1999). Specifically, using embryos of a TIS21-GFP knock-in mouse line (Haubensak et al, 2004), we estimated the size of the apical membrane of mitotic NE cells and investigated the orientation of the cleavage plane and the distribution of the apical membrane in proliferative versus neurogenic divisions.
Results
Identification of the apical membrane of single dividing NE cells: the prominin-1-filled cadherin ‘hole'
To identify the apical membrane of mouse NE cells in mitosis, we explored prominin-1 as a possible marker. Prominin-1 is a pentaspan membrane protein that, in NE cells, is selectively localized in protrusions of the apical membrane (Weigmann et al, 1997) (Figure 1A, a). For comparison, we studied the cell adhesion protein cadherin, a marker of the lateral plasma membrane of NE cells (Nose and Takeichi, 1986; Aaku-Saraste et al, 1996). Analysis of the mouse embryonic brain (E9-14.5; forebrain, midbrain, hindbrain) revealed cadherin immunoreactivity along most of the lateral plasma membrane, being concentrated towards its apical-most end (Figure 1A, b) where the junctional complexes are known to occur (Aaku-Saraste et al, 1996). Double immunofluorescence revealed that this ‘apical' staining for cadherin was very close to, but did not overlap with, the ‘true' apical staining for prominin-1 (Figure 1A, c), consistent with cadherin being concentrated at junctions and prominin-1 in microvilli.
Figure 1.
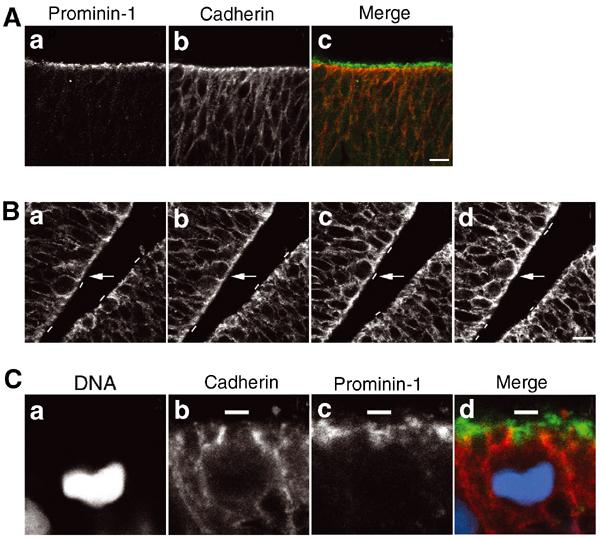
The cadherin ‘hole' as a means of identifying the apical membrane of NE cells. (A) Distinct localization of prominin-1 and cadherin at the apical side of the mouse embryonic neuroepithelium. Double immunolabeling of a frozen section of E9.0 mouse forebrain for prominin-1 (a, c; green) and cadherin (b, c; red). Note that, in the single optical section shown, there is no overlap between the prominin-1 and cadherin staining at the apical side of the neuroepithelium (c), as expected from the specific association of prominin-1 with microvilli of the apical membrane and the concentration of cadherin at the apical-most end of the lateral plasma membrane. Bar in (c)=10 μm. (B) Detection of a cadherin ‘hole' in consecutive optical sections. A frozen section of E10.5 mouse midbrain neuroepithelium was immunostained for cadherin. (a–d) show four consecutive, adjacent 1-μm optical sections obtained by confocal microscopy. The apical membrane, as revealed by the cadherin ‘hole', is indicated by small white bars. Arrows indicate a single cell whose cadherin hole is apparent in only one of the optical sections (c). Bar in (d)=10 μm. (C) The cadherin hole contains the apical membrane-specific protein prominin-1. Triple labeling of a frozen section of an E10.5 mitotic mouse NE cell for DNA (a, d; blue; propidium iodide staining), cadherin (b, d; red) and prominin-1 (c, d; green). White bars indicate the apical membrane of the mitotic NE cell (b–d). Note that, in the single optical section shown, the cadherin staining of the lateral plasma membrane and the prominin-1 staining of the apical membrane are mutually exclusive, and that the cadherin hole is immunostained for prominin-1 (d).
In consecutive optical sections (1 μm intervals) obtained by confocal laser-scanning microscopy, the ‘apical' cadherin staining was found to be interrupted by an unstained segment in one or two of the sections (Figure 1B, a–d, white bars; one example is indicated by arrows). Counterstaining for prominin-1, as shown for a mitotic cell in Figure 1C, revealed that this cadherin-negative segment (Figure 1C, b, white bar) contained prominin-1 immunoreactivity and hence corresponded to the apical membrane proper (Figure 1C, c and d, white bars). We will refer to the cadherin-negative, prominin-1 -positive segments at the apical surface of the neuroepithelium as cadherin ‘holes', and have used these to identify the apical membrane of single mitotic NE cells in this study.
Equal versus unequal distribution of the apical membrane in mitotic NE cells
To determine the distribution of the apical membrane upon division of NE cells, we deduced the orientation of the cleavage plane in mitotic cells in anaphase or telophase. It is known that the final orientation of the mitotic spindle is set before the onset of anaphase and determines the orientation of the cleavage plane, in rodent NE cells (Zieba et al, 1986; Adams, 1996) as in other eukaryotic cells (Reinsch and Karsenti, 1994; Gonczy and Hyman, 1996). Using fixed cryosections of mouse E9.5-E11.5 midbrain and hindbrain stained for DNA using propidium iodide or 4,6-diamidino-2-phenylindole (DAPI), mitotic cells at the apical side of the neuroepithelium were analyzed for the orientation of the sister chromatids in consecutive optical sections. In all experiments described below, a mitotic NE cell was included in the analysis only if (i) in a given optical section, the sister chromatids destined for the prospective daughter cells appeared similar in size and shape and (ii) this similarity was observed in four consecutive sections, indicating that the plane of scanning of the individual optical sections was parallel to the orientation of the mitotic spindle and perpendicular to the cleavage plane (Figure 2A, a–d). In such a case, the orientation of the cleavage plane could then be deduced, as illustrated in Figure 2A, e–h (see figure legend for details). In the example shown in Figure 2A, a–d, the sister chromatids show a parallel orientation (Figure 2A, e and f; deduced cleavage plane indicated by dashed red line in (f), corresponding to dashed white line in (c)). Figure 2A, g and h, shows the deduction of the cleavage plane (dashed red line) in a mitotic cell in which the sister chromatids are oriented at an angle to each other (Figure 2B, a). All of the single optical sections presented below that indicate the orientation of the cleavage plane, as deduced from that of the sister chromatids of mitotic NE cells in anaphase or telophase, are from a series of consecutive sections fulfilling the above two criteria.
Figure 2.
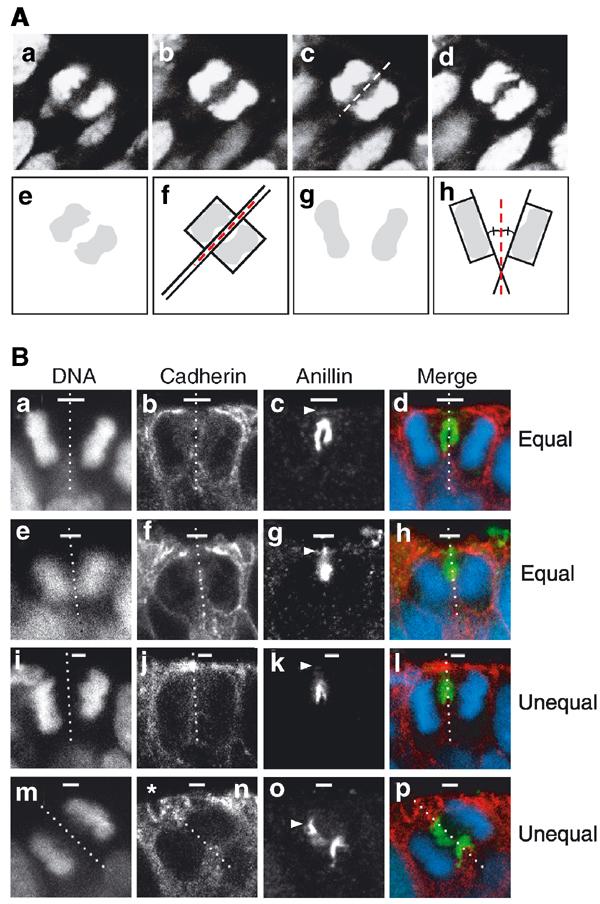
Determination of the cleavage plane and equal versus unequal distribution of the apical membrane during division of NE cells. (A) Determination of the cleavage plane. A frozen section of E9.5 mouse hindbrain neuroepithelium was stained for DNA using propidium iodide. (a–d) Four consecutive, adjacent 1-μm optical sections of a mitotic NE cell in anaphase. The cell shown is a representative example in which, for each optical section, the sister chromatids of the prospective daughter cells appear similar in size and shape, indicating that the planes of scanning of the individual optical sections were perpendicular to the cleavage plane, which in such a case can be deduced accurately (dashed white line in (c)) as illustrated in panels e and f. Only mitotic NE cells fulfilling this criterion (about every fifth cell classified as anaphase or telophase) were included in the subsequent analyses (see Materials and methods). (e–h) Deduction of cleavage plane. The DNA staining as observed in panels c and Ba is shown in (e) and (g) (shaded areas), respectively, and rectangles were designed to fit this staining (f and h, respectively). The orientation of the rectangles relative to each other was deduced (long black lines extending one side of the rectangle). In the example shown in (f), the rectangles are oriented parallel to each other, and the cleavage plane is deduced to be positioned half way in between (dashed red line). In the example shown in (h), the rectangles are oriented at a certain angle to each other, and the cleavage plane is deduced to be positioned such that this angle is halved (dashed red line). (B) Two examples each of an equal (a–d, e–h) and unequal (i–l, m–p) distribution of the apical membrane. (a–d, e–h, i–l and m–p) each show one triple-labeled NE cell in a frozen section from E11.5 mouse midbrain. The cleavage plane (dashed white lines) was deduced from the orientation of the DAPI-stained sister chromatids observed in consecutive optical sections, one of which is shown (a, e, i, m and d, h, l, p; blue), as described in (A). The deduced cleavage plane was corroborated by immunostaining for anillin (c, g, k, o and d, h, l, p; green). The apical membrane, that is, the cadherin hole (white bars), was identified by immunolabeling for cadherin (b, f, j, n and d, h, l, p; red). In the mitotic NE cells shown in the two top rows (a–d and e–h), the apical membrane will be bisected upon cleavage and distributed equally to the daughter cells. In contrast, in the mitotic NE cells shown in the two bottom rows (i–l and m–p), the apical membrane will be bypassed by the cleavage and distributed unequally, that is, to only one daughter cell. Note that, in the mitotic NE cells shown in the second (e–h) and fourth (m–p) rows, the cleavage furrow as revealed by anillin immunostaining has reached the apical (g) and lateral (o) plasma membrane (white arrowheads), respectively, whereas this is not yet the case in the mitotic NE cells shown in the first (a–d) and third (i–l) rows. The asterisk in (n) indicates the cadherin hole of another cell adjacent to the mitotic NE cell shown.
To corroborate the prediction of cleavage plane orientation, mitotic NE cells in anaphase or telophase were analyzed for the subcellular localization of anillin, an actin-binding protein that localizes to the cleavage furrow during cytokinesis (Oegema et al, 2000). Immunostaining for anillin (Figure 2B, c, g, k and o) confirmed that the prediction of the cleavage plane as deduced from the orientation of the sister chromatids (Figure 2B, a, e, i and m) was correct. The anillin immunostaining also revealed that, in NE cells, the cleavage furrow proceeded from basal (Figure 2B, c) to apical (Figure 2B, g), as has been shown for other epithelial cells (Reinsch and Karsenti, 1994).
Next, we determined the position of the apical membrane, identified by the cadherin hole, relative to the orientation of the cleavage plane, deduced from the orientation of the sister chromatids and corroborated by anillin immunostaining. This should reveal whether the distribution of the apical membrane upon division of NE cells is equal or unequal. As illustrated by the four examples of mitotic NE cells shown in Figure 2B, we observed cleavage planes predicted to bisect the cadherin hole, resulting in the distribution of apical membrane to both daughter cells (Figure 2B, a–h), or to bypass the cadherin hole, resulting in the distribution of apical membrane to only one of the daughter cells (Figure 2B, i–p). The prediction of a cleavage plane either bisecting or bypassing the apical membrane was particularly obvious when the cleavage furrow (as revealed by anillin staining) had reached either the apical membrane (Figure 2B, g, arrowhead) or the lateral plasma membrane near the junctional complexes (Figure 2B, o, arrowhead) (see also Figure 5A below).
Figure 5.
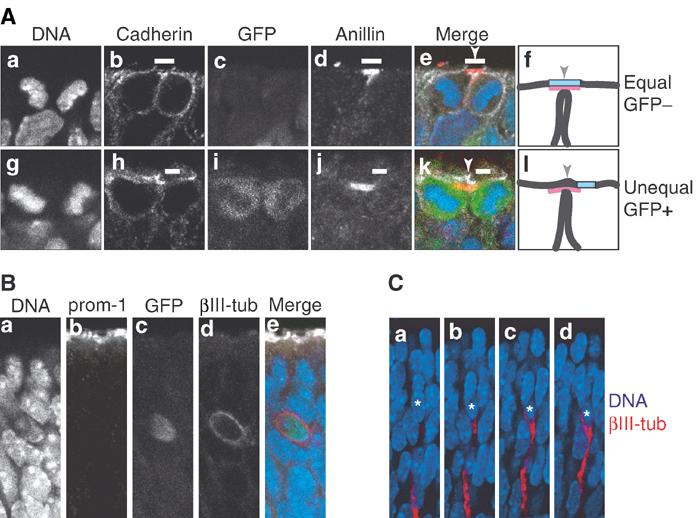
Neurons arising from asymmetric division of NE cells do not inherit apical membrane and prominin-1. (A) Frozen sections of E11.5 hindbrain (a–e) and E11.5 telencephalon (g–k) of heterozygous TIS21-GFP knock-in embryos showing quadruple-labeled dividing NE cells that have almost completed cytokinesis. Single optical sections are shown; DNA, DAPI-stained nuclei (a, e blue, g, k blue). In the NE cell in the top row (a–e), which does not express TIS21-GFP (GFP−) (c, e green) and hence undergoes a proliferative division, the cleavage furrow is about to bisect the apical membrane (i.e. the cadherin hole, white bars), as revealed by immunostaining for cadherin (b, e white) and anillin (d, e red) (which at this stage of cytokinesis indicates the midbody (Oegema et al, 2000)), resulting in its distribution to both daughter cells (equal). In contrast, in the NE cell in the bottom row (g–k), which does express TIS21-GFP (GFP+) (i, k green) and hence undergoes a neurogenic division, the cleavage furrow is about to bypass the apical membrane (i.e., the cadherin hole, white bars), as revealed by immunostaining for cadherin (h, k white) and anillin (j, k red), resulting in its distribution to only one daughter cell (unequal). (f and l) Cartoons summarizing the imminent completion of cytokinesis by plasma membrane fusion in the cell shown in (e) and (k), respectively; black lines, lateral and cleavage furrow plasma membranes; light blue box, apical membrane; red, anillin; arrowheads, site of cleavage. (B) Frozen section of E10.5 telencephalon of heterozygous TIS21-GFP knock-in embryos showing a quadruple-labeled newborn neuron that was identified by immunostaining for βIII-tubulin (βIII-tub, d, e red) and shows GFP fluorescence (c, e green), indicative of its origin from a TIS21-GFP-expressing NE cell (Haubensak et al, 2004). A single optical section is shown; DNA, DAPI-stained nuclei (a, e blue). Note the lack of extension of the neuron to the apical surface immunostained for prominin-1 (prom-1, b, e white). (C) Four consecutive optical sections of a newborn neuron (asterisks), identified by immunostaining for βIII-tubulin (red) in a frozen section of E11.5 hindbrain of heterozygous TIS21-GFP knock-in embryos. Note the extension of the neuron in the basal direction, but not towards the apical side of the neuroepithelium (top). Blue, DAPI-stained nuclei.
For the discussion of the present data, we shall use a cell biological definition of the divisions of NE cells. A division in which the apical membrane is predicted to be bisected by the cleavage and hence distributed to both daughter cells, that is, equally (Figure 2B, a–h), is defined as symmetric in cell biological terms. Conversely, a division in which the apical membrane is predicted to be bypassed by the cleavage and hence distributed to only one daughter cell, that is, unequally (Figure 2B, i–p), is defined as cell biologically asymmetric (Wodarz and Huttner, 2003).
Lack of correlation between cleavage plane orientation and equal versus unequal distribution of the apical membrane
Cleavage planes have been classified according to their orientation relative to the apical surface of the neuroepithelium (Chenn and McConnell, 1995; Heins et al, 2001). Thus, cleavage planes oriented at a 90–60, 60–30 and 30–0° angle relative to the lumenal surface of the neuroepithelium have been referred to as vertical, oblique and horizontal cleavages, respectively. We investigated whether vertical cleavages would always lead to an equal distribution of the apical membrane, and oblique cleavages to its unequal distribution. This was found not to be the case. Not only did vertical cleavages, at any of the developmental stages (E9.5–E14.5) and in any of the brain regions (forebrain, midbrain, hindbrain) analyzed, result (as expected) in an equal distribution of the apical membrane (Table I, Figure 3a, see also Figure 2B, a–h) but also they were found, in as much as ≈30% of the cases, to be accompanied by its unequal distribution (Table I, Figure 3b, see also Figure 2B, i–l). Conversely, oblique cleavages could result not only in an unequal (Figure 2B, m–p) but also equal (Figure 3c) distribution of the apical membrane (Table I). These results indicate that cleavage plane orientation is an insufficient criterion to predict whether a division will be symmetric or asymmetric in cell biological terms. Importantly, a vertical cleavage plane orientation, which was observed in >90% of mitotic NE cells (Table I) not only at early (e.g., E11.5 midbrain; Table II) but also later (e.g., E14.5 forebrain, see below) stages of neurogenesis, does not necessarily mean that a division is symmetric, as has previously been considered to be the case (Chenn and McConnell, 1995).
Table 1.
Cleavage plane orientation and distribution of apical membrane in mitotic mouse NE cells
| Apical membrane distribution | Cleavage plane orientation |
||
|---|---|---|---|
| Vertical | Oblique | Horizontal | |
| (90–60°) | (60–30°) | (30–0°) | |
| Equal | 76 | 1 | 0 |
| Unequal |
30 |
6 |
3 |
| Data for various developmental stages (E9.5–14.5) and brain regions (forebrain, midbrain, hindbrain) were pooled because the vast majority of mitotic NE cells showed a vertical cleavage plane orientation for any of the developmental stages and brain regions analyzed (see below). For the equal versus unequal distribution of apical membrane in various developmental stages and brain regions, see Figure 6. Numbers of cells were: E9.5 forebrain, n=11; E10.5 forebrain, n=4; E11.5 forebrain, n=18; E14.5 forebrain, n=15; E9.5 midbrain, n=2; E10.5 midbrain, n=10; E11.5 midbrain, n=44; E9.5 hindbrain, n=1; E11.5 hindbrain, n=11. Note that even at advanced stages of neurogenesis in the forebrain (E14.5), only one of 15 mitotic NE cells showed a cleavage plane orientation that was not vertical. | |||
Figure 3.
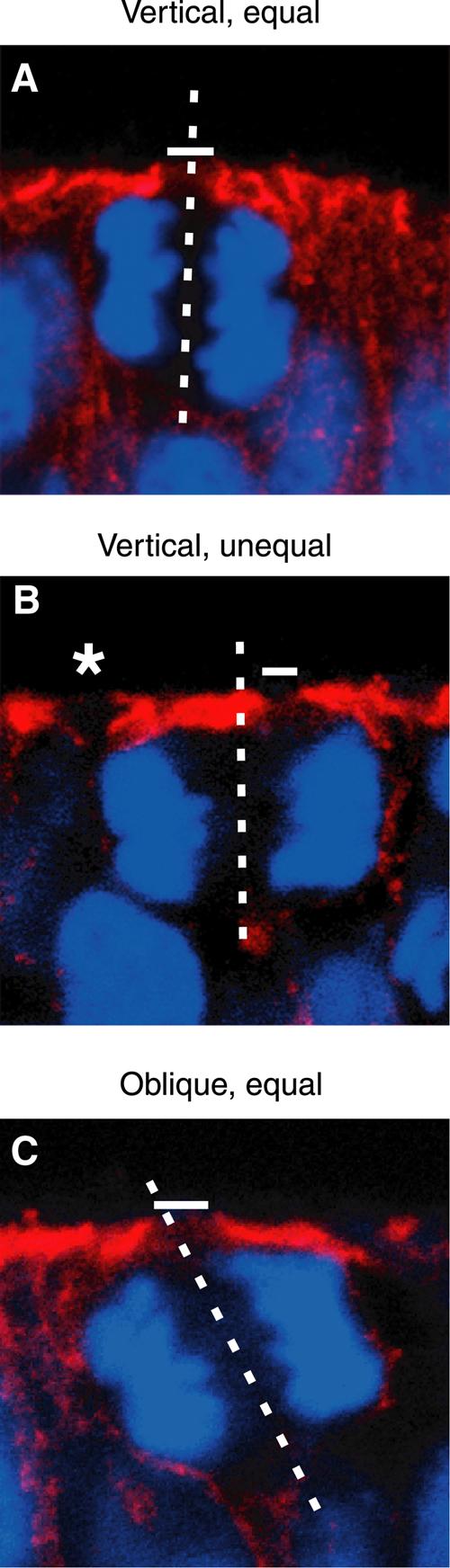
An equal versus unequal distribution of the apical membrane is not necessarily associated with a vertical versus non-vertical cleavage plane of NE cells. Frozen sections of E9.5–10.0 mouse hindbrain neuroepithelium were double-labeled for cadherin (A–C; red) and DNA (A–C; blue; propidium iodide staining). In the single optical section shown, white bars indicate the apical membrane (cadherin hole), and the dashed white lines show the cleavage plane deduced as described in Figure 2A. Note that in (B), the cleavage plane is vertical but the apical membrane distributed unequally, whereas in (C), the cleavage plane is oblique but the apical membrane is distributed equally. The asterisk in (B) indicates the cadherin hole of another cell adjacent to the mitotic NE cell shown.
Table 2.
Cleavage plane orientation, distribution of apical membrane and TIS21-GFP expression in mitotic NE cells
| Cleavage plane orientation |
||
|---|---|---|
| Vertical (90–60°) | Oblique (60–30°)+Horizontal (30–0°) | |
| TIS21-GFP-negative | 22 | 0 |
|
TIS21-GFP-positive |
19 |
3 |
| Distribution of apical membrane |
||
| |
Equal |
Unequal |
| TIS21-GFP-negative | 22 | 0 |
|
TIS21-GFP-positive |
6 |
16 |
| Data are for mouse E11.5 midbrain. | ||
Vertical cleavage planes, but differential inheritance of apical membrane, in proliferative versus neurogenic divisions of NE cells
We next addressed the relation between the distribution of the apical membrane upon division and whether a division is proliferative or neurogenic in nature. To distinguish neurogenic divisions of NE cells from proliferative ones, which at the onset of neurogenesis are known to coexist, we used heterozygous embryos of a TIS21-GFP knock-in mouse line, which express GFP (carrying a nuclear localization signal) from the TIS21 locus, that is, specifically in neurogenic, but not proliferating, NE cells, and which are phenotypically wild type (Haubensak et al, 2004).
Figure 4 shows, for the E11.5 midbrain, examples of proliferative divisions of NE cells, characterized by the absence of TIS21-GFP expression (Figure 4A top, c; Figure 4B, c, right cell), and of neurogenic divisions, characterized by its presence (Figure 4A bottom, g; Figure 4B, c, left cell). In either case, irrespective of whether the division was found to be proliferative or neurogenic in nature, the orientation of the cleavage plane relative to the apical surface of the neuroepithelium was vertical (Figure 4A) or nearly vertical (Figure 4B). Remarkably, however, the divisions of NE cells lacking TIS21-GFP expression were predicted to result in the distribution of apical membrane to both daughter cells (Figure 4A, a–d; Figure 4B, a–d, right cell), whereas the divisions of NE cells showing TIS21-GFP expression were predicted to result in the distribution of apical membrane to only one of the daughter cells (Figure 4A, e–h; Figure 4B, a–d, left cell). Furthermore, the data shown in Figure 4B document that mitotic NE cells showing an equal and unequal distribution of apical membrane can co-exist in close proximity.
Figure 4.
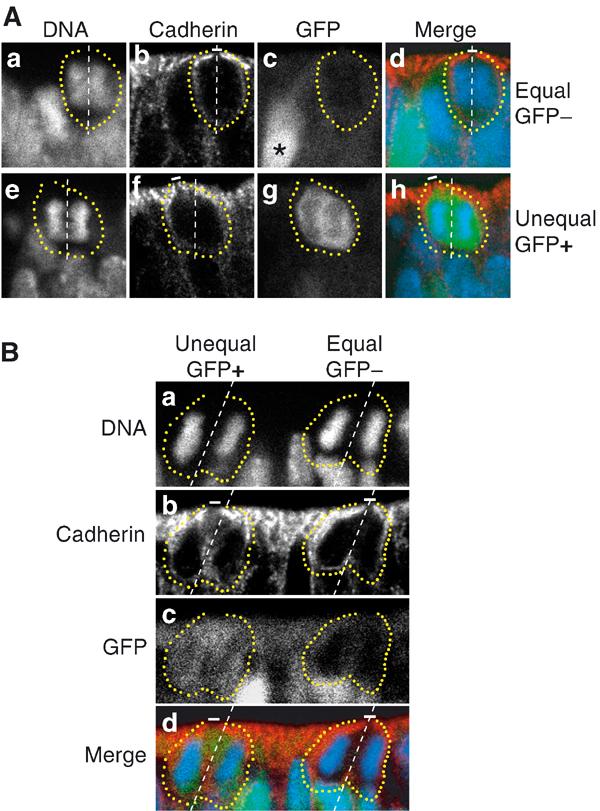
Equal versus unequal distribution of the apical membrane and proliferative versus neurogenic divisions of NE cells in the midbrain of heterozygous E11.5 embryos of TIS21-GFP knock-in mice. (A) An example of an equal distribution of the apical membrane in a proliferative division (a–d) and an example of an unequal distribution of the apical membrane in a neurogenic division (e–h). (a–d) and (e–h) each show one triple-labeled mitotic NE cell, outlined by the yellow dots. The cleavage plane (dashed white lines) was deduced from the orientation of the DAPI-stained sister chromatids observed in consecutive optical sections, one of which is shown (DNA; a, e and d, h; blue), as described in Figure 2A. Note that the cleavage plane in both examples is vertical. The apical membrane, that is, the cadherin hole (white bars), was identified by immunolabeling for cadherin (b, f and d, h; red). In the mitotic NE cell in the top row (a–d), the apical membrane will be bisected by the cleavage and distributed equally to the daughter cells, whereas in the mitotic NE cell in the bottom row (e–h), the apical membrane will be bypassed by the cleavage and distributed unequally, that is, to only one daughter cell. The NE cell showing an equal distribution of the apical membrane (a–d) does not express TIS21-GFP (GFP−) (c, d; green), indicative of a proliferative division, whereas the NE cell showing an unequal distribution (e–h) does (GFP+) (g, h; green), indicative of a neurogenic division. Note the GFP-expressing cell near the mitotic GFP-negative NE cell in (c) (asterisk). (B) Coexistence in close proximity of proliferating NE cells showing an equal distribution of apical membrane and neurogenic NE cells showing an unequal distribution. Analysis of the two mitotic NE cells shown was performed as described for panels A. The mitotic NE cell on the left distributes its apical membrane unequally, that is, to only one daughter cell, and expresses TIS21-GFP (GFP+), indicative of a neurogenic division, whereas the mitotic NE cell on the right distributes its apical membrane equally to the daughter cells and does not express GFP (GFP−), indicative of a proliferative division. Note that for the mitotic NE cell on the right, the cadherin hole is smaller in the optical section shown than in the next optical section (which is not shown).
We analyzed dividing NE cells that had almost completed cytokinesis to corroborate that the equal versus unequal distribution of the apical membrane as predicted for TIS21-GFP-negative versus TIS21-GFP-positive NE cells in anaphase or early telophase (Figure 4), respectively, indeed results in the inheritance of this membrane domain by both versus only one of the daughter cells. Figure 5A, a–e, shows a TIS21-GFP-negative NE cell in which, as revealed by cadherin and anillin staining (which at this stage of cytokinesis indicates the midbody (Oegema et al, 2000)), the plasma membrane of the cleavage furrow is about to fuse with, and bisect, the apical membrane, resulting in its inheritance by both daughter cells (Figure 5A, f). In contrast, Figure 5A, g–k, shows a TIS21-GFP-positive NE cell in which the plasma membrane of the cleavage furrow is about to fuse with the apical-most lateral plasma membrane, thereby bypassing the apical membrane and resulting in its inheritance by only one of the daughter cells (Figure 5A, l).
Together, the finding that TIS21-GFP-positive NE cells generate neurons by asymmetric division (Haubensak et al, 2004) and the lack of inheritance of apical membrane by one of the daughter cells originating from such a division (Figure 5A, g–l) imply that newborn neurons should lack prominin-1. Indeed, newborn neurons (identified by βIII-tubulin staining) were found to lack an extension to the apical surface of the neuroepithelium (stained for prominin-1), even when their perikaryon was still located near this surface (Figure 5B). This was not due to the inability to detect neuronal processes as such, as the basal process of newborn neurons was readily detectable (Figure 5C). We conclude that daughter cells originating from divisions of NE cells predicted to result in an unequal distribution of apical membrane are indeed characterized by a differential inheritance of this membrane domain.
Table II documents that the examples shown in Figures 4 and 5 are indeed representative. Analysis of 44 mitotic NE cells from the E11.5 midbrain (22 GFP-negative and 22 GFP-positive) revealed that 93% showed a vertical cleavage plane (Table II). None of the 22 GFP-negative cells, and <15% of the 22 GFP-positive cells, had an oblique or horizontal cleavage plane (Table II). Thus, cleavage plane orientation does not allow one to distinguish between proliferative (GFP-negative) and neurogenic (GFP-positive) NE cell divisions because, in the overwhelming majority of cases, both types of divisions are associated with a vertical cleavage plane.
In contrast to determining cleavage plane orientation, analysis of the same 22 GFP-negative and 22 GFP-positive mitotic NE cells for the distribution of the apical membrane did reveal a striking relationship between the latter parameter and the type of cell division (Table II). All proliferative (GFP-negative) divisions showed an equal distribution of the apical membrane. In contrast, in the case of neurogenic (GFP-positive) divisions, the vast majority (16 out of 22) showed an unequal distribution (Table II).
Mitotic NE cells showing TIS21-GFP expression and unequal distribution of the apical membrane increase as neurogenesis proceeds
Next, we investigated the relationship between TIS21-GFP expression and the distribution of the apical membrane in mitotic NE cells for various developmental stages (E9.5–14.5) and brain regions (forebrain, midbrain, hindbrain), comparing early and advanced stages of neurogenesis (Figure 6). Irrespective of the stage of neurogenesis, the same principal observation was made. The majority of mitotic NE cells showing an equal distribution of the apical membrane were TIS21-GFP-negative (Figure 6, compare squares below abscissa), whereas the majority of the cells showing an unequal distribution were TIS21-GFP-positive (Figure 6, compare squares above abscissa). In fact, pooling the data from 115 mitotic NE cells revealed that >80% of the 76 cells with an equal distribution of their apical membrane did not yet show TIS21-GFP expression, that is, were still undergoing proliferative divisions (Figure 7, left), whereas almost 90% of the 39 cells with an unequal distribution did express TIS21-GFP, that is, had switched to neurogenic divisions (Figure 7, right).
Figure 6.
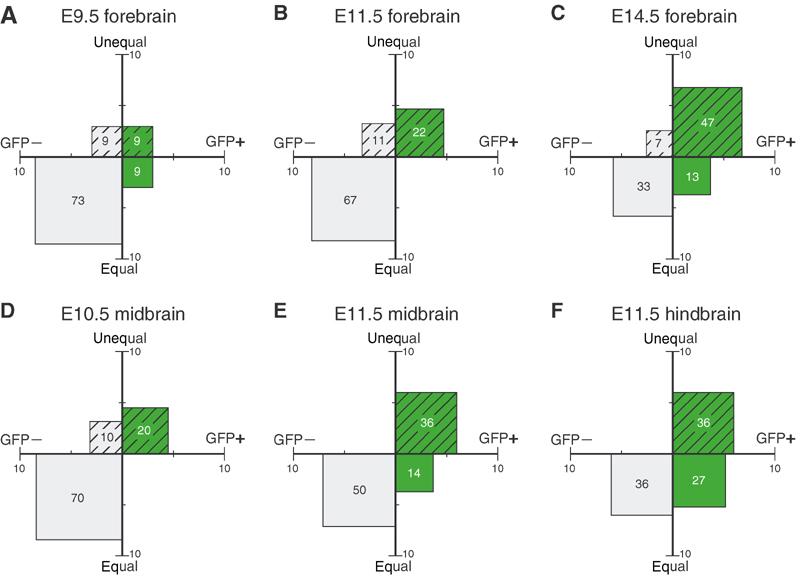
Equal versus unequal distribution of the apical membrane and proliferative versus neurogenic divisions of NE cells at various developmental stages and in various brain regions of heterozygous embryos of TIS21-GFP knock-in mice. The distribution of the apical membrane and the expression of TIS21-GFP in mitotic NE cells were analyzed for the indicated developmental stages and brain regions as exemplified in Figure 4. For a given developmental stage and brain region, mitotic NE cells were classified into four groups: (i) equal distribution of the apical membrane (equal, ordinate) and TIS21-GFP-negative (GFP−, abscissa) (gray, plain), (ii) equal distribution and TIS21-GFP-positive (GFP+, abscissa) (green, plain), (iii) unequal distribution of the apical membrane (unequal, ordinate) and TIS21-GFP-negative (gray, hatched) and (iv) unequal distribution and TIS21-GFP-positive (green, hatched). Cells in each group were expressed as percent of total (sum of the four groups), and the percentage values are indicated by the area of the respective square and the number therein. Numbers of cells were: E9.5 forebrain, n=11; E11.5 forebrain, n=18; E14.5 forebrain, n=15; E10.5 midbrain, n=10; E11.5 midbrain, n=44 (same data as Table II, shown for reference); and E11.5 hindbrain, n=11.
Figure 7.
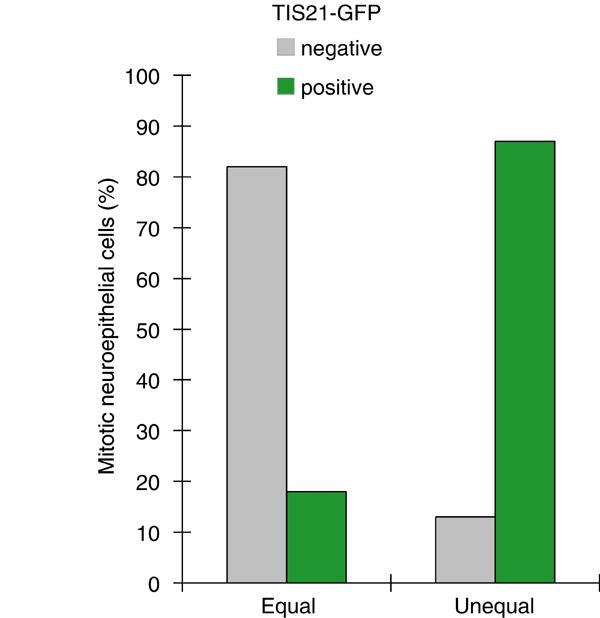
Correlation between the equal versus unequal distribution of the apical membrane and proliferative versus neurogenic divisions of NE cells in heterozygous embryos of TIS21-GFP knock-in mice. The distribution of the apical membrane and the expression of TIS21-GFP in mitotic NE cells were analyzed as exemplified in Figure 4. For both, mitotic NE cells showing an equal distribution of the apical membrane (left columns, n=76) and an unequal distribution (right columns, n=39), the percentage of TIS21-GFP-negative (gray columns) versus TIS21-GFP-positive (green columns) cells was calculated. Data for various developmental stages (E9.5–14.5) and brain regions (forebrain, midbrain, hindbrain) were pooled because the principal observation that the majority of mitotic NE cells showing an equal distribution of the apical membrane are TIS21-GFP-negative, that is, proliferating, and the majority of the cells showing an unequal distribution are TIS21-GFP-positive, that is, neurogenic, was made irrespective of the developmental stage and brain region analyzed (for details, see Figure 6).
Given this correlation between TIS21-GFP expression and the distribution of the apical membrane upon division of NE cells, and in light of the finding that TIS21-GFP expression reflects neurogenesis (Haubensak et al, 2004), one would expect divisions with an unequal distribution to increase concomitant with the progression of neurogenesis during development. Indeed, as exemplified for the forebrain, whereas at E9.5 the vast majority of mitotic NE cells (73%) were TIS21-GFP-negative and showed an equal distribution of the apical membrane (Figure 6A, plain gray square), TIS21-GFP-positive mitotic NE cells with an unequal distribution of the apical membrane became the predominant population (47%) at E14.5 (Figure 6C, hatched green square). It should be noted that, even at this advanced stage of neurogenesis, >85% of the latter cells and >90% of all mitotic NE cells showed a vertical cleavage plane orientation (see the legend to Table I).
Together, our results indicate that proliferative versus neurogenic divisions of mouse NE cells, which can be identified by the absence versus presence of TIS21-GFP expression, are not simply linked to cleavage plane orientation (vertical versus oblique/horizontal) but, rather, to an equal versus unequal distribution of the apical membrane.
Par-3 is associated with the apical cortex of NE cells and differentially distributed to the daughter cells upon neurogenic division
Given these observations, it was of interest to investigate the distribution, upon proliferative versus neurogenic division of NE cells, of par-3, a protein shown to be localized to the apical cortex of mammalian NE cells (Manabe et al, 2002; Takekuni et al, 2003) and implicated in cell polarity and asymmetric division (Lin et al, 2000; Ohno, 2001; Wodarz and Huttner, 2003). Figure 8A shows that par-3 is concentrated at the apical side of the neuroepithelium, being localized more apically than the most apical cadherin and apparently less apical than prominin-1, and showing little overlap with either marker. This is consistent with par-3 being associated with the apical cortex of NE cells at the transition from the lateral to the apical membrane, that is, between the adherens junction and the base of microvilli.
Figure 8.
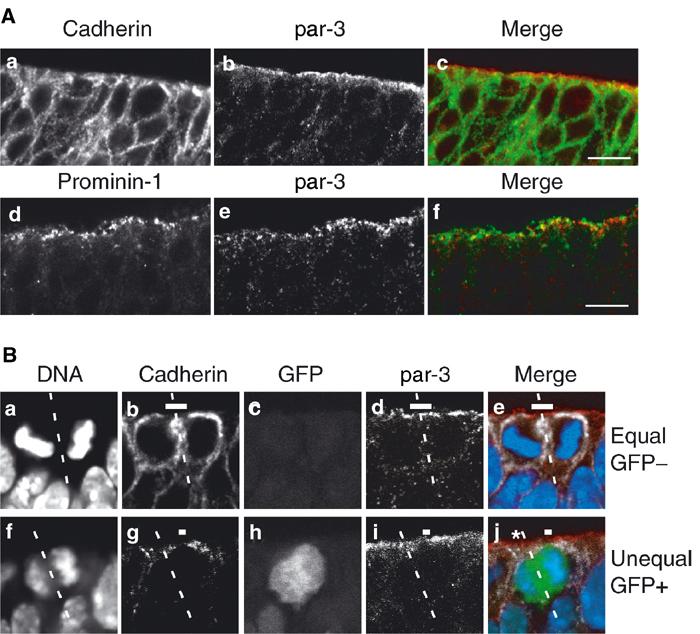
Equal versus unequal distribution of par-3 upon proliferative versus neurogenic divisions of NE cells in the hindbrain of heterozygous E10.5–E12.5 embryos of TIS21-GFP knock-in mice. (A) Frozen section double immunofluorescence showing the localization of par-3 (b, c red, e, f red) in comparison with cadherin (a, c green) and prominin-1 (d, f green) at the apical side of the E10.5 (d–f) and E12.5 (a–c) hindbrain neuroepithelium. Note that in the single optical sections shown, the staining for par-3 appears to be more apical than the most apical staining for cadherin, with little overlap (a–c), and is also largely distinct from the staining for prominin-1, which appears to be more apical than the staining for par-3 (d–f). Bars in (c) and (f)=10 μm. (B) An example of an equal distribution of par-3 in a proliferative division (a–e) and an example of an unequal distribution of par-3 in a neurogenic division (f–j). (a–e) and (f–j) Each show one quadruple-labeled mitotic NE cell in a frozen section. The cleavage plane (dashed white lines) and the apical membrane (i.e. the cadherin hole, white bars) were determined from the orientation of the DAPI-stained sister chromatids (DNA, a, e blue, f, j blue) and by immunolabeling for cadherin (b, e white, g, j white) as in Figures 2 and 4. The single optical section shown reveals that in the mitotic NE cell in the top row (a–e), the apical membrane will be bisected by the cleavage and the apical par-3 (d, e red) distributed to both daughter cells (equal). In contrast, in the mitotic NE cell in the bottom row (f–j), the apical membrane will be bypassed by the cleavage and the apical par-3 (i, j red) distributed to only one daughter cell (unequal). The asterisk in (j) indicates the apical par-3 of another cell adjacent to the mitotic NE cell shown. The NE cell showing an equal distribution of the apical par-3 (a–e) does not express TIS21-GFP (GFP−) (c, e green), indicative of a proliferative division, whereas the NE cell showing an unequal distribution (f–j) does (GFP+) (h, j green), indicative of a neurogenic division.
In mitotic TIS21-GFP-negative NE cells, in which the cleavage bisects the apical membrane, this apical par-3 is predicted to be inherited by both daughter cells (Figure 8B, a–e). In contrast, in mitotic TIS21-GFP-positive NE cells, in which the cleavage bypasses the apical membrane, the apical par-3 is predicted to be inherited by only one daughter cell (Figure 8B, f–j). Hence, the differential distribution of apical membrane to the daughter cells upon the switch of NE cells from proliferative (TIS21-GFP-negative) to neurogenic (TIS21-GFP-positive) divisions indeed results in the unequal inheritance of a protein implicated in asymmetric cell division, par-3.
The switch from an equal to an unequal distribution of apical membrane is accompanied by a halvening of its size
Finally, it was of interest to determine the size of the apical membrane and the proportion it constitutes relative to the entire NE cell plasma membrane. Irrespective of any methodological limitations (see Materials and methods), the apical membrane (ignoring microvilli), estimated from the diameter of the cadherin hole of mitotic NE cells from E11.5 midbrain, was found to be about half the size (3.1 μm2) in cells distributing this membrane domain to only one daughter (i.e., unequally) as compared to cells distributing this membrane domain to both daughters (i.e., equally) (6.6 μm2) (Table III). Relative to the surface of the NE cell body, which we estimated from the diameter of the rounded-up mitotic cell, the apical membrane constituted 2.3 and 1.2% in cells showing an equal and unequal distribution of this membrane domain, respectively (Table III).
Table 3.
Area of apical membrane in relation to its distribution in mitotic NE cells
| Cadherin hole diameter (μm) | Apical membrane (μm2) | Total plasma membrane (μm2) | Apical membrane (% of total) | |
|---|---|---|---|---|
| Equal distribution | 2.9±0.14 (25) | 6.6 | 290 (5) | 2.3 |
| Unequal distribution |
2.0±0.16 (15) |
3.1 |
270 (5) |
1.2 |
| Data are for mouse E11.5 midbrain; mean±s.e.m; numbers in parentheses, n. | ||||
Discussion
The present data reveal that, at the onset as well as advanced stages of neurogenesis in the mouse central nervous system, the switch of NE cells from proliferative to neurogenic divisions is associated with a change in the distribution of the apical membrane from a symmetric to an asymmetric inheritance by the daughter cells, rather than a rotation of the cleavage plane from vertical to horizontal. These observations address a major contradictory issue about mammalian neurogenesis, in particular its early stages. The divisions of NE cells at the apical surface of the ventricular zone that generate neurons are asymmetric (Chenn and McConnell, 1995; Miyata et al, 2001; Noctor et al, 2001; Haubensak et al, 2004); yet, the vast majority of neurogenic NE cells show a vertical cleavage plane orientation (Huttner and Brand, 1997), which has been thought to indicate symmetric division (Chenn and McConnell, 1995). This issue is now resolved by our demonstration that the vertical cleavage planes of mitotic neurogenic NE cells nonetheless allow an unequal distribution of the apical membrane, and hence asymmetric division.
Our study further shows that the orientation of the cleavage plane in mitotic NE cells, that is, vertical versus oblique, is an insufficient parameter to classify their divisions as symmetric or asymmetric. Vertical cleavage planes were observed not only in mitotic NE cells that in terms of cell biological parameters (equal distribution of apical membrane) and daughter cell fate (lack of TIS21 expression) underwent symmetric, proliferative divisions but also in cells that by both criteria (unequal distribution of apical membrane, presence of TIS21 expression) underwent asymmetric, neurogenic divisions. Conversely, an oblique cleavage plane was not necessarily indicative of a cell biologically asymmetric division, but was also found to be compatible with symmetric divisions. In light of our observations, the distribution of the apical membrane in mitotic NE cells appears to be a more reliable parameter in identifying cell biologically asymmetric divisions than cleavage plane orientation.
Time-lapse analysis of TIS21-GFP-expressing cells dividing at the apical surface of the neuroepithelium has revealed that the resulting daughter cells have an asymmetric fate, with one remaining an NE stem cell and the other becoming a post-mitotic neuron that leaves the ventricular zone (Haubensak et al, 2004). In view of these observations, we find it most likely that, in the case of the TIS21-GFP-positive NE cells dividing with an unequal distribution of the apical membrane, the daughter inheriting the apical membrane remains the NE stem cell and the daughter not inheriting it becomes the neuron. This conclusion is consistent with the observations that (i) young neurons lack prominin-1, a marker of the apical membrane of NE cells (Weigmann et al, 1997), and (ii) inheritance of apical membrane by daughter cells is a characteristic feature of divisions that generate more NE cells (i.e. symmetric proliferative divisions).
The observed correlation between the switch of NE cells to neurogenic divisions and the unequal distribution of the apical membrane to only one daughter cell is remarkable, especially if one considers the small proportion that the apical membrane constitutes relative to the entire plasma membrane, which (ignoring the presence of microvilli and the basal process (Miyata et al, 2001)) amounts to only 1–2%. We find it unlikely that this correlation is coincidental, that is, that an equal versus unequal distribution of apical membrane makes no difference for daughter cell fate. Rather, consistent with the notion that the apical membrane of epithelial cells is characterized by a specific protein and lipid compositions (van Meer and Simons, 1988), our observations strongly suggest that certain molecules with a critical role for cell fate are selectively associated with the apical membrane of NE cells. For example, it is conceivable that the apical membrane provides a signal that keeps the inheriting daughter cell in the NE stem cell state and hence in the cell cycle, and that lack of inheritance of apical membrane contributes to an eventually differentiated, postmitotic state of the respective daughter cell. In this context, it is worth noting that par-3, a protein implicated in asymmetric cell division, was found to be associated with the apical cortex of NE cells and differentially inherited by the daughter cells arising from neurogenic divisions. However, other proteins implicated in neurogenesis (e.g., numb (Cayouette and Raff, 2002; Fishell and Kriegstein, 2003)) do not appear to be selectively associated, in mitotic NE cells, with the apical membrane proper.
Consistent with a critical role of apical membrane constituents in NE cell fate, the amount of the apical membrane appeared to be regulated in accordance with its equal versus unequal distribution to the daughter cells. Remarkably, the relative size of the apical membrane of symmetrically dividing NE cells, which need to provide this membrane to both daughter cells, was found to be twice that of asymmetrically dividing NE cells, which need to provide this membrane to only one daughter. Together, our observations imply that the mechanism controlling the switch of NE cells from proliferative to neurogenic divisions regulates, in a concerted manner, (at least) three parameters: (i) the expression of TIS21 (from ‘off' to ‘on'), (ii) the size of the apical membrane (from two units to one) and (iii) the distribution of the apical membrane upon cleavage (from bisecting it to bypassing it).
Finally, the relatively small size of the apical membrane of mammalian NE cells is of relevance when one compares their asymmetric division to that of Drosophila neuroblasts. At first sight, there seems to be a principal difference between these two systems. Asymmetric division of Drosophila neuroblasts is associated with a 90° rotation of the cleavage plane to adopt an orientation perpendicular to the apical–basal axis of the neuroblast, as compared to an orientation parallel to the apical–basal axis in the dividing neuroectodermal progenitors; this change in cleavage plane orientation results in the differential distribution of cell fate determinants between the daughter cells generated by the neuroblast division (Matsuzaki, 2000; Knoblich, 2001; Wodarz and Huttner, 2003). In contrast, as shown in the present study, such rotation is observed only rarely in mouse NE cells, the vast majority of which maintain a cleavage plane orientation roughly parallel to their apical–basal axis as they switch from symmetric, proliferative to asymmetric, neurogenic divisions.
Another view, however, is that the rotation of the cleavage plane in the Drosophila neuroblast simply reflects the fact that its apical-specific cell cortex, like its basal-specific cell cortex, occupies a much larger proportion of the entire cell cortex than the apical membrane of mammalian NE cells does relative to their entire plasma membrane. Thus, the cleavage plane rotation in Drosophila neuroblasts may be required to ensure the inheritance of polarly localized cell constituents by one or the other daughter cell, an asymmetric inheritance that mammalian NE cells can achieve without such rotation, given that their apical domain is relatively small (Huttner and Brand, 1997). In other words, an asymmetric distribution of apical cell constituents appears to be a conserved feature common to both vertebrates and invertebrates, whereas cleavage plane orientation may vary between species depending on the specific geometric requirements of the respective progenitor cell.
Materials and methods
Immunofluorescence confocal microscopy of mouse neuroepithelium
Immunofluorescence on cryosections of E9.5-E14.5 mouse brains from wild-type and heterozygous TIS21-GFP knock-in embryos (Haubensak et al, 2004) was performed according to standard procedures. For details, see Supplementary data.
Analysis of mitotic NE cells for cleavage plane orientation and equal versus unequal distribution of apical membrane
Cleavage plane orientation and equal versus unequal distribution of apical membrane were determined in optical sections of mitotic NE cells stained for DNA, anillin and cadherin, and evaluated (if applicable) for TIS21-GFP fluorescence. For details, see Supplementary data. For the determination of the size and relative proportion of the apical membrane, see Supplementary data.
Supplementary Material
Supplementary data
Acknowledgments
We are greatly indebted to Drs CM Field and T Pawson for providing us with affinity-purified antibodies against anillin and par-3. KR was supported by a fellowship from the Studienstiftung des Deutschen Volkes. WBH was supported by grants from the DFG (SPP 1109, Hu 275/7; SPP 1111, Hu 275/8) and the Fonds der Chemischen Industrie.
References
- Aaku-Saraste E, Hellwig A, Huttner WB (1996) Loss of occludin and functional tight junctions, but not ZO-1, during neural tube closure—remodeling of the neuroepithelium prior to neurogenesis. Dev Biol 180: 664–679 [DOI] [PubMed] [Google Scholar]
- Adams RJ (1996) Metaphase spindles rotate in the neuroepithelium of rat cerebral cortex. J Neurosci 16: 7610–7618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayouette M, Raff M (2002) Asymmetric segregation of Numb: a mechanism for neural specification from Drosophila to mammals. Nat Neurosci 5: 1265–1269 [DOI] [PubMed] [Google Scholar]
- Cayouette M, Raff M (2003) The orientation of cell division influences cell-fate choice in the developing mammalian retina. Development 130: 2329–2339 [DOI] [PubMed] [Google Scholar]
- Cayouette M, Whitmore AV, Jeffery G, Raff M (2001) Asymmetric segregation of Numb in retinal development and the influence of the pigmented epithelium. J Neurosci 21: 5643–5651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenn A, McConnell SK (1995) Cleavage orientation and the asymmetric inheritance of Notch1 immunoreactivity in mammalian neurogenesis. Cell 82: 631–641 [DOI] [PubMed] [Google Scholar]
- Fishell G, Kriegstein AR (2003) Neurons from radial glia: the consequences of asymmetric inheritance. Curr Opin Neurobiol 13: 34–41 [DOI] [PubMed] [Google Scholar]
- Geldmacher-Voss B, Reugels AM, Pauls S, Campos-Ortega JA (2003) A 90-degree rotation of the mitotic spindle changes the orientation of mitoses of zebrafish neuroepithelial cells. Development 130: 3767–3780 [DOI] [PubMed] [Google Scholar]
- Gonczy P, Hyman AA (1996) Cortical domains and the mechanisms of asymmetric cell division. Trends Cell Biol 6: 382–387 [DOI] [PubMed] [Google Scholar]
- Hämmerle B, Vera-Samper E, Speicher S, Arencibia R, Martinez S, Tejedor FJ (2002) Mnb/Dyrk1A is transiently expressed and asymmetrically segregated in neural progenitor cells at the transition to neurogenic divisions. Dev Biol 246: 259–273 [DOI] [PubMed] [Google Scholar]
- Haubensak W, Attardo A, Denk W, Huttner WB (2004) Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc Natl Acad Sci USA 101: 3196–3201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heins N, Cremisi F, Malatesta P, Gangemi RM, Corte G, Price J, Goudreau G, Gruss P, Götz M (2001) Emx2 promotes symmetric cell divisions and a multipotential fate in precursors from the cerebral cortex. Mol Cell Neurosci 18: 485–502 [DOI] [PubMed] [Google Scholar]
- Huttner WB, Brand M (1997) Asymmetric division and polarity of neuroepithelial cells. Curr Opin Neurobiol 7: 29–39 [DOI] [PubMed] [Google Scholar]
- Iacopetti P, Michelini M, Stuckmann I, Oback B, Aaku-Saraste E, Huttner WB (1999) Expression of the antiproliferative gene TIS21 at the onset of neurogenesis identifies single neuroepithelial cells that switch from proliferative to neuron-generating division. Proc Natl Acad Sci USA 96: 4639–4644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich JA (2001) Asymmetric cell division during animal development. Nat Rev Mol Cell Biol 2: 11–20 [DOI] [PubMed] [Google Scholar]
- Kriegstein AR, Götz M (2003) Radial glia diversity: a matter of cell fate. Glia 43: 37–43 [DOI] [PubMed] [Google Scholar]
- Landrieu P, Goffinet A (1979) Mitotic spindle fiber orientation in relation to cell migration in the neo-cortex of normal and reeler mouse. Neurosci Lett 13: 69–72 [DOI] [PubMed] [Google Scholar]
- Lin D, Edwards AS, Fawcett JP, Mbamalu G, Scott JD, Pawson T (2000) A mammalian PAR-3–PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat Cell Biol 2: 540–547 [DOI] [PubMed] [Google Scholar]
- Manabe N, Hirai S, Imai F, Nakanishi H, Takai Y, Ohno S (2002) Association of ASIP/mPAR-3 with adherens junctions of mouse neuroepithelial cells. Dev Dyn 225: 61–69 [DOI] [PubMed] [Google Scholar]
- Matsuzaki F (2000) Asymmetric division of Drosophila neural stem cells: a basis for neural diversity. Curr Opin Neurobiol 10: 38–44 [DOI] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Okano H, Ogawa M (2001) Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron 31: 727–741 [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR (2001) Neurons derived from radial glial cells establish radial units in neocortex. Nature 409: 714–720 [DOI] [PubMed] [Google Scholar]
- Nose A, Takeichi M (1986) A novel cadherin cell adhesion molecule: its expression patterns associated with implantation and organogenesis of mouse embryos. J Cell Biol 103: 2649–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oegema K, Savoian MS, Mitchison TJ, Field CM (2000) Functional analysis of a human homologue of the Drosophila actin binding protein anillin suggests a role in cytokinesis. J Cell Biol 150: 539–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S (2001) Intercellular junctions and cellular polarity: the PAR-aPKC complex, a conserved core cassette playing fundamental roles in cell polarity. Curr Opin Cell Biol 13: 641–648 [DOI] [PubMed] [Google Scholar]
- Rakic P (1988) Specification of cerebral cortical areas. Science 241: 170–176 [DOI] [PubMed] [Google Scholar]
- Reinsch S, Karsenti E (1994) Orientation of spindle axis and distribution of plasma membrane proteins during cell division in polarized MDCKII cells. J Cell Biol 126: 1509–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart IHM (1973) Proliferative characteristics of the ependymal layer during the early development of the mouse neocortex: a pilot study based on recording the number, location and plane of cleavage of mitotic figures. J Anat 116: 67–91 [PMC free article] [PubMed] [Google Scholar]
- Takekuni K, Ikeda W, Fujito T, Morimoto K, Takeuchi M, Monden M, Takai Y (2003) Direct binding of cell polarity protein PAR-3 to cell–cell adhesion molecule nectin at neuroepithelial cells of developing mouse. J Biol Chem 278: 5497–5500 [DOI] [PubMed] [Google Scholar]
- van Meer G, Simons K (1988) Lipid polarity and sorting in epithelial cells. J Cell Biochem 36: 51–58 [DOI] [PubMed] [Google Scholar]
- Weigmann A, Corbeil D, Hellwig A, Huttner WB (1997) Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc Natl Acad Sci USA 94: 12425–12430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A, Huttner WB (2003) Asymmetric cell division during neurogenesis in Drosophila and vertebrates. Mech Dev 120: 1297–1309 [DOI] [PubMed] [Google Scholar]
- Zieba P, Strojny P, Lamprecht J (1986) Positioning and stability of mitotic spindle orientation in the neuroepithelial cell. Cell Biol Int Rep 10: 91–100 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data


