Abstract
The International Parkinson and Movement Disorder Society (MDS)‐sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS) has been developed and is now available in English. Part of the overall program includes the establishment of official non‐English translations of the MDS‐UPDRS. We present the process for completing the official Japanese translation of the MDS‐UPDRS with clinimetric testing results. In this trial, the MDS‐UPDRS was translated into Japanese, underwent cognitive pretesting, and the translation was modified after taking the results into account. The final translation was approved as the Official Working Draft of the MDS‐UPDRS Japanese version and tested in 365 native‐Japanese–speaking patients with PD. Confirmatory analyses were used to determine whether the factor structure for the English‐language MDS‐UPDRS could be confirmed in data collected using the Official Working Draft of the Japanese translation. As a secondary analysis, we used exploratory factor analyses to examine the underlying factor structure without the constraint of a prespecified factor organization. Confirmatory factor analysis revealed that Comparative Fit Index for all parts of the MDS‐UPDRS exceeded the minimal standard of 0.90, relative to the English version, and therefore the Japanese translation met the prespecified criterion to be designated, called an official MDS translation. Secondary analyses revealed some differences between the English‐language MDS‐UPDRS and the Japanese translation; however, these differences were considered to be within an acceptable range. The Japanese version of the MDS‐UPDRS met the criterion as an Official MDS Translation and is now available for use (www.movementdisorders.org).
Keywords: Parkinson's disease, MDS‐UPDRS, UPDRS, Rating scale, validation
The UPDRS has been widely used since the 1980s as a standard clinical rating scale for Parkinson's disease (PD).1, 2 However, increasing evidence indicates that several symptoms frequently experienced by PD patients that affect their quality of life, such as sleep problems, sensory disturbance, urinary problems, constipation, and fatigue, are not adequately evaluated in the original UPDRS.3 In 2001, the International Parkinson and Movement Disorder Society (MDS) sponsored a critique of the UPDRS and subsequently developed a new version of the scale, termed the MDS‐sponsored UPDRS revision. This new version, the MDS‐UPDRS, was intended to be less ambiguous than its predecessor as well as to incorporate a number of clinically pertinent PD‐related problems poorly captured in the original version.4 In 2008, the MDS‐UPDRS successfully passed clinimetric testing with high internal consistency and reliable factor structures for each part of the scale.4 The new MDS‐UPDRS comprises four parts: Part I evaluates nonmotor experiences of daily living, Part II evaluates motor experiences of daily living, Part III evaluates motor function, and Part IV evaluates motor fluctuations and dyskinesia.
After publication of the MDS‐UPDRS, the MDS set forth a specific program to designate successful translations of non‐English‐language versions as official MDS translations. For this purpose, the MDS has set a strict protocol and criteria for testing. Currently, several official translations (Italian,5 Spanish,6 French, Estonian, German, and Slovakian) have already been established, and several other language programs are in progress. Herein, we present the scale translation and clinimetric testing results of the Japanese version of the MDS‐UPDRS.
Patients and Methods
Translation of the MDS‐UPDRS
The MDS‐UPDRS was translated into Japanese by a team of natural Japanese speakers fluent in English who belong to the Department of Neurology of Wakayama Medical University in Japan, led by Kondo. The resultant Japanese translation was further reviewed by a team led by Mizuno from the Movement Disorder Society of Japan to establish the original Japanese translation of the MDS‐UPDRS. The translation was then back‐translated by a team of colleagues fluent in English and Japanese who had not been involved in the original forward translation. The back‐translation was reviewed by the administrative team in charge of the overall translation program (Stebbins, Goetz, LaPelle, and Tilley).
Cognitive Pretesting
Cognitive pretesting is a qualitative approach to assess instrument completion in terms of task difficulty for examiner and respondent as well as respondent interest, attention span, discomfort, and comprehension.7 Where there were observed differences between the back‐translated Japanese and English versions, items were selected for cognitive pretesting, along with questions that had been identified during cognitive pretesting of the English version. Cognitive pretesting was performed on the following sections: Part I Hallucinations and Psychosis; Features of Dopamine Dysregulation Syndrome; and Urinary Problems; Part II Freezing; Part III Postural Stability; and Rest Tremor Amplitude; Part IV Time Spent with Dyskinesia; and Functional Impact of Dyskinesia. Three experienced Japanese movement disorder specialists not involved in the original translation performed cognitive pretesting. Based on the results of the initial cognitive pretesting, additional round(s) of translation, back‐translation, and cognitive pretesting could be required. After taking the cognitive pretesting results into account, the final Japanese translation was obtained.
Testing of the Japanese Version of the MDS‐UPDRS
A total of 30 experienced Japanese movement disorder specialists were recruited as members of the MDS‐UPDRS Japanese version validation team led by Kashihara (members are listed in the Appendix) to examine native‐Japanese–speaking PD patients who had provided informed consent. The sample size for the translation study was based on the need for 5 participants per questionnaire item in order to perform the statistical analysis.8 There are 65 items on the MDS‐UPDRS: Thus, a sample of at least 325 was required. Any participants with missing values within a part were excluded from the analysis of that part only. Hence, the sample size could vary by part. The investigators obtained approval to collect the data in accord with relevant institutional ethics policies regarding human subjects. Anonymized patient data were transferred to the analysis team by a secure website. The protocol for validation of the MDS‐UPDRS Japanese version was approved by the ethics committees of each institute. Informed consent was obtained from all participants before the study.
Data Analysis
Factor Analysis
M‐plus (version 6.11)9 was used to perform confirmatory and exploratory factor analyses (EFA), because the variables are categorical. We used a weighted least squares with mean‐ and variance‐adjusted weighted least square (WLSMV) approach to factor estimation that minimizes the sum of squared differences between observed and estimated correlation matrices not counting diagonal elements. To assist in interpretation of the factors, we used an orthogonal CF‐varimax rotation that constrains the factors to be uncorrelated. These methods were chosen to follow those used in the original examination of the English MDS‐UPDRS.4
Primary Analysis
We conducted a confirmatory factor analysis (CFA)10 as the primary analysis of the Japanese data to determine whether the factor structure for the English‐language MDS‐UPDRS4 could be confirmed in data collected by using the Japanese translation. This was the primary question of interest. The CFA was conducted separately for the MDS‐UPDRS Parts I to IV, with the Japanese data constrained to fall into the factors defined in the English‐language data.4 We evaluated the CFA results based on the comparative fit index (CFI). According to protocol, to establish a successful translation and earn the designation of “official MDS‐UPDRS translation,” the CFI for each part (I–IV) of the translated instrument must be 0.90 or greater, relative to the English‐language version.4 Root mean square error of approximation (RMSEA) was also calculated as another test of model fit. RMSEA values <0.05 were considered to be a good fit, and RMSEA values of 0.1 or more were considered to be a poor fit. WLSMV estimators were used to confirm a model fit.
Secondary Analysis
As a secondary analysis, we conducted an exploratory factor analysis11 for Parts I to IV of the Japanese version of the MDS‐UPDRS to explore the underlying factor structure without the constraints of a prespecified factor structure. We used a Scree plot to choose the number of factors to retain for each part. The subjective Scree test12 is scatter plot of eigenvalues plotted against their ranks with respect to magnitude to extract as many factors as there are eigenvalues that fall before the last large drop (i.e., an “elbow” shape) in the plot. Once the factors were chosen, an item was retained in a factor if the factor loading for the item was 0.40 or greater.
The default estimator for factor analysis in M‐plus is unweighted least squares (ULS). When ULS converges, it yields more‐accurate parameter estimates and standard errors than does WLSMV. However, WLSMV generally outperforms ULS in convergence rates. Thus, Forero et al.13 suggest the use of ULS. In the case of nonconvergence, however, they suggest using WLSMV, because this method might converge when ULS does not. In this case, whereas the ULS algorithm did converge, it converged to an incorrect value (i.e., a percent of variance explained that was greater than 1.0), so WLSMV was used.
The chi‐square test was used to analyze, additionally, the differences in the distribution of responses for each item of the MDS‐UPDRS between PD patients of Japanese and English groups.
Results
Cognitive Pretesting
A total of 12 patients with PD and their examiners were interviewed using a structured interview format typical in cognitive pretesting. During the first round of cognitive pretesting, minor word changes were suggested for features of dopamine dysregulation syndrome, urinary problems, and time spent with dyskinesia. In response to comments from patients and caregivers, we enlarged the size of characters used in questions from Part IB and Part II. No items were identified as problematic during a second round of cognitive pretesting conducted with 10 patients with PD. The modified version of the scale was approved as the Official Working Draft of the Japanese MDS‐UPDRS for testing in a larger group of patients with PD.
Data Analysis
Demographics
Participants' demographic characteristics are shown in Table 1. The Japanese data set included 365 native‐Japanese–speaking patients with PD who were examined using the MDS‐UPDRS. In the Japanese sample, there was a greater proportion of female patients, compared to the English sample. The two cohorts were similar on age and duration of disease, but the distribution of H & Y stages were significantly different between the two cohorts (P < 0.0005; Table 1).
Table 1.
Demographics of Japanese patients with PD in comparison with the MDS‐UPDRS (English version) data
| English | Japanese | P Value | |
|---|---|---|---|
| Total N | 876 | 365 | ns |
| % male | 63.2 | 45.2 | <0.0005 |
| Age (mean ± SD) | 68.2 ± 10.8 | 69.0 ± 9.2 | ns |
| Disease duration (mean years ± SD) | 8.3 ± 6.7 | 7.8 ± 6.1 | ns |
| Years of education | NA | 12.6 ± 2.7 | ns |
| H & Y stage | <0.0005 | ||
| 0 | 0 | 2 | |
| 1 | 63 | 28 | |
| 2 | 467 | 164 | |
| 3 | 174 | 116 | |
| 4 | 109 | 42 | |
| 5 | 53 | 11 |
SD, standard deviation; NA, not available; ns, not significant.
Primary Analysis: CFA
Table 2 displays the CFA models for each part of the MDS‐UPDRS. For all four parts of the Japanese version, the CFI was 0.93 or greater, in comparison to the English‐language factor structure. Our prespecified criterion was a CFI of 0.90 or greater; thus, we conclude that the English factor structure was confirmed in the Japanese data set.
Table 2.
Confirmatory factor analysis model fit
| Part I: Nonmotor aspects of experiences of daily living (a two‐factor model)a | |
| Japanese | CFI = 0.93; RMSEA = 0.09 (351 patients) |
| English language | CFI = 0.97; RMSEA = 0.05 (849 patients) |
| Part II: Motor aspects of experiences of daily living (a three‐factor model) | |
| Japanese | CFI = 0.99; RMSEA = 0.07 (356 patients) |
| English language | CFI = 0.99; RMSEA = 0.05 (851 patients) |
| Part III: Motor examination (a seven‐factor model) | |
| Japanese | CFI = 0.94; RMSEA = 0.08 (336 patients) |
| English language | CFI = 0.95; RMSEA = 0.08 (801 patients) |
| Part IV: Motor complications (a two‐factor model) | |
| Japanese | CFI = 1.00; RMSEA = 0.06 (350 patients) |
| English language | CFI = 1.00; RMSEA = 0.00 (848 patients) |
Dopamine dysregulation syndrome was not included in this analysis because it did not load on any factor in the U.S. version.
Secondary Analysis: EFA
The factor structure of the EFA for the English version has been used as the basis for all CFAs, but our EFA of the Japanese data set differs from that of the English‐language data set in some aspects. The results of the EFA for the English and Japanese versions are shown in Table 3, including the number of factors and their associated eigenvalues and percent variance.
Table 3.
Comparison of English‐language and Japanese exploratory factor structures for parts I to IV of the MDS‐UPDRS
| English | Japanese | |||
|---|---|---|---|---|
| Factor | Eigenvalues | Percent Variance | Eigenvalues | Percent Variance |
| Part I | ||||
| 1 | 4.421 | 34.0 | 5.045 | 42.0 |
| 2 | 1.231 | 9.5 | 1.244 | 10.4 |
| 3 | 1.051 | 8.1 | 1.081 | 9.0 |
| 4 | 1.007 | 7.7 | 0.866 | 7.2 |
| 5 | 0.811 | 6.2 | 0.721 | 6.0 |
| 6 | 0.724 | 5.6 | 0.642 | 5.4 |
| 7 | 0.673 | 5.2 | 0.594 | 5.0 |
| 8 | 0.630 | 4.8 | 0.508 | 4.2 |
| 9 | 0.616 | 4.7 | 0.472 | 3.9 |
| 10 | 0.542 | 4.2 | 0.375 | 3.1 |
| 11 | 0.519 | 4.0 | 0.288 | 2.4 |
| 12 | 0.399 | 3.1 | 0.160 | 1.3 |
| 13 | 0.376 | 2.9 | ||
| Part II | ||||
| 1 | 6.898 | 53.1 | 7.293 | 56.1 |
| 2 | 1.128 | 8.7 | 1.062 | 8.2 |
| 3 | 1.000 | 7.7 | 0.826 | 6.4 |
| 4 | 0.728 | 5.6 | 0.684 | 5.3 |
| 5 | 0.595 | 4.6 | 0.534 | 4.1 |
| 6 | 0.542 | 4.2 | 0.494 | 3.8 |
| 7 | 0.425 | 3.3 | 0.445 | 3.4 |
| 8 | 0.390 | 3.0 | 0.431 | 3.3 |
| 9 | 0.380 | 2.9 | 0.370 | 2.8 |
| 10 | 0.294 | 2.3 | 0.260 | 2.0 |
| 11 | 0.245 | 1.9 | 0.240 | 1.8 |
| 12 | 0.198 | 1.5 | 0.219 | 1.7 |
| 13 | 0.178 | 1.4 | 0.141 | 1.1 |
| Part III | ||||
| 1 | 12.112 | 36.7 | 14.451 | 43.8 |
| 2 | 5.035 | 15.3 | 4.190 | 12.7 |
| 3 | 2.173 | 6.6 | 2.429 | 7.4 |
| 4 | 2.051 | 6.2 | 1.961 | 5.9 |
| 5 | 1.615 | 4.9 | 1.668 | 5.1 |
| 6 | 1.485 | 4.5 | 1.238 | 3.8 |
| 7 | 1.104 | 3.3 | 0.922 | 2.8 |
| 8 | 0.903 | 2.7 | 0.793 | 2.4 |
| 9 | 0.720 | 2.2 | 0.685 | 2.1 |
| 10 | 0.615 | 1.9 | 0.596 | 1.8 |
| 11 | 0.552 | 1.7 | 0.558 | 1.7 |
| 12 | 0.495 | 1.5 | 0.514 | 1.6 |
| 13 | 0.479 | 1.5 | 0.472 | 1.4 |
| 14 | 0.407 | 1.2 | 0.360 | 1.1 |
| 15 | 0.403 | 1.2 | 0.348 | 1.1 |
| 16 | 0.361 | 1.1 | 0.330 | 1.0 |
| 17 | 0.323 | 1.0 | 0.246 | 0.7 |
| 18 | 0.314 | 1.0 | 0.233 | 0.7 |
| 19 | 0.267 | 0.8 | 0.203 | 0.6 |
| 20 | 0.265 | 0.8 | 0.194 | 0.6 |
| 21 | 0.223 | 0.7 | 0.183 | 0.6 |
| 22 | 0.203 | 0.6 | 0.147 | 0.4 |
| 23 | 0.164 | 0.5 | 0.138 | 0.4 |
| 24 | 0.145 | 0.4 | 0.115 | 0.3 |
| 25 | 0.141 | 0.4 | 0.099 | 0.3 |
| 26 | 0.109 | 0.3 | 0.058 | 0.2 |
| 27 | 0.091 | 0.3 | 0.027 | 0.1 |
| 28 | 0.077 | 0.2 | 0.013 | 0.0 |
| 29 | 0.055 | 0.2 | 0.004 | 0.0 |
| Part IV | ||||
| 1 | 3.811 | 63.9 | 3.656 | 60.9 |
| 2 | 0.942 | 15.6 | 1.210 | 20.2 |
| 3 | 0.640 | 10.7 | 0.725 | 12.1 |
| 4 | 0.241 | 4.0 | 0.168 | 2.8 |
| 5 | 0.208 | 3.5 | 0.130 | 2.2 |
| 6 | 0.159 | 2.3 | 0.111 | 1.9 |
Dotted line shows the factors selected in the English cohort.
The Scree plots were used to determine the number of factors to be retained from the EFA. Comparison between the Scree plots for the English and Japanese cohorts revealed similarities in shape of the plots (Fig. 1), but differences were noted in the relationship between factors and their eigenvalues and percent of variance (Table 3): For Part I: Nonmotor aspects of experiences of daily living, we extracted two factors; for Part II: Motor aspects of experiences of daily living, we extracted three components; for Part III: Motor examination, we extracted seven factors; and for Part IV: Motor complications, we extracted two factors.
Figure 1.
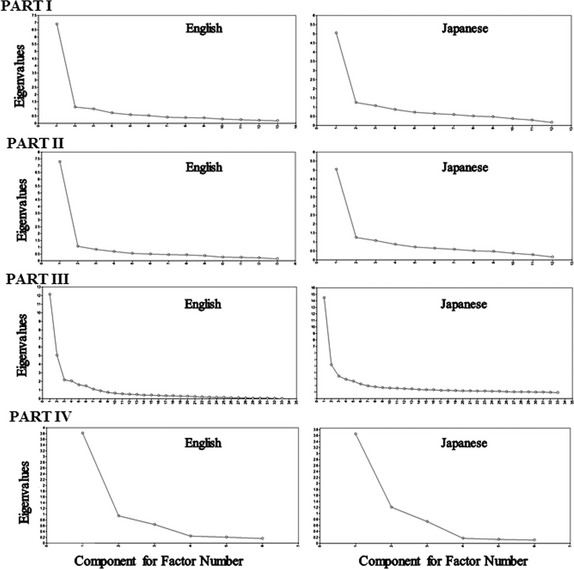
Scree plots for the English and Japanese exploratory factor analyses.
Chi‐square (χ2) test (Table 4) revealed greater distribution of less‐severe scores on the cognitive impairment items (Part I: item 1.1) in the Japanese group, compared to the English group (χ2 = 23.457; df = 4; P = 0.0001). There was no significant difference of the distribution of scores on the hallucinations and psychosis item (Part I: item 1.2) (χ2 = 5.962; df = 4; not significant). In many other items, PD patients in the English group showed greater distribution of more‐severe scores, including depressed mood, pain and other sensations, lightheadedness on standing, fatigue, and sleep problems in Part I; speech, saliva and drooling, doing hobbies and other activities, tremor, and getting out of bed in Part II; speech, facial expression, rigidity, finger tapping, hand movements, pronation supination, toe tapping, leg agility, and tremor in Part III; and time spent with dyskinesia, functional impact of dyskinesias, time spent in the OFF state, complexity of motor fluctuations, and painful OFF‐state dystonia in Part IV. Japanese PD patients showed greater distribution in more‐severe scores than English groups in items constipation problems in Part I and postural stability in Part III.
Table 4.
Distribution of responses by MDS‐UPDRS by languagea
| English | Japanese | English | Japanese | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Part I | |||||||||
| Cognitive impairment* | Frequency | % | Frequency | % | Daytime sleepiness | Frequency | % | Frequency | % |
| 0 | 428 | 48.86 | 227 | 62.19 | 0 | 212 | 24.2 | 104 | 28.49 |
| 1 | 256 | 29.22 | 93 | 25.48 | 1 | 216 | 24.66 | 73 | 20.00 |
| 2 | 121 | 13.81 | 25 | 6.85 | 2 | 364 | 41.55 | 147 | 40.27 |
| 3 | 53 | 6.05 | 17 | 4.66 | 3 | 59 | 6.74 | 32 | 8.77 |
| 4 | 17 | 1.94 | 3 | 0.82 | 4 | 16 | 1.83 | 8 | 2.19 |
| 999 | 1 | 0.11 | 0 | 0.00 | 999 | 9 | 1.03 | 1 | 0.27 |
| Total | 876 | 100 | 365 | 100.00 | Total | 876 | 100 | 365 | 100.00 |
| Hallucinations and psychosis | Frequency | % | Frequency | % | Pain and other sensations* | Frequency | % | Frequency | % |
| 0 | 687 | 78.42 | 280 | 76.71 | 0 | 303 | 34.59 | 148 | 40.55 |
| 1 | 89 | 10.16 | 38 | 10.41 | 1 | 289 | 32.99 | 117 | 32.05 |
| 2 | 51 | 5.82 | 26 | 7.12 | 2 | 130 | 14.84 | 60 | 16.44 |
| 3 | 35 | 4 | 14 | 3.84 | 3 | 106 | 12.1 | 31 | 8.49 |
| 4 | 13 | 1.48 | 4 | 1.10 | 4 | 39 | 4.45 | 4 | 1.10 |
| 999 | 1 | 0.11 | 3 | 0.82 | 999 | 9 | 1.03 | 5 | 1.37 |
| Total | 876 | 100 | 365 | 100.00 | Total | 876 | 100 | 365 | 100.00 |
| Depressed mood* | Frequency | % | Frequency | % | Urinary problems | Frequency | % | Frequency | % |
| 0 | 471 | 53.77 | 223 | 61.10 | 0 | 325 | 37.1 | 144 | 39.45 |
| 1 | 265 | 30.25 | 84 | 23.01 | 1 | 281 | 32.08 | 118 | 32.33 |
| 2 | 81 | 9.25 | 36 | 9.86 | 2 | 137 | 15.64 | 60 | 16.44 |
| 3 | 45 | 5.14 | 21 | 5.75 | 3 | 88 | 10.05 | 32 | 8.77 |
| 4 | 12 | 1.37 | 0 | 0.00 | 4 | 38 | 4.34 | 10 | 2.74 |
| 999 | 2 | 0.23 | 1 | 0.27 | 999 | 7 | 0.8 | 1 | 0.27 |
| Total | 876 | 100 | 365 | 100.00 | Total | 876 | 100 | 365 | 100.00 |
| Anxious mood | Frequency | % | Frequency | % | Constipation problems* | Frequency | % | Frequency | % |
| 0 | 413 | 47.15 | 192 | 52.60 | 0 | 384 | 43.84 | 90 | 24.66 |
| 1 | 307 | 35.05 | 116 | 31.78 | 1 | 287 | 32.76 | 120 | 32.88 |
| 2 | 96 | 10.96 | 39 | 10.68 | 2 | 119 | 13.58 | 74 | 20.27 |
| 3 | 41 | 4.68 | 15 | 4.11 | 3 | 70 | 7.99 | 63 | 17.26 |
| 4 | 17 | 1.94 | 1 | 0.27 | 4 | 9 | 1.03 | 18 | 4.93 |
| 999 | 2 | 0.23 | 2 | 0.55 | 999 | 7 | 0.8 | 0 | 0.00 |
| Total | 876 | 100 | 365 | 100.00 | Total | 876 | 100 | 365 | 100.00 |
| Apathy | Frequency | % | Frequency | % | Lightheadedness on standing* | Frequency | % | Frequency | % |
| 0 | 584 | 66.67 | 249 | 68.22 | 0 | 490 | 55.94 | 238 | 65.21 |
| 1 | 141 | 16.1 | 61 | 16.71 | 1 | 216 | 24.66 | 78 | 21.37 |
| 2 | 88 | 10.05 | 27 | 7.40 | 2 | 103 | 11.76 | 37 | 10.14 |
| 3 | 52 | 5.94 | 20 | 5.48 | 3 | 51 | 5.82 | 10 | 2.74 |
| 4 | 8 | 0.91 | 7 | 1.92 | 4 | 9 | 1.03 | 1 | 0.27 |
| 999 | 3 | 0.34 | 1 | 0.27 | 999 | 7 | 0.8 | 1 | 0.27 |
| Total | 876 | 100 | 365 | 100.00 | Total | 876 | 100 | 365 | 100.00 |
| Features of DDS | Frequency | % | Frequency | % | Fatigue* | Frequency | % | Frequency | % |
| 0 | 747 | 85.27 | 315 | 86.30 | 0 | 217 | 24.77 | 141 | 38.63 |
| 1 | 57 | 6.51 | 23 | 6.30 | 1 | 335 | 38.24 | 128 | 35.07 |
| 2 | 44 | 5.02 | 20 | 5.48 | 2 | 184 | 21 | 57 | 15.62 |
| 3 | 19 | 2.17 | 4 | 1.10 | 3 | 81 | 9.25 | 33 | 9.04 |
| 4 | 6 | 0.68 | 0 | 0.00 | 4 | 50 | 5.71 | 4 | 1.10 |
| 999 | 3 | 0.34 | 3 | 0.82 | 999 | 9 | 1.03 | 2 | 0.55 |
| Total | 876 | 100 | 365 | 100.00 | Total | 876 | 100 | 365 | 100.00 |
| Sleep problems* | Frequency | % | Frequency | % | |||||
| 0 | 280 | 31.96 | 138 | 37.81 | |||||
| 1 | 202 | 23.06 | 103 | 28.22 | |||||
| 2 | 207 | 23.63 | 81 | 22.19 | |||||
| 3 | 140 | 15.98 | 39 | 10.68 | |||||
| 4 | 40 | 4.57 | 3 | 0.82 | |||||
| 999 | 7 | 0.8 | 1 | 0.27 | |||||
| Total | 876 | 100 | 365 | 100.00 | |||||
| Part II | |||||||||
| Speech* | Frequency | % | Frequency | % | Doing hobbies and other activities* | Frequency | % | Frequency | % |
| 0 | 252 | 28.77 | 159 | 43.56 | 0 | 227 | 25.91 | 130 | 35.62 |
| 1 | 236 | 26.94 | 78 | 21.37 | 1 | 289 | 32.99 | 99 | 27.12 |
| 2 | 233 | 26.6 | 82 | 22.47 | 2 | 185 | 21.12 | 65 | 17.81 |
| 3 | 126 | 14.38 | 43 | 11.78 | 3 | 81 | 9.25 | 41 | 11.23 |
| 4 | 22 | 2.51 | 3 | 0.82 | 4 | 84 | 9.59 | 29 | 7.95 |
| 999 | 7 | 0.8 | 0 | 0.00 | 999 | 10 | 1.14 | 1 | 0.27 |
| Total | 876 | 100 | 365 | 100.00 | Total | 876 | 100 | 365 | 100.00 |
| Saliva and drooling* | Frequency | % | Frequency | % | Turning in bed | Frequency | % | Frequency | % |
| 0 | 341 | 38.93 | 186 | 50.96 | 0 | 277 | 31.62 | 122 | 33.42 |
| 1 | 115 | 13.13 | 49 | 13.42 | 1 | 378 | 43.15 | 144 | 39.45 |
| 2 | 203 | 23.17 | 64 | 17.53 | 2 | 111 | 12.67 | 48 | 13.15 |
| 3 | 157 | 17.92 | 46 | 12.60 | 3 | 55 | 6.28 | 31 | 8.49 |
| 4 | 53 | 6.05 | 18 | 4.93 | 4 | 50 | 5.71 | 19 | 5.21 |
| 999 | 7 | 0.8 | 2 | 0.55 | 999 | 5 | 0.57 | 1 | 0.27 |
| Total | 876 | 100 | 365 | 100.00 | Total | 876 | 100 | 365 | 100.00 |
| Chewing and swallowing | Frequency | % | Frequency | % | Tremor* | Frequency | % | Frequency | % |
| 0 | 549 | 62.67 | 241 | 66.03 | 0 | 189 | 21.58 | 118 | 32.33 |
| 1 | 230 | 26.26 | 81 | 22.19 | 1 | 360 | 41.1 | 154 | 42.19 |
| 2 | 54 | 6.16 | 22 | 6.03 | 2 | 212 | 24.2 | 69 | 18.90 |
| 3 | 34 | 3.88 | 18 | 4.93 | 3 | 72 | 8.22 | 17 | 4.66 |
| 4 | 3 | 0.34 | 3 | 0.82 | 4 | 36 | 4.11 | 7 | 1.92 |
| 999 | 6 | 0.68 | 0 | 0.00 | 999 | 7 | 0.8 | 0 | 0.00 |
| Total | 876 | 100 | 365 | 100.00 | Total | 876 | 100 | 365 | 100.00 |
| Eating tasks | Frequency | % | Frequency | % | Getting out of bed* | Frequency | % | Frequency | % |
| 0 | 363 | 41.44 | 158 | 43.29 | 0 | 180 | 20.55 | 101 | 27.67 |
| 1 | 265 | 30.25 | 114 | 31.23 | 1 | 317 | 36.19 | 140 | 38.36 |
| 2 | 187 | 21.35 | 79 | 21.64 | 2 | 199 | 22.72 | 73 | 20.00 |
| 3 | 42 | 4.79 | 8 | 2.19 | 3 | 104 | 11.87 | 35 | 9.59 |
| 4 | 10 | 1.14 | 5 | 1.37 | 4 | 70 | 7.99 | 15 | 4.11 |
| 999 | 9 | 1.03 | 1 | 0.27 | 999 | 6 | 0.68 | 1 | 0.27 |
| Total | 876 | 100 | 365 | 100.00 | Total | 876 | 100 | 365 | 100.00 |
| Dressing | Frequency | % | Frequency | % | Walking and balance | Frequency | % | Frequency | % |
| 0 | 220 | 25.11 | 82 | 22.47 | 0 | 184 | 21 | 74 | 20.27 |
| 1 | 322 | 36.76 | 176 | 48.22 | 1 | 336 | 38.36 | 156 | 42.74 |
| 2 | 211 | 24.09 | 67 | 18.36 | 2 | 105 | 11.99 | 38 | 10.41 |
| 3 | 76 | 8.68 | 28 | 7.67 | 3 | 172 | 19.63 | 61 | 16.71 |
| 4 | 42 | 4.79 | 12 | 3.29 | 4 | 74 | 8.45 | 33 | 9.04 |
| 999 | 5 | 0.57 | 0 | 0.00 | 999 | 5 | 0.57 | 3 | 0.82 |
| Total | 876 | 100 | 365 | 100.00 | Total | 876 | 100 | 365 | 100.00 |
| Hygiene | Frequency | % | Frequency | % | Freezing | Frequency | % | Frequency | % |
| 0 | 342 | 39.04 | 126 | 34.52 | 0 | 453 | 51.71 | 176 | 48.22 |
| 1 | 367 | 41.89 | 160 | 43.84 | 1 | 182 | 20.78 | 74 | 20.27 |
| 2 | 88 | 10.05 | 47 | 12.88 | 2 | 89 | 10.16 | 40 | 10.96 |
| 3 | 33 | 3.77 | 25 | 6.85 | 3 | 90 | 10.27 | 49 | 13.42 |
| 4 | 38 | 4.34 | 7 | 1.92 | 4 | 56 | 6.39 | 25 | 6.85 |
| 999 | 8 | 0.91 | 0 | 0.00 | 999 | 6 | 0.68 | 1 | 0.27 |
| Total | 876 | 100 | 365 | 100.00 | Total | 876 | 100 | 365 | 100.00 |
| Handwriting | Frequency | % | Frequency | % | |||||
| 0 | 161 | 18.38 | 106 | 29.04 | |||||
| 1 | 251 | 28.65 | 151 | 41.37 | |||||
| 2 | 222 | 25.34 | 75 | 20.55 | |||||
| 3 | 146 | 16.67 | 22 | 6.03 | |||||
| Part III | |||||||||
| Speech* | Frequency | % | Frequency | % | Arising from chair | Frequency | % | Frequency | % |
| 0 | 189 | 21.58 | 148 | 40.55 | 0 | 422 | 48.17 | 197 | 53.97 |
| 1 | 379 | 43.26 | 143 | 39.18 | 1 | 245 | 27.97 | 106 | 29.04 |
| 2 | 213 | 24.32 | 53 | 14.52 | 2 | 78 | 8.9 | 24 | 6.58 |
| 3 | 69 | 7.88 | 15 | 4.11 | 3 | 71 | 8.11 | 22 | 6.03 |
| 4 | 22 | 2.51 | 4 | 1.10 | 4 | 55 | 6.28 | 16 | 4.38 |
| 999 | 4 | 0.46 | 2 | 0.55 | 999 | 5 | 0.57 | 0 | 0.00 |
| Total | 876 | 100 | 365 | 100.00 | Total | 876 | 100 | 365 | 100.00 |
| Facial expression* | Frequency | % | Frequency | % | Gait | Frequency | % | Frequency | % |
| 0 | 96 | 10.96 | 88 | 24.11 | 0 | 202 | 23.06 | 81 | 22.19 |
| 1 | 300 | 34.25 | 137 | 37.53 | 1 | 351 | 40.07 | 187 | 51.23 |
| 2 | 361 | 41.21 | 109 | 29.86 | 2 | 167 | 19.06 | 47 | 12.88 |
| 3 | 89 | 10.16 | 23 | 6.30 | 3 | 97 | 11.07 | 36 | 9.86 |
| 4 | 26 | 2.97 | 7 | 1.92 | 4 | 55 | 6.28 | 14 | 3.84 |
| 999 | 4 | 0.46 | 1 | 0.27 | 999 | 4 | 0.46 | 0 | 0.00 |
| Total | 876 | 100 | 365 | 100.00 | Total | 876 | 100 | 365 | 100.00 |
| Rigidity, neck | Frequency | % | Frequency | % | Freezing of gait | Frequency | % | Frequency | % |
| 0 | 260 | 29.68 | 134 | 36.71 | 0 | 655 | 74.77 | 250 | 68.49 |
| 1 | 247 | 28.2 | 97 | 26.58 | 1 | 95 | 10.84 | 50 | 13.70 |
| 2 | 274 | 31.28 | 92 | 25.21 | 2 | 60 | 6.85 | 30 | 8.22 |
| 3 | 73 | 8.33 | 36 | 9.86 | 3 | 26 | 2.97 | 13 | 3.56 |
| 4 | 16 | 1.83 | 4 | 1.10 | 4 | 38 | 4.34 | 19 | 5.21 |
| 999 | 6 | 0.68 | 2 | 0.55 | 999 | 2 | 0.23 | 3 | 0.82 |
| Total | 876 | 100 | 365 | 100.00 | Total | 876 | 100 | 365 | 100.00 |
| Rigidity, RUE* | Frequency | % | Frequency | % | Postural stability* | Frequency | % | Frequency | % |
| 0 | 176 | 20.09 | 93 | 25.48 | 0 | 422 | 48.17 | 150 | 41.10 |
| 1 | 282 | 32.19 | 142 | 38.90 | 1 | 157 | 17.92 | 66 | 18.08 |
| 2 | 342 | 39.04 | 111 | 30.41 | 2 | 60 | 6.85 | 44 | 12.05 |
| 3 | 69 | 7.88 | 14 | 3.84 | 3 | 149 | 17.01 | 84 | 23.01 |
| 4 | 6 | 0.68 | 2 | 0.55 | 4 | 86 | 9.82 | 20 | 5.48 |
| 999 | 1 | 0.11 | 3 | 0.82 | 999 | 2 | 0.23 | 1 | 0.27 |
| Total | 876 | 100 | 365 | 100.00 | Total | 876 | 100 | 365 | 100.00 |
| Rigidity, LUE* | Frequency | % | Frequency | % | Posture | Frequency | % | Frequency | % |
| 0 | 205 | 23.4 | 99 | 27.12 | 0 | 173 | 19.75 | 78 | 21.37 |
| 1 | 268 | 30.59 | 135 | 36.99 | 1 | 337 | 38.47 | 129 | 35.34 |
| 2 | 317 | 36.19 | 121 | 33.15 | 2 | 206 | 23.52 | 84 | 23.01 |
| 3 | 77 | 8.79 | 9 | 2.47 | 3 | 125 | 14.27 | 52 | 14.25 |
| 4 | 7 | 0.8 | 1 | 0.27 | 4 | 33 | 3.77 | 21 | 5.75 |
| 999 | 2 | 0.23 | 0 | 0.00 | 999 | 2 | 0.23 | 1 | 0.27 |
| Total | 876 | 100 | 365 | 100.00 | Total | 876 | 100 | 365 | 100.00 |
| Rigidity, RLE | Frequency | % | Frequency | % | Global spontaneity of movement | Frequency | % | Frequency | % |
| 0 | 272 | 31.05 | 109 | 29.86 | 0 | 108 | 12.33 | 49 | 13.42 |
| 1 | 248 | 28.31 | 125 | 34.25 | 1 | 278 | 31.74 | 155 | 42.47 |
| 2 | 275 | 31.39 | 106 | 29.04 | 2 | 279 | 31.85 | 97 | 26.58 |
| 3 | 67 | 7.65 | 23 | 6.30 | 3 | 184 | 21 | 51 | 13.97 |
| 4 | 10 | 1.14 | 1 | 0.27 | 4 | 27 | 3.08 | 12 | 3.29 |
| 999 | 4 | 0.46 | 1 | 0.27 | 999 | 0 | 0 | 1 | 0.27 |
| Total | 876 | 100 | 365 | 100.00 | Total | 876 | 100 | 365 | 100.00 |
| Rigidity, LLE | Frequency | % | Frequency | % | Postural tremor, right hand | Frequency | % | Frequency | % |
| 0 | 286 | 32.65 | 116 | 31.78 | 0 | 544 | 62.1 | 223 | 61.10 |
| 1 | 227 | 25.91 | 120 | 32.88 | 1 | 262 | 29.91 | 119 | 32.60 |
| 2 | 275 | 31.39 | 100 | 27.40 | 2 | 43 | 4.91 | 19 | 5.21 |
| 3 | 75 | 8.56 | 26 | 7.12 | 3 | 23 | 2.63 | 2 | 0.55 |
| 4 | 11 | 1.26 | 1 | 0.27 | 4 | 1 | 0.11 | 2 | 0.55 |
| 999 | 2 | 0.23 | 2 | 0.55 | 999 | 3 | 0.34 | 0 | 0.00 |
| Total | 876 | 100 | 365 | 100.00 | Total | 876 | 100 | 365 | 100.00 |
| Finger tapping, right hand* | Frequency | % | Frequency | % | Postural tremor, left hand* | Frequency | % | Frequency | % |
| 0 | 122 | 13.93 | 95 | 26.03 | 0 | 518 | 59.13 | 234 | 64.11 |
| 1 | 342 | 39.04 | 167 | 45.75 | 1 | 276 | 31.51 | 98 | 26.85 |
| 2 | 252 | 28.77 | 64 | 17.53 | 2 | 49 | 5.59 | 27 | 7.40 |
| 3 | 144 | 16.44 | 35 | 9.59 | 3 | 29 | 3.31 | 2 | 0.55 |
| 4 | 15 | 1.71 | 3 | 0.82 | 4 | 1 | 0.11 | 1 | 0.27 |
| 999 | 1 | 0.11 | 1 | 0.27 | 999 | 3 | 0.34 | 3 | 0.82 |
| Total | 876 | 100 | 365 | 100.00 | Total | 876 | 100 | 365 | 100.00 |
| Finger tapping, left hand* | Frequency | % | Frequency | % | Kinetic tremor, right hand* | Frequency | % | Frequency | % |
| 0 | 108 | 12.33 | 91 | 24.93 | 0 | 546 | 62.33 | 258 | 70.68 |
| 1 | 298 | 34.02 | 135 | 36.99 | 1 | 265 | 30.25 | 89 | 24.38 |
| 2 | 265 | 30.25 | 96 | 26.30 | 2 | 46 | 5.25 | 15 | 4.11 |
| 3 | 181 | 20.66 | 37 | 10.14 | 3 | 13 | 1.48 | 1 | 0.27 |
| 4 | 22 | 2.51 | 5 | 1.37 | 4 | 2 | 0.23 | 1 | 0.27 |
| 999 | 2 | 0.23 | 1 | 0.27 | 999 | 4 | 0.46 | 1 | 0.27 |
| Total | 876 | 100 | 365 | 100.00 | Total | 876 | 100 | 365 | 100.00 |
| Hand movements, right hand* | Frequency | % | Frequency | % | Kinetic tremor, left hand* | Frequency | % | Frequency | % |
| 0 | 187 | 21.35 | 129 | 35.34 | 0 | 493 | 56.28 | 236 | 64.66 |
| 1 | 346 | 39.5 | 160 | 43.84 | 1 | 293 | 33.45 | 105 | 28.77 |
| 2 | 231 | 26.37 | 57 | 15.62 | 2 | 72 | 8.22 | 22 | 6.03 |
| 3 | 98 | 11.19 | 17 | 4.66 | 3 | 14 | 1.6 | 1 | 0.27 |
| 4 | 12 | 1.37 | 2 | 0.55 | 4 | 0 | 0 | 1 | 0.27 |
| 999 | 2 | 0.23 | 0 | 0.00 | 999 | 4 | 0.46 | 0 | 0.00 |
| Total | 876 | 100 | 365 | 100.00 | Total | 876 | 100 | 365 | 100.00 |
| Hand movements, left hand* | Frequency | % | Frequency | % | Rest tremor amplitude, RUE* | Frequency | % | Frequency | % |
| 0 | 164 | 18.72 | 118 | 32.33 | 0 | 586 | 66.89 | 281 | 76.99 |
| 1 | 311 | 35.5 | 147 | 40.27 | 1 | 112 | 12.79 | 51 | 13.97 |
| 2 | 250 | 28.54 | 78 | 21.37 | 2 | 121 | 13.81 | 26 | 7.12 |
| 3 | 125 | 14.27 | 17 | 4.66 | 3 | 53 | 6.05 | 6 | 1.64 |
| 4 | 25 | 2.85 | 4 | 1.10 | 4 | 3 | 0.34 | 1 | 0.27 |
| 999 | 1 | 0.11 | 1 | 0.27 | 999 | 1 | 0.11 | 0 | 0.00 |
| Total | 876 | 100 | 365 | 100.00 | Total | 876 | 100 | 365 | 100.00 |
| Pronation: supination movements, right hand* | Frequency | % | Frequency | % | Rest tremor amplitude, LUE* | Frequency | % | Frequency | % |
| 0 | 199 | 22.72 | 100 | 27.40 | 0 | 603 | 68.84 | 280 | 76.71 |
| 1 | 335 | 38.24 | 159 | 43.56 | 1 | 120 | 13.7 | 56 | 15.34 |
| 2 | 216 | 24.66 | 64 | 17.53 | 2 | 99 | 11.3 | 20 | 5.48 |
| 3 | 107 | 12.21 | 35 | 9.59 | 3 | 45 | 5.14 | 9 | 2.47 |
| 4 | 17 | 1.94 | 6 | 1.64 | 4 | 5 | 0.57 | 0 | 0.00 |
| 999 | 2 | 0.23 | 1 | 0.27 | 999 | 4 | 0.46 | 0 | 0.00 |
| Total | 876 | 100 | 365 | 100.00 | Total | 876 | 100 | 365 | 100.00 |
| Pronation: supination movements, left hand | Frequency | % | Frequency | % | Rest tremor amplitude, RLE | Frequency | % | Frequency | % |
| 0 | 162 | 18.49 | 76 | 20.82 | 0 | 777 | 88.7 | 319 | 87.40 |
| 1 | 297 | 33.9 | 138 | 37.81 | 1 | 52 | 5.94 | 25 | 6.85 |
| 2 | 235 | 26.83 | 101 | 27.67 | 2 | 35 | 4 | 18 | 4.93 |
| 3 | 150 | 17.12 | 42 | 11.51 | 3 | 9 | 1.03 | 2 | 0.55 |
| 4 | 29 | 3.31 | 8 | 2.19 | 4 | 0 | 0 | 0 | 0.00 |
| 999 | 3 | 0.34 | 0 | 0.00 | 999 | 3 | 0.34 | 1 | 0.27 |
| Total | 876 | 100 | 365 | 100.00 | Total | 876 | 100 | 365 | 100.00 |
| Toe tapping, right foot* | Frequency | % | Frequency | % | Rest tremor amplitude, LLE | Frequency | % | Frequency | % |
| 0 | 168 | 19.18 | 89 | 24.38 | 0 | 795 | 90.75 | 319 | 87.40 |
| 1 | 323 | 36.87 | 149 | 40.82 | 1 | 46 | 5.25 | 24 | 6.58 |
| 2 | 228 | 26.03 | 96 | 26.30 | 2 | 20 | 2.28 | 17 | 4.66 |
| 3 | 129 | 14.73 | 24 | 6.58 | 3 | 12 | 1.37 | 2 | 0.55 |
| 4 | 27 | 3.08 | 6 | 1.64 | 4 | 0 | 0 | 0 | 0.00 |
| 999 | 1 | 0.11 | 1 | 0.27 | 999 | 3 | 0.34 | 3 | 0.82 |
| Total | 876 | 100 | 365 | 100.00 | Total | 876 | 100 | 365 | 100.00 |
| Toe tapping, left foot* | Frequency | % | Frequency | % | Rest tremor amplitude, lip/jaw* | Frequency | % | Frequency | % |
| 0 | 154 | 17.58 | 68 | 18.63 | 0 | 780 | 89.04 | 349 | 95.62 |
| 1 | 251 | 28.65 | 140 | 38.36 | 1 | 63 | 7.19 | 12 | 3.29 |
| 2 | 268 | 30.59 | 111 | 30.41 | 2 | 18 | 2.05 | 3 | 0.82 |
| 3 | 154 | 17.58 | 36 | 9.86 | 3 | 13 | 1.48 | 0 | 0.00 |
| 4 | 46 | 5.25 | 10 | 2.74 | 4 | 1 | 0.11 | 1 | 0.27 |
| 999 | 3 | 0.34 | 0 | 0.00 | 999 | 1 | 0.11 | 0 | 0.00 |
| Total | 876 | 100 | 365 | 100.00 | Total | 876 | 100 | 365 | 100.00 |
| Leg agility, right leg* | Frequency | % | Frequency | % | Constancy of rest* | Frequency | % | Frequency | % |
| 0 | 250 | 28.54 | 119 | 32.60 | 0 | 409 | 46.69 | 219 | 60.00 |
| 1 | 329 | 37.56 | 163 | 44.66 | 1 | 214 | 24.43 | 79 | 21.64 |
| 2 | 190 | 21.69 | 61 | 16.71 | 2 | 91 | 10.39 | 28 | 7.67 |
| 3 | 86 | 9.82 | 18 | 4.93 | 3 | 85 | 9.7 | 21 | 5.75 |
| 4 | 18 | 2.05 | 4 | 1.10 | 4 | 67 | 7.65 | 17 | 4.66 |
| 999 | 3 | 0.34 | 0 | 0.00 | 999 | 10 | 1.14 | 1 | 0.27 |
| Total | 876 | 100 | 365 | 100.00 | Total | 876 | 100 | 365 | 100.00 |
| Leg agility, left leg* | Frequency | % | Frequency | % | |||||
| 0 | 216 | 24.66 | 99 | 27.12 | |||||
| 1 | 298 | 34.02 | 142 | 38.90 | |||||
| 2 | 213 | 24.32 | 90 | 24.66 | |||||
| 3 | 106 | 12.1 | 30 | 8.22 | |||||
| 4 | 38 | 4.34 | 3 | 0.82 | |||||
| 999 | 5 | 0.57 | 1 | 0.27 | |||||
| Total | 876 | 100 | 365 | 100.00 | |||||
| Part IV | |||||||||
| Time spent with dyskinesias* | Frequency | % | Frequency | % | Functional impact of fluctuations | Frequency | % | Frequency | % |
| 0 | 563 | 64.27 | 273 | 74.79 | 0 | 433 | 49.43 | 194 | 53.15 |
| 1 | 173 | 19.75 | 41 | 11.23 | 1 | 165 | 18.84 | 56 | 15.34 |
| 2 | 87 | 9.93 | 30 | 8.22 | 2 | 81 | 9.25 | 32 | 8.77 |
| 3 | 27 | 3.08 | 12 | 3.29 | 3 | 119 | 13.58 | 60 | 16.44 |
| 4 | 17 | 1.94 | 6 | 1.64 | 4 | 63 | 7.19 | 19 | 5.21 |
| 999 | 9 | 1.03 | 3 | 0.82 | 999 | 15 | 1.71 | 4 | 1.10 |
| Total | 876 | 100 | 365 | 100.00 | Total | 876 | 100 | 365 | 100.00 |
| Functional impact of dyskinesias* | Frequency | % | Frequency | % | Complexity of motor fluctuations* | Frequency | % | Frequency | % |
| 0 | 695 | 79.34 | 308 | 84.38 | 0 | 404 | 46.12 | 192 | 52.60 |
| 1 | 90 | 10.27 | 27 | 7.40 | 1 | 291 | 33.22 | 125 | 34.25 |
| 2 | 29 | 3.31 | 19 | 5.21 | 2 | 69 | 7.88 | 21 | 5.75 |
| 3 | 46 | 5.25 | 7 | 1.92 | 3 | 50 | 5.71 | 17 | 4.66 |
| 4 | 5 | 0.57 | 2 | 0.55 | 4 | 46 | 5.25 | 3 | 0.82 |
| 999 | 11 | 1.26 | 2 | 0.55 | 999 | 16 | 1.83 | 7 | 1.92 |
| Total | 876 | 100 | 365 | 100.00 | Total | 876 | 100 | 365 | 100.00 |
| Time spent in the OFF state* | Frequency | % | Frequency | % | Painful OFF state dystonia* | Frequency | % | Frequency | % |
| 0 | 383 | 43.72 | 183 | 50.14 | 0 | 680 | 77.63 | 319 | 87.40 |
| 1 | 341 | 38.93 | 113 | 30.96 | 1 | 114 | 13.01 | 28 | 7.67 |
| 2 | 106 | 12.1 | 50 | 13.70 | 2 | 45 | 5.14 | 4 | 1.10 |
| 3 | 22 | 2.51 | 14 | 3.84 | 3 | 13 | 1.48 | 6 | 1.64 |
| 4 | 14 | 1.6 | 2 | 0.55 | 4 | 15 | 1.71 | 5 | 1.37 |
| 999 | 10 | 1.14 | 3 | 0.82 | 999 | 9 | 1.03 | 3 | 0.82 |
| Total | 876 | 100 | 365 | 100.00 | Total | 876 | 100 | 365 | 100.00 |
a999 = missing.
*P < 0.05 by chi‐square test (df = 4).
DDS, dopamine dysregulation syndrome; RUE, right upper extremity; LUE, left upper extremity; RLE, right lower extremity; LLE, left lower extremity.
Discussion
The overall factor structure of the Japanese version was consistent with the English version based on the CFIs for all four parts of the MDS‐UPDRS in the CFA (all CFI ≥0.93). The Japanese scale was confirmed to share a common factor structure with the English scale. Therefore, this version can be designated as the official Japanese version of the MDS‐UPDRS.
EFA, in which variability from sample to sample is expected, identified isolated item differences of factor structure between the Japanese and English versions of the MDS‐UPDRS. However, the distribution of factors with their associated eigenvalues and percent variances were similar across the two languages.
In our study, female preponderance was noted as the previous study reported from Japan.14 This may, in part, be because of the longer life expectancy (by approximately 6.5 years) in Japanese women, in comparison to men.
Another interesting difference between the Japanese‐ and English‐language versions data sets for the MDS‐UPDRS concerned the pattern of responses to items 1.1 (cognitive impairment) and 1.2 (hallucinations and psychosis). For the hallucination item, the Japanese and English frequencies for each rating option were very similar (77% and 78%, respectively), but cognitive impairment ratings were different in the two cultures. A much greater percentage (62.2%) of Japanese had 0 scores, in comparison to the English‐speaking sample (48.9%). In general, among reports in Western cultures, cognitive impairment and hallucinations are shared or overlapping behaviors and such data have been used to argue shared common pathogeneses.15, 16 Results of the chi‐square test indicate that severity of motor and nonmotor symptoms are generally more severe in patients of English groups than those of Japanese groups. Even after taking these differences into consideration, the present results from the Japanese sample may indicate that cognitive impairment is less frequent or viewed differently and thereby may be underreported for cultural reasons in Japan, in comparison to the Western culture.
Contrary to majority of items, constipation problems and postural stability were rated more severe in Japanese patients than English patients. Differences in genetic factor, eating habits, and amount of daily exercise between two populations are possible factors to produce different response to the former item. The reason why postural stability was rated more severely in Japanese groups remains unknown. Factors including examiner's manner to pull patients may be clarified in future.
In conclusion, the CFI for the Japanese version of the MDS‐UPDRS was 0.93 or greater. Therefore, the Japanese version meets the criterion for designation as an official translation of the MDS‐UPDRS. This is the first Asian‐ or non‐Indo‐European–language translation of the MDS‐UPDRS. The Japanese version of the MDS‐UPDRS is available from the MDS website (http://www.movementdisorders.org/publications/rating_scales/). The establishment of additional non‐English translations will further facilitate the understanding of PD symptoms and help accelerate qualified clinical trials and discussions worldwide.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the First Draft, B. Review and Critique.
K. Kashihara: 1A, 1B, 1C, 2C, 3A, 3B
T. Kondo: 1A, 1B, 1C, 3A, 3B
Y. Mizuno: 1A, 1B, 1C, 3A, 3B
S. Kikuchi: 1B, 1C, 3B
S. Kuno: 1B, 1C, 3B
K. Hasegawa: 1B, 1C, 3A, 3B
N. Hattori: 1B, 1C, 3B
H. Mochizuki: 1B, 1C, 3B
H. Mori: 1B, 1C, 3B
M. Murata: 1B, 1C, 3B
M. Nomoto: 1B, 1C, 3B
R. Takahashi: 1B, 1C, 3B
A. Takeda: 1B, 1C, 3B
Y. Tsuboi: 1B, 1C, 3B
Y. Ugawa: 1B, 1C, 3B
M. Yamamoto: 1B, 1C, 3B
F. Yokochi: 1B, 1C, 3B
F. Yoshii: 1A, 1B, 1C, 3A, 3B
G.T. Stebbins: 1A, 1B, 1C, 2A, 2C, 3B
B.C. Tilley: 2C, 3B
L. Wang: 2B, 2C, 3A, 3B
S. Luo: 2C, 3B
N.R. LaPelle: 2A, 2B, 3B
C.G. Goetz: 1A, 1B, 1C, 2A, 2C, 3A, 3B
Disclosures
Funding Sources and Conflicts of Interest: This work was supported by Boehringer Ingelheim Japan. The administrative core members (G.T.S., B.C.T., S.L., L.W., N.R.L., and C.G.G.) were supported by funds from the Movement Disorder Society.
Financial Disclosures for previous 12 months: Kenichi Kashihara has served on the advisory board of Kyowa Hakko Kirin Co.; has been supported by Health and Labor Sciences Research Grants; has received honoraria from Boehringer Ingelheim, GlaxoSmithKline (GSK), Kyowa Hakko Kirin Co., Novartis, Otsuka Pharmaceutical Co., Dainippon Sumitomo Pharm Co., Ltd., and Fujimoto Pharmaceutical (FP) Pharmaceutical Co.; and has received royalties from Nankodo. Tomoyoshi Kondo has worked as a consultant for Kyowa Hakko Kirin Co. and Novartis and has received honoraria from Boeringer Ingelheim, GSK, Kyowa Hakko Kirin Co., Novartis, Otsuka Pharmaceutical Co., Dainippon Sumitomo Pharm Co., Ltd., and FP Pharmaceutical Co. Yoshikuni Mizuno has held advisory board membership with FP Pharmaceutical Co., Otsuka Pharmaceutical Co., AbbVie Japan, and Kyowa Hakko Kirin Co. and received personal compensation when he attended advisory board meetings and has been supported by grants from Boehringer Ingelheim. Seiji Kikuchi has been supported by grants from the Ministry of Health, Labor and Welfare of Japan and has received honoraria from Boehringer Ingelheim, GSK, Kyowa Hakko Kirin Co., Novartis, Otsuka Pharmaceutical Co., Dainippon Sumitomo Pharm Co., Ltd., FP Pharmaceutical Co., Daiichi‐Sankyo, Takeda Pharmaceutical Co., Biogen Idec Japan, Bayer Yakuhin, Genzyme Japan, Nihon Pharmaceutical Co., and Mitsubishi Tanabe Pharma. Sadako Kuno has served on the advisory board of AbbVie Japan and has received honoraria from Boehringer Ingelheim, GSK, Kyowa Hakko Kirin Co., Novartis, Otsuka Pharmaceutical Co., Dainippon Sumitomo Pharm Co., Ltd., FP Pharmaceutical Co., Ono Pharmaceutical Co., AbbVie Japan, and Alfresa Pharma. Kazuko Hasegawa has received honoraria from Boehringer Ingelheim, GSK, Kyowa Hakko Kirin Co., Novartis, Otsuka Pharmaceutical Co., and Dainippon Sumitomo Pharm Co., Ltd. Nobutaka Hattori has worked as a consultant for Hisamitsu Pharmaceutical; has been supported by grants from Otsuka Pharmaceutical, Boehringer Ingelheim, and Kyowa Hakko‐Kirin Pharmaceutical Company; and has received honoraria from GSK K.K., Nippon Boehringer Ingelheim, Co., Ltd., FP Pharmaceutical Co., Otsuka Pharmaceutical, Co., Ltd., Dainippon Sumitomo Pharma Co., Ltd., Novartis Pharma K.K., Eisai Co., Ltd., Medtronic, Inc., Kissei Pharmaceutical Company, Janssen Pharmaceutical K.K., Nihon Medi‐Physics Co., Ltd., Astellas Pharma Inc., and Kyowa Hakko‐Kirin Co., Ltd. Hideki Mochizuki has been supported by grants from Grant‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, Grant‐in‐Aid for JST‐CREST Basic Research Program from the Ministry of Education, Culture, Sports, Science and Technology of Japan, Grant‐in‐Aid for Scientific Research on Innovative Areas (Brain Environment) from the Ministry of Education, Science, Sports and Culture of Japan, and Grant‐in‐Aid for Research on Applying Health Technology from the Ministry of Health, Labor and Welfare of Japan; has received honoraria from Biogen Idec Japan, Eisai Co., Ltd., FP Pharmaceutical Co., Elsevier Japan, Hisamitsu Pharma, Kyowa Hakko Kirin Co., GSK, Dainippon Sumitomo Pharm Co., Ltd., FP Pharmaceutical Co., Takeda Pharmaceutical Co., Mitsubishi Tanabe Pharma, Nippon Chemiphar Co., Nihon Medi‐Physics Co., Boehringer Ingelheim, Novartis, and UCB Japan; and has received royalties from Nature Japan, Igaku‐Shoin, Iyaku Journal, Nanzando Co., and Kinpodo. Hideo Mori has received honoraria from Boehringer Ingelheim, GSK, Otsuka Pharmaceutical Co., Dainippon Sumitomo Pharm Co., Ltd., and FP Pharmaceutical Co. Miho Murata has been supported by grants from the Ministry of Health, Labor and Welfare of Japan and has received honoraria from Boehringer Ingelheim, GSK, Kyowa Hakko Kirin Co., Novartis, Otsuka Pharmaceutical Co., Dainippon Sumitomo Pharm Co., Ltd., and Nihon Medi‐Physics Co. Masahiro Nomoto has been awarded grants and research support from the Ministry of Health, Labor and Welfare of Japan, Dainippon Sumitomo Pharm Co., Ltd., Boehringer Ingelheim, Novartis, GSK, FP Pharmaceutical Co., Genzyme, and Tsumura & Co.; has worked as a consultant for and held advisory board membership with honoraria with the Japanese Society of Internal Medicine, Takeda Pharm Co., FP Pharmaceutical Co., Kyowa Hakko Kirin Co., Otsuka Pharm Co., Hisamitsu, Ono Pharm Co., and Meiji Seika; has received honoraria from Boehringer Ingelheim, GSK, Dainippon Sumitomo Pharm Co., Ltd., FP Pharmaceutical Co., Novartis, Kyowa Hakko Kirin Co., Otsuka Pharm Co., Genzyme, Panasonic Healthcare Co., and UCB Inc.; and has received royalties from Maruzen, Igaku‐Shoin, and Nishimura. Ryosuke Takahashi has worked as a consultant for KAN Research Institute, Inc., and Daiichi‐Sankyo; has been awarded grants and research support from Dainippon Sumitomo Pharm Co., Ltd., Boehringer Ingelheim, Novartis, Pfizer Co., Ltd., GSK, Takeda Pharmaceutical Co., Mitsubishi Tanabe Pharma, and Kyowa Hakko Kirin Co.; and has received honoraria from Boehringer Ingelheim, GSK, Dainippon Sumitomo Pharm Co., Ltd., FP Pharmaceutical Co., Medical Review, Novartis, Daiichi‐Sankyo, Kyowa Hakko Kirin Co., Mitsubishi Tanabe Pharma, Eisai Co., Ltd., Nihon Pharmaceutical Co., Otsuka Pharmaceutical Co., Janssen Pharmaceutical Company, Sanofi, Alfresa Pharma Co., Japan Blood Products Organization, Asbio Pharma Co., Ltd., and MSD. Atsushi Takeda has been supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan and the Ministry of Health, Labor and Welfare of Japan; has received honoraria from Otsuka Pharmaceutical Co., Kyowa Hakko Kirin Co., Ltd., GSK, Daiichi‐Sankyo, Dainippon Sumitomo Pharm Co., Ltd., FP Pharmaceutical Co., Takeda Pharmaceutical Co., Boehringer Ingelheim, Novartis, and Ono Pharmaceutical; and has received royalties from Iyaku Journal, Chugai‐Igakusha, Igaku‐Shoin, Medical View, Elsevier Japan, and Aruta Shuppan. Yoshio Tsuboi has been supported by grants from the Ministry of Health, Labor and Welfare of Japan and has received honoraria from Eisai Co., Ltd., Otsuka Pharmaceutical Co., Kyowa Hakko Kirin Co., GSK, Daiichi‐Sankyo, Dainippon Sumitomo Pharm Co., Ltd., FP Pharmaceutical Co., Mitsubishi Tanabe Pharma, Teijin Pharma, Boehringer Ingelheim, and Novartis. Yoshikazu Ugawa has been supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan, the Ministry of Health, Labor and Welfare of Japan, the Support Center for Advanced Telecommunications Technology Research, the Association of Radio Industries Businesses, the Uehara Memorial Foundation, Novartis Foundation (Japan) for the Promotion of Science, JST, and Nihon Kohden; has received honoraria from the Taiwan Society of Clinical Neurophysiology, Indonesia Society of Clinical Neurophysiology, Taiwan Movement Disorders Society, Astellas Pharma, Eisai Co. Ltd., Dainippon Sumitomo Pharm Co., Ltd., FP Pharmaceutical Co., Otsuka Pharmaceutical Co., Elsevier Japan, Kissei Pharmaceutical Co., Kyorin Pharma, Kyowa Hakko Kirin Co., GSK, Sanofi, Daiichi‐Sankyo, Takeda Pharmaceutical Co., Mitsubishi Tanebe Pharma, Teijin Pharma, Nippon Chemiphar Co., Nihon Pharmaceutical Co., Boehringer Ingelheim, Novartis, Bayer Yakuhin, and Mochida Pharma; and has received royalties from Chugai‐Igakusha, Igaku‐Shoin Ltd., Medical View, and Blackwell Publishing. Mitsutoshi Yamamoto has received honoraria from Dainippon Sumitomo Pharm Co., Ltd., Boehringer Ingelheim, Novartis, GSK, FP Pharmaceutical Co., Kyowa Hakko Kirin Co., and Otsuka Pharm Co. Fusako Yokochi has received honoraria from GSK, Otsuka Pharmaceutical Co., Medtronic, and AbbVie Japan. Fumihito Yoshii has been supported by grants from Eisai Co., Ltd., Dainippon Sumitomo Pharm Co., Ltd., FP Pharmaceutical Co., Takeda Pharmaceutical Co., Mitsubishi Tanabe Pharma, GSK, Boehringer Ingelheim, Daiichi‐Sankyo, Mitsubishi Tanabe Pharma, and Pfizer and has received honoraria from GSK, Dainippon Sumitomo Pharm Co., Ltd., Boehringer Ingelheim, Novartis, AbbVie Japan, Ono Parmaceutical Co., Otsuka Pharmaceutical Co., and Janssen Pharmaceutical Co. Glenn T. Stebbins has worked as a consultant for and held advisory board membership with honoraria with Adamas Pharmaceuticals, Inc., Ceregene, Inc., Child Health and Development Institute (CHDI) Management, Inc., Ingenix Pharmaceutical Services (i3 Research), and Neurocrine Biosciences, Inc.; has been awarded grants and research support from the National Institutes of Health (NIH), the Michael J. Fox Foundation (MJFF) for Parkinson's Research, and the Dystonia Coalition; has received honoraria from the International Parkinson and Movement Disorder Society (MDS), American Academy of Neurology, and the MJFF; and has received a salary from Rush University Medical Center. Barbara C. Tilley has been awarded grants from the NIH (National Institute of Neurological Disorders and Stroke, National Heart, Lung and Blood Institute, National Institute on Minority Health and Health Disparities, and National Institute of General Medical Sciences), the Pfizer Data and Safety Monitoring Committee and the NIH Data and Safety Monitoring Committees and has received a salary from the University of Texas Health Science Center School of Public Health at Houston, Division of Biostatistics. Sheng Luo and Lu Wang have nothing to declare. Nancy R. Lapelle has worked in cognitive testing, qualitative research, and program/process evaluation consulting for the UMass Medical School (UMMS) Lamar Soutter Library, UMass Medical School Inter‐Professional Development, The Association of Academic Health Sciences Libraries, Medical University of South Carolina (MUSC) College of Nursing and Hollings Cancer Center, and the MDS; Dr. Lapelle is a subcontractor on a variety of research and evaluation grants with principal investigators at UMMS and MUSC. Christopher G. Goetz has worked as a consultant for and held advisory board membership with honoraria with AOP Orphan, Addex Pharma, Advanced Studies of Medicine, Boston Scientific, CHDI, Health Advances, ICON Clinical Research, Inc., Ingenix (i3 Research), the NIH, Neurocrine, Oxford Biomedica, and Synthonics and has been awarded grants and research support with funding from the NIH and the MJFF. Dr. Goetz directs the Rush Parkinson's Disease Research Center that receives support from the Parkinson's Disease Foundation; he directs the translation program for the MDS‐UPDRS and UDysRS and receives funds from the MDS for this effort; has received honoraria from the MDS, the American Academy of Neurology, University of Pennsylvania, University of Chicago, and University of Luxembourg; has received royalties from Oxford University Press, Elsevier Publishers, and Wolters Kluwer Health–Lippincott, Wilkins and Williams; and has received a salary from Rush University Medical Center.
The MDS‐UPDRS Japanese Validation Study Group
| Investigators | Affiliation |
|---|---|
| Takashi Abe, MD | Department of Neurology, Abe Neurological Clinic |
| Kenichi Fujimoto, MD | Department of Neurology, Jichi Medical University Hospital |
| Kazuko Hasegawa, MD | Department of Neurology, National Sagamihara Hospital |
| Nobutaka Hattori, MD | Department of Neurology, Juntendo University School of Medicine |
| Yasuto Higashi, MD | Department of Neurology, Himeji Central Hospital |
| Takaki Imamura, MD | Department of Neurology, Okayama Kyokuto Hospital |
| Hidehumi Ito, MD | Department of Neurology, Wakayama Medical University |
| Kazunori Ito, MD | Department of Neurology, Iwamizawa Neurological Medical Clinic |
| Kenichi Kashihara, MD | Department of Neurology, Okayama Kyokuto Hospital |
| Jyunya Kawada, MD | Department of Neurology, Shonan Kamakura General Hospital |
| Noriko Kawashima, MD | Department of Neurology, Kawashima Neurology Clinic |
| Seiji Kikuchi, MD | National Hospital Organization Hokkaido Medical Center |
| Sadako Kuno, MD | Kyoto Shijyo Hospital |
| Tetsuya Maeda, MD | Department of Neurology, Research Institute for Brain and Blood Vessels‐Akita |
| Hideki Mochizuki, MD | Department of Neurology, Osaka University Graduate School of Medicine |
| Hideo Mori, MD | Department of Neurology, Juntendo University Koshigaya Hospital |
| Kenya Murata, MD | Department of Neurology, Wakayama Medical University |
| Miho Murata, MD | Department of Neurology, National Center of Neurology and Psychiatry, Parkinson Disease and Movement Disorder Center |
| Masahiro Nomoto, MD | Department of Neurology and Clinical Pharmacology, Ehime University Graduate School of Medicine |
| Yasuyuki Okuma, MD | Juntendo University Shizuoka Hospital |
| Hidemoto Saiki, MD | Department of Neurology, Kitano Hospital |
| Hideyuki Sawada, MD | National Hospital Organization Utano Hospital |
| Ryosuke Takahashi, MD | Department of Neurology, Graduate School of Medicine, Kyoto University |
| Atsushi Takeda, MD | Department of Neurology, Tohoku University Medical School |
| Asako Takei, MD | Department of Neurology, Hokuyukai Neurological Hospital |
| Yasuo Terayama, MD | Department of Neurology, Iwate Medical University |
| Masahiko Tomiyama, MD | Department of Neurology, Aomori Prefectural Central Hospital |
| Yoshio Tsuboi, MD | Department of Neurology, Fukuoka University Medical School |
| Yoshikazu Ugawa, MD | Department of Neurology, Fukushima Medical University |
| Mitsutoshi Yamamoto, MD | Takamatsu Neurology Clinic |
| Fusako Yokochi, MD | Department of Neurology, Tokyo Metropolitan Neurological Hospital |
| Kazuto Yoshida, MD | Department of Neurology, Japanese Red Cross Asahikawa Hospital |
| Fumihito Yoshii, MD | Department of Neurology, Tokai University School of Medicine |
Investigators involved in the cognitive pretesting and/or validation and their affiliations.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Fahn S, Elton RL. Unified Parkinson's Disease Rating Scale In: Fahn S, Marsden CD, Goldstein M, Calne DB, eds. Recent Developments in Parkinson's Disease, Vol. 2 Florham Park, NJ: MacMillan Healthcare Information; 1987:153–164. [Google Scholar]
- 2. Movement Disorder Society Task Force on Rating Scales for Parkinson's Disease . The Unified Parkinson's Disease Rating Scale (UPDRS): status and recommendations. Mov Disord 2003;18:738–750. [DOI] [PubMed] [Google Scholar]
- 3. Barone P, Antonini A, Colosimo C, et al. The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson's disease. Mov Disord 2009;24:1641–1649. [DOI] [PubMed] [Google Scholar]
- 4. Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society‐sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23:2129–2170. [DOI] [PubMed] [Google Scholar]
- 5. Antonini A, Abbruzzese G, Ferini‐Strambi L, et al. Validation of the Italian version of the Movement Disorder Society‐Unified Parkinson's Disease Rating Scale. Neurol Sci 2013;34:683–687. [DOI] [PubMed] [Google Scholar]
- 6. Martinez‐Martin P, Rodriguez‐Blazquez C, Alvarez‐Sanchez M, et al. Expanded and independent validation of the Movement Disorder Society‐Unified Parkinson's Disease Rating Scale (MDS‐UPDRS). J Neurol 2013;260:228–236. [DOI] [PubMed] [Google Scholar]
- 7. Fowler FJ. Improving Survey Questions. Thousand Oaks, CA: Sage; 1995. [Google Scholar]
- 8. Hatcher L. Step‐by‐Step Approach to Using the SAS System for Factor Analysis and Structural Equation Modeling. Cary, NC: SAS Institute; 1994. [Google Scholar]
- 9. Muthen LK, Muthen BO. M‐plus User's Guide. 6th ed Los Angeles, CA: Muthen & Muthen; 2010. [Google Scholar]
- 10. Brown TA. Confirmatory Factor Analysis for Applied Research. New York, NY: Guilford SAGE Publications Inc; 2006. [Google Scholar]
- 11. Browne MW. An overview of analytic rotation in exploratory factor analysis. Multivar Behav Res 2001;36:111–150. [Google Scholar]
- 12. Gorsuch RL. Factor Analysis. 2nd ed Hillsdale, NJ: Lawrence Erlbaum Associations Inc; 1983. [Google Scholar]
- 13. Forero CG, Maydeu‐Olivares A, Gallardo‐Pujol D. Factor analysis with ordinal indicators: a Monte Carlo study comparing DWLS and ULS estimation. Struct Equ Model 2009;16:625–641. [Google Scholar]
- 14. Kimura H, Kurimura M, Wada M, et al. Female preponderance of Parkinson's disease in Japan. Neuroepidemiology 2002;21:292–296. [DOI] [PubMed] [Google Scholar]
- 15. Hely MA, Reid WG, Adena MA, et al. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord 2008;23:837–844. [DOI] [PubMed] [Google Scholar]
- 16. Morgante L, Colosimo C, Antonini A, et al. Psychosis associated to Parkinson's disease in the early stages: relevance of cognitive decline and depression. J Neurol Neurosurg Psychiatry 2012;83:76–82. [DOI] [PubMed] [Google Scholar]


