Abstract
Using current treatment regimens, over 90% of patients with acute promyelocytic leukemia will achieve complete remission (CR). However, approximately 30% of these patients will relapse, including a small proportion who will develop extramedullary disease (EMD). In this study, we investigated the incidence of EMD in 263 patients with APL who were treated at our institution from January 1990 to May 2008. With a median follow-up of 31 months (range 2 days–203 months), 8 (3%) patients developed EMD. The most commonly affected site was the central nervous system (n = 7). Before developing EMD, one patient had achieved CR with a chemotherapy-only regimen, six patients had achieved CR with all-trans-retinoic acid (ATRA)-based regimens, and one patient had achieved CR with an ATRA plus arsenic trioxide (ATO)-based regimen. The EMD conferred a poor prognosis; five patients died within 4 months of developing EMD. The molecular status did not predict EMD; four patients had a negative PCR for the PML-RARA transcripts prior to relapse with EMD. In conclusion, the incidence of EMD is low. We were unable to identify any specific factors that could predict the development of EMD.
Keywords: Acute promyelocytic leukemia, Extramedullary disease, All-trans-retinoic acid, Trioxide arsenic
1 Introduction
With the modern treatment regimens, approximately 90% of patients with newly diagnosed acute promyelocytic leukemia (APL) will achieve complete remission (CR) [1, 2]. It is expected that 70–80% of these patients will remain in remission [1, 3]. However, approximately 20–30% of patients will eventually relapse [2, 4]. Factors reported to be associated with a high risk of disease relapse are as follows: presenting white blood cell (WBC) count > 10,000/µL, age over 55 years, CD56 expression, FLT3 gene mutations, evidence of the variable isoform type of the promyelocytic leukemia-retinoic acid receptor alpha (PML-RARA) fusion transcript, and human leukocyte antigen-B13 positivity [2, 3, 5]. Around 5% of patients will have a molecular relapse only, but this is generally followed by a hematologic relapse within 2–20 weeks [2, 3, 6].
Extramedullary disease (EMD) is a rare complication in APL. There are anecdotal cases of EMD at presentation, but this is very uncommon [7]. It is estimated that about 3–5% of patients will have an extramedullary relapse [2, 8]. The occurrence of EMD has been attributed to the use of all-trans-retinoic acid (ATRA), but not all studies support such a higher incidence when compared with the chemotherapy-only treatment era [2, 9–11]. Other factors have been associated with a higher risk of extramedullary relapse, including younger age, a high WBC count at diagnosis, microgranular morphology, expression of CD2 and/or CD56, predominance of the PML-RARA bcr3 isoform, M3 variant disease, differentiation syndrome, use of monotherapy-only regimens, and use of regimens that exclude cytarabine [2, 4, 8, 12, 13]. EMD has been reported to commonly occur within 1 year of achieving CR, but it can occur at any time during the disease course and can be isolated or can precede systemic relapse [2, 9, 10, 12, 14]. The reported sites of extramedullary involvement include the inner ear, central nervous system (CNS), skin, lung, pleura, heart, pericardium lymph nodes, mediastinum, thymus, spine, breast, pelvis, mandible, gingival areas, and vascular access sites, and the most common sites are the skin and CNS [2, 3, 8, 15–17]. Patients with EMD generally have a poor prognosis, with a median survival of only a few months [4].
Patients may experience EMD more frequently because they are achieving longer survival times as a result of current treatment regimens [8, 10]. Another explanation could be the emergence of sanctuary sites where ATRA and arsenic trioxide (ATO) do not achieve therapeutic concentrations in patients who did not receive induction chemotherapy [8, 10]. There is also evidence indicating that ATRA and ATO increase the expression of adhesion molecules in blasts, which could facilitate migration of the cells to different tissues, resulting in EMD [8, 10, 18]. The EMD treatment strategies have been diverse and case-specific and have included radiation for local disease, intrathecal chemotherapy for CNS disease, and combinations of agents used to treat primary APL. After achieving CR, autologous or allogenic stem cell transplants have been used as consolidation therapy, depending on the clinical situation and molecular status of the patients [4, 12, 16, 19–23].
Here, we present our experience with EMD at the University of Texas M. D. Anderson Cancer Center. We sought to determine the incidence of EMD and the possible factors associated with EMD development.
2 Patients
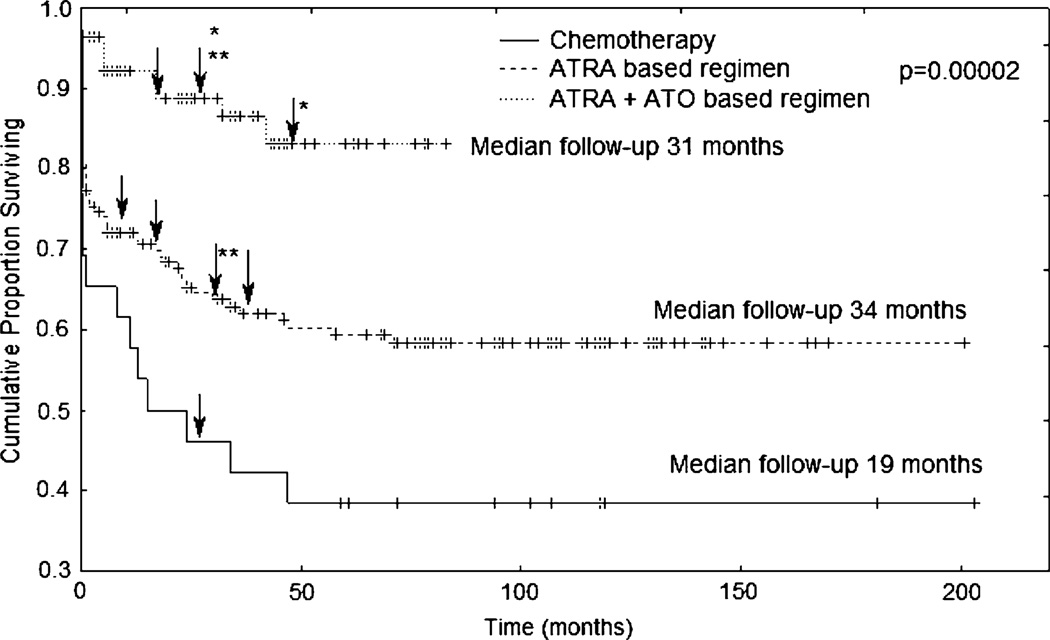
We reviewed the clinical records of patients who were diagnosed with APL between January 1990 and May 2008 at the M. D. Anderson Cancer Center. For our search, we used the Leukemia department’s patient database (VA file manager application) and the M. D. Anderson Clinic Station’s patient reports. Our search included all patients with APL who received treatment at our institution, regardless of whether they were treated with frontline or salvage regimens. From the 263 patients found on our search, 232 (88.2%) received frontline regimens, and 31 (11.8%) were treated with salvage regimens using one of 16 different regimens (Table 1). The median follow-up time for the 263 patients was 31 months (range 2 days–203 months). Follow-up was defined from the time of presentation at our institution until death or last contact with the Leukemia clinic by personal visit, phone call, or scanned document informing the clinical status of the patient. The 16 treatment regimens were divided into three groups: chemotherapy-only regimens, ATRA-based regimens, and ATRA plus ATO-based regimens (Table 1). We produced Kaplan–Meier estimates of overall survival for each treatment group, and we used the log-rank test to determine statistical significance. We considered a P value ≤ 0.05 to be significant (Fig. 1). Eight (3%) cases of extramedullary leukemia were identified. Among these, only five patients had complete immunophenotyping at the time of diagnosis as well as follow-up evaluations with reverse transcriptase polymerase chain reaction (RT-PCR) for the PML-RARA transcript. We describe briefly the history of each patient below. Patient characteristics, detailed treatment, molecular status before developing EMD and outcome are summarized in Table 2.
Table 1.
Treatment groups according to the combination of drugs used
| Number of patients | ||||||||
|---|---|---|---|---|---|---|---|---|
| Death during induction |
Alive first remission |
Alive after relapse |
Death after relapse |
Death in remission |
Total | Median Follow-up (months) |
EMD | |
| Chemotherapy-only regimens | ||||||||
| Amsacrine | – | – | – | 1 | – | 1 | 0.5 | 0 |
| Amsacrine + HDAC | 2 | 6 | 1 | 4 | 1 | 14 | 53 (1–203) | 1 |
| Daunorubicine + HDAC | 1 | – | – | – | – | 1 | 0.5 | 0 |
| Thioguanine + cyclophosphamide + total body iradiation | – | – | 1 | – | – | 1 | 181 | 0 |
| Fludarabine + cytarabine + carboplatine | – | – | 1 | – | – | 1 | 102 | 0 |
| Fludarabine + HDAC | – | – | – | 1 | – | 1 | 0.5 | 0 |
| Idarubicin | 1 | 1 | – | 4 | 1 | 7 | 1 (0.5–118) | 0 |
| Total | 4 (15%) | 7 (27%) | 3 (12%) | 10 (38%) | 2 (8%) | 26 | 19 (0.5–203) | 1 |
| ATRA-based regimens | ||||||||
| ATRA | – | – | 1 | 1 | – | 2 | 13 (0.1–102) | 0 |
| ATRA + idarubicin | 13 | 33 | 8 | 15 | 7 | 76 | 30 (0.1–201) | 3 |
| Liposomal ATRA | 8 | 16 | 12 | 5 | – | 41 | 71 (0.1–142) | 1 |
| ATRA + gemtuzumab ozogamicin | 7 | 21 | 4 | 2 | – | 34 | 24 (0.1–83) | 0 |
| Total | 28 (18.3%) | 70 (46%) | 25 (16%) | 23 (15%) | 7 (4.6%) | 153 | 34 (0.1201) | 4 |
| ATRA + ATO-based regimens | ||||||||
| ATRA + ATO | 1 | 3 | 1 | – | 1 | 6 | 7 (0.1–63) | 0 |
| ATRA + ATO ± idarubicin ± gemtuzumab ozogamicin | 2 | 45 | 1 | 1 | 3 | 52 | 36 (0.2–79) | 1 |
| ATRA + ATO + gemtuzumab ozogamicin | 0 | 3 | – | 1 | – | 4 | 40 (10–78) | 2 |
| ATRA + ATO ± gemtuzumab ozogamicin ± theophylline | 1 | 19 | – | – | – | 20 | 4.5 (0.3–11) | 0 |
| ATO | – | 0 | 1 | 1 | – | 2 | 19 (0.3–83) | 0 |
| Total | 4 (4.7%) | 70 (83.3%) | 3 (3.6%) | 3 (3.6%) | 4 (4.7%) | 84 | 31 (0.1–83) | 3 |
ATO arsenic trioxide, ATRA all-trans-retinoic acid, EMD extramedullary disease, HDAC high-dose cytarabin
Fig. 1.
Overall survival according to the treatment regimens used at the moment patients were diagnosed with extramedullary disease. Arrows indicate when extramedullary disease presented. *Patients were enrolled on this regimen as salvage therapy for previous systemic relapse. **Patients who were still alive at the time of this report
Table 2.
Clinical characteristics of patients with extramedullary disease
| Case | Sex and age (years) | White blood cell count at diagnosis |
Induction/consolidation treatment and salvage treatment |
Time to present to EMD | Site of EMD | Molecular status before EMD |
Treatment and outcome of EMD |
|---|---|---|---|---|---|---|---|
| 1 | Female 54 |
Pancytopenia (exact white blood cell count N/A) |
Induction and consolidation: cytarabine plus amsacrine Reinduction: daunorubicin plus cytarabine |
27 months after diagnosis EMD accompanied second systemic relapse |
CNS | N/A | Systemic and intrathecal cytarabine Death 7 days later |
| 2 | Male 22 |
N/A | Induction: idarubicin, cytarabine plus ATRA. Consolidation: idarubicin, mitoxantrone and etoposide Maintenance: methotrexate plus mercaptopurine |
11 months after diagnosis No systemic relapse |
Scalp, lymph nodes and CNS |
N/A (diploid cytogenetics) |
Intrathecal chemotherapy and autologous transplantation Death 5 weeks later |
| 3 | Male 32 |
3.4 × 109/dL | Induction: ATRA plus Idarubicin Consolidation: idarubicin Reinduction: ATRA, etoposide and mitoxantrone |
19 months after diagnosis EMD presented shortly before second systemic relapse |
CNS | N/A (Southern blot (−) for the PML-RARA gene |
Intratechal and systemic cytarabine plus idrubicine. Death a few days later from infectious complications |
| 4 | Male 51 |
Pancytopenia (exact white blood cell count N/A) |
Induction: idarubicin plus ATRA Consolidation: idarubicin. Maintenance: POMP |
37 months after diagnosis Systemic relapse presented after second and third EMD relapses |
Thoracic spine Second and third relapse: skin and CNS |
RT-PCR (−) PML-RARA transcript |
Radiotherapy and Idarubicn plus ATRA. Gemtuzumab plus ATO Death after third relapse |
| 5 | Female 47 |
Pancytopenia (exact white blood cell count N/A) |
Induction and consolidation: liposomal ATRA Second salvage treatment: gemtuzumab and ATRA plus autologous transplantation |
27 months after diagnosis EMD presented with PCR (+) on bone marrow |
Breasts | RT-PCR (−) PML-RARA transcript |
ATRA plus ATO Remains in remission |
| 6 | Male 79 |
24.2 × 109/dL | Induction: ATRA plus ATO plus gemtuzumab Consolidation: ATRA plus ATO Reinduction: gemtuzumab |
15 months after diagnosis EMD presented three months after starting reinduction |
CNS | RT-PCR (−) PML-RARA transcript |
Intrathecal cytarabine Death after 2 weeks |
| 7 | Female 31 |
N/A | Induction: ATRA Reinduction treatments: ATO, ATRA plus autologous transplantation; ATO, ATRA plus gemtuzumab |
50 months after diagnosis and three systemic relapses |
CNS | RT-PCR (−) PML-RARA transcript |
ATRA plus Idarubicin and intrathecal cytarabine Death one month later from infectious complications |
| 8 | Male 57 |
N/A | Induction: idarubicin, cytarbine and ATRA Consolidation: ATRA plus Idarubicin Maintenance: ATRA, mercaptopurine and methotrexate Reinduction: ATRA, ATO plus gemtuzumab. |
23 months after diagnosis EMD presented during reinduction for systemic relapse |
CNS | RT-PCR (+) PML-RARA transcript |
Intrathecal cytarabine Patient continued reinduction |
EMD Extramedullary disease, N/A not available, CNS central nervous system, ATRA all-trans-retinoic acid, POMP prednisone, vincristine, methotrexate and mercaptopurine, ATO arsenic trioxide
3 Case reports
3.1 Case 1
In November 1988, a 54-year-old female was admitted with a cerebrovascular event and a bone marrow aspiration showed APL. Induction therapy consisted of cytarabine plus amsacrine. Systemic relapse occurred 18 months after achieving CR, and re-induction therapy was initiated. The patient achieved a second CR. In May 1991, she developed altered mental status and lower right-sided paresis. The cerebrospinal fluid (CSF) was positive for leukemic promyelocytes. Five days later, blasts were observed in the patient’s peripheral blood. The patient died as a result of a pontomesencephalic infarct.
3.2 Case 2
In May 1992, a 22-year-old male was diagnosed with APL. The patient achieved CR in July 1992. In April 1993, the patient presented with scalp nodules, which were consistent with an extramedullary relapse on biopsy. Systemic relapse was not confirmed. A CSF evaluation was then performed because of persistent headaches, which revealed CNS leukemia. Intrathecal chemotherapy cleared the blasts from CSF. In May 1993, physical examination revealed cervical lymphadenopathy, and biopsy revealed leukemia.
3.3 Case 3
In February 1993, a 32-year-old male was admitted with a history of bleeding and fever. CBC showed 71% promyelocytes. A cytogenetic evaluation revealed a variant translocation between chromosomes 1, 15, and 17. Induction therapy consisted of ATRA plus idarubicin. Remission was confirmed and Southern blot analysis was negative for the PML-RARA gene. In January 1994, the patient had systemic relapse. Salvage therapy with ATRA was then started. A second remission was documented. In September 1994, the patient presented with neck rigidity, and his CSF showed leukemic promyelocytes. Shortly after, the patient had a systemic relapse.
3.4 Case 4
In March 1996, a 51-year-old male was diagnosed with APL. Immunophenotyping was positive for CD2, CD13, CD38, and CD117. Induction therapy with ATRA and idarubicin was commenced. After 37 months in remission, the patient presented with acute onset of paraplegia. A CT scan demonstrated spinal cord compression at the T7–T8 vertebral levels. Bone marrow evaluation was negative for relapse. The patient then underwent surgical decompression, and pathology was compatible with granulocytic sarcoma. After completing radiotherapy, consolidation therapy was then started. After finishing consolidation therapy a PCR was negative for PML-RARA. In February 2001, he complained of a rash on his back. Circulating promyelocytes were observed on peripheral blood. Skin biopsy showed infiltration by myeloblasts. Therapy with ATO, idarubicin, and gemtuzumab ozogamicin was initiated. He achieved a third CR. Two PCR tests were negative for PML-RARA. In December 2001, he had a seizure followed later by central blindness. A CSF examination showed blasts, and bone marrow aspiration revealed a third relapse. Cytogenetics showed two pseudodiploid clones positive for t(15;17), del(6,7, and 8), and add(3,10, and 17).
3.5 Case 5
In February 2002, a 47-year-old female presented with fever. A bone marrow aspiration was consistent with APL. Immunophenotyping was positive for CD13, CD33, and CD117. RT-PCR showed the long form of the PML-RARA transcript. She started induction therapy with liposomal ATRA and achieved CR. She was followed every 4 months without molecular relapse. After 27 months in remission, a routine mammogram showed multiple masses in both breasts. Ultrasound-guided fine-needle aspiration showed infiltration by cells with granules. On fluorescence in situ hybridization (FISH) 98% of the cells were positive for PML-RARA. Bone marrow RT-PCR was positive for the PML-RARA fusion gene. She was treated with ATRA and ATO. She achieved molecular remission in the marrow. After 9 months, a routine bone marrow exam showed relapse. She began therapy with gemtuzumab ozogamicin and ATRA. She achieved a third CR. In February 2004, the patient underwent an autologous stem cell transplant.
In January 2003, a 79-year-old man was referred with a history of fever and easy bruising. A CBC revealed 5% blasts. Immunophenotyping was positive for myeloperoxidase, CD13, CD33, and CD117. Cytogenetics was positive for a clone with t(15;17) and der(17) i(17). He began induction therapy with gemtuzumab ozogamicin, ATRA, and ATO. After seven courses of consolidation therapy, PCR became negative. In March 2004, cytogenetics showed a pseudodiploid clone with t(15;17), der(17); inv(17). He then received gemtuzumab ozogamicin monthly and after the third course presented with altered mental state. A CSF examination revealed APL. The CBC was within normal parameters. He was treated with intrathecal chemotherapy, but the CSF remained positive for promyelocytes.
3.7 Case 7
A 31-year-old woman was diagnosed with APL in 2003, achieved CR, and received maintenance therapy with ATRA. Her disease relapsed in September 2005, and she was treated with ATO. She achieved a second CR and a quantitative PCR for PML-RARA was negative. In August 2006, she underwent an autologous stem cell transplant. In December 2006, cytogenetics showed a clone with t(15;17) and additional abnormalities (chromosomes 21, 2, and 10 trisomy and chromosomes X and 16 deletion). She started salvage therapy with ATO until achieving a third CR when she started consolidation therapy with ATO, ATRA, and gemtuzumab ozogamicin. After the third course of treatment, PCR was negative for PML-RARA. In February 2008, she complained of headaches and bone pain. Bone marrow exam confirmed third relapse, and a CSF examination was positive for blasts.
3.8 Case 8
A 57-year-old male was initially diagnosed with APL in December 2005 and received induction therapy with idarubicin, cytarabine, and ATRA. He achieved CR and proceeded to receive consolidation and maintenance therapy. The PCR was negative for the PML-RARA transcript after consolidation therapy. In September 2007, the patient experienced a systemic relapse. Immunophenotype was positive for myeloperoxidase, CD2, CD13, CD33, CD34, CD56, and CD117. He started salvage therapy with ATO, ATRA, and gemtuzumab ozogamicin. However, the patient lost consciousness 2 weeks after starting therapy. An MRI showed subacute infarct/hemorrhage involving right inferior basal ganglia and low frontal lesions with peripheral enhancement and possible ependymal involvement. The CSF examination showed a high WBC count, with neutrophil predominance. RT-PCR and FISH were positive for PML-RARA in the CSF. The patient’s brain lesions improved after starting treatment with intrathecal cytarabine. He achieved CR with a negative PCR, 12 weeks after starting therapy. Further CSF examinations performed weekly did not show evidence of CNS leukemia.
4 Discussion
The incidence of EMD in our study is similar to previous reports [2, 8]. This was a heterogeneous cohort of patients with extramedullary APL. The median age was 49 years (range 22–79 years), and they were treated with different regimens prior to developing EMD. However, unlike previous reports, we were unable to identify any specific factors that were associated with EMD. High WBC at disease presentation has been reported to be a risk factor. In our series, only one patient presented with a high WBC; four were pancytopenic, and the presenting CBC was not available for three patients. There were no cases of EMD at diagnosis. One patient presented with EMD without systemic relapse; the other seven patients developed systemic relapse almost concomitantly with EMD. Five patients died within 4 months of developing EMD.
The use of ATRA at induction has been suggested as a potential reason for increased incidence of EMD. However, due to small numbers, we were unable to conclude that there was an association between ATRA use and EMD. Longer survival time has also been suggested to allow for the development of EMD. We found a clear difference in the overall survival of patients according to treatment regimens used (Fig. 1). Three different treatment eras were identified; the time before the use of ATRA, the ATRA era, and more recently the introduction of ATO. Among patients with EMD, one patient (12.5%) received induction treatment with a chemotherapy-only regimen, six patients (75%) received induction with ATRA-based regimens, and one patient (12.5%) received induction with an ATRA plus ATO-based regimen. The median follow-up time for these eight patients was 25 months (range 11–50 months). The patient in the chemotherapy-only group developed EMD at 27 months, the six patients who received first induction with ATRA-based regimens presented with EMD at a median follow-up time of 25 months (range 19–50 months), and the patient who received first induction with ATRA plus ATO presented with EMD at 15 months.
The use of cytarabine has also been suggested to be protective against EMD. Of our eight patients with EMD, three (37.5%) had received cytarabine, including two who had also received ATRA. Therefore, it was difficult to assess the potential role of lack of cytarabine in EMD development.
The most frequent site of EMD in our patients was the CNS, as has been reported previously. Seven of the eight patients in this study presented with CNS leukemia, and a CNS relapse was invariably accompanied by a systemic relapse or preceded a systemic relapse by a few days.
The best treatment for CNS disease is still controversial, because it is unclear whether ATRA and ATO cross the blood-brain barrier; nevertheless, some authors have reported responses to these agents in patients with meningeal disease. This may be due to the disease already disrupting the blood-brain barrier [10, 12, 14]. Some reports have suggested that CNS disease could respond to ATRA had the disease been responsive previously to ATRA [24]. ATO has also been reported to cross the blood-brain barrier and may be able to control disease in patients with CNS relapse. However, other authors have suggested that ATO does not achieve adequate therapeutic concentrations in the CSF [25, 26].
A proposed strategy to improve the cure rate and decrease the possibility of EMD is the application of intrathecal chemotherapy in patients with newly diagnosed APL [12, 27]. However, the available literature does not fully justify this approach, except maybe in patients with high-risk disease [28]. The molecular status in the peripheral blood/bone marrow did not seem to predict the possibility of EMD relapse. In our study, four patients had no evidence of disease on PCR when they finished the first courses of consolidation therapy, and one patient had no evidence of disease on PCR after several courses of consolidation therapy. However, despite their negative molecular status, all of them relapsed. The use of RT-PCR to detect disease in the CSF may be useful for evaluating patients with high-risk disease at diagnosis in order to identify potential candidates for CNS prophylaxis. Studies are needed to confirm the feasibility and utility of this approach. Many patients have a high risk of hemorrhage at diagnosis, but a CSF evaluation could be performed after consolidation therapy is complete.
In the current study, all patients with CNS disease were treated with intrathecal chemotherapy; systemic chemotherapy was used concomitantly or soon after. No single strategy was used for the treatment of CNS relapse. Diverse combinations of agents were used depending on the previous treatment history. Six of the seven patients with CNS disease died within 4 months of its development, with four patients experiencing systemic relapse. Because of this, it is extremely difficult to determine if any therapeutic approach was better than another.
Finally, it is impossible to compare the characteristics of the patients with and without EMD in a meaningful way in this study due to the long study period, large variability of the treatment regimens used, and the small numbers of patients who developed EMD. The purpose of this article was to indicate the low incidence of EMD both in the ATRA and arsenic era as well as the pre-ATRA era which could potentially be due to different factors (e.g., shorter overall survival in the pre-ATRA area or more effective therapy with ATRA and ATO-based regimens).
In conclusion, EMD in APL is uncommon and is associated with a poor prognosis; it typically precedes or is accompanied by systemic relapse. Much has been discussed on whether the differentiating agent ATRA, by changing the natural course of the disease, increases the risk of EMD. However, our findings, along with other reports suggest no clear factors predicting the development of EMD. Effective frontline therapy and preventing systemic relapse is likely to decrease the risk of EMD as these tend to be concurrent. Whether any specific frontline regimen such as ATRA plus ATO-based regimens or those containing cytarabine will decrease the risk of EMD is not clear.
Acknowledgment
There is no financial support for the study.
Footnotes
Conflict of interest statement There is no conflict of interest related to the study for any of the authors.
References
- 1.Wang ZY, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008;111:2505–2515. doi: 10.1182/blood-2007-07-102798. [DOI] [PubMed] [Google Scholar]
- 2.Tallman MS. Treatment of relapsed or refractory acute promyelocytic leukemia. Best Pract Res Clin Haematol. 2007;20:57–65. doi: 10.1016/j.beha.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Lengfelder E, Saussele S, Weisser A, Büchner T, Hehlmann R. Treatment concepts of acute promyelocytic leukemia. Crit Rev Oncol Hematol. 2005;56:261–274. doi: 10.1016/j.critrevonc.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 4.de Botton S, Sanz MA, Chevret S, Dombret H, Martin G, Thomas X, et al. Extramedullary relapse in acute promyelocytic leukemia treated with all-trans retinoic acid and chemotherapy. Leukemia. 2006;20:35–41. doi: 10.1038/sj.leu.2404006. [DOI] [PubMed] [Google Scholar]
- 5.Bolognesi E, Cimino G, Diverio D, Rapanotti MC, D’Alfonso S, Fleischhauer K, et al. HLA class I in acute promyelocytic leukemia (APL): possible correlation with clinical outcome. Leukemia. 2000;14:393–398. doi: 10.1038/sj.leu.2401691. [DOI] [PubMed] [Google Scholar]
- 6.Diverio D, Rossi V, Avvisati G, De Santis S, Pistilli A, Pane F, et al. Early detection of relapse by prospective reverse transcriptase-polymerase chain reaction analysis of the PML-RARA fusion gene in patients with acute promyelocytic leukemia enrolled in the GIMEMA-AIEOP multicenter AIDA trial. Blood. 1998;92:784–789. [PubMed] [Google Scholar]
- 7.Worch J, Ritter J, Fruhwald MC. Presentation of acute promyelocytic leukemia as granulocytic sarcoma. Pediatr Blood Cancer. 2008;50:657–660. doi: 10.1002/pbc.21190. [DOI] [PubMed] [Google Scholar]
- 8.Raanani P, Shpillberg O, Ben-Bassat I. Extramedullary disease and targeted therapies for hematological malignancies—is the association real? Ann Oncol. 2007;18:7–12. doi: 10.1093/annonc/mdl129. [DOI] [PubMed] [Google Scholar]
- 9.Specchia G, Lo Coco F, Vignetti M, Avvisati G, Fazi P, Albano F, et al. Extramedullary involvement at relapse in acute promyelocytic leucemia patients treated or not with all-trans retinoic acid: a report by the Gruppo Italiano Malattie Ematologiche dell’Adulto. J Clin Oncol. 2001;19:4023–4028. doi: 10.1200/JCO.2001.19.20.4023. [DOI] [PubMed] [Google Scholar]
- 10.Evans GD, Grimwade DJ. Extramedullary disease in acute promyelocytic leukemia. Leuk Lymphoma. 1999;33:219–229. doi: 10.3109/10428199909058422. [DOI] [PubMed] [Google Scholar]
- 11.Wiernik PH, De Bellis R, Muxi P, Dutcher JP. Extramedullary acute promyelocytic leukemia. Cancer. 1996;78:2510–2514. doi: 10.1002/(sici)1097-0142(19961215)78:12<2510::aid-cncr10>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 12.Breccia M, Carmosino I, Diverio D, De Santis S, De Propris MS, Romano A, et al. Early detection of meningeal localization in acute promyelocytic leukaemia patients with high presenting leucocyte count. Br J Haematol. 2003;120:266–270. doi: 10.1046/j.1365-2141.2003.04056.x. [DOI] [PubMed] [Google Scholar]
- 13.Lionel Ades, Sanz Miguel A, Chevret S, Montesinos P, Chevallier P, Raffoux E, et al. Treatment of newly diagnosed acute promyelocytic leukemia (APL): a comparison of French-Belgian-Swiss and Pethema results. Blood. 2008;111:1078–1084. doi: 10.1182/blood-2007-07-099978. [DOI] [PubMed] [Google Scholar]
- 14.Patriarca F, Fili C, Antonella G, Sperotto A, Prosdocimo S, Fanin R. Activity of all-trans-retinoic acid in a case of central nervous system extramedullary relapse of acute promyelocytic leukemia. Eur J Haematol. 2002;68:310–313. doi: 10.1034/j.1600-0609.2002.01660.x. [DOI] [PubMed] [Google Scholar]
- 15.Breccia M, Petti MC, Testi AM, Specchia G, Ferrara F, Diverio D, et al. Ear involvement in acute promyelocytic leukemia at relapse: a disease-associated ‘sanctuary’? Leukemia. 2002;16:1127–1130. doi: 10.1038/sj.leu.2402497. [DOI] [PubMed] [Google Scholar]
- 16.Tsimberidou AM, Estey E, Whitman G, Dryden MJ, Ratnam S, Pierce S, et al. Extramedullary relapse in a patient with acute promyelocytic leukemia: successful treatment with arsenic trioxide, all-trans retinoic acid and gemtuzumab ozogamicin therapies. Leuk Res. 2004;28:991–994. doi: 10.1016/j.leukres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Nasilowska-Adamska B, Majewski M, Seferynska I, Szczepinski A, Tomaszewska A, Prochorec-Sobieszek M, et al. Predictive value of RT-PCR PML-RARA transcript monitoring for extra-medullary relapse of acute promyeloytic leukemia in the pleura, heart and pericardium after allogeneic SCT. Ann Transplant. 2007;12:33–38. [PubMed] [Google Scholar]
- 18.Cunha De Santis G, Tamarozzi MB, Sousa RB, Moreno SE, Secco D, Garcia AB, et al. Adhesion molecules and differentiation syndrome: phenotypic and functional analysis of the effect of ATRA, As2O3, phenylbutyrate, and G-CSF in acute promyelocytic leukemia. Haematologica. 2007;92:1615–1622. doi: 10.3324/haematol.10607. [DOI] [PubMed] [Google Scholar]
- 19.Classen CF, Debatin KM, Friedrich W, Schulz AS. Long-term remission of APL with a second allogeneic BMT after CNS relapse following HLA-identical allogeneic BMT. Bone Marrow Transplant. 2003;32:843–846. doi: 10.1038/sj.bmt.1704225. [DOI] [PubMed] [Google Scholar]
- 20.Aribi A, Kantarjian HM, Estey EH, Koller CA, Thomas DA, Kornblau SM, et al. Combination therapy with arsenic trioxide, all-transretinoic acid, and gemtuzumab ozogamicin in recurrent acute promyelocytic leukemia. Cancer. 2007;109:1355–1359. doi: 10.1002/cncr.22524. [DOI] [PubMed] [Google Scholar]
- 21.Kai T, Kimura H, Shiga Y, Ogawa K, Sato H, Maruyama Y, et al. Recurrent extramedullary relapse of acute promyelocytic leukemia after allogeneic stem cell transplantation: successful treatment by arsenic trioxide in combination with local radiotherapy. Int J Hematol. 2006;83:337–340. doi: 10.1532/IJH97.05167. [DOI] [PubMed] [Google Scholar]
- 22.Dvorak CC, Sanders RP, Dahl GV, Donaldson SS, Razzouk BI. Reinduction of relapsed acute promyelocytic leukemia with ATRA and low dose antimetabolite-based therapy. Pediatr Blood Cancer. 2007;48:582–585. doi: 10.1002/pbc.20592. [DOI] [PubMed] [Google Scholar]
- 23.Kharfan-Dabaja MA, Abou Mourad YR, Fernandez HF, Pasquini MC, Sanots ES. Hematopoietic cell transplantation in acute promyelocytic leukemia: a comprehensive review. Biol Blood Marrow Transplant. 2007;13:997–1004. doi: 10.1016/j.bbmt.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Burry LD, Seki JT. CNS relapses of acute promyelocytic leukemia after all-trans retinoic acid. Ann Pharmacother. 2002;36:1900–1906. doi: 10.1345/aph.1A471. [DOI] [PubMed] [Google Scholar]
- 25.Helwig A, Klemm M, Schuttig R, Röllig C, Wassilew N, Ehninger G, et al. Arsenic-induced APL differentiation in cerebrospinal fluid. Leuk Res. 2007;31:703–705. doi: 10.1016/j.leukres.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Knipp S, Gatterman N, Schapira M, Käferstein H, Germing U. Arsenic in the cerebrospinal fluid of a patient receiving arsenic trioxide for relapsed acute promyelocytic leukemia with CNS involvement. Leuk Res. 2007;31:1585–1587. doi: 10.1016/j.leukres.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Ohno R, Asou N, Ohnishi K. Treatment of acute promyelocytic leukemia: strategy toward further increase of cure. Leukemia. 2003;17:1454–1463. doi: 10.1038/sj.leu.2403031. [DOI] [PubMed] [Google Scholar]
- 28.Ravandi F. Prophylactic intrathecal chemotherapy in acute promyelocytic leukemia (APL) Leukemia. 2004;18:879–880. doi: 10.1038/sj.leu.2403306. [DOI] [PubMed] [Google Scholar]