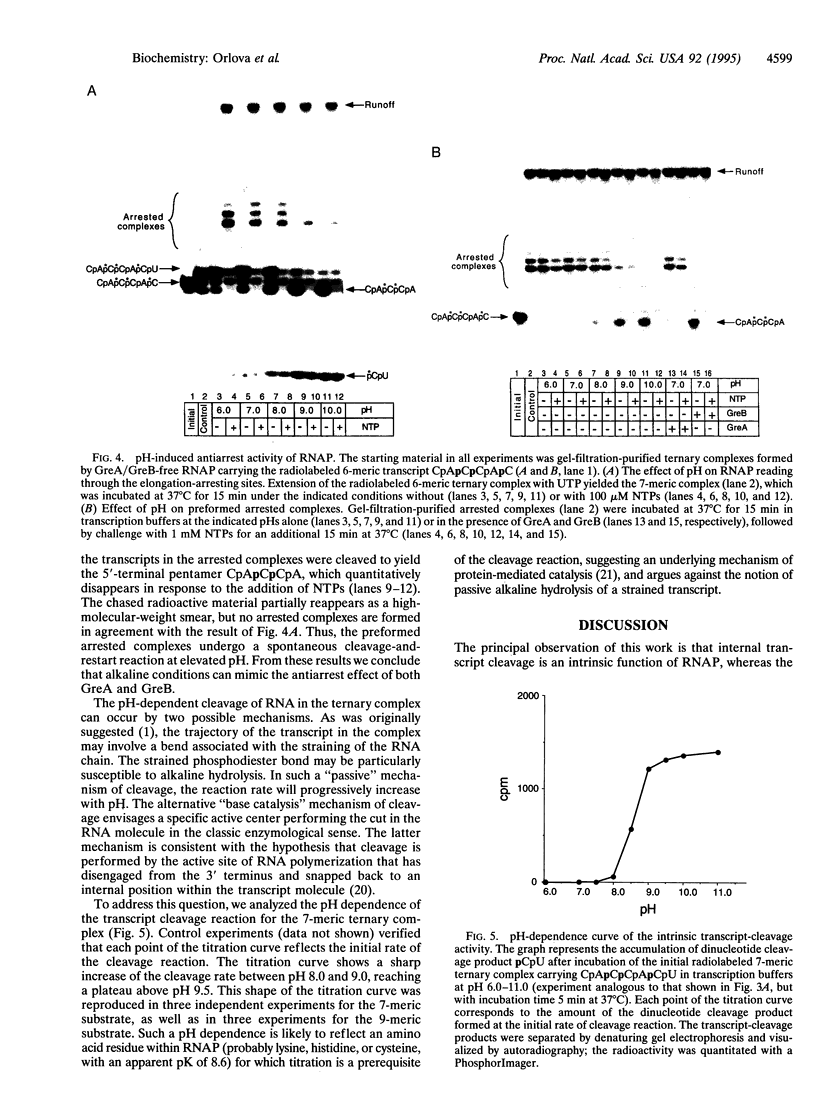
Abstract
The GreA and GreB transcript cleavage factors of Escherichia coli suppress elongation arrest and may have a proofreading role in transcription. With the use of E. coli greA-greB- mutant, RNA polymerase is demonstrated to possess substantial intrinsic transcript cleavage activity. Mildly alkaline pH mimics the effect of the Gre proteins by inducing transcript cleavage in ternary complexes and antagonizing elongation arrest through a cleavage-and-restart reaction. Thus, transcript cleavage constitutes the second enzymological activity of RNA polymerase along with polymerization/pyrophosphorolysis of RNA, whereas the Gre proteins merely enhance this intrinsic property.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borukhov S., Goldfarb A. Recombinant Escherichia coli RNA polymerase: purification of individually overexpressed subunits and in vitro assembly. Protein Expr Purif. 1993 Dec;4(6):503–511. doi: 10.1006/prep.1993.1066. [DOI] [PubMed] [Google Scholar]
- Borukhov S., Polyakov A., Nikiforov V., Goldfarb A. GreA protein: a transcription elongation factor from Escherichia coli. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):8899–8902. doi: 10.1073/pnas.89.19.8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borukhov S., Sagitov V., Goldfarb A. Transcript cleavage factors from E. coli. Cell. 1993 Feb 12;72(3):459–466. doi: 10.1016/0092-8674(93)90121-6. [DOI] [PubMed] [Google Scholar]
- Borukhov S., Sagitov V., Josaitis C. A., Gourse R. L., Goldfarb A. Two modes of transcription initiation in vitro at the rrnB P1 promoter of Escherichia coli. J Biol Chem. 1993 Nov 5;268(31):23477–23482. [PubMed] [Google Scholar]
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- CHAMBERLIN M., BERG P. Deoxyribo ucleic acid-directed synthesis of ribonucleic acid by an enzyme from Escherichia coli. Proc Natl Acad Sci U S A. 1962 Jan 15;48:81–94. doi: 10.1073/pnas.48.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erie D. A., Hajiseyedjavadi O., Young M. C., von Hippel P. H. Multiple RNA polymerase conformations and GreA: control of the fidelity of transcription. Science. 1993 Nov 5;262(5135):867–873. doi: 10.1126/science.8235608. [DOI] [PubMed] [Google Scholar]
- Garrett S., Taylor R. K., Silhavy T. J., Berman M. L. Isolation and characterization of delta ompB strains of Escherichia coli by a general method based on gene fusions. J Bacteriol. 1985 May;162(2):840–844. doi: 10.1128/jb.162.2.840-844.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W., Powell W., Mote J., Jr, Reines D. Nascent RNA cleavage by arrested RNA polymerase II does not require upstream translocation of the elongation complex on DNA. J Biol Chem. 1993 Dec 5;268(34):25604–25616. [PMC free article] [PubMed] [Google Scholar]
- Hagler J., Shuman S. Nascent RNA cleavage by purified ternary complexes of vaccinia RNA polymerase. J Biol Chem. 1993 Jan 25;268(3):2166–2173. [PubMed] [Google Scholar]
- Izban M. G., Luse D. S. The increment of SII-facilitated transcript cleavage varies dramatically between elongation competent and incompetent RNA polymerase II ternary complexes. J Biol Chem. 1993 Jun 15;268(17):12874–12885. [PubMed] [Google Scholar]
- Kassavetis G. A., Geiduschek E. P. RNA polymerase marching backward. Science. 1993 Feb 12;259(5097):944–945. doi: 10.1126/science.7679800. [DOI] [PubMed] [Google Scholar]
- Keleti T. The liberator. J Theor Biol. 1967 Sep;16(3):337–355. doi: 10.1016/0022-5193(67)90060-4. [DOI] [PubMed] [Google Scholar]
- Nudler E., Goldfarb A., Kashlev M. Discontinuous mechanism of transcription elongation. Science. 1994 Aug 5;265(5173):793–796. doi: 10.1126/science.8047884. [DOI] [PubMed] [Google Scholar]
- Reines D., Ghanouni P., Li Q. Q., Mote J., Jr The RNA polymerase II elongation complex. Factor-dependent transcription elongation involves nascent RNA cleavage. J Biol Chem. 1992 Aug 5;267(22):15516–15522. [PMC free article] [PubMed] [Google Scholar]
- Rudd M. D., Izban M. G., Luse D. S. The active site of RNA polymerase II participates in transcript cleavage within arrested ternary complexes. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):8057–8061. doi: 10.1073/pnas.91.17.8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkowski J., Das A. Location of a new gene, greA, on the Escherichia coli chromosome. J Bacteriol. 1991 Sep;173(17):5256–5257. doi: 10.1128/jb.173.17.5256-5257.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkowski J., Das A. Simultaneous gain and loss of functions caused by a single amino acid substitution in the beta subunit of Escherichia coli RNA polymerase: suppression of nusA and rho mutations and conditional lethality. Genetics. 1992 Mar;130(3):411–428. doi: 10.1093/genetics/130.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkowski J., Das A. The nucleotide sequence of greA, a suppressor gene that restores growth of an Escherichia coli RNA polymerase mutant at high temperature. Nucleic Acids Res. 1990 Nov 11;18(21):6443–6443. doi: 10.1093/nar/18.21.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surratt C. K., Milan S. C., Chamberlin M. J. Spontaneous cleavage of RNA in ternary complexes of Escherichia coli RNA polymerase and its significance for the mechanism of transcription. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7983–7987. doi: 10.1073/pnas.88.18.7983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehall S. K., Bardeleben C., Kassavetis G. A. Hydrolytic cleavage of nascent RNA in RNA polymerase III ternary transcription complexes. J Biol Chem. 1994 Jan 21;269(3):2299–2306. [PubMed] [Google Scholar]






