Abstract
Background
Drug use disorders (DUD) have been theorized to share sources of risk variation with other consummatory behaviors. We hypothesized that common mechanisms exist for familial risk for DUD, physiological maturation and nutritional status in girls. Whereas body fat content must exceed a threshold to enable adrenarche and gonadarche, nutritional status may also be a behavior risk indicator. Impaired psychological self-regulation associated with DUD risk may manifest in early overeating, which could in turn accelerate reproductive maturation, resulting in a greater likelihood of affiliation with deviant/older peers and drug use.
Method
The sample consisted of families ascertained through the father who either had (N=95) or did not have (N=130) a DUD, and who had a 10–12 year old daughter and her mother available for study. Correlation, survival and path analyses of three consecutive assessments evaluated the relationships between parental DUD (number of affected parents, NAP), nutritional status (NS, subscapular skinfold measurements and body mass index), sexual maturation (Tanner stage), peer delinquency, and the daughter’s lifetime DUD diagnosis.
Results
NAP was positively related to the girls’ nutritional status. Longitudinal path analysis indicated mediation of the relationship between NAP and peer delinquency by sexual maturation. The relationship between NAP and sexual maturation is mediated by NS. The effect of sexual maturation at age ~11 on the girls’ DUD risk is mediated by peer delinquency.
Conclusion
The data are consistent with mediation of intergenerational transmission of DUD risk in females by elevated nutrition, leading to accelerated maturation, and affiliation with deviant peers.
Keywords: Drug dependence, Gender, Females, Genetics, Eating behavior, Obesity
1. INTRODUCTION
Drug addictions (drug use disorders, DUD) have been theorized to share mechanisms of risk variation with other consummatory behaviors (Vanyukov et al., 2012). These mechanisms span all levels of biological organization and may differ between sexes. It has been previously shown that parental addiction to illicit drugs is related to the rate of sexual maturation in male offspring, which in turn is associated with their disruptive behavior, peer delinquency, and risk for dud (Kirillova et al., 2001, 2008). Extending this line of research, we hypothesized that common mechanisms also exist for familial risk for dud, physiological maturation and nutritional status in girls. Disordered eating behavior and the risk for drug addiction are known to be phenotypically correlated. For example, bulimia, which is particularly prevalent among females, is frequently comorbid with substance use disorders, and the relationship extends to positive genetic correlations between symptoms of bulimia and addictions (Baker et al., 2010; Trace et al., 2013). Moreover, family history of addiction – alcoholism in parents, which is frequently comorbid with other substance use disorders, – has been found to be related to obesity in their offspring (Grucza et al., 2010). Whereas body fat content in females must exceed a certain level to enable adrenarche and gonadarche, nutritional status that does not necessarily reach the diagnostic threshold of an eating disorder may also be an indicator of behavioral risk and contribute to its further increase. In particular, impaired psychological self-regulation, likely to a degree underlying dud risk, may manifest in early-onset overeating and accumulation of fat, which could in turn increase the rate of reproductive maturation, resulting in a greater likelihood of affiliation with deviant/older peers and drug use. Accordingly, nutritional status, as reflected in adiposity, may mediate the relationship between parental dud and, developmentally, daughters’ physiological maturation, peer affiliation and dud. In this study, we tested this system of relationships, illustrated in figure 1.
Figure 1.
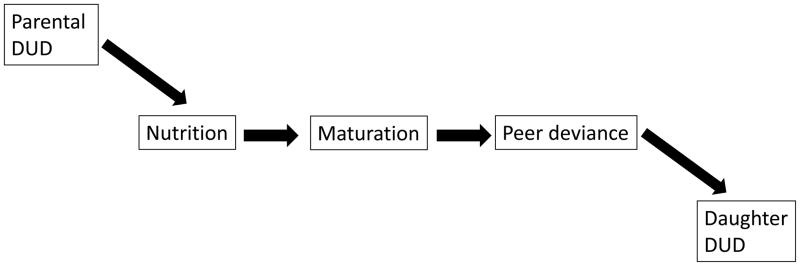
The hypothesized pathway of addiction liability transmission.
2. METHOD
2.1. Participants
The sample, part of a longitudinal family/high-risk study (Center for Education and Drug Abuse Research, CEDAR; Tarter and Vanyukov, 2001), consisted of 225 families ascertained through the father who either had (N=95) or did not have (N=130) a lifetime DSM-III-R diagnosis of drug use disorder (DUD; abuse and/or dependence) related to an illicit drug(s) (alcohol dependence only did not qualify), and who had a ~10–12 year old biological daughter (index child, IC) and her biological mother available for study. Control fathers had no Axis I or II psychiatric disorder. A DSM-III-R diagnosis was used because DSM-IV was introduced after this study started. The girls were self-identified as European-American (71%), African-American (27%), or “other” (2%). Recruitment was via newspaper advertisements, social service agencies, substance abuse treatment programs and various other media. The family was excluded if the father had a history of neurological disorders, schizophrenia or uncorrectable sensory incapacity, or the IC had a history of neurological injury requiring hospitalization, IQ less than 80, chronic physical disability, uncorrectable sensory incapacity or psychosis. The girls are prospectively assessed at regular intervals. For all variables, except the girls’ DUD diagnosis, data from the first three assessment visits were used in this study. In order to capture the maximal information on the main outcome variable, lifetime DUD diagnosis by the last available visit for the individual was used. The girls’ age statistics are presented in Table 1. Because of protracted recruitment, the diminishing sample sizes at the follow-up visits do not per se indicate attrition. Prior analysis in the sample suggests no systematic attrition bias (Kirisci et al., 2012).
Table 1.
Ages at assessments
| Statistics | Visits
|
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| N | 225 | 181 | 170 | 165 | 117 | 57 |
| Mean | 11.5 | 13.7 | 16.2 | 19.0 | 22.0 | 24.8 |
| SE | .06 | .06 | .03 | .03 | .03 | .03 |
| Median | 11.6 | 13.8 | 16.1 | 18.8 | 21.8 | 24.7 |
| Range | 9.6–13.0 | 11.9–15.2 | 15.6–17.4 | 18.2–20.0 | 21.6–23.0 | 24.6–26.0 |
This study was reviewed and approved by the Institutional Review Board of the University of Pittsburgh, Adult participants provided written informed consent prior to implementing the research protocol. Children provided assent and, upon reaching age 18, informed consent.
2.2. Instrumentation
2.2.1. Psychiatric diagnosis
An expanded version of the Structured Clinical Interview for DSM-III-R-outpatient version (SCID-OP; Spitzer et al., 1987) was administered by experienced research associates to obtain psychiatric diagnoses for adults. The expanded SCID evaluates current episode (past 6 months) and worst past episode of psychopathology (before the past 6 months). The children were assessed with the Kiddie—Schedule for Affective Disorders and Schizophrenia for School Age Children—Epidemiological Version (K-SADS-E; Orvaschel and Puig-Antich, 1987), a semistructured diagnostic interview for children and adolescents aged 6–17, covering current and lifetime psychopathology. The standard procedure was to first interview the mother about the psychiatric status of the child. During the subsequent interview of the child, the interviewer attempts to resolve any discrepancies between parent and child in case of disagreement. A summary score is obtained based on positive ratings of either informant. The diagnoses are finalized according to the best estimate procedure (Kosten and Rounsaville, 1992) at a consensus conference chaired by a psychiatrist. The girls’ lifetime DSM-III-R diagnosis of a drug use disorder (abuse/dependence) related to an illicit substance (DUD) was the target outcome variable.
2.2.2. Family addiction load
Because there is no reason to believe that the paternal DUD diagnosis (the ascertainment criterion) would account for the entirety of potential parental effects on the daughters’ DUD liability phenotype (in fact, from data on alcoholism, it is maternal contribution that might have a greater effect for daughters (Bohman et al., 1981), and to keep the analytic approach consistent with prior research on boys (Kirillova et al., 2001, 2008), we constructed a measure of parental DUD load as the sum of parental diagnoses, varying from 0 to 2 (the number of affected parents, NAP). This measure was used to analyze the relationship between parental DUD and phenotypic development (sexual maturation; the rate of disorder development) in offspring. In 54.5% of the couples none of the parents had a DUD (NAP=0), in 29.5% one parent was affected (NAP=1), and in 15.9% both parents were affected (NAP=2).
2.2.3. Sexual maturation
Sexual maturation in girls was assessed at visits 1–3 by a trained registered nurse using Tanner staging (TS; Marshall and Tanner, 1970), based on pubic hair development. To remove the confounding effect of age at a visit as a source of covariation between variables at that assessment, it was regressed out of the visit-specific Tanner stage (the residuals from regression on age at a visit were used in the analysis).
2.2.4. Nutritional status (adiposity)
Nutritional status (NS) was evaluated by the caliper subscapular skinfold (SSF) measurement as well as by the body mass index (BMI). The measurements were taken by a trained registered nurse. The mean of three SSF measurements was recorded. The skewed distributions of SSF measurements were log-transformed for analysis. The study focused on the dimensional measure of nutritional status rather than the diagnosis of obesity. The diagnosis is based on an arbitrary threshold and, statistically, may result in information loss. Therefore, for analyses, we did not dichotomize the BMI distribution by the obesity categorization. In this sample, according to the sex- and age-specific BMI thresholds of 24.6, 26.9 and 29.0 (CDC, 2001), the proportions of girls who qualified as obese were 13.8, 19.8, and 16.9% at the three visits, respectively. These frequencies are consistent with age-specific populational estimates (Ogden et al., 2012).
2.2.5. Peer deviance
Peer deviance (PD) was evaluated at visits 1–3 using the Peer Delinquency Scale (Loeber, 1989), a Likert-type instrument reflecting the child’s assessment of her friends’ involvement in activities of various severity, from skipping school to armed robbery. The skewed score distribution was log-transformed for analysis.
2.3. Statistical analysis
Correlation, logistic regression, and survival (Cox proportional hazard regression) analyses were used to evaluate the relationships between parental DUD, nutritional status, sexual maturation, peer delinquency, and the daughter’s lifetime DUD diagnosis. Path analysis was then performed to test the overall model. Analyses were conducted using SPSS and Amos software for Windows (Version 21), and G*Power 3.1 (Faul et al., 2009). Bayesian structural equation modeling (SEM) implemented in Amos was used for path analysis with the binary dependent variable of the DUD diagnosis. Bayesian estimation method has been shown to be more robust than the maximum likelihood approach in parameter estimation in relatively small samples by using proper or improper prior distributions (Lee and Song, 2004). The associations between the longitudinally tracked variables were tested using autoregressive models. Autoregressive parameters were tested for repeated measures (e.g., BMI at consecutive visits), and cross-lagged parameters for the variables representing different domains, (e.g., BMI and peer delinquency). Posterior predictive p-values are used in Bayesian SEM for model fit evaluation (Lee and Song, 2003). P-values close to 0.5 indicate good fit.
3. RESULTS
3.1. DUD familiality
In this sample, ascertained through fathers, 42% of whom had an illicit drug-related addiction, 20% of mothers (43 out of the 220 with available diagnoses) were also affected with DUD. The mate phenotypic similarity amounts to a tetrachoric correlation of 0.66 (SE=0.08; 95% confidence interval: 0.50–0.82), indicating homogamy for liability to SUD, observed previously among the parents of male index children in the CEDAR sample (Vanyukov et al., 1994,1996). This homogamy is asymmetric, likely related to base rate sex differences: among the affected mothers, only 8 (18.6%) of their husbands were not affected, whereas among affected fathers, 62.0% of wives were not affected.
Although the sample of girls has not yet passed through the age of risk, the high-risk design resulted in 21.3% of them being affected with a DUD related to an illicit substance – most frequently, cannabis (19.2%), followed by opioids (7.6%) and cocaine (2.6%). By the 2×2 χ2 test, the lifetime risk for DUD in the girls was significantly associated with DUD in their mothers (p <.05), but not in fathers (p > .1).
In Cox regression analyses of the girls’ DUD development rate with paternal and maternal DUD diagnoses, similarly, maternal diagnosis was a significant predictor (hazard ratio, HR=2.19; 95% confidence interval, CI: 1.17–4.12; p=.01), while paternal diagnosis was not (HR=1.66; 95% CI: 0.944–2.93; p=.08). NAP was a significant predictor of girls’ DUD development rate, when treated as an ordinal variable (HR=1.59 [1.10–2.29]; p=.01). From the analysis where NAP was treated as a categorical variable, the relationship appeared to be accounted for largely by the effect of having two affected parents (HR=2.51 [1.19–5.28]; p=.015). The effect of single affected parent (mostly, obviously, the father), although trending in the same direction, did not reach significance in this sample (HR=1.63 [0.84–3.17]; p=.151).
3.2. Parental DUD and nutritional status (NS)
In order to test NS as a mediator of the relationships of parental DUD with girls’ characteristics, the NS measures must correlate with NAP. Indeed, the results show that the SSF measurements correlate with the NAP at all three available time points (Spearman’s ρ are 0.18, 0.22 and 0.23, all p< .01, for the sequence of visits). Similar correlations are observed for BMI (0.19, p < .01; 0.28, p < .001; and 0.16, p <.05).
3.3. NS and sexual maturation
Tracking the hypothesized relationship further, NS measured by both SSF and BMI was significantly correlated with TS at visits 1 and 2 (SSF: ρ=0.31, p <.001 for both visits; BMI: 0.38 and 0. 36, p < .001); the correlations at visit 3 were nonsignificant. The latter may be due to diminished TS variation at age 16: indeed, the TS variance across the visits declined from 0.90 to 0.80 to 0.31 (with TS ranging 1–5 at the first two visits, and 3–5 at visit 3).
3.4. Sexual maturation and peer delinquency (PD)
TS correlates with PD cross-sectionally at visit 1 (ρ=0.19, p<.01), with a trend at visit 2 (ρ=0.16, p=.056), but not at visit 3 (p > .2). The visit 1 TS predicted PD at all visits (1: ρ=0.19, p<.01; 2: ρ=0.22, p<.005; 3: ρ=0.17, p <.05), suggesting cross-age effect.
3.5. PD and DUD
PD at all three assessment points predicts the girls’ DUD outcome, with odds ratios, respectively, 2.3 (95% CI: 1.5–3.3, p <.001), 2.2 (1.5–3.4; p < .001), and 2.2 (1.3–3.7; p < .005). PD is also related to NAP (ρ=0.18, p=.009; ρ=0.33, p<.001; and ρ=0.26, p<.001).
3.6. Developmental model testing
The observed relationships can be conjointly considered in a path analysis testing the hypothesized developmental process, using the data from all three consecutive assessments to relate the variable domains of parental DUD, nutritional status (SSF and BMI), sexual maturation (TS), peer delinquency (PD), and the girls’ lifetime DUD risk (DUD). The initial model to test, in accordance with the described set of hypotheses, included all possible paths connecting the developmental tracking of the same variable (its values at different visits) and the paths connecting the variable domains both cross-sectionally and longitudinally. The best-fitting models are presented in Fig. 2 with standardized path (partial regression) coefficients. Only significant direct paths (credibility intervals do not include 0) are shown in the figure. The models have good fit (posterior predictive p=0.50).
Figure 2.
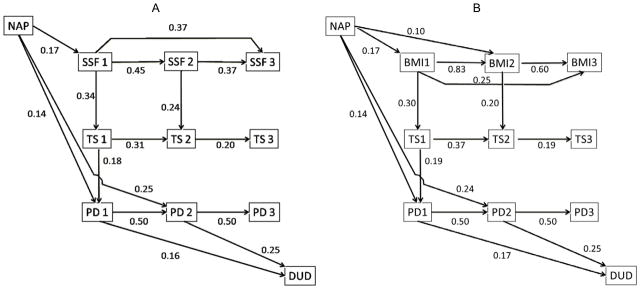
Path analysis of the relationship between parental drug use disorder and the daughters’ nutritional status, sexual maturation, delinquency of peers, and the risk for addiction (A, SSF Model; B, BMI model). Notes: NAP, number of affected parents; SSF, subscapular fold measurement; TS, Tanner stage; PD, peer delinquency; DUD, drug use disorder diagnosis (yes/no). The numbers correspond to the visit (see text).
The models are structurally virtually identical for BMI and SSF, inasmuch as the hypothesis-relevant paths, relating all involved variable domains, are concerned. This is consistent with the substantial correlations between BMI and SSF at all three visits (0.734, 0.535 and 602, respectively; all are highly significant).
As expected, significant relationships are estimated between the values of the same variables over time. The BMI estimates across visits (1–2, 2–3, and 1–3) are somewhat more consistent (path coefficient β=0.83 [95% credibility interval CI: 0.78–0.87]; 0.60 [0.44–0.76]; and 0.25 [0.09–0.42], respectively) than those for SSF (0.45 [0.33–0.55]; 0.37 [0.21–0.52]; 0.37 [0.23–0.51]). In both models, the visit 2 values mediate the relationships between the visit 1 and 3 values. Mediation by the visit 2 values is complete for Tanner staging and peer delinquency.
NAP directly influences peer delinquency at visit 1 (SSF model: 0.14 [0.01–0.26]; BMI model: 0.13 [0.003–0.259]), and, even stronger, at visit 2 (SSF: 0.25 [0.13–0.36]; BMI: 0.24 [0.13–0.37]).
Of greater interest are the relationships corresponding to the study’s hypotheses, joining the variable domains (NAP-NS-TS-PD-DUD) by mediated paths. In addition to direct relationships of both indicators of NS with sexual maturation (TS), the latter mediates BMI’s and SSF’s relationships with PD. The indirect effect of the nutritional status on PD is traced through the visit 1 TS value, with similar total effects on the visit 2 PD value accounted for by the SSF and BMI models: 0.06 (0.014–0.122) vs. 0.03 (0.006–0.056).
Significant indirect effects, in addition to the direct ones noted above, also relate NAP and PD at visits 1 and 2: for the SSF model, respectively, 0.01 [0.001–0.026] and 0.07 [0.009–0.143]; for the BMI model, respectively, 0.01 [0.001–0.023] and 0.07 [0.01–0.14]).
Importantly, the indirect effects of both BMI and SSF at visit 1 on the risk for DUD are significant and mediated by TS at visit 1 and PD at visits 1 and 2. In both models, the NAP-PD relationships, both direct and indirect, ensure the significance of the parent-offspring DUD relationship (NAP-DUD), fully mediated by these effects. The posterior probability for being greater than 0 for all individual indirect paths connecting NAP and offspring’s outcome is close to 1. For instance, for the two hypothesis-relevant indirect paths in the BMI model that relate these variables via nutritional status, Tanner stage and peer delinquency (NAP-BMI1-TS1-PD1-DUD and NAP-BMI1-TS1-PD1-PD2-DUD), this posterior probability is 0.96 and 0.99; it is 0.99 for the sum of these paths as well. The total effect of NAP on the DUD risk in children that is accounted for by this model is 0.10 (0.04–0.17).
Therefore, the data are consistent with mediation of the relationship between NAP and peer delinquency by sexual maturation at age ~11, whereas the relationship between NAP and sexual maturation at that age is mediated by the nutritional status. In turn, a significant indirect effect of the sexual maturation stage on the girls’ DUD risk is mediated by peer delinquency at age ~11–14. The results for both models are similar and consistent with the hypothesis relating the domains represented by the variables, and their role in the mediation of familial DUD risk transmission.
4. DISCUSSION
This study in girls, which complements prior research in boys (Kirillova et al., 2001, 2008), tested the hypothesis that relates the familiality and development of drug addiction to both individual (nutrition/adiposity; physiological maturation) and environmental (peer deviance) factors. The complexity of involvement of the different levels of biological organization calls for placing this hypothesis in a wider context, originating in biological mechanisms of physiological and behavioral development and extending to social behavior.
One source of this hypothesis is the concept that addiction is a facet of consummatory behavior that has common evolutionary roots (Vanyukov, 2004; Vanyukov et al., 2012) and involves a “common fitness metric… along which everything, from apples to oranges to cocaine, can be compared” (Panksepp et al., 2002, p. 460). The mechanisms enabling this common scale are based not only in the dopaminergic system, potentially coding for the general incentive salience of stimuli (Robinson and Berridge, 1993), but also involve virtually all neurotransmitter systems that are recruited by the drugs (Volkow et al., 2011). Eating behavior and liability to addiction share in common the neural circuits subserving reward, motivation, learning, emotion/behavior regulation and executive function (Volkow et al., 2008).
Another potential origin of the hypothesized system is the relationship between the nutritional status and sexual maturation. In boys, familial risk for addiction was shown to be related to the rate of sexual maturation (Kirillova et al., 2001, 2008). The mechanisms of this connection – the relationship between parental DUD load and maturation – are not clear. In girls, sexual maturation is known to be related to their nutritional status (Wang, 2002; Must et al., 2005; Kaplowitz, 2008; Melmed et al., 2011). Similar findings have been obtained for boys (He and Karlberg, 2001), although obesity was associated with earlier maturation in girls but later maturation in boys (Wang, 2002). Pubertal timing is also highly heritable in both males and females (Kaprio et al., 1995; Silventoinen et al., 2008). It is possible that it is the heritability of the rate of physiological maturation that contributes to the intergenerational correlation for DUD liability.
Similar to common mechanisms for consummatory behaviors, evolutionary commonality may also exist for the regulation of nutritional status/adiposity and sexual maturation in females. It could be advantageous if the ability to procreate were related to the availability of resources, which would ensure nutrition for the offspring. This hypothesis is supported by a genetic correlation between nutritional status and pubertal timing (age of menarche), indicating their relationship at the genetic level (Kaprio et al., 1995). At the physiological level, one source of this relationship may be the involvement of leptin in both fat metabolism and sexual maturation in girls (Li et al., 2005). Another important component of the nutrition regulation system, ghrelin, also appears to be involved in reproductive maturation (Navarro and Kaiser, 2013), as well as in reward from both food and addictive substances (Dickson et al., 2011), thus connecting nutritional status and addiction risk both directly and indirectly. The biological mechanisms may also have their roots in the negative relationship between childhood nutritional status and telomere length (Buxton et al., 2011), influencing biological age and the rate of puberty. Interestingly, obesity and substance involvement are negatively associated (Simon et al., 2006; Blüml et al., 2012), suggesting a possibility that they are alternative manifestations of the shared mechanisms.
Earlier maturing girls, but not boys, experience greater stress (Simon et al., 2006), which can also result from parental environment with SUD-affected parents. Stress sensitivity is heightened during adolescence, and stress responsiveness of cortical GABA receptors emerges over adolescence (Walker and Sabuwalla, 2004). In turn, stress and high cortisol reactivity are predictive of increased eating, a coping mechanism, particularly in females (Adam and Epel, 2007). Numerous pathways also relate stress with elevated liability to addiction (Sinha, 2008). Substance use may serve for self-medication (Khantzian, 1985), and drug-dependent females are more likely than men to use drugs for this purpose (Haseltine, 2000). Stress may be also related to accelerated sexual maturation in females (Belsky et al., 1991; Moffitt et al., 1992; Wierson et al., 1993; Ellis and Garber, 2000). Therefore, given the ready availability of food in the US, nutritional status may be a mediator of this relationship. Notably, telomere erosion (and thus potentially biological age) is also related to early-life stress, whereas parenting style moderates this relationship (Asok et al., 2013).
Faster sexual maturation makes likelier the contact with older and more deviant peers, and thus exposure to a higher risk for drug use and addiction. As noted before (Kirillova et al., 2008), the effect of parental SUD liability phenotype on peer deviance, growing over time due to direct and mediated effects, is consistent with growing detachment from parents and the increasingly active homophilic choice of peers, underlying active phenotype-environment correlation. Inasmuch as homophily results from the individual’s selection into, and/or his/her choice of, a behaviorally similar peer group, it will induce higher genetic and/or environmental similarity among the members of the group, such as a gang, —akin to the effects of assortative mating (Vanyukov, 2004; Guo, 2006). Faster maturation combined with behavior dysregulation and homophily for this trait may further lead to behavior contagion (when drug use behavior of a friend/partner influences the girl’s own behavior) and assortative mating. Both contagion and assortative mating may be responsible for the substantial correlation for addiction between the parents, homogamy, observed in this study.
The lack of (or lower) influence of one affected parent – mostly father – on the DUD risk in females, but the significant influence of two affected parents when (largely) mothers are added, is consistent with the data on the familiality of alcoholism among females (Bohman et al., 1981). Whereas there was no excess of alcohol abuse in daughters of alcoholic biological fathers, the rate of alcoholism in daughters of affected mothers was significantly elevated. The data highlight differences between the maternal and paternal contributions to the child’s behavioral development. For instance, mothers’ and fathers’ parenting styles had opposite relationships with their sons’ risk for conduct disorder (Vanyukov et al., 2007). In the current study, the relevant differences in the relationships between parental and offspring phenotypes include that for DUD risk. The effect of two affected parents, while mostly due to the presence of the affected mother, was nevertheless likely contributed by the affected father as well. The latter effect was weaker but the trend was present in the univariate analysis with the father’s diagnosis and when the analysis of the maternal effect was stratified by the father’s diagnosis, suggesting a somewhat higher hazard rate in the presence of the affected father (data not shown). Because the sample, however, was ascertained through the father, while the prevalence of addiction among females is relatively low to enable accrual of the sufficient number of affected wives of nonaffected husbands, it is a limitation of the study that the purely maternal effects cannot be reliably estimated. The selected nature of the sample may also influence parameter estimates.
Other caveats should be noted. The offspring sample has not yet entirely passed through the age of risk for DUD, resulting the likely underestimation of lifetime risk. It is conceivable that this may change the strength of the relationships observed. In an observational study, it is also not possible to control all relevant variables in the analysis of hypothetically causal relationships. Therefore, a third and unaccounted-for variable may be responsible for a relationship, even when the variables are chronologically ordered. It should also be noted that the study considered only one of the many potential pathways relating the variable domains as well as accounting for the intergenerational transmission of liability to addiction. The relationships considered may also be moderated and mediated by numerous processes. It is thus not surprising that the effects observed are relatively low. One of the main general concerns with structural equation models is the large number of potential alternative models that could demonstrate an equal or better fit. For this study, however, the conceptual model supported by the results had a substantial empirical foundation, as it was aligned with the intergenerational transmission of DUD liability and a factual physiological developmental process. The same consideration could mitigate the possibility that the results of the study, obtained in a high-risk design, may be not fully generalizable.
In sum, the data are consistent with the hypothesis that the intergenerational transmission of the elevated DUD risk in females is in part mediated by elevated nutrition, related to an increased rate of sexual maturation and affiliation with deviant peers. These results may have important implications for prevention of substance use disorders, calling for attention to eating behavior, which develops long before drug use becomes possible. Regardless of whether it results in categorically diagnosable consequences such as obesity, targeting regulation of eating behavior as an early manifestation or correlate of liability to addiction may result in a decrease in addiction risk.
Highlights for review.
Results of a longitudinal family study of drug use disorder (DUD) risk in females are reported.
Familial transmission of DUD risk is mediated by nutrition status, the rate of sexual maturation and peer deviancy.
The results call for attention to eating behavior, which develops long before drug use becomes possible.
Targeting regulation of eating behavior as an early manifestation of DUD liability may result in a decrease in DUD risk.
Acknowledgments
Role of funding source
Funding for this study was provided by NIDA Grant P50DA005605. The NIDA had no further role in study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Footnotes
Contributors
All authors must have materially participated in the research and manuscript preparation. G. Kirillova contributed to data analysis and wrote the first draft of the manuscript, M. Reynolds, L. Kirisci, S. Mosovsky, T. Ridenour, R. Tarter and M. Vanyukov participated in data analysis and writing the manuscript. All authors have approved the final manuscript.
Conflict of interest
All authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91:449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Asok A, Bernard K, Roth TL, Rosen JB, Dozier M. Parental responsiveness moderates the association between early-life stress and reduced telomere length. Dev Psychopathol. 2013;25:577–585. doi: 10.1017/S0954579413000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JH, Mitchell KS, Neale MC, Kendler KS. Eating disorder symptomatology and substance use disorders: prevalence and shared risk in a population based twin sample. Int J Eat Disord. 2010;43:648–658. doi: 10.1002/eat.20856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Steinberg L, Draper P. Childhood experience, interpersonal development, and reproductive strategy: an evolutionary theory of socialization. Child Devel. 1991;62:647–670. doi: 10.1111/j.1467-8624.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- Blüml V, Kapusta N, Vyssoki B, Kogoj D, Walter H, Lesch OM. Relationship between substance use and body mass index in young males. Am J Addict. 2012;21:72–77. doi: 10.1111/j.1521-0391.2011.00192.x. [DOI] [PubMed] [Google Scholar]
- Bohman M, Sigvardsson S, Cloninger CR. Maternal inheritance of alcohol abuse. Arch Gen Psychiatry. 1981;38:965–968. doi: 10.1001/archpsyc.1981.01780340017001. [DOI] [PubMed] [Google Scholar]
- Buxton JL, Walters RG, Visvikis-Siest S, Meyre D, Froguel P, Blakemore AIF. Childhood obesity is associated with shorter leukocyte telomere length. Clin Endocrinol Metab. 2011;96:1500–1505. doi: 10.1210/jc.2010-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, Centers for Disease Control and Prevention. [accessed in April 2013];Data Table of BMI-for-age Charts. 2001 Available online: http://www.cdc.gov/growthcharts/html_charts/bmiagerev.htm.
- Dickson SL, Egecioglu E, Landgren S, Skibicka KP, Engel JA, Jerlhag E. The role of the central ghrelin system in reward from food and chemical drugs. Mol Cell Endocrinol. 2011;340:80–87. doi: 10.1016/j.mce.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Garber J. Psychosocial antecedents of variation in girls’ pubertal timing: maternal depression, stepfather presence, and marital and family stress. Child Devel. 2000;71:485–501. doi: 10.1111/1467-8624.00159. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- Grucza RA, Krueger RF, Racette SB, Norberg KE, Hipp PR, Bierut LJ. The emerging link between alcoholism risk and obesity in the United States. Arch Gen Psychiatry. 2010;67:1301–1308. doi: 10.1001/archgenpsychiatry.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G. Genetic similarity shared by best friends among adolescents. Twin Res Hum Genet. 2006;9:113–121. doi: 10.1375/183242706776402920. [DOI] [PubMed] [Google Scholar]
- Haseltine FP. Gender differences in addiction and recovery. J Womens Health Gend Based Med. 2000;9:579–583. doi: 10.1089/15246090050118080. [DOI] [PubMed] [Google Scholar]
- He Q, Karlberg J. BMI in childhood and its association with height gain, timing of puberty, and final height. Pediat Res. 2001;49:244–251. doi: 10.1203/00006450-200102000-00019. [DOI] [PubMed] [Google Scholar]
- Kaplowitz PB. Link between body fat and the timing of puberty. Pediatrics. 2008;121:208–217. doi: 10.1542/peds.2007-1813F. [DOI] [PubMed] [Google Scholar]
- Kaprio J, Rimpela A, Rimpela M, Rose RJ, Viken RJ, Winter T. Common genetic influences on BMI and age at menarche. Hum Biol. 1995;67:739–753. [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. Am J Psychiatry. 1985;142:1259–1264. doi: 10.1176/ajp.142.11.1259. [DOI] [PubMed] [Google Scholar]
- Kirillova GP, Vanyukov MM, Gavaler JS, Pajer K, Tarter RE. Substance abuse in parents and their adolescent offspring: the role of sexual maturation and sensation seeking. J Child Adolesc Subst Abuse. 2001;10:77–89. [Google Scholar]
- Kirillova GP, Vanyukov MM, Kirisci L, Reynolds M. Physical maturation, peer environment, and the ontogenesis of substance use disorders. Psychiatry Res. 2008;158:43–53. doi: 10.1016/j.psychres.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirisci L, Tarter R, Reynolds M, Ridenour T, Stone C, Vanyukov M. Computer adaptive testing of liability to addiction: identifying individuals at risk. Drug Alcohol Depend. 2012;123:79–86. doi: 10.1016/j.drugalcdep.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Rounsaville BJ. Sensitivity of psychiatric diagnosis based on the best estimate procedure. Am J Psychiatry. 1992;149:1225–1227. doi: 10.1176/ajp.149.9.1225. [DOI] [PubMed] [Google Scholar]
- Lee SY, Song XY. Bayesian analysis of structural equation models with dichotomous variables. Stat Med. 2003;22:3073–3088. doi: 10.1002/sim.1544. [DOI] [PubMed] [Google Scholar]
- Lee SY, Song XY. Evaluation of the Bayesian and maximum likelihood approaches in analyzing structural equation models with small sample sizes. Multivar Behav Res. 2004;39:653–686. doi: 10.1207/s15327906mbr3904_4. [DOI] [PubMed] [Google Scholar]
- Li HJ, Ji CY, Wang W, Hu YH. A twin study for serum leptin, soluble leptin receptor, and free insulin-like growth factor-I in pubertal females. J Clin Endocrinol Metab. 2005;90:3659–3664. doi: 10.1210/jc.2004-2079. [DOI] [PubMed] [Google Scholar]
- Loeber R. Peer Delinquency Scale. University of Pittsburgh, Department of Psychiatry; Pittsburgh, PA: 1989. [Google Scholar]
- Marshall W, Tanner I. Variation in pattern of pubertal changes in boys. Arch Dis Childhood. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melmed S, Polonsky KS, Larsen PR, Kronenberg HM. Williams Textbook of Endocrinology. 12. Elsevier Saunders; Philadelphia: 2011. [Google Scholar]
- Moffitt TE, Caspi A, Belsky J, Silva PA. Childhood experience and the onset of menarche: a test of a sociobiological model. Child Devel. 1992;63:47–58. doi: 10.1111/j.1467-8624.1992.tb03594.x. [DOI] [PubMed] [Google Scholar]
- Must A, Naumova EN, Phillips SM, Blum M, Dawson-Hughes B, Rand WM. Childhood overweight and maturational timing in the development of adult overweight and fatness: the Newton Girls Study and its follow-up. Pediatrics. 2005;116:620–627. doi: 10.1542/peds.2004-1604. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Kaiser UB. Metabolic influences on neuroendocrine regulation of reproduction. Curr Opin Endocrinol Diabetes Obes. 2013;20:335–341. doi: 10.1097/MED.0b013e32836318ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvaschel H, Puig-Antich J. Epidemiological version: Kiddie-SADS-E (K-SADS-E) 4. Western Psychiatric Institute and Clinic; Pittsburgh, PA: 1987. Schedule for Affective Disorder and Schizophrenia for School Age Children. [Google Scholar]
- Panksepp J, Knutson B, Burgdorf J. The role of brain emotional systems in addictions: a neuro-evolutionary perspective and new ‘self-report’ animal model. Addiction. 2002;97:459–469. doi: 10.1046/j.1360-0443.2002.00025.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Silventoinen K, Haukka J, Dunkel L, Tynelius P, Rasmussen F. Genetics of pubertal timing and its associations with relative weight in childhood and adult height: the Swedish Young Male Twins Study. Pediatrics. 2008;121:e885–e891. doi: 10.1542/peds.2007-1615. [DOI] [PubMed] [Google Scholar]
- Simon AE, Wardle J, Jarvis MJ, Steggles N, Cartwright M. Examining the relationship between pubertal stage, adolescent health behaviors and stress. Psychol Med. 2006;33:1369–1379. doi: 10.1017/s0033291703008390. [DOI] [PubMed] [Google Scholar]
- Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, Kessler RC. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63:824–830. doi: 10.1001/archpsyc.63.7.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M. Instruction Manual for the Structured Clinical Interview for DSM–III–R. Biometrics Research Department, New York State Psychiatric Institute; New York: 1987. [Google Scholar]
- Tarter RE, Vanyukov MM. Theoretical and operational framework for research into the etiology of substance use disorder. J Child Adolesc Subst Abuse. 2001;10:1–12. [Google Scholar]
- Trace SE, Thornton LM, Baker JH, Root TL, Janson LE, Lichtenstein P, Pedersen NL, Bulik CM. A behavioral-genetic investigation of bulimia nervosa and its relationship with alcohol use disorder. Psychiat Res. 2013;208:232–237. doi: 10.1016/j.psychres.2013.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanyukov MM. Evolution, genes, and the environment: neurobiological outcomes. In: Fishbein DH, editor. The Science, Treatment, and Prevention of Antisocial Behaviors: Application to the Criminal Justice System. Vol. 2. Civic Research Institute; Kingston, NJ: 2004. pp. 4-1–4–29. Chapter 4. [Google Scholar]
- Vanyukov MM, Maher BS, Devlin B, Kirillova GP, Kirisci L, Ferrell RE. The MAOA promoter polymorphism, disruptive behavior disorders, and early onset substance use disorder: gene-environment interaction. Psychiat Genet. 2007;17:323–332. doi: 10.1097/YPG.0b013e32811f6691. [DOI] [PubMed] [Google Scholar]
- Vanyukov MM, Moss HB, Tarter RE. Assortment for the liability to substance abuse and personality traits. Ann N Y Acad Sci. 1994;708:102–107. doi: 10.1111/j.1749-6632.1994.tb24702.x. [DOI] [PubMed] [Google Scholar]
- Vanyukov MM, Neale MC, Moss HB, Tarter RE. Mating assortment and the liability to substance abuse. Drug Alcohol Depend. 1996;42:1–10. doi: 10.1016/0376-8716(96)01255-0. [DOI] [PubMed] [Google Scholar]
- Vanyukov MM, Tarter RE, Kirillova GP, Kirisci L, Reynolds MD, Kreek MJ, Conway KP, Maher BS, Iacono WG, Bierut L, Neale MC, Clark DB, Ridenour TA. Common liability to addiction and “gateway hypothesis”: theoretical, empirical and evolutionary perspective. Drug Alcohol Depend. 2012;123:S3–S17. doi: 10.1016/j.drugalcdep.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cognit Sci. 2011;15:37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Phi Trans R Soc B. 2008;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EF, Sabuwalla Z, Huot R. Pubertal neuromaturation, stress sensitivity, and psychopathology. Dev Psychopathol. 2004;16:807–824. doi: 10.1017/s0954579404040027. [DOI] [PubMed] [Google Scholar]
- Wang Y. Is obesity associated with early sexual maturation? A comparison of the association in American boys versus girls. Pediatrics. 2002;110:903–910. doi: 10.1542/peds.110.5.903. [DOI] [PubMed] [Google Scholar]
- Wierson M, Long PJ, Forehand RL. Toward a new understanding of early menarche: the role of environmental stress in pubertal timing. Adolescence. 1993;28:913–924. [PubMed] [Google Scholar]


