Abstract
Objective:
This study aim to evaluate and compare type and prevalence of drug-drug interactions (DDIs) in prescriptions dispensed in both community and hospital setting in Zabol, Iran.
Methods:
A total of 2796 prescriptions were collected from community and inpatient and outpatient pharmacy of Amir-al-momenin only current acting hospital in Zabol, Iran. The prescriptions were processed using Lexi-Comp drug interaction software. The identified DDIs were categorized into five classes (A, B, C, D, X).
Findings:
Overall 41.6% of prescriptions had at last one potential DDI. The most common type of interactions was type C (66%). The percentage of drug interactions in community pharmacies were significantly lower than hospital pharmacies (P < 0.0001).
Conclusion:
Our results indicate that patients in Zabol are at high risk of adverse drug reactions caused by medications due to potential DDIs. Appropriate education for physicians about potentially harmful DDIs, as well as active participation of pharmacists in detection and prevention of drug-related injuries, could considerably prevent the consequence of DDIs among patients.
Keywords: Community pharmacy services, drug interactions, hospital pharmacy services, Iran, prescriptions
INTRODUCTION
Drug-Drug Interactions (DDIs) are defined as a pharmacokinetic or pharmacodynamic influences of drugs on each other, which may result in undesired effects, reduced efficacy or increased toxicity.[1] DDIs result in many adverse clinical outcomes; they are responsible for 5% of all hospital admissions.[2] Med watch program of Food and Drug Administration reported 6894 fatalities due to adverse drug reactions (ADRs) including DDIs in the United States in 1995.[3] Published studies have reported rates of potential DDIs ranging from 2.2% to 30% in hospitalized and from 9.2% to 70.3% in ambulatory patients.[4] Potential DDIs are defined on the basis of on retrospective chart reviews, and actual DDIs are defined on the basis of clinical evidence, that is, they are confirmed by laboratory tests or symptoms.[5]
The treatment of a disease usually requires the use of more than one drug, but polypharmacy carries high risk of DDIs with serious consequence for health. Some factors such as administration of drugs with low therapeutic index, severity of underlying diseases and patient's age (commonly elderly) could increase the potential of dangerous drug interactions.[6]
Among medication errors, drug interactions could be easily prevented. Unfortunately most physicians are unaware of potential DDIs; therefore in some countries, the pharmacists are responsible for preventing the use of unsafe drug regimens to avoid dispensing of combination therapies that may cause serious DDIs. Since there was not any documented study in drug interactions here in Zabol, South-East of Iran, we decided to investigate the prevalence and type of DDIs in prescriptions of both community and hospital pharmacies of Zabol city, Iran.
METHODS
A prospective, descriptive cross-sectional study was conducted on prescriptions of different community pharmacies and inpatient and outpatient pharmacies of Amir-al-Momenin teaching hospital, affiliated with the Zabol University of Medical Sciences. The hospital is a 260-bed general institution including different wards (Internal, pediatric, surgery, infectious, cardiac care unit [CCU], intensive care unit [ICU], and obstetrics and gynecology) which is also a referral center for hospital care in Zabol. Previous studies were done in Iran[7,8,9] evaluated a mean of 3000 prescriptions, hence during the study period we collected an overall of 2796 prescriptions. All prescriptions from October 2011 to March 2012 were analyzed. Prescriptions with two or more prescribed drugs were selected, and data were extracted on predesigned forms including patient characteristics (gender, age), the number of drugs, physician specialty (general practitioner or medical specialist) and severity and significance of drug interactions. The severity and significance of drug interactions were analyzed using Lexi-Comp on Desktop drug interaction software (Lexi-Interact™, Hudson, Ohio: Lexi-Comp, Inc.; April 29, 2012). Significance of drug interactions was divided into 5 categories (A to X) according to software, which is presented in Table 1.
Table 1.
Significance categories of drug-drug interactions

Demographic data of patients and other data of prescriptions were presented as mean ± standard deviation or percentage of cases. Independent sample t-test and Chi-square test were applied to assess differences among groups. P < 0.05 or less were considered statistically significant. The data were processed using SPSS software (SPSS, Inc. Chicago, IL, USA) version 18.0.
RESULTS
Form 2796 prescriptions, 765 (27.4%) were collected from community pharmacies, 993 (33.5%) from outpatient and 1038 (37.1%) from inpatient pharmacies of Amir-al-Momenin Hospital. Among the prescriptions analyzed, 1163 (41.6%) had at least one drug interaction case. A total of 1576 cases of interactions were found in prescriptions which 66% of interactions were classified as type C.
The frequencies of drug interactions in community and hospital pharmacies are shown in Table 2. The overall prevalence of drug interactions in community pharmacies was significantly lower than hospital pharmacies (inpatient and outpatient) (P < 0.001). The percentage of interactions with significant clinical importance was higher in prescriptions of medical specialists than general practitioners (P = 0.02) (data not shown). About 25.5% and 27.9% of drug interactions in medical specialists’ and general practitioners’ prescriptions were classified as type C, respectively.
Table 2.
Frequencies of drug interactions in community and hospital pharmacies
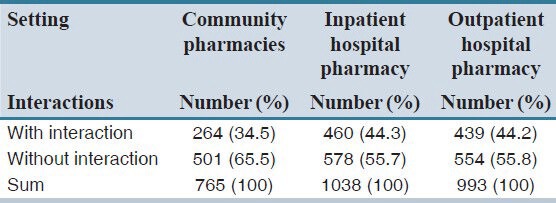
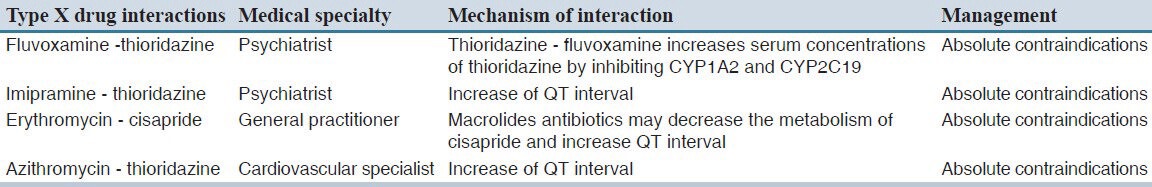
Type C interactions had the highest prevalence among community and hospital pharmacies prescriptions; the percentage was higher in an inpatient pharmacy of Amir-al-Momenin hospital (68.5%). Only four prescriptions (0.25%) from outpatient hospital pharmacy had type X interaction [Table 3].
Table 3.
Observed X-type interactions in prescriptions
Average number of items per prescription was 4.18; three-drug item prescriptions were the most prevalent ones (n = 706, 25.2%). The mean ± standard deviation of items in prescriptions with interactions was 5.47 ± 2.17 compared to 3.26 ± 1.3 in prescriptions without interactions, and the difference was significant (P < 0.001). Increasing the number of drugs per prescription significantly increased the probability of drug interaction (P < 0.001) (data not shown).
From 1038 prescriptions retrieved from inpatient hospital pharmacy, the majority of prescriptions appertained to three different wards as follows: Internal medicine ward (n = 281, 27.1%), ICU and CCU (n = 201, 19.36%). In Infectious disease ward, 51% (n = 200) of prescriptions had at least one drug interaction and the most common drug interactions were between dexamethasone and ranitidine, as well as corticosteroids and nonsteroidal anti-inflammatory drugs (NSAIDs), both categorized as type C interactions. Pattern was completely different in internal medicine ward where aspirin/corticosteroids and aspirin/heparin were the most common interactions (type C) whereas the mean drug items per prescriptions were 5.22. Eighty percent of prescriptions in ICU and CCU had at least one interaction where the amikacin/vancomycin interaction was the most frequent; the mean items per prescriptions were 6.44 in the prescriptions of these wards. About 50.5% and 25% of prescriptions in ICU, CCU and internal medicine ward had ≥7 items per prescription; the relationship between the number of drugs per prescription and DDIs was significant (P < 0.0001).
The overall most observed drug interactions were as follow: Dexamethasone and ranitidine in inpatient hospital pharmacy, corticosteroids and NSAIDs in outpatient hospital pharmacy and community pharmacies. All of the observed X type interactions in retrieved prescriptions were from outpatient hospital pharmacy.
DISCUSSION
In the present study, we found that frequency of potential DDIs in prescriptions (both in community and hospital pharmacies) in Zabol city, Iran, was almost 42%, which is higher than the frequency reported in Kurdistan province, Iran (8.5%)[7] and Yasuj city, Iran (10.5%),[8] but nearly similar to Baft city (42.2%)[9] in Kerman province, Iran, the neighborhood of Zabol city, Iran, so the potential similarity in medical education trends could be a reason. However, generally, rates of drug interactions in Iranian prescriptions are comparable with some other countries.[10] This broad range of prevalence value may be partially explained by factors such as study design, methodology, definitions, and characteristics of the population, number of medications prescribed, and compendium of drug interactions.
Based on our results, the overall prevalence of DDIs were higher in hospital pharmacies than community pharmacies; the potential explanation may be the fact that in hospital settings patients usually have more severe conditions and comorbidities which require multiple medications, while consequently probability of drug interactions will increase. Medical specialists’ prescriptions in comparison with general practitioners’ had more DDIs maybe because medical specialists deal with more severe diseases and more efficient drugs with lower therapeutic index, therefore, more serious side effects and interactions. Results of other studies[11] are consistent with our results regarding the higher rate of DDIs in medical specialist prescriptions.
Polypharmacy is an important factor which leads to DDIs, the more the number of items per prescription, the more the likelihood of drug interactions occurrence. Our survey showed that 48% of prescriptions had 3-4 drug items with an average of 4.18 items per prescription. National committee of rational use of drugs reported the mean items of drug per prescription were 3.2 in 2007;[12] however, it is decreasing, but is still higher than other countries with average of 1.3-2.1 items per prescription.[12] Based on the results of some studies, the rates of potential drug interactions for patients receiving two or more drugs range from 24.3% to 42%,[13] therefore, the greater the number of drugs, the higher the possibility of DDIs. In hospital setting, the mean items per prescription were 4.22; this number was 6.44 for ICU and CCU, which had the most drug interactions (80%).
The most prevalent type of interactions observed in our study was type C, accounting for 66% of all interactions observed regarding the settings. Type C drug interaction will not cause any serious or fatal consequences and just need monitoring. Fortunately, only 0.14% of all interactions were due to type X interactions which all of them were from outpatient hospital pharmacy. Similar studies reported high percentage of major potential drug interactions, ranged from 0.83% to 17%.[14] As this type of interactions could be harmful to patients, physicians and pharmacists should be aware of them and keep the patients under close surveillance.
Our results indicate that patients in Zabol city, Iran, are at risk of ADR due to potential DDIs; however, we did not identify determinants of drug interactions by pharmacies in this study, but possible causes such as lack of knowledge about the DDIs or patient medication history, also lack of communication between primary and secondary health care providers or between the prescribers and patients could be the reasons for the dispensing of unsuitable drug combinations. Thus, adherence to the correct policies of writing prescriptions, reduction the number of prescribed medications, promoting physicians’ knowledge about potentially harmful DDIs, for example, by participating in related educational courses could be helpful in reducing the incidence of drug interactions. Furthermore, appropriate surveillance system for monitoring drug interaction should be implemented. Pharmacists can also contribute in detection and prevention of drug-related injuries and reducing the rate of DDI and its related hazardous consequence.
AUTHORS’ CONTRIBUTION
M.M AND K.T designed and supervised the projects, AR.A and MH.A collect and analyzed data and S.M wrote the manuscript.
ACKNOWLEDGMENTS
The authors are grateful to the assistance of Dr. Mohammad Ashrafi and Dr. Sima Khosravi and all pharmacists in Zabol city who helped us to create this research with their honest cooperation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Edwards IR, Aronson JK. Adverse drug reactions: Definitions, diagnosis, and management. Lancet. 2000;356:1255–9. doi: 10.1016/S0140-6736(00)02799-9. [DOI] [PubMed] [Google Scholar]
- 2.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. JAMA. 1998;279:1200–5. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 3.Chyka PA. How many deaths occur annually from adverse drug reactions in the United States? Am J Med. 2000;109:122–30. doi: 10.1016/s0002-9343(00)00460-5. [DOI] [PubMed] [Google Scholar]
- 4.Jankel CA, Fitterman LK. Epidemiology of drug-drug interactions as a cause of hospital admissions. Drug Saf. 1993;9:51–9. doi: 10.2165/00002018-199309010-00005. [DOI] [PubMed] [Google Scholar]
- 5.Qian Y, Ye X, Du W, Ren J, Sun Y, Wang H, et al. A computerized system for detecting signals due to drug-drug interactions in spontaneous reporting systems. Br J Clin Pharmacol. 2010;69:67–73. doi: 10.1111/j.1365-2125.2009.03557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juurlink DN, Mamdani M, Kopp A, Laupacis A, Redelmeier DA. Drug-drug interactions among elderly patients hospitalized for drug toxicity. JAMA. 2003;289:1652–8. doi: 10.1001/jama.289.13.1652. [DOI] [PubMed] [Google Scholar]
- 7.Rashidi K, Senobar, Tahaee S. Assessment of drug interactions in medical insurance prescriptions in Kurdistan province in 2000. Sci J Kurdestan Univ. 2005;10:78–84. [Google Scholar]
- 8.Nabavizadeh SH, Khoshnevisan F. Drug interactions in prescriptions of general practitioners in yasuj city. Armaghan Danesh. 2003;7:53–9. [Google Scholar]
- 9.Ramezani J. Master Thesis. Kerman University of Medical Sciences; 2010. The rate of drug interactions in prescriptions of baft general practitioners from March 2010 to August 2010. [Google Scholar]
- 10.Leone R, Magro L, Moretti U, Cutroneo P, Moschini M, Motola D, et al. Identifying adverse drug reactions associated with drug-drug interactions: Data mining of a spontaneous reporting database in Italy. Drug Saf. 2010;33:667–75. doi: 10.2165/11534400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Ahmadizar F, Soleymani F, Abdollahi M. Study of drug-drug interactions in prescriptions of general practitioners and specialists in iran 2007-2009. Iran J Pharm Res. 2011;10:921–31. [PMC free article] [PubMed] [Google Scholar]
- 12.Soleymani F, Valadkhani M, Dinarvand R. Challenges and achievements of promoting rational use of drugs in Iran. Iran J Public Health. 2009;38(Suppl 1):166–8. [Google Scholar]
- 13.Dambro MR, Kallgren MA. Drug interactions in a clinic using COSTAR. Comput Biol Med. 1988;18:31–8. doi: 10.1016/0010-4825(88)90054-6. [DOI] [PubMed] [Google Scholar]
- 14.Morteza-Semnani K, Saeedi M, Qari Pour U. Evaluation of drug interactions of Cardiovascular drugs in insurance prescriptions of Sari city – 1998-99. Mazandaran Univ Med Sci J. 2000;11:93–87. [Google Scholar]


