Abstract
Background: Cerebrospinal fluid (CSF) biomarkers’ performance for predicting conversion from mild cognitive impairment (MCI) to Alzheimer’s disease (AD) is still suboptimal.
Objective: By considering several confounding factors we aimed to identify in which situations these CSF biomarkers can be useful.
Data Sources: A systematic review was conducted on MEDLINE, PreMedline, EMBASE, PsycInfo, CINAHL, Cochrane, and CRD (1990–2013).
Eligibility Criteria: (1) Prospective studies of CSF biomarkers’ performance for predicting conversion from MCI to AD/dementia; (2) inclusion of Aβ42 and T-tau and/or p-tau. Several meta-analyses were performed.
Results: Aβ42/p-tau ratio had high capacity to predict conversion to AD in MCI patients younger than 70 years. The p-tau had high capacity to identify MCI cases converting to AD in ≤24 months.
Conclusions: Explaining how different confounding factors influence CSF biomarkers’ predictive performance is mandatory to elaborate a definitive map of situations, where these CSF biomarkers are useful both in clinics and research.
Keywords: mild cognitive impairment, Alzheimer’s disease, CSF biomarkers, confounding factors, meta-analysis, systematic review
Introduction
Mild cognitive impairment (MCI) is a high risk factor for developing dementia, particularly Alzheimer’s disease (AD). About 35% of MCI patients progress to AD, with an annual conversion rate of 5–10% (Mitchell, 2009). Because AD entails severe consequences, an appropriate prediction of MCI outcome is crucial for giving the patients a prognosis and to initiate therapeutical strategies as soon as possible. In this regard, the new MCI diagnostic criteria recommended by the National Institute of Aging – Alzheimer’s Association (NIA-AA) emphasize the use of neuroimaging and cerebrospinal fluid (CSF) biomarkers (Albert et al., 2011). Although significant advances have been made in the field of neuroimaging, biomarkers based on CSF are at present the most convenient for studying disease progression.
The currently validated CSF biomarkers of AD are Aβ42, total tau (T-tau), and phosphorylated tau (p-tau). CSF Aβ42 is reduced, and T-tau and p-tau levels are increased in MCI patients compared to healthy controls (Diniz et al., 2008). In addition, MCI patients with abnormal CSF biomarkers have increased risk to progress to AD (Herukka et al., 2005; Hansson et al., 2006, 2007; Bouwman et al., 2007; Brys et al., 2009; Mattsson et al., 2009; Shaw et al., 2009; Hertze et al., 2010; Buchhave et al., 2012). Buchhave et al. (2012) showed that 90% of MCI patients with pathologic CSF biomarkers developed AD within 9⋅2 years. This knowledge is now incorporated in the new diagnostic criteria for MCI, indicating that positive biomarkers of Aβ accumulation (e.g., CSF Aβ42) and neuronal injury (e.g., CSF T-tau and p-tau) confers the highest likelihood that AD pathophysiological processes are the cause of the cognitive dysfunction; and that individuals with this biomarker profile are more likely to decline or progress to dementia due to AD in relatively short periods (Albert et al., 2011). Regarding predictive capacity, although single CSF biomarkers have shown unsatisfactory results, their combination could be suitable to identify which MCI patients will progress to dementia (Ferreira et al., 2014). In particular, the Aβ42/p-tau ratio has demonstrated high efficiency (Hansson et al., 2006; Mattsson et al., 2009; Buchhave et al., 2012; Parnetti et al., 2012; Roe et al., 2013). Two systematic reviews with meta-analysis have previously been published (Mitchell, 2009; Monge-Argilés et al., 2010). Mitchell (2009) only evaluated p-tau. Monge-Argilés et al. (2010) evaluated the three CSF biomarkers, but the group of MCI patients that converted to AD was compared to a mixed group of stable MCI cases and MCI patients that converted to non-AD dementias. Moreover, their analysis of combined CSF biomarkers was limited to only three studies and the combination procedure was not sufficiently detailed.
Importantly, CSF biomarkers’ predictive performance could be improved by considering different confounding factors such as the MCI subtype, time to AD conversion, and age (Ferreira et al., 2014). Previous studies show that the CSF biomarkers have better predictive capacity in amnestic MCI (Vos et al., 2013), MCI patients that convert to AD in relatively short periods (e.g., <12 months) (Gaser et al., 2013), and young MCI patients (Mattsson et al., 2012). However, most of the studies performed to date do not consider these confounding factors. These aspects together with methodological variability have made it difficult to propose definitive cut-off values for CSF biomarkers. For this reason, the fact of disseminating the use of CSF biomarkers to clinical routine is compromised at present (Ferreira et al., 2014).
The main objective in this study was to carefully evaluate the capacity of the CSF biomarkers to predict conversion from MCI to AD in several clinically relevant situations. In particular, we aimed to identify for which specific MCI patients these CSF biomarkers might be useful in clinical practice. In order to address this question, several meta-analyses were performed for studies that prospectively analyzed the predictive performance of CSF Aβ42 and T-tau and/or p-tau. The design of the included studies is baseline cross-sectional comparisons between MCI patients that convert to AD or dementia (MCI-C) and MCI patients that remain stable (MCI-S) at follow-up. We hypothesized that combined CSF biomarkers would have better predictive performance than single CSF biomarkers, and that this performance could be increased by controlling for different confounding factors such as the MCI subtype, time to AD conversion, and age, among others.
Material and Methods
Search strategy and selection criteria
A systematic review was conducted for the period between January 1990 and September 2013 in the following electronic databases: MEDLINE and PreMedline, EMBASE, PsycInfo, CINAHL, Cochrane Library, and CRD. The search strategy was developed for each database using the following Medical Subject Heading (MeSH) and free-text terms: “Alzheimer’s disease diagnosis” or “Alzheimer’s disease,” and “abeta-42” or “T-tau” or “P-tau” or “tau” or “phospho-tau” or “phosphorylated tau.” Examples for the two major databases are shown in Table S1 in Supplementary Materials (MEDLINE) and Table S2 in Supplementary Materials (EMBASE). In addition, reference sections were searched to identify relevant publications.
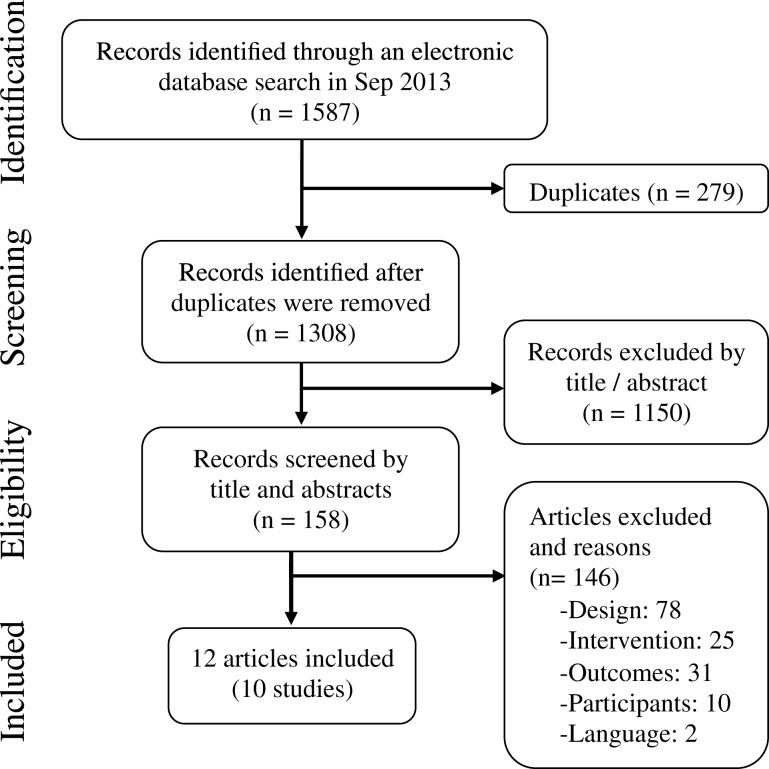
Inclusion criteria for this meta-analysis were studies that (1) performed a prospective analysis of the CSF biomarkers’ performance for predicting conversion to AD or dementia in individuals with MCI at baseline; (2) included at least two CSF biomarkers, being Aβ42 always required along with T-tau and/or p-tau; and (3) were published in English or Spanish. Studies were excluded if they did not report sensitivity or specificity values, or any other data that enabled its calculation. Two reviewers independently performed the study selection (Daniel Ferreira and Amado Rivero-Santana), and in case of doubt and/or disagreements a third reviewer was consulted (Lilisbeth Perestelo-Pérez). The search yielded 1308 references after discarding duplicates. One-hundred fifty-eight articles were selected by title and abstract. After applying eligibility criteria, 12 articles were eventually included (Hampel et al., 2004; Herukka et al., 2005; Parnetti et al., 2006, 2012; Eckerström et al., 2010; Hertze et al., 2010; Monge-Argilés et al., 2011; Buchhave et al., 2012; Ewers et al., 2012; Gaser et al., 2013; Toledo et al., 2013; Vos et al., 2013). Three of these studies included data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI). As these studies represent different analyses of overlapping ADNI subsamples, only one ADNI study was included for each meta-analysis depending on the analyzed biomarker. If two ADNI studies were available for the same biomarker, the one with largest sample was selected. Selection flow including reasons for study exclusion at each phase is shown in Figure 1.
Figure 1.
Study selection flow.
Data collection, risk of bias, and evaluation of methodological quality
A data extraction sheet was developed to collect relevant data by covering: author and publication year, country, objectives, methods (with special attention to participants’ recruitment procedures, study design, follow-up length, CSF biomarkers evaluated including Aβ42/T-tau and Aβ42/p-tau ratios, diagnostic groups characteristics, and statistical analyses), results, and conclusions. Data extraction was carried out by two researchers (Daniel Ferreira and Amado Rivero-Santana), and quality and accuracy of the extraction was verified by a third researcher (Lilisbeth Perestelo-Pérez).
Several strategies were followed in order to reduce the risk of bias related to publication, data availability, and reviewer selection (see Table S3 in Supplementary Materials). The QUADAS-2 scale (Whiting et al., 2011) was used in order to assess the methodological quality of the included studies. The scale was applied by two researchers (Amado Rivero-Santana and Daniel Ferreira), and in case of doubt and/or disagreements a third was consulted (Lilisbeth Perestelo-Pérez). Finally, this study was performed in accordance with the PRISMA statement (Liberati et al., 2009; Moher et al., 2010), which provides a detailed guideline of preferred reporting style for systematic reviews and meta-analyses.
Identification of potential confounding factors of CSF biomarkers’ predictive performance
We hypothesized that CSF biomarkers’ predictive performance might be influenced by different confounding factors. To explore sources of heterogeneity, the following factors were defined a priori based on the literature (see Table 1 for a detailed description of the different factors and considered categories): (1) recruitment setting; (2) MCI subtype; (3) diagnostic criteria for MCI at baseline; (4) diagnostic criteria for AD at follow-up; (5) postmortem confirmation of AD pathology; (6) criteria for conversion from MCI to AD/dementia; (7) diagnosis at follow-up; (8) follow-up length (as rough estimation of time to AD conversion); (9–11) MCI severity at baseline according to mini-mental state examination (MMSE), clinical rating [e.g., clinical dementia rating (CDR), global deterioration scale (GDS)], and magnetic resonance imaging (MRI) rating (i.e., degree of brain atrophy); (12) Age; (13) gender distribution; (14) years of education; (15) family history of AD; (16) APOE e4 status; (17) technology applied for the CSF analysis; and (18) cut-offs for interpreting the CSF levels.
Table 1.
Potential confounding factors of CSF biomarkers’ predictive performance.
| Factors | Considered categories |
|---|---|
| RECRUITMENT | |
| Setting | Specialized centers (9 + 1) vs. primary care (0) vs. general population (0) |
| DIAGNOSTIC ISSUES | |
| MCI subtype | Amnestic MCI (6 + 1) vs. non-amnestic MCI (1) vs. mixed sample (2)a vs. non-specified (1) |
| Diagnostic criteria for MCI at baseline | Petersen et al. (1999, 2001); Petersen, 2004 or Petersen’s group 2006b (8) vs. Winblad et al. (2004) (1) vs. ADNI criteria (+ 1) |
| Diagnostic criteria for AD at follow-up | NINCDS-ADRDA (8 + 1) vs. non-specified (1) |
| Postmortem confirmation of AD pathology | All the studies lacked postmortem confirmation of AD pathology (0) |
| Diagnosis at follow-up | AD (6 + 1) vs. mixed group of dementias (3)c |
| Criteria for conversion | Fulfillment of diagnostic criteria (7 + 1) vs. Decline in clinical scales (e.g., CDR) (1) vs. non-specified (1) |
| TIME TO AD CONVERSION | |
| Time to AD conversion | Follow-up ≤24 months (5 + 1) vs. Follow-up >24 months (4) |
| MCI SEVERITY AT BASELINE | |
| MMSE total score | No enough variability: all studies reporting MMSE have mean scores between 23 and 30 (7 + 1) vs. non-specified (2) |
| Clinical rating | CDR = 0.5/GDS = 3 (5 + 1) vs. Non-specified (4) |
| MRI rating | Only available for 2 (+ 1) studies, with variability in procedures |
| DEMOGRAPHICS/RISK FACTORS AT BASELINE | |
| Age | ≤70 years (4) vs. >70 years (4 + 1) vs. non-specified (1) |
| Gender | Results are never presented separately for men and women (0) |
| Education | ≤12 years (3) vs. >12 years (+ 1) vs. non-specified (6) |
| Family history of AD | Not enough information (1) |
| APOE e4 status | Not enough information (3 + 1) |
| CSF METHODS | |
| Technology for CSF analysis | ELISA (6) vs. xMAP (3 + 1) |
| Cut-offs for interpreting CSF levels | Great variability: Internal (highest value of SN + SP or Youden’s Index) (3) vs. External or independent of clinical diagnosis (Mixture model analysis, obtained from another cohort in the same study; Hulstaert et al., 1999; Sjögren et al., 2001; Shaw et al., 2009; Zetterberg et al., 2003) (4 + 1) vs. both internal and external (2) |
Between brackets, number of studies available for at least one biomarker; +1 refers to ADNI studies (only one ADNI study is included for each subgroup meta-analysis).
aHerukka et al. (2005) included amnestic MCI patients as well as patients with other types of cognitive decline. Monge-Argilés et al. (2011) included a mixed group of amnestic MCI and non-amnestic MCI patients.
bDiagnostic criteria for MCI published by Petersen’s group in Artero et al. (2006).
cBoth in Parnetti et al. (2006) and Monge-Argilés et al. (2011) it is unclear whether MCI converters progressed exclusively to AD.
MCI, mild cognitive impairment; AD, Alzheimer’s disease; MMSE, mini-mental state examination; MRI, magnetic resonance imaging; APOE, apolipoprotein E; CSF, cerebrospinal fluid; ADNI: Alzheimer’s disease neuroimaging initiative; NINCDS-ADRDA, National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association; CDR, clinical dementia rating; GDS, global deterioration scale.
Statistical analysis
For each article, true and false positives/negatives values were calculated from sensitivity, specificity, positive predicted value, negative predicted value, and/or the rate of converters and non-converters. A global meta-analysis was performed for each single CSF biomarker (i.e., Aβ42, T-tau, and p-tau) and two relevant ratios (i.e., Aβ42/T-tau and Aβ42/p-tau). Analyses were performed with the MetaDisc 1.1.1 software (Zamora et al., 2006). Sensitivity and specificity pooled estimates were calculated with random-effects models (DerSimonian and Laird, 1986), which yield more conservative estimates. For a qualitative interpretation of sensitivity and specificity results, values above 80% were considered indicative of satisfactory predictive performance according to international recommendations (The Ronald and Nancy Reagan Research Institute of the Alzheimer’s Association and the national Institute on Aging working Group, 1998). Positive and negative likelihood ratios were calculated from resulting sensitivity and specificity values and interpreted following established guidelines (see these guidelines in Figure 2 footnotes) (Qizilbash, 2002). Likelihood ratios indicate how the pretest probability of disease is increased or decreased by the outcome of a diagnostic test. A positive likelihood ratio [LR + = sensitivity/(1 – specificity)] greater than one increases the probability that the disease is present (in this context progression to AD) and helps to rule-in MCI-C cases. A negative likelihood ratio (LR– = (1 – sensitivity)/specificity) of less than one diminishes the probability that disease is present and helps to rule-out MCI-C cases. Statistical heterogeneity was explored with the Cochran Q-test. As this statistic has low power when few studies are available, a recommended p-value of 0⋅10 was established as statistical significance threshold to detect heterogeneity (Hardy and Thompson, 1998). Differences in sensitivity and specificity values for pairs of subgroup meta-analyses (e.g., MCI cases younger than 70 years vs. older than 70 years) were tested with the formula: QBET = QTOT – (Q1 + Q2). Where QTOT represents the overall inter-study variability, and Q1 and Q2 represents inter-study variability for each subgroup in the comparison (Deeks et al., 2001). The QBET statistic was then compared to a χ2 distribution with J − 1 degrees of freedom using a significance level of 0⋅05, where J is the number of subgroups.
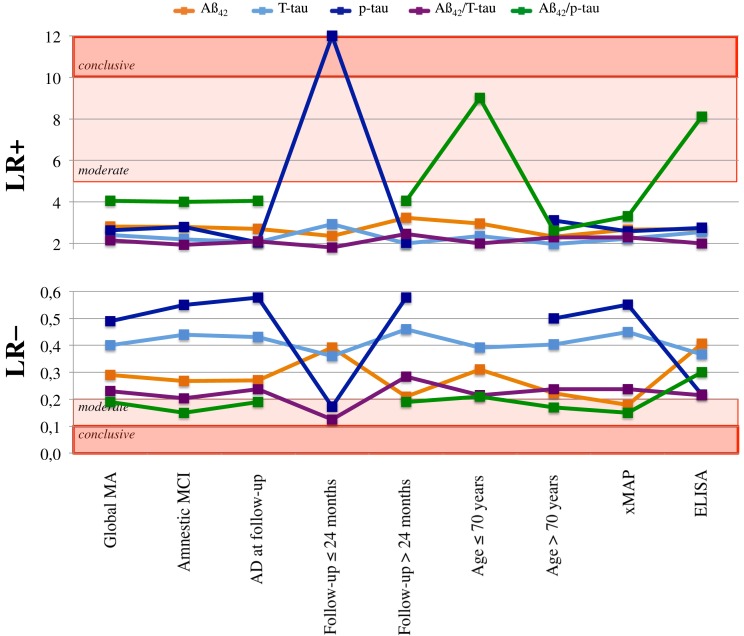
Figure 2.
Positive and negative likelihood ratios. A LR+ greater than one increases the pretest probability that the disease is present [in this context progression from MCI to AD or, in other words, MCI due to AD (Albert et al., 2011)]. A LR– of less than one diminishes the pretest probability that disease is present. The established guidelines (Qizilbash, 2002) states that a LR+ greater than 10 will often make conclusive changes to the pretest probability, indicating that the disease is likely present; a LR+ between 5 and 10 corresponds to moderate increase in probability; and a LR+ between 2 and 5 corresponds to small increase. A LR− of less than 0⋅1 will often make conclusive changes to the pretest probability that the disease is present, indicating that the disease is unlikely present; a LR− between 0⋅1 and 0⋅2 corresponds to moderate decrease in probability; and a LR− between 0⋅2 and 0⋅5 corresponds to small decrease. LR+, positive likelihood ratio; LR−, negative likelihood ratio; Global MA, global meta-analysis; MCI, mild cognitive impairment; AD, Alzheimer’s disease.
Results
Main characteristics of included studies and methodological quality
Among the 12 studies included, 10 offered data about the diagnostic performance of Aβ42, 6 about T-tau, 5 about p-tau, and 6 about the Aβ42/T-tau and Aβ42/p-tau ratios. Main study characteristics are detailed in Table 2. Methodological quality (QUADAS-2) is shown in Table S4 in Supplementary Materials. In summary, (1) Patient selection: only two studies demonstrated low risk of bias; seven did not explicitly state consecutive or random samples; patients could have been inappropriately excluded in nine studies. (2) Diagnostic test: seven studies proved low risk of bias by using external cut-off values or establishing the cut-off in the study sample independently of the clinical diagnosis [i.e., mixture model analysis in Buchhave et al., 2012]. (3) Diagnostic criterion: all the studies were classified as unclear given that postmortem confirmation of AD pathology was never performed. (4) Patients flow and follow-up: three studies demonstrated low risk of bias; all the studies applied the same reference standard to all the patients, but patients were followed during only two years or less in five studies; six studies did not include all baseline patients in the final analyses.
Table 2.
Main characteristics of included studies.
| Biomarkersa | Sample size (N)b | Comparison | Mean age | Women (%) | MCI subtype | Diagnostic criteria for MCI | Diagnostic criteria for AD | CSF method | Cut-off value | Follow-up (years) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Buchhave et al. (2012) | Aβ42, Aβ42/p-tau | 134 | MCI-CAD vs. (MCI-S + MCI-Cother) | 69.8 | 55 | aMCI | Petersen (2004) | NINCDS-ADRDA | xMAP | Mixture model analysis | 9.2 |
| Eckerström et al. (2010) | Aβ42, Aβ42/T-tau | 68 | MCI-C vs. (MCI-S + controls) | 67.8 | 61.8 | n.s. | Winblad et al. (2004) | NINCDS-ADRDA | ELISA | Internal (maximum value of SN + SP) | 2 |
| Ewers et al. (2012) | T-tau, p-tau | 130 | MCI-CAD vs. MCI-S | 73.9 | 63.8 | aMCI | ADNIe | NINCDS-ADRDA | xMAP | n.s. | 2.3 |
| Gaser et al. (2013) | Aβ42, Aβ42/p-tau | 99 | MCI-CAD vs. MCI-S | 75.3 | n.s. | aMCI | ADNIe | NINCDS-ADRDA | xMAP | n.s. | 3 |
| Hampel et al. (2004) | Aβ42, T-tau | 52 | MCI-CAD vs. MCI-S | 72.4 | 59 | aMCI | Petersen et al. (1999) | NINCDS-ADRDA | ELISA | Internal (maximum value of SN + SP) | 0.7 |
| Hertze et al. (2010) | Aβ42, T-tau, p-tau, Aβ42/T-tau Aβ42/p-tau | 159 | MCI-CAD vs. (MCI-S + MCI-Cother) | 71.8 | 57.2 | aMCI | Petersen (2004) | NINCDS-ADRDA | xMAP | AD vs. control in the same study (highest value of Youden’s index) | 4.7 |
| Herukka et al. (2005) | Aβ42, T-tau, p-tau, Aβ42/T-tau, Aβ42/p-tau | 78 | MCI-CAD vs. MCI-S | 70.4 | 59 | aMCI + naMCI | Petersen (2004) | n.s. | ELISA | Internal Sjögren et al. (2001); Hulstaert et al. (1999) | 3 |
| Monge-Argilés et al. (2011) | Aβ42, T-tau, p-tau, Aβ42/T-tau, Aβ42/p-tau | 38 | MCI-C vs. controls | 73.5 | 63.2 | aMCI + naMCI | Artero et al. (2006)d | NINCDS-ADRDA | xMAP | Internal Shaw et al. (2009) | 0.5 |
| Parnetti et al. (2006) | Aβ42, T-tau, p-tau | 44 | MCI-C vs. MCI-S | n.s. | n.s. | aMCI | Petersen et al. (1999) | NINCDS-ADRDA | ELISA | Aβ42, T-tau: Sjögren et al. (2001), p-tau: Zetterberg et al. (2003) | 1 |
| Parnetti et al. (2012) | Aβ42, Aβ42/p-tau | 90 | MCI-CAD vs. MCI-S | 66.7 | 48.9 | aMCI | Petersen et al. (1999) | NINCDS-ADRDA | ELISA | Internal (highest value of Youden’s index) | 3.4 |
| Toledo et al. (2013) | Aβ42/T-tau | 122 | MCI-CAD vs. MCI-S | 74 | 33.2 | aMCI | ADNIe | NINCDS-ADRDA | xMAP | Shaw et al. (2009) | 3.2 |
| Vos et al. (2013) | Aβ42, Aβ42/T-tau | 191 | MCI-CAD vs. MCI-S | 70.7c | 53.6c | aMCI and naMCI | Petersen (2004) | NINCDS-ADRDA | ELISA | Aβ42: Sjögren et al. (2001) Aβ42/T-tau: Hulstaert et al. (1999) | 2 |
aBiomarkers with suitable data for performing meta-analyses.
bSample size included in the meta-analyses.
cResults were only available for the whole MCI sample (N = 625).
dArtero et al. (2006).
eMCI diagnostic criteria in the ADNI cohort are comparable to Petersen et al. (1999).
n.s., non-specified; MCI, mild cognitive impairment; MCI-C, MCI patients that convert to AD or other non-AD dementia form; MCI-CAD, MCI-C patients that exclusively convert to AD; MCI-Cother, MCI-C patients that convert to a non-AD dementia form; MCI-S, MCI patients that remain stable at follow-up; aMCI, amnestic MCI; naMCI, non-amnestic MCI; AD, Alzheimer’s disease; CSF, cerebrospinal fluid; ADNI, Alzheimer’s Disease Neuroimaging Initiative; NINCDS-ADRDA, National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association; SN + SP, sensitivity + specificity.
Global meta-analysis: CSF biomarkers’ predictive performance
Table 3 shows sensitivity and specificity values with 95% CI, heterogeneity, and likelihood ratios. Heterogeneity was significant for the three single biomarkers as well as for the two evaluated ratios. Aβ42/p-tau ratio showed the best performance with 85% sensitivity, 79% specificity, and a negative likelihood ratio of 0⋅19, indicating moderate decrease in the probability that the disease is present.
Table 3.
Global meta-analysis and subgroups meta-analyses.
| Biomarker | Meta-analysis | Sensitivity (%) | Q | Inter-groups difference (QBET) | Specificity (%) | Q | Inter-groups difference (QBET) | LR+ | LR− |
|---|---|---|---|---|---|---|---|---|---|
| Aβ42 | Global (n = 10)a | 79 (75–83) | 47.88*** | – | 72 (68–75) | 60.19*** | – | 2.82 | 0.29 |
| Amnestic MCI (n = 7) | 81 (77–86) | 33.83*** | – | 71 (66–75) | 59.66*** | – | 2.79 | 0.27 | |
| AD at follow-up (n = 7) | 81 (76–85) | 32.03*** | – | 70 (66–74) | 51.90*** | – | 2.70 | 0.27 | |
| Follow-up ≤24 months (n = 5)a | 73 (65–80) | 18.67** | 6.76 (p = 0.009) | 69 (63–75) | 15.69** | 1.61 (p = 0.20) | 2.36 | 0.39 | |
| Follow-up > 24 months (n = 5) | 84 (78–88) | 22.45*** | 74 (69–79) | 42.89*** | 3.23 | 0.21 | |||
| Age ≤ 70 (n = 5)a | 77 (71–82) | 26.20*** | 5.77 (p = 0.02) | 74 (69–79) | 31.33*** | 7.29 (p = 0.007) | 2.96 | 0.31 | |
| Age > 70 (n = 4) | 86 (80–91) | 5.95 | 63 (56–70) | 13.71** | 2.32 | 0.22 | |||
| xMAP (n = 4) | 88 (83–92) | 6.04 | 20.18 (p < 0.001) | 67 (61–73) | 16.41*** | 3.30 (p = 0.07) | 2.67 | 0.18 | |
| ELISA (n = 6)a | 70 (63–77) | 21.66** | 74 (69–79) | 40.48*** | 2.69 | 0.41 | |||
| T-tau | Global (n = 8)a | 72 (66–77) | 18.62* | – | 70 (66–74) | 60.06*** | – | 2.40 | 0.40 |
| Amnestic MCI (n = 5) | 70 (64–77) | 12.34* | – | 68 (63–74) | 30.68*** | – | 2.19 | 0.44 | |
| AD at follow-up (n = 5)a | 72 (66–78) | 13.46* | – | 65 (60–70) | 18.45** | – | 2.06 | 0.43 | |
| Follow up ≤24 months (n = 5)a | 73 (65–80) | 11.94* | 0.32 (p = 0.57) | 75 (69–80) | 43.58*** | 4.83 (p = 0.03) | 2.92 | 0.36 | |
| Follow up > 4 months (n = 3) | 70 (61–78) | 6.36* | 65 (59–72) | 11.65** | 2.00 | 0.46 | |||
| Age ≤70 (n = 3)a | 73 (64–80) | 4.63 | 0 (p = 1) | 69 (63–75) | 31.98*** | 0.35 (p = 0.55) | 2.36 | 0.39 | |
| Age > 70 (n = 4) | 73 (65–79) | 10.58* | 67 (60–73) | 10.70* | 1.97 | 0.40 | |||
| xMAP (n = 3) | 69 (60–77) | 4.45 | 0.97 (p = 0.32) | 69 (62–75) | 6.77* | 0.21 (p = 0.65) | 2.23 | 0.45 | |
| ELISA (n = 5)a | 74 (66–81) | 13.20* | 71 (65–76) | 53.08*** | 2.55 | 0.37 | |||
| p-tau | Global (n = 5) | 63 (55–71) | 21.22*** | – | 76 (70–80) | 51.57*** | – | 2.63 | 0.49 |
| Amnestic MCI (n = 3) | 56 (47–65) | 8.66* | – | 80 (74–85) | 33.41*** | – | 2.80 | 0.55 | |
| AD at follow-up (n = 3) | 59 (51–68) | 15.05*** | – | 71 (65–77) | 35.49*** | – | 2.04 | 0.58 | |
| Follow up ≤24 months (n = 2) | 84 (64–95) | 0.07 | 6.1 (p = 0.01) | 93 (83–98) | 1.92 | 14.16 (p < 0.001) | 12.00 | 0.17 | |
| Follow up > 24 months (n = 3) | 59 (51–68) | 15.05*** | 71 (65–77) | 35.49*** | 2.03 | 0.58 | |||
| Age ≤ 70 | – | – | – | – | – | – | – | – | |
| Age > 70 (n = 3) | 59 (51–68) | 13.79** | 81 (75–86) | 34.23*** | 3.10 | 0.50 | |||
| xMAP (n = 3) | 57 (48–66) | 11.01** | 10.06 (p = 0.001) | 78 (72–84) | 25.48*** | 2.63 (p = 0.10) | 2.59 | 0.55 | |
| ELISA (n = 2) | 85 (69–95) | 0.15 | 69 (59–79) | 23.46*** | 2.74 | 0.22 | |||
| Aβ42/T-tau | Global (n = 5)a | 86 (81–90) | 21.20*** | – | 60 (54–65) | 42.52*** | – | 2.15 | 0.23 |
| Amnestic MCI (n = 3) | 89 (83–93) | 10.62** | – | 54 (48–61) | 22.87*** | – | 1.94 | 0.20 | |
| AD at follow-up (n = 4)a | 86 (81–91) | 21.20*** | – | 59 (54–64) | 41.15*** | – | 2.10 | 0.24 | |
| Follow up ≤ 24 months (n = 2)a | 94 (87–98) | 3.86 | 8.83 (p = 0.003) | 48 (39–56) | 9.00* | 13.50 (p < 0.001) | 1.81 | 0.13 | |
| Follow up > 24 months (n = 3) | 81 (73–87) | 8.51* | 67 (61–73) | 20.02*** | 2.46 | 0.28 | |||
| Age ≤ 70 years (n = 2)a | 88 (79–93) | 18.63*** | 0.27 (p = 0.6) | 56 (48–64) | 29.63*** | 1.83 (p = 0.18) | 2.00 | 0.21 | |
| Age > 70 years (n = 3) | 85 (78–91) | 2.30 | 63 (56–70) | 11.06** | 2.30 | 0.24 | |||
| xMAP (n = 3) | 85 (78–91) | 2.30 | 0.27 (p = 0.6) | 63 (56–70) | 11.06** | 1.83 (p = 0.18) | 2.30 | 0.24 | |
| ELISA (n = 2)a | 88 (79–93) | 18.63*** | 56 (48–64) | 29.63*** | 2.00 | 0.21 | |||
| Aβ42/p-tau | Global (n = 6) | 85 (80–89) | 12.08* | – | 79 (74–83) | 44.03*** | – | 4.05 | 0.19 |
| Amnestic MCI (n = 4) | 88 (83–92) | 2.70 | – | 78 (72–83) | 41.01*** | – | 4.00 | 0.15 | |
| AD at follow-up (n = 5) | 85 (80–89) | 12.08* | – | 79 (74–84) | 43.77*** | – | 4.05 | 0.19 | |
| Follow up ≤ 24 months | – | – | – | – | – | – | – | – | |
| Follow up > 24 months (n = 5) | 85 (80–89) | 12.08* | 79 (74–84) | 43.77*** | 4.05 | 0.19 | |||
| Age ≤70 years (n = 3) | 81 (73–88) | 7.19* | 3.59 (p = 0.06) | 91 (86–95) | 2.07 | 31.79 (p < 0.001) | 9.00 | 0.21 | |
| Age > 70 years (n = 3) | 89 (83–94) | 1.30 | 66 (58–74) | 10.17** | 2.62 | 0.17 | |||
| xMAP (n = 4) | 89 (84–93) | 1.47 | 7.83 (p = 0.005) | 73 (67–79) | 25.23*** | 16.76 (p < 0.001) | 3.30 | 0.15 | |
| ELISA (n = 2) | 73 (59–84) | 2.78† | 91 (84–96) | 2.04 | 8.11 | 0.30 |
†p < 0.10; *p < 0.05; **p < 0.01; ***p < 0.001.
aNumber of estimations is n+1 due to results were reported separately for amnestic MCI and non-amnestic MCI patients in Vos et al. (2013).
Q, heterogeneity (Cochran Q-test); LR+, positive likelihood ratio; LR−, negative likelihood ratio; Global, global meta-analysis; n, number of studies included in the meta-analysis; MCI, mild cognitive impairment; AD, Alzheimer’s disease.
Subgroups meta-analyses: Identification of clinical situations with increased CSF biomarkers’ predictive performance
Table 1 shows the list of potential confounding factors defined a priori based on the literature. Due to the low variability across the studies, it was only possible to perform subgroups meta-analyses for the following factors: (1) MCI subtype (studies exclusively including amnestic MCI cases); (2) diagnosis at follow-up (studies including MCI patients that converted exclusively to AD); (3) follow-up length (as rough estimation of time to AD conversion: ≤24 months vs. > 24 months); (4) age (studies with mean age younger vs. older than 70 years); and (5) technology for CSF analysis (ELISA vs. xMAP). As controlled factors, all the studies included samples from specialized centers; lacked postmortem AD confirmation; used comparable diagnostic criteria for MCI; applied NINCDS-ADRDA criteria for AD diagnosis [except one study (Herukka et al., 2005)]; and included MCI cases with similar global cognitive status (i.e., MMSE). Information on MCI severity according to MRI rating, gender distribution, level of education, family history of AD, and APOE e4 status was scarce or absent.
Table 3 shows sensitivity and specificity values with 95% CI, inter-groups difference (QBET), heterogeneity, and likelihood ratios. Heterogeneity was significant in most of the subgroups meta-analyses. Noteworthy, CSF biomarkers’ predictive performance was optimal (>80%) in two clinically relevant situations, and heterogeneity was no longer significant: (1) P-tau alone had 84% sensitivity and 93% specificity for MCI cases converting to AD in ≤24 months, significantly different from 59% sensitivity (p = 0⋅01) and 71% specificity (p < 0⋅001) in studies with follow-up periods > 24 months; (2) Aβ42/p-tau ratio showed 81% sensitivity and 91% specificity in MCI patients younger than 70 years, significantly different from 66% specificity in MCI patients older than 70 years (p < 0⋅001).
Aβ42/p-tau ratio showed the best performance across the different subgroups meta-analyses. Sensitivity was slightly increased in studies including only amnestic MCI cases (heterogeneity no longer significant), MCI patients older than 70 years (heterogeneity no longer significant), and studies using ELISA. Aβ42/T-tau ratio yielded optimal sensitivity values, but suboptimal specificity. Results were not satisfactory for single CSF biomarkers, except for the remarkably good p-tau diagnostic performance commented above.
The analysis of positive likelihood ratios showed extremely high increase in the probability that the disease is present (LR+ = 12) for p-tau in MCI cases converting to AD in ≤24 months (Figure 2; Table 3). Moreover, there was a moderate increase in the probability that the disease is present (LR+ = 5–10) for the Aβ42/p-tau ratio in two situations: MCI patients younger than 70 years; and studies using ELISA technology. The analysis of negative likelihood ratios showed moderate decrease in the probability that the disease is present (LR– = 0.1–0.2) in several situations: p-tau and Aβ42/T-tau ratio in MCI cases converting to AD in ≤24 months; Aβ42/p-tau ratio in the global meta-analysis as well as in all the subgroups meta-analyses (except in MCI patients younger than 70 years and studies using ELISA technology).
Discussion
The two main findings in this study are that the Aβ42/p-tau ratio has high capacity to predict AD conversion in MCI patients younger than 70 years; and p-tau alone has high capacity to identify MCI cases converting to AD in ≤24 months. The analysis of likelihood ratios showed that, in both situations, a CSF test result indicating pathological values of Aβ42/p-tau or p-tau significantly increase the probability that the disease is present [in this context progression from MCI to AD or, in other words, MCI due to AD (Albert et al., 2011)].
Better predictive performance of the CSF biomarkers in younger MCI patients has been recently shown in a large multi-center study (Mattsson et al., 2012). A fact that may explain this result is that typical AD brain alterations increase with age in individuals without dementia (Green et al., 2000; Bennett et al., 2006), with about a third of cognitively normal elderly evidencing an AD-like pattern of CSF biomarker alterations (Ewers et al., 2007; Bouwman et al., 2009; Mattsson et al., 2009; Shaw et al., 2009). This also occurs in stable MCI cases, therefore obstructing specificity for AD and undermining CSF biomarkers’ performance.
Regarding time to AD conversion, Gaser et al. (2013) showed that the CSF biomarkers had generally better performance for MCI cases that converted to AD in <12 months as compared with MCI cases that converted to AD in >12 months. In the current meta-analysis, this finding is still valid when considering 24 months as threshold. However, Buchhave et al. (2012) reported that the combination of CSF biomarkers might not be recommendable at 60 months before AD conversion. The reason for this is that at that point, many MCI-C have normal T-tau levels but already pathological Aβ42 levels. In another study, the combination of CSF biomarkers with structural MRI showed >80% sensitivity during the first 18 months of follow-up, decreasing to 75% at 24 months, and to 68% at 36 months (Westman et al., 2012). Therefore, predictive value and biomarkers’ utility strongly depend on the stage of the disease and time to conversion. Aβ42 performs better than Tau 5–10 years before conversion to AD, but T-tau and p-tau have better predictive power 0–5 years before conversion (Buchhave et al., 2012). Other biomarkers such as those based on structural MRI have the highest performance the closer to AD diagnosis. Future research should thus pursue in combining the CSF biomarkers not only with each other but also with other biomarkers. Recent studies show an increase in the diagnostic efficiency of CSF biomarkers when combined with neuroimaging biomarkers (Vos et al., 2012; Westman et al., 2012; Choo et al., 2013; Galluzzi et al., 2013; Prestia et al., 2013; Shaffer et al., 2013). The development of new combinations and indexes may contribute not only to predict AD conversion but, importantly, to facilitate prediction of time to conversion, which is still challenging.
Importantly, the Aβ42/p-tau ratio showed satisfactory predictive performance in a heterogeneous group of MCI patients, which better represents the clinical reality (global meta-analysis). Moreover, it is noteworthy that the sensitivity was increased in two specific conditions: amnestic MCI patients and old MCI patients (>70 years). Recently, Vos et al. (2013) showed that the CSF biomarkers are more sensitive in amnestic MCI than in non-amnestic MCI patients. An explanation for this is MCI heterogeneity. Only 30–60% of the MCI patients are affected by prodromal AD, whereas the others may stem from a variety of different etiologies and pathologies (Ritchie et al., 2001; Petersen, 2004). The amnestic subtype is mainly associated with AD pathology. Nonetheless, vascular etiology has also been referred as explicative factor, especially in those cases with cognitive impairment encompassing other domains besides memory (Petersen, 2004; Winblad et al., 2004). On the contrary, the non-amnestic subtype may have higher likelihood of progressing to non-AD dementias such as dementia with Lewy bodies or frontotemporal lobar degeneration (Petersen, 2004; Winblad et al., 2004). In this regard, it seems reasonable that the CSF biomarkers validated for AD perform better in the amnestic MCI cases. In agreement with Vos et al. (2013), this may have implications for clinical implementation of the new revised criteria for MCI (Albert et al., 2011), given that both amnestic and non-amnestic subtypes are considered in this criteria as possible prodromal stages of AD-type dementia. Regarding the finding of better CSF biomarkers’ sensitivity in old MCI patients, this is in line with the discussion above about the age-related increase in AD-like CSF biomarker patterns. Mattsson et al., 2012 also found increased sensitivity in MCI patients older tan 65 years compared to MCI patients younger than 65 years. On the other hand, Aβ42/p-tau specificity was not increased in any of the subgroups meta-analyses except for young MCI patients (≤70 years), as discussed above. The three CSF biomarkers alone and the Aβ42/T-tau ratio showed suboptimal predictive power except p-tau for MCI cases converting to AD in ≤24 months, as already commented.
A better performance of the Aβ42/p-tau ratio over the other CSF biomarkers has been reported in previous studies on MCI prediction (Hansson et al., 2006; Mattsson et al., 2009; Buchhave et al., 2012; Parnetti et al., 2012; Roe et al., 2013) and differential diagnosis between AD and other dementias (Maddalena et al., 2003; Jong et al., 2006; Holtzman, 2011). This finding is likely due to this ratio reflects two aspects of AD pathology, i.e., plaques (Aβ42), and neurodegeneration (tau). Moreover, p-tau usually shows better performance than T-tau (Mitchell, 2009; Bloudek et al., 2011; van Harten et al., 2011), probably because p-tau is not only a marker of axonal damage and neuronal degeneration, as T-tau, but it is more closely related to AD pathophysiology and the formation of neurofibrillary tangles (Anoop et al., 2010; Holtzman, 2011). In addition, CSF p-tau concentrations in dementia with Lewy bodies, frontotemporal lobar degeneration, and vascular dementia have been referred to be more comparable to concentrations in controls than to concentrations in AD patients (van Harten et al., 2011). This positively affects prediction of MCI due to AD.
Regarding the clinical value of the CSF biomarkers, results for negative likelihood ratios were normally better than results for positive likelihood ratios. This means that the CSF biomarkers are more useful to identify MCI patients that remain stable at follow-up (MCI-S) than to rule-in MCI patients that will progress to AD or dementia (MCI-C). This finding supports the consideration made in the new MCI diagnostic criteria in relation to biomarkers profile suggesting that the MCI syndrome is unlikely to be due to AD (point 3.6.4. in Albert et al. (2011): “the definitive absence of evidence of either Aβ deposition or neuronal injury strongly suggests that the MCI syndrome is not due to AD”). Our study shows that a normal result in the Aβ42/p-tau ratio has a moderate decrease in the probability that the disease is present (conversion to AD). This is true in all the situations evaluated in the different subgroups meta-analyses, although we could not confirm this for MCI cases converting to AD in ≤24 months because only one study was available (Monge-Argilés et al., 2011). This single study reported 86% sensitivity and 75% specificity (Monge-Argilés et al., 2011). Therefore, it is quite probable that a meta-analysis of the Aβ42/p-tau ratio in MCI cases converting to AD in ≤24 months would provide a satisfactory negative likelihood ratio, given that both p-tau and the Aβ42/T-tau ratio showed optimal results. On the other hand, positive likelihood ratios were normally within the range of a small increase in the probability that the disease is present. The only two situations where conclusive increase was achieved are those already commented above (Aβ42/p-tau ratio in young MCI patients and p-tau in MCI cases converting to AD in ≤24 months). This finding may have implications for the consideration made in the new MCI diagnostic criteria regarding biomarkers pattern indicating a high likelihood that the MCI syndrome is due to AD [point 3.6.1. in Albert et al., 2011]. In particular, young MCI patients with positive biomarkers of Aβ accumulation and neuronal injury seems to have increased risk to decline or progress to dementia due to AD in relatively short periods.
To determine in which specific situations the CSF biomarkers provide satisfactory predictive performance is of great relevance. In this study, some of those situations have been identified. However, despite these positive results, we acknowledge that much additional work needs to be done to validate the application of the CSF biomarkers as they are proposed in the new revised criteria for MCI (Albert et al., 2011). The main limitation for extending the use of the CSF biomarkers to the clinical routine is the difficulty to establish appropriate cut-offs. There is a big variability in the cut-offs applied across the different studies. This is in part related to differences in methodological aspects as well as absence of technical standardization. In this meta-analysis, two aspects related to variability in CSF methods were considered. First, we tried to analyze the influence of different cut-offs for the CSF biomarkers. Due to the great variability found it was not possible to group the studies in order to perform specific subgroups meta-analyses (Table 1). Second, the technology for the CSF analysis applied was also considered as potential confounding factor. Results showed that sensitivity and specificity values differed depending on whether xMAP (Luminex, Austin, US) or ELISA (Innogenetics, Ghent, Belgium) technology was used. A clear pattern was not found however. Therefore, future research is mandatory to hopefully ascertain universal cut-offs values for the CSF biomarkers. Several studies indicate that the standardization of laboratory procedures could contribute to reduce variability in the results (Hansson et al., 2006; Fagan et al., 2011; Mattsson et al., 2011).
Therefore, standardization of methodological aspects is expected to increase the clinical utility of the CSF biomarkers. In this meta-analysis, we demonstrate that several confounding factors are another source of variability in published diagnostic/predictive performance and cut-offs. We show that CSF biomarkers’ performance can be improved and heterogeneity reduced by carefully considering these confounding factors. In this regard, future studies should be addressed to explain how these factors influence the diagnostic and predictive performance of the CSF biomarkers. This need is reinforced by the fact that we could not evaluate 13 of the 18 identified potentially confounding factors given the lack of studies directly addressing these aspects. A related limitation is the scarce number of studies available for some of the analyses. This causes that certain subgroups meta-analyses could be influenced by some of the other confounding factors. In order to evaluate this, an analysis of coincident studies across factors was performed. Table S5 in Supplementary Material shows that most of the subgroups were rather independent from each other. However, for p-tau and Aβ42/p-tau, studies including follow-up periods >24 months coincided with studies with AD diagnosis at follow-up; and for p-tau and Aβ42/T-tau, studies using xMAP technology coincided with studies including MCI cases older than 70 years (and vice versa only for Aβ42/T-tau: ELISA technology with studies including MCI cases younger than 70 years). Another limitation is that systematic reviews and meta-analyses are essential tools for summarizing evidence accurately and reliably, but might be susceptible of bias if not properly conducted. Following PRISMA recommendations (Liberati et al., 2009; Moher et al., 2010), several strategies were carefully considered in this study to reduce risk of bias related to publication, data availability, and reviewer selection. Evidence was rigorously reviewed and literature was supplemented with manual query of relevant studies in order to minimize both publication and reviewer selection bias. Selected studies were carefully examined for clues suggesting that there may be missing results or data. Moreover, assessments were completed independently by more than one reviewer and consensus was required. Regarding the included studies, QUADAS-2 was applied to evaluate risk of bias and results applicability. It must be noticed that “domain 1” indicated high probability of patient selection bias in six of the included studies, related to inclusion of not completely consecutive or random samples, and not perfect avoidance of inappropriate exclusions. In particular, patients were normally selected from specialized centers on the basis of availability of CSF data, a procedure not generally performed in all incoming patients. This fact, may have certain impact in the applicability of the results, although these six studies scored rather well in the other three domains, indicating that the index test, the standard test, and flow and timing are not compromised. Another drawback is that the follow-up period was used as rough measure of time to AD conversion. Therefore, although it is clear that MCI-C cases in studies with follow-up ≤24 months converted to AD in less than 24 months, it is possible that some MCI-C cases in studies with follow-up >24 months also converted to AD before the threshold of 24 months. Finally, sensitivity and specificity values above 80% were considered indicative of optimal predictive performance according to international recommendations (The Ronald and Nancy Reagan Research Institute of the Alzheimer’s Association and the national Institute on Aging working Group, 1998). Higher levels are not easy to be achieved given that analyses are derived from clinically diagnosed AD cases in which the diagnostic accuracy already approximates 85% when validated by the standard pathologic diagnosis at autopsy (Mendez et al., 1992; Victoroff et al., 1995). None of the studies included in this meta-analysis performed postmortem AD confirmation. It is thus necessary to test CSF biomarkers’ predictive performance in pathologically confirmed AD patients.
In conclusion, this study contributes to define several situations in which the CSF biomarkers seem to be clinically useful for predicting conversion from MCI to AD. In particular, a baseline CSF test result indicating Aβ42/p-tau pathological values in MCI patients younger than 70 years has a moderate increase in the likelihood of developing AD. Moreover, a baseline CSF test result indicating pathological levels of p-tau increases the likelihood of developing AD within the next 24 months. To move forward in the knowledge about how different confounding factors influence the diagnostic and predictive performance of the CSF biomarkers is of utmost importance. Such knowledge will help the elaboration of a map of situations where the CSF biomarkers are useful, so that clinicians and researchers know when the new diagnostic criteria for MCI will be successful or otherwise prone to mistakes. This will be crucial when new disease-modifying treatments are available in the near future. Early prediction of MCI conversion to AD is expected to maximize treatment benefit if applied to the right people and before neuronal degeneration is too widespread and patients are already demented. In addition, this has ethical benefits because it is preferred not to treat patients with low risk of AD in trials that could cause side effects. Finally, this will also be important to enrich the samples with pure AD cases, both for research and clinical trials.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at http://www.frontiersin.org/Journal/10.3389/fnagi.2014.00287/abstract
Acknowledgments
The authors would like to thank the Spanish Ministry of Health, Social Services, and Equality; the Swedish Brain Power; the Strategic Research Programme in Neuroscience at Karolinska Institutet (StratNeuro); the Swedish Medical Society; and the regional agreement on medical training, clinical research (ALF) between Stockholm County Council and Karolinska Institutet.
References
- Albert M. S., DeKosky S. T., Dickson D., Dubois B., Feldman H. H., Fox N. C., et al. (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 270–279. 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anoop A., Singh P. K., Jacob R. S., Maji S. K. (2010). CSF biomarkers for Alzheimer’s disease diagnosis. Int. J. Alzheimers Dis. 2010, 606802. 10.4061/2010/606802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artero S., Petersen R. C., Touchon J., Ritchie K. (2006). Revised criteria for mild cognitive impairment: validation within a longitudinal population study. Dement. Geriatr. Cogn. Disord. 22, 465–470. 10.1159/000096287 [DOI] [PubMed] [Google Scholar]
- Bennett D. A., Schneider J. A., Arvanitakis Z., Kelly J. F., Aggarwal N. T., Shah R. C., et al. (2006). Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 66, 1837–1844. 10.1212/01.wnl.0000219668.47116.e6 [DOI] [PubMed] [Google Scholar]
- Bloudek L. M., Spackman D. E., Blankenburg M., Sullivan S. D. (2011). Review and meta-analysis of biomarkers and diagnostic imaging in Alzheimer’s disease. JAD 26, 627–645. 10.3233/JAD-2011-110458 [DOI] [PubMed] [Google Scholar]
- Bouwman F. H., Schoonenboom N. S., Verwey N. A., van Elk E. J., Kok A., Blankenstein M. A., et al. (2009). CSF biomarker levels in early and late onset Alzheimer’s disease. Neurobiol. Aging 30, 1895–1901. 10.1016/j.neurobiolaging.2008.02.007 [DOI] [PubMed] [Google Scholar]
- Bouwman F. H., Schoonenboom S. N., van der Flier W. M., van Elk E. J., Kok A., Barkhof F., et al. (2007). CSF biomarkers and medial temporal lobe atrophy predict dementia in mild cognitive impairment. Neurobiol. Aging 28, 1070–1074. 10.1016/j.neurobiolaging.2006.05.006 [DOI] [PubMed] [Google Scholar]
- Brys M., Pirraglia E., Rich K., Rolstad S., Mosconi L., Switalski R., et al. (2009). Prediction and longitudinal study of CSF biomarkers in mild cognitive impairment. Neurobiol. Aging 30, 682–690. 10.1016/j.neurobiolaging.2007.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchhave P., Minthon L., Zetterberg H., Wallin A. K., Blennow K., Hansson O. (2012). Cerebrospinal fluid levels of β-amyloid 1-42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch. Gen. Psychiatry 69, 98–106. 10.1001/archgenpsychiatry.2011.155 [DOI] [PubMed] [Google Scholar]
- Choo I. H., Ni R., Schöll M., Wall A., Almkvist O., Nordberg A. (2013). Combination of (18)F-FDG PET and cerebrospinal fluid biomarkers as a better predictor of the progression to Alzheimer’s disease in mild cognitive impairment patients. JAD 33, 929–939. 10.3233/JAD-2012-121489 [DOI] [PubMed] [Google Scholar]
- Deeks J., Altman D., Bradburn M. (2001). “Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis,” in Systematic Reviews in Health Care: Meta-analysis in Context, 2nd Edn, eds Egger M., Davey Smith G., Altman D. G. (London: BMJ Publ. Gr; ), 285–312 [Google Scholar]
- DerSimonian R., Laird N. (1986). Meta-analysis in clinical trials. Control. Clin. Trials 7, 177–188. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- Diniz B. S. O., Pinto Júnior J. A., Forlenza O. V. (2008). Do CSF total tau, phosphorylated tau, and beta-amyloid 42 help to predict progression of mild cognitive impairment to Alzheimer’s disease? A systematic review and meta-analysis of the literature. World J. Biol. Psychiatry 9, 172–182. 10.1080/15622970701535502 [DOI] [PubMed] [Google Scholar]
- Eckerström C., Andreasson U., Olsson E., Rolstad S., Blennow K., Zetterberg H., et al. (2010). Combination of hippocampal volume and cerebrospinal fluid biomarkers improves predictive value in mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 29, 294–300. 10.1159/000289814 [DOI] [PubMed] [Google Scholar]
- Ewers M., Buerger K., Teipel S. J., Scheltens P., Schröder J., Zinkowski R. P., et al. (2007). Multicenter assessment of CSF-phosphorylated tau for the prediction of conversion of MCI. Neurology 69, 2205–2212. 10.1212/01.wnl.0000286944.22262.ff [DOI] [PubMed] [Google Scholar]
- Ewers M., Walsh C., Trojanowski J. Q., Shaw L. M., Petersen R. C., Jack C. R., Jr., et al. (2012). Prediction of conversion from mild cognitive impairment to Alzheimer’s disease dementia based upon biomarkers and neuropsychological test performance. Neurobiol. Aging 33, 1203–1214. 10.1016/j.neurobiolaging.2010.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan A. M., Shaw L. M., Xiong C., Vanderstichele H., Mintun M. A., Trojanowski J. Q., et al. (2011). Comparison of analytical platforms for cerebrospinal fluid measures of β-amyloid 1-42, total tau, and p-tau181 for identifying Alzheimer disease amyloid plaque pathology. Arch. Neurol. 68, 1137–1144. 10.1001/archneurol.2011.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira D., Perestelo-Pérez L., Westman E., Wahlund L.-O., Sarría A., Serrano-Aguilar P. (2014). Meta-review of CSF core biomarkers in Alzheimer’s disease: the state-of-the-art after the new revised diagnostic criteria. Front. Aging Neurosci. 6:1–24. 10.3389/fnagi.2014.00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi S., Geroldi C., Amicucci G., Bocchio-Chiavetto L., Bonetti M., Bonvicini C., et al. (2013). Supporting evidence for using biomarkers in the diagnosis of MCI due to AD. J. Neurol. 260, 640–650. 10.1007/s00415-012-6694-0 [DOI] [PubMed] [Google Scholar]
- Gaser C., Franke K., Klöppel S., Koutsouleris N., Sauer H. (2013). Brain AGE in mild cognitive impaired patients: predicting the conversion to Alzheimer’s disease. PLoS ONE 8:e67346. 10.1371/journal.pone.0067346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. S., Kaye J. A., Ball M. J. (2000). The Oregon Brain Aging Study: Neuropathology accompanying healthy aging in the oldest old. Neurology 54, 105–113. 10.1212/WNL.54.1.105 [DOI] [PubMed] [Google Scholar]
- Hampel H., Teipel S. J., Fuchsberger T., Andreasen N., Wiltfang J., Otto M., et al. (2004). Value of CSF ß-amyloid1–42 and tau as predictors of Alzheimer’s disease in patients with mild cognitive impairment. Mol. Psychiatry 9, 705–710. 10.1038/sj.mp.4001473 [DOI] [PubMed] [Google Scholar]
- Hansson O., Zetterberg H., Buchhave P., Andreasson U., Londos E., Minthon L., et al. (2007). Prediction of Alzheimer’s disease using the CSF Abeta42/Abeta40 ratio in patients with mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 23, 316–320. 10.1159/000100926 [DOI] [PubMed] [Google Scholar]
- Hansson O., Zetterberg H., Buchhave P., Londos E., Blennow K., Minthon L. (2006). Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 5, 228–234. 10.1016/S1474-4422(06)70355-6 [DOI] [PubMed] [Google Scholar]
- Hardy R., Thompson S. (1998). Detecting and describing heterogeneity in meta-analysis. Stat. Med. 17, 841–856. [DOI] [PubMed] [Google Scholar]
- Hertze J., Minthon L., Zetterberg H., Vanmechelen E., Blennow K., Hansson O. (2010). Evaluation of CSF biomarkers as predictors of Alzheimer’s disease: a clinical follow-up study of 4.7 years. JAD 21, 1119–1128. 10.3233/JAD-2010-100207 [DOI] [PubMed] [Google Scholar]
- Herukka S.-K., Hallikainen M., Soininen H., Pirttilä T. C. S. F. (2005). AB42 and tau or phosphorylated tau and prediction of progressive mild cognitive impairment. Neurology 64, 1294–1297. 10.1212/01.WNL.0000156914.16988.56 [DOI] [PubMed] [Google Scholar]
- Holtzman D. M. (2011). CSF biomarkers for Alzheimer’s disease: current utility and potential future use. Neurobiol. Aging 32, S4–S9. 10.1016/j.neurobiolaging.2011.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulstaert F., Blennow K., Ivanoiu A., Schoonderwaldt H.C., Riemen-Schneider M., De Deyn P. P., et al. (1999). Improved discrimination of AD patients using beta-amyloid(1–42) and Tau levels in CSF. Neurology 52, 1555–1562. 10.1212/WNL.52.8.1555 [DOI] [PubMed] [Google Scholar]
- Jong D., Jansen W. M. M., Kremer B. P. H., Verbeek M. M. (2006). Cerebrospinal fluid amyloid beta42/phosphorylated Tau ratio discriminates between Alzheimer’s disease and vascular dementia. J. Gerontol. 61, 755–758. 10.1093/gerona/61.7.755 [DOI] [PubMed] [Google Scholar]
- Liberati A., Altman D. G., Tetzlaff J., Mulrow C., Gøtzsche P. C., Ioannidis J. P., et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann. Intern. Med. 151, 65–94. 10.7326/0003-4819-151-4-200908180-00136 [DOI] [PubMed] [Google Scholar]
- Maddalena A., Papassotiropoulos A., Müller-Tillmanns B., Jung H. H., Hegi T., Nitsch R. M., et al. (2003). Biochemical diagnosis of Alzheimer disease by measuring the cerebrospinal fluid ratio of phosphorylated tau protein to beta-amyloid peptide 42. Arch. Neurol. 60, 1202–1206. 10.1001/archneur.60.9.1202 [DOI] [PubMed] [Google Scholar]
- Mattsson N., Andreasson U., Persson S., Arai H., Batish S. D., Bernardini S., et al. (2011). The Alzheimer’s association external quality control program for cerebrospinal fluid biomarkers. Alzheimers Dement. 7, 386–395. 10.1016/j.jalz.2011.05.2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson N., Rosén E., Hansson O., Andreasen N., Parnetti L., Jonsson M., et al. (2012). Age and diagnostic performance of Alzheimer disease CSF biomarkers. Neurology 78, 468–476. 10.1212/WNL.0b013e3182477eed [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson N., Zetterberg H., Hansson O., Andreasen N., Parnetti L., Jonsson M., et al. (2009). CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA 302, 385–393. 10.1001/jama.2009.1064 [DOI] [PubMed] [Google Scholar]
- Mendez M., Mastri A., Sung J., Frey W. (1992). Clinically diagnosed Alzheimer disease: neuropathologic findings in 650 cases. Alzheimer Dis. Assoc. Disord. 6, 35–43. 10.1097/00002093-199205000-00004 [DOI] [PubMed] [Google Scholar]
- Mitchell A. J. C. S. F. (2009). Phosphorylated tau in the diagnosis and prognosis of mild cognitive impairment and Alzheimer’s disease – a meta-analysis of 51 studies. J Neurol. Neurosurg. Psychiatr. 80, 966–975. 10.1136/jnnp.2008.167791 [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D., the PRISMA Group . (2010). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. 8, 336–341. 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- Monge-Argilés J. A., Muñoz-Ruiz C., Pampliega-Pérez A., Gómez-López M. J., Sánchez-Payá J., Rodríguez Borja E., et al. (2011). Biomarkers of Alzheimer’s disease in the cerebrospinal fluid of Spanish patients with mild cognitive impairment. Neurochem. Res. 36, 986–993. 10.1007/s11064-011-0438-x [DOI] [PubMed] [Google Scholar]
- Monge-Argilés J. A., Sánchez-Payá J., Muñoz-Ruiz C., Pampliega-Pérez A., Montoya-Gutiérrez J., Leiva-Santana C. (2010). Biomarkers in the cerebrospinal fluid of patients with mild cognitive impairment: a meta-analysis of their predictive capacity for the diagnosis of Alzheimer’s disease. Rev. Neurol. 50, 193–200. [PubMed] [Google Scholar]
- Parnetti L., Chiasserini D., Eusebi P., Giannandrea D., Bellomo G., De Carlo C., et al. (2012). Performance of aβ1-40, aβ1-42, total tau, and phosphorylated tau as predictors of dementia in a cohort of patients with mild cognitive impairment. JAD 29, 229–238. 10.3233/JAD-2011-111349 [DOI] [PubMed] [Google Scholar]
- Parnetti L., Lanari A., Silvestrelli G., Saggese E., Reboldi P. (2006). Diagnosing prodromal Alzheimer’s disease: role of CSF biochemical markers. Mech. Ageing Dev. 127, 129–132. 10.1016/j.mad.2005.09.022 [DOI] [PubMed] [Google Scholar]
- Petersen R. C. (2004). Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 256, 183–194. 10.1111/j.1365-2796.2004.01388.x [DOI] [PubMed] [Google Scholar]
- Petersen R. C., Smith G. E., Waring S. C., Ivnik R. J., Tangalos E. G., Kokmen E. (1999). Mild cognitive impairment: clinical characterization and outcome. Arch. Neurol. 56, 303–308. 10.1001/archneur.56.3.303 [DOI] [PubMed] [Google Scholar]
- Petersen R. C., Doody R., Kurz A., Mohs R. C., Morris J. C., Rabins P. V., et al. (2001). Current concepts in mild cognitive impairment. Arch. Neurol. 58, 1985–1992. 10.1001/archneur.58.12.1985 [DOI] [PubMed] [Google Scholar]
- Prestia A., Caroli A., van der Flier W. M., Ossenkoppele R., Van Berckel B., Barkhof F., et al. (2013). Prediction of dementia in MCI patients based on core diagnostic markers for Alzheimer disease. Neurology 80, 1048–1056. 10.1212/WNL.0b013e3182872830 [DOI] [PubMed] [Google Scholar]
- Qizilbash N. (ed.) (2002). Evidenced-based Dementia Practice. Oxford: Blackwell Publishing [Google Scholar]
- Ritchie K., Artero S., Touchon J. (2001). Classification criteria for mild cognitive impairment: a population-based validation study. Neurology 56, 37–42. 10.1212/WNL.56.1.37 [DOI] [PubMed] [Google Scholar]
- Roe C. M., Fagan A. M., Grant E. A., Hassenstab J., Moulder K. L., Maue Dreyfus D., et al. (2013). Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology 80, 1784–1791. 10.1212/WNL.0b013e3182918ca6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer J. L., Petrella J. R., Sheldon F. C., Choudhury K. R., Calhoun V. D., Coleman R. E., et al. (2013). Predicting cognitive decline in subjects at risk for Alzheimer disease by using combined cerebrospinal fluid, MR imaging, and PET biomarkers. Radiology 266, 583–591. 10.1148/radiol.12120010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw L. M., Vanderstichele H., Knapik-Czajka M., Clark C. M., Aisen P. S., Petersen R. C., et al. (2009). Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann. Neurol. 65, 403–413. 10.1002/ana.21610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren M., Vanderstichele H., Agren H., Zachrisson O., Edsbagge M., Wikkelso C., et al. (2001). Tau and Ab42 in cerebrospinal fluid from healthy adults 21–93 years of age: establishment of reference values. Clin. Chem. 47, 1776–1781 [PubMed] [Google Scholar]
- The Ronald and Nancy Reagan Research Institute of the Alzheimer’s Association and the national Institute on Aging working Group. (1998). Consensus report of the working group on: “molecular and biochemical markers of Alzheimer’s disease. Neurobiol. Aging 19, 109–116. [PubMed] [Google Scholar]
- Toledo J. B., Korff A., Shaw L. M., Trojanowski J. Q., Zhang J. C. S. F. (2013). α-Synuclein improves diagnostic and prognostic performance of CSF tau and Aβ in Alzheimer’s disease. Acta Neuropathol. 126, 683–697. 10.1007/s00401-013-1148-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Harten A. C., Kester M. I., Visser P. J., Blankenstein M. A., Pijnenburg Y. A., van der Flier W. M., et al. (2011). Tau and p-tau as CSF biomarkers in dementia: a meta-analysis. Clin. Chem. Lab. Med. 49, 353–366. 10.1515/CCLM.2011.086 [DOI] [PubMed] [Google Scholar]
- Victoroff J., Mack W., Lyness S., Chui H. (1995). Multicenter Clinicopathological Correlation in Dementia. Am. J. Psychiatry 152, 1476–1484. [DOI] [PubMed] [Google Scholar]
- Vos S., van Rossum I., Burns L., Knol D., Scheltens P., Soininen H., et al. (2012). Test sequence of CSF and MRI biomarkers for prediction of AD in subjects with MCI. Neurobiol. Aging 33, 2272–2281. 10.1016/j.neurobiolaging.2011.12.017 [DOI] [PubMed] [Google Scholar]
- Vos S. J., van Rossum I. A., Verhey F., Knol D. L., Soininen H., Wahlund L. O., et al. (2013). Prediction of Alzheimer disease in subjects with amnestic and nonamnestic MCI. Neurology 80, 1124–1132. 10.1212/WNL.0b013e318288690c [DOI] [PubMed] [Google Scholar]
- Westman E., Muehlboeck J.-S., Simmons A. (2012). Combining MRI and CSF measures for classification of Alzheimer’s disease and prediction of mild cognitive impairment conversion. Neuroimage 62, 229–238. 10.1016/j.neuroimage.2012.04.056 [DOI] [PubMed] [Google Scholar]
- Whiting P., Rutjes A., Westwood M., Mallett S., Deeks J. J., Reitsma J. B., et al. (2011). QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 155, 529–536. 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- Winblad B., Palmer K., Kivipelto M., Jelic V., Fratiglioni L., Wahlund L. O., et al. (2004). Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J. Intern. Med. 256, 240–246. 10.1111/j.1365-2796.2004.01380.x [DOI] [PubMed] [Google Scholar]
- Zamora J., Abraira V., Muriel A., Khan K., Coomarasamy A. (2006). Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med. Res. Methodol. 6:31. 10.1186/1471-2288-6-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterberg H., Wahlund L. O., Blennow K. (2003). Cerebrospinal fluid markers for prediction of Alzheimer’s disease. Neurosci. Lett. 352, 67–69. 10.1016/j.neulet.2003.08.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.