Abstract
FGF signaling is essential for mammary gland development, yet the mechanisms by which different members of the FGF family control stem cell function and epithelial morphogenesis in this tissue are not well understood. Here, we have examined the requirement of Fgfr2 in mouse mammary gland morphogenesis using a postnatal organ regeneration model. We found that tissue regeneration from basal stem cells is a multistep event, including luminal differentiation and subsequent epithelial branching morphogenesis. Basal cells lacking Fgfr2 did not generate an epithelial network owing to a failure in luminal differentiation. Moreover, Fgfr2 null epithelium was unable to undergo ductal branch initiation and elongation due to a deficiency in directional migration. We identified FGF10 and FGF2 as stromal ligands that control distinct aspects of mammary ductal branching. FGF10 regulates branch initiation, which depends on directional epithelial migration. By contrast, FGF2 controls ductal elongation, requiring cell proliferation and epithelial expansion. Together, our data highlight a pleiotropic role of Fgfr2 in stem cell differentiation and branch initiation, and reveal that different FGF ligands regulate distinct aspects of epithelial behavior.
Keywords: Fibroblast growth factor, Branching morphogenesis, Collective epithelial migration, Stem cell, Mammary gland, Breast, FGFR2, FGF10
INTRODUCTION
An outstanding question in developmental biology is how different signaling pathways regulate diverse aspects of organ development. The presence of multiple members of a gene family, which are often functionally redundant, and the requirement of an essential gene at different stages of organ development pose a challenge to understanding the molecular basis of vertebrate organogenesis. The mouse mammary gland has become a powerful model for delineating the molecular basis of fundamental developmental processes, including epithelial differentiation and morphogenesis (Lu and Werb, 2008). Consistent with its relatively late emergence during metazoan evolution, the formation of the mammary gland depends on many of the same molecules; for example, components of the WNT and BMP signaling pathways and major transcription factors essential for the ontogeny of other body appendages (Mikkola and Millar, 2006).
Signaling via fibroblast growth factors (FGFs) is another major pathway essential for mammary gland biology (Dillon et al., 2004). The mammalian FGF family consists of 22 members, the majority of which function by binding with low affinity to heparan sulfate and with high affinity to their cognate receptors FGFR1-4 (Itoh and Ornitz, 2008). Binding of paracrine FGFs to their receptors causes receptor dimerization, phosphorylation, and activation via one or more of several downstream signaling sub-branches, including signaling via Ras-Raf-MEK and PI3K (Turner and Grose, 2010). FGF has roles in both breast cancer and mammary gland development. Excessive FGF signaling due to overactive ligand or receptor causes breast tumors (Peters et al., 1983; Welm et al., 2002; Xian et al., 2005, 2007). FGFR2 upregulation as a result of allelic polymorphism is associated with human breast cancer, suggesting a causal role of excessive FGFR2 activities in the disease (Hunter et al., 2007; Meyer et al., 2008). By contrast, a reduction in FGF signaling due to removal of Fgf10 or its receptor Fgfr2 results in a failure to form mammary placodes during embryogenesis (Mailleux et al., 2002; Kim et al., 2013).
We have previously shown that during postnatal mammary gland development a reduction of FGF signaling by conditional removal of Fgfr1 or Fgfr2 severely compromises ductal branching (Lu et al., 2008; Parsa et al., 2008; Pond et al., 2012). Genetic mosaic analysis has indicated that Fgfr2 null cells are outcompeted by wild-type cells when they co-exist during postnatal branching (Lu et al., 2008). Moreover, although Fgfr2 null cells contribute to the branching network of mammary ducts in a mosaic environment, they could not regenerate the mammary gland when a pure population of mutant cells was transplanted into the fat pad. Although these studies have revealed that Fgfr2 promotes cell proliferation at the invasion front, the precise function of Fgfr2 in postnatal mammary development and homeostasis has remained largely elusive. In this study, we have used a combination of in vivo and in vitro models to examine Fgfr2 functions in the developing mouse mammary gland.
RESULTS
Mammary epithelial cells lacking Fgfr2 have reduced self-renewal ability and fail to regenerate the gland
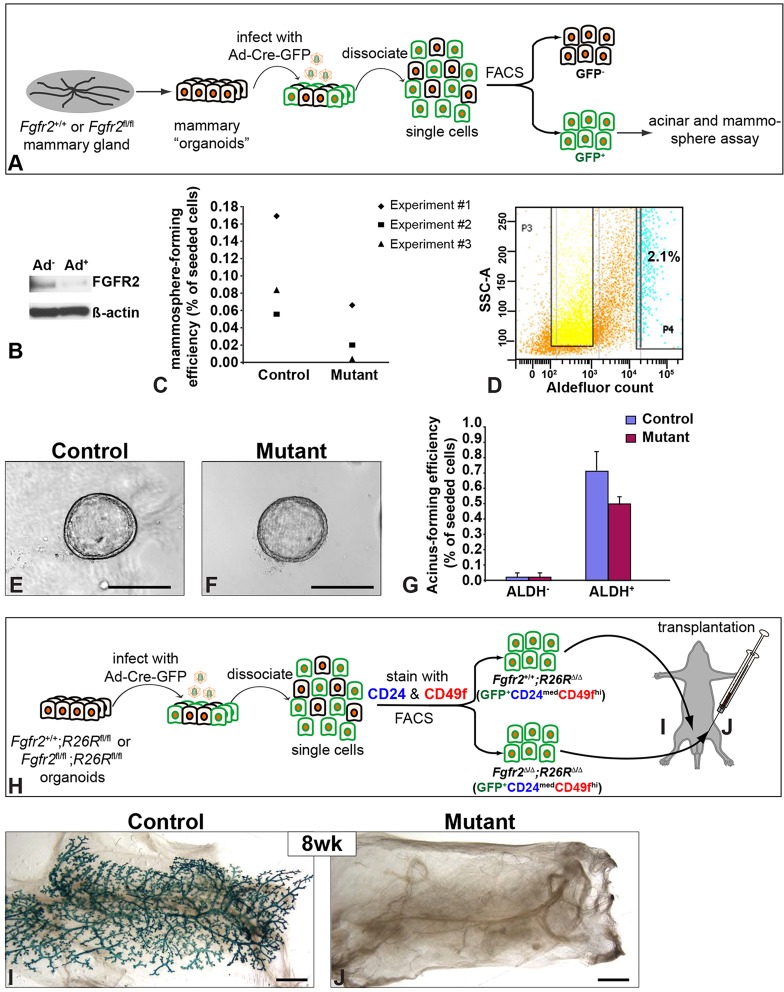
Our previous study showing that FGFR2 is present in basal mammary epithelial cells (MECs) suggests that it plays a role in regulating mammary gland stem cell biology. We examined whether FGFR2 removal affects stem cell self-renewal using the ‘mammosphere’-forming assay, which measures the ability of mammary stem cells to form spheres in suspension (Moraes et al., 2007). We removed the Fgfr2 gene by infecting MECs from either Fgfr2+/+ or Fgfr2fl/fl mice with adenovirus-Cre-GFP to generate control cells (Fgfr2+/+) or mutant (Fgfr2Δ/Δ) cells (Fig. 1A) (Lu et al., 2008). About 80% of the MECs were transduced by adenovirus, as indicated by GFP expression. Immunoblotting revealed that FGFR2 protein levels were greatly reduced in samples that had been infected for 24 h (Fig. 1B). MECs lacking Fgfr2 showed ∼60% reduction in their ability to form primary mammospheres, indicating partially reduced self-renewal ability (Fig. 1C).
Fig. 1.
Mammary epithelial cells lacking Fgfr2 show reduced self-renewal activity and are unable to regenerate the mammary gland. (A) The experimental procedure of sample preparation, adenoviral infection and FACS (for details see the Materials and Methods). (B) Western blotting analysis of FGFR2 expression by mammary gland organoids with (Ad+) or without (Ad−) 24 h infection by adenovirus-Cre-GFP. About 80% of mammary epithelial cells (MECs) were transduced and express GFP protein, whereas ∼20% of MECs remained uninfected, leading to the residual expression of FGFR2 in experimental samples. (C) Quantitative analysis of mammosphere-forming efficiency by control or mutant MECs during primary mammosphere formation. Data were from three independent analyses. (D-G) ALDH+ stem/progenitor cells lacking Fgfr2 function had reduced acini-forming efficiency in a 3D Matrigel assay. ALDH turns a colorless substrate into a green fluorescent product, a reaction that can be blocked by the inhibitor DEAB. Unlike ALDH− cells, ALDH+ progenitor cells that were control (Fgfr2+/+, E) or mutant (Fgfr2Δ/Δ, F) had a relatively high efficiency in forming acini after 15 days culture. (G) Quantitative comparisons of acini-forming efficiency in MECs of different genotypes. Values shown are the mean±s.d. (H) The experimental procedure of basal cell (CD24med CD49fhi) enrichment and transplantation. About 10,000 basal cells were injected into the cleared fat pad. (I,J) Assays for β-Gal activity in the mammary glands 8 weeks after basal cell transplantation of either control (Fgfr2+/+;R26RΔ/Δ, I) or mutant (Fgfr2Δ/Δ;R26RΔ/Δ, n=32, J) cells. Scale bars: 100 µm in E,F; 1 mm in I,J.
We also performed an aldefluor assay in which aldehyde dehydrogenase (ALDH), which is mainly expressed in the luminal epithelium, converts a colorless substrate into a green fluorescent product (Fig. 1D) (Ginestier et al., 2007; Eirew et al., 2012). ALDH+ progenitor cells readily formed hollow cysts after 15 days culture on Matrigel, whereas ALDH− cells did not, indicating that the cyst-forming ability of MECs arises from ALDH+ progenitors (Fig. 1E-G). Fgfr2 null MECs showed a minor (28.5%) reduction in cyst-forming efficiency compared with control MECs (Fig. 1G). Thus, mammary stem cells are compromised but not completely inhibited by FGFR2 removal.
To directly examine the role of Fgfr2 in stem cell biology, we purified basal stem cells lacking Fgfr2 and subjected them to the mammary gland regeneration assay (Fig. 1H). To eliminate the possibility of contamination by control cells, which have a competitive advantage over mutant cells in a mosaic environment, we crossed Fgfr2fl mice with the R26Rfl reporter allele to mark cells transduced by β-Gal adenovirus (Soriano, 1999). MECs were isolated from Fgfr2+/+;R26Rfl/fl and Fgfr2fl/fl;R26Rfl/fl mice, and infected with adenovirus-Cre-GFP to generate control (Fgfr2+/+;R26RΔ/Δ) and mutant (Fgfr2Δ/Δ;R26RΔ/Δ) cells, respectively. Infected MECs were enriched for basal cells (GFP+ CD24med CD49fhi) and transplanted into cleared fat pads of immune-deficient, nude mice (Fig. 1H). Control basal cells readily regenerated an epithelial network (Fig. 1I). However, Fgfr2 mutant basal cells were unable to regenerate a mammary tree (n=32; Fig. 1J).
The complete inability of MECs lacking Fgfr2 to regenerate the gland could not be explained by a reduction in the self-renewal ability of stem cells (Fig. 1A-G). Instead, the data suggest that Fgfr2 plays a hitherto undefined role in mammary gland regeneration from basal cells.
Fgfr2 function is required for luminal cell differentiation from basal stem cells
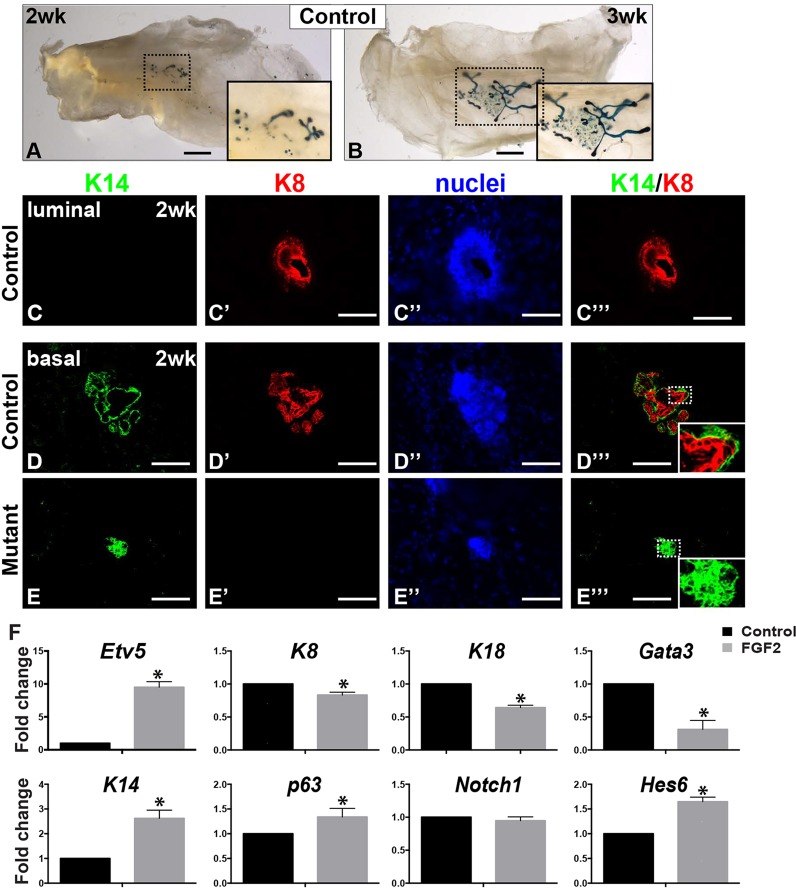
The inability of Fgfr2 null basal cells to regenerate mammary gland could result from a failure to produce luminal cells, a major component of the epithelium; alternatively, Fgfr2 null basal cells could generate luminal cells but their derivatives may not be able to undergo branching morphogenesis. To test these possibilities, we characterized the early morphological changes that basal Fgfr2-deleted cells undergo after transplantation in vivo. Transplants derived from 10,000 control basal cells initially grew in size and started forming small branches by 2 weeks, becoming further elaborated after 3 weeks (Fig. 2A,B). No epithelial outgrowths were observed from Fgfr2 null basal cells or from control luminal cells (not shown).
Fig. 2.
Fgfr2 function is required for luminal cell differentiation from basal stem cells. (A,B) Analysis of epithelial morphogenesis in recombinant glands derived from control basal cells at 2 (A) or 3 (B) weeks after transplantation. Epithelial branching was revealed by β-Gal assays. No epithelial outgrowth was observed in recombinant glands derived from mutant basal cells (not shown). Note that the outgrowths derived from control basal cells contained both branched and unbranched epithelia at 2 weeks and the branching network became progressively more elaborated 3 weeks after transplantation. (C-E‴) Immunofluorescence using anti-K14 (green) or anti-K8 (red) antibodies on frozen sections of recombinant mammary glands derived from control luminal (Fgfr2+/+;R26RΔ/Δ, C-C‴) and basal cells (D-D‴) or mutant basal cells (Fgfr2Δ/Δ;R26RΔ/Δ, E-E‴) 2 weeks after transplantation. Nuclei were counterstained by DAPI. K14 and K8 mark the basal and luminal epithelium, respectively. Note that control luminal cells did not give rise to K14+ basal cells after transplantation, whereas control basal cells had differentiated into luminal cells as marked by K8 expression, and that the overall architecture, in which basal epithelium encapsulates the luminal epithelium, had been established by this stage (D-D‴). Luminal differentiation was not detected in transplants derived from mutant basal cells (E-E‴). (F) Gene expression in basal stem cells in response to FGF2 (500 ng/ml) treatment after 24 h. Values shown are the mean±s.d. *P<0.05, unpaired two-tailed Student's t-tests. Scale bars: 1 mm in A,B; 100 µm in C-E‴.
Consistent with their inability to regenerate mammary gland, K8-expressing control (wild-type) luminal cells were unable to differentiate into K14-expressing basal cells 2 weeks after transplantation (Fig. 2C-C‴) (Shackleton et al., 2006; Stingl et al., 2006; Van Keymeulen et al., 2011). By contrast, K14-expressing control basal cells gave rise to K8-expressing luminal cells at this stage (Fig. 2D-D‴). Accurate tissue architecture was confirmed by the bilayered organization of K14-expressing basal and K8-expressing luminal epithelium. However, in grafts derived from Fgfr2 null basal cells, there were no luminal cells and the growths remained very small (Fig. 2E-E‴).
These results reveal that Fgfr2 is required for the generation of luminal cells. To determine whether this is due to FGFR2 activating genes involved in luminal differentiation, or cooperating with other pathways, we treated FACS-isolated CD24med CD49fhi basal cells with FGF2 and analyzed the expression of genes in the luminal and basal cell differentiation pathway (Fig. 2F). Expression of Etv5, which is a transcriptional target of FGF signaling, was upregulated by FGF2 (Zhang et al., 2009). Target genes of Notch signaling, including Notch1, which promotes luminal differentiation (Bouras et al., 2008; Yalcin-Ozuysal et al., 2010), were not significantly changed, although Hes6 expression was upregulated (Fig. 2F; data not shown). However, whereas expression of basal markers including K14 (also known as Krt14) and p63 (Trp63) was increased by FGF2 treatment, that of luminal markers including K8 (Krt8), K18 (Krt18) and Gata3 was reduced.
These results show that Fgfr2 is required for mammary stem cells to differentiate into luminal cells. This provides one mechanism to explain why Fgfr2 null basal cells are unable to regenerate a functional mammary gland.
Circumvention of Fgfr2 requirement for luminal differentiation reveals its role in epithelial branching
Fgfr2 function might also be required for other aspects of mammary development, such as epithelial branching. To examine this possibility, we took advantage of the observation that basal and luminal lineages remain separate during both normal development and organ regeneration, and that basal-to-luminal differentiation only occurs if luminal cells are under-represented in the epithelium (Van Keymeulen et al., 2011). We tested whether the requirement of Fgfr2 for luminal differentiation could be circumvented by co-transplanting Fgfr2Δ/Δ luminal cells together with basal cells.
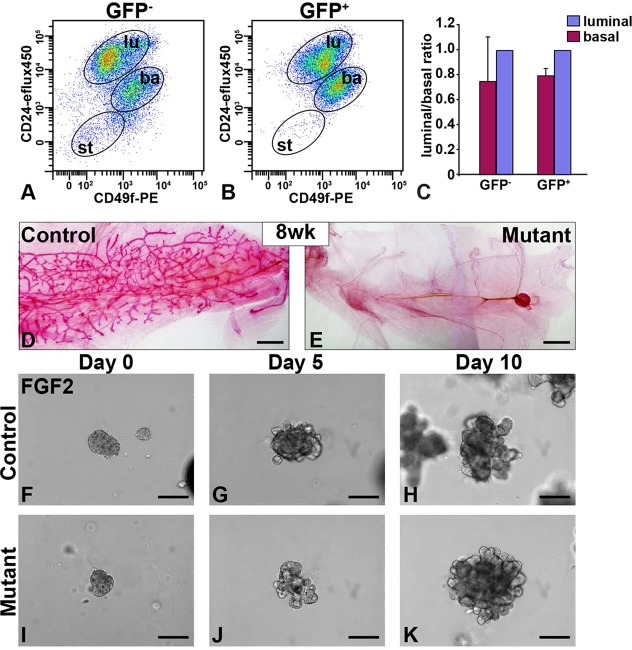
We first determined whether adenovirus-Cre-GFP preferentially infected basal or luminal cells: MECs were infected with adenovirus-Cre-GFP before they were subjected to FACS based on CD24 and CD49f staining. We found that the basal-to-luminal ratio in the uninfected GFP− MECs (0.76±0.34) was similar to that in the infected GFP+ MECs (0.79±0.07; Fig. 3A-C). These data indicate that adenovirus-Cre-GFP does not preferentially infect basal or luminal cells and that Fgfr2Δ/Δ basal and luminal cells would be transplanted in the normal ratio observed under physiological conditions. Next, we transplanted sorted control or Fgfr2 null MECs into the fat pads of nude mice. Based on the above results we concluded that Fgfr2Δ/Δ luminal cells are unlikely to be under-represented when compared with Fgfr2Δ/Δ basal cells, and thus the Fgfr2 null cells would be able to form the mammary epithelial ductal network if luminal differentiation is the only process in mammary gland regeneration that requires Fgfr2 function.
Fig. 3.
MECs lacking Fgfr2 fail to branch in vivo but form branched structures when stimulated by FGF2 in vitro. (A-C) FACS analysis of MECs transduced by adenovirus-Cre-GFP. MECs were sorted based on their expression of integrin α6 (CD49f), CD24 and GFP. CD24med CD49fhi cells were basal, whereas CD24hi CD49flow cells were luminal. The basal-to-luminal ratios were similar between untransduced GFP− MECs (A,C) and transduced GFP+ MECs (B,C), at 0.76±0.34 and 0.79±0.07, respectively. Note that, unlike both luminal (lu) and basal (ba) populations, much of the stromal population (st) that existed in organoid preparations was not transduced well by adenovirus-Cre-GFP. Values shown are the mean±s.d. from at least three independent experiments. (D,E) Epithelial network as revealed by Carmine staining in recombinant mammary glands derived from control (Fgfr2+/+) or mutant (Fgfr2Δ/Δ; n=24) MECs 8 weeks after transplantation. About 10,000 MECs were used for transplantation. (F-K) FGF2-based in vitro branching assay using control (F-H) or mutant (I-K) MEC aggregates. Scale bars: 1 mm in D,E; 100 µm in F-K.
Recombinant glands derived from control MECs readily gave rise to an epithelial tree. However, those from mutant MECs were unable to form a new ductal network (n=24; Fig. 3D,E). The complete inability of Fgfr2 null mammary epithelium, containing both basal and luminal cells, to build an epithelial network could not be explained by a reduction in cell proliferation, which is regulated by Fgfr2 in the terminal end buds (TEBs) (Lu et al., 2008). Rather, the data suggest that Fgfr2 plays additional roles in regulating epithelial branching of the mammary gland.
To further examine the role of Fgfr2 in branching, we used a 3D culture model (Ewald et al., 2008). GFP-expressing control (Fgfr2+/+) and mutant (Fgfr2Δ/Δ) MECs were aggregated, embedded in Matrigel, and cultured in basal medium containing FGF2. Control MEC aggregates remained unbranched without FGF2 (Fig. 4B), but formed nascent epithelial branches after 5 days in the presence of FGF2 and fully branched structures by 10 days (Fig. 3F-H). Surprisingly, the Fgfr2 null MEC aggregates were also able to branch under the same conditions (Fig. 3I-K).
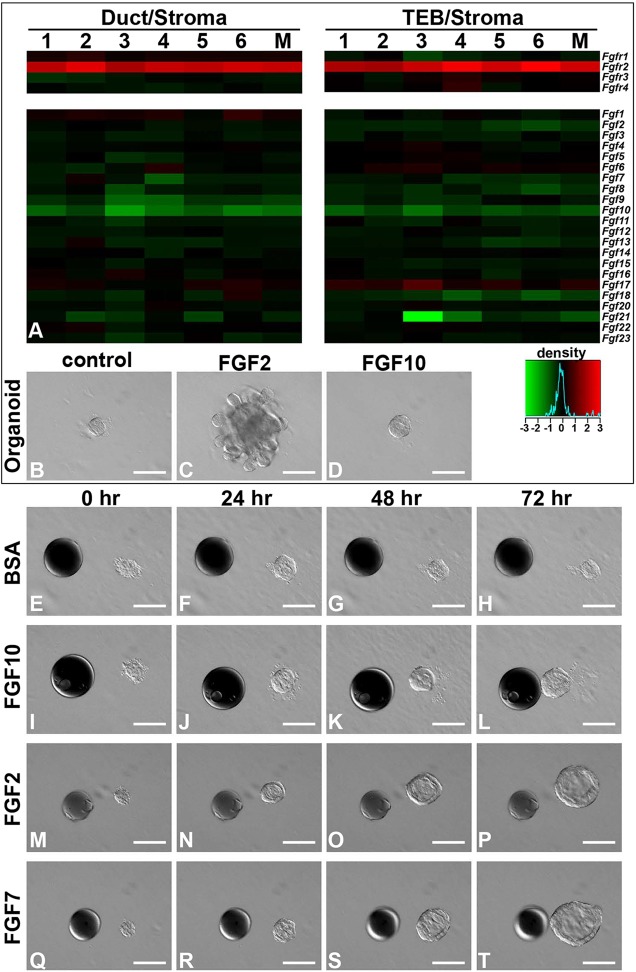
Fig. 4.
FGF10 is a predominant stromal FGF ligand and directs mammary epithelium to undergo collective migration in vitro. (A) Relative expression of FGF genes in 5-week-old mammary glands. Analysis was based on microarray data retrieved from the NCBI Gene Expression Omnibus under accession numbers GSE2988 and GSE5602 (Kouros-Mehr and Werb, 2006). TEB/stroma (Cy5/Cy3) expression ratios and duct/stroma ratios are each shown for six independent experiments (lanes 1-6) and their respective means (M) using a color scale with black indicating no difference in expression, red indicating relative enrichment within the TEB or duct regions and green representing relative enrichment in the pure distal stroma. Intense red indicates a greater than fourfold enrichment in and around TEBs or ducts. (B-D) Differential responses of mammary organoids to basal medium alone (B) or to medium containing either FGF2 (C) or FGF10 (D). Note that FGF2 induced epithelial branching (C) whereas FGF10 did not (D). (E-T) Timecourse of mammary epithelial response to beads pre-soaked in BSA (E-H), FGF10 (I-L), FGF2 (M-P, n=8) or FGF7 (Q-T, n=7). Heparin acrylic beads of ∼100 µm in diameter were juxtaposed with mammary organoids at a distance of ∼100 µm. Scale bars: 100 µm.
These results indicate that FGF2 induces epithelial branching in vitro via a receptor other than FGFR2. Thus, the lack of epithelial branching by Fgfr2 null MECs in vivo is not due to an intrinsic inability of mutant cells to form branched structures.
FGF10 is the predominant stromal FGF ligand during postnatal epithelial branching
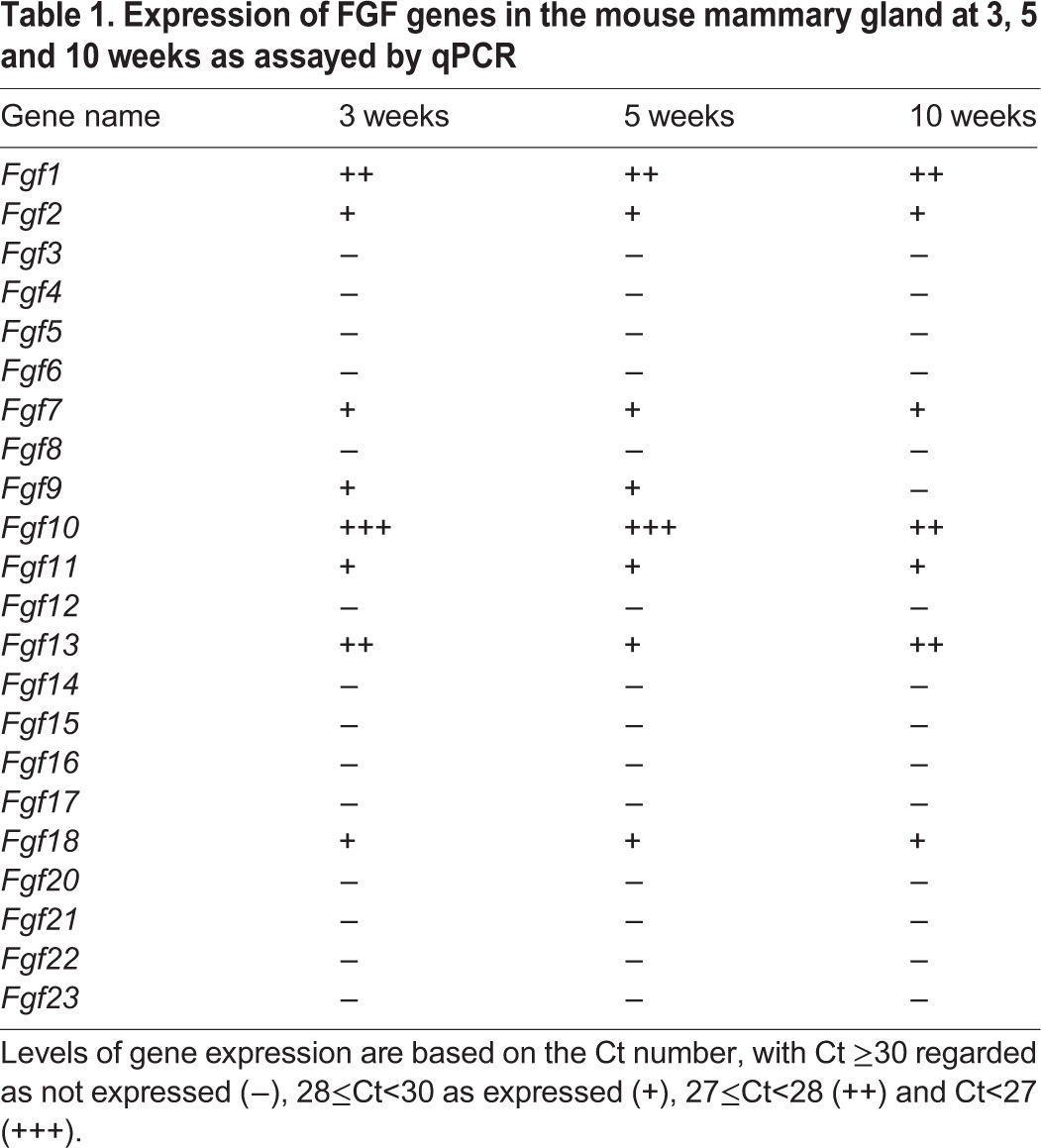
The discrepancy between the requirement of FGFR2 for in vivo and in vitro branching could be explained if ligands other than FGF2 drive morphogenesis during normal development. To examine this possibility, we surveyed a microarray database that compares gene expression between ductal or TEB epithelia with distal stroma in 5-week-old mice (i.e. at a time of rapid branching in vivo) (Kouros-Mehr and Werb, 2006). Fgfr2 is expressed in both the ductal and TEB epithelia (red, Fig. 4A) but not in the stroma. By contrast, FGF genes were detected in the stroma (green, Fig. 4A). Among them, Fgf10 was the most abundantly expressed FGF gene at this developmental stage, although others were also present. qPCR confirmed that Fgf10 was highly expressed during the stages of postnatal mammary ductal branching, and that Fgf2, 7, 9, 13 and 18 were also expressed (Table 1).
Table 1.
Expression of FGF genes in the mouse mammary gland at 3, 5 and 10 weeks as assayed by qPCR
We therefore reasoned that in an in vitro assay FGF10 might influence epithelial branching more than FGF2. Surprisingly, when we replaced FGF2 with FGF10 in the above in vitro branching assay and re-examined the epithelial response, FGF10 did not induce epithelial branching, whereas FGF2 triggered robust epithelial branching from mammary organoids as expected (Fig. 4B-D). These data indicate that mammary epithelium responds differentially to FGF2 and FGF10.
FGF10 and FGF2 trigger distinct epithelial responses when delivered from a point source
In the branching systems of the trachea and air sacs in Drosophila, FGF/Branchless functions as a guidance cue and dictates the branching pattern (Affolter et al., 2003). We therefore reasoned that mammary organoids might only respond to FGF10 when delivered from a point source, rather than uniformly as in the above experiments. To test this, we examined the response of MECs to FGF-soaked beads. When mammary organoids were juxtaposed to beads pre-soaked in BSA, they remained at a relatively constant distance of ∼100 µm from the beads (Fig. 4E-H). By contrast, organoids moved towards FGF10-soaked beads, eventually making contact after 72 h (Fig. 4I-L). However, no epithelial migration was observed towards a point source of FGF2, as would be predicted by the current literature, although the organoids expanded in size and formed a large lumen (Fig. 4M-P; supplementary material Movie 1) (Ewald et al., 2008, 2012). Interestingly, FGF7 induced both cyst formation and movement (Fig. 4Q-T; supplementary material Movie 2). These data reveal that FGF10 promotes epithelial migration and suggest that it functions as a guidance cue for branching.
The differential epithelial responses to FGF2 and FGF10 could result from an insufficient amount of heparan sulfate, which is an essential low-affinity FGF receptor (Makarenkova et al., 2009). If so, then addition of heparan sulfate to the culture system should abolish the differences observed. However, the addition of heparan sulfate had no effect on the response of mammary epithelium to FGF10 (Fig. 5A,B) or FGF2 (Fig. 5C,D). This confirms that the behaviors towards different FGFs are most likely an intrinsic property of the mammary epithelium.
Fig. 5.
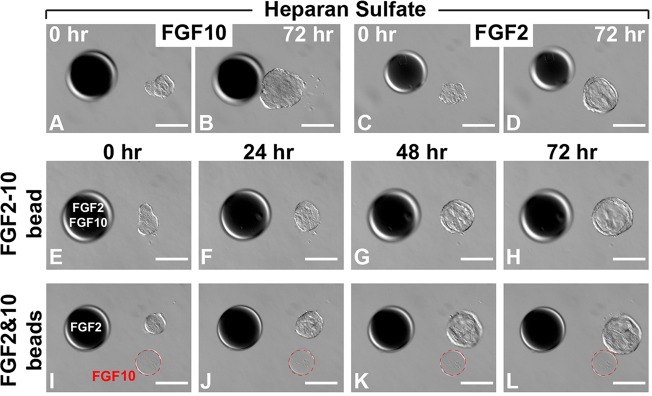
Mammary epithelium responds differentially to localized FGF2 and FGF10. Timecourse of mammary epithelial response toward beads pre-soaked in FGFs in the presence (A-D) or absence (E-L) of heparan sulfate (0.5 µg/ml). (E-L) FGF2 and FGF10 were delivered simultaneously to mammary epithelium either in the same bead (E-H) or in separate beads (I-L); the FGF10 beads are circled (dashed red line). Heparin acrylic beads of ∼100 µm in diameter were juxtaposed with mammary organoids at a distance of ∼100-150 µm. Scale bars: 100 µm.
As an initial step toward developing a complex system that mimics the in vivo stromal environment, in which multiple stromal FGF ligands co-exist, we examined how the presence of FGF2 and FGF10, either delivered together in the same position or separately in different positions, affected epithelial responsiveness. When delivered together in the same position, FGF2 and FGF10 triggered a similar response to that from FGF2 alone (Fig. 5E-H). However, FGF10 triggered directional migration when the ligands were delivered from separate positions (Fig. 5I-L).
Thus, FGF2 and FGF10 elicit distinct responses from mammary epithelium in vitro, suggesting that they regulate different aspects of epithelial branching in vivo. Our data also reveal that the spatial control of ligand delivery influences epithelial behavior. This shows that the spatiotemporal regulation of ligand expression in the postnatal mammary gland stroma is an important determinant of epithelial branching pattern.
Directional migration of stratified epithelium is not a result of localized cell proliferation or death and lacks an obvious front-rear polarity
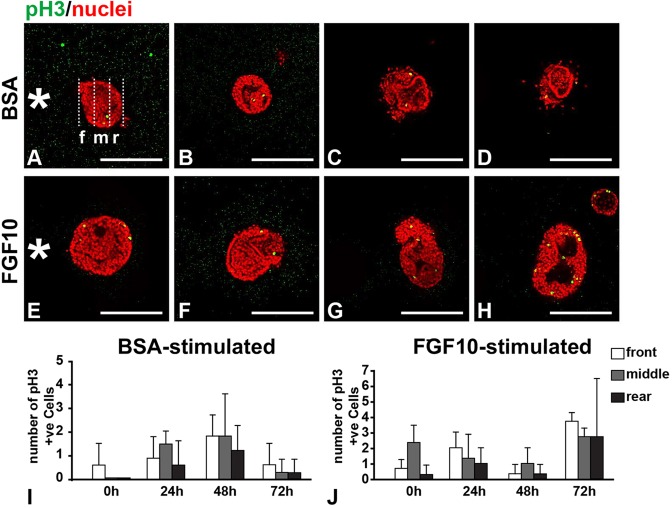
The apparent movement of mammary organoids towards FGF10 beads could result from localized growth and/or apoptosis at the front or rear of the epithelium, or alternatively from collective epithelial migration. To examine these possibilities, we used phospho-histone H3 and Lysotracker staining to detect proliferating and dying cells, respectively (Lu et al., 2006). No localized cell proliferation occurred preferentially in the front region of the mammary organoids (Fig. 6A-J), and similarly there was no preferential death in the rear of mammary organoids juxtaposed with either BSA- or FGF10-soaked beads (supplementary material Fig. S1A-J).
Fig. 6.
Directional migration of stratified epithelium is not a result of localized cell proliferation. (A-H) Cell proliferation as detected by phospho-histone 3 (pH3) immunofluorescence (green) during the timecourse of mammary epithelial migration toward beads soaked in BSA (A-D) or FGF10 (E-H). Cell proliferation was quantified in one of the three evenly divided regions of an organoid – the front (f), middle (m) or rear (r) – depending on the distance between the region and the bead (asterisk). Scale bars: 100 µm. (I,J) Quantification of cell proliferation in different regions of mammary organoids during epithelial migration. Only signals that overlap with nuclei were counted as proliferating cells, and background noise was discounted. No statistically significant differences were apparent among the different regions at the times indicated (unpaired two-tailed Student's t-test). Values shown are the mean±s.d. from at least three independent experiments.
Instead, time-lapse video recording revealed that mammary organoids underwent directional migration toward FGF10-soaked beads (supplementary material Movies 3 and 4). High-magnification imaging showed that the epithelium moved as a coherent entity during the course of its migration (supplementary material Movie 5). We saw no evidence of the existence of lamellipodia, filopodia and other cellular processes that are evident in other systems undergoing directional migration (Friedl and Gilmour, 2009; Ilina and Friedl, 2009). Moreover, we did not detect a front-rear difference in the presence of basal cells, which enveloped luminal cells (supplementary material Fig. S2A-G″). Nor did we observe differential localization of the tissue polarity markers β1-integrin, E-cadherin and ZO-1 (also known as TJP1) (supplementary material Fig. S2H-I″,L-M″). Expression of none of the marker genes examined was affected by whether the bead was pre-soaked in BSA or FGF10 (Fig. 2B-E″; data not shown). Interestingly, E-cadherin was found on all of the cell surfaces in the epithelium (supplementary material Fig. S2J-K″), rather than only on the basal-lateral surfaces, as would be expected for a simple epithelium. Thus, there is a partial loss of tissue polarity in the mammary epithelium during directional migration, presumably as a feature of stratified epithelium.
Collective epithelial migration depends on MEK and PI3K signaling and on MMP activities
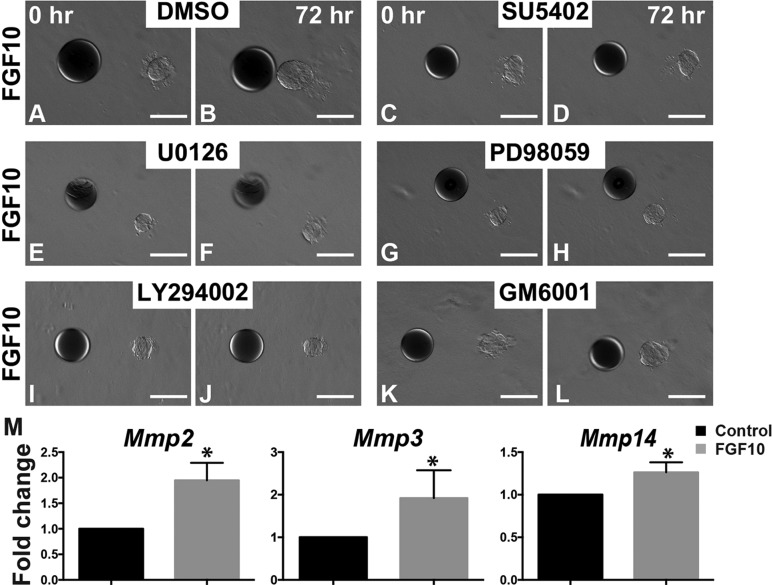
To understand the molecular basis of the directional migration of the stratified epithelium, we examined the effect of several pharmacological inhibitors on the FGF10-induced migration. SU5402, an FGFR inhibitor, blocked the movement of mammary organoids toward FGF10 beads (Fig. 7A-D), confirming that collective epithelial migration requires this FGF. Similarly, the MEK inhibitors U0126 and PD98059 and the PI3K inhibitor LY294002 all inhibited MEC collective migration (Fig. 7E-J). To determine whether these two sub-branches of FGF signaling work independently or cooperatively, we serially diluted the MEK and PI3K inhibitors and found that concentrations that on their own did not affect epithelial migration worked in combination to either completely (1 of 4 samples) or partially (3 of 4 samples) block migration (supplementary material Table S2).
Fig. 7.
FGF10-induced directional epithelial migration depends on MEK/ERK and PI3K/AKT signaling and on MMP activities. (A-L) Timecourse of mammary epithelial migration toward beads soaked in FGF10 in the absence (A,B) or presence (C-H) of various pharmacological inhibitors. Inhibition of signaling via FGFR, MEK or PI3K was achieved using media containing SU5402 (20 μM, C,D), U0126 (2.0 μM, E,F), PD98059 (20 μM, G,H) and LY294002 (200 μM, I,J). Note that collective epithelial migration was blocked in the presence of these inhibitors. GM6001 (100 μM, K,L) was used as a general inhibitor against MMP activities. Collective epithelial migration was only partially blocked in the presence of GM6001; higher concentrations of GM6001 were without additional effect on migration but caused cell death (not shown). Scale bars: 100 µm. (M) qPCR analysis of the expression of several MMP genes that play a role in mammary epithelial branching in response to FGF10 (500 ng/ml) treatment after 24 h. Values are the mean±s.d. *P<0.05, unpaired two-tailed Student's t-tests.
The pan-matrix metallopeptidase (MMP) inhibitor GM6001 partially inhibited epithelial migration (Fig. 7K-L), suggesting that MMP activities play a role in this process. To determine whether FGF signaling regulates MMP activities at the transcriptional level, we analyzed MMP gene expression (Mori et al., 2013; Wiseman et al., 2003) and found that Mmp2, Mmp3 and Mmp14 were upregulated in response to FGF10 treatment (Fig. 7M).
Together, these results show that directional migration of the mammary epithelium depends on MEK/ERK and PI3K/AKT signaling, which cooperate synergistically. Moreover, MMP activities promote epithelial migration and this is in part due to upregulation of MMP gene expression by FGF signaling.
Directional migration of mammary epithelium requires Fgfr2 function
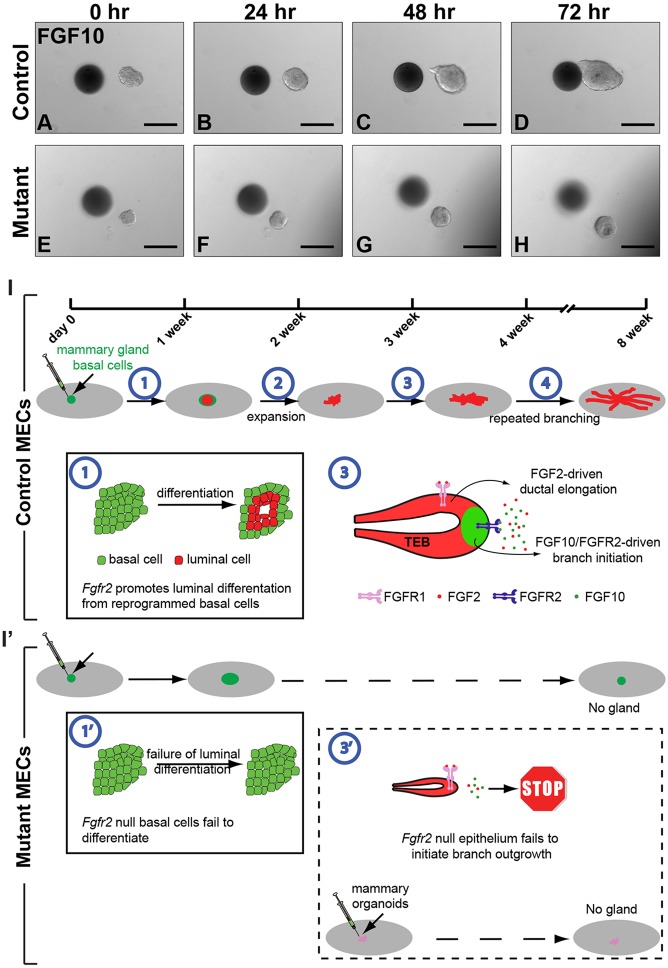
Finally, we hypothesized that the inability of Fgfr2 null epithelium to form a branching ductal network in vivo is due to the inability of the cells to respond to the migratory cues induced by FGF10. Fgfr2+/+ and Fgfr2Δ/Δ MECs were aggregated before being subjected to the in vitro migration assay based on FGF10 stimulation. The control MEC aggregates underwent migration toward FGF10-soaked beads (Fig. 8A-D), whereas the Fgfr2 null aggregates were unable to do so (Fig. 8E-H).
Fig. 8.
Directional migration of mammary epithelium requires Fgfr2 function. (A-H) Timecourse of mammary epithelial migration in response to beads soaked in FGF10. Mammary epithelium of either control (Fgfr2+/+, n=6, A-D) or mutant (Fgfr2Δ/Δ, n=5, E-H) genotype was juxtaposed with FGF10 beads at a distance of ∼100 µm. Note that although Fgfr2Δ/Δ MEC aggregates did not migrate toward FGF10-soaked beads they did grow somewhat in size during culture, as did Fgfr2+/+ MEC aggregates, presumably due to cell proliferation. Scale bars: 100 µm. (I) Model of mammary gland regeneration from basal cell transplantation, which involves at least four stages. (1) FGFR2-dependent luminal cell differentiation from basal cells. (2) Expansion of mammary epithelium composed of both basal and luminal cells. (3) Epithelial budding and ductal elongation. FGF10-FGFR2 function via both MEK and PI3K signaling is essential for collective epithelial migration and branch initiation. By contrast, ductal elongation is primarily driven by FGF2 function in an FGFR2-independent manner. (4) Repeated branching morphogenesis until the mammary fat pad is filled and a functional mammary gland is generated. (1′) In the absence of FGFR2 function, at least two of the above four steps fail to occur: Fgfr2 null basal cells fail to differentiate into luminal cells and, consequently, a functional gland fails to regenerate; (3′) when Fgfr2Δ/Δ basal and luminal cells are transplanted, epithelial migration does not occur, leading to failure in epithelial invasion, budding and thus branch initiation. As a result, a functional gland fails to regenerate.
Thus, the Fgfr2 gene is required for collective epithelial migration induced by FGF10, thereby providing a mechanism to explain the role of FGFR2 in the formation of mammary epithelial ducts in vivo.
DISCUSSION
In the current study, we circumvented the functional requirement of Fgfr2 for embryonic development and studied its various roles during mammary gland formation. We found that mammary gland regeneration from basal cells is a multistep event, including luminal differentiation and subsequent epithelial branching, both of which depend on Fgfr2 function (Fig. 8I). As a result of Fgfr2 deletion, the cells fail to regenerate the mammary gland due to both an inability to generate luminal cells and a failure in directional migration, which is essential for branch initiation and epithelial patterning. We identified FGF10 and FGF2 as two stromal FGFs in the pubertal gland. FGF10 is a guidance cue to direct branch outgrowth, whereas FGF2 provides the driving force for epithelial expansion and ductal elongation. Together, our data highlight the pleotropic roles of Fgfr2 during mammary gland regeneration and underscore the novel phenomenon that FGF ligands regulate distinct aspects of mammary epithelial morphogenesis.
FGFR2 regulates stem cell biology during mammary gland regeneration
Our data show that, whereas basal cells can give rise to luminal cells, the reciprocal process does not occur. These data are consistent with, and provide a functional explanation for, the well-documented observation that basal cells, but not luminal cells, can regenerate the mammary gland when transplanted into the cleared fat pad (Shackleton et al., 2006; Stingl et al., 2006). At present, it remains unclear whether luminal cell differentiation in the transplantation model shares a similar or distinct molecular basis with the in vivo event during normal development and whether what we learn from the former event could be applied to understanding the latter process.
We show that Fgfr2 regulates at least two aspects of stem cell biology in the mammary gland. First, Fgfr2 null cells have a reduced ability to form acini or mammospheres when compared with wild-type cells, suggesting that Fgfr2 promotes self-renewal in adult stem cells of the mammary gland. Second, unlike wild-type basal cells, Fgfr2 null basal cells are unable to differentiate into luminal cells. We conclude that failure in the generation of luminal cells is a cause of the inability to regenerate mammary gland from Fgfr2 null basal cells (Fig. 8I′).
FGF signaling has been shown to regulate cell differentiation in various other contexts, including the differentiation of embryonic stem cells and lens fiber (Zhao et al., 2008; Stavridis et al., 2010). Interestingly, however, FGF2 treatment of mammary basal cells does not upregulate the expression of luminal markers or target genes of Notch signaling, which promotes luminal differentiation (Bouras et al., 2008; Yalcin-Ozuysal et al., 2010). These data suggest that Fgfr2 does not directly promote luminal differentiation at the RNA level. Future studies aimed at addressing the mechanism by which Fgfr2 regulates luminal differentiation could potentially be facilitated by the development of an appropriate in vitro model in which basal cell differentiation occurs.
Mammary epithelium responds differentially to FGF2 and FGF10 stimulation
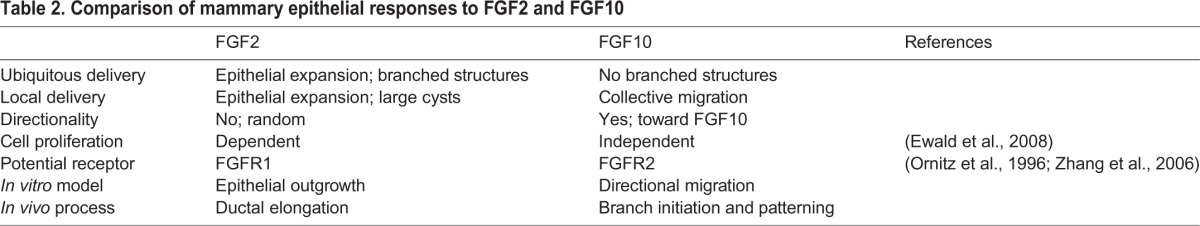
We show that FGF2 and FGF10 elicit distinct responses from the mammary epithelium (Table 2). FGF2 triggers mostly epithelial expansion and the formation of branched structures when delivered ubiquitously or large cysts when delivered locally. By contrast, FGF10 does not trigger an obvious epithelial expansion but instead elicits a conspicuous collective movement of the epithelium. Interestingly, similar differential responses from different FGF ligands have been reported in other developmental settings. For example, FGF4 attracts whereas FGF8 repels gastrulating cells in the early chick embryo (Yang et al., 2002). The basis for such differential responses, however, has remained unknown.
Table 2.
Comparison of mammary epithelial responses to FGF2 and FGF10
The distinct responsiveness of mammary epithelium to FGF2 and FGF10 was unaffected by heparan sulfate, suggesting that it results from intrinsic differences in the downstream events triggered by the ligands. Indeed, FGF2-mediated branch formation is driven by a high rate of cell proliferation, which when inhibited causes a failure in epithelial expansion and, presumably, ductal elongation (Fata et al., 2007; Ewald et al., 2008). This is in contrast to our data showing that cell proliferation is not preferentially localized to the front or rear of the epithelium and thus is unlikely to be a driving force for migration.
Furthermore, FGF2-based epithelial expansion does not depend on Fgfr2 function, whereas FGF10-based directional migration does. Although it has remained unclear, the receptor that FGF2 activates is likely to be FGFR1, which like FGFR2 is expressed in the epithelium and plays a role in mammary branching (Lu et al., 2008; Pond et al., 2012). Interestingly, there is evidence to suggest that FGF2 and FGF10 preferentially activate FGFR1 and FGFR2, respectively (Ornitz et al., 1996; Zhang et al., 2006). Therefore, the distinct responses of mammary epithelium to FGF2 and FGF10 could, at least in part, be ascribed to differential binding to FGF receptors.
FGF ligands regulate distinct aspects of epithelial branching and coordinate organ development
Our data show that the FGF2-based in vitro system, which has been used to study various aspects of epithelial branching, is not suitable for the analysis of collective epithelial migration as has been suggested in the literature (Nguyen-Ngoc et al., 2012). This is because, despite the dynamic movement of individual cells, as a collective group cells do not move in a certain direction. Rather, the FGF2 model is most useful for understanding other aspects of branching morphogenesis, including cell rearrangements and shape changes during epithelial expansion and ductal elongation, an event that depends on cell proliferation (Table 2). By contrast, the FGF10-based system is best suited for understanding the basis of directional migration of stratified epithelium, which most likely employs a very different strategy than that used by single cells, simple epithelial cells or loosely connected fibroblasts, where differences along the front-rear axis appear to be important for directional movement (Friedl and Gilmour, 2009; Ilina and Friedl, 2009). Interestingly, our analysis has detected no such differences in the presence of lamellipodia, filopodia and other cellular extensions or any difference in tissue polarity between the front and rear of the organoid epithelium.
Despite the clear differences between the directional migration of stratified mammary epithelium and that of individual cells in the Drosophila trachea and air sac systems, they share some interesting similarities. In the fly systems, for example, the direction of cell migration and branching pattern is controlled by FGF signaling (Affolter et al., 2003). In the absence of FGF signaling, owing to a lack of ligand (Branchless/FGF) or its receptor (Breathless/FGFR), cell migration does not occur, branches fail to initiate and, consequently, the tracheal and air sac systems fail to develop (Sutherland et al., 1996; Sato and Kornberg, 2002). In the mammary gland, our data show that epithelial cells lacking Fgfr2 fail to undergo epithelial migration, which most likely leads to a failure of branch initiation, as observed in the fly system, and subsequently to the failure to form an epithelial branching tree.
Taken together, our working model is that FGF2 and FGF10 regulate distinct aspects of epithelial branching during postnatal mammary gland development. FGF10 regulates the branch initiation process, which depends on directional epithelial migration, and FGF2 controls the ductal elongation process, which depends on cell proliferation and epithelial expansion. Remarkably, similar situations have been observed in the fly air sac system. Although cell migration and branch initiation are controlled by FGFR signaling, cell proliferation and branch outgrowth are governed by a different receptor tyrosine kinase, EGFR (Cabernard and Affolter, 2005). In both cases, therefore, whether it is FGFR and EGFR in the fly or FGFR1 and FGFR2 in the mammary gland, members of the RTK family regulate distinct epithelial behaviors during branching morphogenesis (Xian et al., 2007).
MATERIALS AND METHODS
Mouse strains
Immunologically deficient female nude (nu/nu) mice were from Charles River Laboratories. Mice carrying the R26Rfl Cre-reporter allele were purchased from the Jackson Laboratory (Soriano, 1999). Mice carrying the Fgfr2fl allele were provided by Dr David Ornitz (Yu et al., 2003). Offspring from crosses of the various lines were genotyped according to methods given in the publications describing the mouse lines. Mice were housed and maintained according to the University of Manchester and UK Home Office guidelines for animal research.
Preparation of mammary gland epithelial cells, adenovirus infection and transplantation
Preparation of MECs was as described (Zhang et al., 2014). Mammary gland fat pads of nude mice at 3 weeks of age were cleared based on an established protocol (Lu et al., 2008). About 10,000 MECs were injected into the cleared fat pad and harvested after 8 weeks.
Fluorescence activated cell sorting (FACS) and quantitative real-time PCR
Single cells were dissociated and labeled by infection using adenovirus-Cre-GFP or by antibody staining. Antibodies against CD24 (FITC; eBioscience, 48-0242), CD49f (PE; eBioscience, 12-0495) and CD45 (PeCy7; BD Biosciences, 552848) were used together with 7-AAD (BD Biosciences) to sort for luminal and basal populations using Aria and FACSCalibur systems (BD Biosciences). Data were processed using FACSDiva software (BD Biosciences). qPCR was performed using the 7500 Fast Real-Time PCR system (Applied Biosystems) and data were normalized to the expression of at least two reference genes, which included Actb, Rn18s, Eef1g and Gapdh (supplementary material Table S1).
In vitro epithelial branching and migration assays
Branching and epithelial invasion assays and live imaging were as described (Ewald et al., 2008; Zhang et al., 2014). FGF2 and FGF10 (2.5 nM; Sigma) were used in the 7- to 10-day culture. Primary antibodies were against smooth muscle actin (Sigma, 1A4, C6198), K8 (DSHB, Troma-1), K14 (Covance, PRB-155P), phospho-histone H3 (Upstate, 06-570), β1-integrin (Millipore, MAB1997), E-cadherin (BD Biosciences, 610181) and ZO-1 (Millipore, MABT11). Samples were counterstained with To-Pro-3 (Invitrogen, T3605) or 4′,6-diamidino-2-phenylindole (DAPI; Sigma, 32670). Assays for cell death using LysoTracker (Molecular Probes, L-7528) staining were as previously reported (Lu et al., 2006).
Statistical data were analyzed using two-factorial analysis of variance (ANOVA), having time and section of organoid as the two factors. Student's t-test was used to compare the number of phospho-histone H3-positive nuclei between control (BSA-soaked beads) and experimental (FGF10-soaked beads) groups.
Data mining of expression microarray
Microarray data were from the NCBI Gene Expression Omnibus under the accession numbers GSE2988 and GSE5602 (Kouros-Mehr and Werb, 2006). Expression of FGF genes was determined by calculating M=log2(Cy5/Cy3), where Cy5 values of TEB/ductal epithelium were compared with Cy3 values of the distal stroma from female mice at 5 weeks of age. TEB/stroma (Cy5/Cy3) expression ratios and duct/stroma ratios are each shown for six independent experiments.
Supplementary Material
Acknowledgements
We thank Dr Yaoyong Li for bioinformatics analyses on FGF gene expression, and Drs Zena Werb, Rob Clark and Karel Dorey for critical reading of the manuscript.
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
X.Z. and P.L. designed the research. X.Z., D.M., Z.K., G.Q. and P.L. performed the experiments. All authors analyzed the data. P.L. wrote the paper.
Funding
This work was supported by grants from the Wellcome Trust [no. 081203/Z/06/Z] to C.H.S.; and the National Institutes of Health [R03 HD060807] and Breakthrough Breast Cancer to P.L. The Wellcome Trust Centre for Cell-Matrix Research, University of Manchester, is supported by core funding from the Wellcome Trust [088785/Z/09/Z]. Deposited in PMC for release after 6 months.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.106732/-/DC1
References
- Affolter M., Bellusci S., Itoh N., Shilo B., Thiery J.-P., Werb Z. (2003). Tube or not tube: remodeling epithelial tissues by branching morphogenesis. Dev. Cell 4, 11-18 10.1016/S1534-5807(02)00410-0 [DOI] [PubMed] [Google Scholar]
- Bouras T., Pal B., Vaillant F., Harburg G., Asselin-Labat M.-L., Oakes S. R., Lindeman G. J., Visvader J. E. (2008). Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell Stem Cell 3, 429-441 10.1016/j.stem.2008.08.001 [DOI] [PubMed] [Google Scholar]
- Cabernard C., Affolter M. (2005). Distinct roles for two receptor tyrosine kinases in epithelial branching morphogenesis in Drosophila. Dev. Cell 9, 831-842 10.1016/j.devcel.2005.10.008 [DOI] [PubMed] [Google Scholar]
- Dillon C., Spencer-Dene B., Dickson C. (2004). A crucial role for fibroblast growth factor signaling in embryonic mammary gland development. J. Mammary Gland Biol. Neoplasia 9, 207-215 10.1023/B:JOMG.0000037163.56461.1e [DOI] [PubMed] [Google Scholar]
- Eirew P., Kannan N., Knapp D. J. H. F., Vaillant F., Emerman J. T., Lindeman G. J., Visvader J. E., Eaves C. J. (2012). Aldehyde dehydrogenase activity is a biomarker of primitive normal human mammary luminal cells. Stem Cells 30, 344-348 10.1002/stem.1001 [DOI] [PubMed] [Google Scholar]
- Ewald A. J., Brenot A., Duong M., Chan B. S., Werb Z. (2008). Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev. Cell 14, 570-581 10.1016/j.devcel.2008.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald A. J., Huebner R. J., Palsdottir H., Lee J. K., Perez M. J., Jorgens D. M., Tauscher A. N., Cheung K. J., Werb Z., Auer M. (2012). Mammary collective cell migration involves transient loss of epithelial features and individual cell migration within the epithelium. J. Cell Sci. 125, 2638-2654 10.1242/jcs.096875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata J. E., Mori H., Ewald A. J., Zhang H., Yao E., Werb Z., Bissell M. J. (2007). The MAPK(ERK-1,2) pathway integrates distinct and antagonistic signals from TGFalpha and FGF7 in morphogenesis of mouse mammary epithelium. Dev. Biol. 306, 193-207 10.1016/j.ydbio.2007.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P., Gilmour D. (2009). Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 10, 445-457 10.1038/nrm2720 [DOI] [PubMed] [Google Scholar]
- Ginestier C., Hur M. H., Charafe-Jauffret E., Monville F., Dutcher J., Brown M., Jacquemier J., Viens P., Kleer C. G., Liu S., et al. (2007). ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 1, 555-567 10.1016/j.stem.2007.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter D. J., Kraft P., Jacobs K. B., Cox D. G., Yeager M., Hankinson S. E., Wacholder S., Wang Z., Welch R., Hutchinson A., et al. (2007). A genome-wide association study identifies alleles in FGFR2 associated with risk of sporadic postmenopausal breast cancer. Nat. Genet. 39, 870-874 10.1038/ng2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilina O., Friedl P. (2009). Mechanisms of collective cell migration at a glance. J. Cell Sci. 122, 3203-3208 10.1242/jcs.036525 [DOI] [PubMed] [Google Scholar]
- Itoh N., Ornitz D. M. (2008). Functional evolutionary history of the mouse Fgf gene family. Dev. Dyn. 237, 18-27 10.1002/dvdy.21388 [DOI] [PubMed] [Google Scholar]
- Kim E.-J., Jung H.-S., Lu P. (2013). Pleiotropic functions of fibroblast growth factor signaling in embryonic mammary gland development. J. Mammary Gland Biol. Neoplasia 18, 139-142 10.1007/s10911-013-9278-4 [DOI] [PubMed] [Google Scholar]
- Kouros-Mehr H., Werb Z. (2006). Candidate regulators of mammary branching morphogenesis identified by genome-wide transcript analysis. Dev. Dyn. 235, 3404-3412 10.1002/dvdy.20978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P., Werb Z. (2008). Patterning mechanisms of branched organs. Science 322, 1506-1509 10.1126/science.1162783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P., Minowada G., Martin G. R. (2006). Increasing Fgf4 expression in the mouse limb bud causes polysyndactyly and rescues the skeletal defects that result from loss of Fgf8 function. Development 133, 33-42 10.1242/dev.02172 [DOI] [PubMed] [Google Scholar]
- Lu P., Ewald A. J., Martin G. R., Werb Z. (2008). Genetic mosaic analysis reveals FGF receptor 2 function in terminal end buds during mammary gland branching morphogenesis. Dev. Biol. 321, 77-87 10.1016/j.ydbio.2008.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailleux A. A., Spencer-Dene B., Dillon C., Ndiaye D., Savona-Baron C., Itoh N., Kato S., Dickson C., Thiery J. P., Bellusci S. (2002). Role of FGF10/FGFR2b signaling during mammary gland development in the mouse embryo. Development 129, 53-60. [DOI] [PubMed] [Google Scholar]
- Makarenkova H. P., Hoffman M. P., Beenken A., Eliseenkova A. V., Meech R., Tsau C., Patel V. N., Lang R. A., Mohammadi M. (2009). Differential interactions of FGFs with heparan sulfate control gradient formation and branching morphogenesis. Sci. Signal. 2, ra55 10.1126/scisignal.2000304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K. B., Maia A.-T., O'Reilly M., Teschendorff A. E., Chin S.-F., Caldas C., Ponder B. A. J. (2008). Allele-specific up-regulation of FGFR2 increases susceptibility to breast cancer. PLoS Biol. 6, e108 10.1371/journal.pbio.0060108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkola M. L., Millar S. E. (2006). The mammary bud as a skin appendage: unique and shared aspects of development. J. Mammary Gland Biol. Neoplasia 11, 187-203 10.1007/s10911-006-9029-x [DOI] [PubMed] [Google Scholar]
- Moraes R. C., Zhang X., Harrington N., Fung J. Y., Wu M.-F., Hilsenbeck S. G., Allred D. C., Lewis M. T. (2007). Constitutive activation of smoothened (SMO) in mammary glands of transgenic mice leads to increased proliferation, altered differentiation and ductal dysplasia. Development 134, 1231-1242 10.1242/dev.02797 [DOI] [PubMed] [Google Scholar]
- Mori H., Lo A. T., Inman J. L., Alcaraz J., Ghajar C. M., Mott J. D., Nelson C. M., Chen C. S., Zhang H., Bascom J. L., et al. (2013). Transmembrane/cytoplasmic, rather than catalytic, domains of Mmp14 signal to MAPK activation and mammary branching morphogenesis via binding to integrin beta1. Development 140, 343-352 10.1242/dev.084236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Ngoc K.-V., Cheung K. J., Brenot A., Shamir E. R., Gray R. S., Hines W. C., Yaswen P., Werb Z., Ewald A. J. (2012). ECM microenvironment regulates collective migration and local dissemination in normal and malignant mammary epithelium. Proc. Natl. Acad. Sci. USA 109, E2595-E2604 10.1073/pnas.1212834109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz D. M., Xu J., Colvin J. S., McEwen D. G., MacArthur C. A., Coulier F., Gao G., Goldfarb M. (1996). Receptor specificity of the fibroblast growth factor family. J. Biol. Chem. 271, 15292-15297 10.1074/jbc.271.25.15292 [DOI] [PubMed] [Google Scholar]
- Parsa S., Ramasamy S. K., De Langhe S., Gupte V. V., Haigh J. J., Medina D., Bellusci S. (2008). Terminal end bud maintenance in mammary gland is dependent upon FGFR2b signaling. Dev. Biol. 317, 121-131 10.1016/j.ydbio.2008.02.014 [DOI] [PubMed] [Google Scholar]
- Peters G., Brookes S., Smith R., Dickson C. (1983). Tumorigenesis by mouse mammary tumor virus: evidence for a common region for provirus integration in mammary tumors. Cell 33, 369-377 10.1016/0092-8674(83)90418-X [DOI] [PubMed] [Google Scholar]
- Pond A. C., Bin X., Batts T., Roarty K., Hilsenbeck S., Rosen J. M. (2012). Fibroblast growth factor receptor signaling is essential for normal mammary gland development and stem cell function. Stem Cells 31, 178-189 10.1002/stem.1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Kornberg T. B. (2002). FGF is an essential mitogen and chemoattractant for the air sacs of the drosophila tracheal system. Dev. Cell 3, 195-207 10.1016/S1534-5807(02)00202-2 [DOI] [PubMed] [Google Scholar]
- Shackleton M., Vaillant F., Simpson K. J., Stingl J., Smyth G. K., Asselin-Labat M.-L., Wu L., Lindeman G. J., Visvader J. E. (2006). Generation of a functional mammary gland from a single stem cell. Nature 439, 84-88 10.1038/nature04372 [DOI] [PubMed] [Google Scholar]
- Soriano P. (1999). Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70-71 10.1038/5007 [DOI] [PubMed] [Google Scholar]
- Stavridis M. P., Collins B. J., Storey K. G. (2010). Retinoic acid orchestrates fibroblast growth factor signalling to drive embryonic stem cell differentiation. Development 137, 881-890 10.1242/dev.043117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl J., Eirew P., Ricketson I., Shackleton M., Vaillant F., Choi D., Li H. I., Eaves C. J. (2006). Purification and unique properties of mammary epithelial stem cells. Nature 439, 993-997. [DOI] [PubMed] [Google Scholar]
- Sutherland D., Samakovlis C., Krasnow M. A. (1996). branchless encodes a Drosophila FGF homolog that controls tracheal cell migration and the pattern of branching. Cell 87, 1091-1101 10.1016/S0092-8674(00)81803-6 [DOI] [PubMed] [Google Scholar]
- Turner N., Grose R. (2010). Fibroblast growth factor signalling: from development to cancer. Nat. Rev. Cancer 10, 116-129 10.1038/nrc2780 [DOI] [PubMed] [Google Scholar]
- Van Keymeulen A., Rocha A. S., Ousset M., Beck B., Bouvencourt G., Rock J., Sharma N., Dekoninck S., Blanpain C. (2011). Distinct stem cells contribute to mammary gland development and maintenance. Nature 479, 189-193 10.1038/nature10573 [DOI] [PubMed] [Google Scholar]
- Welm B. E., Freeman K. W., Chen M., Contreras A., Spencer D. M., Rosen J. M. (2002). Inducible dimerization of FGFR1: development of a mouse model to analyze progressive transformation of the mammary gland. J. Cell Biol. 157, 703-714 10.1083/jcb.200107119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman B. S., Sternlicht M. D., Lund L. R., Alexander C. M., Mott J., Bissell M. J., Soloway P., Itohara S., Werb Z. (2003). Site-specific inductive and inhibitory activities of MMP-2 and MMP-3 orchestrate mammary gland branching morphogenesis. J. Cell Biol. 162, 1123-1133 10.1083/jcb.200302090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian W., Schwertfeger K. L., Vargo-Gogola T., Rosen J. M. (2005). Pleiotropic effects of FGFR1 on cell proliferation, survival, and migration in a 3D mammary epithelial cell model. J. Cell Biol. 171, 663-673 10.1083/jcb.200505098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian W., Schwertfeger K. L., Rosen J. M. (2007). Distinct roles of fibroblast growth factor receptor 1 and 2 in regulating cell survival and epithelial-mesenchymal transition. Mol. Endocrinol. 21, 987-1000 10.1210/me.2006-0518 [DOI] [PubMed] [Google Scholar]
- Yalcin-Ozuysal O., Fiche M., Guitierrez M., Wagner K.-U., Raffoul W., Brisken C. (2010). Antagonistic roles of Notch and p63 in controlling mammary epithelial cell fates. Cell Death Differ. 17, 1600-1612 10.1038/cdd.2010.37 [DOI] [PubMed] [Google Scholar]
- Yang X., Dormann D., Münsterberg A. E., Weijer C. J. (2002). Cell movement patterns during gastrulation in the chick are controlled by positive and negative chemotaxis mediated by FGF4 and FGF8. Dev. Cell 3, 425-437 10.1016/S1534-5807(02)00256-3 [DOI] [PubMed] [Google Scholar]
- Yu K., Xu J., Liu Z., Sosic D., Shao J., Olson E. N., Towler D. A., Ornitz D. M. (2003). Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development 130, 3063-3074 10.1242/dev.00491 [DOI] [PubMed] [Google Scholar]
- Zhang X., Ibrahimi O. A., Olsen S. K., Umemori H., Mohammadi M., Ornitz D. M. (2006). Receptor specificity of the fibroblast growth factor family: the complete mammalian FGF family. J. Biol. Chem. 281, 15694-15700 10.1074/jbc.M601252200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Verheyden J. M., Hassell J. A., Sun X. (2009). FGF-regulated Etv genes are essential for repressing Shh expression in mouse limb buds. Dev. Cell 16, 607-613 10.1016/j.devcel.2009.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Qiao G., Lu P. (2014). Modulation of fibroblast growth factor signaling is essential for mammary epithelial morphogenesis. PLoS ONE 9, e92735 10.1371/journal.pone.0092735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Yang T., Madakashira B. P., Thiels C. A., Bechtle C. A., Garcia C. M., Zhang H., Yu K., Ornitz D. M., Beebe D. C., et al. (2008). Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev. Biol. 318, 276-288 10.1016/j.ydbio.2008.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.