Abstract
IMPORTANCE
Mutations in the SQSTM1 gene, coding for p62, are a cause of Paget disease of bone and amyotrophic lateral sclerosis (ALS). Recently, SQSTM1 mutations were confirmed in ALS, and mutations were also identified in 3 patients with frontotemporal dementia (FTD), suggesting a role for SQSTM1 in FTD.
OBJECTIVE
To evaluate the exact contribution of SQSTM1 to FTD and FTD with ALS (FTD-ALS) in an independent cohort of patients.
DESIGN
A SQSTM1 mutation was first identified in a multiplex family with FTD by use of whole-exome sequencing. To evaluate the frequency of SQSTM1 mutations, we sequenced this gene in a cohort of patients with FTD or FTD-ALS, with no mutations in known FTD and ALS genes.
SETTING
Primary care or referral center.
PARTICIPANTS
An overall cohort of 188 French patients, including 132 probands with FTD and 56 probands with FTD-ALS.
MAIN OUTCOMES AND MEASURES
Frequency of SQSTM1 mutations in patients with FTD or FTD-ALS; description of associated phenotypes.
RESULTS
We identified 4 heterozygous missense mutations in 4 unrelated families with FTD; only 1 family had clinical symptoms of Paget disease of bone, and only 1 family had clinical symptoms of FTD-ALS, possibly owing to the low penetrance of some of the clinical manifestations.
CONCLUSIONS AND RELEVANCE
Although the frequency of the mutations is low in our series (4 of 188 patients [2%]), our results, similar to those already reported, support a direct pathogenic role of p62 in different types of FTD.
Mutations in the SQSTM1 gene, coding for the p62 (sequestosome 1) protein, were initially identified as a cause of Paget disease of bone (PDB).1 The protein p62 is an adaptor protein that contains several protein-protein interaction motifs and has multiple functions in receptor-mediated signal transduction, regulating osteoclast differentiation, activity, and survival. It also acts as a shuttling factor that targets ubiquitinated proteins for degradation by autophagy or by the proteasome pathways.2
There is growing evidence implicating p62 in neurodegeneration. The p62 protein aggregates in neurons in various neurodegenerative disorders, including frontotemporal dementia (FTD; OMIM 105550) and amyotrophic lateral sclerosis (ALS).3 It plays a critical role in the formation of ubiquitinated protein inclusions in autophagic-deficient neurons.2
In 2011, SQSTM1 mutations were identified in ALS.4,5 SQSTM1 mutations were later confirmed in ALS by Rubino and colleagues,6 who also identified mutations in 3 patients with FTD, but owing to the small number of cases and the lack of segregation information in families with FTD, the link between SQSTM1 and FTD needed confirmation. Independently, we identified a SQSTM1 mutation by use of whole-exome sequencing in a large French family with FTD. To further investigate the role of SQSTM1 in the FTD spectrum, we analyzed an independent cohort of 187 additional French probands with FTD or FTD with ALS (FTD-ALS).
Methods
A cohort of 429 unrelated French probands with FTD or FTD-ALS (including 310 familial cases)7 was recruited between 1998 and 2012, through a national network of neurologists experts in FTD and FTD-ALS from 15 French university hospitals. The diagnosis of FTD was based on the revised Neary et al criteria,8 and the diagnosis of associated ALS was based on the El Escorial criteria.9
DNA was extracted from blood samples of each of the probands after informed consent was obtained for genetic studies. Our study was approved by the ethics committee of “AP-HP de Paris.” Known FTD genes (C9orf72, MAPT, PGRN, VCP, and CHMP2B) and autosomal dominant ALS genes (SOD1, TARDBP, FUS/TLS, PFN1, and UBQLN2) were first analyzed in probands; a total of 241 probands carried one of these mutations: C9orf72 expansions (n = 151), PGRN mutations (n = 47), MAPT mutations (n = 27), VCP mutations (n = 10), TARDBP mutations (n = 5), and FUS/TLS mutation (n = 1). Finally, no known mutations were identified in 188 probands, including 134 probands with FTD (68 familial cases) and 54 probands with FTD-ALS (37 familial cases). Notably, PDB aggregated with FTD (n = 9) or with FTD-ALS (n = 3) in a subset of 12 families.
Exome sequencing was performed on 3 affected sibs (003, 005, and 018) from family F297 (Figure 1). In brief, genomic DNA was prepared according to Illumina’s TruSeq Sample Preparation, version 3, and sequence capture, enrichment, and elution were performed according to the manufacturer’s instructions and protocols (Illumina’s TruSeq Exome Enrichment). Sequencing was performed on Illumina’s HiSeq2000 using 100–base pair paired-end reads. Sequence alignment and variant calling were performed against the reference human genome (UCSC hg19) using the Burrows-Wheeler Alignment tool10 and the Genome Analysis Toolkit.11
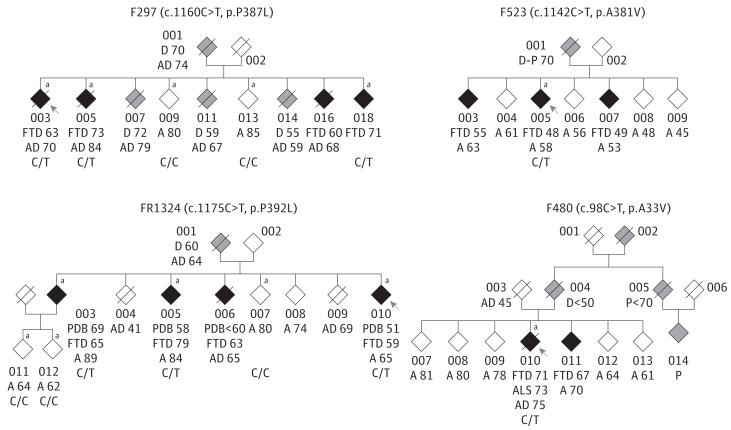
Figure 1. Pedigrees of Family F297 Carrying c.1160C>T, p.P387L Mutation, Family F523 Carrying c.1142C>T, p.A381V Mutation, Family FR1324 Carrying c.1175C>T, p.P392L Mutation, and Family F480 Carrying c.98C>T, p.A33V Mutation.
The individuals are represented by diamonds for confidentiality. The probands are indicated by arrows. The black diamonds indicate individuals with a behavioral variant of frontotemporal dementia; the gray diamonds indicate individuals with dementia with no clinical information; and the white diamonds indicate nonsymptomatic individuals. The ages of individuals (in years) are indicated at onset of frontotemporal dementia (FTD), at onset of Paget disease of bone (PDB), at onset of amyotrophic lateral sclerosis (ALS), at onset of unspecified dementia (D), at onset of parkinsonism (P), and at death (AD), along with the current ages of alive individuals (A) and genotypes. In family F297, individuals 009 and 013, who did not carry the mutation, had no neurological symptoms at 80 and 85 years of age, respectively.
aDNA samples are available.
Approximately 32 000 to 34 000 heterozygous single-nucleotide variants and 3400 to 4300 heterozygous insertions/deletions (indels) were identified by case. We assumed a dominant mode of inheritance in which shared variants could be determined in the affected cases. Based on the hypothesis that the mutation underlying this rare familial disease was not present in the general population, we excluded all known polymorphisms identified in the 1000 Genomes project (www.1000genomes.org/), in the Exome Variant Server database (evs.gs.washington.edu/EVS/), in the database of single-nucleotide polymorphisms (dbSNP; www.ncbi.nlm.nih.gov/projects/SNP/, Build 132), and in 50 in-house exomes of controls.
After filtering the results according to the selected criteria (heterozygous variants, exonic nonsense or missense non-synonymous changes, indels, or splice site mutations) and the tissular expression (all genes expressed in the central nervous system and all ubiquitously expressed genes), and after analyzing segregation, we found that only 17 variants (16 non-synonymous and 1 indel) were present in all the 3 affected sibs (003, 005, and 018) and were predicted to be deleterious by at least 2 in silico software programs. The list is given in eTable 1 in Supplement. Among these variants, the p.P387L of the SQSTM1 gene was probably one of the most deleterious according to predictions by the following in silico software programs: SIFT, MutationTaster, and PolyPhen2 (eTable 1 in Supplement). It was the best candidate gene considering the function of p62 and the presence of p62-positive neuronal inclusions in a subset of FTD and ALS. Because individuals 009 and 013 were unaffected at 80 and 85 years of age, respectively, the fact that they do not carry the mutation found in the 3 affected sibs reinforces the evidence for its pathogenicity. Therefore, we have sequenced the entire coding sequence of SQSTM1 using the Sanger method in the 187 remaining probands, as previously described.4 The entire coding sequence of the SQSTM1 gene was also sequenced in 352 age-matched healthy French controls. The frequency of rare variants in patients was compared with that of controls using the χ2 test. In addition, the exons 1, 6, and 7, in which mutations were identified, have been sequenced in 187 extra French controls (539 controls in total).
Results
Molecular Analyses
We found 4 heterozygous missense mutations in the SQSTM1 gene (p.A33V, p.P387L, p.A381V, and p.P392L) in 4 unrelated families (Figure 1). Three mutations were identified in familial FTD cases (3 of 68 individuals [ie, 4.4% of familial FTD cases]). In family F297, the c.1160C>T, p.P387L mutation (NM_003900.4, NP_003891.1) segregated with the disease. The 3 patients analyzed carried the mutation, whereas 2 asymptomatic individuals (009 and 013, who were 80 and 85 years of age, respectively) had no mutation (Figure 1). The c.1175C>T, p.P392L mutation in exon 8 was found in a family (FR1324) with both FTD and PDB, and it also segregated with the dementia (Figure 1): 2 affected relatives carried the p.P392L mutation, whereas 3 older and unaffected individuals (62, 64, and 80 years of age, respectively) did not carry the mutation (Figure 1). The proband 005 of the third family (F523) carried a c.1142C>T, p.A381V mutation in exon 7, and 1 mutation (c.98C>T, p.A33V in exon 1) was found in 1 proband with FTD-ALS (F480) and a familial history of dementia (1 of 37 cases with familial FTD-ALS [2.7%]). Segregation could not be analyzed in the 2 latter families.
Ala33, Pro387, Ala381, and Pro392 are conserved residues across species. Pro387, Ala381, and Pro392 are located in or close to the ubiquitin-associated domain of the protein. The p.A33V mutation was previously identified as a disease-causing mutation in patients with ALS.4 The p.A381V, p.P387L, and p.P392L mutations were predicted to be deleterious by at least 2 in silico prediction software programs (SIFT, Polyphen2, or Mutation-Taster) (eTable 2 in Supplement). The p.A33V, p.A381V, p.P387L, and p.P392L mutations were also absent from 539 French controls, therefore supporting their pathogenicity in the disease. The p.A381V and p.P387L mutations were also absent from 6503 individuals from the Exome Variant Server database.
In addition, 2 rare missense variants of unknown pathogenicity (p.R110C and p.R321H) were identified in 4 unrelated patients. The c.962G>A, p.R321H variant in exon 6 was identified in a family with FTD, and the c.328C>T, p.R110C variant was identified in a patient with FTD and PDB with a family history of dementia, but their causative roles could not be firmly established. The p.R110C affected a highly conserved residue and is predicted to be deleterious, but it was present, although rare, in controls from the Exome Variant Server database (minor allele frequency, 0.04%). The p.R321H variant was predicted to be tolerated and affected a lowly conserved residue, but, of note, other mutations affecting the residue Ala321 have been found in patients with ALS.4 Finally, 2 other rare missense variants identified in patients were probably not pathogenic because they were found at the same frequency in patients and in our French controls: p.K238E (1 of 188 patients [5.3%] and 2 of 352 controls [5.7%]) and p.E274D (9 of 188 patients [4.8%] and 14 of 352 controls [4.0%]).
In our 352 French controls, 4 other rare variants were identified, including 3 synonymous changes p.G61G (3 controls), p.S152S (1 control), and p.A426A (1 control) and 1 missense change p.T278I (1 control) (eTable 3 in Supplement). In patients, notably, there was clearly an excess of rare missense variants (including disease-causing mutations in SQSTM1) that were 2-fold higher (12%) than the variants in the 352 French controls (5%; χ2 = 6.57, P < .01). This clearly argues in favor of a role for SQSTM1 as a causative gene or a susceptibility gene in frontotemporal lobar degeneration. The complete list of variants identified in our study is provided in eTable 4 in Supplement.
Clinical Description of the Patients
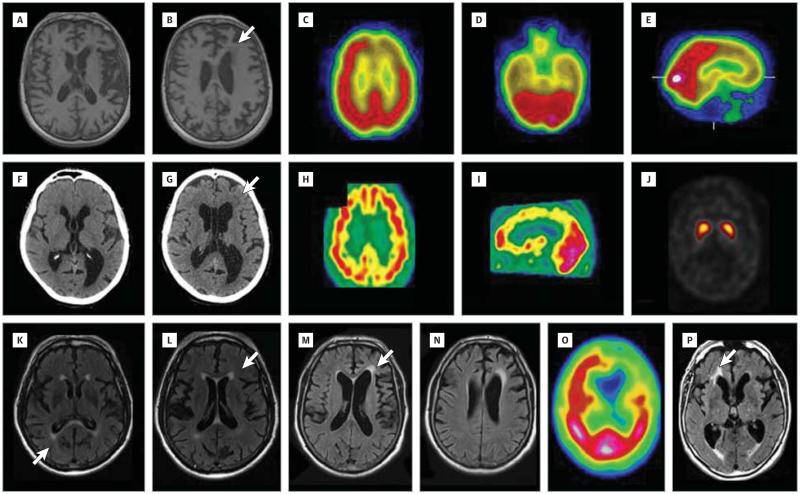
The clinical characteristics of the patients and their neuropsychological scores are summarized in Table 1 and Table 2, respectively. At 63 years of age, the proband 003 of family F297 initially presented with indifference, disinhibited behavior, and bulimia. Her speech was characterized by a paucity of spontaneous verbal output, as well as echolalia and palilalia. Bilateral grasping, rigidity, and akinesia were present, but there were no symptoms of ALS. Her serum alkaline phosphatase levels were normal. Neuropsychological testing revealed executive dysfunction (Table 2). Magnetic resonance imaging (MRI) of the brain showed diffuse cortical atrophy with left frontal predominance and bilateral basal ganglia calcifications associated with a septum pellucidum cyst (Figure 2A and B). Technetium (Tc) 99m ethyl cysteinate dimer (ECD) single-photon emission computed tomography (SPECT) of the brain revealed severe hypoperfusion of the prefrontal, predominantly left frontal, and bilateral temporal lobes (Figure 2C-E). Subsequently, she developed abulia, combined with perseverative behavior. Her speech progressively worsened until it was reduced to mutism, with buccofacial apraxia. She eventually experienced increasing difficulties in swallowing (these difficulties were of pseudobulbar origin) and a limitation of voluntary downward gaze, and she developed extrapyramidal signs. The patient died at 70 years of age. Three sibs (005, 016, and 018) presented with a behavioral variant of FTD (bvFTD) at 60 to 73 years of age (Table 1); 5 other relatives (001, 007, 011, and 014) had behavioral disorders and dementia, but no more clinical information was obtained. None of the patients that carried mutations had overt clinical signs of Paget disease or ALS.
Table 1. Clinical Characteristics of 13 SQSTM1 Mutation Carriers.
| Age at Onset, y | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Family/Patient | First Symptoms | First Symptoms | bvFTD | Clinical Symptoms of PDB | Clinical Symptoms of ALS | Disease Duration, y | Age at Last Evaluation, y | Additional Clinical Symptoms | Results of ENMG |
| F297/003 | Behavioral disorders | 63 | 63 | Absent | Absent | 7 | 70a | Oculomotor limitation, buccofacial apraxia, parkinsonism | Not performed |
| F297/005 | Behavioral disorders | 73 | 73 | Absent | Absent | 11 | 84a | Absent | Normal (at 79 y) |
| F297/016 | Behavioral disorders | 60 | 60 | Absent | Absent | 8 | 68a | Absent | Not performed |
| F297/018 | Behavioral disorders | 71 | 71 | Absent | Absent | 2 | 73 | Absent | Not performed |
| F523/005 | Behavioral disorders | 48 | 48 | Absent | Absent | 10 | 58 | Absent | Not performed |
| F523/003 | Behavioral disorders | 55 | 49 | Absent | Absent | 8 | 63 | Absent | Not performed |
| F523/007 | Behavioral disorders | 49 | 49 | Absent | Absent | 4 | 53 | Absent | Not performed |
| F480/010 | Behavioral disorders, dysarthria | 71 | 71 | Absent | 73 | 4 | 75a | Absent | Motor neuron disease (at 74 y) |
| F480/011 | Behavioral disorders | 67 | 67 | Absent | Absent | 3 | 70 | Absent | Not performed |
| FR1324/010 | PDB | 51 | 59 | 51 | Absent | 14 | 65 | Absent | Normal (at 63 y) |
| FR1324/005 | PDB | 55 | 79 | 58 | Absent | 29 | 84 | Absent | Not performed |
| FR1324/006 | PDB | <60 | 63 | <60 | Absent | NA | 65a | Absent | Not performed |
| FR1324/003 | Dementia | 65 | 65 | 69 | Absent | 23 | 89 | Absent | Not performed |
Abbreviations: ALS, amyotrophic lateral sclerosis; bvFTD, behavioral variant of frontotemporal dementia; ENMG, electroneuromyography; PDB, Paget disease of bone.
Age at death (in years).
Table 2. Neuropsychological Scores of the Probandsa.
| Patient 003 From Family F297b | Patient 005 From Family F523c | Evaluation of Patient 010 From Family F480d | Patient 010 From Family FR1324e | ||||
|---|---|---|---|---|---|---|---|
| First Evaluation | Second Evaluation | First Evaluation | Second Evaluation | First Evaluation | Second Evaluation | ||
| Age at evaluation, y | 67 | 68 | 54 | 56 | 74 | 62 | 63 |
| Mini-Mental Status Examination12 | |||||||
| Total score (30) | 26 | 13 | NA | NA | 22 | 18 | 18 |
| Orientation (10) | 9 | NA | NA | NA | 10 | NA | NA |
| Attention (5) | 3 | NA | NA | NA | 1 | NA | NA |
| Encoding (3) | 3 | NA | NA | NA | 3 | NA | NA |
| Recall (3) | 3 | NA | NA | NA | 3 | NA | NA |
| Language (8) | 7 | NA | NA | NA | 5 | NA | NA |
| Praxies (1) | 1 | NA | NA | NA | 0 | NA | NA |
| Mattis Dementia Rating Scale13 | |||||||
| Total score (144) | NA | 67 | NA | 74 | 101 | 108 | 114 |
| Initiation (37) | NA | 11 | NA | 6 | 18 | 20 | 22 |
| Concept (39) | NA | 16 | NA | 31 | 33 | 30 | 31 |
| Attention (37) | NA | 30 | NA | 28 | 32 | 32 | 32 |
| Construction (6) | NA | 4 | NA | 3 | 3 | 5 | 5 |
| Memory (25) | NA | 6 | NA | 6 | 15 | 11 | 14 |
| Fluency tasks (2 min)14 | |||||||
| Categories (animals) | NA | 4 | NA | NA | 2 | 4 | 1 |
| Letter (P) | NA | 0 | NA | NA | 5 | 2 | 2 |
| Frontal Assessment Battery15 | |||||||
| Total score (18) | 12 | 4 | 7 | NA | 3 | 7 | 8 |
| Free and cued recall test16 | |||||||
| Encoding (16) | NA | 0 | 11 | NA | 16 | 0 | 0 |
| Free recall (48) | NA | NA | 2 | NA | 14 | NA | NA |
| Total recall (48) | NA | NA | 24 | NA | 31 | NA | NA |
| Delayed free recall (16) | NA | NA | NA | NA | 4 | NA | NA |
| Delayed total recall (16) | NA | NA | NA | NA | 12 | NA | NA |
| No. of intrusions | NA | NA | 3 | NA | 1 | NA | NA |
| Rey’s figure copy (36)17 | 26 | 16 | NA | NA | NA | 31 | 29 |
| Oral confrontation naming (80)18 | NA | 60 | 70 | NA | 76 | 65 | 65 |
Abbreviations: bvFTD, behavioral variant of frontotemporal dementia; NA, not available.
The maximum score of each test and subtest is indicated in parentheses, unless otherwise specified.
Age at onset of bvFTD, 63 years.
Age at onset of bvFTD, 48 years.
Age at onset of bvFTD, 71 years.
Age at onset of bvFTD, 59 years.
Figure 2. Brain Imaging of Patients Carrying SQSTM1 Mutations.
Axial T1-weighted magnetic resonance imaging (MRI) scans of the brain of proband 003 of family F297 reveal left-sided predominant frontal and temporal atrophy (A and B), a septum pellucidum cyst (A), and moderate periventricular hyposignals (B [arrow]). Technetium (Tc) 99m ethyl cysteinate dimer (ECD) single-photon emission computed tomographic (SPECT) scans on the axial (C and D) and sagittal (E) sections of proband 003 of family F297 reveal hypoperfusion of predominantly left frontal and bilateral temporal lobes. Computed tomographic scans of the brain of patient 005 of family F523 reveal moderate left-sided perisylvian (F) and bilateral frontal atrophy (G) associated with moderate white matter hypodensities (G [arrow]) and a septum pellucidum cyst (F and G). Tc 99m ECT-SPECT scans of the brain of patient 005 of family F523 reveal diffuse cerebral hypoperfusion on the axial (H) and sagittal (I) sections; the dopamine transporter (DaT) scan is normal (J). Axial fluid-attenuated inversion recovery (FLAIR) MRI scans of patient 010 of family F480 reveal predominantly right-sided perisylvian atrophy associated with moderate periventricular and callosal hypersignals (K and L [arrows]). Axial FLAIR MRI scans of patient 010 from family F1324 reveal predominantly left-sided frontal and perisylvian atrophy associated with moderate periventricular and callosal hypersignals (M [arrow] and N). Tc 99m ECT-SPECT scan of the brain of patient 010 from family F1324 reveals severe, predominantly left-sided frontal and temporal hypoperfusion (O). Axial FLAIR MRI scan of patient 005 from family FR1324 reveals bilateral frontal and temporal atrophy, with periventricular hypersignals (P [arrow]).
In family F523, the proband 005 presented with bvFTD at 48 years of age. He developed disinhibition, rituals, verbal stereotypies, indifference, social avoidance, apathy, and reduced verbal fluency but no ALS symptoms. Neuropsychological testing revealed executive dysfunction (Table 2). A computed tomographic scan of the brain revealed moderate left-sided perisylvian and bilateral frontal lobe atrophy associated with diffuse white matter hypodensities and a septum pellucidum cyst (Figure 2F and G). Tc 99m ECD-SPECT of the brain revealed diffuse hypoperfusion (Figure 2H and I). The dopamine transporter (DaT) scan was normal (Figure 2J). Two sibs (003 and 007) had symptoms of bvFTD at 49 to 55 years of age. One of their parents (001) had behavioral disorders associated with parkinsonism, but the cause of death for this parent was myocardial infarct at 70 years of age.
The proband 010 of family F480 developed behavioral symptoms and dysarthria at 71 years of age. He presented with disinhibition, joviality, and irritability. An MRI scan of the brain revealed predominantly right-sided perisylvian atrophy associated with discrete periventricular and callosal hypersignals (Figure 2K and L). At 73 years of age, he secondarily developed a distal motor deficit and amyotrophy of the right upper limb. At 74 years of age, he was examined and was revealed to have dysarthria and dysphonia with stuttering and buccofacial apraxia. He also had diffuse enhanced reflexes, a right-sided Babinski sign, a bilateral motor deficit, fasciculations, and amyotrophy predominantly affecting the upper limbs. Neuropsychological testing revealed cognitive deterioration with predominant frontal executive dysfunction (Table 2). Electroneuromyograms confirmed the diagnosis of ALS at 74 years of age. He died at 75 years of age. He had no clinical symptoms and no familial history of PDB. A brother of his had FTD characterized by predominant behavioral disorders, bulimia, and collectionism at 67 years of age. An MRI scan of the brain revealed bilateral frontal atrophy and white matter lesions, and an SPECT scan of the brain revealed bilateral frontal hypoperfusion. The proband’s father had behavioral disorders and dementia and died younger than 50 years of age. A paternal uncle (005) had unspecified dementia and died younger than 70 years of age. His daughter (014) had Parkinson disease.
The proband 010 of family FR1324 had PDB diagnosed at 51 years of age. He presented with behavioral disorders, familiarity, apathy, and reduced speech output at 59 years of age. Neuropsychological testing confirmed cognitive deterioration (Table 2). An MRI of the brain revealed predominantly left-sided perisylvian atrophy associated with moderate periventricular and callosal hypersignals (Figure 2M and N). Tc 99m ECD-SPECT revealed severe, predominantly left-sided frontotemporal hypoperfusion. He had no clinical symptoms of ALS or PDB. At 63 years of age, the results of electroneuromyography were normal, and no biomarkers of Alzheimer disease were detected in cerebrospinal fluid samples. Three relatives (003, 005, and 006) had bvFTD and PDB, 2 of whom carried the p.P392L mutation (Figure 1). The DNA of 006 was not available. An MRI scan of the brain of patient 005 revealed bilateral frontal and temporal atrophy and periventricular hyper-signals (Figure 2P).
Discussion
We describe 4 novel families with FTD, FTD-ALS, or FTD-PDB carrying SQSTM1 mutations. To our knowledge, this is the first study that clearly demonstrates a segregation of a SQSTM1 mutation with dementia in 2 families, F297 and FR1324. Clinically, all the families were affected by bvFTD,19 which was associated with ALS in family F480 (8% of the patients) and with PDB in family FR1324 (30% of the patients). The familial aggregation of FTD with ALS or with PDB in 2 families suggests intrafamilial clinical variability, as in families carrying the VCP mutations. The age at onset of dementia was late (≥70 years) in 4 of 13 cases with documented bvFTD, as previously reported (Table 1).6 Notably, brain imaging revealed bilateral frontal or predominantly perisylvian atrophy, which was associated with callosal and periventricular T2-weighted white matter hypersignals that were moderate but present in all the cases. Two patients had a septum pellucidum cyst.
The p.A33V4 and the p.P392L4,20 mutations, identified in family F480 (FTD-ALS) and in family FR1324 (FTD-PDB), were previously shown to cause familial ALS; p.P392L, p.P387L, and p.A381V mutations were also previously identified in PDB.21 Frontotemporal dementia and PDB aggregated in family FR1324 (p.P392L mutation), but none of the patients in family F297 (p.P387L mutation) or family F523 (p.A381V mutation) had clinically symptomatic PDB. However, they were not investigated specifically for this aspect using biological tests or bone scintigraphy. Likewise, the members of families with Paget disease were not interviewed for dementia.21,22 Our results could be due to the low penetrance of Paget disease,21,22 and probably of ALS, which more than half of the SQSTM1 mutation carriers are sporadic cases of.4 A larger series of patients will be needed to precisely evaluate the penetrance of the various clinical features and their relation with the different types of mutations.
The p62 protein is a multifunctional protein that interacts with misfolded and ubiquitinated proteins and that acts as a cargo receptor for the degradation of ubiquitinated proteins through autophagic or proteasomal pathways. Most of the SQSTM1 mutations identified in PDB, ALS, and FTD, as well as the 3 mutations (p.P387L, p.A381V, and p.P392L) identified in our study, are located in the ubiquitin-associated domain of the protein that binds to ubiquitinated proteins; therefore, it is possible that these 3 mutations might eventually abrogate the binding of p62 to ubiquitinated proteins. Interestingly, p62 physiologically binds to TDP-43 and is possibly involved in its degradation, thereby establishing a possible functional link between p62 and TDP-43 proteinopathies.23 Of note, pathological studies in a subset of SQSTM1 mutation carriers showed macroscopic frontal atrophy associated with TDP-43–positive and p62-positive aggregates in neurons, not only in the spinal cord but also in neurons of the frontal cortex. Our study strongly supports the possibility that neuronal degeneration in the frontal cortex may be associated with spinal cord degeneration in SQTM1 mutation carriers.20
The disease spectrum associated with SQSTM1 mutations, which includes PDB, FTD, and ALS, establishes a clinical link between the proteins p62, VCP, and OPTN. Indeed, VCP mutations are responsible for a complex phenotype (inclusion body myopathy with early-onset Paget disease and FTD) that variably is associated with inclusion body myopathy, PDB, FTD, and, rarely, ALS.24 Similarly, the OPTN gene is a genetic cause of ALS25 and a genetic risk factor for PDB.26 VCP, p62, and OPTN are all involved in protein degradation via autophagy and might be possibly associated with neurodegeneration through a unif ying biologic al pathway.21,27
Rapid advances were recently made in our understanding of FTD and ALS with the identification of TDP-43 and FUS proteins in neuronal aggregates, as well as with the discovery of C9orf72 expansions in both disorders. The recent identification of SQSTM1 mutations in 3 patients with FTD has suggested that the p62 protein is implicated in FTD as well. We now report 4 additional families carrying SQSTM1 mutations, and we showed a segregation of the mutation with FTD in 2 of these families, thereby supporting the results of Rubino and colleagues.6 Although the frequency of the mutations in our series of familial cases is low (4 of 105 individuals [3.8%]), it is close to the frequency found in the study by Rubino and colleagues6 (1.8%) and is in the same range as in populations with pure ALS (2%-3%).4-6,20 Taken together, our study and the study by Rubino and colleagues6 support a pathogenic role for the p62 protein in FTD disorders.
Supplementary Material
Acknowledgments
Funding/Support: The research leading to these results has received funding from the program “Investissements d’avenir” ANR-10-IAIHU-06. This study was supported by grant ANR R06363DS (to Dr Brice), by the France-Alzheimer Association (to Dr Le Ber), and, in part, by Alzheimer’s Research UK, by an anonymous donor, by the Wellcome Trust/MRC Joint Call in Neurodegeneration award (WT089698) to the UK Parkinson’s Disease Consortium whose members are from the University College London/Institute of Neurology, the University of Sheffield, and the MRC Protein Phosphorylation Unit at the University of Dundee, Scotland, and by a fellowship from Alzheimer’s Research UK to Dr Guerreiro.
Role of the Sponsor: The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions: We thank Cyril Pottier and Anne-Claire Richard at CNR-MAJ, Rouen University Hospital for their technical expertise, Bérangère Beauplet, MD (CHU Rouen), Gerard Bouvard, MD (CHU Rouen), and Eric Guedj, MD, PhD (CHU Marseille), for providing imaging data, and Sophie Rivaud-Pechoux, PhD (Centre de Recherche de l’Institut du Cerveau et de la Moelle Epinière, Paris), for statistical analyses. We also thank the staff at the DNA and cell bank of Centre de Recherche de l’Institut du Cerveau et de la Moelle Epinière, Hôpital de la Salpêtrière, Paris, France, for their excellent technical assistance.
Appendix
Group Information: The French Clinical and Genetic Research Network on FTD/FTD-ALS members are Sophie Auriacombe (CHU Pellegrin, Bordeaux), Alexis Brice (Hôpital de la Salpêtrière, Paris), Frédéric Blanc (Hôpitaux Civils, Strasbourg), Françoise Clerget-Darpoux (Hôpital Paul Brousse, Villejuif), Philippe Couratier (CHU Limoges), Mira Didic (CHU La Timone, Marseille), Bruno Dubois (Hôpital de la Salpêtrière, Paris), Charles Duyckaerts (Hôpital de la Salpêtrière, Paris), Marie-Odile Habert (Hôpital de la Salpêtrière, Paris), Véronique Golfier (CHU Rennes), Eric Guedj (CHU Marseille), Didier Hannequin (CHU Charles Nicolle, Rouen), Lucette Lacomblez (Hôpital de la Salpêtrière, Paris), Isabelle Le Ber (Hôpital de la Salpêtrière, Paris), Richard Levy (CHU St Antoine, Paris), Vincent Meininger (Hôpital de la Salpêtrière, Paris), Bernard-François Michel (CH Sainte-Marguerite, Marseille), Florence Pasquier (CHU Roger Salengro, Lille), Catherine Thomas-Anterion (CHU Bellevue, Saint-Etienne), Michèle Puel (CHU Rangueil, Toulouse), François Salachas (Hôpital de la Salpêtrière, Paris), François Sellal (CH Colmar), Martine Vercelletto (CHU Laennec, Nantes), and Patrice Verpillat (Hôpital de la Salpêtrière, Paris).
Footnotes
Conflict of Interest Disclosures: None reported.
REFERENCES
- 1.Laurin N, Brown JP, Morissette J, Raymond V. Recurrent mutation of the gene encoding sequestosome 1 (SQSTM1/p62) in Paget disease of bone. Am J Hum Genet. 2002;70(6):1582–1588. doi: 10.1086/340731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Komatsu M, Kageyama S, Ichimura Y. p62/SQSTM1/A170: physiology and pathology. Pharmacol Res. 2012;66(6):457–462. doi: 10.1016/j.phrs.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Al-Sarraj S, King A, Troakes C, et al. p62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS. Acta Neuropathol. 2011;122(6):691–702. doi: 10.1007/s00401-011-0911-2. [DOI] [PubMed] [Google Scholar]
- 4.Fecto F, Yan J, Vemula SP, et al. SQSTM1 mutations in familial and sporadic amyotrophic lateral sclerosis. Arch Neurol. 2011;68(11):1440–1446. doi: 10.1001/archneurol.2011.250. [DOI] [PubMed] [Google Scholar]
- 5.Hirano M, Nakamura Y, Saigoh K, et al. Mutations in the gene encoding p62 in Japanese patients with amyotrophic lateral sclerosis. Neurology. 2013;80(5):458–463. doi: 10.1212/WNL.0b013e31827f0fe5. [DOI] [PubMed] [Google Scholar]
- 6.Rubino E, Rainero I, Chiò A, et al. TODEM Study Group SQSTM1 mutations in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Neurology. 2012;79(15):1556–1562. doi: 10.1212/WNL.0b013e31826e25df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Ber I, Guedj E, Gabelle A, et al. French research network on FTD/FTD-MND Demographic, neurological and behavioural characteristics and brain perfusion SPECT in frontal variant of frontotemporal dementia. Brain. 2006;129(pt 11):3051–3065. doi: 10.1093/brain/awl288. [DOI] [PubMed] [Google Scholar]
- 8.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 9.Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. J Neurol Sci. 1994;124(suppl):96–107. doi: 10.1016/0022-510x(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 10.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKenna A, Hanna M, Banks E, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20(9):1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 13.Mattis S. Dementia Rating Scale. Psychological Assessment Resources; Odessa, FL: 1988. [Google Scholar]
- 14.Thuillard F, Assal G. Données neuropsychologiques chez le sujet âgé normal. In: Habib M, Joanette Y, Puel M, editors. Démences et syndromes démentiels: approche neuropsychologique. Masson; Paris, France: 1991. pp. 125–133. [Google Scholar]
- 15.Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology. 2000;55(11):1621–1626. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- 16.Van der Linden M, Coyette F, Poitrenaud J, et al. L’épreuve de rappel libre/rappel indicé de 16 items (RL/RI-16) In: Van der Linden M, editor. L’évaluation des troubles de la mémoire. Solal; Marseille, France: 2004. pp. 25–47. [Google Scholar]
- 17.Rey A. Test de copie et de reproduction de mémoire de figures géométriques complexes. ECPA; Paris, France: 1959. [Google Scholar]
- 18.Deloche G, Hannequin D, Dordain M, et al. Picture confrontation oral naming: performance differences between aphasics and normals. Brain Lang. 1996;53(1):105–120. doi: 10.1006/brln.1996.0039. [DOI] [PubMed] [Google Scholar]
- 19.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(pt 9):2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teyssou E, Takeda T, Lebon V, et al. Mutations in SQSTM1 encoding p62 in amyotrophic lateral sclerosis: genetics and neuropathology. Acta Neuropathol. 2013;125(4):511–522. doi: 10.1007/s00401-013-1090-0. [DOI] [PubMed] [Google Scholar]
- 21.Ralston SH, Layfield R. Pathogenesis of Paget disease of bone. Calcif Tissue Int. 2012;91(2):97–113. doi: 10.1007/s00223-012-9599-0. [DOI] [PubMed] [Google Scholar]
- 22.Michou L, Morissette J, Gagnon ER, et al. Novel SQSTM1 mutations in patients with Paget’s disease of bone in an unrelated multiethnic American population. Bone. 2011;48(3):456–460. doi: 10.1016/j.bone.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Tanji K, Zhang HX, Mori F, Kakita A, Takahashi H, Wakabayashi K. p62/sequestosome 1 binds to TDP-43 in brains with frontotemporal lobar degeneration with TDP-43 inclusions. J Neurosci Res. 2012;90(10):2034–2042. doi: 10.1002/jnr.23081. [DOI] [PubMed] [Google Scholar]
- 24.Johnson JO, Mandrioli J, Benatar M, et al. ITALSGEN Consortium. Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron. 2010;68(5):857–864. doi: 10.1016/j.neuron.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maruyama H, Morino H, Ito H, et al. Mutations of optineurin in amyotrophic lateral sclerosis. Nature. 2010;465(7295):223–226. doi: 10.1038/nature08971. [DOI] [PubMed] [Google Scholar]
- 26.Albagha OM, Visconti MR, Alonso N, et al. Genome-wide association study identifies variants at CSF1, OPTN and TNFRSF11A as genetic risk factors for Paget’s disease of bone. Nat Genet. 2010;42(6):520–524. doi: 10.1038/ng.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fecto F, Siddique T. UBQLN2/P62 cellular recycling pathways in amyotrophic lateral sclerosis and frontotemporal dementia. Muscle Nerve. 2012;45(2):157–162. doi: 10.1002/mus.23278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.