Abstract
Individuals who die of traumatic brain injury show damage to the lungs mediated by the HMGB1-RAGE axis, which renders the lungs suboptimal for transplantation (Weber et al.).
There are nearly 1700 patients currently awaiting lung and heart-lung transplants in the United States (http://optn.transplant. hrsa.gov/data/). The largest source of donor lungs is from deceased patients who have suffered traumatic brain injury (TBI). Unfortunately, TBI can severely compromise pulmonary function, and thus TBI restricts the total number of organs available for the many patients awaiting this life-saving therapy. Every year, there are nearly 1.4 million incidents of TBI, leading to approximately 50,000 deaths. Only one-third of severe TBI patients are considered eligible organ donors, of which less than 20% actually donate lungs (1). Given the relative scarcity of viable donor lungs and the high risk of death for potential recipients awaiting a transplant, it is important to understand and limit damage caused to potential donor lungs through TBI. However, to date, the pathophysiology involving brain-pulmonary interactions remains poorly understood. In a new study, Weber et al. use mouse models of TBI and lung transplantation to elucidate the mechanisms through which TBI causes lung injury and the impact of this injury on the health of transplanted donor lungs (2).
Patients with TBI often have concomitant injuries to the thorax that are obvious causes of pulmonary compromise, including trauma to the chest wall (such as rib fractures) and to the lung parenchyma (such as, contusions, pneumothorax, or hemothorax) (3). Patients with TBI frequently require mechanical ventilation and suffer pulmonary complications associated with life support, including pneumonia, acute lung injury, and acute respiratory distress syndrome (ARDS). TBI appears to independently increase the risk to mechanically ventilated patients. Neurogenic pulmonary edema, which is associated with acute lung injury and ARDS, can occur even when the chest X-ray is normal on admission, suggesting that the brain injury itself contributes to pulmonary compromise. Neurogenic pulmonary edema is the extravasation of fluid from the blood into the lungs in patients who have sustained a sudden neurological event and is a common contributor to compromised pulmonary function following TBI. Several theories have been proposed to explain capillary leak in this setting, including increased vascular permeability and hydrostatic forces occurring in association with damage to specialized alveolar epithelial cells, called type II pneumocytes (4). Acute lung injury and ARDS occur in 20–25% of TBI patients and may be related to higher intracranial pressures and lower cerebral perfusion pressures. Here, a “double hit” has been proposed in which a strong adrenergic and inflammatory response to TBI is exacerbated by a second factor, such as mechanical ventilation or infection resulting in neurogenic pulmonary edema (5). However, a more specific mechanism that links TBI to pulmonary compromise has not been explored, and this is where the Weber et al. study comes in.
HMGB1/RAGE, TBI, AND LUNG TRANSPLANTATION
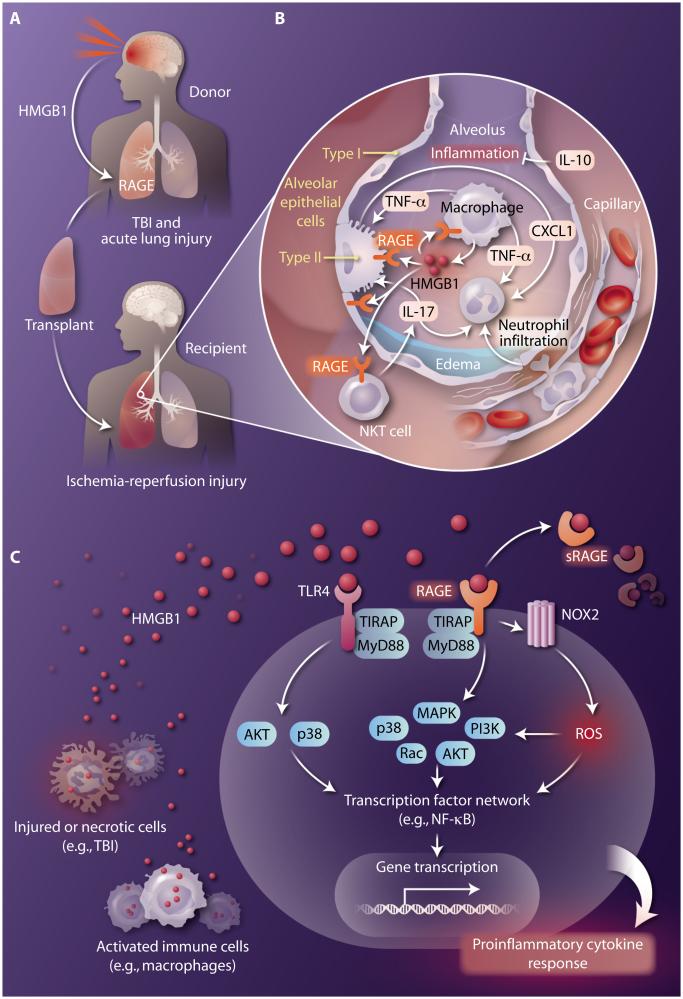
Many potential lung donors suffer TBI, which results in injury to the cerebral vasculature leading to disruption of the blood-brain barrier, thus permitting entry of immune cells and inflammation. The ensuing food of secreted inflammatory mediators such as cytokines and chemokines as well as damage-associated molecular patterns (DAMPs) released by injured cells trigger further brain inflammation and also affect distal organs including the lungs (Fig. 1A). Unfortunately, mechanisms of systemic inflammation and multi-organ injury following TBI have not been well studied. DAMPs are endogenous molecules released by stressed or injured cells that act as danger signals to promote an inflammatory response. The most extensively studied DAMP is high-mobility group box-1 (HMGB1), a ubiquitous nuclear protein that, among a variety of functions, serves as an early mediator of inflammation and plays a key role in many pathogenic states, including TBI and ischemia-reperfusion injury (6). Ischemia-reperfusion injury is a rapid inflammatory response that occurs in transplanted lungs when pulmonary tissues that have not been perfused for several hours (and hence are ischemic) are suddenly perfused with oxygenated blood at the time of transplantation. In these instances, HMGB1 can be passively released by necrotic cells or actively secreted by inflammatory cells (such as macrophages) and endothelial cells. In the case of TBI, HMGB1 is released by necrotic neurons and promotes neuroinflammatory responses through activation of microglia (7). In lung ischemia-reperfusion injury, in addition to passive release from damaged cells, HMGB1 appears to be actively released by alveolar macrophages and mediates ischemia-reperfusion injury by enhancing interleukin-17 (IL-17) production by invariant natural killer T (NKT) cells, leading to chemokine production (for example, CXCL1 by epithelial cells) and subsequent neutrophil infiltration (8) (Fig. 1B).
Fig. 1. HMGB1, TBI, and lung transplantation.
HMGB1 released in response to traumatic brain injury (TBI) or ischemia-reperfusion injury in the donor mediates lung dysfunction after transplantation. (A) TBI in a potential lung donor disrupts the blood-brain barrier, releasing HMGB1, which leads to acute lung injury and the binding of HMGB1 to RAGE. Transplantation of lungs from donors with TBI results in an increase in ischemia-reperfusion injury in the donor lungs in recipients. (B) Lung ischemia-reperfusion injury after transplant is mediated, in part, by the HMGB1/RAGE axis and can result in primary graft dysfunction involving edema formation and impaired gas exchange. HMGB1 (produced by alveolar macrophages) augments production of the proinflammatory cytokine IL-17 by NKT cells and CXCL1 production by type 2 alveolar epithelial cells, resulting in neutrophil infiltration and activation, which are key events in inflammation and tissue injury. Enhanced production of the anti-inflammatory cytokine IL-10 (from various sources) can deter innate immune cell activation and inflammation. (C) Common HMGB1-mediated signaling pathways in a target cell. HMGB1, released from necrotic cells or secreted by activated immune cells, signals by binding to RAGE and/or TLR4 (with associated regulators such as TIRAP and MyD88) to stimulate various signaling cascades that activate proinflammatory transcription factors such as NF-κB. The enzymatic cleavage of membrane-bound RAGE releases soluble RAGE (sRAGE), which also can bind to HMGB1 to serve as a decoy receptor. HMGB1-RAGE signaling can also induce production of reactive oxygen species (ROS) through activation of NADPH oxidase (NOX2), which also leads to activation of NF-κB. The end result is rapid transcription of genes encoding proinflammatory cytokines to elicit an inflammatory, injurious response.
HMGB1 activates inflammatory responses primarily through binding to receptor for advanced glycation end products (RAGE) and/or Toll-like receptors (TLRs), such as TLR4. It has been suggested that RAGE and TLRs might interact to coordinate immune and inflammatory responses through synergistic effects, and this certainly may be the case for lung ischemia-reperfusion injury in which HMGB1, RAGE and TLR4 have all been implicated. RAGE activation induces a multitude of signaling pathways, including generation of reactive oxygen species via activation of the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, which culminate in the activation of transcription factors such as nuclear factor κB (NF-κB) and subsequent expression of pro-inflammatory cytokines and chemokines (Fig. 1C). RAGE is expressed by many cell populations, including macrophages and NKT cells, but is most highly expressed on lung epithelial cells, which is one reason why the lung is so sensitive to damage after TBI or ischemia-reperfusion injury. In fact, circulating sRAGE (a soluble, truncated form that competes with membrane-bound RAGE for ligand binding) is considered to be a marker of epithelial cell injury, and plasma sRAGE concentrations have been shown to correlate with primary graft dysfunction after lung transplantation (9).
The new study by Weber and colleagues now demonstrates that TBI results in acute lung injury (increased alveolar hemorrhage, neutrophil infiltration, and vascular leak) and dysfunction (increased lung stiffness and poor oxygenation) in the first 24 hours after TBI. This acute lung injury was accompanied by a doubling in serum HMGB1 concentrations along with evidence that neocortical brain cells were a source of HMGB1 following TBI. Furthermore, TBI-induced lung injury was attenuated in mice lacking RAGE as well as in wild-type mice treated with anti-HMGB1 neutralizing antibody. Mice lacking TLR4 were also protected but not to the same extent as animals lacking RAGE. This is not surprising, as TLRs share several intracellular signaling pathways with RAGE. These results demonstrate that the HMGB1/RAGE axis serves as a central signaling mechanism for pulmonary injury and dysfunction after TBI.
The authors then addressed the core question of the study: Does TBI in lung donors enhance ischemia-reperfusion injury (primary graft dysfunction) after lung transplantation? To answer this question, the researchers utilized an elegant mouse lung transplantation model. In short, the authors demonstrated that recipients of donor lungs from wild-type mice that suffered TBI showed greater ischemia-reperfusion injury than did recipients of donor lungs from control wild-type mice with no TBI. This difference was largely eliminated if the recipient mice received donor lungs from animals with TBI that lacked RAGE. Although the expression of many cytokines after transplantation was similar between transplanted donor lungs from wild-type and RAGE-deficient mice, two key diferences stood out: In RAGE-deficient donor lungs, there was an increase in the anti-inflammatory cytokine IL-10 and a decrease in the pro-inflammatory cytokine IL-17. These results suggest that RAGE activation regulates the IL-10/IL-17 axis and confirms previous findings that HMGB1/RAGE signaling enhances IL-17 production by NKT cells, resulting in lung ischemia-reperfusion injury (8). Last, the authors measured HMGB1 concentrations in blood from brain-dead human donors, including 11 of 18 who died from TBI. They found that elevated HMGB1 concentrations in blood correlated with poor blood oxygenation both in the donor lung prior to transplantation as well as in the recipient after transplantation. Taken together, the findings of Weber et al. reveal a HMGB1/RAGE-mediated mechanism for TBI-induced lung injury.
INTO THE CLINIC
Lung transplants currently have a dismal 5-year survival rate of ~55%, and improvements are desperately needed, not only to increase the donor lung pool size but also to improve long-term outcomes. In this regard, one of the most exciting recent developments in the field involves ex vivo lung perfusion for the evaluation and rehabilitation of donor lungs prior to transplantation (10). In this process, suboptimal lungs that may be infected, inflamed or compromised in other ways (such as poorly inflated areas of the organ) can be rendered suitable for transplantation. Thus, from this perspective, preconditioning or rehabilitation of donor lungs from individuals who died of TBI using ex vivo lung perfusion combined with therapeutic targeting of the HMGB1/RAGE axis could, in addition to expanding the donor lung pool size, also improve long-term outcomes following lung transplantation. Of even greater significance to the general population, the targeting of the HMGB1/RAGE axis has the potential to prevent the pulmonary complications of TBI and thus improve the survival of the many patients who currently succumb to head wounds. In conclusion, therapeutic strategies to block RAGE activation soon after brain trauma may not only save more lives by increasing available organs for transplantation but also limit the progression of systemic manifestations that currently make TBI such a lethal process.
REFERENCES
- 1.Kemp CD, Cotton BA, Johnson JC, Ellzey M, Pinson CW. Donor conversion and organ yield in traumatic brain injury patients: Missed opportunities and missed organs. J. Trauma. 2008;64:1573–1580. doi: 10.1097/TA.0b013e318068fc2f. [DOI] [PubMed] [Google Scholar]
- 2.Weber DJ, Gracon ASA, Ripsch MS, Fisher AJ, Cheon BM, Pandya PH, Vittal R, Capitano ML, Kim Y, Allete YM, Riley AA, McCarthey BP, Territo PR, Hutchins GD, Broxmeyer HE, Sandusky GE, White FA, Wilkes DS. The HMGB1-RAGE axis mediates traumatic brain injury–induced pulmonary dysfunction in lung transplantation. Sci. Transl. Med. 2014;6:251ra118. doi: 10.1126/scitranslmed.3009443. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Lee K, Rincon F. Pulmonary complications in patients with severe brain injury. Crit. Care Res. Pract. 2012;2012:207247. doi: 10.1155/2012/207247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yildirim E, Kaptanoglu E, Ozisik K, Beskonakli E, Okutan O, Sargon MF, Kilinc K, Sakinci U. Ultrastructural changes in pneumocyte type II cells following traumatic brain injury in rats. Eur. J. Cardiothorac. Surg. 2004;25:523–529. doi: 10.1016/j.ejcts.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 5.Mascia L. Acute lung injury in patients with severe brain injury: A double hit model. Neurocrit. Care. 2009;11:417–426. doi: 10.1007/s12028-009-9242-8. [DOI] [PubMed] [Google Scholar]
- 6.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu. Rev. Immunol. 2011;29:139–162. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laird MD, Shields JS, Sukumari-Ramesh S, Kimbler DE, Fessler RD, Shakir B, Youssef P, Yanasak N, Vender JR, Dhandapani KM. High mobility group box protein-1 promotes cerebral edema after traumatic brain injury via activation of toll-like receptor 4. Glia. 2014;62:26–38. doi: 10.1002/glia.22581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma AK, LaPar DJ, Stone ML, Zhao Y, Kron IL, Laubach VE. Receptor for advanced glycation end products (RAGE) on iNKT cells mediates lung ischemia-reper-fusion injury. Am. J. Transplant. 2013;13:2255–2267. doi: 10.1111/ajt.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christie JD, Shah CV, Kawut SM, Mangalmurti N, Lederer DJ, Sonett JR, Ahya VN, Palmer SM, Wille K, Lama V, Shah PD, Shah A, Weinacker A, Deutschman CS, Kohl BA, Demissie E, Bellamy S, Ware LB, Lung Transplant Outcomes Group Plasma levels of receptor for advanced glycation end products, blood transfusion, and risk of primary graft dysfunction. Am. J. Respir. Crit. Care Med. 2009;180:1010–1015. doi: 10.1164/rccm.200901-0118OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cypel M, Yeung JC, Liu M, Anraku M, Chen F, Karolak W, Sato M, Laratta J, Azad S, Madonik M, Chow CW, Chaparro C, Hutcheon M, Singer LG, Slutsky AS, Yasufuku K, de Perrot M, Pierre AF, Waddell TK, Keshavjee S. Normothermic ex vivo lung perfusion in clinical lung transplantation. N. Engl. J. Med. 2011;364:1431–1440. doi: 10.1056/NEJMoa1014597. [DOI] [PubMed] [Google Scholar]