Abstract
Objective
Currently, accurately identifying endometrial cancer patients at high risk for recurrence remains poor. To ascertain if changes in the endoplasmic reticulum (ER) stress marker, glucose-regulated-protein-78 (GRP78) can serve as a prognosticator in endometrial cancer, we examined GRP78 expression in patient samples to determine its association with clinical outcome.
Methods
A retrospective cohort study was conducted in endometrial cancer patients. Archived specimens of visceral adipocytes and paired endometrial tumors were analyzed by immunohistochemistry for GRP78 and another ER stress marker, C/EBP homologous protein (CHOP). Expression of these markers was correlated with clinico-pathological information and outcomes.
Results
GRP78 expression in visceral adipocytes was detected in 95% of the 179 endometrial cancer patients with analyzable visceral adipocytes. Within individual samples, 24% of adipocytes (range, 0-90%, interquartile range 18%-38%) exhibited GRP78 expression. High visceral adipocyte GRP78 expression positively correlated with advanced-stage disease (p=0.007) and deep myometrial invasion (p=0.004). High visceral adipocyte GRP78 expression was significantly associated with decreased disease-free survival (DFS) in multivariate analyses (hazard ratio 2.88, 95% CI 1.37-6.04, p=0.005). CHOP expression paralleled the GRP78 expression in adipocytes (r=0.55, p<0.001) and in the tumor (p=0.018).
Conclusions
Our study demonstrates that the ER stress markers, GRP78 and CHOP, are elevated in endometrial cancer patients. Furthermore, GRP78 expression levels in visceral adipocytes from these patients were significantly correlated to disease stage and patient survival. Our results demonstrate, for the first time, that the GRP78 levels in endometrial cancer patients may be a prognosticator and aid with clinical risk stratification and focused surveillance.
Keywords: endometrial cancer, endoplasmic reticulum (ER) stress, adipocyte
Introduction
In 2012, approximately 47,130 women in the United States will be diagnosed with endometrial cancer (EC) with approximately 8,010 succumbing to the disease, mostly due to relapse and metastasis [1]. Currently, histological characteristics of endometrial tumors provide marginal insight into patients' recurrence risk. This highlights the need for indicators that reflect unique molecular processes in EC to allow for focused surveillance, early intervention, and improved patient survival.
Obesity is a well-recognized EC risk factor [2,3]. However, the prognostic utility of obesity for EC development remains poor [4,5]. Some have proposed an incremental increase in EC risk for every 5 kg/m2 increase in body mass index (BMI); however, whether traditional anthropometric measurements appropriately reflect the putative mechanisms of obesity is still unknown [6,7]. With nearly two-thirds of all US female adults considered to be obese or overweight, the impending escalation of obesity-associated health problems poses a major health issue [8,9]. Therefore, understanding mechanisms linking obesity to EC development is critical [10]. Although unopposed estrogen due to the peripheral conversion of androstenedione to estrone has been a classical paradigm for EC development in obese women, increasing data now highlight the critical and putative role of the endoplasmic reticulum (ER) and ER stress in obesity [11-13]. The ER is an organelle with important roles in the folding and processing of membrane-bound and secretory proteins.
During cellular perturbations, such as those encountered by adipocytes in obesity [14], the protein load in the ER exceeds its folding capacity, resulting in the retention of misfolded proteins within the ER, and consequently, ER stress. Activation of the unfolded protein response (UPR) alleviates ER stress by decreasing general protein translation and increasing the protein folding capacity of the ER [11]. One of the most critical UPR regulators is the 78-kDa glucose-regulated protein (GRP78), a molecular chaperone protein and member of the heat shock protein (HSP)-70 family, also referred to as BiP/HSPA5 [11,15]. In addition to its association with obesity, ER stress and GRP78 overexpression also occur in many human cancers, including breast, lung, gastric, prostate, hematologic, and head and neck [11,16-32]. GRP78 expression has been shown to be critical for tumor progression and chemoresistance, and is negatively associated with survival [11,15,33]. How GRP78 contributes to cancer progression is under investigation, but its overexpression is associated with UPR activation, including upregulation of associated proteins: protein kinase RNA-like ER kinase (PERK), activating transcription factor-6 (ATF6), and CCAAT/enhancer-binding protein homologous protein (CHOP). These factors can regulate key signaling pathways, including JNK and NF-κB, and can enhance production of secreted factors thereby increasing cell survival and proliferation [11].
Despite the strong association between obesity and EC, it is unclear if ER stress occurs in EC cells and/or adipocytes in these patients. While the role of ER stress and activation of the UPR in enhancing the oncogenic potential of tumor cells is well established, it is uncertain if adipocytes in close proximity to tumor cells mediate similar changes in response to ER stress and UPR activation. This would be of particular interest particularly since emerging reports now demonstrate close cross-talk between tumor cells and cancer-associated adipocytes that may promote a more aggressive and invasive phenotype in these tumor cells [34]. To further elucidate the relationship between visceral adipocytes and EC, we hypothesize that increased ER stress in visceral adipocytes is an important determinant of disease progression in patients with EC. Here, we demonstrate for the first time that ER stress markers in adipocytes are elevated in EC patients, and that GRP78 levels in visceral adipocytes correlate with disease state and survival. Our findings suggest that the level of the ER stress marker, GRP78, in visceral adipocytes may represent molecular prognosticators for EC patients that may help inform clinical management.
Patients and Methods
Clinical samples and data
After Institutional Review Board (IRB) approval, archived formalin-fixed, paraffin-embedded (FFPE) specimens of both the uterus and visceral adipose tissue (omentum or peri-nodal adipose tissue) from patients who underwent primary surgical treatment for endometrial adenocarcinoma of the endometrium from January 2000 to July 2010 were retrieved. A section of normal endometrium from the same patient served as an internal control. Data about patient demographics, surgery, pathological diagnoses, treatment, and clinical outcomes were culled from patient records. All visceral adipose samples were evaluated by study pathologists to assure no tumor within the tissue block prior to analyses. Endometrial samples from 15 hysterectomies performed for benign non-uterine indications were evaluated as controls; patients with no cancer served as negative controls for comparative analyses.
Immunohistochemistry staining
Immunohistochemical analyses for GRP78 (H-129, Santa Cruz Biotechnology, Santa Cruz, CA) and the additional ER stress marker, CHOP/GADD153 (F-168, Santa Cruz Biotechnology) were performed on FFPE samples. Briefly, freshly cut 5-μm samples were deparaffinized and rehydrated in declining grades of ethanol. Antigen retrieval was performed using the Retrievagen A kit (BD Pharmingen, Carpenteria, CA) with pressure cooker. Endogenous peroxidases were blocked with 3% H2O2-PBS (Thermo-Fisher Scientific, Pittsburg, PA) for 12-min at room temperature (RT). Non-specific epitopes were blocked with 5% normal horse serum for 20-min at RT. Sections were incubated with primary antibodies (GRP78, 1:100; CHOP, 1:50) at 4°C overnight with appropriate negative controls. Vectastain Elite® avidin-biotin complex (ABC) kit (Vector Laboratories, Burlingame, CA) was used with the species-specific secondary antibodies (45-min, RT) per manufacturer instructions followed by 3,3′-diaminobenzidine (DAB, Phoenix Biotechnologies, Huntsville, AL) and counterstaining with Gill's No. 3 hematoxylin (Sigma-Aldrich, St. Louis, MO).
Evaluation of GRP78 overexpression
Immunostaining intensity within the endometrial tumor, normal endometrium, and visceral adipocytes was defined as follows: 0=absent, 1=weak, 2=moderate, and 3=strong. The percent distribution of GRP78 expression throughout the tumor and normal endometrium was scored: 0=0%; 1=1 to <10%, 2=10-50%, and 3=>50%. Figure 1 illustrates representative staining patterns for both the endometrium and adipocytes. As before, overexpression was defined when both intensity and distribution scores are ≥2 [34]. Because the adipocyte cytoplasm is generally displaced peripherally by a lipid droplet, adipocytes expressing GRP78 of any intensity (1, 2, or 3) were defined as positive. The extent of GRP78 distribution within visceral adipocytes was determined by the ratio of GRP78-expressing adipocytes divided by the total number of adipocytes quantified over 5 separate high-power-fields (HPF, 40×). Quantification of nuclear staining for CHOP in adipocytes and tumor cells was performed similarly to GRP78.
Fig. 1.
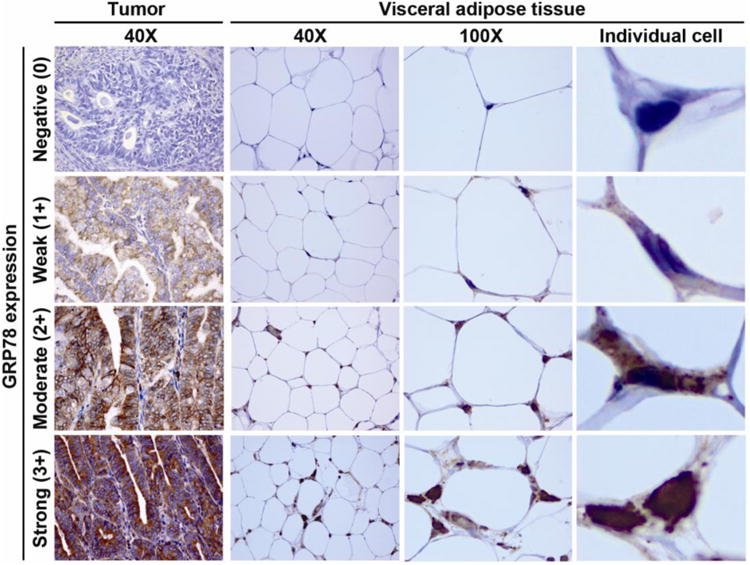
GRP78 immunohistochemistry in endometrial tumor and visceral adipocytes.
Intensity is determined as negative (0), weak (1+), moderate (2+), and strong (3+) in tumor and adipose tissue. Individual cell: adipocyte from 100× image is magnified. Abbreviation: GRP78, glucose-regulated-protein-78.
GRP78 and CHOP expression were independently determined by two surgical pathologists (SAS, LD) blinded to clinical information. A κ-statistic of 0.801 ± 0.064 (p<0.0001) confirmed excellent agreement between pathologists. GRP78 expression in adipocytes digitally calculated using the product of density- and pixel-measurements (Adobe Photoshop CS2, San Jose, CA) also correlated well with immunohistochemistry scores by the study pathologists (Spearman's correlation r=0.55, p<0.001). All microscopy was performed using the Olympus-BX51 microscope with the Olympus-DP25 camera (Olympus America, Inc., Center Valley, PA).
Statistical analysis
Disease-free survival (DFS), defined as the time from the date of hysterectomy to the date of tumor recurrence or death, or the date of last contact if the patient was alive and disease-free, was the primary endpoint. Overall survival (OS), as defined by the date of hysterectomy to the date of death from any cause or date of last contact, was also evaluated. Associations between GRP78 expression and clinic-pathological parameters were examined using Chi-square, Fisher's exact, Wilcoxon, or Kruskal- Wallis tests, as appropriate. Associations between GRP78 and survival were evaluated using Kaplan-Meir curves and log-rank tests in the univariate analysis, and multivariable Cox proportional hazards regression models to account for other potential prognostic factors. All statistical tests were 2-sided at a significance level of 0.05 and conducted using the Statistical Analysis System statistical package (SAS Institute Inc. version 9.2, Cary, NC).
Results
Patient characteristics and GRP78 expression
Among 246 EC patients with available tissue, 227 cases had sections of endometrial tumor, 120 had sections of normal endometrium, and 179 had visceral adipose tissue available for immunohistochemical analysis. Demographic characteristics of the study population are listed in Table 1. Most patients were Hispanic (72%), had stage I disease (65%), and had endometrioid histology (85%). The majority of the study patients were obese (median BMI 34.6 kg/m2, range 20.0-74.1kg/m2). Thirty-five patients developed disease recurrence during this follow-up period (median 2.3 years, range 0.01-10.6 years). The 5-year DFS was 78% (± 0.04%) for this study population. On univariate analysis, stage, grade, histology, presence of lymphovascular space invasion (LVSI), deep myometrial invasion, and elevated CA-125 were associated with decreased DFS and OS (Table 1).
Table 1. Demographic characteristics of endometrial cancer patients.
| No. (%) | Disease-free survival | Overall survival | |||
|---|---|---|---|---|---|
|
| |||||
| HR (95% CI)* | P*-value | HR (95% CI) | P*-value | ||
| Age (median, range) | 53 (24-80) | 0.13 | 0.4 | ||
| <50 | 91 (37%) | 1 | 1 | ||
| ≥50 | 155 (63%) | 1.77 (0.83, 3.78) | 1.63 (0.52, 5.11) | ||
|
| |||||
| Race | 0.49 | 0.24 | |||
| Hispanic | 177 (72%) | 1 | 1 | ||
| Asian | 36 (15%) | 1.24 (0.50, 3.07) | |||
| White | 22 (9%) | 1.65 (0.62, 4.35) | 1.62 (0.46, 5.75) | ||
| Black | 11 (4%) | 2.52 (0.59, 10.77) | |||
|
| |||||
| Nulligravida | 0.98 | 0.42 | |||
| No | 155 (69%) | 1 | 1 | ||
| Yes | 69 (31%) | 0.99 (0.47, 2.10) | 0.59 (0.16, 2.14) | ||
|
| |||||
| BMI (kg/m2) (median, range) | 34.6 (20.0, 74.1) | 0.21 | 0.34 | ||
| <30 | 68 (31%) | 1 | 1 | ||
| ≥30 | 149 (69%) | 0.65 (0.32, 1.30) | 0.59 (0.20, 1.77) | ||
|
| |||||
| Diabetes | 0.24 | 0.3 | |||
| No | 143 (60%) | 1 | 1 | ||
| Yes | 94 (40%) | 0.64 (0.30, 1.36) | 0.52 (0.14, 1.85) | ||
|
| |||||
| CA-125 (IU/L) (median, range) | 19 (2, 5421) | <.001 | 0.032 | ||
| <35 | 156 (72%) | 1 | 1 | ||
| ≥35 | 61 (28%) | 4.63 (2.25, 9.55) | 3.10 (1.04, 9.24) | ||
|
| |||||
| FIGO Stage | <.001 | <.001 | |||
| I | 161 (65%) | 1 | 1 | ||
| II | 31 (13%) | 5.34 (1.20, 23.73) | 6.92 (0.63, 76.28) | ||
| III | 39 (16%) | 23.90 (7.02, 81.39) | 31.18 (3.96, 245.6) | ||
| IV | 15 (6%) | 61.73 (17.22, 221.4) | 33.09 (3.45, 317.3) | ||
|
| |||||
| Histology | <.001 | <.001 | |||
| Endometrioid | 208 (85%) | 1 | 1 | ||
| Non-endometrioid | 38 (15%) | 6.27 (3.23, 12.18) | 10.65 (3.64, 31.17) | ||
|
| |||||
| FIGO grade | <.001 | <.001 | |||
| 1 | 125 (51%) | 1 | 1 | ||
| 2 | 73 (30%) | 2.95 (1.09, 7.96) | 1.48 (0.21, 10.44) | ||
| 3 | 48 (20%) | 8.67 (3.44, 21.84) | 12.49 (2.78, 56.15) | ||
|
| |||||
| LVSI | <.001 | <.001 | |||
| Absence | 193 (79%) | 1 | 1 | ||
| Presence | 51 (21%) | 6.55 (3.30, 13.00) | 20.86 (4.71, 92.39) | ||
|
| |||||
| Deep myometrium invasion | <.001 | <.001 | |||
| No | 168 (71%) | 1 | 1 | ||
| Yes | 69 (29%) | 8.00 (3.72, 17.20) | 6.72 (2.11, 21.42) | ||
Log-rank test for p-values. Total number may not be 246 due to missing data. Deep myometrial invasion defined as tumor invading ≥50% of the myometrium. Abbreviations: HR, hazard ratio; 95% CI, 95% confidence interval; BMI, body mass index; CA-125, cancer antigen 125; FIGO, the International Federation of Gynecology and Obstetrics; and LVSI, lymphovascular space invasion.
To determine whether or not EC itself might be associated with differential GRP78 overexpression in normal endometrium elsewhere in the uterine cavity, the extent of endometrial GRP78 expression was examined in sections of endometrium from patients with and without cancer. The percentage of GRP78 overexpression in histologically normal endometrium from EC patients (n=120) was significantly higher than that seen in normal endometrium from patients without cancer (n=15) (88% vs. 60%, p=0.011, Figure 2A), suggesting that, compared to patients without cancer, EC patients may have higher baseline levels of ER stress within their endometrium. When GRP78 expression was evaluated in tumor sections from patients with EC, we found that approximately two-thirds of tumors overexpressed GRP78 (66%). Non-endometrioid histology was associated with higher GRP78 overexpression in visceral adipocytes when compared to endometrioid histology (non-endometrioid vs. endometrioid, 28% vs. 22%, p=0.031, Figure 2B). Obesity (BMI ≥30 kg/m2) was observed in significantly more patients with tumoral GRP78 overexpression compared to those who did not (BMI <30 vs. ≥30, 74% vs. 62%, p=0.042, Figure 2C). In contrast, obesity was not related to GRP78 overexpression in visceral adipocytes (BMI <30 vs. ≥30, 23% vs. 23%, p=0.56, Figure 2D).
Fig. 2.
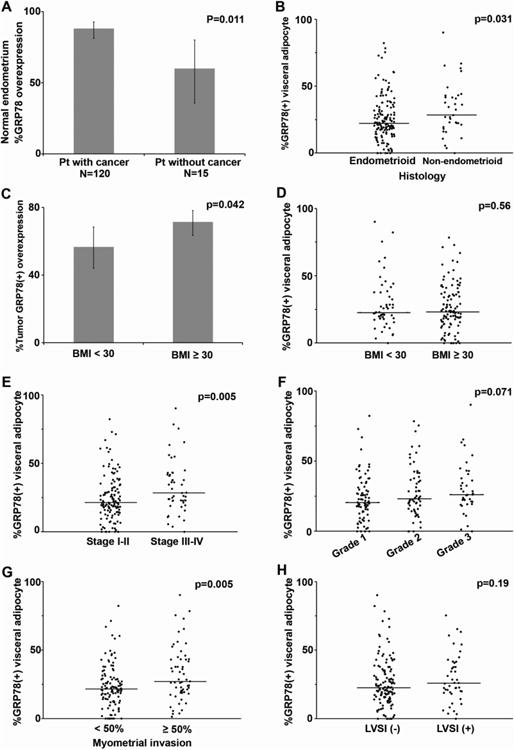
GRP78 expression in visceral adipocytes in endometrial cancer.
P-values were based on Fisher's exact test, Chi-square test, Wilcoxon two-sample test, or Kruskal-Wallis test whenever appropriate. (A) Proportion of GRP78 overexpression in normal endometrium is shown for patient with EC and for patient without cancer. (B) Proportion of GRP78 (+) expression in visceral adipocytes by histology. (C) Proportion of tumoral GRP78 overexpression by obesity. Proportion of GRP78 (+) expression in visceral adipocytes based on obesity (D), FIGO stage (E), FIGO grade (F), deep myometrium invasion (G), and LVSI (+) (H). Abbreviations: GRP78, glucose-regulated protein 78; BMI, body mass index (kg/m2); FIGO, the International Federation of Gynecology and Obstetrics; and LVSI, lymphovascular space invasion.
Assessment of visceral adipocyte GRP78 levels in EC
Among the 179 patients who had available visceral adipocytes for analysis, GRP78 expression was detectable in 171 (95%); 8 (5%) patients had no GRP78 expression within their adipocytes. The median percentage of GRP78-positive-adipocytes per sample was 24% (range, 0-90%; interquartile range 18%-38%). Univariate analysis of GRP78 expression in visceral adipocytes with clinico-pathologic variables showed that increasing percentages of GRP78-expressing-adipocytes significantly correlated with advanced-stage disease (stage III-IV vs. I-II, 28% vs. 21%, p=0.005, Figure 2E). There was a trend of increasing GRP78-expressing adipocytes with grade 3 tumors, but this did not achieve statistical significance (Grade 1, 2, and 3, 21%, 23%, and 26%, p=0.071, Figure 2F).
We then examined GRP78 expression in visceral adipocytes in relation to histological characteristics of the uterus, such as depth of invasion and LVSI, which traditionally represent the aggressiveness of endometrial tumors, and guide clinical management. We found that increasing percentages of GRP78-expressing adipocytes significantly corresponded with deeply invasive tumors (≥50% vs. <50% myometrial invasion, 27% vs. 22%, p=0.005, Figure 2G); however, GRP78 expression in visceral adipocytes did not appear to be as closely associated with LVSI (presence vs. absence, 26% vs. 23%, p=0.19, Figure 2H).
We next examined the impact of GRP78 expression in visceral adipocytes on patient survival. Patients with the highest quartile of GRP78-expressing-adipocytes (quartile 4) had the shortest 5-year disease-free survival (27.9%) compared to those in the 1st, 2nd and 3rd quartiles (84.3%, 77.0%, and 61.3%, respectively, p<0.001, Figure 3A).
Fig. 3.
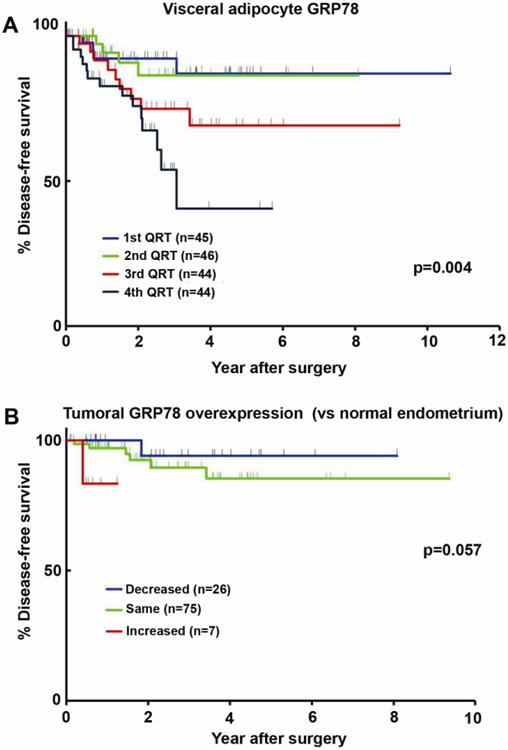
GRP78 expression and endometrial cancer free survival.
Log-rank test. Disease-free survival curves were constructed by visceral adipocyte GRP78 expression using Kaplan-Meier method (A) based on quartiles (B) Survival significance was evaluated for tumoral GRP78 overexpression based on the relative expression to normal endometrium as internal control.
As the primary factor involved in conditions of ER stress, GRP78 was used as the representative marker of ER stress in multivariate analyses. Multivariate analysis with Cox proportional hazard regression models showed that, in addition to stage and grade, the level of visceral adipocyte GRP78 expression was also a significant independent prognostic factor for DFS (hazard ratio 2.877, 95% CI 1.370-6.044, p=0.005, Table 2).
Table 2. Multivariate analysis for disease-free survival outcome in endometrial cancer.
| HR (95% CI) | P-value | |
|---|---|---|
| FIGO stage | 9.192 (3.540, 23.869) | <.0001 |
| FIGO grade | 1.847 (0.873, 3.907) | 0.11 |
| Visceral adipocyte GRP78 | 1.491 (1.017, 2.185) | 0.041 |
Multivariate Cox proportional hazards regression model. Hazard ratio represents stage (III-IV vs. I-II), grade (3 vs. 1-2), and visceral adipocyte GRP78 (quartiles from 1 to 4), respectively. Abbreviations: HR, hazard ratio; 95% CI, 95% confidence interval; FIGO, the International Federation of Gynecology and Obstetrics stage; and GRP78, glucose-regulated protein 78.
Assessment of tumor GRP78 and CHOP in EC
Since histologically normal endometrium away from malignant cells had some degree of GRP78 expression, we examined whether or not malignant endometrium was associated with higher levels of GRP78 expression than observed in contiguous histologically normal endometrium. In 6.48% of patients, there was more GRP78 expression in the primary tumor than that observed in normal endometrium from the same patient. On the other hand, 69.45% of patients had comparable GRP78 expression levels both in the tumor and in the histologically normal endometrium, and 24.07% of patients had decreased tumoral GRP78 expression compared to histologically normal endometrial expression in the same patient. Patients with advanced stage disease in whom tumoral GRP78 expression was relatively higher than that in histologically normal endometrium demonstrated somewhat shorter time to progression compared to those with the same or decreased tumoral GRP78 expression relative to histologically normal endometrium, although this was of borderline statistical significance (p=0.057, Figure 3B). The relatively sparse number of patients with early stage disease who progressed precluded this same evaluation of time to progression. While higher tumoral expression of GRP78 suggests a trend towards worse DFS without reaching statistical significance, GRP78 levels in visceral adipocytes did retain significant prognostic ability in predicting DFS and may represent a more pertinent EC marker than GRP78 levels in the endometrium itself.
To obtain further evidence supporting the presence of ER stress within endometrial tumors and adipocytes, expression of another ER stress marker, CHOP, was examined in endometrial tumors and adipocyte. CHOP expression correlated well to GRP78 overexpression within adipocytes (Spearman's r=0.55, p<0.001, Figure 4A) and in endometrial tumors (GRP78 overexpression in CHOP overexpression vs. non-overexpression, 75% vs. 33%, p=0.018, Figure 4B), suggesting that ER stress occurs in EC and may be a factor in its progression.
Fig. 4.
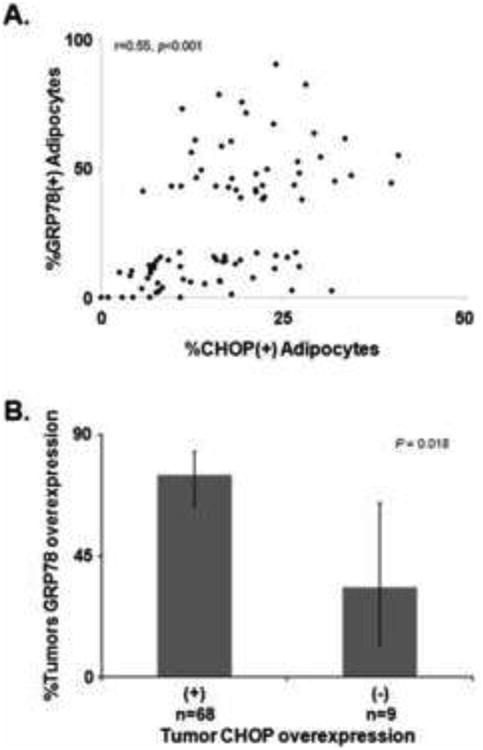
GRP78 and other ER stress markers in endometrial cancer.
(A) Spearman's correlation between percent expression of CHOP and GRP78 in visceral adipocytes. (B) Proportion of GRP78 overexpression in tumor based on CHOP overexpression.
Discussion
Visceral adipocytes as prognostic markers of EC progression are unexplored. Our results suggest that GRP78 expression in visceral adipocytes of EC patients is predictive of clinical outcome. Higher GRP78 levels in visceral adipocytes appear to closely reflect advanced stage and deep myometrial invasion. More specifically, we showed that patients with the highest quartile of GRP78-expressing adipocytes experienced worse overall survival. While interpretation of the full impact of visceral GRP78 expression on overall survival is limited due to the infrequent deaths observed during the study period, the association of visceral GRP78 expression with disease recurrence remains robust. GRP78 levels in visceral adipocytes retained important prognostic utility as a significant predictor of DFS. In short, patients with lower levels of visceral adipocyte GRP78 fared better than those with higher levels.
Precisely how obesity contributes to EC development and progression remains unclear. The causal mechanism for this association has rested on observational studies showing that a hyperestrogenic state in obesity leads to increased conversion of androgens to estrone and estradiol within adipose tissues and mitogenic effects on the endometrium [5,35]. However, increasing evidence suggests that obesity-related estrogen is unlikely to be the single driver for EC development [5]. This suggests other factors including estrogen independent ones may contribute to EC progression, such as those triggered by UPR activation in response to ER stress in EC patients.
To further investigate the effects of obesity on the endometrial tumor, and specifically the relationship of the UPR marker GRP78 in the endometrial tumor itself, particularly among obese women. In doing so, we identified that high tumoral GRP78 expression was more frequently observed among obese patients. Furthermore, we also demonstrated that histologically normal endometrium can variably express GRP78, a finding that is consistent with previously reports [36]. To determine whether or not estrogen plays a direct effect on ER stress in human EC cells, Guzel and colleagues directly applied estradiol to Ishikawa cells, and showed no GRP78 induction [37]. In fact, pre-treatment of both Ishikawa cells and primary endometrial stromal cells appeared to abrogate chemically-induced ER stress, further supporting that estrogen exposure may not directly increase endometrial ER stress [37]. These findings seem to contradict an earlier study by Ray and colleagues that showed that GRP78 levels in murine uteri are up-regulated by exogenously delivered estrogen and estrogen-like compounds. However, these effects seen in mice appear to be independent of canonical estrogen-receptor mediated pathways [38,39]. Recently, Bifulco and colleagues reported increased GRP78 protein levels in Stage I endometrial tumors from 12 patients; however, limited data regarding the inherent variability of GRP78 expression in adjacent normal endometrium, as well as the endometrial histology of control samples make full understanding of these results difficult [40].
While changes in subcutaneous adipocyte GRP78 levels have been noted in obesity studies, our work is the first to record elevated levels of GRP78 in the visceral adipocytes in EC patients [14]. Obesity, also referred to as nutritional excess, is a well-characterized inducer of ER stress [13]. Studies examining the biochemical effects of nutritional excess have shown UPR induction and GRP78 activation in the liver and subcutaneous adipocytes in oncogenic and non-oncogenic murine and human models [14]. While GRP78 is a key component of the UPR, other components of the UPR are likely to also play a role in conditions associated with obesity and metabolic stress. These factors may include CHOP, whose expression in visceral adipocytes, as demonstrated, closely reflected that observed with GRP78 in our patient population. While CHOP activity is typically associated with promoting ER stress induced apoptosis, its function in chronic diseases like cancer remains unclear, especially when occurring in conjunction with GRP78 overexpression.
Recently, the role and function of visceral adipocytes in various cancers has come under increased scrutiny, and evidence suggests that they are not simply benign bystanders [5,41-44]. Nieman and colleagues recently used in vitro and in vivo models to show that adipocytes may directly alter the metastatic phenotype of ovarian cancer cells by metabolic and lipolytic alterations [44]. These findings provide further mechanistic support for new antitumor therapeutic studies focusing upon adipocyte-targeting, and obesity reduction, including a novel, ongoing therapeutic clinical trial [43,45].
Our results demonstrate a link between EC and GRP78 levels in visceral adipocytes. While the conclusions and findings from our study are subject to the limitations of single-institution, retrospective studies (e.g., applicability in other patient populations), we believe the biological process of ER stress seen in visceral adipocytes and their role in endometrial cancer growth merit additional investigation in other patient cohorts. Some of the statistically significant differences we observe in adipocyte GRP78 expression and disease stage or depth of invasion may appear modest in magnitude. However, we believe that exploring these relationships still provides an important framework upon which to better understand the clinical implications of this process. Until now, the contribution of omental adipocytes to cancer development and progression may have been underestimated. We show that the levels of ER stress markers analyzed in visceral adipocytes may potentially play a more predictive role than their levels within the endometrium itself. Indeed, while we did observe more GRP78 overexpression in normal endometrium from patients with cancer than from patients without cancer, we also acknowledge that intrinsic differences between these two populations, including BMI or menopausal status, may also contribute to this difference, further highlighting the dynamic role of ER stress in the endometrium.
Precisely how GRP78 expression in visceral adipocytes undergoing ER stress contributes to EC progression remains unclear. Although tumor aggressiveness and even ER stress may be, in part, due to increased angiogenesis, tumor microvessel density (MVD) in our patient cohort did not appear to correlate with GRP78 expression in the tumor (Wilcoxon test, p=0.53) or in the adipocytes (Spearman's coefficient=0.099, p=0.5; data not shown). While the production of cytokines and adipokines by adipocytes is well-reported in the literature [44,46] the specific inciting factor or molecular event triggering this production and release is not understood. Given the critical role GRP78 plays in the tumor development and progression, one may speculate that ER stress and GRP78 in adipocytes may elicit increased adipokine production that acts on adjacent tumor cells and/or the local microenvironment and enhances progression. Conversely, EC cells experiencing ER stress may be undergoing changes in expression of factors that impacts their local environment, giving rise to ER stress in neighboring cells including visceral adipocytes.
Currently, the precise relationship between UPR activation in the endometrium in EC development and progression are not clearly understood. We demonstrate that GRP78 levels in visceral adipocytes correlate with disease stage and patient survival, and may have clinical utility as a prognosticator for EC. Lastly, our findings may represent a unique opportunity to assay for ER stress and GRP78 induction in easily accessible tissues, which may guide clinical management for EC patients.
Research Highlights.
Increased GRP78 expression in visceral adipocytes is associated with advanced endometrial cancer stage and shortened survival.
Further understanding of the endoplasmic reticulum stress within the large reservoir of visceral adipocytes in the omentum may provide insight into clinical management.
Acknowledgments
The authors acknowledge Kyle T. Pfaffenbach, PhD for his thoughtful discussion during the study inception.
Funding sources: This work was supported by the USC Department of Obstetrics/Gynecology Seed Grant (YGL), Stop Cancer Career Development Award (YGL), and NCI Cancer Center Support Grant (P30CA014089, ASL/YGL).
Footnotes
Conflict of Interest statement: The authors declare that there are no conflicts of interest in all authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Burke TW, Fowler WC, Jr, Morrow CP. Clinical aspects of risk in women with endometrial carcinoma. J Cell Biochem Suppl. 1995;23:131–6. doi: 10.1002/jcb.240590917. [DOI] [PubMed] [Google Scholar]
- 3.Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev. 2002;11:1531–43. [PubMed] [Google Scholar]
- 4.McTiernan A, Irwin M, Vongruenigen V. Weight, physical activity, diet, and prognosis in breast and gynecologic cancers. J Clin Oncol. 2010;28:4074–80. doi: 10.1200/JCO.2010.27.9752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmandt RE, Iglesias DA, Co NN, Lu KH. Understanding obesity and endometrial cancer risk: opportunities for prevention. Am J Obstet Gynecol. 2011;205:518–25. doi: 10.1016/j.ajog.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munstedt K, Wagner M, Kullmer U, Hackethal A, Franke FE. Influence of body mass index on prognosis in gynecological malignancies. Cancer Causes Control. 2008;19:909–16. doi: 10.1007/s10552-008-9152-7. [DOI] [PubMed] [Google Scholar]
- 7.Temkin SM, Pezzullo JC, Hellmann M, Lee YC, Abulafia O. Is body mass index an independent risk factor of survival among patients with endometrial cancer? Am J Clin Oncol. 2007;30:8–14. doi: 10.1097/01.coc.0000236047.42283.b8. [DOI] [PubMed] [Google Scholar]
- 8.CDC. Vital signs: state-specific obesity prevalence among adults --- United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:951–5. [PubMed] [Google Scholar]
- 9.Modesitt SC, van Nagell JR., Jr The impact of obesity on the incidence and treatment of gynecologic cancers: a review. Obstet Gynecol Surv. 2005;60:683–92. doi: 10.1097/01.ogx.0000180866.62409.01. [DOI] [PubMed] [Google Scholar]
- 10.Grodin JM, Siiteri PK, MacDonald PC. Source of estrogen production in postmenopausal women. J Clin Endocrinol Metab. 1973;36:207–14. doi: 10.1210/jcem-36-2-207. [DOI] [PubMed] [Google Scholar]
- 11.Pfaffenbach KT, Lee AS. The critical role of GRP78 in physiologic and pathologic stress. Curr Opin Cell Biol. 2011;23:150–6. doi: 10.1016/j.ceb.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–61. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 14.Sharma NK, Das SK, Mondal AK, Hackney OG, Chu WS, Kern PA, et al. Endoplasmic reticulum stress markers are associated with obesity in nondiabetic subjects. J Clin Endocrinol Metab. 2008;93:4532–41. doi: 10.1210/jc.2008-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang M, Wey S, Zhang Y, Ye R, Lee AS. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal. 2009;11:2307–16. doi: 10.1089/ars.2009.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiu CC, Lin CY, Lee LY, Chen YJ, Lu YC, Wang HM, et al. Molecular chaperones as a common set of proteins that regulate the invasion phenotype of head and neck cancer. Clin Cancer Res. 2011;17:4629–41. doi: 10.1158/1078-0432.CCR-10-2107. [DOI] [PubMed] [Google Scholar]
- 17.Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496–9. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- 18.Pootrakul L, Datar RH, Shi SR, Cai J, Hawes D, Groshen SG, et al. Expression of stress response protein Grp78 is associated with the development of castration-resistant prostate cancer. Clin Cancer Res. 2006;12:5987–93. doi: 10.1158/1078-0432.CCR-06-0133. [DOI] [PubMed] [Google Scholar]
- 19.Daneshmand S, Quek ML, Lin E, Lee C, Cote RJ, Hawes D, et al. Glucose-regulated protein GRP78 is up-regulated in prostate cancer and correlates with recurrence and survival. Hum Pathol. 2007;38:1547–52. doi: 10.1016/j.humpath.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 20.de Ridder G, Ray R, Misra UK, Pizzo SV. Modulation of the unfolded protein response by GRP78 in prostate cancer. Methods Enzymol. 2011;489:245–57. doi: 10.1016/B978-0-12-385116-1.00014-5. [DOI] [PubMed] [Google Scholar]
- 21.Langer R, Feith M, Siewert JR, Wester HJ, Hoefler H. Expression and clinical significance of glucose regulated proteins GRP78 (BiP) and GRP94 (GP96) in human adenocarcinomas of the esophagus. BMC Cancer. 2008;8:70. doi: 10.1186/1471-2407-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim SO, Park SG, Yoo JH, Park YM, Kim HJ, Jang KT, et al. Expression of heat shock proteins (HSP27, HSP60, HSP70, HSP90, GRP78, GRP94) in hepatitis B virus-related hepatocellular carcinomas and dysplastic nodules. World J Gastroenterol. 2005;11:2072–9. doi: 10.3748/wjg.v11.i14.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin CY, Chen WH, Liao CT, Chen IH, Chiu CC, Wang HM, et al. Positive association of glucose-regulated protein 78 during oral cancer progression and the prognostic value in oral precancerous lesions. Head Neck. 2010;32:1028–39. doi: 10.1002/hed.21287. [DOI] [PubMed] [Google Scholar]
- 24.Marcinak SJ, Ron D. The unfolded protein response in lung disease. Proc Am Thorac Soc. 2010;7:356–62. doi: 10.1513/pats.201001-015AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Misra UK, Payne S, Pizzo SV. Ligation of prostate cancer cell surface GRP78 activates a proproliferative and antiapoptotic feedback loop: a role for secreted prostate-specific antigen. J Biol Chem. 2011;286:1248–59. doi: 10.1074/jbc.M110.129767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uematsu K, Ogata S, Nakanishi K, Hiroi S, Tominaga S, Aida S, et al. Glucose-regulated protein 78 expression in urothelial carcinoma of the upper urinary tract. BJU Int. 2010;106:873–8. doi: 10.1111/j.1464-410X.2009.09144.x. [DOI] [PubMed] [Google Scholar]
- 27.Uramoto H, Sugio K, Oyama T, Nakata S, Ono K, Yoshimastu T, et al. Expression of endoplasmic reticulum molecular chaperone Grp78 in human lung cancer and its clinical significance. Lung Cancer. 2005;49:55–62. doi: 10.1016/j.lungcan.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 28.Wang Q, He Z, Zhang J, Wang Y, Wang T, Tong S, et al. Overexpression of endoplasmic reticulum molecular chaperone GRP94 and GRP78 in human lung cancer tissues and its significance. Cancer Detect Prev. 2005;29:544–51. doi: 10.1016/j.cdp.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Jiang Y, Jia Z, Li Q, Gong W, Wang L, et al. Association of elevated GRP78 expression with increased lymph node metastasis and poor prognosis in patients with gastric cancer. Clin Exp Metastasis. 2006;23:401–10. doi: 10.1007/s10585-006-9051-9. [DOI] [PubMed] [Google Scholar]
- 30.Zhang LH, Zhang X. Roles of GRP78 in physiology and cancer. J Cell Biochem. 2010;110:1299–305. doi: 10.1002/jcb.22679. [DOI] [PubMed] [Google Scholar]
- 31.Zheng HC, Takahashi H, Li XH, Hara T, Masuda S, Guan YF, et al. Overexpression of GRP78 and GRP94 are markers for aggressive behavior and poor prognosis in gastric carcinomas. Hum Pathol. 2008;39:1042–9. doi: 10.1016/j.humpath.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Zhuang L, Scolyer RA, Lee CS, McCarthy SW, Cooper WA, Zhang XD, et al. Expression of glucose-regulated stress protein GRP78 is related to progression of melanoma. Histopathology. 2009;54:462–70. doi: 10.1111/j.1365-2559.2009.03242.x. [DOI] [PubMed] [Google Scholar]
- 33.Lee AS. The Par-4-GRP78 TRAIL, more twists and turns. Cancer Biol Ther. 2009;8:2103–5. doi: 10.4161/cbt.8.22.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71:2455–65. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- 35.Lee E, Nichols P, Spicer D, Groshen S, Yu MC, Lee AS. GRP78 as a novel predictor of responsiveness to chemotherapy in breast cancer. Cancer Res. 2006;66:7849–53. doi: 10.1158/0008-5472.CAN-06-1660. [DOI] [PubMed] [Google Scholar]
- 36.Byers T, Sedjo RL. Does intentional weight loss reduce cancer risk? Diabetes Obes Metab. 2011;13:1063–72. doi: 10.1111/j.1463-1326.2011.01464.x. [DOI] [PubMed] [Google Scholar]
- 37.Guzel E, Basar M, Ocak N, Arici A, Kayisli UA. Bidirectional interaction between unfolded-protein-response key protein HSPA5 and estrogen signaling in human endometrium. Biol Reprod. 2011;85:121–7. doi: 10.1095/biolreprod.110.089532. [DOI] [PubMed] [Google Scholar]
- 38.Ray S, Hou X, Zhou HE, Wang H, Das SK. Bip is a molecular link between the phase I and phase II estrogenic responses in uterus. Mol Endocrinol. 2006;20:1825–37. doi: 10.1210/me.2006-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ray S, Xu F, Li P, Sanchez NS, Wang H, Das SK. Increased level of cellular Bip critically determines estrogenic potency for a xenoestrogen kepone in the mouse uterus. Endocrinology. 2007;148:4774–85. doi: 10.1210/en.2007-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bifulco G, Miele C, Di Jeso B, Beguinot F, Nappi C, Di Carlo C, et al. Endoplasmic reticulum stress is activated in endometrial adenocarcinoma. Gynecol Oncol. 2012;125:220–5. doi: 10.1016/j.ygyno.2011.11.045. [DOI] [PubMed] [Google Scholar]
- 41.Adham SA, Coomber BL. Glucose is a key regulator of VEGFR2/KDR in human epithelial ovarian carcinoma cells. Biochem Biophys Res Commun. 2009;390:130–5. doi: 10.1016/j.bbrc.2009.09.081. [DOI] [PubMed] [Google Scholar]
- 42.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–17. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kolonin MG, Saha PK, Chan L, Pasqualini R, Arap W. Reversal of obesity by targeted ablation of adipose tissue. Nat Med. 2004;10:625–32. doi: 10.1038/nm1048. [DOI] [PubMed] [Google Scholar]
- 44.Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.U.S. National Institutes of Health; 2011. NCT 01262664: Prohibitin Targeting Peptide 1 [database on the Internet] Available from http://www.clinicaltrials.gov/ct2/show/study/NT01262664. [Google Scholar]
- 46.Klopp AH, Zhang Y, Solley T, Amaya-Manzanares F, Marini F, Andreeff M, et al. Omental adipose tissue-derived stromal cells promote vascularization and growth of endometrial tumors. Clin Cancer Res. 2012;18:771–82. doi: 10.1158/1078-0432.CCR-11-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]


