Abstract
Endogenous retroviruses (ERVs) are present in the genome of all vertebrates and have coevolved with their hosts for millions of years. Some ERVs play a critical role in placental development, contribute to genome plasticity, and protect the host against infection of related pathogenic and exogenous retroviruses, thus some ERVs have been positively selected and maintained in the host genome. The sheep genome contains 27 endogenous retroviruses (en-JSRVs) related to the pathogenic Jaagsiekte sheep retrovirus (JSRV), the causative agent of a transmissible lung cancer in sheep. en-JSRVs are able to protect their host against JSRV infection by blocking different steps of the viral replication cycle. In addition, en-JSRVs are absolutely required for sheep placental development. Thus, en-JSRVs-JSRV provides a unique and interesting model to study the symbiotic relationship and interplay between host ERVs and evolution. This review will provide some examples of the biological functions of ERVs. In particular, the role of ERVs in reproductive biology and in protecting the host against pathogenic retrovirus infections will be emphasized using en-JSRVs/JSRV and the sheep as a model.
Keywords: JSRV, en-JSRVs, placenta, endogenous retroviruses, virus evolution, virus–host coevolution
Introduction
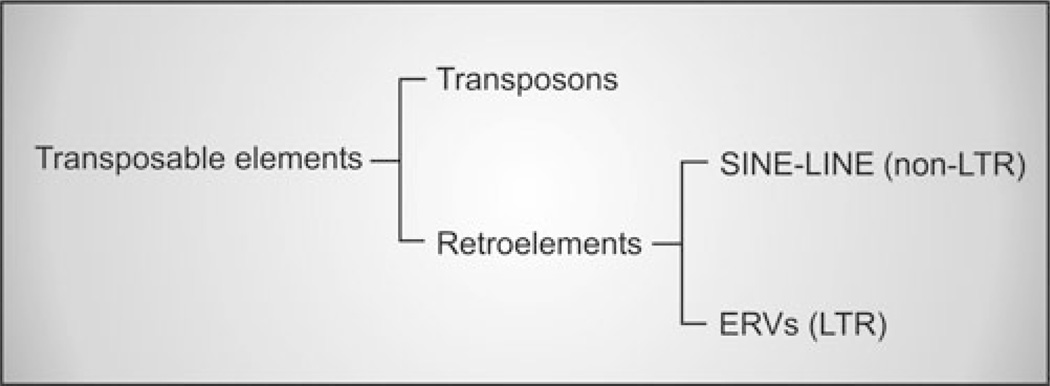
Endogenous retroviruses (ERVs) are present in the genome of all vertebrates and are vertically transmitted as stable inherited Mendelian genes.1 The obligatory integration step of the retroviral replication cycle allows the incorporation of the viral genome (provirus) into the host genome. ERVs are thought to arise from ancient infections of the germline of the host by exogenous retroviruses. Retrotransposition or reinfection of the germline can generate further insertions augmenting the number of a particular lineage in the genome.2 ERVs have heavily colonized the genome of all animal species; for example, they account for approximately 8% of the human genome (Fig. 1).3
Figure 1.
Endogenous retroviruses are transposable elements. Approximately 45% of the human genome is composed of transposable elements.3 Transposable elements can be divided into DNA transposons (Class II), which act via a DNA intermediate, and retrotransposons (Class I) that use an RNA intermediate. DNA transposons constitute 2.8% of the transposable elements of the human genome, while the remaining 42.2% are retrotransposons. Retrotransposons can be further divided into non-LTR (long terminal repeats) elements (33.9%), comprising the long and short interspersed elements (LINEs and SINEs respectively), and LTR elements (8.3%) that are endogenous retroviruses (ERVs).
A complete ERV “provirus” (i.e., the retroviral genome integrated into the host cell genome) shares the same genomic structure of an exogenous retrovirus: four viral genes (gag, pro, pol, and env) flanked by two long terminal repeats (LTRs). The gag gene encodes for the major viral structural protein, while pro and pol encode for the viral enzymatic machinery necessary for the viral replication cycle. The env gene encodes for the envelope glycoprotein that is inserted in the lipid bilayer of the cell membrane to form the viral envelope and mediates entry of the virus into susceptible cells. The LTRs contain enhancer elements that direct expression of the viral genes. ERVs are destined to extinction if their expression brings deleterious consequences for the host. Thus, their persistence in the host genome is the result of a fine balance reached throughout evolution which usually renders them replication defective due to the accumulation of mutations, deletions, rearrangements, and methylation.1
ERVs are widespread throughout vertebrate genomes.4 Most ERVs do not have an exogenous counterpart, but some are highly related to exogenous retroviruses, including Jaagsiekte sheep retrovirus (JSRV), mouse mammary tumor virus (MMTV), feline leukemia virus (FeLV), and avian leukemia virus (ALV) which are currently active and infect sheep, mice, cats, and chickens, respectively.1 These ERVs are generally referred to as “modern” ERVs as they integrated into the host genome after speciation and are closely related to exogenous viruses that are still infectious. Some modern ERVs are still able to produce infectious virus due to the lack of inactivating mutations. Modern ERVs can also have insertionally polymorphic loci because they are not completely fixed in a particular population and are still undergoing endogenization. This is the case for koalas and sheep which are currently being invaded by the koala retrovirus (KoRV)5 and endogenous JSRVs (en-JSRVs),6 respectively. In contrast, “ancient” ERVs invaded the genomes before speciation and, consequently, are present in every individual at the same genomic location of phylogenetically related species. Ancient ERVs are replication defective due to the accumulation of mutations and genetic damage.7
The biological significance of ERVs has been debated for several decades and were generally thought to be “junk DNA.”8 However, recent studies suggest that ERVs have a variety of beneficial roles to their host. At the very least, the abundance of these elements in the host genome suggests that they contribute to genome plasticity. Further, the presence of transcriptionally active ERVs with intact open reading frames conserved million of years after integration support the idea that some ERVs were co-opted by the host for specific biological roles.
This review will first provide some examples on how ERVs have deleterious and beneficial effects on their hosts and then focus on the protection of the host against exogenous retroviruses by ERVs and their critical role in placental morphogenesis. These aspects of ERV biology will be highlighted using recent information generated from the analysis of en-JSRVs in domestic sheep.
ERVs and Disease
In general, ERVs do not have major deleterious effects to their hosts, otherwise they would be counterselected during evolution. However, ERVs may persist if the deleterious effects they induce are intermittent or if they are counterbalanced by beneficial consequences. Until recently, associations between disease (e.g., autoimmunity) and ERVs have remained speculative. However, ERVs seem to play a role in a human autoimmune disease called Aicardi-Goutieres syndrome (AGS), which has been experimentally addressed in elegant mice models. Mutations in the human TREX1 gene are responsible for AGS. TREX1 is a 3′–5′ exonuclease that acts as a negative regulator of the interferon stimulatory DNA pathway. In the absence of a functional TREX1 protein, reverse transcribed DNA products of transposons and ERVs accumulate in the cell and induce an interferon stimulatory DNA pathway that results in inflammation, production of autoantibodies, and autoimmune diseases.9 Other examples supporting a role for ERVs in disease are from strains of mice selected for high incidence of tumors. In the absence of exogenous virus, AKR mice develop spontaneous lymphoma as a result of retroviral insertional activation of oncogenes. The oncogenic agents are a group of viruses referred to as mink cell focus forming viruses (MCF) that arise by recombination events from three different ERVs.10 Another example is provided by the proviral loci Mtv1 and Mtv2 which induce mammary tumors in mice not exposed to exogenous MMTV. Expression of these loci releases infectious virus in the lactating mammary gland that subsequently reinfects and transforms cells of the mammary gland.1 It is important to stress that most examples of pathogenic ERVs derive from experimental models in which the desired phenotype (e.g., lymphoma) was selected for.
Roles of ERVs in Host Biology
ERVs have been found to play a major role in a variety of physiological processes. Some ERVs have the capacity to regulate expression of cellular genes. A specific example is given by the ERV-induced expression of the α-amylase gene in humans. The α-amylase gene family in humans comprises five active genes clustered in chromosome 1, including two pancreatic and three salivary genes, which are all associated with insertions of two transposable elements, a γ-actin pseudogene and an ERV. Using transgenic mice, it was shown that the endogenous provirus contains specific enhancer sequences that promote expression in the salivary gland. However, other mechanisms regulating gene expression might be present because Old World monkeys show high levels of salivary amylase and lack proviral insertion.11,12
Interactions between Endogenous and Exogenous Retrovirus
ERVs can modulate the outcome of infection by exogenous retroviruses both to the benefit or detriment to the host. Some ERVs confer resistance to superinfection by receptor block-age, where the expression of endogenous env genes impedes interaction of exogenous viruses with their receptors. An example of this interference mechanism is provided by mice expressing the Fv4 locus that encodes a mutated Env protein whose receptor binding domain resembles the one of ecotropic murine leukemia virus (MLV) and blocks infection by exogenous MLVs.1 Another mechanism by which ERVs modulate exogenous retroviral infections is the Fv1 locus in particular strains of mice. Fv1 is the mouse counterpart of the Gag protein of human endogenous retrovirus L (HERV-L) and determines susceptibility to MLV infection.13
Some ERVs can shape the immune response toward retroviral infections or other microorganisms such as bacteria.14 For example, MMTV is transmitted to newborn pups through the milk of infected females. The virus enters the small intestine and infects B lymphocytes and dendritic cells of the underlying lymphoid tissue and expresses a protein known as superantigen (sag). The expression of superantigen induces a T cell response that results in the proliferation of cells susceptible to MMTV. These cells act as a reservoir of infection and transmit the virus to the developing mammary gland during puberty. Laboratory mice harbor between 2 to 8 replication defective endogenous MMTVs (Mtv) which express sag genes early in life leading to a clonal deletion of responsive T cells and thus preventing infection by exogenous MMTV. However, the interaction between Mtv and their host is complex. For example, laboratory mice lacking Mtvs are not only resistant to mammary tumors but also to Vibrio cholerae, and this phenomenon is reverted by the restoration of any of the endogenous MMTVs present in that particular mice strain.14 Another example is provided by ALV-related ERVs, which reduce the immune response in chickens against exogenous ALV, thereby augmenting the risk of infection, although their expression prevents the development of wasting syndrome.2
In a cell expressing both exogenous and endogenous retroviruses, the ERV RNAs can be copackaged with the genomes of exogenous viruses, resulting in recombination and the appearance of novel pathogenic variants as is the case for avian, murine, and feline leukemia viruses.1 This phenomenon generates significant concern with the use of retroviral vectors for gene therapy, because ERVs present in packaging cell lines can potentially contaminate therapeutic vectors.
ERVs in Reproductive Tissues
ERVs are highly expressed in the genital tract and placenta of various animal species.15,16 The presence of intact env genes that are expressed in syncytiotrophoblast and have been preserved over thousands of years, together with the observation that they elicit fusion of cells in vitro, led to the speculation that ERVs play an essential role in placental morphogenesis and were positively selected for a fundamental role in the evolution of placental mammals and the development of viviparity.17–20
HERV-W, HERV-FRD, and ERV-3 are three human ERVs (HERV) whose intact env genes are expressed in the human placenta.21–23 HERV-W is not present in the human genome as a complete provirus, however its env gene, encoding a protein termed syncytin-1, is preferentially expressed in the syncytiotrophoblast. The syncytiotrophoblast is a multinuclear cell that lines the outer surface of the placenta, derived by intercellular fusion of trophoblast cells, and is responsible for the transport of oxygen, nutrients, and waste products, as well as produces hormones and is involved in immune tolerance.24 Syncytin is an 80 kDa glycosylated protein and possesses characteristic features of a retroviral Env protein, such as the presence of a leader peptide, a potential furin cleavage site, a fusion peptide-like sequence, and a putative immunosuppressive region. It also contains a hydrophobic membrane-spanning domain, suggesting it could be inserted into the plasma membrane.23 There is considerable in vitro information suggesting that syncytin is involved in the fusion of mononuclear cytotrophoblasts to form syncytiotrophoblast. Transfection of a variety of cell lines with HERV-W env induced cellular fusion that was reduced when the cell cultures were treated with an antibody against the HERV-W Env protein.18,22 In addition, induction of fusion of BeWo cells (a human trophoblastic choriocarcinoma cell line)25 by forskolin was associated with increased expression of syncytin.18 Moreover, inhibition of syncytin expression in primary trophoblast cells reduced the number and size of syncytia formed during culture.26
The Env glycoprotein of HERV-FRD, termed syncytin-2, is structurally similar to syncytin-1, however it entered the primate genome before the split of the New World and the Old World monkeys more than 40 million years ago, while syncytin-1 entered the primate genome approximately 25 millions years ago and is not present in Old World monkeys.27 Syncytin-2 also elicits cell fusion when transiently transfected into several cell lines.28 However, the two syncytins display different properties. Indeed, while both are fusogenic, syncytin-2 is immunosuppressive unlike syncytin-1.29
The Env protein of ERV-3 is also expressed in syncytiotrophoblast and was the first ERV Env for which a potential physiological function was described.30 Although it has a long open reading frame, the protein is prematurely terminated by the presence of a stop codon in the transmembrane region that truncates the hydrophobic domain that is required for anchoring to the cell membrane.31 It also lacks a leader and a fusion peptide and, although it harbors a region with the characteristics of an immunosuppressive domain, its function is likely diminished by the lack of membrane anchorage.32 ERV-3 Env does not elicit cell fusion, although its expression increases in BeWo cells treated with forskolin. When ERV-3 Env is stably expressed in undifferentiated BeWo cells, it induces changes characteristic of trophoblast differentiation, such as increased levels of β-human chorionic gonadotropin, growth inhibition, and altered morphology.33 Considering that ERV-3 Env is expressed in a variety of normal tissues and particularly in hormone producing organs, including adrenal and sebaceous glands and testis, it may play a general role in hormone production.32 However, 1% of 150 healthy Caucasian individuals were found to be homozygous for a premature stop codon that would theoretically result in a severely truncated nonfunctional protein.34 Thus, it is debatable whether the ERV-3 Env has a critical biological function.
Recently, two murine ERV env genes were identified and found to be expressed in the syncytiotrophoblast component of the labyrinthine zone of the placenta. They both are highly fusogenic in transfection assays and are present in all Muridae tested, which suggests positive selection.17 ERVs are also expressed in the male reproductive tract although their expression has not been associated with any biological function.35
Endogenous and Exogenous Betaretrovirus of the Domestic Sheep: en-JSRVs/JSRV
Domestic sheep harbor in their genome at least 27 copies of ERVs, termed en-JSRVs, because they are highly related to the exogenous and pathogenic Jaagsiekte sheep retrovirus (JSRV).6,36 JSRV is the causative agent of ovine pulmonary adenocarcinoma (OPA), a transmissible lung cancer of sheep.37 A unique feature of JSRV among oncogenic retroviruses is that its Env glycoprotein is the main determinant of cell transformation both in vitro and in vivo.38–44 Expression of the JSRV Env alone is able to transform a variety of cell lines in vitro, including mouse, rat, and chicken fibroblasts, as well as human bronchial, canine and rat epithelial cells.38,40–42,45,46 More importantly, the JSRV Env is able to induce lung adenocarcinomas in immunocompetent sheep when expressed by a JSRV-based vector under the control of the JSRV LTR. Thus, JSRV Env is a dominant oncoprotein. Moreover, JSRV can induce OPA without viral spread, a unique feature among oncogenic retroviruses.43 The mechanisms of cell transformation induced by the JSRV Env are not completely understood. The cytoplasmic tail of the transmembrane domain of the JSRV Env plays a critical role in cell transformation. In particular, mutations of a tyrosine in position 590 present within a YXXM motif abolish cell transformation in vivo.47 Although the Ras-MEK-MAPK and the PI3K-AKT pathways have been implicated in JSRV-induced cell transformation, it still remains to be determined how the cytoplasmic tail engages the cell signalling network to activate these pathways.46,48–50
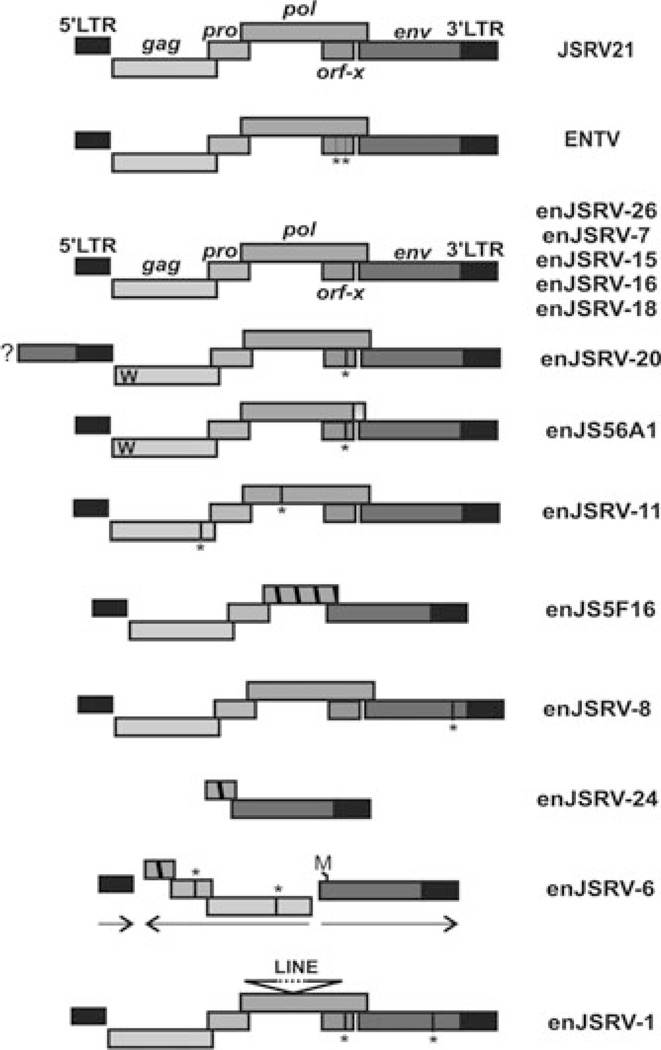
The majority of enJSRV proviruses harbor defective genomes as a result of deletions, nonsense mutations, and recombinations; however, five enJSRV proviruses contain intact genomes with uninterrupted open reading frames for all the retroviral genes (Fig. 2). These enJSRV loci are insertionally polymorphic in the sheep population.
Figure 2.
Representative endogenous Jaagsiekte sheep retrovirus (en-JSRVs) proviruses present within the sheep genome. Five en-JSRVs display an intact genomic organization typical of replication competent proviruses (top). The ″W″ present in the Gag protein of the two transdominant proviruses enJS56A1 and enJSRV-20 indicates the R21W substitution. enJSRV-20 5′ flanking region contains an env gene indicated by a box and a question mark (?). Vertical lines and an asterisk (*) represent stop codons, while hatched boxes indicate deletions. enJSRV-6 harbors a recombined structure with internal sequence in the opposite direction compared to the 5′ and 3′ long terminal repeats of the provirus. The first methionine (indicated by the letter M) of the env gene of enJSRV-6 is present after the usual start codon.
Overall, JSRV and en-JSRVs have a high degree of similarity (~85–89% identity at the nucleotide level). The major differences between JSRV and en-JSRVs are in U3 region within the LTR and in three regions in gag and env referred to as variable regions 1, 2, and 3 (VR1–2–3).51 The VR1 and VR2 reside in the matrix domain within Gag while VR3 comprises the last 67 amino acids of the transmembrane domain of Env.
The evolutionary history of these proviruses, together with ruminants, suggests that integration of en-JSRVs began before the split between the genus Ovis and the genus Capra, approximately 5 to 7 million years ago, and continued after sheep domestication (~10,000 years ago). Interestingly, one enJSRV provirus, enJSRV-26, is thought to have integrated in the host less than 200 years ago and may be a unique integration event that occurred in a single animal.6
en-JSRVs Viral Interference
en-JSRVs can block JSRV replication at both early and late stages of the retroviral cycle. Both JSRV and en-JSRVs use the same cellular receptor for entry called HYAL2 (hyaluronidase2), a glycosylphosphatidylinositol (GPI)-anchored protein. Thus, en-JSRVs Env could prevent JSRV entry by a classic mechanism of receptor interference as described for other viruses.52 In addition, two enJSRV loci (enJS56A1 and enJSRV-20) can block JSRV replication at a late stage of the retroviral replication cycle by a block referred to as JSRV late restriction (JLR).53 These two transdominant proviruses entered the host genome 3 million years ago before and during speciation within the genus Ovis. In two temporally distinct events, they subsequently acquired a defective Gag polyprotein via a substitution of an arginine at position 21 (typical of a replication competent virus) to a tryptophan residue. JLR likely occurs via the production of defective Gag proteins by the transdominant proviruses that form viral particles and/or multimers with the functional Gag proteins, which then accumulate in the cytoplasm as preaggresome structures that are subsequently degraded by the proteasome. Therefore, the transdominant proviruses prevent Gag proteins of the competent virus to interact with the trafficking cellular machinery and ultimately the release of viral particles.54,55 A recent study from our laboratory strongly supports the hypothesis that selection of trans-dominant enJSRV loci has protected sheep against infection with related exogenous retroviruses. Indeed, both proviruses with transdominant (protective) phenotypes became fixed in the host genome of the domestic sheep (Ovis aries) supporting the idea of their positive selection during or immediately before sheep domestication (9,000 years ago). These observations highlight the idea that some en-JSRVs act as restriction factors and were selected during sheep domestication, supporting the hypothesis that ERVs could help the host to fight retroviral infections.6
en-JSRVs have also been shown to be essential for sheep conceptus development and placenta morphogenesis. Thus, en-JSRVs furnish an attractive model to experimentally investigate the role of ERVs in the host reproduction.
The Sheep Placenta and Blastocyst Development
In sheep, the morula-stage embryo enters the uterus by day 5 after mating and form a blastocyst by day 6 that contains a blastocoele surrounded by a monolayer of trophectoderm.56,57 By day 9, the blastocyst hatches from the zona pellucida, develops into an ovoid conceptus (embryo/fetus and associated extraembryonic membranes) by day 12 and then begins to elongate, reaching 25 cm or more by day 17. Elongation of the conceptus is critical for the production of interferon tau (IFNT), which is the pregnancy recognition signal needed to maintain progesterone production by the corpus luteum, and also for the onset of implantation.58 Implantation of the conceptus involves the apposition, attachment and adhesion of the conceptus trophectoderm to the endometrial luminal epithelium (LE) of the uterus. Within the outer layer of the conceptus, termed the chorion, trophoblast giant binucleate cells (BNC) begin to appear on day 14.59 The BNC are likely derived from the mononuclear trophectoderm cells (MTC) by a process referred to as mitotic polyploidy, which involves consecutive nuclear divisions without cytokinesis.60 BNC then fuse with uterine LE to form trinucleate fetomaternal hybrid cells.59 Other BNCs fuse with the trinucleate cells (and likely each other) to form plaques of multinucleated syncytiotrophoblast that have 20–25 nuclei. Trophoblast BNCs of the sheep placenta are analogous in many ways to the giant cells of the syncytiotrophoblast of the human placenta.61 The syncytial plaques and BNC form specialized structures on the placenta termed cotyledons that develop into endometrial caruncles of the maternal uterus to form a structure termed a placentome.62 Blood flow to the uterus and from the fetus is predominantly routed to the placentomes, which provides hematrophic nutrition to the conceptus. Other functions of BNC and multinucleated syncytia include production and synthesis of proteins and hormones, such asplacental lactogen, pregnancy associated glycoproteins (PAGs) and progesterone that are involved in growth of the uterus and mammary gland and other maternal functions.60
Role of en-JSRVs in the Host Reproductive Biology
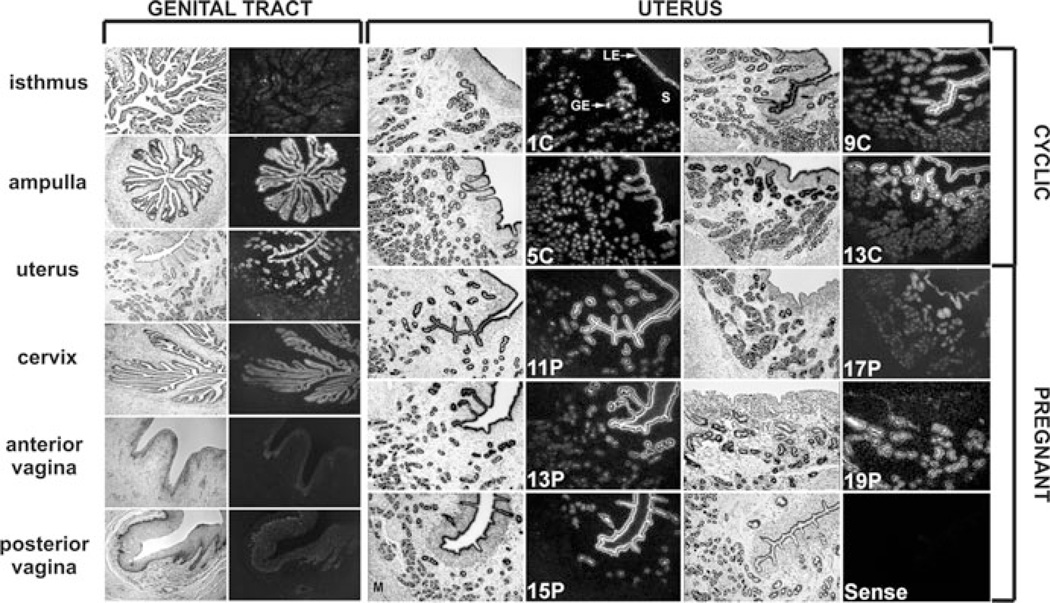
In sheep, en-JSRVs are abundantly expressed in the epithelia lining the different tissues of the female reproductive tract (vagina, cervix, uterus, and oviduct) (Fig. 3).51,63 In the uterus, both en-JSRVs RNA and protein are detected specifically in the endometrial LE and in the glandular epithelium (GE) (Fig. 3).51,64,65 In addition, en-JSRVs are expressed in the trophectoderm cells of the placenta in a temporal fashion that is coincident with key events in conceptus development.63 Within the trophectoderm, en-JSRVs are most abundant in the trophoblast giant BNC and multinucleated plaques of syncytiotrophoblast within the placentomes.
Figure 3.
Expression of endogenous Jaagsiekte sheep retrovirus (en-JSRVs) env RNA in the female genital tract. Various tissues of the reproductive tract as well as the uteri of cyclic and pregnant ewes were hybridized with antisense cRNA probes of the en-JSRVs env. Note that en-JSRVs env RNA is observed in the epithelia lining the reproductive tract tissues and is particularly abundant in the luminal epithelial cells (LE) and glandular epithelial cells (GE) of the endometrium of the uterus. Figure modified from Palmarini et al65 and printed with permission from the American Society for Microbiology.
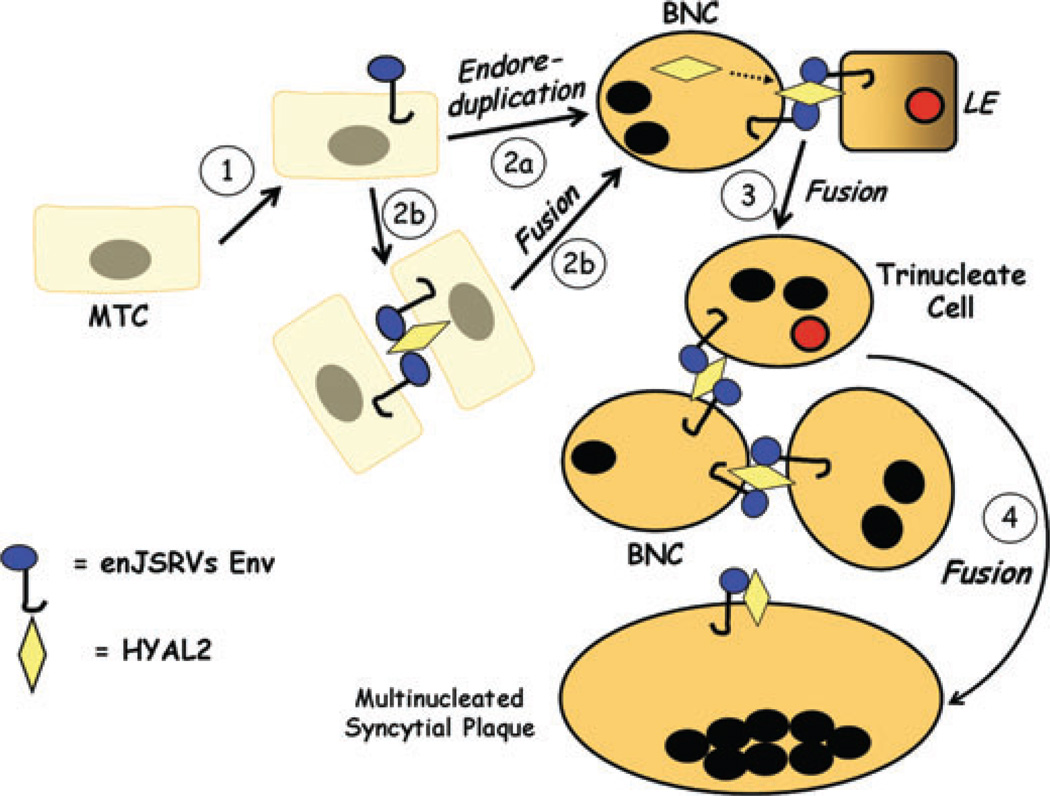
The RNA of en-JSRVs is first detected in the conceptus on day 12 that is the onset of trophoectoderm elongation and production of IFNT. Interestingly, HYAL2, a cellular receptor for both JSRV and en-JSRVs Env,6,40 is detected exclusively in the BNC and the multinucleated syncytial plaques of the placenta.63 These observations led to the hypothesis that en-JSRVs and HYAL2 are important for placental growth and differentiation in sheep.58 The precise cellular mechanisms whereby BNC and multinucleated syncytial plaques are formed within the placenta are not known. One possibility is that BNC derive by mitotic polyploidy that involves division of MTC without cytokinesis (Fig. 4). Another possibility is that BNC derive from the fusion of two MTC. In the latter scenario, some MTC would begin to express HYAL2 and fusion would take place by the interaction of HYAL2 with the Env of en-JSRVs expressed in another MTC and then form a BNC. In both cases, the newly formed BNC fuse with the LE to form trinucleate cells. Interestingly, both BNC and the LE cells express enJSRV Env while only BNC express HYAL2. Thus, the formation of trinucleate cells could also be the result of cell fusion elicited by the interaction between enJSRV Env and HYAL2. Finally, multinucleated syncytial plaques form by the fusion of BNC with trinucleate cells as well as each other to form cells with 20–25 nuclei. Indeed, en-JSRVs Env and HYAL2 are coexpressed in all of those three different cell types (BNC, trinucleate cells, and multinucleated syncytial plaques).58 The biological role of HYAL2 in sheep conceptus development and differentiation has not been reported, but en-JSRVs Env do have a fundamental role in conceptus growth and trophectoderm differentiation.
Figure 4.
Hypothesis on the biological role of endogenous Jaagsiekte sheep retrovirus (en-JSRVs) Env and HYAL2 in trophoblast differentiation in sheep. During pregnancy, trophoblast giant binucleate cells (BNC) begin to differentiate from mononuclear trophoblast cells (MTC) on day 14. First, MTC begin to express en-JSRVs envelope (Env) in the conceptus on day 12. Second, results from microscopy studies support the idea that trophoblast giant BNC are derived from karyokinesis without cytokinesis (endoreduplication) (Step 2a). Alternatively, BNC could also form by fusion of MTC (Step 2b). In this scenario, some MTC would begin to express HYAL2, the cellular receptor for en-JSRVs Env. The interaction of an enJSRV Env and HYAL2 would elicit MTC cell fusion to form a BNC. Indeed, the onset of BNC differentiation coincides with the appearance of HYAL2 mRNA in the conceptus. Third, the newly formed BNC that are coexpressing en-JSRVs env and HYAL2 initially fuse with en-JSRVs env-expressing endometrial luminal epithelial (LE) cells, forming a trinucleate cell (Step 3). During this period, the BNC and LE cells express enJSRV env RNA, whereas only the BNC express HYAL2; HYAL2 mRNA is not detectable in uterine cells. By days 20 to 25, virtually all of the endometrial LE cells are fused with the BNC. Fourth, other newly formed BNC fuse with trinucleate cells to form a multinucleated syncytial plaque (Step 4). During most of gestation, the BNC continue to differentiate from the MTC and then fuse with each other and existing multinucleated syncytia to form multinucleated syncytial plaques with 20–25 nuclei. The multinucleated syncytial plaques and BNC form the basis of the cotyledons of the placenta which interact with caruncles of the endometrium to develop and form placentomes.
Collective evidence from studies of primates, rodents, and sheep supports the idea that independent ERVs influenced mammalian evolution and were positively selected for a convergent physiological role in placental morphogenesis. To determine the biological role of en-JSRVs Env in sheep conceptus development, morpholino antisense oligonucleotides were used in utero to inhibit the splicing and/or translation of en-JSRVs env RNA in the conceptus.66 Inhibitory and control morpholinos were injected into the lumen of the ovine uterus on day 8 after mating. On day 16, conceptuses recovered from ewes injected with control morpholino were elongated and filamentous. In contrast, conceptuses recovered from ewes injected with the inhibitory morpholinos were growth retarded, had fewer MTC that was documented by lower amounts of IFNT in the uterine lumen, and contained very few or no BNC. These data strongly support the hypothesis that en-JSRVs play a critical role in MTC growth as well as trophoblast giant BNC differentiation in sheep.67
Recently, we reported that 16 of the 27 enJSRV loci contain an intact env ORF.6 Sequence alignment revealed 92.2% to 99.7% identity among the enJSRV Envs and 87.7% and 92.4% similarity between exogenous and en-JSRVs Envs at the amino acid level. Comparison of the sequence and predicted hydrophobic profiles of the Env from JSRV and en-JSRVs can be used to define several different domains including (1) a signal peptide present at the amino terminus portion of Env (the surface domain starts at amino acid 81);55 (2) a furin consensus cleavage site (R-X-[R/K]-R)68 that is present between the surface (SU) and the transmembrane (TM) domains; (3) a hydrophobic segment at the amino terminus of TM, which in other retroviral Envs maps to the location of the fusion peptide;69 (4) a hydrophobic transmembrane anchor domain; and (5) a cytoplasmic tail at the carboxy terminus of the TM of en-JSRVs that lack the characteristic transformation domains present in the JSRV Env (Fig. 5). Considering that the hydrophobic profile of enJSRV Envs revealed the possible presence of a fusion peptide, the fusogenic properties of the Env were investigated in a variety of cell lines, including 293, COS, HeLa, NIH-3T3, and ovine trophoblast cells from day 16 conceptuses (M. Varela and M. Palmarini, unpublished). In contrast to what was observed with the Env of HERV-W, HERV-FRD and two murine ERV (syncytin-A and syncytin-B), induction of syncytia by cell fusion was not observed under neutral pH conditions. These results were unexpected, but limitations of the in vitro systems used should be considered. First, it is possible that other molecules beside enJSRV Env and HYAL2 are required for the induction of cell fusion and may be absent in the cell lines used for the cell fusion assays. Second, another consideration is that syncytia formation by exogenous JSRV Env is enhanced by low-pH treatment,70 a condition that has not been investigated for the Env of the en-JSRVs. A better understanding of the cellular and molecular mechanisms governing sheep trophectoderm differentiation and the role of ERVs in this process would provide invaluable information for comparative physiology and pathology, considering that for ethical reasons similar experiments can not be performed in humans.
Figure 5.
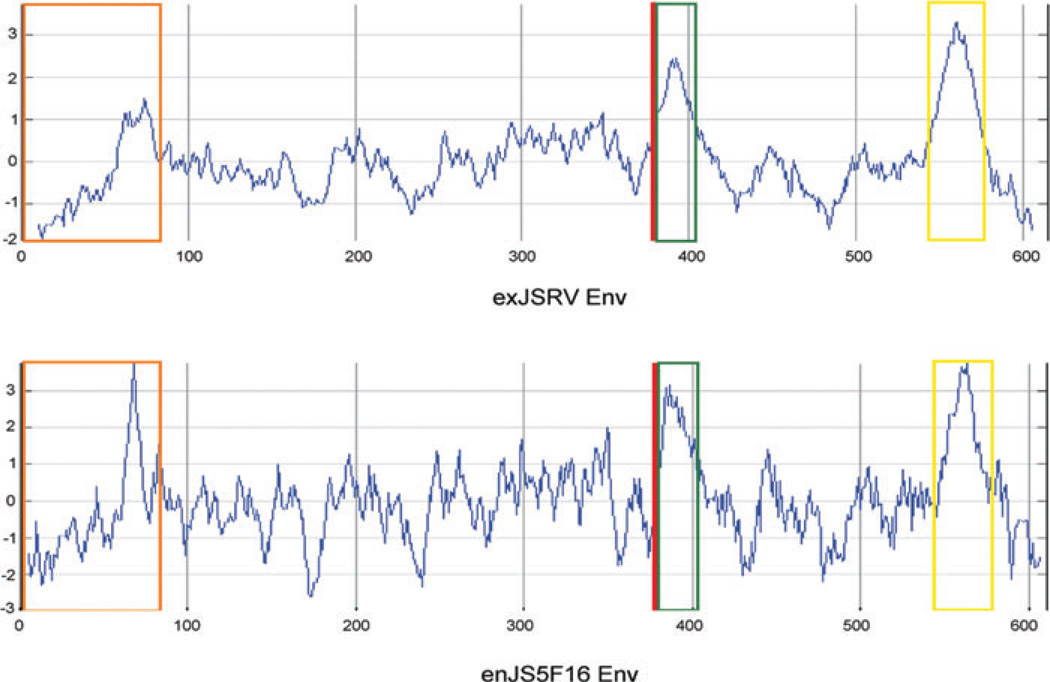
Hydrophobicity profiles and characteristics of exogenous Jaagsiekte sheep retrovirus (JSRV) envelope (exJSRV Env) and an endogenous JSRV envelope (enJS5F16 Env). The hydrophobic profile of endogenous Jaagsiekte sheep retrovirus (en-JSRVs) Env and exJSRV Env was calculated by the Kyte and Doolittle method. The hydrophobic profile of enJS5F16 is shown as an example of the 16 of 27 en-JSRVs with an intact envelope. The orange box indicates the signal peptide; the red line indicates the consensus proteolytic cleavage site separating SU from TM; the green and yellow boxes correspond to hydrophobic regions associated with the fusion peptide and the membrane spanning domain respectively.
It is interesting that en-JSRVs Env have a high degree of similarity with the oncogenic exogenous JSRV Env. It is tempting to speculate that both endogenous and exogenous JSRV Env share similar mechanisms to induce trophoblast proliferation/differentiation and cell transformation, respectively, because placental morphogenesis has features similar to tumorigenesis and metastasis.71,72 Although many of these parallels come from comparisons made with the human placenta, trophoblast cells in general have a high proliferation rate, are migratory and invasive, and have the capacity to evade the immune system, which are also characteristics of cancer cells. Interestingly, human cytotrophoblast cells express functional tumor-associated genes and are capable of engaging in autocrine stimulatory loops, rendering them less dependent on survival and growth factors from the surrounding tissue.71 Moreover, growth stimulatory effects can be amplified by signals provided by the neighboring cells through paracrine loops.71 However, the ultimate fate of trophoblast cells is terminal differentiation, which regulates their tumor-like attributes and the progression to senescence and apoptosis. The difference between malignant cell transformation and normal trophoblast development is that in the latter, the cellular and molecular events leading to cell proliferation/migration/invasion are spatially and temporally regulated, following a highly controlled plan. Thus, trophoblast cells are an ideal model for the study of the regulation of cell growth, differentiation, migration/invasion, and carcinogenesis. Thus, it is likely that enJSRV and JSRV Env mediate their effects through the activation of similar, albeit not identical, pathways.73
An obligatory step in the understanding of the role of en-JSRVs in placenta morphogenesis and the cellular and molecular mechanisms underpinning their effects is the identification of the enJSRV loci expressed in the placenta. In particular, it would be interesting to know the specific enJSRV loci that are expressed in the conceptus and uterine LE during the periimplantation period. A role of en-JSRVs Env that are unable to encode full-length products should not be discounted because of the presence of stop codons as is the case of enJSRV1. As previously mentioned, the Env protein of ERV-3 that is expressed in the developing human placenta contains a premature stop codon that prevents the expression of the membrane spanning domain, it also lacks signal and fusion peptides. Moreover, the LTRs of en-JSRVs may also be involved in conceptus development by the creation of alternative promoters, enhancers, and polyadenylation signals. The insertion of HERV-E in the 5′ UTR of the growth factor pleiotrophin (PTN) provides an example of a trophoblast-specific promoter created upon retroviral insertion which seems to contribute to the invasive phenotype of the trophoblast.74 Another example is provided by the expression of the insulin-like 4 (INSL4) gene in the placenta which seems to be driven by a HERV-K insertion.75
Another angle to investigate is the possible role of ERV Envs in maternal immune tolerance to the fetus. Many retroviral Envs seem to mediate immunosuppression by means of the expression of a stretch of conserved amino acids present in the TM domain.76 Syncytin-2 is immunosuppressive and may create an immune tolerant environment for the fetus although the mechanism remains unclear.18,20 On the other hand, it is unlikely that full immune suppression is due to the expression of a single retroviral gene. None of the currently known enJSRV Envs harbor a known immunosuppression domain, although this does not exclude the possibility that they do play a role in maternal tolerance to the semi-allogeneic fetus.
Conclusions
ERVs are present in the genomes of all vertebrates2 and can be used as DNA fossils to unravel virus–host coevolution over millions of years.7 The domestic sheep constitutes a powerful model to study the biological significance of ERVs given the contemporary presence in this animal species of a pathogenic exogenous retrovirus (JSRV) and the biologically active en-JSRVs. ERVs have been speculated to play a physiological role in placenta morphogenesis for almost three decades considering that retroviral particles have been frequently observed in the reproductive tract of several animal species.15,77–81 The study of en-JSRVs has provided the first in vivo evidence of a physiological role for ERVs in conceptus and placental development.67
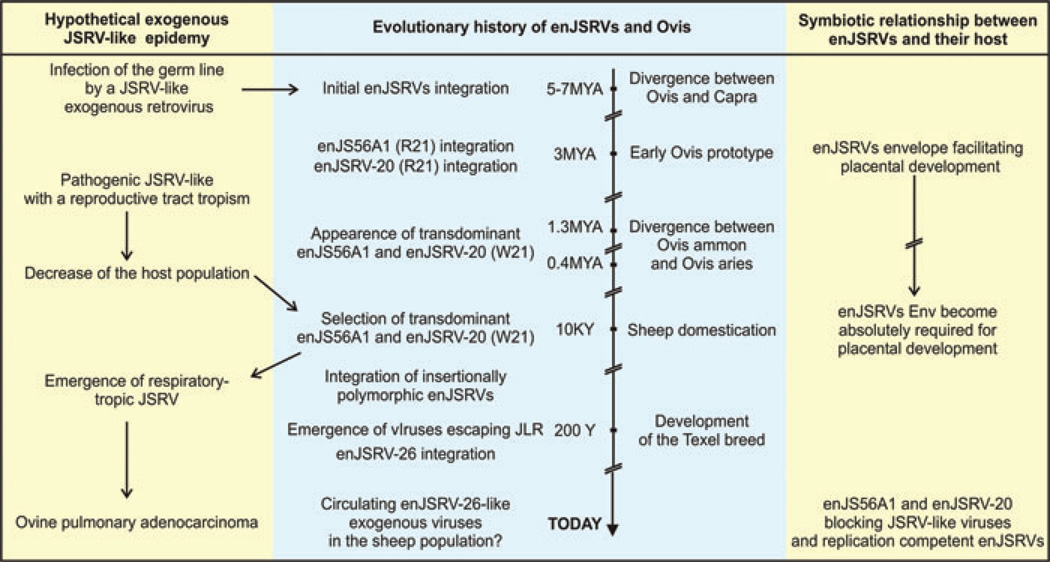
The mammalian placenta predates the first en-JSRVs integration in the genome of the Caprinae. Therefore, we speculate that during evolution the first driving force that positively selected and fixed en-JSRVs in the sheep population was their ability to protect their host against infection by related pathogenic retroviruses (Fig. 6). Initially, en-JSRVs arose from retroviral infection of the germline by a JSRV-like exogenous retrovirus. Most likely, this exogenous retrovirus had a reproductive tract tropism like en-JSRVs today. Next, natural selection allowed for evolution of sheep containing “friendly en-JSRVs” that were able to protect their host. As a countermeasure, JSRV would have acquired a tropism for the respiratory tract (where little en-JSRVs expression occurs) leading to the retrovirus that we know today responsible for lung cancer in sheep. Further, en-JSRVs expressed in the reproductive tract may have resulted in a gain-of-function event that enhanced placental development and pregnancy success, which altered evolution of sheep and became an essential part of their reproductive strategy. Therefore, en-JSRVs and the domestic sheep possess a symbiotic relationship.15 This idea is supported by findings that independently acquired ERVs were positively selected for a convergent biological role in evolution and development of the placenta in a number of different species. Finally, the transdominant proviruses enJS56A1 and enJSRV-20 might also prevent related betaretroviruses from infecting the germline. Evidence of such restriction occurring in vivo is given by the fact that the most recent enJSRV isolated is able to escape the restriction induced by the transdominant en-JSRVs. Furthermore, this data shows that events of adaptation and counter-measure between JSRV and en-JSRVs and their host are still in progress today.
Figure 6.
Theoretical adaptation events between endogenous Jaagsiekte sheep retrovirus (en-JSRVs), Jaagsiekte sheep retrovirus (JSRV), and their host during evolution. See conclusion remarks for a complete description.
Acknowledgments
We are grateful to the members of the Laboratory of Viral Pathogenesis of the University of Glasgow Faculty of Veterinary Medicine for stimulating discussions. Work in the laboratory of the authors is supported by a program grant of the Wellcome Trust, by a Strategic Research Developmental Grant by the Scottish Funding Council, and by NIH grant HD052745. MP is a Wolfson-Royal Society Research Merit Awardee.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Boeke JD, Stoye JP. Retrotransposons, endogenous retroviruses and the evolution of retroelements. In: Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses. Plainview, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 343–436. [PubMed] [Google Scholar]
- 2.Gifford R, Tristem M. The evolution, distribution and diversity of endogenous retroviruses. Virus Genes. 2003;26:291–315. doi: 10.1023/a:1024455415443. [DOI] [PubMed] [Google Scholar]
- 3.Lander ES, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 4.Herniou E, et al. Retroviral diversity and distribution in vertebrates. J. Virol. 1998;72:5955–5966. doi: 10.1128/jvi.72.7.5955-5966.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tarlinton RE, Meers J, Young PR. Retroviral invasion of the koala genome. Nature. 2006;442:79–81. doi: 10.1038/nature04841. [DOI] [PubMed] [Google Scholar]
- 6.Arnaud F, et al. A paradigm for virus-host coevolution: Sequential counter-adaptations between endogenous and exogenous retroviruses. PLoS Pathog. 2007;3:e170. doi: 10.1371/journal.ppat.0030170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coffin JM. Evolution of retroviruses: Fossils in our DNA. Proc. Am. Philos. Soc. 2004;148:264–280. [PubMed] [Google Scholar]
- 8.Bock M, Stoye JP. Endogenous retroviruses and the human germline. Curr. Opin. Genet. Dev. 2000;10:651–655. doi: 10.1016/s0959-437x(00)00138-6. [DOI] [PubMed] [Google Scholar]
- 9.Stetson DB, et al. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134:587–598. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoye JP, Moroni C, Coffin JM. Virological events leading to spontaneous AKR thymomas. J. Virol. 1991;65:1273–1285. doi: 10.1128/jvi.65.3.1273-1285.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ting CN, et al. Endogenous retroviral sequences are required for tissue-specific expression of a human salivary amylase gene. Genes Dev. 1992;6:1457–1465. doi: 10.1101/gad.6.8.1457. [DOI] [PubMed] [Google Scholar]
- 12.Samuelson LC, Phillips RS, Swanberg LJ. Amylase gene structures in primates: Retroposon insertions and promoter evolution. Mol. Biol. Evol. 1996;13:767–779. doi: 10.1093/oxfordjournals.molbev.a025637. [DOI] [PubMed] [Google Scholar]
- 13.Nethe M, Berkhout B, Van Der Kuyl AC. Retroviral superinfection resistance. Retrovirology. 2005;2:52. doi: 10.1186/1742-4690-2-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhadra S, et al. Endogenous MMTV proviruses induce susceptibility to both viral and bacterial pathogens. PLoS Pathog. 2006;2:e128. doi: 10.1371/journal.ppat.0020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris JR. The evolution of placental mammals. FEBS Lett. 1991;295:3–4. doi: 10.1016/0014-5793(91)81370-n. [DOI] [PubMed] [Google Scholar]
- 16.Harris JR. Placental endogenous retrovirus (ERV): Structural, functional, and evolutionary significance. Bioessays. 1998;20:307–316. doi: 10.1002/(SICI)1521-1878(199804)20:4<307::AID-BIES7>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 17.Dupressoir A, et al. Syncytin-A and syncytin-B, two fusogenic placenta-specific murine envelope genes of retroviral origin conserved in Muridae. Proc. Natl. Acad. Sci. USA. 2005;102:725–730. doi: 10.1073/pnas.0406509102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mi S, et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature. 2000;403:785–789. doi: 10.1038/35001608. [DOI] [PubMed] [Google Scholar]
- 19.Voisset C, et al. Chromosomal distribution and coding capacity of the human endogenous retrovirus HERV-W family. AIDS Res. Hum. Retroviruses. 2000;16:731–740. doi: 10.1089/088922200308738. [DOI] [PubMed] [Google Scholar]
- 20.Villarreal LP. On viruses, sex, and motherhood. J. Virol. 1997;71:859–865. doi: 10.1128/jvi.71.2.859-865.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venables PJ, et al. Abundance of an endogenous retroviral envelope protein in placental trophoblasts suggests a biological function. Virology. 1995;211:589–592. doi: 10.1006/viro.1995.1442. [DOI] [PubMed] [Google Scholar]
- 22.Blond JL, et al. An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor. J. Virol. 2000;74:3321–3329. doi: 10.1128/jvi.74.7.3321-3329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Parseval N, et al. Survey of human genes of retroviral origin: Identification and transcriptome of the genes with coding capacity for complete envelope proteins. J. Virol. 2003;77:10414–10422. doi: 10.1128/JVI.77.19.10414-10422.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benirschke K, Kaufmann P. Pathology of the Human Placenta. New York: Springer Verlag; 2000. [Google Scholar]
- 25.Pattillo RA, et al. Human hormone production in vitro . Science. 1968;159:1467–1469. doi: 10.1126/science.159.3822.1467. [DOI] [PubMed] [Google Scholar]
- 26.Frendo JL, et al. Direct involvment of HERV-W Env glycoprotein in human trophoblast cell fusion and differentiation. Mol. Cell. Biol. 2003;23:3566–3574. doi: 10.1128/MCB.23.10.3566-3574.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Parseval N, Heidmann T. Human endogenous retroviruses: From infectious elements to human genes. Cytogenet. Genome Res. 2005;110:318–332. doi: 10.1159/000084964. [DOI] [PubMed] [Google Scholar]
- 28.Blaise S, et al. Genomewide screening for fusogenic human endogenous retrovirus envelopes identifies syncytin 2, a gene conserved on primate evolution. Proc. Natl. Acad. Sci. USA. 2003;100:13013–13018. doi: 10.1073/pnas.2132646100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mangeney M, et al. Placental syncytins: Genetic disjunction between the fusogenic and immunosuppressive activity of retroviral envelope proteins. Proc. Natl. Acad. Sci. USA. 2007;104:20534–20539. doi: 10.1073/pnas.0707873105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyd MT, et al. The human endogenous retrovirus ERV-3 is upregulated in differentiating placental trophoblast cells. Virology. 1993;196:905–909. doi: 10.1006/viro.1993.1556. [DOI] [PubMed] [Google Scholar]
- 31.Cohen M, et al. The nucleotide sequence of the env gene from the human provirus ERV3 and isolation and characterization of an ERV3-specific cDNA. Virology. 1985;147:449–458. doi: 10.1016/0042-6822(85)90147-3. [DOI] [PubMed] [Google Scholar]
- 32.Rote NS, Chakrabarti S, Stetzer BP. The role of human endogenous retroviruses in trophoblast differentiation and placental development. Placenta. 2004;25:673–683. doi: 10.1016/j.placenta.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Lin L, Xu B, Rote NS. Expression of endogenous retrovirus ERV-3 induces differentiation in BeWo, a choriocarcinoma model of human placental trophoblast. Placenta. 1999;20:109–118. doi: 10.1053/plac.1998.0337. [DOI] [PubMed] [Google Scholar]
- 34.de Parseval N, Heidmann T. Physiological knockout of the envelope gene of the single-copy ERV-3 human endogenous retrovirus in a fraction of the Caucasian population. J. Virol. 1998;72:3442–3445. doi: 10.1128/jvi.72.4.3442-3445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prudhomme S, Bonnaud B, Mallet F. Endogenous retroviruses and animal reproduction. Cytogenet. Genome Res. 2005;110:353–364. doi: 10.1159/000084967. [DOI] [PubMed] [Google Scholar]
- 36.York DF, et al. Nucleotide sequence of the Jaaksiekte retrovirus, an exogenous and endogenous type D and B retrovirus of sheep and goats. J. Virol. 1992;66:4930–4939. doi: 10.1128/jvi.66.8.4930-4939.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmarini M, et al. Jaagsiekte sheep retrovirus is necessary and sufficient to induce a contagious lung cancer in sheep. J. Virol. 1999;73:6964–6972. doi: 10.1128/jvi.73.8.6964-6972.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allen TE, et al. The Jaagsiekte sheep retrovirus envelope gene induces transformation of the avian fibroblast cell line DF-1 but does not require a conserved SH2 binding domain. J. Gen . Virol. 2002;83(Pt 11):2733–2742. doi: 10.1099/0022-1317-83-11-2733. [DOI] [PubMed] [Google Scholar]
- 39.Maeda N, et al. Direct transformation of rodent fibroblasts by Jaagsiekte sheep retrovirus DNA. Proc. Natl. Acad. Sci. USA. 2001;98:4449–4454. doi: 10.1073/pnas.071547598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rai SK, et al. Candidate tumor suppressor HYAL2 is a glycosylphosphatidylinositol (GPI)-anchored cell-surface receptor for Jaagsiekte sheep retrovirus, the envelope protein of which mediates oncogenic transformation. Proc. Natl. Acad. Sci. USA. 2001;98:4443–4448. doi: 10.1073/pnas.071572898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu SL, Miller AD. Transformation of madin-darby canine kidney epithelial cells by sheep retrovirus envelope proteins. J. Virol. 2005;79:927–933. doi: 10.1128/JVI.79.2.927-933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zavala G, et al. Relevance of Akt phosphorylation in cell transformation induced by Jaagsiekte sheep retrovirus. Virology. 2003;312:95–105. doi: 10.1016/s0042-6822(03)00205-8. [DOI] [PubMed] [Google Scholar]
- 43.Caporale M, et al. Expression of the Jaagsiekte sheep retrovirus envelope glycoprotein is sufficient to induce lung tumors in sheep. J. Virol. 2006;80:8030–8037. doi: 10.1128/JVI.00474-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wootton SK, Halbert CL, Miller AD. Sheep retrovirus structural protein induces lung tumours. Nature. 2005;434:904–907. doi: 10.1038/nature03492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Danilkovitch-Miagkova A, et al. Hyaluronidase 2 negatively regulates RON receptor tyrosine kinase and mediates transformation of epithelial cells by Jaagsiekte sheep retrovirus. Proc. Natl. Acad. Sci. USA. 2003;100:4580–4585. doi: 10.1073/pnas.0837136100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varela M, et al. Association of RON tyrosine kinase with the Jaagsiekte sheep retrovirus envelope glycoprotein. Virology. 2006;350:347–357. doi: 10.1016/j.virol.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 47.Cousens C, et al. In vivo tumorigenesis by Jaagsiekte sheep retrovirus (JSRV) requires Y590 in Env TM, but not full-length orfX open reading frame. Virology. 2007;367:413–421. doi: 10.1016/j.virol.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maeda N, et al. Roles of the Ras-MEK-mitogen-activated protein kinase and phosphatidylinositol 3-kinase-Akt-mTOR pathways in Jaagsiekte sheep retrovirus-induced transformation of rodent fibroblast and epithelial cell lines. J. Virol. 2005;79:4440–4450. doi: 10.1128/JVI.79.7.4440-4450.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Las Heras M, et al. In-situ demonstration of mitogen-activated protein kinase Erk 1/2 signalling pathway in contagious respiratory tumours of sheep and goats. J. Comp. Pathol. 2006;135:1–10. doi: 10.1016/j.jcpa.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Palmarini M, et al. A phosphatidylinositol-3-kinase (PI-3K) docking site in the cytoplasmic tail of the Jaagsiekte sheep retrovirus transmembrane protein is essential for envelope-induced transformation of NIH3T3 cells. J. Virol. 2001;75:11002–11009. doi: 10.1128/JVI.75.22.11002-11009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palmarini M, et al. Molecular cloning and functional analysis of three type D endogenous retroviruses of sheep reveals a different cell tropism from that of the highly related exogenous Jaagsiekte sheep retrovirus. J. Virol. 2000;74:8065–8076. doi: 10.1128/jvi.74.17.8065-8076.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spencer TE, et al. Receptor usage and fetal expression of ovine endogenous betaretroviruses: Implications for coevolution of endogenous and exogenous retroviruses. J. Virol. 2003;77:749–753. doi: 10.1128/JVI.77.1.749-753.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mura M, et al. Late viral interference induced by transdominant Gag of an endogenous retrovirus. Proc. Natl. Acad. Sci. USA. 2004;101:11117–11122. doi: 10.1073/pnas.0402877101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arnaud F, Murcia PR, Palmarini M. Mechanisms of late restriction induced by an endogenous retrovirus. J. Virol. 2007;81:11441–11451. doi: 10.1128/JVI.01214-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murcia PR, Arnaud F, Palmarini M. The transdominant endogenous retrovirus enJS56A1 associates with and blocks intracellular trafficking of Jaagsiekte sheep retrovirus Gag. J. Virol. 2007;81:1762–1772. doi: 10.1128/JVI.01859-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guillomot M. Cellular interactions during implantation in domestic ruminants. J. Reprod. Fertil. Suppl. 1995;49:39–51. [PubMed] [Google Scholar]
- 57.Spencer TE, et al. Implantation mechanisms: Insights from the sheep. Reproduction. 2004;128:657–668. doi: 10.1530/rep.1.00398. [DOI] [PubMed] [Google Scholar]
- 58.Spencer TE, et al. Pregnancy recognition and conceptus implantation in domestic ruminants: Roles of progesterone, interferons and endogenous retroviruses. Reprod. Fertil. Dev. 2007;19:65–78. doi: 10.1071/rd06102. [DOI] [PubMed] [Google Scholar]
- 59.Wooding FB. Role of binucleate cells in fetomaternal cell fusion at implantation in the sheep. Am. J. Anat. 1984;170:233–250. doi: 10.1002/aja.1001700208. [DOI] [PubMed] [Google Scholar]
- 60.Wooding FB. Current topic: The synepitheliochorial placenta of ruminants: Binucleate cell fusions and hormone production. Placenta. 1992;13:101–113. doi: 10.1016/0143-4004(92)90025-o. [DOI] [PubMed] [Google Scholar]
- 61.Hoffman LH, Wooding FB. Giant and binucleate trophoblast cells of mammals. J. Exp. Zool. 1993;266:559–577. doi: 10.1002/jez.1402660607. [DOI] [PubMed] [Google Scholar]
- 62.Igwebuike UM. Trophoblast cells of ruminant placentas—a minireview. Anim. Reprod. Sci. 2006;93:185–198. doi: 10.1016/j.anireprosci.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 63.Dunlap KA, et al. Sheep endogenous betaretroviruses (en-JSRVs) and the hyaluronidase 2 (HYAL2) receptor in the ovine uterus and conceptus. Biol. Reprod. 2005;73:271–279. doi: 10.1095/biolreprod.105.039776. [DOI] [PubMed] [Google Scholar]
- 64.Spencer TE, et al. Discovery and characterization of endometrial epithelial messenger ribonucleic acids using the ovine uterine gland knockout model. Endocrinology. 1999;140:4070–4080. doi: 10.1210/endo.140.9.6981. [DOI] [PubMed] [Google Scholar]
- 65.Palmarini M, et al. Expression of endogenous betaretroviruses in the ovine uterus: Effects of neonatal age, estrous cycle, pregnancy and progesterone. J. Virol. 2001;75:11319–11327. doi: 10.1128/JVI.75.23.11319-11327.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Summerton J. Morpholino antisense oligomers: The case for an RNase H-independent structural type. Biochim. Biophys. Acta. 1999;1489:141–158. doi: 10.1016/s0167-4781(99)00150-5. [DOI] [PubMed] [Google Scholar]
- 67.Dunlap KA, et al. Endogenous retroviruses regulate periimplantation placental growth and differentiation. Proc. Natl. Acad. Sci. USA. 2006;103:14390–14395. doi: 10.1073/pnas.0603836103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakayama K. Furin: A mammalian subtilisin/Kex2p–like endoprotease involved in processing of a wide variety of precursor proteins. Biochem. J. 1997;327(Pt 3):625–635. doi: 10.1042/bj3270625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hunter E. Viral entry and receptors. In: Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses. New York: Cold Spring Harbor Laboratory Press; 1997. pp. 71–119. [PubMed] [Google Scholar]
- 70.Cote M, et al. Fusogenicity of Jaagsiekte sheep retrovirus envelope protein is dependent on low pH and is enhanced by cytoplasmic tail truncations. J. Virol. 2008;82:2543–2554. doi: 10.1128/JVI.01852-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ferretti C, et al. Molecular circuits shared by placental and cancer cells, and their implications in the proliferative, invasive and migratory capacities of trophoblasts. Hum. Reprod. Update. 2007;13:121–141. doi: 10.1093/humupd/dml048. [DOI] [PubMed] [Google Scholar]
- 72.Soundararajan R, Rao AJ. Trophoblast ‘pseudo-tumorigenesis’: Significance and contributory factors. Reprod. Biol. Endocrinol. 2004;2:15. doi: 10.1186/1477-7827-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pollheimer J, Knofler M. Signalling pathways regulating the invasive differentiation of human trophoblasts: A review. Placenta. 2005;26(Suppl A):S21–S30. doi: 10.1016/j.placenta.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 74.Schulte AM, et al. Human trophoblast and choriocarcinoma expression of the growth factor pleiotrophin attributable to germ-line insertion of an endogenous retrovirus. Proc. Natl. Acad. Sci. USA. 1996;93:14759–14764. doi: 10.1073/pnas.93.25.14759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bieche I, et al. Placenta-specific INSL4 expression is mediated by a human endogenous retrovirus element. Biol. Reprod. 2003;68:1422–1429. doi: 10.1095/biolreprod.102.010322. [DOI] [PubMed] [Google Scholar]
- 76.Cianciolo GJ, et al. Inhibition of lymphocyte proliferation by a synthetic peptide homologous to retroviral envelope proteins. Science. 1985;230:453–455. doi: 10.1126/science.2996136. [DOI] [PubMed] [Google Scholar]
- 77.Kalter SS, et al. Brief communication: C-type particles in normal human placentas. J. Natl. Cancer Inst. 1973;50:1081–1084. doi: 10.1093/jnci/50.4.1081. [DOI] [PubMed] [Google Scholar]
- 78.Kalter SS, et al. A comparative study on the presence of C-type viral particles in placentas from primates and other animals. Bibl. Haematol. 1975;40:391–401. doi: 10.1159/000397557. [DOI] [PubMed] [Google Scholar]
- 79.Vernon ML, McMahon JM, Hackett JJ. Additional evidence of type-C particles in human placentas. J. Natl. Cancer Inst. 1974;52:987–989. doi: 10.1093/jnci/52.3.987. [DOI] [PubMed] [Google Scholar]
- 80.Smith CA, Moore HD. Expression of C-type viral particles at implantation in the marmoset monkey. Hum. Reprod. 1988;3:395–398. doi: 10.1093/oxfordjournals.humrep.a136714. [DOI] [PubMed] [Google Scholar]
- 81.DeHaven JE, et al. Novel retroviral sequences are expressed in the epididymis and uterus of Syrian hamsters. J. Gen . Virol. 1998;79(Pt 11):2687–2694. doi: 10.1099/0022-1317-79-11-2687. [DOI] [PubMed] [Google Scholar]