Abstract
Background
Primary cervical dystonia is the most common form of adult-onset focal dystonia. Although most frequently sporadic, 15–20% of patients report a positive family history, suggesting a possible genetic cause. Head tremor is often present in patients with cervical dystonia and may be a prominent symptom.
Objective
To describe the clinical characteristics of patients with tremulous cervical dystonia.
Methods
Patients with primary cervical dystonia attending our botulinum toxin clinic were assessed with an interview and neurological examination and their notes reviewed. Patients were classified as having either tremulous or non-tremulous cervical dystonia, according to the presence or absence of head tremor on examination. Clinical and demographic data were compared between groups.
Results
From 273 patients included (190 females, 83 males), 125 (46%) were classified as tremulous and 148 (54%) as non-tremulous. Tremulous patients were more likely to have a segmental distribution (61% vs 25%), often involving the arms (48%), and had more frequently associated arm tremor (55% vs 10%). A positive family history of dystonia and/or tremor was more frequent in tremulous patients (50% vs 18%).
Conclusions
Patients with cervical dystonia with associated head tremor are more likely to have a segmental distribution (with frequent arm involvement), associated arm tremor and a positive family history, suggesting a genetic etiology in this subgroup of patients.
Keywords: Tremor, Dystonia, Familial tremor, Familial dystonia
1. Introduction
Primary cervical dystonia is the most common form of adult-onset dystonia, with a prevalence ranging between 23 and 130 cases/million [1,2]. It usually presents between the 4th and 6th decade of life and it affects women 1.5–1.9 times more frequently than men [1]. Although most commonly sporadic, approximately 15–20% of patients report a positive family history [3], suggesting a genetic cause in a subgroup of patients. Further supporting the influence of genetic factors are the observation that cervical dystonia occurs in monozygotic twins [4,5] and the association of this phenotype with certain loci in linkage studies, namely DYT7 [6] and DYT13 [7], and, more recently, with mutations in CIZ1 and GNAL genes [8,9].
Patients with cervical dystonia often have associated tremor, involving head and/or arms [1]. Head tremor can be seen in 28 to 68% of patients, depending on the series [10–13], and it can be a prominent symptom, contributing to disability. This feature seems to be relatively specific of primary dystonia [13,14]. Recently, mutations in the gene ANO3, have been identified as a cause for familial tremulous cervical dystonia [15].
In this series, one of the largest of its kind, we present a description of the phenotype of patients with tremulous cervical dystonia, identifying their specific clinical features. We found that these patients have a frequent family history of tremor, confirming findings in previous reports [12], but also of dystonia, suggesting a genetic predisposition.
2. Methods
We recruited consecutive patients with a diagnosis of primary cervical dystonia [16] attending the botulinum toxin clinic at the National Hospital for Neurology and Neurosurgery, London, United Kingdom, between the preset dates of April 2010 and November 2011. Secondary forms were excluded by history, examination and ancillary tests, when appropriate [16]. This study is based solely on information and investigations that are carried out as part of the routine clinical care of the patients. As such, our institution does not require ethical approval.
All patients were assessed by one of the authors at least three months after their last set of injections, with an interview and neurological examination, focused on tremor and dystonia. Assessment of tremor included observation of head tremor (seated upright, with the head in neutral position and while turning the head to either side), arm tremor (rest tremor-arms relaxed in the lap; postural tremor-arms outstretched in pronation and supination; flexed postural tremor–shoulders abducted and elbows flexed, placing both hands, palms down, in front of the chest; kinetic tremor-performing the finger-to-nose maneuver, writing a standardized sentence and drawing a spiral) and voice tremor (holding a note, reading aloud). Following current recommendations [17], tremor was defined as a rhythmic/semirhythmic involuntary oscillatory movement of a body part. Irregular amplitudes and superimposed jerks, recognized as characteristic of dystonic tremor [17–19], were accepted, but patients with isolated head jerks, were not classified as tremulous. Tremor could be present in the neutral position or be position sensitive, but patients who displayed tremor only while rotating the head to an extreme position, away from the direction towards which their dystonia pulled, were not considered tremulous. The diagnosis of dystonia and classification by distribution were done following current recommendations [16]. Brachial involvement included the presence of dystonic features in the fingers, hand or arm. Shoulder elevation was not considered as brachial involvement. Patient’s notes were reviewed for relevant clinical information, including age at onset, presenting complaint, improvement of symptoms with alcohol, presence of a sensory trick, associated neck pain and family history of movement disorders.
Patients were classified as having tremulous cervical dystonia (TCD) or non-tremulous cervical dystonia (NTCD), according to the presence or absence of head tremor.
Clinical and demographic data were compared between these groups. Statistical analysis was performed with IBM SPSS Statistics 19.0. Categorical variables were expressed as percentages and were compared using Chi-square test or Fisher’s exact test, as appropriate. Numerical data were expressed as means, with the standard deviation (SD), or as medians, with the interquartile range (IQR), if they were not normally distributed. Comparison of numerical variables was made with independent sample Student’s t-test or with U-Mann–Whitney test, if parametric assumptions were not met. Two-tailed probability was used for all comparisons and a statistical significance of p < 0.05 was assumed. Effect size was reported with the odds ratio (OR).
3. Results
3.1. Cohort characteristics
A total of 273 patients with cervical dystonia were included, 190 females and 83 males (female/male ratio: 2.3). Mean age at the time of assessment was 60.5 years (12.3). Median time of follow-up was 8.1 years (3.9–13.9). Regarding ethnicity, 261 patients were Caucasian (239 English, 7 Irish, 3 Welsh, 3 German, 2 Italian, 2 South African, 1 Scottish, 1 Austrian, 1 Dutch, 1 Greek, 1 Hungarian), 5 patients were African (2 Kenyan, 2 Moroccan, 1 Zimbabwean) and 7 Asian (3 Turkish, 2 Pakistani, 1 Iranian, 1 Yemeni).
Mean age at onset of symptoms was 41.4 years (13.6). Distribution of dystonic features was segmental in 113 patients (41%) and focal in 160 (59%). Head tremor was present in 125 patients (46%) and arm tremor in 84 patients (31%). Head tremor was irregular/jerky in 114/125 patients (91%).
3.2. Classification and demographic data
Table 1 A total of 125 patients (46%) were classified as TCD and 148 (54%) as NTCD.
Table 1.
Demographic and clinical characteristics.
| TCD | NTCD | p-value | |
|---|---|---|---|
| Number of patients, n (%) | 125 (46) | 148 (54) | |
| Sex distribution, n | 0.02 | ||
| Female/male | 96/29 | 94/54 | |
| Ratio F:M | 3.3:1 | 1.7:1 | |
| Age at onset, mean (SD), years | 39.9(14.1) | 42.7 (13.1) | 0.10 |
| Distribution of dystonia, n (%) | <0.01 | ||
| Focal | 49 (39) | 111 (75) | |
| Segmental | 76 (61) | 37 (25) | |
| Upper face | 15 (12) | 11 (7) | 0.2 |
| Lower face | 14 (11) | 16 (11) | 0.92 |
| Jaw | 5 (4) | 5 (3) | 0.99 |
| Larynx | 19 (15) | 11 (7) | 0.04 |
| Hand | 60 (48) | 23 (16) | <0.01 |
| Arm tremor, n (%) | 69 (55) | 15 (10) | <0.01 |
| Rest | 22 (18) | 1 (1) | <0.01 |
| Postural | 67 (54) | 15 (10) | <0.01 |
| Kinetic | 11 (9) | 1 (1) | <0.01 |
| Other tremor, n (%) | 12 (10) | 1 (1) | <0.01 |
| Voice tremor | 8 (6) | 1 (1) | 0.01 |
| Jaw tremor | 5 (4) | 1 (1) | 0.09 |
| Neck pain, n (%) | 73(58) | 102 (69) | 0.07 |
| Alcohol response, ratio (%)a | 41/108 (38) | 32/118 (27) | 0.08 |
| Sensory trick, n (%) | 87 (70) | 113 (76) | 0.21 |
TCD: tremulous cervical dystonia. NTCD: non-tremulous cervical dystonia. F: female. M: male.
Information regarding alcohol response was not available for 17 patients. Of the remaining 256, 30 denied use of alcohol. Information regarding improvement of symptoms with alcohol was thus available for 226 patients.
Although sex distribution favored females in both groups, this difference was greater in TCD patients (3.3:1 vs 1.7:1; X2(1) 5.7; p = 0.02). There were no significant differences in age at onset between groups.
3.3. Clinical features
Table 1, video TCD patients had a segmental distribution of dystonic symptoms more often than NTCD patients (61% vs 25%; X2(1) = 35.8; p < 0.01; OR: 4.7). This difference was mainly due to a higher frequency of brachial involvement (48% vs 16%; X2(1) 33.7; p < 0.01; OR:5), but also partly to laryngeal involvement (15% vs 7%; X2(1) 4.2; p = 0.04; OR:2.2).
Supplementary video related to this article can be found online at 10.1016/j.parkreldis.2013.02.017
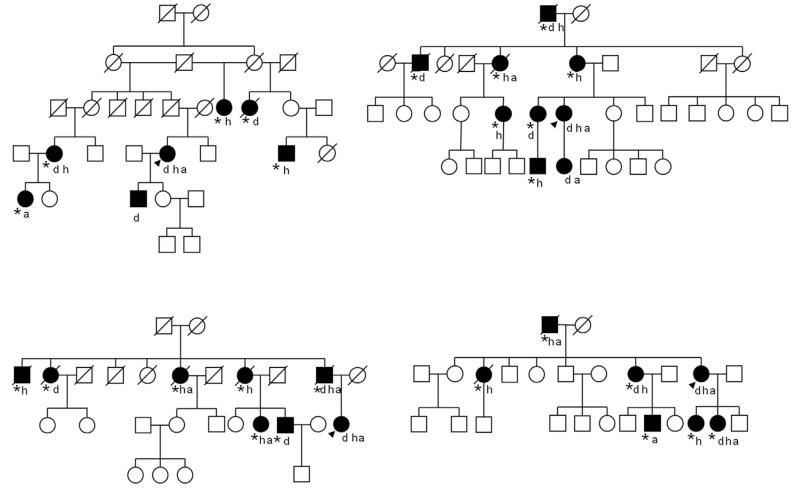
Fig 1. Pedigrees.
Selected pedigrees of unrelated families of tremulous cervical dystonia patients. Affected family members are shown as filled shapes. The index case is indicated by an arrow. A “d” bellow the shape indicates dystonia, an “h” indicates head tremor, an “a” arm tremor. An asterisk indicates involvement reported by history, but not confirmed by examination. Females are represented by circular shapes and males by square shapes. Deceased individuals are indicated by a diagonal line over the shape. The index cases had been screened negative for TOR1A and THAP-1 mutations.
TCD patients were more likely to have associated arm tremor than NTCD patients (55% vs 10%; X2(1) = 64.6; p < 0.01; OR: 10.3). Arm tremor in these patients was most frequently postural (60%), but 20% also had a rest component and 10% a kinetic component. Kinetic and rest tremor were only seen in one patient in the NTCD group (1%). Tremor involving other areas in the cranio-cervical region, such as the jaw or vocal cords, occurred rarely, but was also more frequent in TCD patients (10% vs 1%; X2(1) = 11.9; p < 0.01; OR: 15.6).
There were no significant differences between groups regarding the frequency of associated neck pain, improvement of symptoms with alcohol (information available for 226 patients) or presence of a sensory trick. A total of 200 patients (73%) reported at least one sensory trick. These included: touching the chin (97/200), touching the cheek (71/200), touching the occiput (43/200) and other (18/200). Some patients had more than one sensory trick. There were no significant differences regarding the type of sensory trick between groups.
3.4. Family history
Table 2 Information regarding family history was not available for one NTCD patient, who was adopted.
Table 2.
Reported family history of movement disorders.
| TCD (n = 125) | NTCD (n = 147)a | p-value | |
|---|---|---|---|
| Family history of any MD, n (%) | 71 (57) | 37 (25) | <0.01 |
| Tremor and/or dystonia, n (%) | 63 (50) | 26 (18) | <0.01 |
| Tremor | 56 (45) | 14 (10) | <0.01 |
| Head tremor | 47 (38) | 12 (8) | <0.01 |
| Arm tremor | 25 (20) | 6 (4) | <0.01 |
| Dystonia | 24 (19) | 15 (10) | 0.03 |
| Cervical dystonia | 20 (16) | 13 (9) | 0.07 |
| Brachial dystonia | 8 (6) | 2 (1) | 0.04 |
| Other | 6 (5) | 4 (3) | 0.52 |
| Probable AD inheritance, ratio (%)b | 47/63 (75) | 19/26 (73) | 0.88 |
| ≥3 family members affected, ratio (%)c | 16/63 (25) | 3/26 (12) | 0.15 |
| 3 family members | 8/63 (13) | 1/26 (4) | |
| 4–5 family members | 3/63 (5) | 1/26 (4) | |
| >5 family members | 5/63 (8) | 1/26 (4) | |
| Other MD, (%) | 15 (12) | 15 (10) | 0.64 |
| Parkinsonism | 14 (11) | 14 (10) | 0.65 |
| Tics | 1 (1) | 2 (1) | 0.99 |
TCD: tremulous cervical dystonia. NTCD: non-tremulous cervical dystonia. MD: movement disorders. AD: autosomal dominant.
Family history was not known in one patient, who was adopted.
A probable autosomal dominant pattern of inheritance was assumed when affected members were present in two consecutive generations.
Families with three or more family members affected, as reported by history and excluding the index case. Only patients with a positive family history of dystonia and or tremor (n = 89) were considered for these comparisons.
A total of 89 patients reported a positive family history of dystonia and/or tremor. Of them, 40 reported familial tremor (all 40 reporting familial head tremor, 12 also reporting additional familial arm tremor), 20 tremor and dystonia and 19 familial dystonia.
Familial dystonia (39 patients) included cervical dystonia (33 patients), brachial dystonia (writer’s cramp; 10 patients), and dystonia involving other areas (10 patients; six with cranial dystonia, two with laryngeal dystonia, two with foot dystonia).
TCD patients more commonly reported a positive family history of dystonia and/or tremor (50% vs 18%; X2(1) = 32.8; p < 0.01; OR: 4.7). This difference was more pronounced for familial tremor (45% vs 10%; X2(1) = 44; p < 0.01; OR: 7.7), but was also clear for familial dystonia (19% vs 10%; X2(1) = 4.5; p = 0.03; OR: 2.1).
Pedigrees with two consecutive generations affected by dystonia and/or tremor, suggesting a probable autosomal dominant pattern of inheritance, were seen in 47/63 (75%) of the TCD patients who had a positive family history.
We also identified 19 families with three or more members affected by dystonia and/or tremor, excluding the index case. Sixteen of them belonged to the TCD group. Pedigrees of the largest families are shown in Fig. 1.
Of the 89 patients with a positive family history of tremor and/or dystonia, genetic testing for pathogenic mutations had been performed in 18 patients for TOR1A (DYT1) and in 12 for THAP-1 (DYT6), and was negative.
The frequency of a positive family history of other movement disorders, such as parkinsonism or tics, was similar in both groups.
4. Discussion
Here we report the clinical characteristics of the largest reported cohort of patients with TCD. We found that these patients are more likely to have a positive family history of dystonia and/or tremor (OR: 4.7), with pedigrees suggesting an autosomal dominant pattern of inheritance in up to 75% of cases. Large pedigrees, with more than three affected family members, were also more common in this group.
TCD patients had specific clinical features. They had a stronger female preponderance than NTCD patients (3.3:1 vs 1.7:1) and were more likely to have a segmental distribution of their symptoms (OR: 4.7), with more frequent arm (OR: 5) and laryngeal involvement (OR:2.2). Arm tremor, most frequently postural, was more frequent in this group (55% vs 10%; OR: 10.3), and occasionally included a rest and kinetic component, which were almost never seen in NTCD patients. Other forms of tremor, such as jaw tremor and voice tremor were also more common in the TCD group. There were no significant differences regarding other features, such as the presence or type of sensory trick, associated neck pain or improvement with alcohol. We found a high frequency of alcohol responsiveness (32%). Other large series of patients with cervical dystonia have not systematically assessed this clinical trait. However, one small series including 18 patients with primary dystonia involving neck or ams, reported a rate of alcohol responsiveness of 39%, similar to that observed in our study [20].
Although Pal et al. reported that patients with cervical dystonia and head tremor were more likely to have associated arm tremor (40% vs 8.3%) and a positive family history of tremor (21.8% vs 5.5%) [12] in their smaller series (114 patients; 78 head tremor positive patients), they did not find other clinical differences and concluded that “the clinical characteristics are not different in CD patients with or without tremor” [12]. However, in our larger sample, we have found several differences that help defining a specific phenotype within cervical dystonia, as detailed above. This includes a stronger tendency for spread of dystonic features, a higher proportion of tremor in other cranio-cervical regions, a different arm tremor phenotype and a higher frequency of a positive family history, not only of tremor, but also of familial dystonia, with a pattern of inheritance suggesting an autosomal dominant mode in most cases.
Taken together, our results suggest that TCD patients have specific clinical characteristics that are frequently familial, and, in most cases, transmitted as an autosomal dominant trait, suggesting that mutations in one or more Mendelian genes might be responsible for the phenotype in these families. Adequate identification of this phenotype may allow a greater yield of genome-wide association screening studies.
From the previously known causes of monogenic dystonia, TOR1A (DYT1) and THAP-1 (DYT6), none has been specifically associated with head tremor. Patients with TOR1A mutations typically present during childhood with limb involvement, spreading subsequently to a generalized distribution [21] and cervical involvement occurs only rarely [22]. Patients harbouring THAP-1 mutations also tend to present with early-onset generalized dystonia, but cervical and laryngeal involvement are more frequent [21]. Recently, mutations in the GNAL gene have been demonstrated in nine out of 39 families with autosomal dominant primary dystonia. Similar to our patients, most patients with GNAL mutations have focal or segmental dystonia presenting in adulthood with cervical involvement, which may spread to involve the arms or other cranial structures [9].
Although head tremor has not been specifically described as a common trait in these patients, it is possible that some TCD patients might carry mutations in these genes.
Two other loci have been linked with adult-onset focal dystonia, predominantly involving the cranio-cervical region [6,7,21]. However, the existence of DYT7 (chromosome 18p) is currently debated. A recent study using deep sequencing failed to identify any mutation within this locus [23]. In any case, head tremor has not been reported as a feature of DYT7 [6]. On the contrary, in their description of patients with DYT13 (chromosome 1p), the authors report head tremor, arm tremor and laryngeal involvement, including voice tremor [7], resembling the phenotype described in our patients. It is thus possible that some of them might link to the same region.
At least five other large pedigrees with adult-onset cervical dystonia have been reported, without linkage to a specific chromosome [24–28]. In the description of these large families, head tremor, arm tremor and segmental distribution, often with brachial or laryngeal involvement, are commonly reported, lending support to the idea of a genetic component for this phenotype. Mutations in ANO3 (chromosome 11) have been identified as the cause of dystonia in one of these previously reported large kindreds and in a smaller family [15]. The phenotype, including tremulous cervical dystonia with laryngeal involvement and associated arm tremor corresponds to that seen in our patients. It is thus possible that some of them will carry mutations in this gene. However, ANO3 mutations seem to be rare. In their study, the authors screened 384 cases with cervical dystonia and/or upper limb dystonic tremor, with 137 having a positive family history, and found only one additional case. ANO3 codes for anoctamin 3, which belongs to a family of calcium-activated chloride channels and expresses highly in the striatum. Alterations in this protein may result in abnormal intracellular calcium signaling leading to altered striatal neuronal excitability [15].
To date there is no accurate way of distinguishing essential tremor from dystonic tremor apart from observation of additional features i.e. dystonia. To avoid confusion, we have focused on an accurate phenotypic description regarding the type of tremor. Based on accepted consensus [17] all our patients had dystonic head tremor and, either dystonic arm tremor (when dystonic features were present in the arms) or tremor associated with dystonia (when clear dystonic features were not present in the arms). Given that their family members were not directly examined, it is even more difficult to draw conclusions about the nature of their tremor and the possibility that some of them have essential tremor cannot be entirely ruled out. In those in which dystonic features were also reported or with isolated head tremor, dystonic tremor would be more likely [29,30].
5. Limitations
We acknowledge limitations to our study. As a tertiary center for movement disorders, we cannot exclude a referral bias. We did not use objective measures for tremor (tremor recordings), which are not used as part of routine clinical care. Our project was conceived as a clinical study, with methods available to any clinician, underscoring the importance of simple and careful clinical observations. Further research may focus not only on identifying possible genetic alterations behind this phenotype, but also on describing the neurophysiological characteristics. Some tests were not performed systematically in all patients, such as genetic testing for TOR1A and THAP-1. It is possible that some TCD patients might carry mutations of these genes. However, the yield of THAP-1 testing seems to be low in the context of adult-onset cervical dystonia [31] and we believe that the phenotype of TOR1A differs sufficiently to make it unlikely that mutations in this gene will account for a large number of the familial TCD. Finally, because information regarding familial involvement was obtained by history alone, the frequency of affected family members may be under- or overestimated, but this should have affected both groups similarly. It has been reported that patients with dystonia underestimate the frequency of family members affected [6,32].
6. Conclusion
Head tremor is a frequent finding in patients with cervical dystonia. Patients with tremulous cervical dystonia are more likely to have a segmental distribution (often involving the arms) and arm tremor. They frequently have a positive family history, suggesting a genetic cause. It is likely that genetic studies will identify additional Mendelian gene(s) associated with this particular phenotype.
Supplementary Material
Acknowledgments
Funding sources for study
This work was undertaken at UCLH/UCL, which received a proportion of funding from the Department of Health’s NIHR Biomedical Research Centres’ funding scheme. MJE and TAS are supported by fellowships from the National Institute of Health Research (NIHR).
Footnotes
Financial disclosure/Conflict of interest
Nothing to declare by any of the authors.
References
- [1].Dauer WT, Burke RE, Greene P, Fahn S. Current concepts on the clinical features, aetiology and management of idiopathic cervical dystonia. Brain. 1998;121(Pt 4):547–60. doi: 10.1093/brain/121.4.547. [DOI] [PubMed] [Google Scholar]
- [2].Defazio G, Berardelli A, Hallett M. Do primary adult-onset focal dystonias share aetiological factors? Brain. 2007;130:1183–93. doi: 10.1093/brain/awl355. [DOI] [PubMed] [Google Scholar]
- [3].Leube B, Kessler KR, Goecke T, Auburger G, Benecke R. Frequency of familial inheritance among 488 index patients with idiopathic focal dystonia and clinical variability in a large family. Mov Disord. 1997;12:1000–6. doi: 10.1002/mds.870120625. [DOI] [PubMed] [Google Scholar]
- [4].Wunderlich S, Reiners K, Gasser T, Naumann M. Cervical dystonia in monozygotic twins: case report and review of the literature. Mov Disord. 2001;16:714–8. doi: 10.1002/mds.1128. [DOI] [PubMed] [Google Scholar]
- [5].Factor SA. Cervical dystonia in twins. Mov Disord. 2002;17:846–7. doi: 10.1002/mds.10215. [DOI] [PubMed] [Google Scholar]
- [6].Leube B, Rudnicki D, Ratzlaff T, Kessler KR, Benecke R, Auburger G. Idiopathic torsion dystonia: assignment of a gene to chromosome 18p in a German family with adult onset, autosomal dominant inheritance and purely focal distribution. Hum Mol Genet. 1996;5:1673–7. doi: 10.1093/hmg/5.10.1673. [DOI] [PubMed] [Google Scholar]
- [7].Bentivoglio AR, Ialongo T, Contarino MF, Valente EM, Albanese A. Phenotypic characterization of DYT13 primary torsion dystonia. Mov Disord. 2004;19:200–6. doi: 10.1002/mds.10634. [DOI] [PubMed] [Google Scholar]
- [8].Xiao J, Uitti RJ, Zhao Y, Vemula SR, Perlmutter JS, Wszolek ZK, et al. Mutations in CIZ1 cause adult onset primary cervical dystonia. Ann Neurol. 2012;71:458–69. doi: 10.1002/ana.23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fuchs T, Saunders-Pullman R, Masuho I, Luciano MS, Raymond D, Factor S, et al. Mutations in GNAL cause primary torsion dystonia. Nat Genet. 2013;45:88–92. doi: 10.1038/ng.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chan J, Brin MF, Fahn S. Idiopathic cervical dystonia: clinical characteristics. Mov Disord. 1991;6:119–26. doi: 10.1002/mds.870060206. [DOI] [PubMed] [Google Scholar]
- [11].Jankovic J, Leder S, Warner D, Schwartz K. Cervical dystonia: clinical findings and associated movement disorders. Neurology. 1991;41:1088–91. doi: 10.1212/wnl.41.7.1088. [DOI] [PubMed] [Google Scholar]
- [12].Pal PK, Samii A, Schulzer M, Mak E, Tsui JK. Head tremor in cervical dystonia. Can J Neurol Sci. 2000;27:137–42. [PubMed] [Google Scholar]
- [13].Molho ES, Feustel PJ, Factor SA. Clinical comparison of tardive and idiopathic cervical dystonia. Mov Disord. 1998;13:486–9. doi: 10.1002/mds.870130319. [DOI] [PubMed] [Google Scholar]
- [14].Svetel M, Ivanovic N, Marinkovic J, Jovic J, Dragasevic N, Kostic VS. Characteristics of dystonic movements in primary and symptomatic dystonias. J Neurol Neurosurg Psychiatry. 2004;75:329–30. doi: 10.1136/jnnp.2003.017632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Charlesworth G, Plagnol V, Holmstrom KM, Bras J, Sheerin UM, Preza E, et al. Mutations in Ano3 cause dominant craniocervical dystonia: ion channel implicated in pathogenesis. Am J Hum Genet. 2012 doi: 10.1016/j.ajhg.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Albanese A, Asmus F, Bhatia KP, Elia AE, Elibol B, Filippini G, et al. EFNS guidelines on diagnosis and treatment of primary dystonias. Eur J Neurol. 2011;18:5–18. doi: 10.1111/j.1468-1331.2010.03042.x. [DOI] [PubMed] [Google Scholar]
- [17].Deuschl G, Bain P, Brin M, Ad Hoc Scientific Committee Consensus statement of the Movement Disorder Society on Tremor. Mov Disord. 1998;13(Suppl. 3):2–23. doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- [18].Deuschl G. Dystonic tremor. Rev Neurol (Paris) 2003;159:900–5. [PubMed] [Google Scholar]
- [19].Jedynak CP, Bonnet AM, Agid Y. Tremor and idiopathic dystonia. Mov Disord. 1991;6:230–6. doi: 10.1002/mds.870060307. [DOI] [PubMed] [Google Scholar]
- [20].Valente EM, Edwards MJ, Mir P, DiGiorgio A, Salvi S, Davis M, et al. The epsilon-sarcoglycan gene in myoclonic syndromes. Neurology. 2005;64:737–9. doi: 10.1212/01.WNL.0000151979.68010.9B. [DOI] [PubMed] [Google Scholar]
- [21].Muller U. The monogenic primary dystonias. Brain. 2009;132:2005–25. doi: 10.1093/brain/awp172. [DOI] [PubMed] [Google Scholar]
- [22].Valente EM, Warner TT, Jarman PR, Mathen D, Fletcher NA, Marsden CD, et al. The role of DYT1 in primary torsion dystonia in Europe. Brain. 1998;121(Pt 12):2335–9. doi: 10.1093/brain/121.12.2335. [DOI] [PubMed] [Google Scholar]
- [23].Winter P, Kamm C, Biskup S, Kohler A, Leube B, Auburger G, et al. DYT7 gene locus for cervical dystonia on chromosome 18p is questionable. Mov Disord. 2012 doi: 10.1002/mds.25219. [DOI] [PubMed] [Google Scholar]
- [24].Munchau A, Valente EM, Davis MB, Stinton V, Wood NW, Quinn NP, et al. A yorkshire family with adult-onset cranio-cervical primary torsion dystonia. Mov Disord. 2000;15:954–9. doi: 10.1002/1531-8257(200009)15:5<954::aid-mds1028>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- [25].Uitti RJ, Maraganore DM. Adult onset familial cervical dystonia: report of a family including monozygotic twins. Mov Disord. 1993;8:489–94. doi: 10.1002/mds.870080413. [DOI] [PubMed] [Google Scholar]
- [26].Bressman SB, Warner TT, Almasy L, Uitti RJ, Greene PE, Heiman GA, et al. Exclusion of the DYT1 locus in familial torticollis. Ann Neurol. 1996;40:681–4. doi: 10.1002/ana.410400421. [DOI] [PubMed] [Google Scholar]
- [27].Brancati F, Defazio G, Caputo V, Valente EM, Pizzuti A, Livrea P, et al. Novel Italian family supports clinical and genetic heterogeneity of primary adult-onset torsion dystonia. Mov Disord. 2002;17:392–7. doi: 10.1002/mds.10077. [DOI] [PubMed] [Google Scholar]
- [28].O’Riordan S, Lynch T, Hutchinson M. Familial adolescent-onset scoliosis and later segmental dystonia in an Irish family. J Neurol. 2004;251:845–8. doi: 10.1007/s00415-004-0444-x. [DOI] [PubMed] [Google Scholar]
- [29].Jain S, Lo SE, Louis ED. Common misdiagnosis of a common neurological disorder: how are we misdiagnosing essential tremor? Arch Neurol. 2006;63:1100–4. doi: 10.1001/archneur.63.8.1100. [DOI] [PubMed] [Google Scholar]
- [30].Schrag A, Munchau A, Bhatia KP, Quinn NP, Marsden CD. Essential tremor: an overdiagnosed condition? J Neurol. 2000;247:955–9. doi: 10.1007/s004150070053. [DOI] [PubMed] [Google Scholar]
- [31].Groen JL, Ritz K, Contarino MF, van de Warrenburg BP, Aramideh M, Foncke EM, et al. DYT6 dystonia: mutation screening, phenotype, and response to deep brain stimulation. Mov Disord. 2010;25:2420–7. doi: 10.1002/mds.23285. [DOI] [PubMed] [Google Scholar]
- [32].Martino D, Aniello MS, Masi G, Lamberti P, Lucchese V, Lamberti S, et al. Validity of family history data on primary adult-onset dystonia. Arch Neurol. 2004;61:1569–73. doi: 10.1001/archneur.61.10.1569. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.