Abstract
Boron-doped diamond (BDD) has seen a substantial increase in interest for use as electrode coating material for electrochemistry and studies of deep brain stimulation mechanism. In this study, we present an alternative method for determining important characteristics, including conductivity, carrier concentration, and time constant, of such material by the signature of Drude-like metallic behavior in the far-infrared (IR) spectral range. Unlike the direct determination of conductivity from the four-point probe method, using far-IR transmittance provides additional information, such as whether the incorporation of boron results in a large concentration of carriers or in inducing defects in the diamond lattice. The slightly doped to medium-doped BDD samples that were produced using chemical vapor deposition and analyzed in this work show conductivities ranging between 5.5 and 11 (Ω cm)−1. Different growth conditions demonstrate that increasing boron concentration results in an increase in the carrier concentration, with values between 7.2 × 1016 and 2.5 × 1017 carriers/cm3. Addition of boron, besides leading to a decrease in the resistivity, also resulted in a decrease in the time constant, limiting BDD conductivity. Investigations, by confocal Raman mapping, of the induced stress in the material due to interaction with the substrate or to the amount of doping are also presented and discussed. The induced tensile stress, which was distributed closer to the film-substrate interface decreased slightly with doping.
Introduction
Due to boron-doped diamond’s (BDD) important role as an electrode material in biosensing applications such as clinical studies of deep brain stimulation (DBS), conductive transport measurements in thin films of this material have been a subject of active interest in recent years [1–8]. In this p-type semiconductor, where the boron dopant acts as electron acceptor, two main conduction mechanisms occur depending on doping levels and temperature. While conduction through holes in the conduction band is expected at low doping levels (i.e., boron atoms <1017 cm−3) or low temperatures, nearest-neighbor and variable range hopping of carriers between ionized boron sites occurs at higher doping levels, with the ultimate formation of an impurity band that gives rise to metal-like conductivity for very high doping levels [2, 9]. This transition in the conduction mechanism from localized hopping to band conduction was observed to shift toward higher temperatures with increasing doping level [9]. Furthermore, an increase in the amount of doping not only results in an increase in the density of electronic states and in a widening of the acceptor band, but also in a reduction of the activation energy of carriers and in an increase in their mobility [2].
For polycrystalline BDD films grown by chemical vapor deposition (CVD), which are the type of samples analyzed here, accurate estimation of electrical conductivity is far from being a simple task. Even though the effects of the contact resistance between the spring-loaded probes and the surface of the film can be largely removed by four-point probe measurements, conductivity values are still influenced by other causes. One cause is the existence of nondiamond, sp2 type impurities that accumulate at the boundaries of crystallites. The inherent inhomogeneity of the samples arising from film thickness variation and from preferential incorporation of boron at the grain surfaces is another reason [10]. These causes lead to measurements of effective surface conductivity that does not correspond to bulk conductivity.
In this study, we present an alternative method for determining the conductivity or resistivity of such thin films by the signature of their Drude-like metallic behavior in the far-infrared (IR) spectral range. The transmittance of a thin film in the far-IR is related to its electrical conductivity, which in turn is a function of the frequency of the applied IR field. Since the characteristic frequency (the inverse of the average time between two carrier-core collisions) is typically in the far-IR range, transmission measurements in this spectral region are particularly suited to determining the above-mentioned material characteristics.
The reason why the Drude theoretical approach works so well in the case of polycrystalline, inhomogeneous films such as those of BDD is that it relies on the characteristic transport length scale, , which is inversely proportional to the square root of frequency (where D is the diffusion coefficient). Since in the far-IR frequency range Lω is smaller than the typical length scales encountered in dc transport measurements, a frequency-dependent weak localization correction to the conductivity can be considered [11, 12]. Consequently, in this frequency range, the material can be treated as homogeneous, since the energy lost to transport through grain boundaries can be neglected.
A feature of the Drude model is that the carrier density is temperature independent. The experimentally observed change in the material conductivity with temperature variation relates to the temperature dependence of the relaxation rate or the characteristic transport length scale (e.g., the charge carrier’s mean free path) [13, 14]. Since part of the scattering of the charge carriers is due to their interaction with the thermally generated excitations in the material, phonon-related analysis becomes important in understanding such phenomena. Furthermore, because charge-transfer and interband transitions usually take place at higher energies [15, 16], resulting in strong absorption in the mid-IR spectral range, the Drude model is applicable mainly in the far-IR optical range (i.e., up to the THz frequency range, such as below ~250 cm−1 or 30 meV). The generalized Drude model with a frequency-dependent scattering rate and effective mass should be considered for mid-IR absorption analysis, which is beyond the scope of this work.
By the Drude approach, additional information about the material, such as whether the incorporation of boron resulted in a large concentration of acceptors (hence, a concentration of carriers) or in inducing defects in the diamond lattice (hence, a decrease in the time constant), is obtained; this information being unattainable if the fourpoint probe method is employed.
If development of high-quality diamond-based electrodes is envisioned for their further use in fast-scan cyclic voltammetry (FSCV) applications, another aspect that could influence the quality of the grown BDD is the stress induced in the material by the substrate or by the amount of doping employed. While the lattice mismatch between the deposited material and the substrate (i.e., the lattice constant for diamond is 3.567 Å and for silicon is 5.430 Å) can induce tensile stress that will result in defects such as splitting and cracking of the films, the unwanted existence of impurities produces compressive intrinsic stress that could result in potential delamination of the film from the substrate [17–19]. These considerations were investigated using confocal Raman mapping measurements and are also discussed in this work.
Materials and methods
Experimental details
The BDD films were grown in a hot filament chemical vapor deposition (CVD) reactor using a mixture of CH4/H2 gases at a pressure of 20 Torr. Trimethylborane (TMB), 1000 ppm in H2, was introduced in the chamber at various flow rates ranging from 0 to 2.5 sccm and metered using an Alicat mass flow controller. The volumes of H2 and CH4 gases of 196 sccm and 2 sccm, respectively, were metered using FloCat flow controllers. Undoped single crystal (100) Si that was initially abraded by immersion in a slurry mixture of 8 nm diamond powder and isopropyl alcohol, and ultrasonically agitated, was used as the substrate material. The filament temperature was kept constant at 2,050 °C during film deposition and monitored with a Spectrodyne DFP 2000 optical pyrometer. An Omega Engineering type K thermocouple was used to monitor the substrate temperature. Uniform films with crystallite sizes varying between 0.3 and 2.4 microns were obtained as a result of these growth conditions.
The far-IR transmission measurements were acquired at room temperature with a vacuum-based Bruker IFS 66v FT-IR spectrometer equipped with a Ge-coated mylar beam splitter and a deuterated triglycine sulfate (DTGS) detector. Each IR spectrum resulted from an accumulation of 256 scans at a resolution of 4 cm−1. An alpha 300R WITec system using the 532 nm excitation of a Nd:YAG laser and a 100× objective lens was employed for confocal Raman mapping measurements, which were performed at ambient conditions with a resolution of 4 cm−1. The mapping was acquired with a pixel number of 22,500 and an integration time of 50 ms per pixel. The WITec Control software, which also controls the piezoelectric stage for sample scanning, was employed for data acquisition. The strain data analysis was performed using the Advanced Fitting Tool of the WITec Project Plus software.
Drude theoretical approach
If multiple reflections are negligible, the ratio T(ω) of the transmission through a thin conductive film deposited on a substrate, TC(ω), to the transmission through the substrate alone, TS(ω), is provided by [20, 21]
| (1) |
where σ(ω) is the conductivity, Zo = 377 Ω is the impedance of free space, d is the thickness of the thin film, and nS is the index of refraction of the substrate (in this case, for Si, nS = 3.4).
Assuming a free electron model in the presence of scattering (the Drude model), the conductivity is
| (2) |
where is the Drude dc conductivity, n is the electron density, e and m are the electron charge and mass, respectively, and s is the mean free time between collisions of the carriers with the lattice ions (i.e., time constant).
Combining (1) and (2) gives [12]
| (3) |
and
| (4) |
The fitted parameters a and b provide
| (5a) |
| (5b) |
| (5c) |
Results and discussion
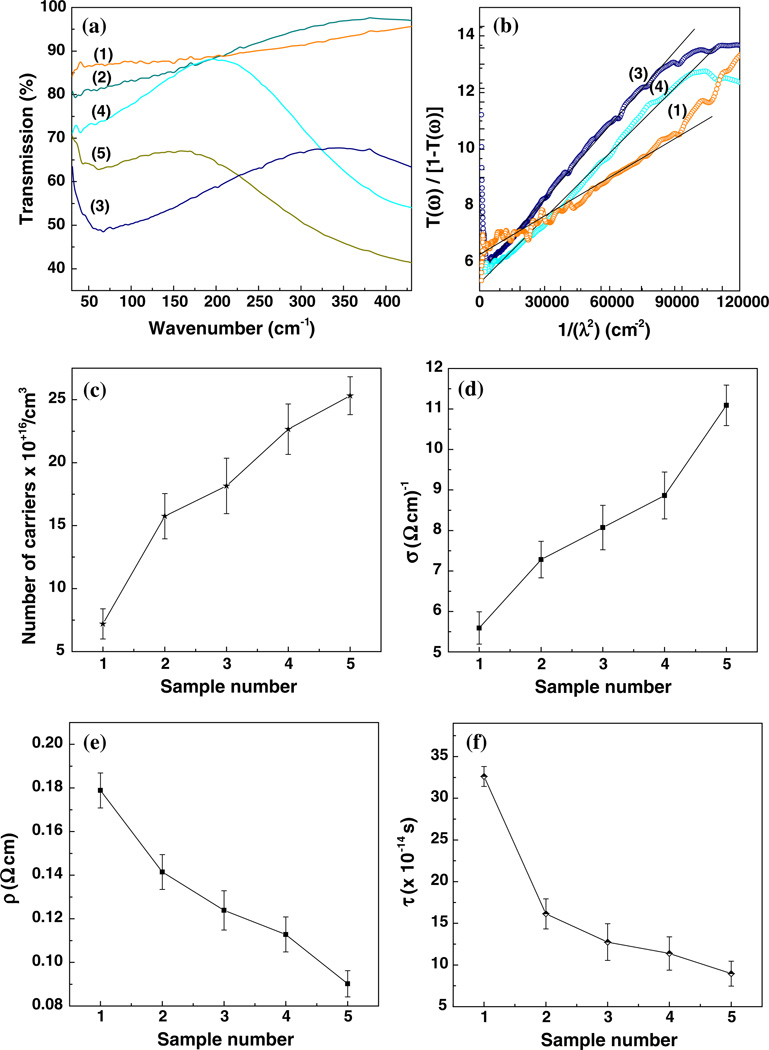
The far-IR experimental transmission measurements of five BDD samples grown on Si substrates with various trim-ethylborane (TMB) dopant flow rates ranging from 0 to 2.5 sccm, and their derived results based on the Drude model analysis, are presented in Fig. 1a–f. Although similar information could be obtained through reflectance measurements [22], the reason we consider transmission measurements in this work (Fig. 1a) is to avoid potential experimental systematic errors. Transmission is measured relative to 0 %, whereas reflectance is measured relative to 100 %; thus, it is harder to accurately determine a 100 % reference for the latter. Also, the linearity of the photodetector response with the intensity of the incident radiation is better for lower signals, such as those transmitted through the samples, than for the signals obtained from reflectance, where detector saturation effects are possible.
Fig. 1.
a Far-IR transmission results of five BDD samples grown on Si substrates with various trimethylborane (TMB) doping gas admissions ranging from 0 to 2.5 sccm, as labeled. b Representative fits demonstrating linear dependence of the ratio of transmittance, T(ω), to [1– T(ω)] as a function of 1/λ2. c–f The number of carriers, n, conductivity, σ, resistivity, ρ, and the mean free time between collisions of the carriers with the lattice ions, τ, respectively, as functions of sample number. Higher number labels of samples correspond to higher amounts of TMB
To account for the transmission just through each BDD conductive film, appropriate subtraction of the transmission of the Si substrate was performed on the spectra shown in Fig. 1a. In this way, the variations observed in the overall transmission behavior of these spectra depend mainly on the inherent differences in BDD film thicknesses, which vary from 1.5 to 4.5 µm, and on the amount of boron doping. While the thickness of the film contributes to an overall transmission decrease, the boron doping influences the range over which the transmission increases with frequency without the presence of additional absorption features. Thus, the latter determines the frequency region of Drude model applicability. In this context, it is also worth mentioning that saturation effects in absorption, which increases with increasing boron doping level (i.e., BDD becomes less transparent with increasing boron concentration [1, 23]), limit the transmission spectral measurements.
As revealed in Fig. 1b, where three representative ratios of transmittance, T(ω), to [1–T(ω)] as a function of ω2 are presented, these two physical parameters (i.e., film thickness and boron doping level) also influence the values of the fitting parameters a and b (i.e., the intercept and the slope), as well as the range suitable for a linear fit. The characteristic Drude falloff at higher frequencies, namely the deviation from the linear fit in the 30–50 wavenumber region, is due to the noise induced by reaching the system detection limit (e.g., of the DTGS detector).
The expected increase in the number of carriers with higher boron doping, which corresponds to higher sample number, is presented in Fig. 1c. Since unwanted contamination with boron is frequently encountered in the fabrication of BDD samples, as boron could reside on the walls and other components of the reactor, we prefer to present the results versus the number of the sample, not versus TMB flow gas. An obvious example that supports this affirmation is the sample labeled 1, where no TMB (0 sccm TMB) was added during growth. However, as can be seen in Fig. 1c, the estimated number of carriers for this sample is about 7.2 × 1016/cm−3. Another reason for this quite high value of carrier density for an undoped sample is the potential contribution of sp2 carbon impurities. The similar effect of increasing conductivity of these films with increasing boron doping level is revealed in Fig. 1d. Values ranging from 5.5 to 11 (ω cm)−1 were derived using the Drude model for conductivities of these samples. Given that the overall sample conductivity is a result of both bulk conductivity within diamond grains and surface conductivity, the potential influence of sp2 carbon should be considered as part of the latter. It is not unlikely that higher amounts of TMB flow gas would be correlated with increases in sp2 contamination, also. Behavior opposite to that of conductivity is observed in Fig. 1e for the resistivity, which decreases from 0.2 to 0.09 (ω cm). While, for these low doping levels, the current results for conductivity are comparable with the ones reported in the literature from four-point probe measurements [23–25], there is a difference of slightly more than two orders of magnitude between the number of carriers obtained in this work (7.2 × 1016 –2.5 × 1017/cm−3) and the literature-reported boron content (~1019–1020/cm3).
A potential explanation of these observations is as follows: at low boron doping levels, the boron atoms substitute for the carbon atoms, occupying neutrally interstitial positions in a carbon sp3 environment. Thus, the holes (carriers) provided by the boron atoms are energetically bound (i.e., binding energy of ~0.38 eV) in one of the three-fold degenerate impurity states. Also, since the average distance between the boron atoms decreases with increasing boron concentration, to a value close to that of the Bohr radius (around 3.5 × 10−8 cm when a hydrogenic model for the bound hole is considered), a metallic type of conduction should be expected with increasing boron concentration. However, even at high doping levels (i.e., 1020 atoms/cm−3), no metallic conduction takes place [9]. Instead, hopping conduction through nearest-neighbor (more distant impurities) and the formation of an impurity band, and of boron atom pairs, occur. Metallic conduction could appear only if the energy levels in this impurity band broaden enough so that this band crosses the Fermi level.
The nearly linear trend of increasing conductivity seen in Fig. 1d suggests that the metallic threshold is not yet reached in the current samples, and conduction through hopping between ionized and neutral acceptors within the impurity band is more probably taking place. This information, combined with the decreasing trend of the mean free time between collisions of the carriers, τ, that is presented in Fig. 1f, is the key that elucidates the discrepancy between the values of the number of carriers obtained using the Drude model and the boron content reported in the literature [23–26]. Even if higher boron doping concentrations is achievable, the number of carriers that contribute to the conductive properties of BDD samples is limited by this time constant, which has the tendency of leveling out at about 5 × 10−14 s (see Fig. 1f). Consequently, not necessarily all of the boron atoms incorporated in the diamond lattice contribute to the conductivity of BDD.
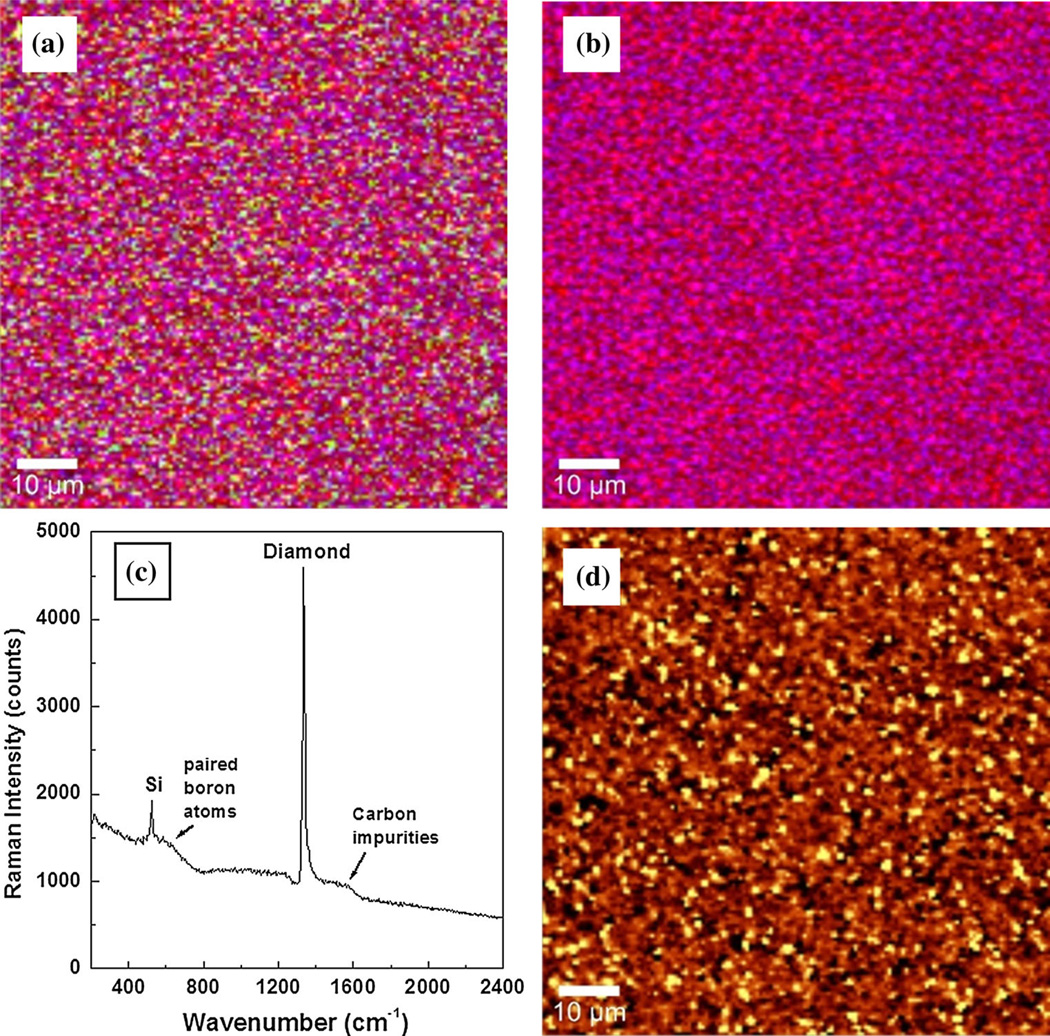
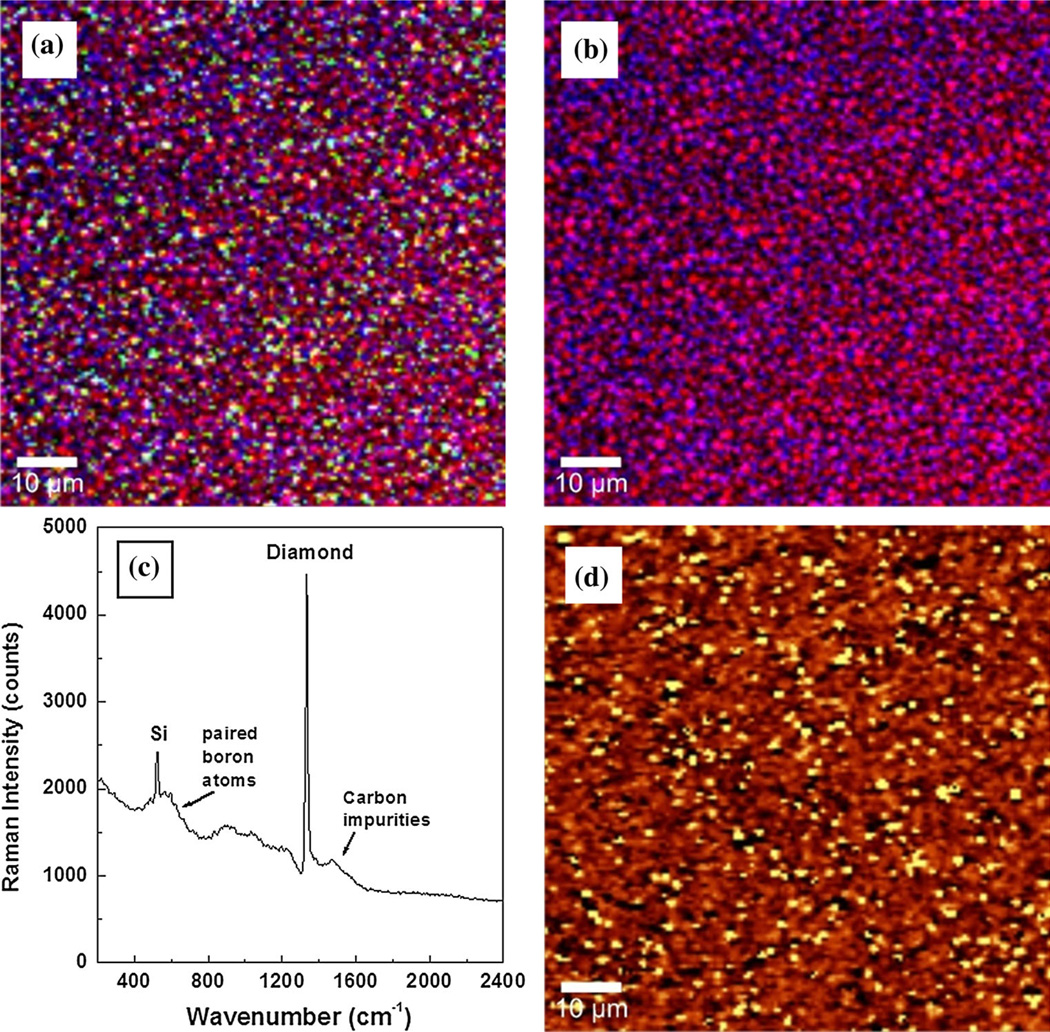
Investigations by confocal Raman mapping of the internal stress induced in these thin films by the substrate or by the amount of boron doping level are presented in Figs. 2a–d and 3a–d. Two different doping levels, a low (Fig. 2a–d) and a medium one (Fig. 3a–d), were considered. Besides being a qualitative and a relative quantitative method for determining the constituents of materials (e.g., in this case, pure diamond, boron incorporation and accumulation, and non-diamond, carbon sp2 type of impurities), Raman spectroscopy can be also used in stress and strain analyses [13–15]. In this context, it has been shown that the characteristic optical-phonon band of diamond at 1,332 cm−1 shifts around 3 cm−1 under a stress of 1 GPa [13, 14]. A higher or lower frequency shift corresponds to a compressive or a tensile stress, respectively.
Fig. 2.
a and b Confocal Raman mapping images of low-doped BDD film. Red, blue, and green pseudo-colors are used for diamond, boron, and amorphous carbon impurities, respectively. c Raman integrated spectrum and d confocal Raman stress mapping image
Fig. 3.
a and b Confocal Raman mapping images of medium-doped BDD film. Red, blue, and green pseudo-colors are used for diamond, boron, and amorphous carbon impurities, respectively. c Raman integrated spectrum, and d confocal Raman stress mapping image
The employment of confocal Raman mapping in determining the material constituents is presented in Figs. 2a, b and 3a, b. These measurements were performed by selecting characteristic Raman vibrational lines at 1,332 cm−1 for diamond, around 1,230 cm−1 for boron in the diamond lattice, and the band centered at 1,500 cm−1 corresponding to amorphous carbon impurities [1, 7, 9, 10, 27]. Three pseudo-colors (i.e., red for diamond, blue for BDD, and green for carbon impurities) were assigned to these constituents and used for relative quantitative visualization. While the presence of carbon impurities is considered in the Raman mapping images presented in Figs. 2a and 3a, for easier examination of film uniformity as related to boron incorporation, this option is eliminated in Figs. 2b and 3b. In addition to the expected color trend toward blue with increasing boron doping level (Fig. 3b), the use of less TMB feed gas during growth might result in more uniform incorporation of boron (Fig. 2b). However, this observation is debatable since both Raman mapping images show the existence of pure diamond crystallites (red color). There is also a very slight increase of sp2 carbon (green color) in Fig. 3a in comparison with Fig. 2a, which is confirmed in Figs. 2c and 3c by the slight increase in the intensity of the feature around 1,500 cm−1. The integrated Raman spectra of these images are presented in Figs. 2c and 3c.
The qualitative identification of the induced stress in the samples due to boron incorporation was achieved by performing confocal Raman mapping on their surfaces. These images, which are presented in Figs. 2d and 3d, account for the shift of the 1,332 cm−1 diamond vibrational line. At every image pixel, a Raman spectrum was recorded in milliseconds (for an overall Raman mapping in a few minutes) and the entire spectral data set was fitted with appropriate Lorentz functions using the Advanced Fitting Tool of the WITec Project Plus software. Shifts of about 4 ± 2 cm−1 to 6 ± 2 cm−1 (i.e., 1.3 ± 0.7–2.0 ± 0.7 GPa) to lower frequency, which indicate a tensile stress, were obtained. The indicated error accounts for the change in the width of the diamond Raman lines and the unevenness of the side lobes due to the presence of the Fano effect. The full widths at half maximum (FWHM) of the Raman lines are of the order of 10–20 cm−1 and, although the sampling resolution is 4 cm−1, fitting the peaks allows the maxima to be determined with a precision better than 1 cm−1; hence small shifts can be detected. The light yellow pseudo-color in these images corresponds to a more strained structure. A slight decrease of the stress with increasing boron concentration is observed (i.e., slightly more yellow in Fig. 2d versus that in Fig. 3d). Thus, as boron incorporates into the diamond lattice, it expands it, weakening the induced tensile stress. Due to interstitial boron incorporation and its accumulation at the grain boundaries, an opposite effect is expected at higher boron doping levels [18, 19]. An increase in the dislocation density with increasing boron concentration was also observed and reported [18, 19].
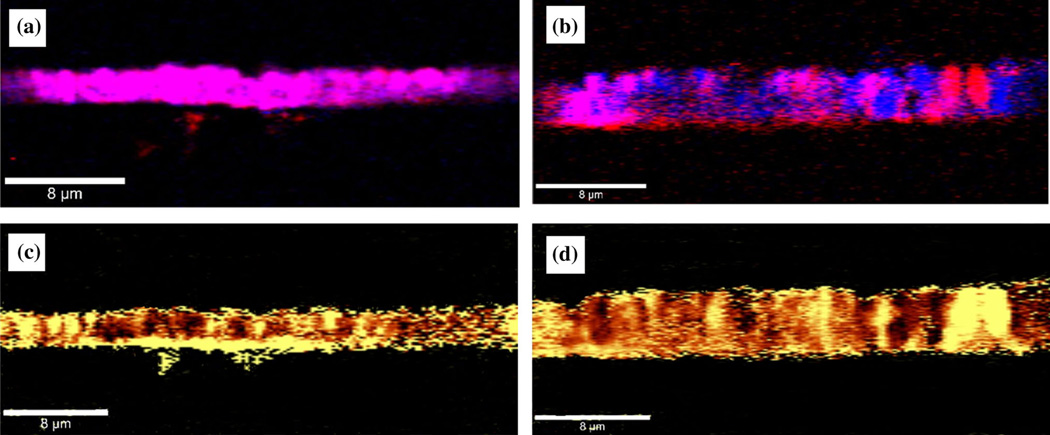
The induced tensile stress due to the lattice mismatch between the materials is presented in Fig. 4a–d. To properly analyze the dominance of this effect at the interface, a side-wall confocal Raman mapping was performed in this case. While a more uniform incorporation of boron throughout the entire film thickness can be observed in Fig. 4a for a slightly doped sample (purple color is a combination of red and blue), a columnar, preferential incorporation of boron with distinct blue and red areas corresponding to boron and pure diamond, can be seen in Fig. 4b for a medium-doped sample. More importantly, as revealed in Fig. 4c, d, there is substantially increased tensile stress (bright yellow pseudo-color) at the interface between the BDD material and the Si substrate. Comparison of Fig. 4c, d with their corresponding images presented in Fig. 4a, b respectively, demonstrates that pure diamond regions (red color) are associated with more induced stress. Not only does this observation support our previous affirmation of less induced stress with increasing boron concentration (e.g., less stress observed at the material surfaces), but, indirectly, also assesses the quality of the samples. There is no evidence of defects or dislocations that could degrade material quality and create other types of internal stresses that could lower its performance in future FSCV measurements.
Fig. 4.
a and b Side-wall Raman mapping images of a slightly and a medium-doped BDD film. Red and blue pseudo-colors are used for diamond and boron, respectively. c and d side-wall confocal Raman stress mapping images of the corresponding films presented in (a) and (b)
Conclusions
Boron-doped diamond samples produced by chemical vapor deposition and analyzed in this work using the Drude model in the far-IR spectral region show conductivities ranging between 5.5 and 11 (ω cm)−1. An increase in boron concentration resulted either in an increase in the carrier concentration (between 7.2 × 1016 and 2.5 × 1017 carriers/cm3), leading to a decrease in the resistivity, or in a decrease in the mean free time between collisions of the carriers (i.e., time constant). Although the threshold of metallic-type conduction has not been reached for the samples analyzed here, the decreasing trend of the time constant (with a tendency of leveling out at about5 × 10−14 s) suggests that for even higher doping amounts, the number of carriers that contribute to the conductive properties of BDD is limited. This trend also explains the observed difference between the numbers of carriers obtained in this work and the ones reported in the literature [23–26]; not necessarily do all the boron atoms incorporated in the diamond lattice contribute to material conductivity.
Analysis by confocal Raman mapping of the induced stress in the samples due to boron incorporation and to the lattice mismatch between the materials demonstrates a decrease of the interfacial tensile stress with the addition of boron, as well as of that farther away from the interface between the materials. The confocal Raman mapping investigation of the material constituents shows a relatively more uniform incorporation of boron if a lower doping level is used (e.g., more uniform purple color with less distinct red and blue areas).
With the goal of optimizing and improving BDD characteristics through understanding phenomena at the molecular level, the far-IR and Raman spectroscopic experimental studies presented here and combined with a Drude theoretical approach represent innovative methods of acquiring valuable information for developing highquality diamond-based electrodes for their further use in FSCV applications.
Acknowledgements
This work was supported by The Grainger Foundation, the NIH R01 NS075013 award, and by a research agreement between the University of Texas at El Paso and the Mayo Clinic.
Contributor Information
Felicia S. Manciu, Email: fsmanciu@utep.edu, Department of Physics, University of Texas at El Paso, El Paso, TX 79968, USA.
Marian Manciu, Department of Physics, University of Texas at El Paso, El Paso, TX 79968, USA.
William G. Durrer, Department of Physics, University of Texas at El Paso, El Paso, TX 79968, USA
Jessica G. Salazar, Department of Physics, University of Texas at El Paso, El Paso, TX 79968, USA
Kendall H. Lee, Department of Neurosurgery, Mayo Clinic, Rochester, MN 55905, USA
Kevin E. Bennet, Department of Neurosurgery, Mayo Clinic, Rochester, MN 55905, USA Division of Engineering, Mayo Clinic, Rochester, MN 55905, USA.
References
- 1.Kraft A. Doped diamond: a compact review on a new. Versatile Electrode Material, Int Electrochem Sci. 2007;2:355–385. [Google Scholar]
- 2.Holt KB, Bard AJ, Show Y, Swain GM. Scanning electrochemical microscopy and conductive probe atomic force microscopy studies of hydrogen-terminated boron-doped diamond electrodes with different doping levels. J Phys Chem B. 2004;108:15117–15127. [Google Scholar]
- 3.Shang F, Zhou L, Mahmoud KA, Hrapovic S, Liu Y, Moynihan HA, Glennon JD, Luong JHT. Selective nanomolar detection of dopamine using a boron-doped diamoind electrode modified with an electropolymerized sulfobutylether-β-cyclodextrin-doped poly(N-acetyltyramine) and polypyrrole composite film. Anal Chem. 2009;81:4089–4098. doi: 10.1021/ac900368m. [DOI] [PubMed] [Google Scholar]
- 4.Smith NP, Ashfold MNR, Smith DJ, Pearce TRA. Manufacture and performance of diamond-coated thermocouples. Diam Relat Mater. 1999;8:856–960. [Google Scholar]
- 5.Luong JHT, Male KB, Glennon JD. Boron-doped diamond electrode: synthesis, characterization, functionalization and analytical applications. Analysts. 2009;134:1965–1979. doi: 10.1039/b910206j. [DOI] [PubMed] [Google Scholar]
- 6.Nebel CE, Shin D, Rezek B, Tokuda N, Uetsuka H, Watanabe H. Diamond and biology. J R Soc Interface. 2007;4:439–461. doi: 10.1098/rsif.2006.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soh KL, Kang WP, Davidson JL, Wong YM, Wisitsoraat A, Swain G, Cliffel DE. CVD diamond anisoptropic film as electrode for electrochemical sensing. Sensors Actuator B. 2003;91:39–45. [Google Scholar]
- 8.Volpe PN, Pernot J, Muret P, Omnes F. High hole mobility in boron-doped diamond for power device application. Appl Phys Lett. 2009;94:092102-1. [Google Scholar]
- 9.Massarani B, Bourgoin JC, Chrenko RM. Hopping conduction in semiconducting diamond. Phys Rev B. 1978;17:1758–1769. [Google Scholar]
- 10.May PW, Ludlow WJ, Hannaway M, Heard PJ, Smith JA, Rosser KN. Raman and conductivity studies of boron-doped microcrystalline diamond, facetted nanocrystalline diamond and cauliflower diamond films. Diam Relat Mater. 2008;17:105–107. [Google Scholar]
- 11.Gorkov LP, Larkin AI, Khmelnitskii DE. Particle conductivity in a two-dimensional random potential. JETP Lett. 1979;30(4):228–232. [Google Scholar]
- 12.Henning PF, Homes CC, Maslov S, Carr GL, Basov DN, Nikolic B, Strongin M. Infrared Studies of the Onset of Conductivity in Utrathin Pb Films. Phys Rev Lett. 1999;83(23):4880–4883. [Google Scholar]
- 13.Ziman JM. Principles of the theory of solids. Cambridge: Cambridge Press; 1964. [Google Scholar]
- 14.Allen PB, Beaulac TP, Khan FS, Butler WH, Pinski FJ, Swihart JC. Dc transport in metals. Phys Rev B. 1986;34:4331–4333. doi: 10.1103/physrevb.34.4331. [DOI] [PubMed] [Google Scholar]
- 15.Dordevic SV, Basov DN. Electrodynamics of correlated electron matter. Ann Phys. 2006;15(7–8):545–570. [Google Scholar]
- 16.Gurvitch M, Fiory AT. Resistivity of La1.825Sr0.175CuO4 and YBa2Cu3O7 to 1100 K: absence of saturation and its implications. Phys Rev Lett. 1987;59:1337–1339. doi: 10.1103/PhysRevLett.59.1337. [DOI] [PubMed] [Google Scholar]
- 17.Boppart H, Straaten JV, Silvera IF. Raman spectra of diamond at high pressures. Phys Rev B. 1985;32:1423–1425. doi: 10.1103/physrevb.32.1423. [DOI] [PubMed] [Google Scholar]
- 18.Wang WL, Liao K, Gao J, Liu A. Internal stress analysis in diamond films formed by d.c. plasma chemical vapour deposition. Thin Solid Films. 1992;215:174–178. [Google Scholar]
- 19.Wang WL, Polo MC, Sanchez G, Cifre J, Esteve J. Internal stress and strain in heavily boron-doped diamond films grown by microwave plasma and hot filament chemical vapor deposition. J Appl Phys. 1996;80(3):1846–1850. [Google Scholar]
- 20.Tinkham M. Introduction to superconductivity. New York: McGraw- Hill; 1975. p. 70. [Google Scholar]
- 21.Glover RE, III, Tinkham M. Conductivity of Superconducting Films for Photon Energies between 0.3 and 40kTc. Phys Rev Lett. 1957;108:243–256. [Google Scholar]
- 22.Liu HL, Quijada M, Romero BD, Tanner DB, Zibold A, Carr GL, Berger H, Forro L, Mihaly L, Cao G, Markert JT, Rice JP, Burns MJ, Delin KA. Drude behavior in the far-infrared conductivity of cuprate superconductors. Ann Phys. 2006;15(7–8):606–618. [Google Scholar]
- 23.Ramamurti R, Becker M, Schuelke T, Grotjohn T, Reinhard D, Asmussen J. Synthesis of boron-doped homoepitaxial single crystal diamond by microwave plasma chemical vapor deposition. Diam Relat Mater. 2008;17:1320–1323. [Google Scholar]
- 24.Lagrange JP, Deneuville A, Gheeraert E. Activation energy in low compensated homoepitaxial boron-doped diamond films. Diam Relat Mater. 1998;7:1390–1393. [Google Scholar]
- 25.Teraji T, Wada M, Yamamoto M, Arima K, Ito T. Highly efficient doping of boron into high-quality homoepitaxial diamond films. Diam Relat Mater. 2006;15:602–605. [Google Scholar]
- 26.Bennet KE, Lee KH, Kruchowski JN, Chang SY, Marsh MP, Van Orsow AA, Paez A, Manciu FS. Development of conductive boron-doped diamond electrode: a microscopic. Spectroscopic, and Voltammetric Study, Materials. 2013;6:5726–5741. doi: 10.3390/ma6125726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernard M, Baron C, Deneuville A. About the origin of the low wave number structures of the Raman spectra of heavily boron doped diamond films. Diam Relat Mater. 2004;13:896–899. [Google Scholar]