Summary
Chemotherapy has yielded minimal clinical benefit in pancreatic and biliary tract cancer. A high-dose, short course capecitabine schedule with oxaliplatin, has shown some efficacy with a lower incidence of palmar-plantar erythrodysesthesia. Achieving high exposures of the targeted agent sorafenib may be possible with this shorter schedule of capecitabine by avoiding dermatologic toxicity. All patients had pancreatic or biliary tract cancer. Patients in both cohorts received oxaliplatin 85 mg/m2 followed by capecitabine 2250 mg/m2 PO every 8 hours × 6 doses starting on days 1 and 15 of a 28 day cycle, or 2DOC (2 Day Oxaliplatin/Capecitabine). Cohort 1 used sorafenib 200 mg BID, and cohort 2 used sorafenib 400 mg BID. Sixteen patients were enrolled. Across all cycles the most common grade 1 or 2 adverse events were fatigue (10 pts), diarrhea (10 pts), nausea (9 pts), vomiting (8 pts), sensory neuropathy (8 pts), thrombocytopenia (7 pts), neutropenia (5 pts), and hand-foot syndrome (5 pts). Grade 3 toxicites included neutropenia, mucositis, fatigue, vomiting and diarrhea. Cohort 1 represented the MTD. Two partial responses were seen, one each in pancreatic and biliary tract cancers. The recommended phase II dose of sorafenib in combination with 2DOC is 200 mg BID. There were infrequent grade 3 toxicities, most evident with sorafenib at 400 mg BID.
Keywords: Pancreatic cancer, bile duct cancer, oxaliplatin, sorafenib, capecitabine, chemotherapy
Introduction
Pancreas cancer is a disease with high rates of metastatic disease at diagnosis, poor prognosis and high rates of recurrence even after attempts at cure with surgery. Gemcitabine with or without erlotinib has demonstrated a response rate of 8–9%, with an associated modest improvement in survival [1,2]. A combination of 5-FU, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX) has demonstrated a significant improvement over gemcitabine in patients with a good performance status; while the response rates and overall survival improvements are significant, the regimen is associated with significant toxicity, particularly myelosuppression [3,4].
Similarly, biliary tract malignancies (cholangiocarcinoma/gallbladder adenocarcinoma) are often metastatic at diagnosis, and portend a poor prognosis in spite of extensive resection/transplantation/chemotherapy. Combining gemcitabine and capecitabine has demonstrated activity in the phase II setting [5,6], as has oxaliplatin plus capecitabine [7]. The phase III ABC-02 study established gemcitabine/cisplatin as a standard of care, with an overall survival of 11.7 months [8]. Patients with unresectable disease face a median survival of slightly over one year with combination chemotherapy based on existing data [5,6]. Newer, more effective, and more convenient chemotherapeutic combinations for pancreaticobiliary malignancies are warranted.
A novel chemotherapy regimen designed to more closely replicate FOLFOX-6, substituting high-doses of capecitabine over a shortened period of time for continuous infusion 5-FU was developed at the University of Wisconsin Carbone Cancer Center and has been described [9]. This regimen has been called oral FOLFOX-6 or 2DOC (Two-Day Oxaliplatin/Capecitabine). The recommended phase II dose is as follows: oxaliplatin 85 mg/m2 intravenously on days one and fourteen of a 28 day cycle and capecitabine 2250 mg/m2 orally every 8 hours for six doses starting on days one and fourteen of a 28 day cycle [9]. During the phase I study of this combination, modest disease activity was seen in both biliary and pancreas cancer patients. Of six patients with pancreatic adenocarcinoma, there was one minor response (21% shrinkage, with a reduction in CA 19-9 from 14,370 to 887 U/mL on therapy) and one patient with stable disease. Notably, both of these patients had gemcitabine-refractory disease. Among biliary tract tumors, a total of nine patients were treated, with one complete response and two minor responses. Notably, there were no reported grade 2 or greater palmar-plantar erythrodysesthesia events.
Other centers have evaluated FOLFOX-type regimens in pancreaticobiliary tumors. FOLFOX-6 produced a 34.5% disease control rate, with time to progression of 4 months, and median overall survival of 7.5 months in the first line setting in pancreatic cancer [10]. In 30 gemcitabine-pretreated patients with advanced pancreatic cancer, a bolus 5-FU/oxaliplatin regimen showed 23% partial responses, 30% stable disease, and an improvement in performance status from baseline in 43% of patients [11]. The CONKO-003 trial randomized 165 patients with pancreatic cancer to 5-FU/leucovorin versus oxaliplatin plus 5-FU-leucovorin, and demonstrated an improvement in OS (20 wks vs 13 weeks, p=0.014) for the oxaliplatin-containing arm [12]. Traditional dose capecitabine and erlotinib demonstrated a 10% overall response rate in gemcitabine refractory pancreatic cancer, with minimal excess toxicity [13].
Sorafenib (Nexavar, BAY 43-9006) is an oral multi-kinase inhibitor with effects on tumor proliferation and tumor angiogenesis. Sorafenib has activity against c-raf, b-raf, VEGFR-2, PDGFR-β, c-kit and FLT3 [14]. Both pancreas cancer and bilary tract cancers have disruptions in cellular mechanisms where sorafenib could be considered an attractive targeted agent. Seventy to 90% of pancreas cancers have Ras activation [15]. In addition, 76% of cholangiocarcinoma tumor specimens express VEGF [16], 46% express PDGFR-beta, and 31% express kit [17]. There has also been activity seen with sorafenib in pancreas and biliary cancer in animal models [18,19]. Adding sorafenib to gemcitabine was tested in a phase I trial and showed stable disease in 13 of 23 patients, but no radiographic responses. No added toxicity was observed by combining sorafenib with gemcitabine [18], however a phase II study did not show any promising responses in either the gemcitabine plus sorafenib arm or the sorafenib alone arm [28].
One of the major dose-limiting toxicities of sorafenib is palmar-plantar erythrodysesthesia [20–23]. This toxicity has made it difficult to successfully combine sorafenib with capecitabine, another chemotherapeutic with palmar-plantar erythrodysesthesia [23]. However, given the low rate of capecitabine-related hand-foot syndrome observed with 2DOC, there is a lessened likelihood of cumulative toxicity combining sorafenib with this unique capecitabine schedule. Further, given the signal of activity of oxaliplatin and capecitabine in pancreas and biliary tract cancers and the preclinical activity of sorafenib in pancreas and biliary tract cancers, a phase Ib, two dose level dose escalation study was initiated.
Methods
Study design and sample size
The study was designed as an open label, multicenter phase I/II trial. This report details the phase I dose escalation component of the trial. Two dose levels of sorafenib were explored, 200 mg PO BID and 400 mg PO BID. Dose escalation was planned in groups of three patients. If none of the first three patients experienced a dose limiting toxicity (DLT), subsequent patients could be treated at the next higher cohort of sorafenib (from 200 mg BID to 400 mg BID). Once a DLT was observed, the cohort would expand to include at least 6 patients. If two or more toxicities were observed, then the maximum tolerated dose (MTD) was exceeded and subsequent subjects were enrolled at the lower cohort, expanding to at least 6 patients. The recommended phase II dose level was defined as the highest level at which there were less than two DLTs observed in at least six patients. The 400 mg dose was preset as the maximum dose of sorafenib that would be explored. Sample size was estimated at up to nine patients per cohort to detect a true toxicity rate of 10–30%.
This study accrued through the Wisconsin Oncology Network (WON), a network of academic and private practice institutions across the state of Wisconsin and the upper Midwest. The study was approved by the scientific review committees, if applicable, and the institutional review boards at all participating institutions and in concordance with good clinical practice guidelines. All patients provided informed consent at the time of enrollment.
Objectives
The primary objectives of this phase I study were to assess the overall safety, to define the DLTs and recommended phase II dose of sorafenib, oxaliplatin and capecitabine given in combination. A secondary objective was the clinical response rate (complete response, partial response, and stable disease rates) in patients with pancreaticobiliary primary tumors treated with 2DOC/sorafenib. RECISTv1.0 was used to assess for clinical response.
Definition of DLT and MTD
Dose limiting toxicity was defined as toxicity probably or definitely related to capecitabine, oxaliplatin or sorafenib including the following: any grade 4 neutropenia of greater than or equal to five days, febrile neutropenia of any duration, grade 4 anemia, grade 4 thrombocytopenia, grade 4 sensory neuropathy, grade 3 nausea/vomiting with maximal antiemetic treatment, grade 3 diarrhea with maximal antidiarrheal therapy or grade 3 or greater non-hematologic toxicity. All grades were according to NCI Common Terminology Criteria for Adverse Events, Version 3.0. Patients were defined as being evaluable for toxicity (e.g., for DLT) if they did not miss more than the intended 24 hours of dosing of sorafenib (2 doses) or capecitabine (three doses).
The MTD was specified as the dose level at which less than one-third of patients experience DLT. For MTD determination, only attributable toxicities that occur during treatment days 1 through 28 were utilized, including toxicities present pretreatment on Cycle 2 Day 1. Patients whose treatments were delayed due to toxicities from cycle 1, but not considered a DLT, were replaced.
Patient selection
To be eligible for participation in this study, patients were required to have histologically or cytologically confirmed adenocarcinoma of the pancreas or biliary tract without more than one prior systemic treatment for his or her disease. Eligible patients were required to have at least one measurable lesion as defined by RECISTv1.0 [24]. All participating patients had to be at least 18 years of age, have an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2, and have adequate organ and marrow function (including leukocytes ≥3,000/μl, absolute neutrophil count ≥1,500/μl, platelets ≥ 100,000/μl, total bilirubin ≤ 2.5 × institutional upper limit of normal (ULN), AST (SGOT)/ALT (SGPT)≤ 5 × ULN, creatinine clearance ≥50 mL/min as calculated by the Cockcroft-Gault formula).
Exclusion criteria included concomitant radiation therapy, or other systemic cancer therapies, known brain metastases, allergy to any of the study drugs, pregnancy or nursing, HIV, second malignancy within three years, gastrointestinal malabsorption or uncontrolled intercurrent illness. Patients were also excluded if he or she had a grade 2 or greater peripheral neuropathy (as defined in the Common Toxicity Criteria, version 3.0) [25]. In addition, the protocol required that prior systemic therapy, radiotherapy, major surgery, open biopsy or significant traumatic injury be completed more than 4 weeks from first dose of study drug. Because of drug interactions with sorafenib, use of St. John’s Wort or rifampin (rifampicin) was contraindicated. Patients were allowed to discontinue the use of these drugs to become eligible.
Treatment
Study treatment consisted of oxaliplatin 85 mg/m2 intravenously over two hours on days one and fifteen, followed by capecitabine 2250 mg/m2 orally every eight hours for six doses starting on days one and fifteen, and sorafenib 200–400 mg orally twice daily taken continuously without breaks. Cohort 1 received 200 mg BID of sorafenib and Cohort 2 received 400 mg BID of sorafenib. The oxaliplatin and capecitabine dosing was the same between the two cohorts. A cycle was defined as 28 days, and disease was evaluated every two cycles with computed tomography. Standard antiemetics were used. Dose reductions of oxaliplatin for grade 3 or greater neuropathy to 65 mg/m2 were allowed. Two dose reductions were allowed for sorafenib. If sorafenib was not tolerated after two dose reductions, sorafenib was discontinued, but patients were allowed to continue on the oxaliplatin and capecitabine if they were thought to be having clinical benefit.
Statistical analysis
Descriptive statistics are provided for all endpoints discussed. Continuous measurements are summarized using means, standard deviations, and absolute number of patients. Graphical assessments are presented by dose cohort and individual patients.
Results
Patient Characteristics
Sixteen patients enrolled in the phase I portion of the study. Nine patients were enrolled on the 200 mg sorafenib/2DOC cohort, and seven were enrolled on the 400 mg sorafenib/2DOC cohort. Demographics are summarized in Table 1. Eleven patients had received prior therapy either in the adjuvant or metastatic setting; all prior regimens contained gemcitabine.
Table 1.
Demographics of participants
| Characteristic | # per cohort | % patients (n=16) | |
|---|---|---|---|
| 2DOC + sorafenib 200mg BID | 2DOC + sorafenib 400 mg BID | Total | |
| Gender | # (%) | ||
| Female | 5 | 3 | 8 (50%) |
| Male | 4 | 4 | 8 (50%) |
| Race | |||
| Caucasian | 9 | 6 | 15 (94%) |
| Asian | 0 | 1 | 1 (6%) |
| Age Group(years) | |||
| 40–49 | 1 | 2 | 3 (19%) |
| 50–59 | 1 | 1 | 2 (12%) |
| 60–69 | 3 | 4 | 7 (44%) |
| 70–79 | 3 | 0 | 3 (19%) |
| 80–89 | 1 | 0 | 1 (6%) |
Dose Escalation and Safety
Toxicity data are summarized in Table 2. All sixteen patients were evaluable for safety. Three patients were enrolled at the 200 mg cohort. No DLTs were seen, and the 400 mg cohort was opened to three patients. One DLT was seen (grade 3 pneumonia) and the 200 mg cohort was expanded to an additional 6 patients to gather further toxicity data. When no additional DLTs were seen at 200 mg, four additional patients were enrolled at 400 mg, comprising the entire study population. Additional DLTs seen at the 400 mg dose level included grade 3 fatigue, grade 3 diarrhea and grade 4 vomiting. Thus, 200 mg of sorafenib plus 2DOC was defined as the recommended phase II dose.
Table 2.
Related Toxicities by Grade (% of patients by arm by grade)
| Cohort 1 sorafenib 200 mg bid | n (%) | n (%) | Cohort 2 Sorafenib 400 mg BID | n (%) | n (%) |
|---|---|---|---|---|---|
| Toxicity | Grade 2 | Grade 3 | Grade 2 | Grade 3 | |
| Gastrointestinal | |||||
| Nausea | 2 (22%) | 0(0%) | 2 (28%) | 0(0%) | |
| Vomiting | 1 (11%) | 1 (11%) | 2 (28%) | 1 (14%) | |
| Diarrhea | 1 (11%) | 1 (11%) | 0(0%) | 2 (28%) | |
| Hematologic | |||||
| Anemia | 0(0%) | 0(0%) | 1 (14%) | 0(0%) | |
| Thrombocytopenia | 1 (11%) | 0(0%) | 2 (28%) | 0(0%) | |
| Neutropenia | 0(0%) | 3 (33%) | 0(0%) | 0(0%) | |
| Fatigue | 3 (33%) | 0(0%) | 1 (14%) | 1 (14%) | |
| Dermatologic | |||||
| Hand-Foot | 1 (11%) | 0(0%) | 0(0%) | 0(0%) | |
| Acneiform Rash | 1 (11%) | 0(0%) | 0(0%) | 0(0%) | |
| Neuropathy | 2 (22%) | 0(0%) | 0(0%) | 0(0%) | |
| Hypertension | 0(0%) | 0(0%) | 0(0%) | 0(0%) |
Thirteen patients (81%) experienced at least one adverse event. The most common side effects at least possibly related to the study drug across all cycles were fatigue (10 patients), diarrhea (10 patients), nausea (9 patients), vomiting (8 patients), sensory neuropathy (8 patients), thrombocytopenia (7 patients), neutropenia (5 patients), and hand-foot syndrome (5 patients). In dose level 1, the most severe adverse event was neutropenia (3 patients [pts] with grade 3 toxicity). Grade 3 toxicities for dose level 2 were diarrhea (1 pt), vomiting (2 pts, with 1 felt to not be related to treatment), and fatigue (1 pt).
The primary reason for treatment discontinuation was disease progression (8 patients). One patient with metastatic pancreas cancer died from disease progression while on treatment at dose level 1. This death was not thought to be related to study treatment. Three patients discontinued treatment because of side effects (one with hyperbilirubinemia, one with nausea/abdominal pain, and one with an unspecified adverse event)
Efficacy
Two partial responses were seen; one in pancreatic cancer (200 mg cohort), the other in biliary tract cancer (400 mg cohort). These partial responses were independantly confirmed. The patient with pancreatic cancer had been pretreated with gemcitabine, cisplatin, and radiotherapy, all completed more than one month before enrollment. The patient with biliary tract (gallbladder) cancer had a cholecystectomy and pathologic confirmation of peritoneal metastatic disease, but had not received chemotherapy prior to enrollment. Stable disease was seen in six patients, with one unconfirmed partial response (with progression noted at the follow up scan two months later). The patients whose best responses were able to be assessed radiographically are summarized in the waterfall plot labeled Figure 1.
Figure 1.
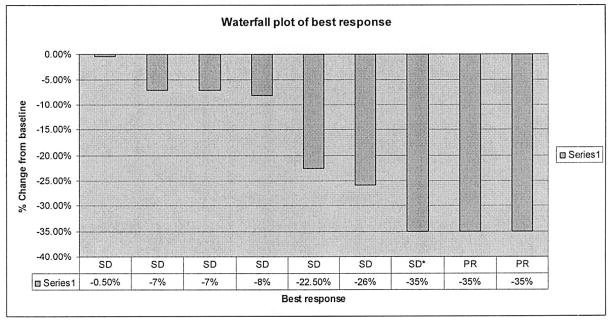
Waterfall plot of best response. SD= Stable disease; PR=Partial response; SD*=Unconfirmed PR, classified as SD. Patients who died, withdrew consent, or had symptomatic disease progression and were not imaged are not included in this plot.
Discussion
The recommended phase II dose for sorafenib in combination with 2DOC is sorafenib 200 mg orally BID, oxaliplatin 85 mg/m2 and capecitabine 2250 mg/m2 orally every 8 hours for six doses on days one and fifteen of a 28 day cycle. This dose was associated with one partial response in a patient with pancreas cancer, two grade 3 nonhematologic toxicities and no grade 4 toxicities. Toxicities were expected and predictable from these agents. The most frequent adverse events were fatigue, diarrhea, nausea, vomiting, and sensory neuropathy. Given the rate and severity of palmar-plantar erythrodysesthesia was similar to that expected from single agent sorafenib, there did not appear to be synergistic palmar-plantar erythrodysesthesia between this schedule of capecitabine and sorafenib.
The suggestion of disease activity of 2DOC and sorafenib in this analysis prompted further study with this drug combination into a phase II study in pancreaticobiliary malignancies. Combinations of 5-FU and oxaliplatin have demonstrated efficacy for many GI malignancies. The novel design of the 2DOC regimen takes advantage of the dosing characteristics of capecitabine, and looks to reduce some of the toxic adverse events. Whether or not the 2DOC regimen is as efficacious as traditional FOLFOX will of course require additional testing.
Other future directions for the regimen include expansion into hepatocellular carcinoma or colorectal cancer. Preclinical models suggest that sorafenib may have activity in colorectal cancer[26], however clinical studies have not shown any responses in oxaliplatin-refractory patients [29]. However, it is notable that regorafenib (or fluro-sorafenib or BAY 73-4506), which is similar in its molecular structure to sorafenib, has demonstrated modest activity in survival and progression-free survival in oxalplatin and irinotecan refractory colorectal cancer [30]. Sorafenib has been approved in hepatocellular carcinoma, and FOLFOX has demonstrated safety in patients for whom full-dose sorafenib was considered too toxic [21,27]. The continued testing of various biologic agents in gastroenterologic cancers requires attention to potential added/synergistic toxicities, and modification of either the cytotoxic ‘backbone’ therapies or the biologic agents themselves. 2DOC continues to be an attractive regimen for other active agents in colorectal cancer that have overlapping toxicities, such as regorafenib with its noted palmar-plantar erythrodysesthesia.
This is a small phase I study looking at this novel combination. While we do not have biomarker data for this study population, future studies looking into the effects of this combination on the Ras/Raf/Erk pathway may elucidate a disease or population for whom this combination has potential efficacy. While there exist multiple standard of care options for those with pancreas and biliary cancers, less toxic regimens with incremental improvements in efficacy will be of benefit.
Acknowledgments
Funding for this study was provided by Bayer-Onyx Pharmaceuticals, Sanofi-Aventis Pharmaceuticals and University of Wisconsin Carbone Cancer Center Core Grant NCI P30 CA014520.
Footnotes
Conflict of Interest:
The authors declare that they have no conflict of interest.
References
- 1.Moore M, Goldstein J, Hamm A. Erlotinib plus gemcitabine compared to gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Cancer Clinical Trials Group (NCIC-CTG) Proc Amer Soc Clin Oncol. 2005 [Google Scholar]
- 2.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–6. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 3.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. The New England journal of medicine. 2011;364:1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 4.Conroy T, Desseigne F, Ychou M, et al. Randomized phase III trial comparing FOLFIRINOX versus gemcitabine as first-line treatment for metastatic pancreatic adenocarcinoma: Preplanned interim analysis results of the PRODIGE 4/ACCORD 11 trial. Proc Amer Soc Clin Oncol. 2010;28:4010 (abstract). [Google Scholar]
- 5.Knox JJ, Hedley D, Oza A, et al. Combining gemcitabine and capecitabine in patients with advanced biliary cancer: a phase II trial. J Clin Oncol. 2005;23:2332–8. doi: 10.1200/JCO.2005.51.008. [DOI] [PubMed] [Google Scholar]
- 6.Riechelmann RP, Townsley CA, Chin SN, Pond GR, Knox JJ. Expanded phase II trial of gemcitabine and capecitabine for advanced biliary cancer. Cancer. 2007;110:1307–12. doi: 10.1002/cncr.22902. [DOI] [PubMed] [Google Scholar]
- 7.Glover K, Thomas M, Brown T, et al. A phase II study of oxaliplatin and capecitabine in patients with unresectable cholangiocarcinoma, including carcinoma of the gallbladder and biliary tract. Proc Amer Soc Clin Oncol. 2005 [Google Scholar]
- 8.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. The New England journal of medicine. 2010;362:1273–81. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 9.Mulkerin D, LoConte N, Holen K, et al. A phase I study of an oral simulated FOLFOX with high dose capecitabine. Investigational New Drugs. 2009 doi: 10.1007/s10637-008-9210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosn M, Farhat F, Kattan J, et al. FOLFOX-6 combination as the first-line treatment of locally advanced and/or metastatic pancreatic cancer. Am J Clin Oncol. 2007;30:15–20. doi: 10.1097/01.coc.0000235997.18657.a6. [DOI] [PubMed] [Google Scholar]
- 11.Tsavaris N, Kosmas C, Skopelitis H, et al. Second-line treatment with oxaliplatin, leucovorin and 5-fluorouracil in gemcitabine-pretreated advanced pancreatic cancer: A phase II study. Invest New Drugs. 2005;23:369–75. doi: 10.1007/s10637-005-1446-y. [DOI] [PubMed] [Google Scholar]
- 12.Pelzer U, Kubica K, Stieler J, et al. A randomized trial in patients with gemcitabine refractory pancreatic cancer. Final results of the CONKO-003 study. Proc Amer Soc Clin Oncol. 2008;26:4508. [Google Scholar]
- 13.Kulke MH, Blaszkowsky LS, Ryan DP, et al. Capecitabine plus erlotinib in gemcitabine-refractory advanced pancreatic cancer. J Clin Oncol. 2007;25:4787–92. doi: 10.1200/JCO.2007.11.8521. [DOI] [PubMed] [Google Scholar]
- 14.Reuben DB, Herr KA, Pacala JT. Geriatrics At Your Fingertips. 7. American Geriatrics Society; 2005. [Google Scholar]
- 15.Hruban RH, van Mansfeld AD, Offerhaus GJ, et al. K-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. Am J Pathol. 1993;143:545–54. [PMC free article] [PubMed] [Google Scholar]
- 16.Tang D, Nagano H, Yamamoto H, et al. Angiogenesis in cholangiocellular carcinoma: expression of vascular endothelial growth factor, angiopoietin-1/2, thrombospondin-1 and clinicopathological significance. Oncol Rep. 2006;15:525–32. [PubMed] [Google Scholar]
- 17.Holcombe RF, Gu M, Imagawa D, Milovanovic T. Expression of Kit and platelet-derived growth factor receptors alpha and beta in cholangiocarcinoma, and case report of therapy with imatinib mesylate (STI571) Anticancer Drugs. 2003;14:651–7. doi: 10.1097/00001813-200309000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Siu LL, Awada A, Takimoto CH, et al. Phase I trial of sorafenib and gemcitabine in advanced solid tumors with an expanded cohort in advanced pancreatic cancer. Clin Cancer Res. 2006;12:144–51. doi: 10.1158/1078-0432.CCR-05-1571. [DOI] [PubMed] [Google Scholar]
- 19.Yu C, Bruzek LM, Meng XW, et al. The role of Mcl-1 downregulation in the proapoptotic activity of the multikinase inhibitor BAY 43-9006. Oncogene. 2005;24:6861–9. doi: 10.1038/sj.onc.1208841. [DOI] [PubMed] [Google Scholar]
- 20.Richly H. Results of a Phase I trial of sorafenib (BAY 43-9006) in combination with doxorubicin in patients with refractory solid tumors. Annals of Oncology. 2006;17:866–73. doi: 10.1093/annonc/mdl017. [DOI] [PubMed] [Google Scholar]
- 21.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 22.Furuse J, Ishii H, Nakachi K, Suzuki E, Shimizu S, Nakajima K. Phase I study of sorafenib in Japanese patients with hepatocellular carcinoma. Cancer Sci. 2008;99:159–65. doi: 10.1111/j.1349-7006.2007.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baselga J, Roche H, Costa F, et al. SOLTI-0701: A multinational double-blind, randomized phase 2b study evaluating the efficacy and safety of sorafenib compared to placebo when administered in combination with capecitabine in patients with locally advanced or metastatic breast cancer. Cancer Research. 2009;69:Abstract 45. [Google Scholar]
- 24.Therasse P, Arbuck S, Elizabeth A, Eisenhauer JW, Richard S, Kaplan LR, Verweij Jaap, Van Glabbeke Martine, Allan T, van Oosterom MCC, Gwyther Steve G. New Guidelines to Evaluate the Response to Treatment in Solid Tumors. Journal of the National Cancer Institute. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 25.Common Terminology Criteria for Adverse Events v3.0 (CTCAE) CTEP; 2006. (Accessed at http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.) [Google Scholar]
- 26.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–40. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coriat R, Mir O, Cessot A, et al. Feasibility of oxaliplatin, 5-fluorouracil and leucovorin (FOLFOX-4) in cirrhotic or liver transplant patients: experience in a cohort of advanced hepatocellular carcinoma patients. Investigational New Drugs. 2010 doi: 10.1007/s10637-010-9525-0. [DOI] [PubMed] [Google Scholar]
- 28.El-Khoueiry AB, Ramanathan RK, Yang DY, Zhang W, Shibata S, Wright JJ, Gandara D, Lenz HJ. A randomized phase II of gemcitabine and sorafenib versus sorafenib alone in patients with metastatic pancreatic cancer. Invest New Drugs. 2012;39(3):1175–83. doi: 10.1007/s10637-011-9658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kupsch P, Henning BF, Passarge K, et al. Results of a phase I trial of sorafenib (BAY 43-9006) in combination with oxaliplatin in patients with refractory solid tumors, including colorectal cancer. Clin Colorectal Cancer. 2005;5:188–196. doi: 10.3816/ccc.2005.n.030. [DOI] [PubMed] [Google Scholar]
- 30.Grothey A, Cutsem EV, Sobrero A, et al. Regorafenib monotherapy for previously treatment metastatic colorectal cancer (CORRECT): an international, multicentre, randomized, placebo-controlled, phase 3 trial. Lancet. 2012 doi: 10.1016/S0140-6736(12)61900-X. e-pub Nov. 21,2012. [DOI] [PubMed] [Google Scholar]


