Abstract
Introduction
Chronic stress is known to have negative effects on reproduction, but little is known about how it affects the sexual response cycle. The present study examined the relationship between chronic stress and sexual arousal and the mechanisms that mediate this relationship.
Aim
To test the relationship between chronic stress and sexual arousal and identify mechanisms that may explain this relationship. We predicted that women experiencing high levels of chronic stress would show lower levels of genital arousal & DHEAS and higher levels of cortisol and cognitive distraction compared to women with average levels of stress.
Methods
Women who were categorized as high in chronic stress (high stress group, n = 15) or average in chronic stress (average stress group; n = 15) provided saliva samples and watched an erotic film while having their genital and psychological arousal measured.
Main Outcome Measures
Main outcome measures were vaginal pulse amplitude, psychological arousal, salivary cortisol, salivary DHEAS, and heart rate and compared them between women with high and average levels of chronic stress.
Results
Women in the high stress group had lower levels of genital, but not psychological arousal, had higher levels of cortisol, and reported more distraction during the erotic film than women in the average stress group. The main predictor of decreased genital sexual arousal was participants’ distraction scores.
Conclusions
High levels of chronic stress were related to lower levels of genital sexual arousal. Both psychological (distraction) and hormonal (increased cortisol) factors were related to the lower levels of sexual arousal seen in women high in chronic stress, but distraction was the only significant predictor when controlling for other variables.
Introduction
Chronic stress has been linked to problems with female reproduction both in human and non-human animals in numerous studies. By contrast, much less research attention has been focused on the relationship between chronic stress and sexual functioning. It is likely that the negative effects of chronic stress also extend to sexual functioning, which is an integral part of reproductive functioning, but also of women’s quality of life and relationship satisfaction.1,2 Many women experience hectic daily lives in which they are regularly exposed to small, chronic stressors. The accumulation of constant small stressors can potentially contribute to problems with sexual functioning.
Chronic stress can be defined in many ways, but for the purposes of research in North America, we focused on psychosocial stress (as opposed to more physiological stressors, such as starvation.) In the research literature, chronic psychosocial stress generally defined as either 1) major life events that induce an extended period of stress, such as a death in the family,3 or 2) as the accumulation of small stressors that are constantly or frequently present, such as deadlines that never seem to be met, traffic, or financial worries.4,5 Kanner et al. were the first to find that these small stressors, which they called daily hassles, have more of a negative effect on health than the more severe but less common stressors.4 The negative effects of daily hassles have been replicated in several studies across multiple health domains (e.g.,6–9), including a survey study on the effects of daily hassles and major life events on sexual function.10 It was found that daily hassles, not major life events, were related to sexual difficulties. The link between chronic daily stressors and sexual function has also been examined in additional survey research which found that for women, higher levels of chronic daily stressors were related to higher levels of sexual problems lower levels of sexual satisfaction.11 To our knowledge, only one study has found a positive relationship between stress and sexual functioning. The sample in this study included a large portion of unemployed men and women, and the researchers found that daily hassles stress combined with unemployment actually resulted in higher levels of desire; however this did not translate into higher levels of sexual activity.12
In addition to survey studies of the relationship between chronic stress and sexual function, there has been one laboratory-based study that examined the relationship between chronic stress and women’s sexual function.13 In this study, women completed a daily stressor checklist (similar to Kanner et al.’s Hassles Scale4) while in the lab. Based on a median split of their scores on the checklist, researchers categorized participants as having high or low levels of chronic daily stress. Those in the high stress group had lower levels of genital arousal (measured by vaginal photoplethysmography), but not psychological arousal (measured continuously) than those in the low chronic daily stress group when shown an erotic film. This laboratory study added to the survey-based findings that chronic stress is linked to lower levels of sexual responding.
While the evidence of the negative effects of chronic stress on sexual function is fairly consistent, the mechanisms involved in this relationship are not well defined, particularly in humans. There has been extensive research on the effects of chronic stress on reproductive function in non-human animals.14 These studies document the hormonal mechanisms involved in the suppression of reproductive function by both acute and chronic stressors, but they do not directly address sexual response, nor do they take into account the psychological components of stress that may play a role in the relationship between stress and sexual function. In humans, there are both psychological and physiological components that can potentially be involved in the relationship between stress and sexual function. For the purposes of the present study, we were interested in testing the effects of two physiological mechanisms (hormonal effects and sympathetic nervous system activity) and one psychological mechanism (distraction) in women. Each of these mechanisms is outlined in more detail below.
Chronic stressors, whether they are daily stressors or major life events, increase an individual’s allostatic load (i.e., The body’s response to the accumulation of chronic or recurring stressors). In turn, the individual’s physiological systems need to change and adapt in an attempt to maintain allostasis, which is defined as maintaining stability under changing demands.15 Increased chronic stress leads to higher levels of cortisol, which can cause harmful effects when elevated over extended periods of time.16 There has been extensive research (primarily in animal models) demonstrating how hormones released from the hypothalamic-pituitary-adrenal (HPA) axis in response to stress can interfere with hormonal secretion from the hypothalamic-pituitary-gonadal (HPG) axis, which is involved in the control of reproduction and sexual response. Briefly, glucocorticoids (i.e., cortisol in humans) released from the adrenal gland inhibit the HPG through interfering with the release of gonadotropin releasing hormone (GnRH), luteinizing hormone (LH), and follicle stimulating hormone (FSH) at the hypothalamic and pituitary levels. This model or similar disruptions of the HPG axis by hormones released from the HPA axis has been demonstrated in several species.17–20 A reduction in gonadotropin release results in less production of gonadal steroids, such as testosterone and estradiol, both of which have been shown to have facilitatory effects on women’s genital arousal,21–23 and may be involved in subjective arousal as well.24 Given this evidence, we would expect that elevated levels of cortisol in women experiencing high levels of chronic, daily stressors would be linked to lower levels of sexual arousal.
In addition to adrenal glucocorticoids, adrenal androgens have also been implicated as playing a key role in sexual arousal and desire in women.25 Of particular interest to the relationship between stress and sexual arousal are both dehydroepiandrosterone (DHEA), an androgenic prohormone, and its sulfated metabolite (DHEAS). Low levels of endogenous DHEAS have been implicated in arousal and desire problems in women.26,27 DHEAS is also released in response to acute stress28,29 and may remain high in response to chronic stress, as demonstrated in non-human primates.29 A recent study of prolonged stress in humans showed no change in DHEA in response to prolonged stress, but did not measure DHEAS,30 while another study found lower levels of DHEAS in those who were chronically stressed.31 Research on the relationship between DHEAS and chronic stress is relatively new, and so the exact nature of the relationship is unclear. Endogenous DHEAS appears to be beneficial for sexual function, given that low levels of DHEAS have been related to both arousal and desire problems26,27 and are thought to be protective against the negative effects of cortisol in other domains, where the cortisol:DHEAS ratio is often used as a predictor.32 If DHEAS is higher in women with high levels of chronic stress, it could be beneficial for sexual function, but if it is suppressed by high levels of cortisol, then we would expect to see negative effects on sexual functioning.
Chronic stress is also linked to increases in sympathetic nervous system (SNS) activity that are harmful to health over time (e.g., increased blood pressure), which can inhibit blood flow to all areas of the body over the long term.33 Inhibited blood flow to the genitals, by definition, interferes with genital arousal. In contrast, acute, moderate increases in SNS have been shown to facilitate women’s genital arousal, while inhibition of SNS activity impairs genital arousal.34 With chronic stress, there will not be an acute spike of SNS activity that has shown to be beneficial for women’s arousal, so any differences in SNS activity related to chronic stress will likely correspond with decreases in sexual arousal.
Psychologically, stress can interfere with sexual activity through both emotional and cognitive changes that distract the individual from focusing on sexual cues. These psychological responses can distract the focus of the participant toward the stressful stimuli and away from the appropriate stimulus35. This would be relevant to any stress or anxiety-related stimuli participants experience that draws focus away from sexual stimuli and towards negative outcomes, as outlined by Barlow in his theory of anxiety and sexual dysfunction in men.36 When experiencing chronic stress, we would expect participants to be distracted in daily life by these small chronic stressors, which would extend to distraction from sexual cues. This distraction from sexual stimuli would result in lower levels of arousal because the participants experiencing higher levels of stress may not be attending to the sexual stimuli at the same level of participants with lower levels of stress. Distraction from sexual cues has been shown to have deleterious effects on both genital and subjective arousal in women. 37–39 However, these studies have manipulated distraction experimentally. To our knowledge, there have been no previous studies that measured naturally-occurring distraction (e.g., as a result of stress) and its relationship with sexual arousal.
Aims
The present study had two primary aims. The first was to replicate the finding that those high in daily, chronic stressors (referred to hereafter as just “chronic stress/chronic stressors”) would have lower levels of genital arousal than women with an average level of stress exposure. The second was to examine potential physiological (i.e., cortisol, DHEA-S, heart rate, heart rate variability) and psychological (i.e., distraction) mechanisms that might explain the effects of chronic stress on sexual arousal in women. We hypothesized that women with high levels of chronic stress would be more distracted, have lower levels of DHEAS and genital arousal, and higher levels of cortisol compared to women with average levels of chronic stress. We were also interested in testing which mechanisms were most related to differences in genital and subjective arousal.
Method
Participants
Seventy-nine participants completed an online prescreening survey. Of these participants, 18 were excluded because they did not fit into our predefined stress groups (five were too low and 13 fell between the cutoffs for high and average stress), seven were excluded for health or medication reasons, and nine did not return the initial screening phone call. After completing a more detailed phone screening two participants did not feel comfortable participating and five were excluded for not meeting inclusion criteria. Of the 38 who were scheduled to come into the lab, eight did not keep their appointments, resulting in a total sample size of 30. Participants were recruited until the study included 15 women categorized as having high levels of chronic stress (High Stress group) and 15 women categorized as having average levels of chronic stress (Average Stress group) based on scores from the Hassles Scale (see below for details).4
Women were recruited from the community via flyers and online advertisements. Participants were required to have been sexually active with a male partner in the four weeks prior to the study to ensure that any differences were not because of one group of participants not engaging in sexual activity for an extended period of time. We limited the sample to those currently sexually active with men because our stimuli involved heterosexual couples, so we wanted participants who would be psychologically aroused by heterosexual sexual activity. Previous research has shown that while women who are primarily attracted to women report some psychological arousal to erotic heterosexual stimuli, it is lower that the psychological arousal reported by women primarily attracted to men.40 Relationships ranged in length from 3 months to 10 years with an average of 2.8 (SD = 2.2) years. Twenty-nine women were heterosexual; one woman reported bisexual orientation. See Table 1 for more detailed demographic information.
Table 1.
Demographic & Stress-Related Factors
| Average Stress (n = 15) | High Stress (n = 15) | |
|---|---|---|
| Age in years (SD) | 24.8 (3.8) | 26.27 (6.8) |
| Ethnicity | ||
| Asian | 3 | 3 |
| Black or AA | 0 | 2 |
| Latina | 3 | 1 |
| White | 9 | 9 |
| Marital Status | ||
| Single/Dating | 2 | 4 |
| Long term relationship | 9 | 6 |
| Cohabiting/Married | 4 | 5 |
| Hassles Scale score (SD) | 25.1 (6.3)* | 68.0 (6.0)* |
| Life Experiences Scale Score | ||
| Positive Events (SD) | 5.1 (2.1) | 4.1 (6.2) |
| Negative Events (SD) | 6.2 (4.1) | 6.0 (10.1) |
| CES-D Score (SD) | 10.9 (8.3) | 13.0 (7.9) |
indicates significant group difference at p <. 05
Participants were screened over the phone before coming into the lab to verify that they met study requirements. Participants were excluded if they reported using exogenous hormones or medications known to affect sexual or cardiovascular response (e.g., sildenafil, beta-blockers), being pregnant or breastfeeding within the past three months, or currently experiencing distress from sexual abuse or assault. Participants were also excluded if they reported a disease or disorder known to affect sexual, cardiovascular, or hormonal functioning. These included diabetes, heart disease, any previous problems or injuries that could have caused nerve damage or neurological impairments, and any hormonal abnormalities.
Materials and apparatus
Stimuli
Film sequences
Two 12 minute film sequences were used for this study to ensure results were not solely from the effects of one particular film. Each participant saw only one of the film sequences, and the film sequences were counterbalanced between the High and Average stress groups. Both sequences consisted of a one minute display of the word “Relax” on a black screen, three minutes of a neutral film, which was a Lewis & Clark documentary, followed by eight minutes of an erotic film which depicted a heterosexual couple engaging in foreplay and vaginal intercourse. These films have been previously shown to elicit sexual arousal in women.41
Questionnaires
Life Experiences Survey (LES)
The LES survey assessed 47 major life events for the general population and an additional 10 major school-related events for students. Participants indicated whether or not they had experienced each event in the past year and reported the effect it had on them. The 7-point scale ranged from −3 (extremely negative) to +3 (extremely positive). Scores for all items participants identified as positive stressors were summed and then scores for all items participants identified as negative stressors were summed separately (Table 1).3 There were four participants in each group who indicated that they had school related stress, so school-related stress was equally distributed across the Average and High stress groups.
Hassles Scale
The Hassles Scale measures 117 possible hassles. Examples of these included “Social obligations,” “Concerns about job security” and “Problems with your lover.” If the hassle occurred during the past month, participants indicated how severe of a stressor they found the hassle to be on a scale from 1 (Somewhat severe) to 3 (Extremely severe). The scale was scored summing the total number of hassles (Table 1). In the original scale development, the monthly average score for this measure in an adult population (ages 45–64) over a period of nine months is 20.5, and there were no significant differences between the men and women.4 Previous research used a post hoc median split to divide participants into high and low stress groups.13 For the present study, we wanted to ensure discrete stress-level categories to better examine differences between those experiencing average levels of stress and those experiencing high levels of stress. To do so, we defined the average stress and high stress categories a priori. Women reporting a score of 15–30 were considered to be experiencing average levels of stress, while those reporting a score of over 45 (approximately 1.5 SDs above the mean of 20.5) were categorized as having high levels of stress. Participants with scores below 15 or between 30 and 45 were excluded from the study.
We compared women with average levels of stress to women with high levels of stress because several studies have demonstrated that there is an inverted-U shaped relationship between stress or sympathetic nervous system (SNS) activity and genital sexual arousal.42,43 As such, if the same relationship applies to chronic stress as has been seen with acute stress and anxiety, we would expect that women low in chronic stress and high in chronic stress would have similar, low levels of arousal, while women with average levels of stress would have the optimal level of stress to facilitate sexual arousal. Based on this relationship, the best comparison for women with high levels of chronic stress would be women with moderate levels of chronic stress, so we excluded women reporting low levels of chronic stress. Additionally, women with average levels of chronic stress are also more representative of women, since statistically we would expect a majority of women to have average levels of stress.
Pre-Study Screening
Prior to being contacted for the phone screen, participants completed an online Pre-Study Screening survey that included basic demographics and health information along with the Hassles Scale. Previously determined means from the Hassles scale were used to categorize participants into Average Stress and High Stress groups.
Center for Epidemiological Studies – Depression (CES-D)
To ensure the findings were the results of stress and not depression, we included a measure of depressive symptoms. The CES-D scale is a 20-item measure designed to measure depressive symptomatology in the general population.44
Female Sexual Function Index (FSFI)
The FSFI is a validated 19-item questionnaire designed to assess sexual functioning in women.45 In addition to a total score, the FSFI measures sexual functioning in six domains: Desire, Arousal, Lubrication, Orgasm, Satisfaction, and Pain. A clinical cut-off score of 26.55 has been established to reliably discriminate between women with and without sexual dysfunction.46
Distraction Quiz
Cognitive distraction was assessed with a multiple-choice quiz administered following the erotic film. The test consisted of five questions from the neutral portion of the film sequence and seven questions from the erotic portion of the film sequence to assess participants’ attention to the content of each film. Each question had three multiple-choice response options from which participants were instructed to choose the best answer.47 A recent study that manipulated distraction levels found that these multiple choice quizzes were able to discriminate between participants exposed to a low level of distraction and participants exposed to high levels of distraction.48 The quizzes were scored by calculating the percent correct. Higher scores indicated that participants were less distracted because they were able to answer more questions about the film sequence correctly. The average score for the distraction quiz was 8.7 out of 12 for Film 1 and 8.2 out of 12 for Film 2. For both quizzes, the scores ranged from 5–12.
Apparatus
Vaginal photoplethysmograph
Genital arousal was measured using a vaginal photoplethysmograph.49 The vaginal photoplethysmograph is a clear, acrylic, tampon-shaped device that contains an infrared light-emitting diode as a light source, and a photosensitive light detector. When inserted into the vagina, the light source illuminates the capillary bed of the vaginal wall and the blood circulating within it. Upon contact with the vaginal wall, some of the light is absorbed, while the rest is backscattered. The amount of backscattered light is related to the transparency of engorged tissue and serves as an indirect measure of vasoengorgement. The measure of interest from the photoplethysmograph is the pulse amplitude (VPA), which is received through the A/C signal and band pass filtered at 0.5 to 30 Hz. VPA was sampled 200 times per second. Results were measured in millivolts (mV). VPA was acquired using the software program AcqKnowledge III, Version 3.7.3 (BIOPAC Systems, Inc., Santa Barbara, CA) and a Model MP100WS data acquisition unit (BIOPAC Systems, Inc., Santa Barbara, CA) for analog/digital conversion.
Electrocardiograph (ECG)
Heart rate and heart rate variability (HRV) were calculated from an ECG. Participants had three disposable electrodes attached to their bodies (upper right chest, lower left chest, and right ankle) and connected by cables to a BIOPAC Systems ECG100 module. The signal from the ECG100 module was recorded in real time using the AcqKnowledge software program. ECG was also sampled at 200 times per second. HRV is believed to be a more accurate measure of autonomic nervous system (ANS) activity than heart rate. For this study we used the standard deviation of the R-R intervals as our measure of HRV. The Standard Deviation of the R-R interval (SDRR) is a measure of vagal or parasympathetic activity, and increases in response to parasympathetic activation.50
Arousometer
Continuous subjective sexual arousal was measured during the erotic film using a hand-controlled device that consists of an optical computer mouse mounted on a wooden track divided into seven equally spaced intervals, where 0 indicated neutral, and 1–7 reflected increasingly higher levels of sexual arousal.51 A software program written in MatLab (The MathWorks, Inc, Natick, MA, USA) detected the position of the pointer with respect to the y-axis of the computer’s monitor twice per second. Data were recorded as a proportion between 0 and 1.
Saliva samples
In order to establish basal levels of hormones, participants were asked to provide a saliva sample prior to eating dinner (between 2:00 pm and 6:00 pm) the day before their session, and were instructed to freeze the sample in the test tube provided until their appointment. Cortisol levels are relatively stable in the afternoons(e.g.,52), which is why we chose this time frame to collect saliva samples. Participants were provided with the following instructions with their test tubes:
This sample needs to be taken between the hours of 2:00 pm and 6:00 pm the day before your appointment at the XXXX Lab. Please do not eat (includes gum or candy), drink anything but water, smoke, exercise, or brush your teeth for one hour before providing this sample.
To provide the sample, you can remove the lid and drool or spit directly into the test tube. Please fill it until at least the 2 ml mark (not including bubbles). It helps if you think of something sour, like lemons, which should bring saliva into your mouth.
While providing the saliva sample, please record how long it took you to complete.
When the saliva sample is complete, please place it in your freezer until you bring it to the lab.
Below the instructions was a brief questionnaire that asked questions about compliance, stressors, and problems with the sample. In addition to the instructions, participants had all been instructed over the phone not to eat, drink, or exercise within an hour before providing the samples, and all of them reported complying. They were also asked to report any major stressors they had experienced that day. Only one participant, from the Average Stress group, indicated a major stressor, which was a speeding ticket from earlier in the day. Her cortisol was not elevated compared to other participants in the Average Stress condition. There was also an open ended question in which they were asked to report any problems they had with collecting the sample, and none of the participants indicated any problems.
Two participants in the High Stress group and one in the Average Stress group forgot to bring their samples to their appointment and were asked to provide a baseline sample after sitting in the lab for 30 minutes. Samples were assayed in duplicate for cortisol and DHEA-S using enzyme immunoassay kits from Salimetrics. Interassay variability was below 10% for the high and low control samples for both hormones, and intraassay variability was below 5% for all samples.
Procedures
All procedures were approved by the XXXXX Institutional Review Board. After completing the online Pre-Study Screening, qualified women in the High Stress or Average Stress categories were contacted for Phone Screen interview. Those who qualified were scheduled to come to the lab for psychophysiological testing between days 5–10 of their menstrual cycle. They were also mailed a test tube with instructions on how and when to provide the saliva sample. Once in the lab, participants had the study procedures explained to them and provided written consent to participate. Before leaving the room, the researcher attached the three ECG electrodes to them. Participants completed the questionnaire packet that consisted of the Demographics questionnaire, LES, Hassles Scale, CES-D and FSFI. These questionnaires took approximately 20–30 minutes to complete. After 30 minutes, participants were instructed to insert the photoplethysmograph and attach the electrode leads. The researcher verified that the photoplethysmograph and ECG signals were clear and then began the film sequence. During the film sequence, participants moved the Arousometer to indicate their continuous level of subjective sexual arousal. After the film sequence, participants completed the distraction quiz, removed the photoplethysmograph and electrodes and got dressed.
Data analysis
Preprocessing
VPA data
VPA data were reduced by calculating the total change in amplitude for each heart beat. This was done by finding the peak and nadir for each pulse wave and computing the differences between the two, using AcqKnowledge software. Artifacts in the data were identified visually by the researcher and removed manually. VPA was averaged across the neutral film and the erotic film for use in the ANOVAs. In order to control for individual variability in VPA signals, a VPA difference score for each person and each condition was calculated as the percent change in VPA during the experimental film over the neutral film. To compute correlations between VPA and subjective arousal, VPA data were also averaged every 10 seconds and then each 10 second interval was calculated as a percent change over the average of the neutral condition.
Subjective Arousal
Data gathered from the Arousometer were calculated as a percent increase over the neutral video. In order to calculate correlations with VPA data, the subjective arousal data were averaged every 10 seconds and then multiplied by 100 to get the percent increase.
EGC data
Heart rate was determined by calculating the average beats per minute from the ECG signal for the neutral and erotic portions of each film for each participant. Heart rate variability was calculated from the ECG signal by determining the time interval between each heart beat (R-R interval). The R-R intervals from the neutral segment (three minutes) and from the experimental segment (final three minutes) were entered into a MATLAB based program, Biosignal.53 This program analyzed several aspects of heart rate variability, including the SDRR. For heart rate and heart rate variability, raw scores were entered into the analyses instead of difference scores. This allowed for detection of differences between the Average Stress and High Stress groups at baseline.
Main Outcome Measures
The present study had main outcome measures related to sexual arousal and to the proposed mechanisms that can explain the relationship between stress and sexual arousal. For sexual arousal, outcome measures were vaginal pulse amplitude (VPA; genital arousal) and subjective sexual arousal. Measures related to mechanisms included salivary cortisol and DHEAS levels, and level of cognitive distraction.
Results
Demographic and stress-related variables
As defined by the study design, scores on the Hassles scale were significantly different between the High Stress and Average Stress groups t(28) = −4.2, p < .001 (Table 1). There were no significant differences between the High Stress and Average Stress groups on their age, LES scores, or CES-D scores (Table 1).
Genital and subjective arousal
Women in each group saw one of two different film sequences, and there were no significant differences between the two films in genital arousal, t(28) = 1.5 p = .15, or subjective arousal, t(22) = 1.0, p = .32. As such, data from both videos were collapsed for all subsequent analyses. In response to the erotic film, women in the High Stress group showed significantly lower levels of genital arousal than women in the Average Stress group, t(28) = 2.08, p = .04, d = .78 (Figure 1). Three women from each group indicated that they forgot to move the Arousometer while watching the film. There was no significant difference between the groups on their subjective arousal, t(22) = −.38, p = .71, d = .17 (Figure 1). The average within-subject Pearson correlation between VPA and subjective arousal was r = .24 (SD = .37) for the High stress condition and r = .35 (SD = .35) for the Average Stress condition. We conducted a hierarchical linear model analysis for the relationship between VPA and subjective arousal using VPA and group as the predictor and subjective arousal as the dependent. The following is a summary of the model.
Figure 1.
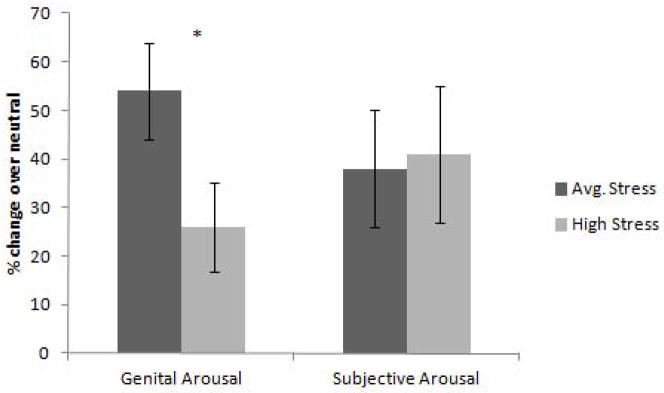
Genital and Subjective Arousal for High and Average Stress groups reported as percent change over baseline (+/− SEM). The women in the High Stress group showed significantly lower levels of genital arousal than the Average Stress group. There was no significant difference between the groups on their reported subjective arousal.
In the model, γ00 is the mean subjective arousal for all participants, γ01 is the change in the subjective sexual arousal associated with a one unit increase in group status (from 0 to 1), γ10 is the average slope for the relationship between subjective arousal and VPA for all participants, γ11 is the change in the subjective-VPA slope associated with one unit increase in group (from 0 to 1), and the two ζ variables are the unique effects of each participant. Results indicated that there was a significant effect of VPA on the slope of subjective sexual arousal, β = .16, t(11.1) = 2.5, p = .03 but that there was no effect of group on the intercept, β = 7.2, t(24.7) = .89, p = .38 or on the slope β = .03, t(11.6) = .21, p = .84, indicating that the relationship between genital and subjective arousal was not affected by group membership. See Figure 2 for the average predicted subjective arousal for each group.
Figure 2.
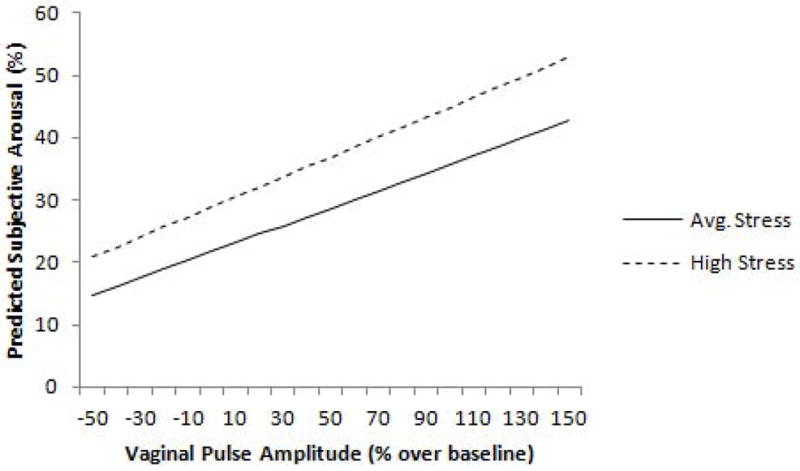
Regressions for High and Average Stress groups for vaginal pulse amplitude (VPA) predicting subjective sexual arousal. The groups are not significantly different from one another.
Autonomic activity
The women in the High and Average Stress groups did not show any significant differences in heart rate, F(1, 24) = .06, p = .81, ηp2 = .03 or in HRV, F(1, 24) = .99, p = .33, ηp2 = .04. There was also no significant change in heart rate, F(1, 24) = 3.58, p = .07, ηp2 = .13 or HRV, F(1, 24) = 3.69, p = .07, ηp2 = .14 across film type (neutral vs. erotic) (Table 2).
Table 2.
Heart Rate and Heart Rate Variability during neutral and erotic films for women in the High Stress and Average Stress groups.
| Heart Rate – BPM (SD) | Standard Deviation of the R-R interval (SD) | |||
|---|---|---|---|---|
| Neutral | Erotic | Neutral | Erotic | |
| Average Stress | 71.4 (6.4) | 72.7 (6.5) | .061 (.017) | .056 (.013) |
| High Stress | 71.0 (7.0) | 71.8 (8.8) | .054 (.014) | .053 (.014) |
Distraction Quiz
Participants in the High Stress group scored 66.6 % (SD = 16.4) on the distraction quiz, which was significantly lower than the Average Stress group who scored 78.8 % (SD = 14.7), indicating that the High Stress group had higher levels of distraction as they answered fewer questions about the film correctly, t(28) = 2.15, p = .04, d = .81.
Basal Hormones
The saliva sample for one participant in the Average Stress group was unusable due to visible blood contamination. Cortisol levels were significantly higher in the High Stress group compared to the Average Stress group, t(27) = −2.5, p = .02, d = .96 (Figure 3). There was no significant difference between the High Stress group (M = 5019 pg/min, SD = 4193) or the Average stress group (M = 4050 pg/min, SD = 2272) on DHEAS levels t(27) = .77, p = .45, d = .29. There was also no significant difference between the groups on their cortisol: DHEAS ratios, t(27) = 1.7, p = .10, d = 60. The ratio for the High Stress group was .69 (SD = .66) and the ratio for the Average Stress group was .36 (SD = .27).
Figure 3.
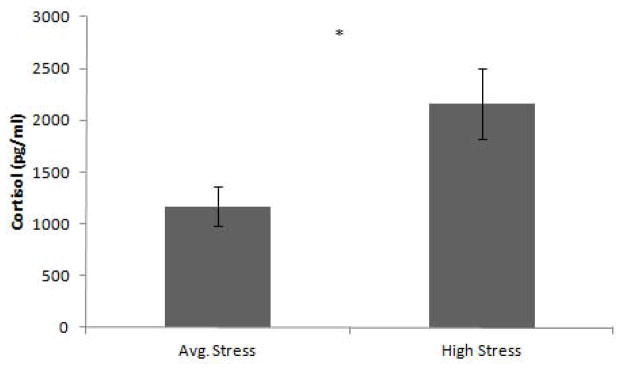
Salivary Cortisol (pg/ml) (+/− SEM). The High Stress group had significantly higher basal cortisol levels than the Average Stress group.
Sexual Functioning
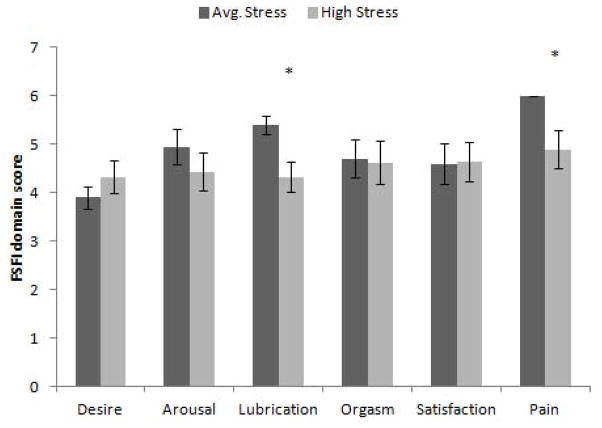
There were no significant differences in sexual function as measured by the FSFI total score between the High Stress (M = 27.26) and Average Stress (M = 29.55) groups t(28) = 1.44, p = .27, d = .42. Analyses done on the six domains of the FSFI found that the High Stress group reported more problems with Lubrication, t(28) = 2.11, p = .03, d = .36 and Pain t(28) = 2.4, p = .02, d = .33, than the women in the Average Stress group (Figure 4).
Figure 4.
Mean Female Sexual Function Index domain scores (+/− SEM). There were no significant differences between the groups on the Desire, Arousal, Orgasm, and Satisfaction domains. Lubrication and Pain were reported as more problematic for women in the High Stress group.
Variables predicting arousal
To test which of the proposed mechanisms had an effect on genital and psychological arousal, correlations were conducted between the arousal measures (VPA percent change and subjective arousal percent) and the hormonal measures and the Distraction Quiz scores. The only significant correlation was between genital arousal and distraction quiz scores, r(28) = .55, p = .002. To assess the relationship between these variables while controlling for the other variables, the hormonal measures, the Distraction Quiz score, and group membership (High or Average Stress) were entered as predictor variables into a multiple linear regression with either VPA percent change or average Arousometer score as the dependent variable. This VPA model had an adjusted R2 of .32, p = .009. The only variable that significantly predicted VPA was score on the distraction quiz (Table 3). The model for the Arousometer data was not significant, and none of the variables were related to subjective sexual arousal.
Table 3.
Predictors of VPA signal
| Predictor | B | t | p |
|---|---|---|---|
| Stress Group | −.221 | −1.20 | .24 |
| Cortisol | .249 | 1.49 | .17 |
| DHEAS | −.248 | −1.54 | .14 |
| Distraction Score | .552 | 3.14 | .004 |
Dependent Variable = Vaginal Pulse Amplitude
Discussion
The present study examined sexual arousal, ANS activity, basal hormones, and cognitive distraction in women categorized as high and average in chronic stress. The goal was to understand the role of chronic stress in women’s sexual arousal and the mechanisms that might explain the relationship between the two. As expected, women reporting high levels of stress (as measured by daily stressors) had lower levels of genital arousal in response to an erotic film than women reporting average levels of stress. The women high in chronic stress also had higher levels of cortisol and distraction.
In addition to measuring women’s sexual arousal response in the laboratory, the present study also measured sexual functioning over the past four weeks using the FSFI. In the laboratory, women in the High Stress group showed impaired genital but not subjective arousal, and this finding corresponds with results of the FSFI. Women in the High Stress group reported lower scores on the FSFI domains associated with genital arousal (Lubrication and Pain domains), but not subjective arousal (the Arousal domain). Both the laboratory results and the FSFI results indicate that chronic stress may affect the physiological components of arousal more than the psychological components.
The finding of decreased genital, but not subjective arousal in women high in chronic stress replicated the results of ter Kuile et al.7 Understanding the degree to which chronic stress impacted genital arousal in the previous study was complicated by the fact that half of the participants were also exposed to an acute stressor. The present study isolated the effects of chronic stress to verify the effect of chronic stress alone on sexual arousal. The similar findings for both of the laboratory studies, paired with results from survey-based research, indicate that the negative relationship between sexual functioning and chronic stress is fairly robust.
Cortisol levels were higher for women in the High Stress group than for women in the Average Stress group. Cortisol is known to impair reproductive function and likely plays a role in reducing women’s genital sexual arousal response. The higher levels of cortisol seen in women with high levels of stress in the present study did not impair their subjective arousal, however. Although cortisol was higher in the High Stress group and genital arousal was lower, there was no direct correlation between cortisol levels and VPA.
There were no differences in DHEAS levels or in the cortisol:DHEAS ratios between the High Stress and Average Stress groups. If DHEAS is co-released with cortisol as has been suggested,18 it is possible that the increase only occurs in response to an acute stressor and is not sustained over time in response to chronic stress. DHEAS has been shown to have health benefits that potentially counteract the negative effects of cortisol.32 Therefore, having increased cortisol without the benefit of increased DHEAS could be an additional factor contributing to the negative effect of chronic stress on genital arousal.
There was no evidence for the role of the ANS in the relationship between genital arousal and chronic stress. SNS activation is the first stage in the stress response and occurs immediately in response to a threatening situation. With chronic stress, there is no immediate threat, so it is reasonable that we did not see an elevated level of heart rate or heart rate variability. The hormones released by the SNS, specifically norepinephrine (NE), are generally considered to enhance genital arousal in women.34 In acute stress situations, increased SNS activity can facilitate genital arousal. The absence of increased SNS activity in the High Stress group compared to the Average Stress group could contribute to the negative effect of chronic stress on genital arousal.
Surprisingly, the higher level of distraction seen in the High Stress group did not correspond with lower levels of subjective arousal as was expected. Inducing distraction experimentally has been repeatedly shown to have a significant negative effect on both genital and subjective arousal.37–39 Although the present study did find a relationship between increased distraction and lower levels of genital arousal, we expected that cognitive distraction would interfere more directly with the cognitive component of arousal. There are several possible explanations for why the present study did not find an effect of distraction on subjective arousal. One is that the measure of subjective arousal is not as sensitive as the measure of genital arousal to detect differences between groups. A second possibility is that chronic stress elicits a lower level of distraction compared to laboratory manipulations in which participants are required to attend to multiple sources of cognitive input at the same time. A third, related explanation is that in the present study distraction was not manipulated; it was simply measured as a response to stress. The measurement was also indirect and inferred through participants’ scores on the distraction quiz. It is possible that participants were able to pay attention to the arousing stimuli in the film clip, but perhaps not the smaller details they were asked about in the distraction quiz. A more direct measure of attention, such as participant self-report or even an eye-tracking device may show a stronger link between distraction and subjective sexual arousal. Although distraction was not related to subjective arousal, when stress level, cortisol, DHEAS, and distraction scores were entered into a regression as predictors for VPA, the only significant predictor was distraction score.
Using a quiz to assess attention/distraction from erotic material has been used in previous studies and scores have been shown to be related to the level of manipulated distraction.38,48 Even with evidence that this type of measure is related to distraction, it is difficult to identify exactly what this type of measure is assessing. It could be that stress or distraction are causing deficits in memory for recall of the material and not necessarily attention at the time the erotic stimuli is presented. Ideally, future studies could measure attention during presentation of erotic stimuli with technologies such as eye-tracking.
A key issue with the present study, and with most studies of chronic stress in humans, is the lack of experimental manipulation. Most studies of the relationship between stress and sexual arousal have looked at acute stressors that are manipulated in the laboratory, which allows for more experimental control, but is less representative of women’s actual experiences. The goal of the study was to look at the naturally occurring levels of stress in order to get an ecologically valid representation of chronic stress in women’s daily lives. Of course, the trade off is that these data are strictly correlational, and there could be other unmeasured factors that are contributing to the decreased levels of arousal seen in women experiencing high levels of chronic stress.
The sample for this study was also fairly small, and post-hoc power analyses indicated that there was only sufficient power to detect large effect sizes (above .7) for many of the variables. In particular, the DHEAS data had higher variability than the cortisol data, and so any differences between groups could have been masked by the within-group variability. It is possible that the differences in DHEAS were too small to detect with this small sample.
In conclusion, chronic daily stressors are correlated with impairment of women’s genital but not subjective sexual arousal responses both in the laboratory, and in the women’s reports of their sexual functioning over the past four weeks. Increased distraction is the most strongly correlated factor, but increased cortisol, and a lack of increase in SNS activity and DHEAS are mechanisms that may contribute to this effect.
Acknowledgments
This research was supported, in part, by Grant Number R01 HD51676 from the National Institute for Child Health and Human Development to Cindy M. Meston. Lisa Dawn Hamilton was supported by a postgraduate doctoral fellowship from the Natural Sciences and Engineering Research Council (Canada). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute for Child Health and Human Development or the Natural Sciences and Engineering Research Council. This project was part of Lisa Dawn Hamilton’s doctoral dissertation while at the University of Texas at Austin. The authors thank Ashlyn Abell, Kathy Chung, Mallory Koai, and Michelle Milberger for their assistance with data collection for this project.
Footnotes
Conflict of Interest: “None”
References
- 1.Bancroft J, Loftus J, Long JS. Distress about sex: A national survey of women in heterosexual relationships. Arch Sex Behav. 2003;32:193–209. doi: 10.1023/a:1023420431760. [DOI] [PubMed] [Google Scholar]
- 2.Kingsberg SA, Janata JW. Female sexual disorders: Assessment, diagnosis, and treatment. Urol Clin N Am. 2007;34:497–506. doi: 10.1016/j.ucl.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life changes: Development of the Life Experiences Survey. J Consult Clin Psychol. 1978;46:932–946. doi: 10.1037//0022-006x.46.5.932. [DOI] [PubMed] [Google Scholar]
- 4.Kanner AD, Coyne JC, Schaefer C, Lazarus RS. Comparison of two modes of stress measurement: Daily hassles and uplifts versus major life events. J Behav Med. 1981;4:1–39. doi: 10.1007/BF00844845. [DOI] [PubMed] [Google Scholar]
- 5.Lazarus R. Puzzles in the study of daily hassles. J Behav Med. 1984;7:375–389. doi: 10.1007/BF00845271. [DOI] [PubMed] [Google Scholar]
- 6.De Benedittis G, Lorenzetti A. The role of stressful life events in the persistence of primary headache: major events vs. daily hassles. Pain. 1992;51:35–42. doi: 10.1016/0304-3959(92)90006-W. [DOI] [PubMed] [Google Scholar]
- 7.Landreville P, Vezina J. A comparison between daily hassles and major life events as correlates of well-being in older adults. Can J Aging. 11:137–149. [Google Scholar]
- 8.Mair C, Cutchin M, Kristen Peek M. Allostatic load in an environmental riskscape: the role of stressors and gender. Health & Place. 2011;17:978–87. doi: 10.1016/j.healthplace.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McIntosh E, Gillanders D, Rodgers S. Rumination, goal linking, daily hassles and life events in major depression. Clin Psychol Psychother. 2010;17:33–43. doi: 10.1002/cpp.611. [DOI] [PubMed] [Google Scholar]
- 10.Bodenmann G, Ledermann T, Blattner D, Galluzzo C. Associations among everyday stress, critical life events, and sexual problems. J Nerv Ment Dis. 2006;194:494. doi: 10.1097/01.nmd.0000228504.15569.b6. [DOI] [PubMed] [Google Scholar]
- 11.Bodenmann G, Atkins D, Schär M, Poffet V. The association between daily stress and sexual activity. J Fam Psychol. 2010;24:271–9. doi: 10.1037/a0019365. [DOI] [PubMed] [Google Scholar]
- 12.Morokoff P, Gillilland R. Stress, sexual functioning, and marital satisfaction. J Sex Res. 1993;30:43–53. [Google Scholar]
- 13.ter Kuile M, Vigeveno D, Laan E. Preliminary evidence that acute and chronic daily psychological stress affect sexual arousal in sexually functional women. Behav Res Ther. 2007;45:2078–2089. doi: 10.1016/j.brat.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Welsh TH, Kemper-Green KN, Livingston KN. Stress and Reproduction. In: Knobil E, Neill JD, editors. Encyclopedia of Reproduction. San Diego, CA: Academic Press; 1999. pp. 662–674. [Google Scholar]
- 15.McEwen B, Wingfield J. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 16.McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breen KM, Karsch FJ. Does cortisol inhibit pulsatile luteinizing hormone secretion at the hypothalamic or pituitary level? Endocrinology. 2004;145:692–698. doi: 10.1210/en.2003-1114. [DOI] [PubMed] [Google Scholar]
- 18.Gore AC, Attardi B, DeFranco DB. Glucocorticoid repression of the reproductive axis: Effects on GnRH and gonadotropin subunit mRNA levels. Mol Cell Endocrinol. 2006;256:40–48. doi: 10.1016/j.mce.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Olster DH, Ferin M. Corticotropin-releasing hormone inhibits gonadotropin secretion in the ovariectomized rhesus monkey. J Clin Endocrinol Metab. 1987;65:262–267. doi: 10.1210/jcem-65-2-262. [DOI] [PubMed] [Google Scholar]
- 20.Rivier C, Rivest S. Effect of stress on the activity of the hypothalamic-pituitary-gonadal axis: peripheral and central mechanisms. Biol Reprod. 1991;45:523–532. doi: 10.1095/biolreprod45.4.523. [DOI] [PubMed] [Google Scholar]
- 21.Traish A, Kim N, Min K, Munarriz R, Goldstein I. Role of androgens in female genital sexual arousal: receptor expression, structure, and function. Fertil Steril. 2002;77(Suppl 4):S11–8. doi: 10.1016/s0015-0282(02)02978-3. [DOI] [PubMed] [Google Scholar]
- 22.Traish AM, Botchevar E, Kim NN. Biochemical factors modulating female genital sexual arousal physiology. J Sex Med. 2010;7:2925–2946. doi: 10.1111/j.1743-6109.2010.01903.x. [DOI] [PubMed] [Google Scholar]
- 23.Tuiten A, van Honk J, Verbaten R, et al. Can sublingual testosterone increase subjective and physiological measures of laboratory-induced sexual arousal? Arch Gen Psychiatry. 2002;59:465–6. doi: 10.1001/archpsyc.59.5.465. [DOI] [PubMed] [Google Scholar]
- 24.Caldwell JD. A sexual arousability model involving steroid effects at the plasma membrane. Neurosci Biobehav Rev. 2002;26:13–30. doi: 10.1016/s0149-7634(01)00035-5. [DOI] [PubMed] [Google Scholar]
- 25.Spark RF. Dehydroepiandrosterone: A springboard hormone for female sexuality. Fertil Steril. 2002;77(Suppl 4):S19–S25. doi: 10.1016/s0015-0282(02)02987-4. [DOI] [PubMed] [Google Scholar]
- 26.Basson R, Brotto LA, Petkau A, Labrie F. Role of androgens in women’s sexual dysfunction. Menopause. 2010;17:962–971. doi: 10.1097/gme.0b013e3181d59765. [DOI] [PubMed] [Google Scholar]
- 27.Guay A, Jacobson J, Munarriz R, Traish A, Talakoub L, Quirk F, Goldstein I, Spark R. Serum androgen levels in healthy premenopausal women with and without sexual dysfunction: Part B: Reduced serum androgen levels in healthy premenopausal women with complaints of sexual dysfunction. Int J Impot Res. 2004;16:121–9. doi: 10.1038/sj.ijir.3901176. [DOI] [PubMed] [Google Scholar]
- 28.Lennartsson A-K, Kushnir MM, Bergquist J, Jonsdottir IH. DHEA and DHEA-S response to acute psychosocial stress in healthy men and women. Biol Psychol. 2012;90:143–149. doi: 10.1016/j.biopsycho.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Maninger N, Capitanio JP, Mason WA, Ruys JD, Mendoza SP. Acute and chronic stress increase DHEAS concentrations in rhesus monkeys. Psychoneuroendocrinology. 2010;35:1055–1062. doi: 10.1016/j.psyneuen.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Izawa S, Saito K, Shirotsuki K, Sugaya N, Nomura S. Effects of prolonged stress on salivary cortisol and dehydroepiandrosterone: A study of a two-week teaching practice. Psychoneuroendocrinology. 2012;37:852–858. doi: 10.1016/j.psyneuen.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Moriguchi Jeckel CM, Lopes RP, Berleze MC, et al. Neuroendocrine and immunological correlates of chronic Stress in “strictly healthy” populations. Neuroimmunomodulation. 2010;17:9–18. doi: 10.1159/000243080. [DOI] [PubMed] [Google Scholar]
- 32.Bonne O, Grillon C, Vythilingam M, Neumeister A, Charney DS. Adaptive and maladaptive psychobiological responses to severe psychological stress: implications for the discovery of novel pharmacotherapy. Neurosci Biobehav Rev. 2004;28:65–94. doi: 10.1016/j.neubiorev.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Lambert E, Lambert G. Stress and its role in sympathetic nervous system activation in hypertension and the metabolic syndrome. Curr Hypertens Rep. 2011;13:244–248. doi: 10.1007/s11906-011-0186-y. [DOI] [PubMed] [Google Scholar]
- 34.Meston CM. Sympathetic nervous system activity and female sexual arousal. Am J Cardiol. 2000;86:30–34. doi: 10.1016/s0002-9149(00)00889-4. [DOI] [PubMed] [Google Scholar]
- 35.Lazarus RS. Psychological Stress and the Coping Process. New York: McGraw Hill; 1966. [Google Scholar]
- 36.Barlow DH. Causes of sexual dysfunction: The role of anxiety and cognitive interference. J Consult Clin Psychol. 1986;54:140–148. doi: 10.1037//0022-006x.54.2.140. [DOI] [PubMed] [Google Scholar]
- 37.Adams AE, Haynes SN, Brayer MA. Cognitive Distraction in Female Sexual Arousal. Psychophysiology. 1985;22:689–696. doi: 10.1111/j.1469-8986.1985.tb01669.x. [DOI] [PubMed] [Google Scholar]
- 38.Elliott AN, O’Donohue WT. The effects of anxiety and distraction on sexual arousal in a nonclinical sample of heterosexual women. Arch Sex Behav. 1997;26:607–624. doi: 10.1023/a:1024524326105. [DOI] [PubMed] [Google Scholar]
- 39.Salemink E, van Lankveld J. The effects of increasing neutral distraction on sexual responding of women with and without sexual problems. Arch Sex Behav. 2006;35:175–186–186. doi: 10.1007/s10508-005-9014-2. [DOI] [PubMed] [Google Scholar]
- 40.Chivers ML, Rieger G, Latty E, Bailey JM. A sex difference in the specificity of sexual arousal. Psychol Sci. 2004;15:736–744. doi: 10.1111/j.0956-7976.2004.00750.x. [DOI] [PubMed] [Google Scholar]
- 41.Harte CB, Meston CM. The inhibitory effects of nicotine on physiological sexual Arousal in nonsmoking Women: Results from a randomized, double-blind, placebo-controlled, cross-over trial. J Sex Med. 2008;5:1184–1197. doi: 10.1111/j.1743-6109.2008.00778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bradford A, Meston CM. The impact of anxiety on sexual arousal in women. Behav Res Ther. 2006;44:1067–1077. doi: 10.1016/j.brat.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lorenz TA, Harte CB, Hamilton LD, Meston CM. Evidence for a curvilinear relationship between sympathetic nervous system activation and women’s physiological sexual arousal. Psychophysiology. 2012;49:111–117. doi: 10.1111/j.1469-8986.2011.01285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Measure. 1977;1:385–401. [Google Scholar]
- 45.Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): A Multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 46.Wiegel M, Meston CM, Rosen R. The Female Sexual Function Index (FSFI): Cross-validation and development of clinical cutoff scores. J Sex Marital Ther. 2005;31:1–20. doi: 10.1080/00926230590475206. [DOI] [PubMed] [Google Scholar]
- 47.Seal BN, Meston CM. The impact of body awareness on sexual arousal in women with sexual dysfunction. J Sex Med. 2007;4:990–1000. doi: 10.1111/j.1743-6109.2007.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson AA, Hamilton LD. The assessment of distraction from erotic stimuli by non-erotic interference. Poster presented at the annual meeting of the Canadian Sex Research Forum; 2012. [Google Scholar]
- 49.Sintchak G, Geer JH. A vaginal plethysmograph system. Psychophysiology. 1975;12:113–115. doi: 10.1111/j.1469-8986.1975.tb03074.x. [DOI] [PubMed] [Google Scholar]
- 50.Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 51.Rellini AH, McCall KM, Randall PK, Meston CM. The relationship between women’s subjective and physiological sexual arousal. Psychophysiology. 2005;42:116–124. doi: 10.1111/j.1469-8986.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 52.Nicolson NA. Measurement of cortisol. In: Luecken LJ, Gallo LC, editors. Handbook of physiological research methods in health psychology. Los Angeles, CA: Sage; 2008. pp. 37–74. [Google Scholar]
- 53.Niskanen J-P, Tarvainen MP, Ranta-aho PO, Karjalainen PA. Software for advanced HRV analysis. Comput Methods Programs Biomed. 2004;76:73–81. doi: 10.1016/j.cmpb.2004.03.004. [DOI] [PubMed] [Google Scholar]