Abstract
Background:
Inflammatory processes are implicated in the etiology of cardiovascular disease (CVD). Data on the association of inflammatory markers with cardiovascular risk factors in Indian patients with CVD are limited.
Aim:
This study was conducted with the aim to evaluate the association of inflammatory markers with traditional and nontraditional cardiovascular risk factors in angiographically proven coronary artery disease (CAD) patients.
Subjects and Methods:
We studied the association of serum highly sensitive C-reactive protein (hsCRP) (0.1-37.9 mg/l), interleukin-6 (IL-6) (2-253.2 pg/ml) and tumor necrosis factor-alpha (TNF-α) (8-525.8 pg/ml) with cardiovascular risk factors in 300 (M: 216, F: 84; mean age: 60.9 (12.4) years) CAD patients. All patients were evaluated for anthropometry and cardiovascular risk factors, and blood samples were collected for biochemical and inflammatory markers. Statistical analysis was carried out using SPSS Version 20.
Results:
Mean hsCRP, IL-6 and TNF-α in study population were 11.7 (9.7) mg/l, 64.5 (75.2) pg/ml, and 25.3 (40.9) pg/ml respectively. A total of 73.6% (221/300) patients had hsCRP levels >3.0 mg/l. All inflammatory markers were significantly higher and showed a positive correlation with dyslipidemia, diabetes mellitus, and/or hypertension (HTN). TNF-α had a negative correlation with age and positive correlation with smoking. Only IL-6 and hsCRP had a positive correlation with insulin resistance and negative correlation with insulin secretion. Among lipid parameters, triglyceride had a positive correlation, and high density lipoprotein had a negative correlation with all inflammatory markers. There was a progressive increase in the percentage of subjects with diabetes, HTN, and dyslipidemia with increasing levels of inflammatory markers.
Conclusions:
Indian patients with CAD had significantly high levels of inflammatory markers, which were related to cardiovascular risk factors.
Keywords: C-reactive protein, Coronary artery disease, Inflammatory markers, Interleukin-6, Tumor necrosis factor-α
Introduction
Epidemiologists have been sounding an alarm on the rapidly rising burden of cardiovascular disease (CVD) for the past 15 years in world over including India.[1] It was projected that by 2020, CVD will be the largest cause of disability and death in India.[2] The etiology of CVD is multifactorial and certainly there are many traditional and nontraditional risk factors that are associated with CVD.[3,4] High incidence of CVD in Indians cannot be explained by traditional risk factors. Hence, many researchers investigated and reported other novel risk factors to explain the increasing incidence and prevalence of CVD that is Vitamin B12, homocysteine, inflammatory markers, macro and micronutrients and presence of metabolic syndrome.[3,4,5,6,7]
Past decades have witnessed a spurt in research activity that has shown a key role of inflammation in coronary artery disease (CAD) and other manifestations of atherosclerosis. Immune cells dominate early atherosclerotic lesions and their effector molecules accelerate progression of the lesions, and can elicit acute coronary syndromes.[6,7,8,9] The inflammatory process associated with atherosclerosis lead to increased blood levels of inflammatory cytokines and other acute-phase reactants. Tumor necrosis factor-α (TNF-α) is a cytokine involved in systemic inflammation and is a member of a group of cytokines that stimulate the acute phase reaction. Interleukin-6 (IL-6) is an IL that acts as both a pro-inflammatory and antiinflammatory cytokine.[5] C-reactive protein (CRP) is a member of the class of acute-phase reactants as its levels rise dramatically during inflammatory processes. IL-6 and TNF-α are inflammatory cytokines and the main inducers of the secretion of CRP in the liver.[6] CRP is a marker of low grade inflammation, and few studies suggest that this protein has a role in the pathogenesis of atherosclerotic lesions in humans.[10,11,12,13,14,15] Levels of CRP and IL-6 are elevated in patients with unstable angina and myocardial infarction (MI), with high levels predict worse prognosis.[8] Highly sensitive CRP (hsCRP) and IL-6 are also associated with increased risk of all-cause mortality, and their measurement improves the ability to predict the risk of cardiovascular events.[8,16,17,18,19] Inflammation and related cytokines also interact with various metabolic factors which are related to the atherosclerotic process such as hyperglycemia, dyslipidemia, and insulin resistance (IR).
In patients with CAD, variable association of inflammatory markers with traditional and nontraditional risk factors has been described in the literature.[20,21,22,23,24,25,26] Few studies have analyzed inflammatory markers and its association with CVD risk factors in subjects with CAD from India.[10,27,28] Sharma et al.[27] studied hsCRP, dyslipidemia and oxidative stress in a small group of premature CAD. Goswami et al.[28] evaluated interplay between dyslipidemia, hsCRP and TNF-α in patients with acute MI. These all studies have studied only one or two inflammatory markers and none have studied all markers simultaneously and their relationship with all traditional and nontraditional risk factors. Combination of inflammatory markers will help to know the importance of inflammatory markers in patients with CVD. Therefore, we conducted this study to evaluate the association of inflammatory markers namely serum hsCRP, IL-6 and TNF-α with traditional and nontraditional cardiovascular risk factors in angiographically proven CAD patients.
Subjects and Methods
Three hundred patients with known CAD were included in this cross-sectional study. Patients, who were admitted in Cardiology Department of a single tertiary care center for evaluation of chest pain, and found angiography positive, were selected in the study consecutively. Exclusion criteria were the presence of chronic kidney disease; hepatic dysfunction; known endocrinal (except diabetes mellitus [DM]) or rheumatologic diseases or chronic infections. All cases were interviewed using a questionnaire, which included data on smoking, physical activity. Height, weight, waist, and hip circumference were measured. Body mass index (BMI) was calculated by dividing weight in kg with square of height in meters. Waist hip ratio (WHR) was calculated. Data on clinical history of hypertension (HTN) and DM and medications was also acquired.
Definitions
Traditional (conventional) risk factors were defined as follows: BMI < 25-normal, ≥25 overweight/obese, DM (by history and American Diabetes Association; 2011), HTN (systolic and diastolic blood pressures above 140 and 90 mm Hg, respectively). Dyslipidemia was defined as triglyceride level ≥150 mg/dl and HDL Cholesterol level <40 mg/dl (National Cholesterol Education Program Adult Treatment Panel III).
Initially, we thought of including the control group with normal coronary artery. However, we found that most of the patients had lesion ranging from 20% to <70% labeled as nonsignificant lesion. Hence, these patients if included as control, due to underlying pathology we will not be comparing our patients with individuals with absence of CAD. Moreover, age matched asymptomatic controls may not necessarily exclude underlying CAD. Hence, we have taken 25 asymptomatic apparently healthy young physically active controls from our previous study,[11] where only data for hsCRP was available.
Fasting blood samples were collected after 14 h fasting. Total cholesterol, triglyceride, and high density lipoprotein (HDL) were measured by using cholesterol oxidase para-aminoantipyrine, lipase glycerol kinase, the enzymatic reaction respectively, and low density lipoprotein (LDL), and very LDL (VLDL) were calculated by Freidwald formula. Inter assay 3.8%, and intra precision was 2% respectively for all biochemical parameters. TNF-α (linearity: 200 pg/ml, sensitivity: 2 pg/ml), IL-6 (linearity: 800 pg/ml, sensitivity 8 pg/ml), and hsCRP (linearity: 119.3 mg/l, sensitivity: 0.1 mg/l) were measured by enzyme linked immunosorbent assay method with kits manufactured by Gen-probe Diaclone, France and Biochek, CA, USA. Insulin was done by microparticle enzyme immunoassay with commercial kits supplied by Abbott laboratory, USA. Intra assay and inter assay precision was <5% and <10% respectively for above parameters. IR and sensitivity was calculated by using homeostatic model analysis (HOMA) model (HOMA-IR = Fasting insulin (μIU/ml) × Fasting glucose (mmol/l)/22.5; and quantitative insulin check index (QUICKI) (QUICKI = 1/(log [fasting insulin μU/ml] + log [fasting glucose mg/dl]) respectively. The study was approved by Institutional ethics committee. The study period was from 2009 to 2011. Informed consent was obtained from all subjects.
Sample size calculated with 95% confidence (1-α), 90% power (1-β), population prevalence of 10% and relative risk of 2 was 268 subjects. Statistical analysis was carried out using SPSS Version 20.0 (IBM, SPSS Inc. Chicago, USA). Data were presented as mean (standard deviation, median (range) or number (%) unless specified. Significance levels were analyzed by Student's t-test between two parameters and ANOVA test when parameters were more than two. If Bartlett's Chi-square test for equality of population variances was <0.05, then Kruskal–Wallis test was applied. Pearson correlation was used to evaluate the correlation between traditional and nontraditional risk factors such as markers of IR (Insulin, HOMA-IR, QUICKI) and inflammatory markers (hsCRP, IL-6 and TNF-α). Multiple regression analysis was performed after adjustment for age, sex, BMI, and presence of HTN. All nonparametric data were analyzed by Chi-square test. P < 0.05 was considered as statistically significant.
Results
Three hundred patients with known CAD (male: 216; female: 84, mean age 60.9 [12.4] years, 95% confidence interval [CI] 59.50-62.34 years) were studied. Table 1 shows baseline characteristics of the subjects studied. Mean hsCRP, IL-6 and TNF-α in study population were 11.7 (9.7) mg/l (median 12 mg/l), 64.6 (75.3) pg/ml (median 25.0 pg/ml), and 25.3 (40.9) pg/ml (median 10.0 pg/ml), respectively. Mean hsCRP levels (age adjusted) were higher in study population than controls (2.0 (0.9) mg/L, P < 0.001).
Table 1.
Basic characteristics of study population
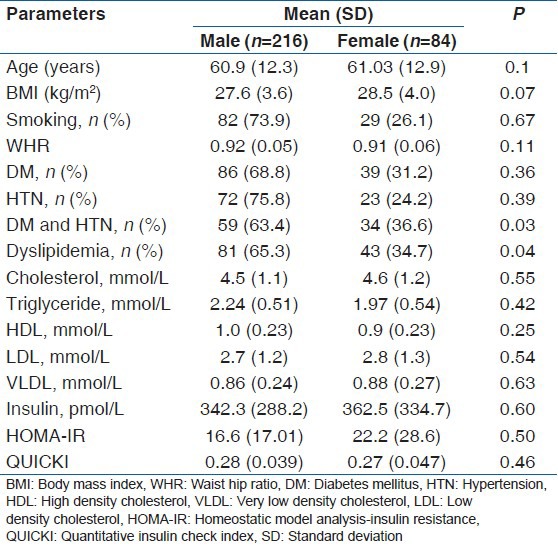
A total of 73.6% (221/300) of the patients had hsCRP levels >3.0 mg/l. There was no gender difference in hsCRP levels [Table 2]. hsCRP, IL-6, and TNF-α levels were higher in the subject with dyslipidemia, diabetes, and/or HTN compared to nondiabetics and normotensives. It was positively correlated with total and LDL cholesterol, triglyceride, insulin levels, HOMA-IR, and negatively correlated with insulin secretion and HDL cholesterol in univariate analysis [Table 3 and Figure 1]. However, after adjustment with other risk factors, the association between hsCRP and HTN was lost, but the significance was maintained with dyslipidemia and DM (HTN; beta coefficient 1.4, P = 0.15, DM; beta coefficient 2.5, P = 0.01, Dyslipidemia; beta coefficient 9.0, P < 0.001). A similar observation was noted for total cholesterol, triglyceride, LDL, and VLDL with quartiles of hsCRP except HDL that decreased [Table 4].
Table 2.
Inflammatory markers according to traditional risk factors
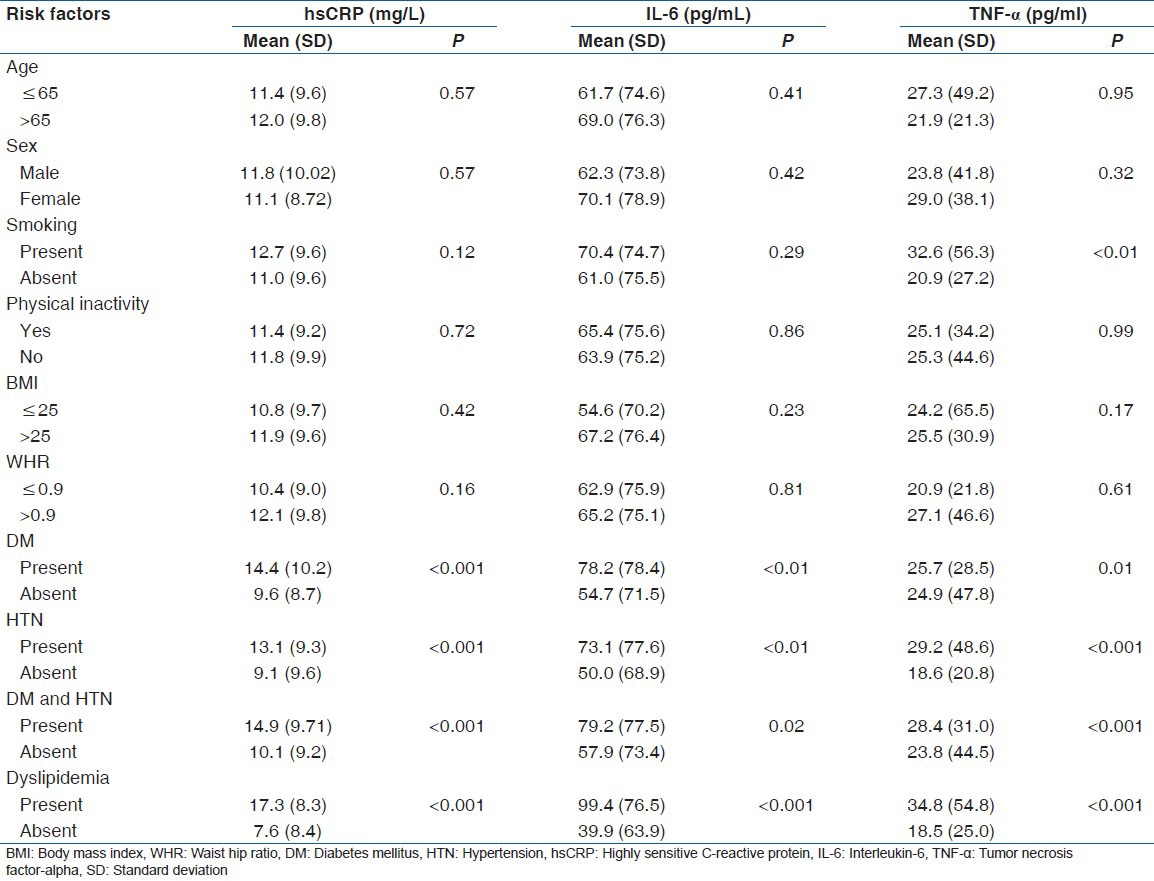
Table 3.
Correlation of inflammatory markers with risk factors
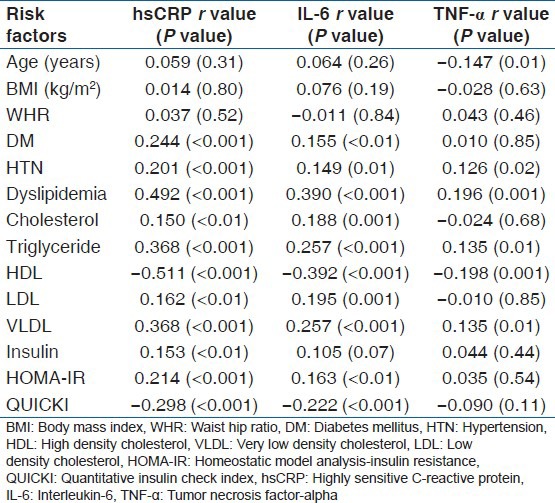
Figure 1.
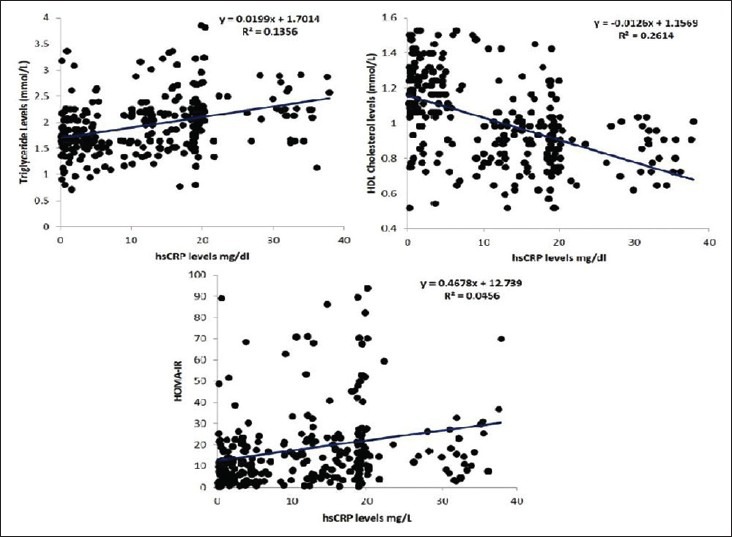
Correlation of hsCRP levels with triglyceride, HDL and HOMA-IR
Table 4.
Cardiovascular risk factors according to quartiles of hsCRP
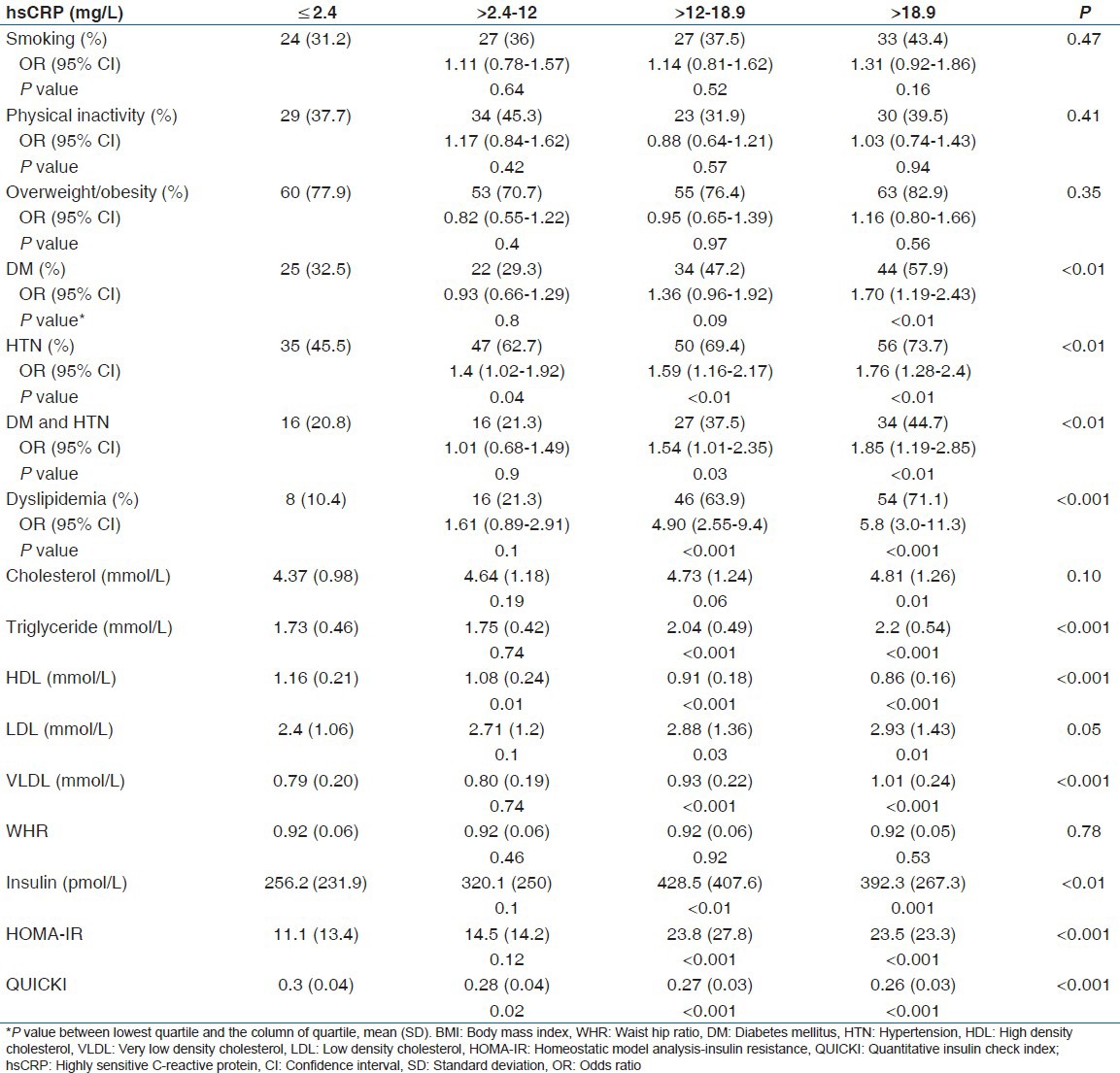
Odds ratio of DM (1.70, 95% CI: 1.19–2.43), HTN (1.76, 95% CI: 1.28-2.4), and dyslipidemia (5.8, 95% CI: 3.0-11.3) increased from lowest to the highest quartile of hsCRP. A similar trends were seen with IL-6 (Odds ratio of DM [1.73, 95% CI: 1.18–2.52], HTN (1.65, 95% CI: 1.2-2.27), and dyslipidemia (4.5, 95% CI: 2.5-8.1)].
Subjects were also categorized into subject with modifiable risk factor (DM, HT, and dyslipidemia) and without modifiable risk factors. Subjects with modifiable risk factors had significantly higher inflammatory levels (hsCRP-12.8 [9.5] vs. 4.8 [7.2] mg/l, IL-6-72 [77.1] vs. 21.0 [42.5] pg/ml, TNF-α - 27.4 [43.5] vs. 12.5 [13.9] pg/ml) compared with without modifiable risk factors.
Discussion
Atherosclerosis is a chronic inflammatory state and many studies have demonstrated an association of inflammatory markers with CAD. Elevated levels of these markers predict future ischemic events, and mortality from CAD.[5,12] In the present study, we have measured inflammatory markers in three hundred patients with angiographically proven CAD. Among inflammatory markers, hsCRP has been extensively studied and was part of two meta-analysis.[3,13]
Mean hsCRP levels were higher in present study than those reported in the literature[10,27] except a study from Brazil (male 18.2 [2.3] mg/l and female 12.0 [2.1] mg/l) in angiographically proven patients with CAD.[14] In the present study, 73.6% of the patients had hsCRP levels >3.0 mg/l, whereas other studies reported above this level in 50% patients of CAD.[3,15] This can be explained by differences in age, sex ratio, BMI, associated morbidities, and dietary differences among various populations studied. Dietary differences as high carbohydrate-fat and low protein, mineral, and fiber diet,[29] Vitamin B12 deficiency, and hyperhomocysteinemia[4] and hypomagnesaemia[30] are associated with inflammation in Indian patients. IR and associated metabolic syndrome is also associated with increased levels of inflammatory markers[15] and Indians have higher IR than other population.[31]
Interleukin-6 is the main inducer of hsCRP production and secretion from liver. It has been reported to predict future risk of CAD and mortality in patients with CAD.[16] Mean IL-6 level in the present study was similar to the level reported in a study from Pakistan in patients with CAD with diabetes and without diabetes.[17] Other studies have also reported higher level of IL-6 in patients with CAD.[8,16] Serum IL-6 levels are increased in diabetic, and hypertensive subjects[24] compared with those without these morbidities. Bautista[18] reported that after adjusting for other risk factors; age, sex, and BMI, IL-6 was not significantly associated with HTN that is similar to the present study. Serum IL-6 levels were not correlated with age, BMI, WHR, and smoking in our study. There are contradictory reports of relation of IL-6 with age[9] BMI[9,15,19,20] and smoking.[8,9]
Tumor necrosis factor-alpha is a pro-inflammatory cytokine, which is prerequisite for initiation of inflammation and production of other cytokine. TNF-α has been associated with recurrent MI and cardiovascular death.[32] TNF-α was negatively correlated with age and was positively correlated with smoking. Similar observation has been made by others.[33,34] Elevated plasma TNF-α level have been observed in obese compared to lean subjects,[9,23] which is in contrast to our study. In the present study, TNF-α level was significantly higher in subjects with dyslipidemia compared with those without it. TNF-α level showed a positive association with increased triglyceride and reduced HDL, but was not related to total and LDL cholesterol. Mendall et al.[20] also observed a similar association. In contrast, Khan et al.[24] reported a strong association of TNF-α with LDL, but not with triglyceride and HDL cholesterol, whereas Goswami et al.[28] did not find any correlation with any lipid parameter. The reason for a strong association of TNF-α with a reduced serum level of HDL is uncertain. It has been speculated that the reduced HDL seen in the inflammation result from increased serum concentration of serum amyloid A protein replacing apo-A1 as an apolipoprotein in HDL particles and that this leads to increased catabolism.[35] In experimental studies administration of TNF-α administration into mice and human increased triglyceride concentration by ~85%.[36] TNF-α increases plasma triglyceride by increasing the concentration of free fatty acids, which acts as substrate for triglyceride synthesis, and by diminishing the clearance of triglyceride rich lipoproteins (VLDLs) from the circulation.[36]
In this study, patients without modifiable risk factors (smoking, DM, HT, and dyslipidemia), had higher hsCRP levels than reported in healthy young Indian population.[27,28] This may suggest role of inflammation in patients without modifiable risk factors.[5,15] Low grade inflammation in these patients may be related to subclinical infection,[9] dietary factors[29] or environmental factors.[37] Genetic and epigenetic factors have also been implicated in inflammation. DNA methylation is emerging as a primary regulator of inflammation. DNA methylation states may vary over an individual's lifetime, and have been shown to regulate biological processes underlying CVD, such as atherosclerosis, inflammation, HTN, and diabetes.[38] Altered DNA methylation may be related to dietary factors such as sufficiency of folic acid in presence of vitamin B12 deficiency in Indian population.[39]
The main strength of the study is that all subjects had angiographically proven CAD and all inflammatory markers were simultaneously measured along with traditional and nontraditional risk factors. There were some limitations of our study. First, we have not taken a control group. We wanted to study population with confirmed CAD, and healthy asymptomatic controls do not certainly means absence of underlying asymptomatic CAD. Second, being a cross-sectional study, long-term follow-up data were not available. Third, males were more than females as CAD is more common in males, this may have affected results. Finally, it was single center study.
Conclusion
the present study showed that inflammatory markers (hs-CRP, IL-6 and TNF-α) have a strong association with diabetes, dyslipidemia, HTN, and IR in subjects with underlying CVD. The current study suggests that Indians have an underlying proinflammatory state, which may contribute to increased cardiovascular risk factors and predispose them to CVD. These markers may identify the future risk of CAD in Indian subjects if strong association is confirmed by future longitudinal prospective studies.
Acknowledgments
We thank Deenanath Mangeshkar Hospital and Research Centre, Pune, for providing necessary facilities.
Footnotes
Source of Support: We thank Deenanath Mangeshkar Hospital and Research Centre, Pune, India for providing necessary facilities.
Conflict of Interest: None declared.
References
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alwan A. Geneva: World Health Organization; 2011. World Health Organization: Noncommunicable diseases country profiles. [Google Scholar]
- 3.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–97. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 4.Bhagwat VR, Yadav AS, Rathod IM. Homocysteine, lipid indices and antioxidants in patients with ischaemic heart disease from Maharashtra, India. Singapore Med J. 2009;50:418–24. [PubMed] [Google Scholar]
- 5.Tedgui A, Mallat Z. Cytokines in atherosclerosis: Pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–81. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 6.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: Is interleukin-6 the link? Atherosclerosis. 2000;148:209–14. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 7.Zakynthinos E, Pappa N. Inflammatory biomarkers in coronary artery disease. J Cardiol. 2009;53:317–33. doi: 10.1016/j.jjcc.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Gotsman I, Stabholz A, Planer D, Pugatsch T, Lapidus L, Novikov Y, et al. Serum cytokine tumor necrosis factor-alpha and interleukin-6 associated with the severity of coronary artery disease: Indicators of an active inflammatory burden? Isr Med Assoc J. 2008;10:494–8. [PubMed] [Google Scholar]
- 9.de Maat MP, Pietersma A, Kofflard M, Sluiter W, Kluft C. Association of plasma fibrinogen levels with coronary artery disease, smoking and inflammatory markers. Atherosclerosis. 1996;121:185–91. doi: 10.1016/0021-9150(95)05716-1. [DOI] [PubMed] [Google Scholar]
- 10.Young D, Camhi S, Wu T, Hagberg J, Stefanick M. Relationships among changes in C-reactive protein and cardiovascular disease risk factors with lifestyle interventions. Nutr Metab Cardiovasc Dis. 2013;23:857–63. doi: 10.1016/j.numecd.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garg MK, Dutta MK, Brar KS. Inflammatory markers in metabolic syndrome. Int J Diabetes Dev Ctries. 2012;32:131–7. [Google Scholar]
- 12.Iso H, Cui R, Date C, Kikuchi S, Tamakoshi A JACC Study Group. C-reactive protein levels and risk of mortality from cardiovascular disease in Japanese: The JACC Study. Atherosclerosis. 2009;207:291–7. doi: 10.1016/j.atherosclerosis.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 13.Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, et al. Low grade inflammation and coronary heart disease: Prospective study and updated meta-analyses. BMJ. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monteiro CM, Pinheiro LF, Izar MC, Barros SW, Vasco MB, Fischer SM, et al. Highly sensitive C-reactive protein and male gender are independently related to the severity of coronary disease in patients with metabolic syndrome and an acute coronary event. Braz J Med Biol Res. 2010;43:297–302. doi: 10.1590/s0100-879x2010005000008. [DOI] [PubMed] [Google Scholar]
- 15.Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- 16.Lindmark E, Diderholm E, Wallentin L, Siegbahn A. Relationship between interleukin-6 and mortality in patients with unstable coronary artery disease: Effects of an early invasive or noninvasive strategy. JAMA. 2001;286:2107–13. doi: 10.1001/jama.286.17.2107. [DOI] [PubMed] [Google Scholar]
- 17.Yaseen F, Jaleel A, Aftab J, Zuberi A, Alam E. Circulating levels of resistin, IL-6 and lipid profile in elderly patients with ischemic heart disease with and without diabetes. Biomark Med. 2012;6:97–102. doi: 10.2217/bmm.11.104. [DOI] [PubMed] [Google Scholar]
- 18.Bautista LE. Inflammation, endothelial dysfunction, and the risk of high blood pressure: Epidemiologic and biological evidence. J Hum Hypertens. 2003;17:223–30. doi: 10.1038/sj.jhh.1001537. [DOI] [PubMed] [Google Scholar]
- 19.Liuzzo G, Biasucci LM, Gallimore JR, Grillo RL, Rebuzzi AG, Pepys MB, et al. The prognostic value of C-reactive protein and serum amyloid a protein in severe unstable angina. N Engl J Med. 1994;331:417–24. doi: 10.1056/NEJM199408183310701. [DOI] [PubMed] [Google Scholar]
- 20.Mendall MA, Patel P, Asante M, Ballam L, Morris J, Strachan DP, et al. Relation of serum cytokine concentrations to cardiovascular risk factors and coronary heart disease. Heart. 1997;78:273–7. doi: 10.1136/hrt.78.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spranger J, Kroke A, Möhlig M, Hoffmann K, Bergmann MM, Ristow M, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: Results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–7. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 22.Bo M, Raspo S, Morra F, Isaia G, Cassader M, Fabris F, et al. Body fat and C-reactive protein levels in healthy non-obese men. Nutr Metab Cardiovasc Dis. 2004;14:66–72. doi: 10.1016/s0939-4753(04)80012-7. [DOI] [PubMed] [Google Scholar]
- 23.Dupuy AM, Jaussent I, Lacroux A, Durant R, Cristol JP, Delcourt C. Waist circumference adds to the variance in plasma C-reactive protein levels in elderly patients with metabolic syndrome. Gerontology. 2007;53:329–39. doi: 10.1159/000103555. [DOI] [PubMed] [Google Scholar]
- 24.Kim ES, Im JA, Kim KC, Park JH, Suh SH, Kang ES, et al. Improved insulin sensitivity and adiponectin level after exercise training in obese Korean youth. Obesity (Silver Spring) 2007;15:3023–30. doi: 10.1038/oby.2007.360. [DOI] [PubMed] [Google Scholar]
- 25.Khan SA, Aslam M, Owais M, Zaheer MS. Correlation between tumour necrosis factor-alpha and other co-variates and different grades of blood pressure in essential hypertensive patients. Biomed Res. 2010;21:184–8. [Google Scholar]
- 26.Syvänen K, Korhonen P, Jaatinen P, Vahlberg T, Aarnio P. High-sensitivity C-reactive protein and ankle brachial index in a finnish cardiovascular risk population. Int J Angiol. 2011;20:43–8. doi: 10.1055/s-0031-1272551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma SB, Garg S, Veerwal A, Dwivedi S. hs-CRP and oxidative stress in young CAD patients: A pilot study. Indian J Clin Biochem. 2008;23:334–6. doi: 10.1007/s12291-008-0073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goswami B, Rajappa M, Singh B, Ray PC, Kumar S, Mallika V. Inflammation and dyslipidaemia: A possible interplay between established risk factors in North Indian males with coronary artery disease. Cardiovasc J Afr. 2010;21:103–8. [PMC free article] [PubMed] [Google Scholar]
- 29.Mahalle N, Kulkarni MV, Naik SS, Garg MK. Association of dietary factors with insulin resistance and inflammatory markers in subjects with diabetes mellitus and coronary artery disease in Indian population. J Diabetes Complications. 2014;28:536–41. doi: 10.1016/j.jdiacomp.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Mahalle N, Kulkarni MV, Naik SS. Is hypomagnesaemia a coronary risk factor among Indians with coronary artery disease? J Cardiovasc Dis Res. 2012;3:280–6. doi: 10.4103/0975-3583.102698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snehalatha C, Ramachandran A. Insulin resistance in Asians Indians. Pract Diabetes Int. 1999;16:19–22. [Google Scholar]
- 32.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–72. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 33.Mio T, Romberger DJ, Thompson AB, Robbins RA, Heires A, Rennard SI. Cigarette smoke induces interleukin-8 release from human bronchial epithelial cells. Am J Respir Crit Care Med. 1997;155:1770–6. doi: 10.1164/ajrccm.155.5.9154890. [DOI] [PubMed] [Google Scholar]
- 34.Petrescu F, Voican SC, Silosi I. Tumor necrosis factor-alpha serum levels in healthy smokers and nonsmokers. Int J Chron Obstruct Pulmon Dis. 2010;5:217–22. doi: 10.2147/copd.s8330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han CY, Chiba T, Campbell JS, Fausto N, Chaisson M, Orasanu G, et al. Reciprocal and coordinate regulation of serum amyloid A versus apolipoprotein A-I and paraoxonase-1 by inflammation in murine hepatocytes. Arterioscler Thromb Vasc Biol. 2006;26:1806–13. doi: 10.1161/01.ATV.0000227472.70734.ad. [DOI] [PubMed] [Google Scholar]
- 36.Feingold KR, Marshall M, Gulli R, Moser AH, Grunfeld C. Effect of endotoxin and cytokines on lipoprotein lipase activity in mice. Arterioscler Thromb. 1994;14:1866–72. doi: 10.1161/01.atv.14.11.1866. [DOI] [PubMed] [Google Scholar]
- 37.Delfino RJ, Staimer N, Tjoa T, Gillen DL, Polidori A, Arhami M, et al. Air pollution exposures and circulating biomarkers of effect in a susceptible population: Clues to potential causal component mixtures and mechanisms. Environ Health Perspect. 2009;117:1232–8. doi: 10.1289/ehp.0800194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turunen MP, Aavik E, Ylä-Herttuala S. Epigenetics and atherosclerosis. Biochim Biophys Acta. 2009;1790:886–91. doi: 10.1016/j.bbagen.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 39.Yajnik CS, Deshmukh US. Fetal programming: Maternal nutrition and role of one-carbon metabolism. Rev Endocr Metab Disord. 2012;13:121–7. doi: 10.1007/s11154-012-9214-8. [DOI] [PubMed] [Google Scholar]


