Abstract
Bacteria belonging to the genera Rhizobium, Mesorhizobium, Sinorhizobium, Bradyrhizobium, and Azorhizobium (collectively referred to as rhizobia) grow in the soil as free-living organisms but can also live as nitrogen-fixing symbionts inside root nodule cells of legume plants. The interactions between several rhizobial species and their host plants have become models for this type of nitrogen-fixing symbiosis. Temperate legumes such as alfalfa, pea, and vetch form indeterminate nodules that arise from root inner and middle cortical cells and grow out from the root via a persistent meristem. During the formation of functional indeterminate nodules, symbiotic bacteria must gain access to the interior of the host root. To get from the outside to the inside, rhizobia grow and divide in tubules called infection threads, which are composite structures derived from the two symbiotic partners. This review focuses on symbiotic infection and invasion during the formation of indeterminate nodules. It summarizes root hair growth, how root hair growth is influenced by rhizobial signaling molecules, infection of root hairs, infection thread extension down root hairs, infection thread growth into root tissue, and the plant and bacterial contributions necessary for infection thread formation and growth. The review also summarizes recent advances concerning the growth dynamics of rhizobial populations in infection threads.
INTRODUCTION
Prior to agriculture, biological fixation of atmospheric nitrogen is estimated to have accounted for ∼90% of the 100 to 140 Tg of nitrogen (1 Tg = 1012 g [106 metric tons]) fixed annually in terrestrial environments. The remaining 10% was fixed abiotically, primarily by lightning. Now human activity, especially the generation of ammonium compounds for agricultural fertilizers, fossil fuel consumption, and increased planting of legumes, contributes an estimated 140 Tg of additional fixed nitrogen each year (179). Biological nitrogen fixation is catalyzed by prokaryotes only, so far as is known. The group of prokaryotes that do this is large and diverse and contains both eubacteria and archaea (186, 193). Nitrogenase, the enzyme complex responsible for nitrogen reduction, is irreversibly inactivated by oxygen; therefore, this process requires conditions that are anoxic or nearly anoxic. In oxic environments nitrogenase is protected from inactivation by being sequestered in differentiated cells with morphological and biochemical characteristics that limit exposure of nitrogenase to oxygen. In some plants, root nodules develop to house nitrogen-fixing bacteria in a microaerobic environment. This process, a type of symbiotic nitrogen fixation, is, for the most part, restricted to a limited number of bacterial groups, including the genera Rhizobium, Mesorhizobium, Sinorhizobium, Bradyrhizobium, and Azorhizobium (collectively referred to in this review as rhizobia) and Frankia. All but the last of these are from the α-proteobacterial Rhizobiaceae family and induce nodules on plants from the Leguminosae family. Frankia is a filamentous gram-positive actinomycete that induces nodules on a variety of woody plants from the Betulaceae, Casuarinaceae, Myricaceae, Elaegnaceae, Rhamnaceae, Rosaceae, Coriariaceae, and Datisticaceae families (10, 11). Rhizobia carry most of the genes specifically required for nodulation either on large (500-kbp to 1.5-Mbp) plasmids or on symbiosis islands (4, 58, 85, 86). Interestingly, it has been recently discovered that bacteria from outside the Rhizobiaceae can induce nodules on legumes. For example, a strain of Methylobacterium, an α-proteobacterium, can nodulate Crotalaria, and β-proteobacteria related to Burkholderia can nodulate Machaerium lunatum and Aspalathus carnosa (110, 159). Apparently these species have acquired, by horizontal gene transfer, plasmids or islands that contain many of the same genes used by typical Rhizobiaceae to induce nodule formation and catalyze nitrogen fixation (110, 159).
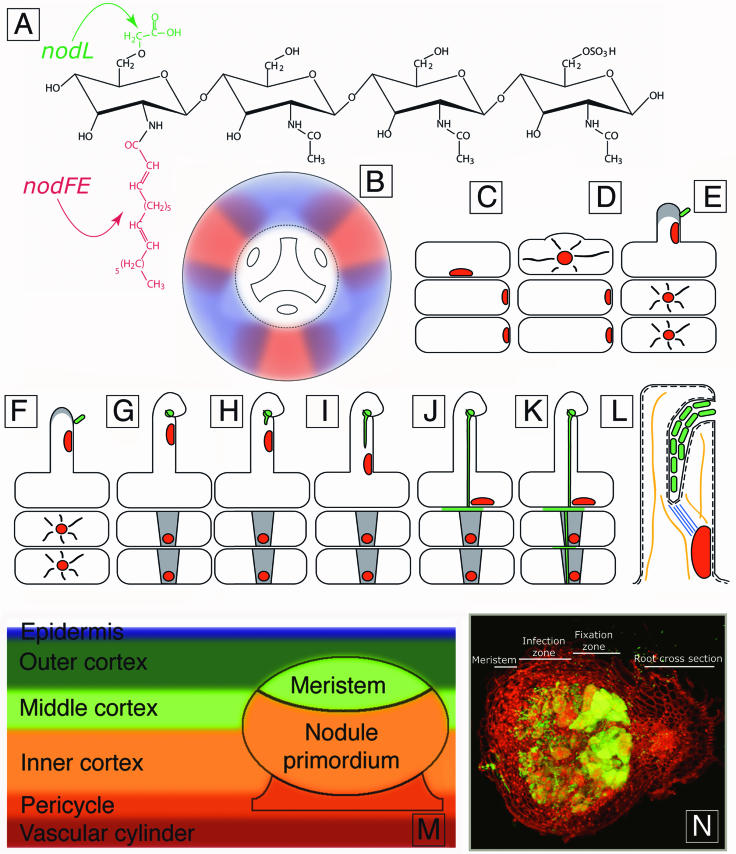
Nodules induced by rhizobia are of two general kinds, determinate and indeterminate. These differ in a number of respects, one of the most important being that indeterminate nodules are elongated and have a persistent meristem that continually gives rise to new nodule cells that are subsequently infected by rhizobia residing in the nodule. These newly infected cells, and the bacteria inside them, develop further and form new nodule tissue that actively fixes nitrogen. This process results in a gradient of developmental stages, from the young meristem at the nodule tip to the older senescent tissue near the root (Fig. 1).
FIG. 1.
Overview of the nodulation process in plants that form indeterminate nodules. (A) One form of Nod factor synthesized by S. meliloti. The upper arrow indicates the acetyl group added by NodL, and the lower arrow indicates the lipid moiety, the length and degree of saturation of which is modified by NodF and NodE. (B) Diagrammatic cross section of a root, showing gradients of an activating factor at protoxylem poles (blue) and an inhibitor at protophloem poles (red). Together such gradients may determine which root cells can become activated in response to infecting rhizobia. Nodules are typically formed next to the protoxylem poles, which are at the ends of the Y-shaped structure depicted in the center the diagram, rather than above the protophloem poles, which are depicted as ovals. (C) An epidermal cell and two underlying outer cortical cells. The epidermal cell has a nucleus positioned across from the place where a new root hair will form. (D) Root hair initiation in the epidermal cell. (E) Binding of a rhizobial cell to a type I root hair and activation of the underlying cortical cells in response to Nod factor. (F) Continued growth of the root hair, shown as stage II. (G) Curling of the stage II root hair under the influence of Nod factor and growth of a rhizobial microcolony in the curl. The underlying cortical cells have become polarized, and cytoplasmic bridges (PITs) have formed and are shown in grey. (H) Infection thread initiation. (I) Growth of the infection thread down the root hair. The nucleus moves down the root hair in front of the thread. (J) Fusion of the infection thread with the epidermal cell wall and growth of rhizobia into the intracellular space between the epidermal cell and the underlying cortical cell. (K) Growth of the infection thread through PITs in the outer cortical cells. (L) Enlarged view of the root hair shown in panel I. The curl has been unrolled to show that topologically, the bacteria in the infection thread are still outside the root hair. The plant cell wall and plant cell membrane are shown as black and dashed lines, respectively. Microtubules are located between the nucleus and the infection thread tip (blue). Actin cables are depicted as orange strands. These are likely to be found where indicated in the diagram because cytoplasmic streaming is seen in these areas during the progression of infection threads down root hairs. Bacteria are topologically outside the root until they later bud from the tip of the thread and enter nodule cells as membrane-enclosed bacteria. (M) Diagram showing root tissues and a young nodule not yet emerged from the root. The derivation of nodule tissues from root tissues is indicated. (N) A longitudinal section of 10-day-old alfalfa nodule. The nodule was infected with GFP-expressing S. meliloti, and the infection thread network can be seen behind the meristem region of the nodule. The initial infection site that gave rise to the bacteria in the nodule can be seen on the nodule periphery at the left. Propidium iodide (red) was used to counterstain the plant tissue. The root from which the nodule emerged is seen in cross-section at the right.
Medicago sativa (alfalfa), Medicago truncatula, Pisum sativum (pea), Vicia species (vetches), and Trifolium species (clovers) are have historically been used as models for studying the formation of indeterminate nodules. M. truncatula has recently become a favored model for studies focusing on the genetics and cell biology of indeterminate nodule formation because it is diploid, has a small genome, and can be readily inbred to form genetically homogenous lines (32).
Determinate nodules lack a persistent meristem, are usually round, and do not display an obvious developmental gradient as do indeterminate nodules. Legumes that form determinate nodules are typically tropical in origin and include Glycine max (soybean), Vicia faba (bean), and Lotus japonicus. L. japonicus, like M. truncatula, has genetic characteristics that make it particularly suitable as a model to study the formation of determinate-type nodules (71).
Topics Covered in This Review
The body of knowledge generated by studies of nodulation over the last 150 years is so large that a review of the entire field, even if covered superficially, would present a Herculean task for both reader and writer. Therefore, this review will present an overview of a limited, but fascinating, part of symbiotic nodulation: how rhizobia infect and invade the root tissues of legume hosts that form indeterminate nodules. While much of the work on infection and invasion has been done with legumes that form determinate nodules, the present focus on indeterminate nodules is warranted because of the large amount of cellular and molecular work on infection that has been done recently with legumes that form indeterminate nodules. In addition, indeterminate-type nodules present a unique opportunity to observe many developmental stages in a single nodule because their meristems continually generate new cells that undergo infection and invasion.
Brief Outline of the Infection Process in Plants That Form Indeterminate Nodules
In order to initiate a productive symbiosis, rhizobia must recognize and then respond to the presence of host plant roots. During growth in the rhizosphere of a host plant, rhizobia sense compounds such as flavonoids and betaines secreted by the host root and respond by inducing nod genes (29, 35, 34). The nod genes encode approximately 25 proteins required for the bacterial synthesis and export of Nod factor. Nod factor is a lipooligosaccharide signal consisting of a chitin backbone, four to five N-acetylglucosamine units in length, with a lipid attached to the nonreducing end and host-specific modifications on the backbone (Fig. 1). Nod factor initiates many of the developmental changes seen in the host plant early in the nodulation process, including root hair deformation, membrane depolarization, intracellular calcium oscillations, and the initiation of cell division in the root cortex, which establishes a meristem and nodule primordium.
Other responses of rhizobia upon encountering a host root undoubtedly involve changes in the expression of genes other than those involved in Nod factor synthesis. Such genes are likely to be important for rhizobia to compete effectively with other organisms for access to growth substrates emanating from the host root, to adhere to the root surface, and to become resistant to toxic substances such as phytoalexins secreted by the root.
Early during symbiosis, rhizobia must get from the root surface to the inner root tissue where they will populate cells in the incipient nodule. To do this, they grow and divide inside a tubule called an infection thread. Infection thread formation is most often initiated when rhizobia become trapped between two root hair cell walls. This usually occurs when a deformed root hair forms a sharp bend or curl, and bacteria bound to the root hair become trapped between appressed cell walls (26). Invagination of plant cell wall in the curl, or degradation of the wall and invagination of the cell membrane, followed by tip growth of the invagination results in the initiation of an infection thread that grows down the inside of the root hair and into the body of the epidermal cell. Rhizobia inside the thread grow and divide, thereby keeping the tubule filled with bacteria. If the infection thread exits the epidermal cell, it does so by fusing with the distal cell wall, and bacteria enter the intercellular space between the epidermal cell and the underlying cell layer. Invagination and tip growth, similar to those seen at the beginning of infection thread growth, occur in the underlying cell, and a thread filled with bacteria is propagated further toward the root interior (170, 173, 175). Branching of the thread as it grows through the root and enters the nodule primordium increases the number of sites from which bacteria can exit the thread and enter nodule cells, ensuring that a sufficient number of nodule cells are colonized. Bacteria inside the infection thread eventually exit it and enter nodule cells. Once inside nodule cells, the bacteria continue to differentiate and synthesize proteins required for nitrogen fixation and for the maintenance of the mutualistic partnership. The processes described above are outlined in Fig. 1.
In the sections that follow, the growth of root hairs, their curling under the influence of Nod factor, initiation and growth of infection threads, growth of infection thread networks through the root cortex and in nodules, and plant control of infection are covered in detail. Some related areas are not covered, but the reader is provided with citations to current reviews on those topics.
GROWTH OF ROOT HAIRS
Root hairs and pollen tubes are the best-studied plant cells that elongate through the process of tip growth (28, 75, 156). Infection threads develop from growing root hairs, are thought to be tip-growing structures, and therefore most likely elongate by using at least some of the machinery that was supporting root hair growth before infection took place. Thus, understanding the processes that contribute to tip growth in root hairs and pollen tubes should shed much needed light on processes involved in infection thread growth.
Cytology and Development of Root Hairs
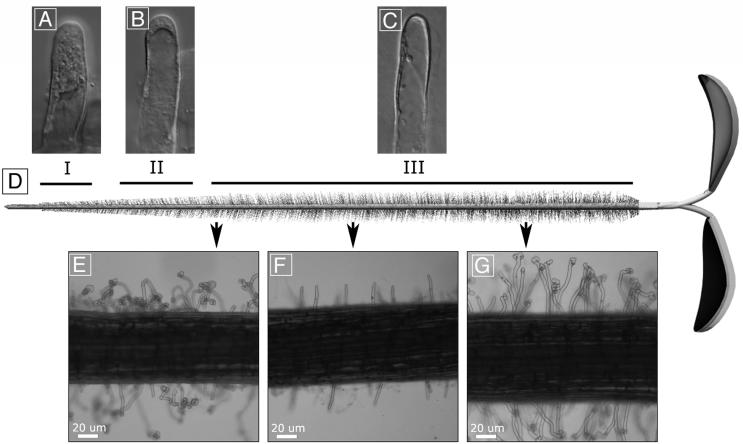
In M. truncatula the process of root hair development begins with the nucleus of an epidermal cell moving to the center of the inner wall opposite of the position on the outer wall where a nascent root hair bulge develops (149) (Fig. 1). The bulge is initially full of cytoplasm, and it grows out from the root surface via tip growth. The extending root hair contains a mass of cytoplasm at its tip, a large vacuole in its shank, and a thin sheath of cytoplasm between the vacuole membrane and the plasma membrane of the root hair. As growth proceeds, the epidermal cell nucleus leaves its position on the inner periclinal wall, enters the root hair, and takes a position in the cytoplasmic dense region, at a point about 30 μm from the tip (these are zone I root hairs [Fig. 2]). The nucleus remains this distance from the tip as the tip extends away from the root surface. As the root hair approaches its mature length, the vacuole enters the cytoplasmic dense region behind the tip, and the nucleus ceases to follow the tip (these are zone II root hairs [Fig. 2]). In fully grown cells the vacuole extends nearly to the tip of the root hair, and the nucleus may be found anywhere in the cell (these are zone III root hairs [Fig. 2]) (149). Detailed measurements of the growth of M. truncatula root hair tips show that the highest rates of tip growth are in a ring centered on, but 2 to 4 μm away from, the very tip of the root hairs. Tips resulting from this growth are not truly hemispherical but rather are slightly flattened at the apex with slight shoulders where wall growth rate is the highest (145).
FIG. 2.
Root hair morphology on uninoculated and inoculated roots. (A to C) Typical root hairs from zones I, II, and III, respectively, of an uninoculated alfalfa plant. (D) Diagram of an alfalfa seedling, showing the locations of root hair zones I, II, and III. (E to G) Photographs showing how root hair responses to S. meliloti can vary along the length of a single root. The three images were taken from a single inoculated seedling at the locations indicated on the central diagram.
Root hairs and pollen tubes elongate through polarized secretion of vesicles to the tip region, with concomitant yielding of the tip wall under the influence of internal turgor pressure (reviewed in references 75, 87, and 156). Thus, spatial gradients in material properties of growing tips can play a role in their growth characteristics and shapes. For example, in pollen tubes esterified pectin is secreted at the tip and subsequently de-esterified. The de-esterified pectin can be cross-linked by Ca2+ ions via the newly generated carboxyl groups (62, 98, 148). The resulting gradient of cross-linked pectin sets up a gradient in the ability of the tip to deform under internal pressure, with the tip being more extensible and regions farther back being less extensible.
Vesicles that fuse at the tip of root hairs are derived from Golgi bodies that are a short distance behind the growing tip. The vesicles deliver membrane and cell wall components that are incorporated into the plasma membrane, cell wall, and extracellular matrix. During tip growth, actin-dependent cytoplasm streaming brings vesicles and other organelles to the apical region of the cell. The cytoplasm typically moves toward the growing tip along the outside of the cell and then moves back toward basal regions through the center of the cell. This pattern, most obvious in pollen tubes, is referred to as reverse fountain streaming (75, 83). The region immediately adjacent to the tip does not exhibit cytoplasmic streaming and is free of organelles that can be resolved by typical light microscopy. This region, termed the clear zone, contains the vesicles that fuse with the tip and deliver the material required for growth (reviewed in reference 107). Because the vesicles are delivered to the base of the clear zone and are consumed at its apex, it has been suggested that diffusion alone may suffice to transport the vesicles from the base of the clear zone to their site of fusion near the tip of the root hair (97, 107).
Roles of Actin and Microtubule Cytoskeleton in Tip-Growing Cells
There is a large body of work devoted to understanding the roles played by actin filaments and microtubules in cytoplasmic streaming of plant cells (28, 75, 87, 135, 149, 178). In the case of tip-growing cells, the accumulated evidence suggests that vesicle and organelle movement in cytoplasmic streams involves myosin-based motors moving along actin filaments. The fact that agents that inhibit actin polymerization (cytochalasin D, latrunculin B, DNase I, and profilin) (157, 178) and agents that inhibit myosin (butanedione monoxime) (78, 163) halt cytoplasmic streaming and inhibit tip growth is consistent with this idea. Electron micrographs show that actin in Hydorchais dubia root hairs is oriented such that if myosins moved along actin from the pointed to barbed ends, as they do in muscle and lower plants (89), then cytoplasmic streaming should move toward the root hair apex along the cell edges and away from the apex at the center of the root hair cylinder (164). Thus, the orientation of the actin filaments in root hairs is consistent with the idea that cytoplasmic streaming is driven by myosin motors traveling along actin filaments from the pointed to barbed ends. The relationship between actin and tip growth can be more complex, however, than is intimated in the preceding discussion, because it has been shown that the growth of Lilium logiflorum (lily) pollen tubes is more sensitive to actin polymerization inhibitors than is cytoplasmic streaming (178). This has been interpreted to mean that tip growth in these cells requires a step other than cytoplasmic streaming that is also dependent on actin polymerization.
The role of microtubules in tip growth is less clear than is the role of actin. This is because microtubule-stabilizing and -destabilizing drugs can cause dramatic growth distortions in root hairs and pollen tubes but often do not stop tip growth (13, 102, 150). Work with Arabidopsis root hairs led Bibikova et al. to the conclusion that actin filaments were required for tip growth and microtubules were required for the proper directional control of the extending tips (13). The effects of microtubule-stabilizing and -destabilizing drugs on the morphology of growing M. truncatula root hairs have shown that microtubules are involved in maintaining the normal structure of the subapical cytoplasmic dense region and are involved in maintaining a normal distance between the nucleus and the growing tip of the root hair (150). Microtubules may also organize actin filaments during root hair growth (102, 162). This has been suggested because when both the actin and microtubule networks are depolymerized, and then actin filaments, but not microtubules, are allowed to reform, cytoplasmic streaming is abnormal (162).
Roles of Calcium in Tip-Growing Cells
Studies with ion-specific dyes have shown that growing root hairs and pollen tubes exhibit tip-focused gradients of Ca2+ (28, 75). In growing root hairs the concentration of this ion at the tip, just below the plasma membrane, is about 1 μM and decays to a basal concentration of about 100 nM within 20 μm (13, 188). Manipulation of the calcium gradient by a variety of methods has shown that the gradient is needed for normal tip growth of pollen tubes. Disruption of the gradient by a variety of methods results in pollen tube tip growth that is isodiametric (recently reviewed in references 28 and 75). Blocking of Ca2+ channels pharmacologically causes root hairs to stop growing (141, 188). Manipulation of the Ca2+ gradients and observations made during reestablishment of Ca2+ gradients indicate that the direction of tip growth is toward regions of highest calcium ion concentration in both pollen tubes and root hairs (13, 14, 40).
In spite of the accumulating data correlating the Ca2+ gradient with tip growth, the actual roles played by Ca2+ in tip growth are still somewhat obscure. Suggested roles for Ca2+ include influencing cell wall strength and extensibility, controlling actin dynamics, and controlling vesicle fusion at the growing tip (28, 75, 138). Of course, these roles need not be mutually exclusive.
Roles of Other Proteins in Tip-Growing Cells
NADPH oxidase.
The rhd2 (root hair defective) mutant of Arabidopsis develops unusually short root hairs. Recent work with this mutant showed that RHD2 encoded a NADPH oxidase. Such enzymes are usually capable of catalyzing the formation of reactive oxygen species (ROS) (55). The phenotype of the rhd2 mutant could mimicked in wild-type plants by the addition of compounds that inhibit ROS formation, suggesting that generation of ROS is needed for the normal elongation of root hairs in Arabidopsis. rhd2 mutants were deficient in establishing a root hair tip-focused Ca2+ gradient, because of an altered activity of a Ca2+ influx channel. Exposure to exogenous hydroxyl radicals partially restored the ability of rhd2 root hairs to establish a Ca2+ gradient. Thus, the RHD2 NADPH oxidase may be responsible for generating ROS that in turn help establish tip-associated calcium gradients by activating Ca2+ channels needed for root hair elongation. This gives a mechanistic explanation for earlier observations that root hair length and root hair density along the root is influenced by redox conditions (28, 139).
Small GTPases.
Rac/Rho-like small GTPases (called ROPs, for Rho of plants) are found in plants and have been implicated in a variety of functions, including signal transduction, control of actin dynamics, and control of tip growth in root hairs and pollen tubes (59, 70, 92, 192). These proteins have been localized to growing tips of root hairs and pollen tubes. Interference with normal activity of some of the family members affects polarized growth of both of these cell types. For example, in Arabidopsis, which has 11 ROPs (156), expression of constitutively active ROP4 or ROP6 in Arabidopsis causes depolarized growth of root hair tips resulting in swollen, distorted tips; the distortions were correlated with delocalized calcium gradients in the tips (108). Similar experiments have implicated ROP2 and ROP7 in control of tip growth of Arabidopsis root hairs (84). Thus, the evidence suggests that at least some of the ROPs involved in control of tip growth may act by affecting actin dynamics and/or calcium gradient formation in the apical region of growing of root hairs and pollen tubes.
INITIATION OF INFECTION
For the purpose of this review, initiation of infection is considered to encompass the steps from bacterial adhesion to root tips through the beginning of infection thread growth. Many of these infection events are mediated by Nod factor. Recent papers have covered Nod factor structures, the synthesis of Nod factors, Nod factor signal transduction, and the relationship between host range and Nod factor structure. The reader is directed to previously published reviews (20, 38, 63, 97, 146, 165, 181, 183) for a comprehensive discussion of these topics.
Adhesion of Rhizobia to Root Hairs
Rhizobia are capable of binding tightly to host root hairs. With Rhizobium leguminosarum this binding consists of two steps. The first is a weak, Ca2+-dependent binding step to root hairs that is mediated by a protein called rhicadhesin, which is thought to be present in most rhizobia (152, 154). The rhicadhesin protein has been purified from R. leguminosarum, but its gene has not been cloned or otherwise identified (155). Recently an R. leguminosarum bv. trifolii protein, RapA1, that has many of the properties attributed to rhicadhesin was described. Both are secreted proteins that can bind calcium, bind at bacterial cell poles, mediate calcium-dependent agglutination, and bind to root hairs. However, rhicadhesin and RapA1 most likely are not the same proteins, because they are different sizes and RapA1, being found only in R. leguminosarum and Rhizobium etli, does not appear to have the broad phylogenetic distribution of rhicadhesin (3).
Following weak binding, a tight binding step that is mediated by the bacterial synthesis of cellulose fibrils is initiated (153, 154). The synthesis of these fibrils was shown to be required for R. leguminosarum to form biofilm-like caps on the tips of pea root hairs. Mutants that did not form the fibrils did not form caps, but they were able form nitrogen-fixing nodules, which indicated that capping and cellulose-mediated tip binding are not absolutely required for a successful symbiosis to occur (154). However, binding and capping may be needed for rhizobia to effectively colonize root hairs under natural conditions where competition for access to root surfaces and grazing pressure by eukaryotes are likely to be intense.
Host lectins have also been shown to play roles in rhizobial adhesion to plants that form determinate and indeterminate nodules. These lectins localize to root hair tips and are thought to help convey host-symbiont specificity by binding simultaneously to the plant cell wall and to saccharide moieties on the surfaces of compatible bacteria (41, 43, 79). A series of experiments in which a variety of transgenic plants expressed lectins from other species of legumes has shown that the presence of heterologous lectins often allows transgenic plants to respond to symbionts that are usually noncompatible, provided that the heterologous lectin can bind to the noncompatible bacteria and provided that the noncompatible bacteria make the proper Nod factor (42, 79, 171, 172). For example, transgenic alfalfa that expressed pea lectin formed nodules and infection threads when inoculated with low numbers of R. leguminosarum bv. viciae that synthesized Sinorhizobium meliloti Nod factors. Control plants and transgenic plants that expressed mutant pea lectin did not form nodules or infection threads when inoculated with low numbers of the same strain (171). Results such as these suggest that cell-cell contact and specific binding of compatible bacteria to root tips are important for infection and infection thread formation, because they result in the exposure of infectible root hair tips to the proper symbiont and hence to a high localized concentration of the Nod factors needed to trigger root hair curling and infection thread formation (79, 171). A direct test of the importance of host lectins in promoting infection and invasion would be very interesting. Such a test may be difficult, however, because down regulation of lectin expression causes severe developmental defects in alfalfa and may (or may not) also do so in other legumes (19).
Root Hair Deformation and Curling
Root hairs of host plants deform under the influence of purified Nod factors from compatible rhizobial species. This phenomenon has been studied in detail in many plant species including alfalfa, M. truncatula, pea, clover, and vetch. It has been shown that a subset of root hairs are particularly susceptible to Nod factor-induced deformation (40, 72, 149). The most susceptible root hairs are those that that have nearly finished growing (root hair zone II [Fig. 2]). Root hairs that have finished growing (root hair zone III) and root hairs that are actively growing with a strongly polarized internal organization (root hair zone I) are refractory to the deforming activity of Nod factor. Zone II root hairs are terminating growth and are different morphologically from actively growing root hairs in zone I. They do not display the large plug of cytoplasm below the root hair tip, the large vacuole is nearer the tip, and reverse fountain streaming is still present, but as growth termination nears it may switch to circulation-type streaming several times before displaying permanently the circulation streaming characteristic of zone III root hairs (149).
Nod factor induced deformation of zone II root hairs begins with root hair tips swelling isodiametrically; this is followed by the establishment of a new growing tip that resembles highly polarized, actively growing tips of zone I root hairs (40, 72, 106, 149). Thus, purified Nod factor can induce new tip growth in zone II cells. It is not yet clear why vesicle deposition becomes temporarily isodiametric following exposure to Nod factor. Isodiametric deposition may come about because of the disruption of the cytoskeleton. An alternative explanation, set forward by Sieberer and Emons, is that zone II root hairs normally no longer have a tip-directed vesicle delivery system and, upon Nod factor addition, Golgi vesicles are incorporated randomly into the tip region until a new tip-focused delivery system is reestablished (149).
It is interesting to consider why zone I and zone III cells do not deform in response to the addition of purified Nod factor. Zone III cells may be unable to deform because they have a secondary cell wall or because they no longer have the machinery in place to catalyze tip growth. Zone I cells may not respond to Nod factor by deforming because they are already highly polarized and actively growing (97). It has been pointed out that root hairs in zones I and III change in other ways after exposure to Nod factor and are thus responsive to its presence. For example, zone I hairs exhibit depolarization, Ca2+ influx, and Ca2+ spiking (47, 48, 53, 146, 183), and the subapical region vetch root hairs in zones I, II, and III exhibit a characteristic increase in fine bundles of actin following addition of Nod factor (39).
When added to the external medium, purified compatible Nod factors are sufficient to cause root hair deformation and branching, but they are not sufficient to cause the formation of tightly curled root hairs (shepherd's crooks) that are usually the sites of bacterial entry into plants. It had been hypothesized that the reason for this is that a localized source of Nod factor is needed to continually redirect the off-axis tip growth needed to form a tight curl (49, 135, 168). This idea has intuitive appeal. Recent experiments in which purified Nod factor from S. meliloti was applied in a highly localized fashion to root hairs of M. truncatula have shown that a point source of Nod factor can cause root hairs to grow into structures resembling shepherd's crooks (50).
Following inoculation with compatible bacteria, root hairs on a single plant can show a wide range of deformation morphologies. Hairs in some regions of the root show no deformation at all, other regions have wavy root hairs with swellings, and other regions are populated with tightly curled root hairs (shepherd's crooks) that are able to support the development of infection threads (Fig. 2) (26). These various morphologies are often seen in a single plant whose root hairs went through all phases of development in the presence Nod factor-secreting bacteria. Assuming that Nod factor was continually present, these observations suggest that root hair responsiveness toward Nod factor changes as the root grows. The responsiveness of root hairs to deform in the presence of Nod factor can be modulated by plant hormones such as ethylene, which inhibits Nod factor signal transduction and can influence the degree of root hair deformation and the frequency of productive infections (119) (see below). Thus, changes in ethylene levels, in ethylene signal transduction, or in other hormone signaling systems during root growth may explain the observed variability in root hair responsiveness to Nod factor.
It was shown by Cardenas et al. that Nod factor causes the fragmentation of the actin microfilament network upon addition to bean root hairs (27). The actin bundles normally seen in bean root hairs injected with fluorescent phalloidin disappeared within 5 min after the application of Nod factor, and an area of diffuse fluorescent staining concomitantly appeared in the apical region of the root hair. Because phalloidin binds to filamentous actin, this diffuse staining presumably represented filamentous, fragmented microfilaments. Actin bundles reappeared within an hour of Nod factor addition, indicating that cells were able to at least partially recover a microfilament network even when Nod factor was present (27).
Membrane depolarization, Ca2+ influx at the root hair tip, and Ca2+ spiking in the perinuclear region occur within seconds to minutes upon addition of compatible Nod factors to growing legume root hairs. It has been hypothesized that the influx of calcium associated with Nod factor addition may be related to the fragmentation of the actin network at the root hair tip, as is the case with pollen tubes (90).
Work done by de Ruijter et al. with vetch leads to a different picture of how the actin network responds to the addition of Nod factor. In this case, quantitation of actin bundles in root hairs fixed and then stained with phalloidin 3 to 15 min after addition of Nod factor revealed that the root hairs responded to Nod factor by increasing the number of fine actin filaments in the subapical region. The authors suggested that such an increase could have come about by unbundling actin cables or by polymerization of actin filaments (39). The conflicting observations of Cardenas et al. and de Ruijter et al. have yet to be reconciled. Species differences or the fact that one study employed injected phalloidin for visualization and the other employed phalloidin staining after fixation may explain the different responses observed by the two groups. Given the critical role of the actin network in tip growth, it will be important to understand exactly how this network responds to Nod factor and how that response relates to root hair deformation and curling.
In addition to changes in the actin component of the root hair cytoskeleton, the microtubule component also changes following exposure to compatible rhizobia. A detailed study, employing antibodies against tubulin, showed that arrays of microtubules changed after M. truncatula root hairs were exposed to wild-type S. meliloti. Initially microtubules were arranged in the root hairs in helical cortical arrays. Following exposure to S. meliloti, the microtubules in root hair cells rearranged to form endoplasmic networks around nuclei and cortical networks that were no longer helical but were more closely aligned with long axes of the root hairs. Nuclei and their associated microtubules then moved into the tip region of the root hairs, and the microtubule array became positioned between the tip of the root hair and the nucleus. As curling commenced, the endoplasmic microtubule arrays became associated with the center of the curl. If the curl developed an infection thread, the microtubular array became disconnected from the root hair tip and instead connected the tip of the infection thread to the nucleus (161). This arrangement likely remains as nuclei advance down root hairs in front of infection threads.
It is too soon to integrate information about root hair growth and the events of Nod factor-induced deformation into a detailed model that explains the molecular and cellular events leading to the formation of the tightly curled root hairs that support rhizobial infection. However, any such model must take into account how rearrangements of the actin and microtubule networks lead to deposition of cell material at off-axis sites that are away from the ring of deposition that is normally near the root hair tip (145). Given that (i) actin directs cell material to the tip of the growing root hair, (ii) actin may fragment and reassemble after the addition of Nod factor, (iii) the microtubule network rearranges to become centered on the growing curl, and (iv) the microtubule network is involved in stabilizing the growth site at the root hair tip, it is reasonable to propose that redirection of tip growth may require the breakup of the preexisting actin network at the root hair tip, followed by its reassembly at on off-center site that is stabilized, or selected, by the microtubule network, which is itself is rearranged during the early steps of rhizobial infection. The ultimate signal for these spatial reorganizations would be bacteria bound to the root hair tip, acting as a point source of Nod factor and perhaps as a point source of other bioactive signals as well (49, 50, 135, 168).
Is There a Nod Factor-Mediated Signal That Is Specifically Required for the Initiation of Infection Threads?
Ardourel et al. have shown that a nodF nodL double mutant of S. meliloti, which synthesizes Nod factors that lack the acetyl modification at the nonreducing end and contain an alternative lipid (vaccenic acid C18:1 Δ11) at the same end, did not form shepherd's crooks or initiate infection threads when inoculated onto alfalfa seedlings (2). The changes at the nonreducing end did not alleviate the capacity for Nod factor to cause deformation; in fact, its ability to do so appeared to be enhanced, with many root hairs exhibiting multiple bulbous structures indicative of multiple growth points. Nonhaired epidermal cells also exhibited deformations, indicating that the Nod factor made by the nodF nodL double mutant was abnormally active in terms of inducing or allowing tip growth. Also, the nodF nodL mutant caused hyperactive cell division in the root inner cortex. These results suggested that the nonreducing end of wild-type S. meliloti Nod factor contains information essential for triggering and control of some early steps of nodulation.
Ardourel et al. (2) proposed a model in which root hairs have at least two receptors or signal transduction pathways devoted to Nod factor perception. One is less stringent and, when activated by Nod factor or Nod factor with an altered nonreducing end, causes root hair deformation and cortical cell division. The other is more stringent and is activated by wild-type Nod factor but not by Nod factor synthesized by the nodF nodL strain. This more stringent pathway is required for shepherd's crook formation and infection thread initiation, and it was postulated to inhibit the deformations and cortical cell divisions induced by the first pathway. Thus, Nod factors made by nodF nodL strains result in deformation and cortical cell division but are not sufficient for infection thread formation or for down regulation of deformation and induced cortical cell division.
In a related study, Walker and Downie inoculated a nodFEMNTLO deletion mutant of R. leguminosarum bv. viciae onto vetch seedlings (182). The mutant synthesized Nod factor molecules that contained no host-specific decorations. Microscopic observation of roots showed that inoculated plants contained overly deformed root hairs and occasionally formed shepherd's crooks that often contained large aggregations of bacteria, but they failed to initiate infection threads from such crooks. Interestingly, this was similar to the phenotype of the nodF nodL double mutants of S. meliloti when they were inoculated onto M. truncatula. Note that this was different than the response seen with the nodF nodL mutant on alfalfa, where shepherd's crooks were not initiated. The phenotype seen when the nodFEMNTLO stain was inoculated onto vetch is particularly intriguing result because it again shows that Nod factor decorations are not needed for root hair deformation but are required for the initiation of infection threads.
Walker and Downie (182) showed that overexpression of nodO rescued nodFEMNTLO phenotypes to some extent. With nodO overexpression the mutant no longer accumulated in large masses, some root hairs initiated infection threads, and some nodules formed. NodO protein is known to be secreted from bacterial cells, has been shown to form pores in membranes, and can be a host range determinant (180). It may be that NodO rescued the nodFEMNTLO phenotype by allowing ion flow across a root hair membrane and thus amplifying a weaker-than-normal signal transmitted by the stripped-down version of Nod factor.
Recently, strong candidates for the stringent Nod factor receptor required for infection thread initiation in M. truncatula have been identified by Limpens et al. (101). Putative genes (LYK3 and LYK4) for this receptor encode two similar histidine kinases with extracellular LysM domains. The LysM domains are most similar to LysM domains of a Volvox carteri chitinase gene. This similarity is interesting given that the undecorated backbone of Nod factor is a small molecule of chitin. Using RNA interference, the Limpens et al. down regulated the expression of LYK3 and LYK4. The root hairs of transgenic LYK3 and LYK4 knockdown mutants had aberrant infection threads when inoculated with S. meliloti nodFE mutants, which synthesize Nod factors with an alternative lipid (vaccenic acid C18:1 Δ11) at the nonreducing end. The structure of the aberrant threads was very similar of those of threads induced by the S. meliloti nodF nodL double mutant discussed above (2). On wild-type roots the S. meliloti nodFE mutant was able to induce infections and infection threads that were indistinguishable from those induced by wild-type S. meliloti. Wild-type S. meliloti also induced aberrant threads on the LYK3 mutant, although not at the same frequency as the nodFE mutant, indicating that the LYK3 knockdown phenotypes were not induced only in response to the nodFE mutant. In addition, 20% of the nodFE microcolonies in shepherd's crooks on LYK3 knockdown roots initiated infection threads, whereas 80% of the microcolonies initiated infection threads on wild-type roots. Together, the data presented by Limpens et al. are compatible with the hypothesis that the nonreducing end of S. meliloti Nod factor interacts with LYK3 or LYK4 and that the signal resulting from such an interaction is required to initiate infection threads and for proper polar growth of those infection threads.
Two L. japonicus genes encoding histidine kinases with LysM domains, NFR1 (Nod factor receptor) and NFR5, were recently shown to be required for the earliest plant responses to Nod factor and the microsymbiont of L. japonicus, Mesorhizobium loti (103, 129). The genetic and physiological characteristics of the NFR mutants are consistent with their encoding a Nod factor receptor needed for the first steps of symbiosis. The NFR1 and NFR3 proteins are very similar to the LYK3 and LYK4 proteins of M. truncatula, but the phenotypes associated with the NFR mutants are much more extreme. At this point it is not clear if the phenotypic differences between the NFR mutants and the LYK mutants are because the proteins, although similar, are required at different steps of the symbiosis, because the LYK knockdown mutants retained some residual activity that allowed steps such as root hair deformation to occur, or because the signal transduction pathways of the two plants may be different in terms of redundancies or other key properties.
Degradation of Cell Wall Associated with Infection Thread Initiation
Infection threads usually initiate at tightly curled root hairs, at junctions of branched root hairs, or where two root hairs are pressed together. It has been hypothesized that either the initial ingrowth starts as an invagination of the root hair cell wall (116) or new cell wall is laid down as bacteria approach the cell membrane following localized degradation of root hair wall at the site of infection. Most evidence favors the latter hypothesis. Degradation of root hair cell wall at the site of infection thread initiation and subsequent bacterial approach toward the underlying membrane have been clearly demonstrated in a number of cases in a variety of plant-host pairs (26, 113, 136, 166).
The question of which partner, plant or bacterium, is responsible for the degradation remains open. Rhizobial bacteria have enzymes that are capable of degrading cellulose and other plant cell wall polysaccharides (82, 105, 109, 127, 194). Studies by Mateos et al., employing electron microscopy, have shown that R. leguminosarum bv. trifolii and S. meliloti induce the formation of pits on root surfaces of their respective hosts (104). These pits are the same size and shape as the bacteria and are found directly under bacteria bound to roots, indicating that if the bacteria are producing the enzymes responsible, the enzymes are bound to the bacterial surface and are not secreted. It should be noted that electron micrographs depicting the degradation associated specifically with infection thread initiation show that degradation, while localized, is more widespread and diffuse than that shown by Mateos et al. Plants clearly have the capability to degrade, or otherwise alter, their cell walls. For example, cell walls are weakened or degraded during root hair initiation, fruit ripening, pollen tube transit down the pistil, and leaf abscission (21, 34, 35, 137). Observations that cell wall-degrading enzyme activities, induced by compatible bacteria, do not occur in the presence of nitrate are easiest to explain if the host has at least some control over degradation in response to rhizobial infection (52, 105, 173).
It was recently shown that an alfalfa polygalacturonase gene (MsPG3) was induced specifically in roots in response to S. meliloti (111). The gene was induced as early as 1 day after spot inoculation, and high levels of expression of this gene were seen in the nodule meristem, infection zone, and interzone II-III of nodules. Those authors did not determine whether the gene was induced in root hairs following inoculation. Given that cell wall degradation likely occurs before infection thread initiation, it would be interesting to know if MsPG3 is induced in root hairs following inoculation with S. meliloti.
Questions about Initiation of Infection Threads
While the processes that precede infection thread initiation are beginning to be understood, the steps that constitute the actual initiation of infection threads are not understood at all, and many steps probably remain to be identified. A few outstanding questions are as follows (i) How is cell wall material in growing root hairs redirected from its normal deposition site near the apical dome to the site of infection thread initiation some distance away at the center of the curled root hair? (ii) Are there mechanical or structural constraints associated with the deposition of cell wall material at the inward-growing, convex infection thread tip that are not required for cell wall deposition at the outward-growing, concave root hair apex during root hair extension? (iii) Is an inward pressure differential required for the infection thread to initiate and extend down the root hair? If so, what is responsible for that differential? (iv) Why aren't infection threads initiated from all microcolonies enclosed in shepherd's crooks—what are the extra requirements needed for initiation of infection thread growth?
EXTENSION OF INFECTION THREADS THROUGH ROOT HAIRS
Given that infection threads initiate only in growing root hairs, it is likely that some of the machinery required for root hair extension is also required for infection thread extension. During growth of infection threads down root hairs, there is an association between the extending infection thread tip and the root hair nucleus, much as there is a connection between the root hair tip and the root hair nucleus during growth of root hairs.
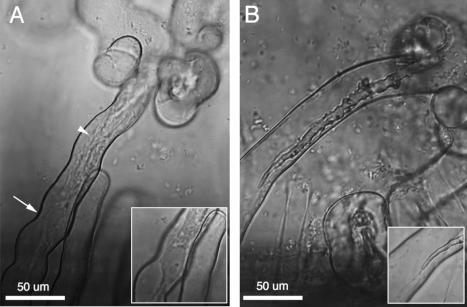
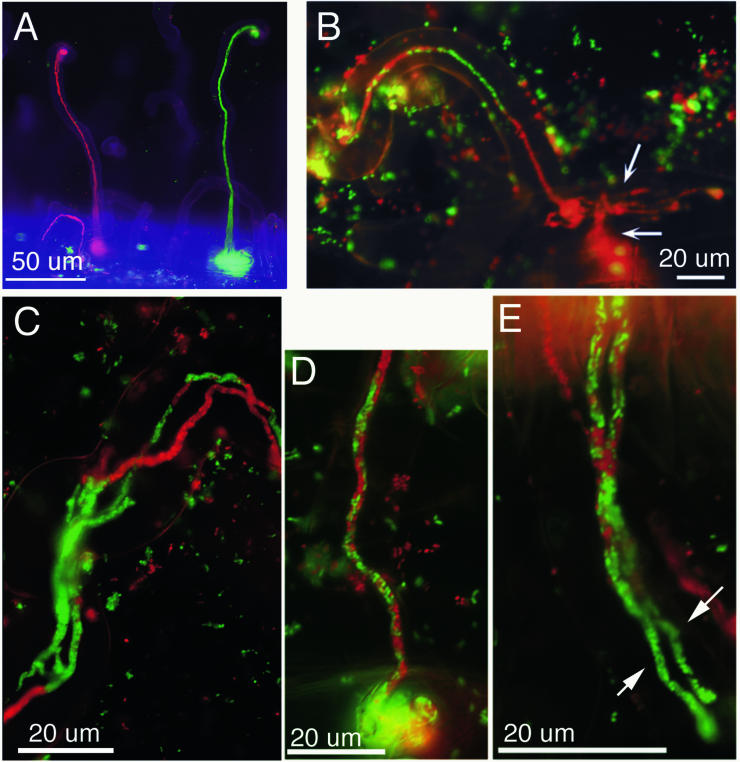
During growth of infection threads down root hairs, the nucleus is connected to the extending infection thread tip by a thick and actively streaming column of cytoplasm (Fig. 3A). While the roles of actin and microtubules in root hair growth and associated cytoplasmic streaming have been investigated, their roles in infection thread growth remain uninvestigated. Because of the active cytoplasmic streaming in the tip region of extending threads, plant cytoskeleton is likely to have an important role in growth of infection threads. In alfalfa, the large amount of active cytoplasm in the tip region and immature infection thread cell wall makes discerning the tips of growing threads difficult. In some cases only thin cytoplasmic strands can be seen connected to threads with well-defined tips (Fig. 3B). In alfalfa, such threads with well-defined tips have invariably stopped growing through root hairs (C. Arango and D. J. Gage, unpublished data).
FIG. 3.
Infection threads in alfalfa root hairs. (A) A growing infection thread, with an active column of cytoplasm between the nucleus (arrow) and the tip region of the thread (arrowhead). The actual tip of the thread cannot be discerned because it is obscured by the cytoplasmic column or because it has not yet been enclosed with thick, mature cell wall. The inset shows the nucleus and its associated column of active cytoplasm. (B) An infection thread that has terminated growth. Note that the tip is easily discerned and the tip region has only very thin cytoplasmic streams connected to it (inset).
As described above, root hairs that are curling and undergoing infection exhibit changes in their microtubule arrays. The arrays change from helical, cortical arrays to ones in which new, nucleus-associated endoplasmic microtubules and cortical microtubules become aligned with the long axis of the root hair. Next, the endoplasmic array appears to connect the nucleus to the tip of the root hair. As curling commences, the microtubules become centered on the curl and eventually become disconnected from the root hair tip and distributed between the root hair nucleus and the growing tip of the infection thread (161). In alfalfa, these microtubule arrays likely colocalize with the column of actively streaming cytoplasm that is between the nucleus and the thread. These microtubules could be involved in directing growth of the thread tip in much the same way that microtubules are involved in directing the tip growth of root hairs (13, 102, 150).
Studies of the hcl (hair curling) mutant of M. truncatula have shown that its root hairs deform and branch after inoculation with S. meliloti but that subsequent curling and infection steps do not occur. Shepherd's crook formation, infection thread initiation, infection thread extension, and the concomitant reorganization of microtubule arrays were not present following inoculation, suggesting that morphological changes of root hairs during infection and reorganization of microtubule arrays are directly or indirectly linked (30). Those authors hypothesize that hcl mutants are deficient in establishing or responding to the organizational signals that emanate from S. meliloti microcolonies and that are normally needed for shepherd's crook formation, infection thread initiation, reorganization of microtubule arrays in root hairs, and polarization of root cortical cells (30).
Plant Contributions to Infection Threads
Bacteria inside infection threads are topologically outside the root hair (Fig. 1), and the infection thread wall is contiguous with the cell wall of the root hair. Not surprisingly, then, the structure of infection thread walls is similar to that of root hair cell walls. In particular, esterified and unesterified pectins, xyloglucans, and cellulose are likely components of infection thread walls (130). The matrix inside the lumen of the thread contains material that is normally found as part of the extracellular matrix of plant cell walls. A monoclonal antibody, MAC 265, has been shown to react with a glycoprotein found in homogenized pea nodules, the lumens of infection threads in pea nodules, cell walls and apoplastic spaces of rapidly expanding pea root cortical cells, and the material released from pea root tips (131, 170). Partial sequencing of the MAC 265 antigen and cloning of its cDNA revealed that the antibody recognizes one or more members of a specific group of extensin proteins found so far only in legumes (133). Extensins are hydroxyproline-rich cell wall proteins involved in cell wall synthesis that can be cross-linked by peroxidation (17, 29). Cross-linking of the MAC 265 antigen occurs as part of plant defense responses and probably serves to inhibit invasion of pathogens (18). During symbiosis its role may not be defensive, or it may have roles in addition to defense. It has been suggested that hydrogen peroxide, which is known to be present in infection threads, may cross-link the legume-specific extensins recognized by MAC 265 and thus modulate the material properties of the infection thread matrix (77, 133, 140, 187). A role in establishing the oxygen barrier in the nodule cortex that helps protect nitrogenase from inactivation has also been suggested for the MAC 265 antigen (120).
Bacterial Contributions to Infection Threads
Rhizobial exopolysaccharides are required for the development of nitrogen-fixing nodules on plants that form indeterminate nodules. However, plants that form determinate nodules do not have such a requirement (16, 31, 54, 64, 67, 68, 81, 88, 94, 95, 121, 134, 175). The roles of rhizobial exopolysaccharides in symbiotic nodulation have been most thoroughly studied in S. meliloti. This species makes at least five symbiotically important polysaccharides: succinoglycan (EPS I), EPS II, K antigen, lipopolysaccharide, and cyclic β-glucans. It turns out that the first three of these are involved in the extension of infection threads. These exopolysaccharides and the phenotypes of mutants unable to make them are the subject of a recent review (57). Readers are referred there for details that are omitted from the summary below.
Alfalfa and M. truncatula plants fail to nodulate properly when inoculated with S. meliloti Rm1021 exo mutants that are unable to produce succinoglycan, an exopolysaccharide consisting of repeating acidic octcasaccharide subunits (54, 95). Nodules induced by such strains are small, lack a persistent meristem, contain few or no bacteroids, and cannot fix nitrogen (54, 95). The nodules are empty, because S. meliloti exo mutants are inefficient at initiating infection threads. Those that do initiate are bloated and grossly misshapen and usually cannot extend beyond the basal part of the root hair (Fig. 4) (31, 121, 190). Infection threads also cannot initiate normally in vetch root hairs inoculated with R. leguminosarum bv. viciae exo mutants (175). In the S. meliloti-alfalfa symbiosis and other symbioses, the Fix− phenotypes of exo mutants can be reversed by the addition to roots of small amounts of purified, low-molecular-weight succinoglycan (6, 44, 65, 167). Fractionation studies of S. meliloti succinoglycan indicated that small quantities of the trimeric form of the repeating octasaccharide were sufficient for extracellular complementation of exo mutants. Two conclusions can be drawn from the fact that only small quantities of succinoglycan were sufficient for complementation of exo mutants: (i) that signaling is an important function of trimeric form of succinoglycan and (ii) that succinoglycan is unlikely to be a required structural component of the infection thread matrix (6, 44, 65, 167, 184).
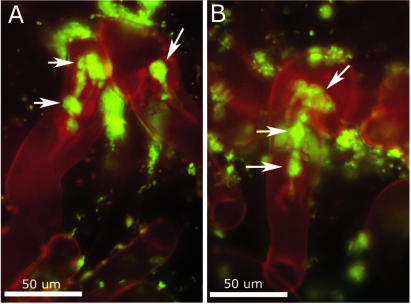
FIG. 4.
Infection threads induced by an exoF mutant of S. meliloti. (A) Two swollen and misshapen infection threads induced by a GFP-expressing exoF mutant of S. meliloti. Arrows point to sections of the threads that are particularly swollen. (B) Another example of an infection thread induced by the exoF mutant. For infection threads induced by wild-type S. meliloti on alfalfa, see Fig. 5.
EPS II, a second class of exopolysaccharide synthesized by some S. meliloti strains, consists of repeating galactose-glucose disaccharide subunits with acetyl and pyruvyl side groups (76, 96). EPS II is normally not present at detectable levels in the S. meliloti wild-type strain Rm1021 because of an insertion into expR, a luxR-type transcriptional activator that is required for EPS II synthesis. Derivatives of strain Rm1021 with a nondisrupted expR gene produce large quantities of EPS II (7, 122). Interestingly, EPS II production can substitute for succinoglycan and support infection thread formation and nodule invasion by S. meliloti exo mutants (66, 121). The forms of EPS II that are able to promote nodule formation are low-molecular-weight polymers consisting of 15 to 20 disaccharide repeats. EPS II can act extracellularly, and the addition of as little as 7 pmol of the active forms of EPS II to an alfalfa seedling will allow S. meliloti mutants that are unable to make either succinoglycan or EPS II to form functioning nodules (66). This indicates that EPS II, like succinoglycan, probably acts as a signaling molecule and not a structural one.
K antigen, a capsular polysaccharide, is not synthesized by S. meliloti strain Rm1021 but is made by other S. meliloti strains. Analysis of strain Rm41, a strain that makes succinoglycan and K antigen, and of isogenic mutants that make only K antigen shows that this exopolysaccharide can also support infection thread initiation, infection thread extension, and nodule development (121).
The roles that succinoglycan, EPS II and K antigen might have in infection thread initiation and growth are not clear. The misshapen infection threads, overfilled with bacteria, that are often seen in the absence of succinoglycan (Fig. 4) suggest that the growth of the bacteria inside the threads is not matched to the growth of the tube (31, 121). This may be because the rate at which the tube is synthesized, or extends, is abnormally low, or its growth polarity may be altered. Pellock et al. suggested that succinoglycan may be involved in organizing the root hair cytoskeleton in such a way that tip growth of the infection thread is rapid and highly polarized (121). Others have shown that exopolysaccharides are able to modulate (down regulate) defense responses in plants (57, 114, 115, 174, 175). Thus, rhizobial mutants lacking exopolysaccharides may cause abnormal infection thread growth because the plant mounts an inappropriate defense response to the invading rhizobia.
Nod factor is required for the redirection of root hair growth that leads to shepherd's crook formation. If infection thread initiation and growth result from a continual redirection of root hair cell wall growth, then Nod factor may play an important role in these processes as well. The fact that mutations that alter the nonreducing end of Nod factor affect infection thread initiation and extension supports this idea (2, 182). That the LYK3 and LYK4 knockdown mutants are altered in infection thread initiation and extension may also indicate that Nod factor production is important for processes beyond root hair deformation (101). Finally, work showing that nod genes are expressed by bacteria in tissues of the nodule that contain actively growing infection threads supports the idea that infection thread propagation probably requires Nod factor-dependent targeting of material to infection thread tips in these cells as well (143, 144).
Growth Dynamics of Bacterial Populations inside Infection Threads
Infection threads are composite structures in the sense that they are composed of parts derived from the two symbiotic partners. During growth down root hairs, the plant-derived and bacterium-derived parts normally grow in unison. That is, the extension rate of the plant-derived part of the infection thread matches the extension rate of the bacterial columns inside the thread. As described above, the morphology of infection threads containing S. meliloti exo mutants may be a result of a disruption of these normally synchronized growth rates.
The use of S. meliloti tagged with green fluorescent protein (GFP) and similar proteins has rendered bacterial growth inside the infection threads particularly amenable to study (31, 60, 61, 121, 189). Upon mixing fluorescent and nonfluorescent S. meliloti, infection threads that contain both types of bacteria develop. Tagged bacteria often enter the infection thread and form alternating light and dark sectors with sharp boundaries between the sectors (Fig. 5) (61). These sectors apparently originate from the clonal expansion of founder cells which enter the thread very early. Recent work has confirmed these observations and extended them by showing that even though sectored infection threads are the most common type of mixed infection thread, other types of mixed threads can form, including ones in which strains appear to be randomly mixed (Fig. 5) (60). Observations of infections arising from bacterial strains tagged with different fluorescent proteins have led to insights into the infection and invasion process because they allow spatially distinct subpopulations of bacteria to be discerned. Growth patterns of the whole population in infection threads can be inferred from the growth and behavior of the observed subpopulations. For example, observation of threads populated with two S. meliloti strains that could be differentiated by fluorescence microscopy indicated that most of the bacterial cells in infection threads were not growing. Bacteria near the extending tips of threads were growing; the rest, near the back of the threads, appeared to be static (60, 61). The sizes of these bacterial growth zones were estimated to be about one-quarter to one-third of the length of a typical root hair (60).
FIG. 5.
Examples of mixed infection threads following coinoculation of alfalfa with red- and green-fluorescent bacteria. (A) Infection threads containing only gfp-expressing or DsRed-expressing S. meliloti. (B) A sectored infection thread in which the mixed population gave rise to a series of sectors which increase in length along the thread. The tipmost sector advanced into the epidermal cell body, branched (top arrow), and penetrated the underlying cell (bottom arrow), leaving the distal sectors behind. (C) A root hair infected with dual infection threads. Each thread contains sectors of gfp-expressing and DsRed-expressing bacteria. (D) A jumbled type of mixed infection thread. gfp-expressing and DsRed-expressing cells appear randomly mixed throughout the infection thread. (E) Dual infection threads inside a root hair. The threads began as jumbled-type threads but later gave rise to green sectors at their tips (arrows show the sectors in each thread). (Reprinted from reference 60.)
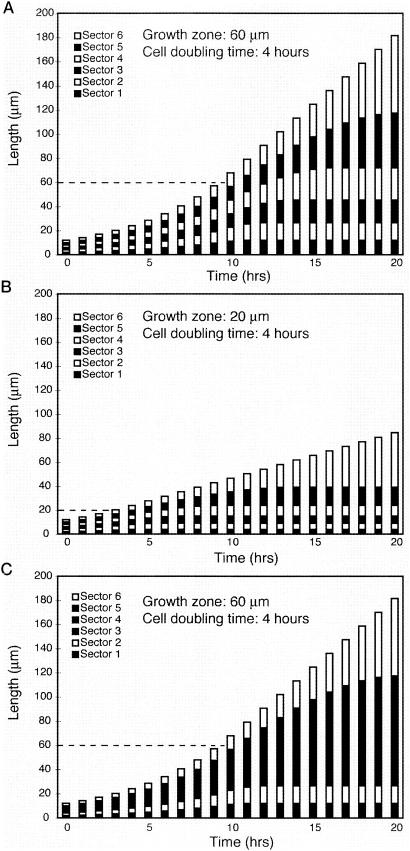
A model describing the dynamics of bacterial growth inside infection threads has been developed based on the observations that sectored infection threads are populated by descendants of only a few founder cells and that growing cells are present only in a zone near the tip of the infection thread (60, 61). The model is presented in graphical form in Fig. 6. The model is shown for three cases in which the host plant is inoculated with a mixture of bright, GFP-tagged S. meliloti and dark nontagged S. meliloti. In the cases shown in Fig. 6A and B, it is assumed that the thread is small at the start (time zero) and contains six alternating light and dark sectors of equal size. For Fig. 6A it is assumed that bacteria which are 60 μm or less from the tip of the thread can grow and divide. Those that are more than 60 μm from the tip are nongrowing; thus, only sectors, or parts of sectors, which are 60 μm or less from the tip grow between time points. For Fig. 6B it is assumed that the growth zone is 20 μm. In each case it is assumed that growing cells can double every 4 h when they are in the growth zone. A number of testable predictions follow from the model, as follows.
FIG. 6.
Model of infection thread growth. (A). A computer model was used to simulate sectored infection thread growth. For these cases, it was assumed that the thread started with six alternating bright and dark sectors of equal size. The doubling time for the growing bacteria (those 60 μm or less from the growing tip of the thread) was 4 h. Each bar is a representation of the length of the thread and its six sectors at each time point. Growth of the thread is exponential until it reaches 60 μm in length (dashed line) and becomes linear thereafter. Once the tipmost sector is more than 60 μm in length, it will be the only sector which continues to increase in size (see the last bar as an example). (B) Same as panel A, except the size of the growth zone is 20 μm. The thread grows at the same rate as for panel A while the thread is less than 20 μm long. After that, only cells in the tipmost 20 μm contribute to subsequent growth, and the overall rate of thread extension is lower than in the case where the growth zone is 60 μm long. (C). Same as panel A, except bacteria entering the thread are not assumed to have strictly alternated in terms of fluorescence. The entry pattern shown was dark-light-dark-dark-dark-light. This gives a total of four sectors, with the large dark sector in the middle of the thread having arisen from the loading of three dark cells in a row.
(i) The model predicts that sectors will increase in size along the length of the thread, with smaller sectors at the rear of the thread and longer ones near the growing tip. This pattern will arise because sectors at the rear of the thread stop growing earlier than sectors located closer to the growing tip. Such increases in sector length are commonly seen in sectored threads (60, 61) (Fig. 5).
(ii) The model predicts that sectored infections should give rise to nodules that are populated with only one of the two infecting strains. This outcome occurs because the only subpopulation of bacterial cells which continues to advance down the infection thread indefinitely are the descendants of the bacteria which were at the very tip of the infection thread when it initiated.
(iii) The model predicts that growth of the bacterial population in the thread will be exponential while the infection thread is shorter than the growth zone. After that, growth of the bacterial population will be linear and the extension rate will be proportional to the size of the growth zone (Fig. 6B). This implies that the growth zone must relatively large in order to ensure quick transit of the infection thread through root tissue.
It is most parsimonious to assume that most infection threads, whether mixed or not, grow in the same manner as sectored threads, with bacteria near their tips actively growing, more distal bacteria not growing, and only descendants of the tipmost bacterium going on to populate the nodule. The random distribution of colored bacteria in some infection threads would appear to be in conflict with this idea and with the growth model outlined in Fig. 6. However, mixed infection threads in which the infecting strains are seemingly randomly distributed often become sectored after a time. This suggests that the growth patterns of these two types of threads are not too different. An addition to the model may explain the apparently random growth pattern seen in some threads (60).
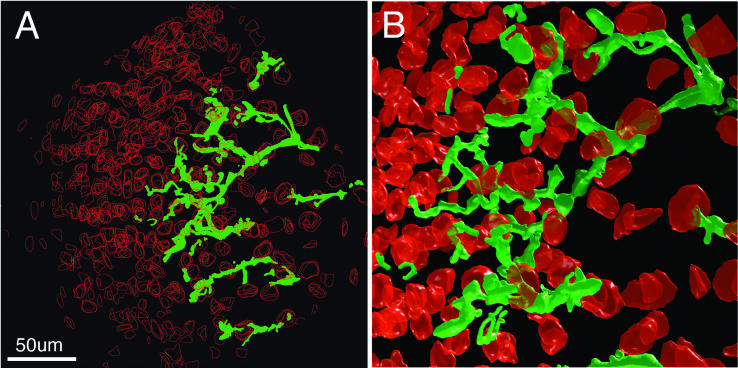
It should be noted that the model outlined here holds when the increase in bacterial number inside infection threads is manifested as an increase only in the length, and not in the width, of the space occupied by the bacteria. This is the case for most infection threads that are actively growing down alfalfa root hairs. Infection threads developing in the infection zone of alfalfa nodules are much broader, and less uniform in width, than those seen in root hairs. The growth behavior of such bacterial populations is likely to be different than the growth behavior of the bacterial populations in the model above (Gage, personal observation) (Fig. 7).
FIG. 7.
Architecture of the infection thread network inside a 10-day-old alfalfa nodule. An alfalfa nodule, induced by wild-type S. meliloti strain Rm1021, was fixed, embedded, and sliced into 1-μm-thick sections. Fifteen sections were dyed to reveal the bacteria and plant cell structures, photographed, and reassembled into a three-dimensional volume. (A) Projection of all 15 sections onto a single plane. The nuclei in each section are outlined in red, and the infection threads are filled in with green. The infection thread network appears to be polarized and growing toward the meristem, which is in the left side of the image. (B) Three-dimensional reconstruction of the nuclei and infection threads, showing a volume from the central part of the data set shown in panel A.
INFECTION THREAD INVASION OF THE DEVELOPING NODULE
Early Steps of Infection Thread Invasion: Activation of the Pericycle and Root Cortex
While our understanding of the plant-based cellular and molecular mechanisms of infection thread extension is limited, the cytological and molecular changes of cortical cells made in preparation for infection thread passage are currently better understood. Examination of sections of fixed material from a variety of legumes that form indeterminate nodules has shown that during the initiation of indeterminate nodules, two series of events take place concurrently. One begins on the surface and moves inward (46, 99, 112, 160, 161, 169). This series of events includes root hair curling, infection thread initiation, infection thread growth, and the reentry of outer cortical cells into the cell cycle. The other series of events begins near the center of the root, moves outward, and consists of a wave of activation in which cells reenter the cell cycle. Pericycle cells are the first to respond to the presence of compatible rhizobia. They become activated about 12 h after inoculation, and activated cells are typically located next to protoxylem poles (Fig. 1) (161). Other cells involved in this outwardly propagating wave of activation are also arranged in fairly narrow columns above the protoxylem poles of the root (161, 169, 191). Following pericycle activation, cells of the inner cortex reenter the cell cycle, and the resulting cell mass constitutes the nodule primordium. These cells will be the first to receive the intracellular bacteria that eventually differentiate into nitrogen-fixing bacteroids. The wave of cell activation continues to move outward, and cells of the middle cortex become activated next. These divide, and some of them (those that are not infected) will form the nodule meristem.
The spatial localization of pericycle activation next to protoxylem poles may require an inducing factor, termed stele factor and thought to perhaps be uridine, coming from the protoxylem, or it may be caused by inhibition of the response of cells near the protophloem poles (100, 142, 151). mRNA encoding 1-aminocyclopropane-1-carboxylic acid (ACC) oxidase, which catalyzes the final step of ethylene synthesis, has been shown by in situ hybridization to be enriched in cells adjacent to the protophloem poles (74). Given that ethylene can inhibit nodulation at a variety of steps (see below), it may be that expression of ACC oxidase at the protophloem poles increases ethylene production there and prevents activation of pericycle cells and nodule formation at these sites. Of course, an activating factor such as uridine at protoxylem poles may act in concert with an inhibitor such as ethylene at protophloem poles to provide the spatial information that results in columns of activated cells aligned over the protoxylem poles (see Fig. 1B for an example).
Concomitant with the outward-propagating wave of cell activation, a column or two of cells in the outer cortex also begin to divide. The cytoplasm moves from the cell periphery to a central position, as it normally does during cell division, but the cells usually progress no further through the cell cycle (161, 169). The cytoplasms in these activated outer cortical cells align with each other, giving rise to columns of cytoplasmic bridges called preinfection threads (PITs) through which the inwardly growing infection thread propagates. The PITs have a polarized structure in that nuclei are against the inner wall, and the column of cytoplasm is wider where it meets the outer wall than it is at the inner wall (169).
The future path of the growing infection thread through the outer cortex is marked by the aligned PITs. Micrographs show that as infection threads leave epidermal cells, they fuse with the innermost cell wall at a point that is marked by a cell wall weakening, a cytoplasmic bridge (PIT) in the underlying cell, and a bundle of endoplasmic microtubules that run along the length of the PIT (161, 169). Once the infection thread penetrates the epidermal cell wall, the bacteria spill into the intracellular space. The weakened outer wall of the underlying cell invaginates, and the infection thread propagates inward (169).
In pea, the differences between cell activation of the outer cortical cells, which reenter the cell cycle but do not typically divide, and of the pericycle, inner cortical, and middle cortical cells, which do divide, is reflected in the expression of some cell cycle genes. Most cortical cells in the columns of activated cells express an S-phase-specific histone H4 gene, indicating that they passed from G0/G1 into S phase after inoculation with R. leguminosarum. Middle and inner cortical cells also express cyc2, encoding a mitotic cyclin B, indicating that they progress from G2 to mitosis. However outer cortical cells do not express this gene suggesting that they are arrested in G2 (112, 191). Activated outer cortical cells can reenter the cell cycle and complete division. When PIT-containing outer cortical cells in M. truncatula divide, the PIT architecture is retained by daughter cells (161). A comprehensive review of nodule development and its attendant cell cycle gene regulation has been recently published (56).
On alfalfa, S. meliloti nodF nodL double mutants induce very few infection threads and cannot induce PIT formation (161). In contrast, activation of inner cortical cells does occur. This indicates, perhaps, that in these plants the inward-propagating wave of cellular changes requires that the nonreducing end of Nod factor be in a wild-type configuration, while the outward-propagating wave of activation has a less stringent dependency on that aspect of Nod factor structure. When exposed to wild-type S. meliloti Nod factors, alfalfa is able to induce activation of pericycle and inner cortical cells, as well as to form nodule primordia and meristems, but PITs do not form in the outer cortex (161), suggesting that an active bacterial substance was lost or inactivated during purification of Nod factor. Succinoglycan is not likely to be that substance, because exoA mutants of S. meliloti are able to form PITs on alfalfa (161). Interestingly, vetch and pea have been shown to form PITs in response to R. leguminosarum Nod factors, showing that the response of these plants may be different from that of alfalfa in this regard (or differences in the Nod factor purification protocols may account for the differing activities of the Nod factors).
Later Steps: Growth and Architecture of Infection Thread Networks in Young Nodules
Early during infection with S. meliloti, the growth of the infection thread network is highly polarized in alfalfa plants. Threads initiate in root hairs and then branch and ramify to some degree as they grow inward toward the developing nodule primordium. The cortical cells through which they pass exhibit polarized cytoplasm. The polarized cytoplasm is probably responsible for the polarized growth of the infection thread network at this point. After the thread network enters the nodule primordium, some of the uninfected, activated cells in the middle cortex organize into a nodule meristem (161). The meristem is located on the distal side of the nodule primordium, and it grows outward toward the root surface. At this time, the infection thread network is located behind (proximal to) the meristem (Fig. 1M). As the nodule grows, the infection thread network behind the meristem continues to develop, and eventually it forms a highly branched network in a region of the nodule termed the infection zone (Fig. 1N) (176). In indeterminate nodules such as those of alfalfa, the meristem continually grows away from the interior of the root. Clearly, as the meristem moves outward, the infection thread network must grow into the region behind the meristem that contains the newly divided and expanding cells that need to be infected.
How infection thread networks propagate in the nodule is currently unknown. It may be that the infection thread network in the infection zone is polarized and extends by tip growth toward the outward-moving meristem. Such polarization would be reminiscent of the polarized growth of infection threads in the early stages of nodulation. However, the infection thread network in the infection zone would exhibit outwardly polarized growth rather than inwardly polarized growth, although in both cases growth would be toward dividing plant cells. Polarized network growth could be supported if cells between the meristem and the infection zone were some how polarized or gave rise to a diffusible targeting signal. The hypothesis of a response to a diffusible signal and the hypothesis of directionally polarized cells directing infection thread growth in the nodule are not mutually exclusive, because cells between the meristem and infection zone could theoretically be polarized in response to diffusible meristem signals.
Alternatively, it may be that the infection thread network, as a whole, is not polarized at all and grows in the infection zone in a directionally random fashion, with only some of the threads invading newly formed cells behind the meristem. Preliminary work on serial sections of young nodules suggests that in the S. meliloti-alfalfa symbiosis, growth of the infection thread network is polarized and directed toward the nodule meristem (Fig. 7 and unpublished results).
PLANT CONTROL OF INFECTION
The number of infection threads initiated after inoculation of a host with a compatible rhizobial strain usually far exceeds the number of nodules that eventually develop (51, 117, 128, 177). In 1962 Nutman documented the infection and nodulation dynamics of a variety of plant-bacterium pairs, and he observed that infection proceeded in two phases (117). There was an early phase in which infection rate was high, followed by a second phase when that rate was much lower. The start of the second phase corresponded to the time just before nodules appeared, suggesting that nodules were perhaps involved in the subsequent decline in infection rate. Experiments utilizing nodule excision and split root systems showed that nodules themselves were responsible for the decline in infection rates, as opposed to the decline being time or plant growth phase dependent (12, 22-25, 91, 118). The addition of ammonium or nitrate has similar inhibitory effects on infection (24, 73, 158). Nutman also noted that early infections were not evenly distributed along the seedling root. Rather there were zones that were particularly susceptible early on, and later infections arose first in root hairs near these susceptible zones and then in zones father away (117). All of these observations indicate that there is plant control over infection rates, spatial distribution of infections, and how many infections are allowed to progress down the developmental pathway to nodules. That the plant controls these aspects of infection makes intuitive sense because infection may be an inherently risky process and because nodule number should be regulated such that it is high enough to meet the demands of plant growth without fixing unneeded nitrogen.
The fact that infection threads often outnumber nodules suggests that either infection thread progression is actively stopped by the plant or infection thread extension and movement out of root epidermal cells are inherently inefficient. Recent work suggests that the plant can actively stop thread growth, resulting in a small number of nodules relative to infections. Plant control over infection and nodulation has been shown to involve plant defense responses, the gaseous hormone ethylene, and a third signaling system. These plant control mechanisms are outlined below.
Plant Defense Responses following Infection Thread Formation
Plants have the capacity to limit the spread of viral, bacterial, or fungal pathogens by having plant cells in the vicinity of the pathogen generate ROS and reactive nitrogen species, synthesize phenolic compounds and protective proteins, alter their cell wall composition, and undergo programmed cell death (1, 37, 45, 69, 77, 80). This collection of defensive activities is called the hypersensitive response. Some of these activities have been noted to occur during the establishment of symbioses between legumes and rhizobial bacteria (5, 8, 125). Interestingly, cortical cells adjacent to epidermal cells containing infection threads and thread-containing epidermal cells often display characteristics reminiscent of a defense response (177). Such cells are necrotic, are autofluorescent (indicating that they likely are enriched in phenolics and phytoalexins), and by immunolocalization appear to contain defense-induced proteins such as hydroxyproline-rich glycoproteins, phenylalanine ammonia lyase, chalcone synthase, and chitinase (177). The presence of cells exhibiting this defense reaction has been correlated with termination of infection thread growth (177). Infection threads were often coiled within cells displaying signs of having mounted a defense response, suggesting that growth of such infection threads was not impaired but that exit from the host cells was inhibited. This is consistent with the idea that the defense-like response was not merely induced around threads that had already terminated growth. However, it may be that the defense response developed secondarily in cells that had already managed to contain infection threads but had not terminated their growth. Cells through which infection threads had successfully grown on their way toward the developing nodule did not display the defense-like response. Thus, it appeared that in some infected cells the host mounted a response, the end result of which may have been thread termination. The authors of that work suggested that this defensive response may be one of the mechanisms that regulates how many infections are allowed to progress down the pathway to nodule formation.
The generation of ROS can occur very early in the nodulation process. The M. truncatula rip1 peroxidase gene is induced within 3 h of S. meliloti addition (33). Ramu et al. have shown that the induction of this gene after inoculation with bacteria or purified Nod factor appears to be in response to a Nod factor-dependent increase in superoxide synthesis in the zone of the root most susceptible to root hair deformation and infection (132). Surprisingly, sulfation of the S. meliloti Nod factor was required for the induction of rip1 and for the preceding generation of superoxide radical. This implied that specific recognition of Nod factor was required for generation of ROS during nodulation. The fact that an M. truncatula dmi1-1 mutant that could not respond to Nod factor also could not induce rip1 or produce superoxide in response to inoculation reinforces the idea that generation of ROS during symbiosis requires specific Nod factor perception.
Other work shows that Nod factor is able to down regulate the generation of ROS, specifically hydrogen peroxide, when M. truncatula root tips are exposed to oligosaccharide elicitors of plant defense responses (147). This down regulation was not seen in the M. truncatula nfp (Nod factor perception) mutant, which is completely unresponsive to Nod factor (9). Interestingly, it was also shown that hydrogen peroxide production was inhibited by diphenylene iodonium, implicating NADPH oxidase in its generation.
Given that ROS and NADPH oxidase are required for normal root hair tip development in Arabidopsis (55), it is not unreasonable to postulate that modulation of hydrogen peroxide generation during symbiosis may be involved in controlling growth, deformation, or curling of root hairs. In addition, modulating the production of ROS may also affect defense responses in M. truncatula, which could affect infection thread initiation or progression of infection threads into the underlying cortical cell layers. The authors of the work described above point out that their data appear to conflict with that of Ramu et al. (132), but they suggest that the changes in ROS may be complex during symbiosis and that the differences seen may be due to differences in the times when the measurements were made following exposure to Nod factor or bacteria.
Ethylene and Control of Infection and Nodulation
Genetic and physiological experiments indicate that the gaseous hormone ethylene plays an important role in controlling nodule number in legumes and that application of exogenous ethylene can inhibit nodulation (93, 126). Modulation of in planta synthesis of ethylene in M. truncatula by using aminoethoxyvinylglycine (AVG) and ACC (an inhibitor of the ethylene-producing enzyme ACC oxidase and the substrate of ACC oxidase, respectively) has shown that the nodule number is low in the presence of ACC (high in planta levels of ethylene) and higher in the presence of AVG (low in planta levels of ethylene) (119). Molecular analyses indicated that ethylene inhibited early plant responses to nodulating bacteria. In particular, the presence of ACC decreased the effectiveness of S. meliloti Nod factor in causing calcium spiking and decreased transcription of the early nodulins encoded by RIP1 and ENOD11. Ethylene itself was shown to cause attenuation of calcium spiking when applied to root hairs in the process of spiking. Finally, down regulation of nodule number by ACC was shown to be due to a decreased number of infections rather than to a decrease in the number of infection threads allowed to enter the extension phase. This result is concordant with the results indicating that ethylene down regulates early steps in the infection process. Those authors point out that ethylene levels were likely to be fairly constant in these experiments because of the continuous presence of AVG and ACC and that under more natural conditions, where ethylene levels are under plant control and could fluctuate over time, ethylene may influence not only infection thread initiation but also infection thread extension (119). This caveat is important because experiments with M. truncatula skl mutants that cannot respond to ethylene imply that infection thread extension is down regulated by ethylene (124) (see below).
The M. truncatula sickle (skl) mutant is a dramatic example of how the infection regulation process can go awry (124). Upon inoculation with S. meliloti, this mutant displayed an extraordinary number of extended infection threads in zones of the root where root hairs were maturing and were most susceptible to infection. Many of these infections proceeded slowly along the developmental pathway toward nodules and resulted in only small, nodule-like, bumps. Spatial placement of infections and nodule promordia in the skl mutant was such that nodules were present around the circumference of the root in the susceptible zone. This was unusual because nodule primordia are typically found only next to the protoxylem poles, and this precludes circumferential placement of nodules (15, 99, 124). Physiological analysis of calcium spiking showed that skl plants were more sensitive to Nod factor than were wild-type plants (119). The initial analysis of the skl mutant showed that it displayed other phenotypes that were indicative of a defect in ethylene synthesis or perception. Because the mutant did not alter these phenotypes or its unusual nodulation phenotypes in response to ACC or externally applied ethylene, skl mutants were most likely defective in perception of, or response to, these compounds (123). The work with skl and the work done in modulating in planta ethylene levels with ACC and AVG indicate that ethylene can inhibit (i) the number of infections, (ii) the progression of those infections, (iii) the number of nodules, and (iv) activation of cortical cells next to protophloem poles (119, 123, 124). Some of these effects appear to be caused, at least in part, by the ability of ethylene to inhibit Nod factor perception or Nod factor signal transduction in the host.
ECOLOGICAL QUESTIONS ABOUT RHIZOBIAL INFECTION
Plant hosts growing in nitrogen-poor environments clearly benefit from being infected with nitrogen-fixing rhizobia. How the bacteria benefit from the association is less clear. As described above, infection threads on alfalfa are populated with the descendants of only a few founder S. meliloti cells, and the population in nodules may often be descendants of the bacterium that first entered the infection thread. Nodules are populated with many bacterial cells, but many of those are terminally differentiated bacteroids that can no longer give rise to daughter cells. Nevertheless, a few infecting bacteria can give rise to ten thousand or so viable bacteria that can be released from a mature alfalfa nodule upon crushing. Does such an increase in cell number occur in natural environments where nodules are not routinely crushed but rather senesce slowly over time? How do the numbers of bacteria released under natural conditions compare with the number of descendants that would have been left after the same amount of time if the bacteria had not entered the nodule and had instead grown in the rhizosphere, where grazing pressure from nematodes and single-celled eukaryotes and competition from other microbes may have acted to limit growth? Is infection an altruistic act in the sense that it benefits related bacteria in the soil by supporting plant growth, which in turn results in increased resources (mainly carbon) being returned to the rhizosphere where those related bacteria may live?
OUTLOOK
Investigation of cellular and molecular aspects of infection thread initiation and growth has been difficult because two partners are involved and their individual contributions are highly integrated with each other. Traditional reductionistic approaches have been hard to apply to such an integrated process. However, the introduction of new techniques such as microinjection into root hairs, image analysis, and transgenic plant generation are providing new avenues for investigating symbiotic infection. In addition, the development of appropriate genetic models on the plant side (L. japonicus and M. truncatula) is providing a wealth of opportunities by allowing the full power of genetic analysis to be brought to bear on host plant biology. Sequencing of several rhizobial species, and the eventual sequencing of their plant partners, will provide essential data that should allow investigators to more quickly link mutant phenotypes to genes.
Several important areas of infection thread biology are still opaque more than 100 years after the earliest depictions and discussions of infection threads were published (36, 185). Three of these areas are (i) The nature of the machinery that builds the infection thread, (ii) how bacteria leave the infection thread, and (iii) the nature of bacterial growth control that prevents bacteria from overgrowing their host both in infection threads and inside nodule cells. It is hoped that progress on these questions will be made in the near future, given the new techniques and tools now at hand.
Acknowledgments
This work was supported by a grant from the National Science Foundation (IBN9974483) and by the University of Connecticut Research Foundation.
I thank Catalina Arango and Devanshi Pandya for help in generating some of the figures and for providing unpublished data.
REFERENCES
- 1.Able, A. J. 2003. Role of reactive oxygen species in the response of barley to necrotrophic pathogens. Protoplasma 221:137-143. [DOI] [PubMed] [Google Scholar]
- 2.Ardourel, M., N. Demont, F. Debelle, F. Maillet, F. de Billy, J. C. Prome, J. Denarie, and G. Truchet. 1994. Rhizobium meliloti lipooligosaccharide nodulation factors: different structural requirements for bacterial entry into target root hair cells and induction of plant symbiotic developmental responses. Plant Cell 6:1357-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausmees, N., K. Jacobsson, and M. Lindberg. 2003. A unipolarly located, cell-surface-associated agglutinin, RapA1, belongs to a family of Rhizobium-adhering proteins (Rap) in Rhizobium leguminosarum bv. trifolii. Microbiology 147:549-559. [DOI] [PubMed] [Google Scholar]
- 4.Barnett, M. J., R. F. Fisher, T. Jones, C. Komp, A. P. Abola, F. Barloy-Hubler, L. Bowser, D. Capela, F. Galibert, J. Gouzy, M. Gurjal, A. Hong, L. Huizar, R. W. Hyman, D. Kahn, M. L. Kahn, S. Kalman, D. H. Keating, C. Palm, M. C. Peck, R. Surzycki, D. H. Wells, K. C. Yeh, R. W. Davis, N. A. Federspiel, and S. R. Long. 2001. Nucleotide sequence and predicted functions of the entire Sinorhizobium meliloti pSymA megaplasmid. Proc. Natl. Acad. Sci. USA 98:9883-9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baron, C., and P. C. Zambryski. 1995. The plant response in pathogenesis, symbiosis, and wounding: variations on a common theme? Annu. Rev. Genet. 29:107-129. [DOI] [PubMed] [Google Scholar]
- 6.Battisti, L., J. Lara, and J. A. Leigh. 1992. Specific oligosaccharide form of the Rhizobium meliloti exopolysaccharide promotes nodule invasion in alfalfa. Proc. Natl. Acad. Sci. USA 89:5625-5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker, A., S. Ruberg, B. Baumgarth, P. A. Bertram-Drogatz, L. Quester, and A. Puhler. 2002. Regulation of succinoglycan and galactoglucan biosynthesis in Sinorhizobium meliloti. J. Mol. Microbiol. Biotechnol. 4:187-190. [PubMed] [Google Scholar]
- 8.Benaben, V., G. Duc, V. Lefebvre, and T. Huguet. 1995. TE7, an inefficient sybiotic mutant of Medicago truncatula Gaertn. cv Jemalong. Plant Physiol. 107:53-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ben Amor, B., S. L. Shaw, G. Oldroyd, M. F., R. V. Penmetsa, D. Cook, S. R. Long, J. Denarie, and C. Gough. 2003. The NFP locus of Medicago truncatula controls an early step of Nod factor signal transduction upstream of a rapid calcium flux and root hair deformation. Plant J. 34:495-596. [DOI] [PubMed] [Google Scholar]
- 10.Benson, D. R., and M. L. Clawson. 2000. Evolution of the actinorhizal plant nitrogen-fixing symbiosis, p. 207-224. In E. Triplett (ed.), Prokaryotic nitrogen fixation: a model system for the analysis of a biological process. Horizon Scientific Press, Wymondham, England.
- 11.Benson, D. R., and W. B. Silvester. 1993. Biology of Frankia strains, actinomycete symbionts of actinorhizal plants. Microbiol. Rev. 57:293-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhuvaneswari, T. V., A. A. Bhagwat, and W. D. Bauer. 1981. Transient susceptibility of root cells in four common legumes to nodulation by Rhizobia. Plant Physiol. 68:1144-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bibikova, T. N., E. B. Blancaflor, and S. Gilroy. 1999. Microtubules regulate tip growth and orientation in root hairs of Arabidopsis thaliana. Plant J. 17:657-665. [DOI] [PubMed] [Google Scholar]
- 14.Bibikova, T. N., A. Zhigilei, and S. Gilroy. 1997. Root hair growth in Arabidopsis thaliana is directed by calcium and an endogenous polarity. Planta 203:495-505. [DOI] [PubMed] [Google Scholar]
- 15.Bond, L. 1948. Origin and developmental morphology of root nodules of Pisum sativum. Botan. Gaz. 109:411-434. [Google Scholar]
- 16.Borthakur, D., R. F. Barker, J. W. Latchford, L. Rossen, and A. W. Johnston. 1988. Analysis of pss genes of Rhizobium leguminosarum required for exopolysaccharide synthesis and nodulation of peas: their primary structure and their interaction with psi and other nodulation genes. Mol. Gen. Genet. 213:155-162. [DOI] [PubMed] [Google Scholar]
- 17.Bradey, J. D., and S. C. Fry. 1997. Formation of di-isodityrosine and loss of isodityrosine in the cell walls of tomaot cell-suspension cultures treated with fungal elicitors or H2O2. Plant Physiol. 115:87-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradley, D. J., P. Kjellbom, and C. J. Lamb. 1992. Elicitor- and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: a novel, rapid defense response. Cell 70:21-30. [DOI] [PubMed] [Google Scholar]
- 19.Brill, L. M., C. J. Evans, and A. M. Hirsch. 2001. Expression of MsLEC1- and MsLEC2-antisense genes in alfalfa plant lines causes severe embryogenic, developmental and reproductive abnormalities. Plant J. 25:453-461. [DOI] [PubMed] [Google Scholar]
- 20.Broughton, W. J., S. Jabbouri, and X. Perret. 2000. Keys to symbiotic harmony. J. Bacteriol. 182:5641-5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brownleader, M. D., P. Jackson, A. Mobasheri, A. T. Pantelides, S. Sumar, M. Trevan, and P. M. Dey. 1999. Molecular aspects of cell wall modifications during fruit ripening. Criti. Rev. Food Sci. Nutr. 39:149-164. [DOI] [PubMed] [Google Scholar]
- 22.Caetano-Anolles, G., and W. D. Bauer. 1988. Feedback regulation in nodule formation in alfalfa. Planta 175:546-557. [DOI] [PubMed] [Google Scholar]
- 23.Caetano-Anolles, G., and P. M. Gresshoff. 1991. Alfalfa controls nodulation during the onset of Rhizobium-induced cortical cell division. Plant Physiol. 95. [DOI] [PMC free article] [PubMed]
- 24.Caetano-Anolles, G., and P. M. Gresshoff. 1991. Plant genetic control of nodulation. Annu. Rev. Microbiol. 45:345-382. [DOI] [PubMed] [Google Scholar]
- 25.Caetano-Anolles, G., P. A. Johshi, and P. M. Gresshoff. 1991. Spontaneous nodules induce feedback suppression of nodulation in alfalfa. Planta 188:77-92. [DOI] [PubMed] [Google Scholar]
- 26.Callaham, D. A., and J. G. Torrey. 1981. The structural basis for infection of root hairs of Trifolium repens by Rhizobium. Can. J. Bot. 59:1647-1664. [Google Scholar]
- 27.Cardenas, L., L. Vidali, J. Dominguez, H. Perez, F. Sanchez, P. K. Hepler, and C. Quinto. 1998. Rearrangement of actin microfilaments in plant root hairs responding to Rhizobium etli nodulation signals. Plant Physiol. 116:871-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carol, R. J., and L. Dolan. 2002. Building a hair: tip growth in Arabidopsis thaliana root hairs. Philos. Trans. R. Soc. London Ser. B 357:815-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cassab, G. I. 1998. Plant cell wall proteins. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49:281-309. [DOI] [PubMed] [Google Scholar]
- 30.Catoira, R., A. C. J. Timmers, F. Maillet, C. Galera, R. V. Penmetsa, D. Cook, J. Denarie, and C. Gough. 2001. The HCL gene of Medicago truncatula controls Rhizobium-induced root hair curling. Development 128:1507-1518. [DOI] [PubMed] [Google Scholar]
- 31.Cheng, H. P., and G. C. Walker. 1998. Succinoglycan is required for initiation and elongation of infection threads during nodulation of alfalfa by Rhizobium meliloti. J. Bacteriol. 180:5183-5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cook, D. 2000. Medicago truncatula—a model in the making! Curr. Opin. Plant Biol. 2:301-304. [DOI] [PubMed] [Google Scholar]
- 33.Cook, D., D. Dreyer, D. Bonnet, M. Howell, E. Nony, and K. VandenBosch. 1995. Transient induction of a peroxidase gene in Medicago truncatula precedes infection by Rhizobium meliloti. Plant Cell 7:43-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cosgrove, D. J., P. Bedinger, and D. M. Durachko. 1997. Group I allergens of grass pollen as cell wall-loosening agents. Proc. Natl. Acad. Sci. USA 94:6559-6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cosgrove, D. J., L. C. Li, H. T. Cho, S. Hoffmann-Benning, R. C. Moore, and D. Blecker. 2002. The growing world of expansins. Plant Cell Physiol. 43:1436-1444. [DOI] [PubMed] [Google Scholar]
- 36.Dawson, M. 1900. ”Nitragin“ and the nodules of Leguminous plants. Philos. Trans. R. Soc. London Ser. B 192:1-28. [Google Scholar]
- 37.Delledonne, M., A. Polverari, and I. Murgia. 2003. The functions of nitric oxide-mediated signaling and changes in gene expression during the hypersensitive response. Antioxid. Redox Signal. 5:33-41. [DOI] [PubMed] [Google Scholar]
- 38.Denarie, J., F. Debelle, and J. C. Prome. 1996. Rhizobium lipo-chitooligosaccharide nodulation factors: signaling molecules mediating recognition and morphogenesis. Annu. Rev. Biochem. 65:503-535. [DOI] [PubMed] [Google Scholar]
- 39.de Ruijter, N. C. A., T. Bisseling, and A. M. C. Emons. 1999. Rhizobium Nod factors induce an increase in sub-apical fine bundles of actin filaments in Vicia sativa root hairs within minutes. Mol. Plant-Microbe Interact. 12:829-832. [Google Scholar]
- 40.de Ruijter, N. C. A., M. B. Rook, T. Bisseling, and A. M. C. Emons. 1998. Lipochito-oligosaccharides re-initiate root hair tip growth in Vicia sativa with high calcium and spectrin-like antigen at the tip. Plant J. 13:341-350. [Google Scholar]
- 41.Diaz, C. L., T. Logman, H. C. Stam, and J. W. Kijne. 1995. Sugar-binding activity of pea lectin expressed in white clover hairy roots. Plant Physiol. 109:1167-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diaz, C. L., L. S. Melchers, P. J. J. Hooykaas, B. J. J. Lugtenberg, and J. W. Kijne. 1989. Root lectin as a determinant of host-plant specificity in the Rhizobium-legume symbiosis. Nature 338:579-581. [Google Scholar]
- 43.Diaz, C. L., P. C. Van Spronsen, R. Bakhuizen, G. J. J. Logman, E. J. J. Lugtenberg, and J. W. Kijne. 1986. Correlation between infection by Rhizobium leguminosarum and lectin on the surface of Pisum sativum roots. Planta 168:350-359. [DOI] [PubMed] [Google Scholar]
- 44.Djordjevic, S. P., H. Chen, M. Batley, J. W. Redmond, and B. G. Rolfe. 1987. Nitrogen fixation ability of exopolysaccharide synthesis mutants of Rhizobium sp. strain NGR234 and Rhizobium trifolii is restored by the addition of homologous exopolysaccharides. J. Bacteriol. 169:53-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doke, N., Y. Miura, L. M. Sanchez, H. J. Park, T. Noritake, H. Yoshioka, and K. Kawakita. 1996. The oxidative burst protects plants against pathogen attack: mechanism and role as an emergency signal for plant bio-defence. Gene 179:45-51. [DOI] [PubMed] [Google Scholar]
- 46.Dudley, M. E., T. W. Jacobs, and S. R. Long. 1987. Microscopic studies of cell divisions induced in alfalfa roots by Rhizobium meliloti. Planta 171:289-301. [DOI] [PubMed] [Google Scholar]
- 47.Ehrhardt, D. W., E. M. Atkinson, and S. R. Long. 1992. Depolarization of Alfalfa root hair membrane potential by Rhizobium meliloti Nod factors. Science 256:998-1000. [DOI] [PubMed] [Google Scholar]
- 48.Ehrhardt, D. W., R. Wais, and S. R. Long. 1996. Calcium spiking in plant root hairs responding to Rhizobium nodulation signals. Cell 85:673-681. [DOI] [PubMed] [Google Scholar]
- 49.Emons, A. M. C., and B. Mulder. 2000. Nodulation factors trigger an increase of fine bundles of subapical actin filaments in Vicia root hairs: implications for root hair curling around bacteria, p. 44-49. In J. G. M. De Wit, T. Bisseling, and W. Stiekema (ed.), Biology of plant-microbe interaction, vol. 2. International Society for Molecular Plant-Microbe Interactions, St. Paul, Minn. [Google Scholar]
- 50.Esseling, J. J., F. G. Lhuissier, and A. M. Emons. 2003. Nod factor-induced root hair curling: continuous polar growth towards the point of Nod factor application. Plant Physiol. 132:1982-1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fahraeus, G. 1957. The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. J. Gen. Microbiol. 16:374-381. [DOI] [PubMed] [Google Scholar]
- 52.Fahraeus, G., and H. Ljunggren. 1959. The possible significance of pectic enzymes in root hair infection by nodule bacteria. Physiol. Plant 12:145-154. [Google Scholar]
- 53.Felle, H. H., E. Kondorosi, A. Kondorosi, and M. Schultze. 1999. Elevation of the cytosolic free [Ca2+] is indispensable for the transduction of the Nod factor signal in alfalfa. Plant Physiol. 121:273-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Finan, T. M., A. M. Hirsch, J. A. Leigh, E. Johansen, G. A. Kuldau, S. Deegan, G. C. Walker, and E. R. Signer. 1985. Symbiotic mutants of Rhizobium meliloti that uncouple plant from bacterial differentiation. Cell 40:869-877. [DOI] [PubMed] [Google Scholar]
- 55.Foreman, J., V. Demidchik, J. H. Bothwell, P. Mylona, H. Miedema, M. A. Torres, P. Linstead, S. Costa, C. Brownlee, J. D. Jones, J. M. Davies, and L. Dolan. 2003. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422:442-446. [DOI] [PubMed] [Google Scholar]
- 56.Foucher, F., and E. Kondorosi. 2000. Cell cycle regulation in the course of nodule organogenesis in Medicago. Plant Mol. Biol. 43:773-786. [DOI] [PubMed] [Google Scholar]
- 57.Fraysse, N., F. Couderc, and V. Poinsot. 2003. Surface polysaccharide involvement in establishing the rhizobium-legume symbiosis. Eur. J. Biochem. 270:1365-1380. [DOI] [PubMed] [Google Scholar]
- 58.Freiberg, C., R. Fellay, A. Bairoch, W. J. Broughton, A. Rosenthal, and X. Perret. 1997. Molecular basis of symbiosis between Rhizobium and legumes. Nature 387:394-401. [DOI] [PubMed] [Google Scholar]
- 59.Fu, Y., H. Li, and Z. B. Yang. 2002. The ROP2 GTPase controls the formation of cortical fine F-actin and the early phase of directional cell expansion during Arabidopsis organogenesis. Plant Cell 14:777-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gage, D. J. 2002. Analysis of infection thread development using Gfp- and DsRed-expressing Sinorhizobium meliloti. J. Bacteriol. 184:7042-7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gage, D. J., T. Bobo, and S. R. Long. 1996. Use of green fluorescent protein to visualize the early events of symbiosis between Rhizobium meliloti and alfalfa (Medicago sativa). J. Bacteriol. 178:7159-7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Geitmann, A., J. Hudak, F. Vennigerholz, and B. Walles. 1995. Immunogold localization of pectin and callose in pollen grains and pollen tubes of Brugmansia suaveolens—implications for the self-incompatibility reaction. J. Plant Physiol. 147:225-235. [Google Scholar]
- 63.Geurts, R., and T. Bisseling. 2002. Rhizobium nod factor perception and signalling. Plant Cell 14:S239-S249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Glazebrook, J., J. W. Reed, T. L. Reuber, and G. C. Walker. 1990. Genetic analyses of Rhizobium meliloti exopolysaccharides. Int. J. Biol. Macromol. 12:67-70. [DOI] [PubMed] [Google Scholar]
- 65.Gonzalez, J. E., et al. 1998. Biosynthetic control of molecular weight in the polymerization of the octasaccharide subunits of succinoglycan, a symbiotically important exopolysaccharide of Rhizobium meliloti. Proc. Natl. Acad. Sci. USA 95:13477-13482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gonzalez, J. E., B. L. Reuhs, and G. C. Walker. 1996. Low molecular weight EPS II of Rhizobium meliloti allows nodule invasion in Medicago sativa. Proc. Natl. Acad. Sci. USA 93:8636-8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gonzalez, J. E., G. M. York, and G. C. Walker. 1996. Rhizobium meliloti exopolysaccharides: synthesis and symbiotic function. Gene 179:141-146. [DOI] [PubMed] [Google Scholar]
- 68.Gray, J. X., and B. G. Rolfe. 1990. Exopolysaccharide production in Rhizobium and its role in invasion. Mol. Microbiol. 4:1425-1431. [DOI] [PubMed] [Google Scholar]
- 69.Greenberg, J. T. 1997. Programmed cell death in plant-pathogen interactions. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48:525-545. [DOI] [PubMed] [Google Scholar]
- 70.Gu, Y., V. Vernoud, Y. Fu, and Z. B. Yang. 2003. ROP GTPase regulation of pollen tube growth through the dynamics of tip-localized F-actin. J. Exp. Bot. 54:93-101. [DOI] [PubMed] [Google Scholar]
- 71.Handberg, K., and J. S. Stougaard. 1992. Lotus japonicus, an autogamous, diplod legume species for classical and molecular genetics. Plant J. 2:487-496. [Google Scholar]
- 72.Heidstra, R., R. Geurts, H. Franssen, H. Spaink, A. van Kammen, and T. Bisseling. 1994. Root hair deformation activity of nodulation factors and their fate on Vicia sativa. Plant Physiol. 105:787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heidstra, R., G. Nilsen, F. Martinea-Abarca, A. van Kammen, and T. Bisseling. 1997. Nod factor-induced expression of leghemoglobin to study the mechanism of NH4NO3 inhibition on root hair deformation. Mol. Plant-Microbe Interact. 10:215-220. [DOI] [PubMed] [Google Scholar]
- 74.Heidstra, R., W. C. Yang, Y. Yalcin, S. Peck, A. M. Emons, A. van Kammen, and T. Bisseling. 1997. Ethylene provides positional information on cortical cell division but is not involved in Nod factor-induced root hair tip growth in Rhizobium-legume interaction. Development 124:1781-1787. [DOI] [PubMed] [Google Scholar]
- 75.Hepler, P. K., L. Vidali, and A. Y. Cheung. 2001. Polarized cell growth in higher plants. Annu. Rev. Cell. Dev. Biol. 17:159-187. [DOI] [PubMed] [Google Scholar]
- 76.Her, G. R., J. Glazebrook, G. C. Walker, and V. N. Reinhold. 1990. Structural studies of a novel exopolysaccharide produced by a mutant of Rhizobium meliloti strain Rm1021. Carbohydr. Res. 198:305-312. [DOI] [PubMed] [Google Scholar]
- 77.Herouart, D., E. Baudouin, P. Frendo, J. Harrison, R. Santos, A. Jamet, G. Van de Sype, D. Touati, and A. Puppo. 2002. Reactive oxygen species, nitric oxide and glutathione: a key role in the establishment of the legume-Rhizobium symbiosis? Plant Physiol. Biochem. 40:619-624. [Google Scholar]
- 78.Herrmann, C., J. Wray, F. Travers, and T. Barman. 1992. Effect of 2,3-butanedione monoximie on myosin and myofibrillar ATPases: an example of an uncompetitive inhibitor. Biochemistry 31:12227-12232. [DOI] [PubMed] [Google Scholar]
- 79.Hirsch, A. M. 1999. Role of lectins (and rhizobial exopolysaccharides) in legume nodulation. Curr. Opin. Plant Biol. 2:320-326. [DOI] [PubMed] [Google Scholar]
- 80.Hoeberichts, F. A., and E. J. Woltering. 2003. Multiple mediators of plant programmed cell death: interplay of conserved cell death mechanisms and plant-specific regulators. BioEssays 25:47-57. [DOI] [PubMed] [Google Scholar]
- 81.Hotter, G. S., and D. B. Scott. 1991. Exopolysaccharide mutants of Rhizobium loti are fully effective on a determinate nodulating host but are ineffective on an indeterminate nodulating host. J. Bacteriol. 173:851-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hubbell, D., V. M. Morales, and M. Umali-Garcia. 1978. Pectolytic enzymes in Rhizobium. Appl. Environ. Microbiol. 35:210-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Iwanami, Y. 1956. Protoplasmic movement in pollen grains and pollen tubes. Phytomorphology 6:288-295. [Google Scholar]
- 84.Jones, M. A., J. J. Shen, Y. Fu, H. Li, Z. B. Yang, and C. S. Grierson. 2002. The Arabidopsis Rop2 GTPase is a positive regulator of both root hair initiation and tip growth. Plant Cell. 14:763-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaneko, T., Y. Nakamura, S. Sato, E. Asamizu, T. Kato, S. Sasamoto, A. Watanabe, K. Idesawa, A. Ishikawa, K. Kawashima, T. Kimura, Y. Kishida, C. Kiyokawa, M. Kohara, M. Matsumoto, A. Matsuno, Y. Mochizuki, S. Nakayama, N. Nakazaki, S. Shimpo, M. Sugimoto, C. Takeuchi, M. Yamada, and S. Tabata. 2000. Complete genome structure of the nitrogen-fixing symbiotic bacterium Mesorhizobium loti. DNA Res. 7:38-406. [DOI] [PubMed] [Google Scholar]
- 86.Kaneko, T., Y. Nakamura, S. Sato, K. Minamisawa, T. Uchiumi, S. Sasamoto, A. Watanabe, K. Idesawa, M. Iriguchi, M. Matsumoto, S. Shimpo, H. Tsuruoka, T. Wada, M. Yamada, and S. Tabata. 2002. Complete genomic sequence of nitrogen-fixing symbiotic bacterium Bradyrhizobium japonicum USDA110. DNA Res. 9:189-197. [DOI] [PubMed] [Google Scholar]
- 87.Ketelaar, T., and A. M. C. Emons. 2001. The cytoskeleton in plant cell growth: lessons from root hairs. New Phytol. 152:409-418. [DOI] [PubMed] [Google Scholar]
- 88.Ko, Y. H., and R. Gayda. 1990. Nodule formation in soybeans by exopolysaccharide mutants of Rhizobium fredii USDA 191. J. Gen. Microbiol. 136:105-113. [DOI] [PubMed] [Google Scholar]
- 89.Kohno, T., S. Chaen, and T. Shimmen. 1990. Characterization of the translocator associated with pollen tube organelles. Protoplasma 154:179-183. [Google Scholar]
- 90.Kohno, T., and T. Shimmen. 1987. Ca2+ induced fragmentaion of actin filaments in pollen tubes. Protoplasma 141:177. [Google Scholar]
- 91.Kosslak, R. M., and B. B. Bohlool. 1984. Supression of nodule development on one side of a split root system of soybeans caused by prior inoculaton of the other side. Plant Physiol. 75:125-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kost, B., E. Lemichez, P. Spielhofer, Y. Hong, K. Tolias, C. Carpenter, and N. H. Chua. 1999. Rac homologues and compartmentalized phosphatidylinositol 4,5-bisphosphate act in a common pathway to regulate polar pollen tube growth. J. Cell Biol. 145:317-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee, K. H., and T. A. La Rue. 1992. Exogenous ethylene inhibits nodulation of Pisum sativum L. cv Sparkle. Plant Physiol. 100:1759-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leigh, J. A., and D. L. Coplin. 1992. Exopolysaccharides in plant-bacterial interactions. Annu. Rev. Microbiol. 46:307-346. [DOI] [PubMed] [Google Scholar]
- 95.Leigh, J. A., E. R. Signer, and G. C. Walker. 1985. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc. Natl. Acad. Sci. USA 82:6231-6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Levery, S. B., H. Zhan, C. C. Lee, J. A. Leigh, and S. Hakomori. 1991. Structural analysis of a second acidic exopolysaccharide of Rhizobium meliloti that can function in alfalfa root nodule invasion. Carbohydr. Res. 210:339-347. [DOI] [PubMed] [Google Scholar]
- 97.Lhuissier, F. G. P., N. C. A. De Ruijter, B. J. Sieberer, J. J. Esseling, and A. M. C. Emons. 2001. Time course of cell biological events evoked in legume root hairs by Rhizobium Nod factors: state of the art. Ann. Bot. 87:289-302. [Google Scholar]
- 98.Li, Y. Q., C. Faleri, A. Geitmann, H. Q. Zhang, and M. Cresti. 1995. Immunogold localization of arabinogalactan proteins, unesterified and esterified pectins in pollen grains and pollen tubes of Nicotiana tabacum-L. Protoplasma 189:26-36. [Google Scholar]
- 99.Libbenga, K. R., and P. A. A. Harkes. 1973. Initial proliferation of cortical cells in the formation of root nodules in Pisum sativum. Planta 114:17-28. [DOI] [PubMed] [Google Scholar]
- 100.Libbenga, K. R., F. van Iren, R. J. Bogers, and M. F. Schrag-Lamers. 1973. The role of hormones and gradients in the initiation of cortex proliferation and nodule formation in Pisum sativum L. Planta 114:29-39. [DOI] [PubMed]
- 101.Limpens, E., C. Franken, P. Smit, J. Willemse, T. Bisseling, and R. Geurts. 2003. LysM domain receptor kinases regulating rhizobial Nod factor-induced infection. Science 302:630-633. [DOI] [PubMed] [Google Scholar]
- 102.Lloyd, C., K. Pearce, D. J. Rawlins, R. W. Ridge, and P. J. Shaw. 1987. Endoplasmic microtubules connect the advancing nucleus to the tip of legume root hairs, but F-actin is involved in basipetal migration. Cell Motil. Cytoskel. 8:27-36. [Google Scholar]
- 103.Madsen, E. B., L. H. Madsen, S. Radutoiu, M. Olbryt, M. Rakwalska, K. Szczyglowski, S. Sato, T. Kaneko, S. Tabata, N. Sandal, and J. Stougaard. 2003. A receptor kinase gene of the LysM type is involved in legume perception of rhizobial signals. Nature 425:637-640. [DOI] [PubMed] [Google Scholar]
- 104.Mateos, P. F., D. L. Baker, M. Petersen, E. Velazquez, J. I. Jimenez-Zurdo, E. Martinez-Molina, A. Squartini, G. Orgambide, D. H. Hubbell, and F. B. Dazzo. 2001. Erosion of root epidermal cell walls by Rhizobium polysaccharide-degrading enzymes as related to primary host infection in the Rhizobium-legume symbiosis. Can. J. Microbiol. 47:475-487. [DOI] [PubMed] [Google Scholar]
- 105.Mateos, P. F., J. I. Jimenez-Zurdo, J. Chen, A. S. Squartini, S. K. Haack, E. Martinez-Molina, D. H. Hubbell, and F. B. Dazzo. 1992. Cell-associated pectinolytic and cellulolytic enzymes in Rhizobium leguminosarum biovar trifolii. Appl. Environ. Microbiol. 58:1816-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Miller, D. D., N. C. A. de Ruijter, T. Bisseling, and A. M. C. Emons. 1999. The role of actin in root hair morphogenesis: studies with lipochito-oligosaccharide as a growth stimulator and cytochalasin as an actin perturbing drug. Plant J. 17:141-154. [Google Scholar]
- 107.Miller, D. D., N. C. A. deRuijter, and A. M. C. Emons. 1997. From signal to form: aspects of the cytoskeleton plasma membrane cell wall continuum in root hair tips. J. Exp. Bot. 48:1881-1896. [Google Scholar]
- 108.Molendijk, A. J., F. Bischoff, C. S. V. Rajendrakumar, J. Friml, M. Braun, S. Gilroy, and K. Palme. 2001. Arabidopsis thaliana Rop GTPases are localized to tips of root hairs and control polar growth. EMBO J. 20:2779-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Morales, V. M., E. Martinez-Molina, and D. H. Hubbell. 1984. Cellulase production by Rhizobium. Plant Soil 80:407-416. [Google Scholar]
- 110.Moulin, L., A. Munive, B. Dreyfus, and C. Boivin-Masson. 2001. Nodulation of legumes by members of the beta-subclass of Proteobacteria. Nature 411:948-950. [DOI] [PubMed] [Google Scholar]
- 111.Munoz, J. A., C. Coronado, J. Perez-Hormaeche, A. Kondorosi, P. Ratet, and A. J. Palomares. 1998. MsPG3, a Medicago sativa polygalacturonase gene expressed during the alfalfa Rhizobium meliloti interaction. Proc. Natl. Acad. Sci. USA 95:9687-9692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mylona, P., K. Pawlowski, and T. Bisseling. 1995. Symbiotic nitrogen fixation. Plant Cell 7:869-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Napoli, C. A., and D. H. Hubbell. 1975. Ultrastructure of Rhizobium-induced infection threads in clover root hairs. Appl. Microbiol. 30:1003-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Niehaus, K., R. Baier, B. Kohring, E. Flashl, and A. Puhler. 1997. Symbiotic suppression of the Medicago sativa plant defense system by Rhizobium meliloti oligosaccharides, p. 110-114. In A. Legoki, H. Bothe, and A. Puhler (ed.), Biological fixation of nitrogen for ecology and sustainable agriculture. Springer Verlag, Heidelberg, Germany.
- 115.Niehaus, K., D. Kapp, and A. Puhler. 1993. Plant defense and delayed infection of alfalfa pseudonodules induced by an exopolysaccharide (EPS-I) deficient Rhizobium meliloti mutant. Planta 190:415-425. [Google Scholar]
- 116.Nutman, P. S. 1956. The influence of the legume in root nodule symbiosis: a comparative study of host determinants. Biol. Rev. Camb. Philos. Soc. 31:109-151. [Google Scholar]
- 117.Nutman, P. S. 1962. The relation between root hair infection by Rhizobium and nodulation in Trifolium and Vicia. Proc. R. Soc. London Ser. B 156:122-137. [Google Scholar]
- 118.Nutman, P. S. 1952. Studies on the physiology of nodule formation. III. Experiments on the excision of root-tips and nodules. Ann. Bot. 16:79-101. [Google Scholar]
- 119.Oldroyd, G. E. D., E. M. Engstrom, and S. R. Long. 2001. Ethylene inhibits the Nod factor signal transduction pathway of Medicago truncatula. Plant Cell 13:1835-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Olsson, P. A., P. Kjellbom, and L. Rosendahl. 2002. Rhizobium colonization induced changes in membrane-bound and soluble hydroxyproline-rich glycoprotein composition in pea. Physiol. Plant 114:652-660. [DOI] [PubMed] [Google Scholar]
- 121.Pellock, B. J., H. P. Cheng, and G. C. Walker. 2000. Alfalfa root nodule invasion efficiency is dependent on Sinorhizobium meliloti polysaccharides. J. Bacteriol. 182:4310-4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pellock, B. J., M. Teplitski, R. P. Boinay, W. D. Bauer, and G. C. Walker. 2002. A LuxR homolog controls production of symbiotically active extracellular polysaccharide II by Sinorhizobium meliloti. J. Bacteriol. 184:5067-5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Penmetsa, R. V., and D. R. Cook. 1997. A legume ethylene-insensitive mutant hyperinfected by its rhizobial symbiont. Science 275:527-530. [DOI] [PubMed] [Google Scholar]
- 124.Penmetsa, R. V., J. A. Frugoli, L. S. Smith, S. R. Long, and D. R. Cook. 2003. Dual genetic pathways controlling nodule number in Medicago truncatula. Plant Physiol. 131:998-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Perotto, S., N. Brewin, and E. L. Kannenberg. 1994. Cytological evidence for a host-defense response that reduces cell and tissue invasion in pea nodules by lipopolysaccharide-defective mutants of Rhizobium leguminosarum strain 3841. Mol. Plant-Microbe Interact. 7:99-112. [Google Scholar]
- 126.Peters, N. K., and D. K. Crist Estes. 1989. Nodule formation is stimulated by the ethylene inhibitor aminoethoxyvinylglycine. Plant Physiol. 91:690-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Plazinski, J., and B. G. Rolfe. 1985. Analysis of the pectolytic activity of Rhizobium and Azospirillium strains isolated from Trifolium repens. J. Plant Physiol. 120:181-187. [Google Scholar]
- 128.Purchase, H. F. 1958. Restriction of infection threads in nodulaton of clover and lucerne. Aust. J. Biol. Sci. 11:155-161. [Google Scholar]
- 129.Radutoiu, S., L. H. Madsen, E. B. Madsen, H. H. Felle, Y. Umehara, M. Gronlund, S. Sato, Y. Nakamura, S. Tabata, N. Sandal, and J. Stougaard. 2003. Plant recognition of symbiotic bacteria requires two LysM receptor-like kinases. Nature 425:585-592. [DOI] [PubMed] [Google Scholar]
- 130.Rae, A. L., P. Bonfante-Fasolo, and N. J. Brewin. 1992. Structure and growth of infection threads in the legume symbiosis with Rhizobium leguminosarum. Plant J. 2:385-395. [Google Scholar]
- 131.Rae, A. L., S. Perrotto, J. P. Knox, E. L. Kannenberg, and N. J. Brewin. 1991. Expression of extracellular glycoproteins in the uninfected cells of developing pea nodule tissue. Mol. Plant-Microbe Interact. 4:563-570. [Google Scholar]
- 132.Ramu, S. K., H. M. Peng, and D. R. Cook. 2002. Nod factor induction of reactive oxygen species is correlated with expression of the early nodulin gene rip1 in Medicago truncatula. Mol. Plant-Microbe Interact. 15:522-528. [DOI] [PubMed] [Google Scholar]
- 133.Rathbun, E. A., M. J. Naldrett, and N. J. Brewin. 2002. Identification of a family of extensin-like glycoproteins in the lumen of Rhizobium-induced infection threads in pea root nodules. Mol. Plant-Microbe Interact. 15:350-359. [DOI] [PubMed] [Google Scholar]
- 134.Reuber, T. L., J. Reed, J. Glazebrook, M. A. Glucksmann, D. Ahmann, A. Marra, and G. C. Walker. 1991. Rhizobium meliloti exopolysaccharides: genetic analyses and symbiotic importance. Biochem. Soc. Trans. 19:636-641. [DOI] [PubMed] [Google Scholar]
- 135.Ridge, R. W. 1992. A model of legume root hair growth and Rhizobium infection. Symbiosis 14:349-373. [Google Scholar]
- 136.Ridge, R. W., and B. G. Rolfe. 1985. Rizobium sp. degradation of legume root hair cell wall at the site of infection thread origin. Appl. Environ. Microbiol. 50:717-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Roberts, J. A., C. A. Whitelaw, Z. H. Gonzalez-Carranza, and M. T. McManus. 2000. Cell separation processes in plants—models, mechanisms and manipulation. Ann. Bot. 86:223-235. [Google Scholar]
- 138.Roy, S. J., T. L. Holdaway-Clarke, G. R. Hackett, J. G. Kunkel, E. M. Lord, and P. K. Hepler. 1999. Uncoupling secretion and tip growth in lily pollen tubes: evidence for the role of calcium in exocytosis. Plant J. 19:379-386. [DOI] [PubMed] [Google Scholar]
- 139.SanchezFernandez, R., M. Fricker, L. B. Corben, N. S. White, N. Sheard, C. J. Leaver, M. VanMontagu, D. Inze, and M. J. May. 1997. Cell proliferation and hair tip growth in the Arabidopsis root are under mechanistically different forms of redox control. Proc. Natl. Acad. Sci. USA 94:2745-2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Santos, R., D. Herouart, S. Sigaud, D. Touati, and A. Puppo. 2001. Oxidative burst in alfalfa-Sinorhizobium meliloti symbiotic interaction. Mol. Plant-Microbe Interact. 14:86-89. [DOI] [PubMed] [Google Scholar]
- 141.Schiefelbein, J. W., A. Shipley, and P. Rowse. 1992. Calcium influx at the tip of growing root hair cells of Arabidopsis thaliana. Planta 187:455-459. [DOI] [PubMed] [Google Scholar]
- 142.Schlaman, H. R., A. A. Gisel, N. E. Quaedvlieg, G. V. Bloemberg, B. J. Lugtenberg, J. W. Kijne, I. Potrykus, H. P. Spaink, and C. Sautter. 1997. Chitin oligosaccharides can induce cortical cell division in roots of Vicia sativa when delivered by ballistic microtargeting. Development 124:4887-4895. [DOI] [PubMed] [Google Scholar]
- 143.Schlaman, H. R. M., B. Horvath, E. Vijgenboom, R. J. H. Okker, and B. J. J. Lugtengerg. 1991. Suppression of nodulation gene expression in bacteroids of Rhizobium leguminosarum biovar Viciae. J. Bacteriol. 173:4277-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sharma, S. B., and E. R. Signer. 1990. Temporal and spatial regulation of the symbiotic genes of Rhizobium meliloti in planta revealed by transposon Tn5-gusA. Genes Dev. 4:344-356. [DOI] [PubMed] [Google Scholar]
- 145.Shaw, S. L., J. Dumais, and S. R. Long. 2000. Cell surface expansion in polarly growing root hairs of Medicago truncatula. Plant Physiol. 124:959-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shaw, S. L., and S. R. Long. 2003. Nod factor elicits two separable calcium responses in Medicago truncatula root hair cells. Plant Physiol. 131:976-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shaw, S. L., and S. R. Long. 2003. Nod factor inhibition of reactive oxygen efflux in a host legume. Plant Physiol. 132:2196-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sherrier, D. J., and K. A. Vandenbosch. 1994. Secretion of cell-wall polysaccharides in Vicia root hairs. Plant J. 5:185-195. [Google Scholar]
- 149.Sieberer, B., and A. M. C. Emons. 2000. Cytoarchitecture and pattern of cytoplasmic streaming in root hairs of Medicago truncatula during development and deformation by nodulation factors. Protoplasma 214:118-127. [Google Scholar]
- 150.Sieberer, B. J., A. C. J. Timmers, F. G. P. Lhuissier, and A. M. C. Emons. 2002. Endoplasmic microtubules configure the subapical cytoplasm and are required for fast growth of Medicago truncatula root hairs. Plant Physiol. 130:977-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Smit, G., C. C. de Koster, J. Schripsema, H. P. Spaink, A. A. van Brussel, and J. W. Kijne. 1995. Uridine, a cell division factor in pea roots. Plant Mol. Biol. 29:869-873. [DOI] [PubMed] [Google Scholar]
- 152.Smit, G., J. W. Kijne, and B. J. Lugtenberg. 1989. Roles of flagella, lipopolysaccharide, and a Ca2+-dependent cell surface protein in attachment of Rhizobium leguminosarum biovar vicae to pea root hair tips. J. Bacteriol. 171:569-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Smit, G., J. W. Kijne, and B. J. J. Lugtenberg. 1986. Correlation between extracellular fibrils and attachment of Rhizobium leguminosarum to pea root hair tips. J. Bacteriol. 168:821-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Smit, G., J. W. Kijne, and B. J. J. Lugtenberg. 1987. Involvement of both cellulose fibrils and a Ca2+-dependent adhesin in the attchment of Rhizobium leguminosarum to pea root hair tips. J. Bacteriol. 169:4294-4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Smit, G., T. J. J. Logman, E. T. I. Boerrigter, J. W. Kijne, and B. J. J. Lugtenberg. 1989. Purification and partial characterization of the Rhizobium leguminosarum biovar vicae Ca2+-dependent adhesin, which mediates the first step in attachment of cells of the family Rhizobiaceae to plant root hair tips. J. Bacteriol. 171:4054-4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Smith, L. G. 2003. Cytoskeletal control of plant cell shape: getting the fine points. Curr. Opin. Plant. Biol. 6:63-73. [DOI] [PubMed] [Google Scholar]
- 157.Staiger, C. J., M. Yuan, R. Valenta, P. J. Shaw, R. M. Warn, and C. W. Lloyd. 1994. Microinjected profilin affects cytoplasmic streaming in plant-cells by rapidly depolymerizing actin microfilaments. Curr. Biol. 4:215-219. [DOI] [PubMed] [Google Scholar]
- 158.Streeter, J. 1988. Inhibition of legume nodule formation and nitrogen fixation by nitrate. CRC Crit. Rev. Plant Sci. 7:1-23. [Google Scholar]
- 159.Sy, A., E. Giraud, P. Jourand, N. Garcia, A. Willems, P. de Lajudie, Y. Prin, M. Neyra, M. Gillis, C. Boivin-Masson, and B. Dreyfus. 2001. Methylotrophic Methylobacterium bacteria nodulate and fix nitrogen in symbiosis with legumes. J. Bacteriol. 183:214-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Timmers, A. C., M. C. Auriac, F. de Billy, and G. Truchet. 1998. Nod factor internalization and microtubular cytoskeleton changes occur concomitantly during nodule differentiation in alfalfa. Development 125:339-349. [DOI] [PubMed] [Google Scholar]
- 161.Timmers, A. C., M. C. Auriac, and G. Truchet. 1999. Refined analysis of early symbiotic steps of the Rhizobium-Medicago interaction in relationship with microtubular cytoskeleton rearrangements. Development 126:3617-3628. [DOI] [PubMed] [Google Scholar]
- 162.Tominaga, M., K. Morita, S. Sonobe, E. Yokota, and T. Shimmen. 1997. Microtubules regulate the organization of actin filaments at the cortical region in root hair cells of Hydrocharis. Protoplasma 199:83-92. [Google Scholar]
- 163.Tominaga, M., E. Yokota, S. Sonobe, and T. Shimmen. 2000. Mechanism of inhibition of cytoplasmic streaming by a myosin inhibitor, 2,3-butanedione monoxime. Protoplasma 213:46-54. [Google Scholar]
- 164.Tominaga, M., E. Yokota, L. Vidali, S. Sonobe, P. K. Hepler, and T. Shimmen. 2000. The role of plant villin in the organization of the actin cytoskeleton, cytoplasmic streaming and the architecture of the transvacuolar strand in root hair cells of Hydrocharis. Planta 210:836-843. [DOI] [PubMed] [Google Scholar]
- 165.Tsyganov, V. E., V. A. Voroshilova, U. B. Priefer, A. Y. Borisov, and I. A. Tikhonovich. 2002. Genetic dissection of the initiation of the infection process and nodule tissue development in the Rhizobium-pea (Pisum sativum L.) symbiosis. Ann. Bot. 89:357-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Turgeon, B. G., and W. D. Bauer. 1985. Ultrastructure of infection-thread development during the infection of soybean by Rhizobium japonicum. Planta 163:328-349. [DOI] [PubMed] [Google Scholar]
- 167.Urzainqui, A., and G. C. Walker. 1992. Exogenous suppression of the symbiotic deficiencies of Rhizobium meliloti exo mutants. J. Bacteriol. 174:3404-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Van Batenburg, F. D. H., R. Jonker, and J. W. Kijne. 1986. Rhizobium induces marked root hair curling by redirection of tip growth, a computer simulation. Physiol. Plant 66:476-480. [Google Scholar]
- 169.van Brussel, A. A. N., R. Bakhuizen, P. C. van Spronsen, H. P. Spaink, T. Tak, B. J. J. Lugtenberg, and J. W. Kijne. 1992. Induction of pre-infection thread structures in the leguminous host plant by mitogenic lipo-oligosaccharidess of Rhizobium. Science 257:70-72. [DOI] [PubMed] [Google Scholar]
- 170.Van den Bosch, K. A., D. J. Bradley, J. P. Knox, S. Perotto, G. W. Butcher, and N. J. Brewin. 1989. Common components of the infection thread matrix and intercellular space identified by immunocytochemical analysis of pea nodules and uninfected roots. EMBO J. 8:335-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.van Rhijn, P., N. A. Fujishige, P. O. Lim, and A. M. Hirsch. 2001. Sugar-binding activity of pea lectin enhances heterologous infection of transgenic alfalfa plants by Rhizobium leguminosarum biovar viciae. Plant Physiol. 126:133-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.van Rhijn, P., R. B. Goldberg, and A. M. Hirsch. 1998. Lotus corniculatus nodulation specificity is changed by the presence of a soybean lectin gene. Plant Cell 10:1233-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.van Spronsen, P. C., R. Bakhuizen, A. A. N. van Brussel, and J. W. Kijne. 1994. Cell-wall degradation during infection thread formation by the root-nodule bacterium Rhizobium leguminosarum is a 2-step process. Eur. J. Cell Biol. 64:88-94. [PubMed] [Google Scholar]
- 174.van Workum, W. A. T., A. A. N. van Brussel, T. Tak, C. A. Wijffelman, and J. W. Kijne. 1995. Ethylene prevents nodulation of Vicia sativa Ssp Nigra by exopolysaccharide-deficient mutants of Rhizobium-Leguminosarum Bv Viciae. Mol. Plant-Microbe Interact. 8:278-285. [Google Scholar]
- 175.van Workum, W. A. T., S. van Slageren, A. A. N. van Brussel, and J. W. Kijne. 1998. Role of exopolysaccharides of Rhizobium leguminosarum bv. viciae as host plant-specific molecules required for infection thread formation during nodulation of Vicia sativa. Mol. Plant-Microbe Interact. 11:1233-1241. [Google Scholar]
- 176.Vasse, J., F. de Billy, S. Camut, and G. Truchet. 1990. Correlation between ultrastructural differentiation of bacteroids and nitrogen fixation in alfalfa nodules. J. Bacteriol. 172:4295-4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Vasse, J., F. de Billy, and G. Truchet. 1993. Abortion of infection during the Rhizobium meliloti-alfalfa sybiotic interaction is accompanied by a hypersensitive reaction. Plant J. 4:555-566. [Google Scholar]
- 178.Vidali, L., S. T. McKenna, and P. K. Hepler. 2001. Actin polymerization is essential for pollen tube growth. Mol. Biol. Cell 12:2534-2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Vitousek, P. M., J. D. Aber, R. W. Howarth, G. E. Likens, P. A. Matson, D. W. Schindler, W. H. Schlesinger, and D. G. Tilman. 1997. Human alteration of the global nitrogen cycle: sources and consequences. Ecol. Appl. 7:737-750. [Google Scholar]
- 180.Vlassak, K. M., E. Luyten, C. Verreth, P. van Rhijn, T. Bisseling, and J. Vanderleyden. 1998. The Rhizobium sp. BR816 can function a a determinant for nodulation of Leucaena leucocephala, Phaseolus vulgaris and Trifolium repens by a diversity of Rhizobium spp. Mol. Plant-Mirobe Interact. 11:383-392. [Google Scholar]
- 181.Wais, R. J., D. H. Keating, and S. R. Long. 2002. Structure-function analysis of Nod factor-induced root hair calcium spiking in rhizobium-legume symbiosis. Plant Physiol. 129:211-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Walker, S. A., and J. A. Downie. 2000. Entry of Rhizobium leguminosarum bv. viciae into root hairs requires minimal nod factor specificity, but subsequent infection thread growth requires nodO or nodE. Mol. Plant-Microbe Interact. 13:754-762. [DOI] [PubMed] [Google Scholar]
- 183.Walker, S. A., V. Viprey, and J. A. Downie. 2000. Dissection of nodulation signaling using pea mutants defective for calcium spiking induced by nod factors and chitin oligomers. Proc. Natl. Acad. Sci. USA 97:13413-13418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wang, L. X., Y. Wang, B. Pellock, and G. C. Walker. 1999. Structural characterization of the symbiotically important low-molecular-weight succinoglycan of Sinorhizobium meliloti. J. Bacteriol. 181:6788-6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ward, H. M. 1887. On the turbercular swellings on the roots of Vicia faba. Philos. Trans. R. Soc. London Ser. B 178:539-562. [Google Scholar]
- 186.Widmer, F., B. T. Shaffer, L. A. Porteous, and R. J. Seidler. 1999. Analysis of nifH gene pool complexity in soil and litter at a Douglas fir forest site in the Oregon Cascade mountain range. Appl. Environ. Microbiol. 65:374-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wisniewski, J. P., E. A. Rathbun, J. P. Knox, and N. J. Brewin. 2000. Involvement of diamine oxidase and peroxidase in insolubilization of the extracellular matrix: implications for pea nodule initiation by Rhizobium leguminosarum. Mol. Plant-Microbe Interact. 13:413-420. [DOI] [PubMed] [Google Scholar]
- 188.Wymer, C. L., T. N. Bibikova, and S. Gilroy. 1997. Cytoplasmic free calcium distributions during the development of root hairs of Arabidopsis thaliana. Plant J. 12:427-439. [DOI] [PubMed] [Google Scholar]
- 189.Xi, C., G. Dirix, J. Hofkens, F. C. Schryver, J. Vanderleyden, and J. Michiels. 2001. Use of dual marker transposons to identify new symbiosis genes in Rhizobium. Microb. Ecol. 41:325-332. [DOI] [PubMed] [Google Scholar]
- 190.Yang, C., E. R. Signer, and A. M. Hirsch. 1992. Nodules initiated by Rhizobium meliloti exopolysaccharide mutants lack a discrete, persistent nodule meristem. Plant Physiol. 98:143-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yang, W. C., C. de Blank, I. Meskiene, H. Hirt, J. Bakker, A. van Kammen, H. Franssen, and T. Bisseling. 1994. Rhizobium Nod factors reactivate the cell cycle during infection and nodule primordium formation, but the cycle is only completed in primordium formation. Plant Cell 6:1415-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yang, Z. B. 2002. Small GTPases: versatile signaling switches in plants. Plant Cell 14:S375-S388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zehr, J. P., B. D. Jenkins, S. M. Short, and G. F. Steward. 2003. Nitrogenase gene diversity and microbial community structure: a cross-system comparison. Environ. Microbiol. 5:539-554. [DOI] [PubMed] [Google Scholar]
- 194.Zorreguieta, A., C. Finnie, and J. A. Downie. 2000. Extracellular glycanases of Rhizobium leguminosarum are activated on the cell surface by an exopolysaccharide-related component. J. Bacteriol. 182:1304-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]