Abstract
Delivery and effects of therapeutics remain suboptimal. Most drugs do not have affinity to their targets. Biotherapeutics including enzymes and genetic materials require specific sub-cellular addressing not attainable naturally. Endothelium, lining the luminal surface of blood vessels, represents a key therapeutic target in many diseases. Studies in cell culture and animal models revealed that targeted delivery of therapeutics to, into and across endothelium can be achieved using carriers targeted to specific molecules expressed on the surface of the endothelial cells. For example, cell adhesion molecules represent attractive targets for drug delivery. Rational design of the drug delivery systems (e.g., selection of optimal geometry and affinity to specific epitopes) provides an unprecedented level of control of such parameters of drug delivery as pharmacokinetics, circulation in blood, binding to selected endothelial cell phenotypes, anchoring on cell surface or internalization into the endothelium, subsequent intracellular addressing and duration of the effects. We discusse here key aspects of design of endothelium-targeted drug delivery systems with potential for translation into the clinical domain.
Keywords: Vascular endothelium, cell adhesion molecules, drug targeting, drug carriers, stealth nanocarriers
INTRODUCTION: DRUG CARRIERS
Effects of most drugs are suboptimal and marred by harmful side effects, in major part due to lack of mechanisms for targeted delivery to desired sites of therapeutic action. Most drugs do not have natural affinity to their intended targets in the body. Furthermore, biotherapeutics including proteins, enzymes and nucleic acids require specific sub-cellular addressing, not attainable for these drugs naturally. For example, DNA should be delivered into the nucleus, RNA into cytosol, whereas protein therapeutics may require diverse localizations: cell surface, cytosol or organelles including mitochondria, endosomes, lysosomes, endoplasmic reticulum, the Golgi and other cellular vacuoles. Delivery of drugs to selected subcellular locations in specific cell types is the Holy Grail of modern medicine.
In order to achieve this goal, drugs can be coupled to or encapsulated in a carrier optimizing their delivery to selected cell types and phenotypes (for example, activated, dividing or pathologically altered cells). Such carriers can be introduced in the body via various pharmacological routes. Intratracheal, intracranial, intraperitoneal and some other routes favor focal deposition of the administered materials and often seem optimal for treatment of local processes. Oral delivery of biotherapeutics is limited by their degradation in the gastro-intestinal tract and ineffective permeation of barriers separating its lumen from blood.
Except for those areas where the blood-brain barrier poses an obstacle to drug transport, the intravascular route seems the most useful for delivery of drug carriers to diverse organs and therapeutic targets. In order to avoid occlusion of blood vessels, carriers for vascular use should circulate through capillaries, for which the sub-micron size of nanocarriers is ideal. Functions of drug carriers include protection of therapeutics from inactivation en route to the target sites, optimization of pharmacokinetics and blood clearance, prevention of premature, potentially harmful and systemic side effects, control of the kinetics and duration of drug activity, and control of drug release. Further, carriers help to target drugs to the cells and subcellular compartments of interest. Carrier parameters including stability, surface charge and amphiphilic features all influence targeting.
In order to enhance circulation properties of drug carriers in the bloodstream, the typical course of action is to coat a carrier with polyethylene glycol or PEG. For example, PEG-ylation of liposomes increased their half-life from less than 30 minutes to approximately five hours in mice (Klibanov, Maruyama, Torchilin, & Huang, 1990). Other benefits, such as reduced interaction with the immune system, have also been found. For example, due to their dense PEG surface brush, polymersomes and filomicelles are compatible with blood (Bermudez, Brannan, Hammer, Bates, & Discher, 2002; Lee, Wong, & Discher, 1999; Photos, Bacakova, Discher, Bates, & Discher, 2003), as they: i) remain suspended and flexible in plasma; ii) do not adhere to red blood cells and leukocytes in blood; iii) do not fix opsonins or activate complement (Bermudez et al., 2002; Photos et al., 2003) and iv) do not cause hemolysis (Dalhaimer, Bates, & Discher, 2003).
Liposomes arguably represent the most extensively studied drug carriers (Mainardes & Silva, 2004; Moghimi & Szebeni, 2003), where amphiphilic phospholipids form a bilayer in aqueous media providing capsular vehicles. The capsule’s internal aqueous space can be used for delivery of hydrophilic drugs, while the lipid bilayer can be loaded with small hydrophobic drugs. Polymersomes, the polymer analogue of liposomes, consist of amphiphilic di-block copolymers such as degradable PEG-poly(caprolactone). Polymersomes are more robust than liposomes and circulate for days (B. M. Discher et al., 1999). The extent of stealth effects depends on PEG molecular weight and surface density on the carrier. Liposomes can only contain up to ~15 mole% PEG, while higher extents of hydrophilic PEG grafting destroys the phospholipid bilayer (B. M. Discher et al., 1999; D. E. Discher & Eisenberg, 2002). In contrast, each polymer chain in polymersomes can contain a PEG group, hence 100% surface coverage is possible, resulting in a circulation half life of days vs hours for PEG-liposomes. Also, the membrane of polymersomes is thicker than that of liposomes (~8 nm compared to ~3 nm), providing highly durable carriers that are able to resist deforming forces that destroy liposomes (B. M. Discher et al., 1999; D. E. Discher & Eisenberg, 2002). By changing the ratio between the hydrophobic and hydrophilic polymer blocks (e.g., ~42–50% PEG content in co-polymers), the polymer chains can self-assemble into cylindrical, flexible structures known as worm micelles, with widths of ~40 nm and lengths up to 20–40 μm (D. E. Discher & Eisenberg, 2002). These species have been only recently conceived as a new prospective class of drug carriers. A unique and highly attractive feature of worm micelles is their ability to align with flow (Croce et al., 2005), which presumably may enhance circulation even further by avoiding collisions with vascular cells.
This review will discuss recent advances in exploration of carriers for drug targeting to the vascular endothelium, both a prime therapeutic target and barrier for drug delivery. For the sake of focus, we will pay specific attention to carrier affinity and geometry, features that govern targeting in the most profound fashion (E. Simone, Ding, & Muzykantov, 2009; E. A. Simone, Dziubla, & Muzykantov, 2008).
TARGETING DRUGS TO ENDOTHELIUM
After vascular injection, nanocarriers encounter interactions with several cell types including blood cells, phagocytes in the reticuloendothelial tissues (first of all, hepatic and spleenic sinuses) and vascular endothelium, a highly specialized cellular monolayer lining the luminal surface of blood vessels. Endothelial cells control many vital functions in the body, including maintenance of optimal vascular tone and blood pressure, blood fluidity, and transport of blood components to the tissues and vice versa. The endothelium also separates the blood from the extravascular tissues, a barrier especially difficult to permeate in the brain, retina and some other tissues. Therefore, endothelium is an important therapeutic target and/or barrier in many cardiovascular, pulmonary and systemic diseases and tumor growth. Studies in cell culture and animal models revealed that targeted delivery of therapeutics to, into and across endothelial cells can be facilitated using nanocarriers targeted to specific molecules expressed on the surface of the endothelium.
To achieve targeting, molecules including antibodies and their fragments, affinity peptides, hormones, receptor ligands and other affinity moieties can be coupled to the carrier surface (V. R. Muzykantov, 2005). Both affinity of individual molecules and their arrangement on a carrier (e.g., surface density, presence of spacers, carrier plasticity) dictate the resultant affinity and efficiency of a carrier binding to the target cells.
Endothelial cell phenotypes differ in various types of vasculature (arteries, veins, capillary and lymphatic vessels), organs and physiological states. For example, pulmonary capillary endothelium is enriched in angiotensin-converting enzyme and some other surface molecules, whereas hepatic and cerebrovascular endothelial cells are relatively enriched in transferrin receptor. Capillary endothelium expresses a surface glycoprotein, thrombomodulin, at much higher density than its counterpart in large arteries.
Inflammation, tumor growth and abnormal changes in shear stress cause pathological activation of endothelial cells leading to expression of selectins, cytokine receptors and cell adhesion molecules that are normally absent on the endothelial surface. These “pathological” determinants represent especially attractive targets for diagnostic and therapeutic drug delivery. Selective proteomics of the endothelial plasmalemma (Durr et al., 2004; Oh et al., 2004) and in vivo phage display (Rajotte et al., 1998) are attractive methods to define new targets, as they identify binding sites accessible from circulation in normal and pathological vascular areas (Hajitou et al., 2006).
Of note, the pulmonary vasculature represents a privileged target for circulating carriers displaying endothelial affinity, because this vascular area: i) contains between one fifth and one third of the total endothelial surface in the body; ii) receives 50% of blood output, contrary to other organs; iii) represents the first pass surface for some types of intravenous administration; and, iv) experiences relatively low flow rate via high capacity vasculature, which favors binding to endothelium.
In order to be useful for drug delivery, binding epitopes on a target surface molecule must be spatially accessible to harbor circulating nanocarrier and their engagement should not cause harmful adverse effects. In many cases, anchoring to certain target epitopes initiates carrier endocytosis via pathways including clathrin- and caveoli-mediated mechanisms, phagocytosis and pinocytosis (fluid phase uptake). Materials entering cells via these routes travel through a series of intracellular vesicular compartments. The endocytic route used for cellular entry affects the final destination; most often carriers are eventually delivered to and reside in the lysosomes, acidic vesicles filled with proteolytic enzymes and other hydrolases (Muro, Koval, & Muzykantov, 2004).
In particular, cell adhesion molecules expressed on the surface of senescent or pathologically activated endothelium, namely, Platelet-Endothelial Cell Adhesion Molecule 1 (PECAM-1) and InterCellular Adhesion Molecule 1 (ICAM-1) represent attractive targets for drug delivery to endothelium. These molecules support adhesion and migration of activated leukocytes in sites of inflammation. Therefore, blocking of PECAM-1 and ICAM-1 by bound drug carriers may provide a secondary anti-inflammatory benefit. Studies in vitro and in animal models by our labs and several others showed that conjugation with PECAM-1 or ICAM-1 antibodies provides targeting of drugs and drug carriers to the vascular endothelium (Muro & Muzykantov, 2005).
ENDOTHELIAL UPTAKE OF DRUG CARRIERS
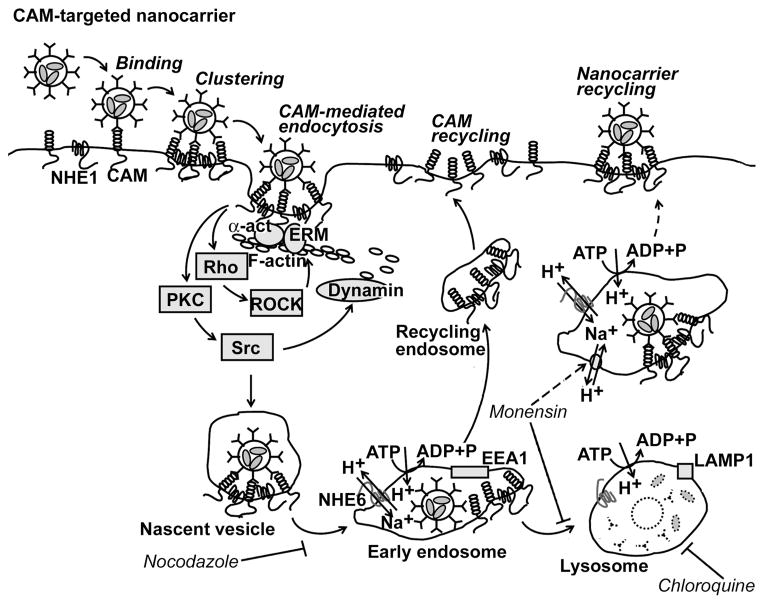
Endothelial cells do not internalize PECAM-1 and ICAM-1 antibodies, which reside for a prolonged time in the vascular lumen (Vladimir R. Muzykantov et al., 1999). However, endothelial cells internalize very effectively (time ½ ~15–20 min) multimolecular, multivalent complexes of proteins, nucleic acids and polymeric nanocarriers conjugated to these antibodies, i.e. anti-PECAM and anti-ICAM conjugates, via a uniquely regulated endocytic pathway, CAM-mediated endocytosis (Muro, Wiewrodt et al., 2003). Endocytosis and intracellular trafficking of carriers internalized via this route are associated with marked rearrangements of actin and microtubule elements of cytoskeleton, respectively. Figure 1 describes schematically the main elements of regulation of the endocytosis and intracellular trafficking of CAM-targeted carriers.
Figure 1. Drug delivery into endothelial cells by anti-CAM nanocarriers.
Multivalent anti-CAM/nanocarriers and conjugates bind to and cluster cell surface CAMs. This activates PKC, Src kinases, and Rho dependent kinase (ROCK), enzymes which regulate the activity of the GTPase, dynamin, and the amiloride-sensitive sodium/proton exchanger, NHE1, which induces formation of actin stress fibers. CAMs and NHE1 form a complex, which may help cross-link actin fibers to the CAM cytosolic tail through α-actinin (α-act) and ezrin/radixin/moesin (ERM) family proteins. After internalization, anti-CAM/nanocarriers traffic via a microtubule-dependent process to endosomes (1–2 h), that can be identified by early endosome antigen-1 (EEA1). Endosomes are enriched in NHE6, an intracellular ion exchanger that helps regulate endosome acidification by vacuolar H+/ATPase. Anti-CAM/nanocarriers dissociate from CAMs and NHE1 in endosomes and CAM recycles to the plasma membrane. Nanocarriers arrive to lysosomes about 3 h after their internalization, which can be identified by co-localization with lysosome associated membrane protein 1 (LAMP1). In lysosomes, NHE6 becomes inactive, favoring further acidification and degradation of delivered proteins by acidic proteases. Nocodazole (which disrupts the cell microtubule network), chloroquine (a mild base that inhibits lysosomal acidification), and monensin (which enhances Na+/H+ exchange in endosomes and induces recycling of anti-CAM nanocarriers to the plasma membrane) all alter intracellular itinerary of anti-CAM/nanocarriers and prolong their effects. For further explanation see Ding et al, 2006. (Reproduced with permission from Ding et al, 2006).
Both static and flow adapted endothelial cells in culture and endothelial cells in vivo in diverse vessels internalize anti-PECAM and anti-ICAM carriers without signs of cellular damage or discomfort (Muro, Dziubla et al., 2006). Endothelial cells internalize anti-PECAM and anti-ICAM carriers ranging from 50 nm to 5 μm in diameter for spherical polymer nanocarriers coated by multiple copies of anti-PECAM or anti-ICAM. Furthermore, effectiveness of internalization and subsequent intracellular trafficking of spherical anti-PECAM coated nanocarriers depend on anchoring to specific binding epitopes in the extracellular domain of PECAM-1 (Garnacho, Albelda, Muzykantov, & Muro, 2008). The extracellular segment of human PECAM-1 consists of a 574 amino acids containing six Ig-like domains, numbered from 1 to 6, from the most membrane-distal to the most membrane-proximal domain. We have compared endothelial binding, uptake and trafficking of spherical carriers (100 nm diameter) coated with antibodies directed to different PECAM-1 extracellular epitopes: mAb4G6 binds to membrane-proximal domain 6, mAbGi34 binds between domains 2 and 3, and mAb 62, mAb35 and mAb37 bind to membrane-distal domain 1.
Non-conjugated mAb62 and mAb37 displayed the highest binding level, mAb35 and mAb4G6 (recognizing the most distal and proximal PECAM-1 domains, respectively), bound slightly less effectively, whereas mAbGi34 recognizing a middle area of PECAM-1 showed the lowest level of binding to endothelial cells. However, nanocarriers coated with these antibodies (mAb/NC, ~200 nm diameter) showed a binding pattern different from that of their corresponding free counterparts: mAb62/NC showed the highest binding, followed by mAb37/NC, mAb35/NCs and mAbGi34/NC, whereas mAb4G6/NC did not bind to endothelial cells, despite the effective binding of the corresponding antibody. This could reflect low accessibility of the most membrane-proximal PECAM-1 domain to the carriers. However, among anti-PECAM/nanocarriers that effectively bound to endothelial cells, mAb35/NC, mAb62/NC and mAbGi34/NC were quickly internalized, whereas mAb37/NC was internalized rather slowly and incompletely. Following internalization, the majority of mAb62/NC were transported to lysosomes within 3 h after transient and rapid passage through endosomes. In contrast, a small fraction of the internalized mAb35/NC arrived to the lysosomes at this time, whereas a major fraction resided in pre-lysosomal compartments for prolonged periods of time [23].
Therefore, endowing nanocarriers with a multivalent affinity to specific binding determinants exposed on PECAM-1 and ICAM-1 provides a strategy to control both drug targeting to and subcellular addressing in endothelial cells (Dziubla et al., 2008). A series of prospective biotherapeutics including antioxidant enzymes catalase (Kozower et al., 2003) and SOD (Shuvaev, Tliba, Nakada, Albelda, & Muzykantov, 2007), and enzyme replacement therapies for lysosomal storage diseases (Muro, Schuchman, & Muzykantov, 2006) using this strategy are being designed and tested in animal models of human diseases.
CARRIER GEOMETRY MODULATES TARGETING
Carrier’s geometry greatly modulates endothelial targeting of drug carriers. Recent advances in polymer biomaterials have provided carriers of various shapes (spherical, discoid, rode-like, tubes, filamentous) and sizes (from few nanometers for quantum dots to several micrometers for biodegradable microspheres). From the standpoint of size, many of these carriers, traditionally called “nanocarriers,” exceed the NSF dimensional limits defined for “nanoscale” (<100 nm), yet fit within broader nanoscale definitions (<1,000 nm). Furthermore, the term nanostructure refers to any non-spherical carrier with at least one dimension that is less than one micrometer, typically less than 100 nm.
The most basic and common geometry of nanocarriers is spherical. Diameters of these carriers range from 50–300 nm liposomes (Lasic, 1996), polymersomes (Photos et al., 2003) (the synthetic polymer analogue of a liposome), dendrimers and polyplexes (Saad et al., 2008), to ~5–50 nm gold (Predescu & Palade, 1993), silica (Vallet-Regi, Balas, & Arcos, 2007), or iron oxide (Neumaier, Baio, Ferrini, Corte, & Daga, 2008) nanoparticles and quantum dots, used as the core of dual-imaging/treatment carriers (Misra, 2008). Sub-micrometer carriers include 300–1,000 nm diameter polymer spheroids (Dziubla, Karim, & Muzykantov, 2005). Polystyrene spheroids from sub-100 nm to several micrometers have been utilized to explore cellular mechanisms of carrier uptake and processing (Muro, Cui et al., 2003; Muro, Dziubla et al., 2006; Muro, Gajewski, Koval, & Muzykantov, 2005).
Asymmetric carriers include single wall carbon tubes (SWNT), filomicelles produced from variety of biocompatible polymers and polymer with diverse shapes manufactured using PRINT process, i.e., Particle Replication In Non-wetting Templates (for review see (E. Simone et al., 2009; E. A. Simone et al., 2008)). Figure 2 provides selected examples of carrier geometries, some of which are currently explored for drug delivery in animals and human patients.
Figure 2. Carrier geometries.
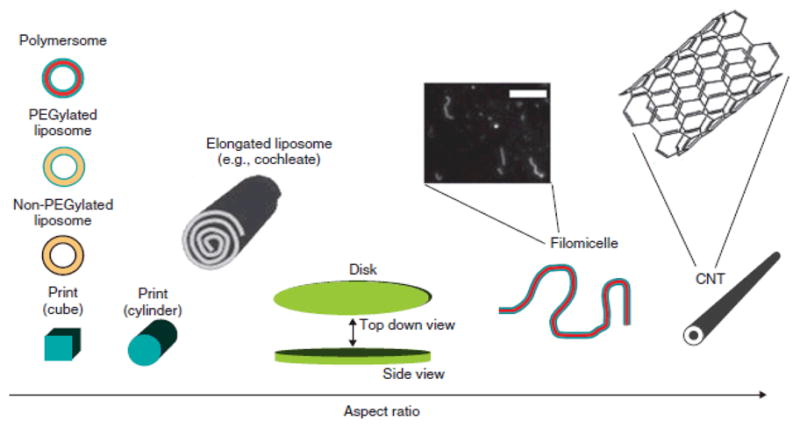
Schematics of different geometry carriers are shown in order of increase of their aspect ratio. Blue and red colors indicate PEG coating and hydrophobic polymer, respectively. Thick black lines in liposomes represent polar groups of phospholipids, while yellow indicates non-polar tails. Blown up fluorescence microscopy image of labeled filomicelles is shown. Scale bar is 10 μm. Common chemical structure is shown in blow up of carbon nano-tubes, CNT (5,5-armchair conformation for a non-spiraled SWNT). For further explanations, see E. Simone et al, 2008 (Reproduced with permission from Simone at al, 2008).
Carrier geometries modulate their circulation
The geometry of carrier and their physical properties regulate their circulation in vivo. Most extensively studied has been the effect of size of spherical carriers on their half-life in the circulation. Particles over 100 nm are typically retained mechanically by the spleen (Moghimi, Porter, Muir, Illum, & Davis, 1991). Small spherical structures (<20 nm) permeate through all fenestrated endothelia (e.g., in kidneys, liver, spleen), resulting in an increased rate of clearance from the circulation. For large spherical structures (e.g., >1μm) clearance rates are also high for multiple reasons. Large structures possess an increased tendency to aggregate in and be retained, mechanically, within capillaries. From a hydrodynamic standpoint, as the size increases, momentum forces begin to dominate and wall collisions become more common, resulting in an increased rate of clearance by cellular uptake. However, when spherical structures reside within the “sweet spot” (20 nm<d<1 μm), these clearance mechanisms are minimized and circulation times are prolonged.
For spherical carriers, optimal size range for specific targeting in the vasculature is restricted by few hundred nanometers, as larger spheres get non-specifically mechanically retained in the capillary networks (for example, in the lungs after intravenous injection). In contrast, discoid and filamentous nanocarriers can extend several micrometers in one direction, avoid mechanical retention in the vasculature due to flow alignment and yet display even higher specificity of targeting to endothelial cells [39].
Comparison of polymersomes and filomicelles based on the same polymer chemistry provides unique insight into the role of carrier geometry in circulation. The current maximum in terms of circulation appears to be highly flexible filomicelles, with half lives on the order of a week (Geng et al., 2007). This is thought to be due primarily to filomicelles’ ability to align with blood flow and avoid vascular collisions, filtration and phagocytosis. It was shown that within the 1–20 micrometer length scale, longer filomicelles circulate for longer periods of time and that they are less readily internalized by macrophages, particularly in flow conditions. This is attributable to their extensive length in that they provide a difficult substrate for macrophages to engulf and further, that the carrier’s flow-aligning body experiences extensive drag forces from directional flow. These drag forces are in opposition to phagocytosis, thus providing significant competition to cell-enveloping mechanisms. Filomicelles’ internalization was observed under static conditions with cultured epithelial cells, thus suggesting that flow conditions are the primary barrier to phagocytosis.
To study the effects of carrier geometry on targeting to endothelium, we compared organ distribution of antibody-coated (anti-ICAM) polystyrene disks and spheres injected intravenously in mice. While the circulation half life was nowhere near as prolonged as in the case of filomicelles, anti-ICAM coated disks (dimensions 3 × 1 × 0.1 μm) persisted in the circulation markedly longer than spheres ≥ 1 μm (Muro et al., 2008a). In fact, disk-shaped carriers remained in circulation for comparable (or even longer) times to 100 nm spheres, despite having one dimension ~3 μm. It is possible that these disks align with flow, similarly to filomicelles or discoid erythrocytes, and therefore circulate in the mainstream, avoiding vascular collisions, RES uptake and stacking in capillaries.
Furthermore, disks coated with anti-ICAM showed excellent targeting to the pulmonary endothelium (Muro et al., 2008a). Interestingly, the immunospecificity index, or ratio of targeted to non-targeted carriers in an organ, was three fold greater for disks than the best spherical preparation (100 nm polystyrene particles in this study). In fact, both specificity and efficacy of pulmonary targeting of the disks were higher than that of their spherical counterparts. This likely reflects both the superior circulation features of disks, discussed in the previous section, and the higher affinity of anchoring on the target cells. The latter advantage may come from the preferable shape of the contact surface (i.e., large numbers of antibodies immobilized on the flat disk surface can multivalently engage numerous copies of cellular their targets) and the hydrodynamic features (i.e., drag force detaching initially adhered carrier from cell surface is much less in the case of thin flat disks relative to spheroids).
Optimal circulation time depends on clinical objectives, drug delivery system stability and drug release profile. Thus, in order to effectively permeate into extravascular targets and treatment of chronic conditions, carriers should circulate for a prolonged time (from hours to days and even weeks), whereas only minutes are required for immediate delivery to blood and endothelial cells. A mismatch between the clinical need and longevity in the circulation may compromise drug delivery. Thus, a long-circulating polymer carrier with fast kinetics of drug release would not be of clinical benefit. On the other hand, optimal balance should be found between longevity in the circulation (in part defined by the carrier ability to avoid collisions with cells) and specific affinity to the target cells. Intuitively, 100% stealth carriers that completely avoid contacts with cells would not be useful for drug delivery to any cell type including endothelium and blood cells. However, absolute stealth properties can not be achieved: drug carriers and other objects collide with the vascular walls at some rate and, therefore, if they display high affinity to specific targets, they will anchor. This is the field in which rigorous interdisciplinary analysis of the dynamic interactions between carriers and vascular cells, including computational modeling of the process and defining the role of the hydrodynamic forces ruling such events, is a pivotal and timely endeavor.
Carrier geometry modulates uptake and trafficking by cells
Geometry of anti-CAM carriers modulates their fate after binding to target cells. Thus, endothelial endocytosis of nanoparticles targeted to PECAM-1 and ICAM-1 seems to be dependent on their geometry. Thus, upon attachment of anti-CAM targeted nanocarriers, the rate of internalization by endothelial cells is markedly faster for spheres relative to disks (Muro et al., 2008a). Fluorescence and electron microscopy showed that endothelial cells effectively internalize anti-CAM disks in cell culture and in vivo in pulmonary vessels without detectable cellular and pulmonary damage (Muro et al., 2008a).
Intracellular trafficking of CAM-targeted carriers internalized via CAM-endocytosis is modulated by carrier size. Regardless of the shape, small (100–200 nm diameter) carriers get trafficked via endosomes to the lysosomes within 2–3 hours, whereas large micrometer-size counterparts reside in endosomal compartment for many hours. Endosomal vs lysosomal addressing of anti-ICAM carriers, regulated by modulating carrier size, highlights the variability and potential utility this paradigm for therapeutic applications that require corresponding intracellular localization. As an example with relevant implications in the design of antioxidant therapies, utilization of micrometer-size anti-ICAM carriers delivering catalase provided prolonged antioxidant protection by catalase residing in endosomes. As a distinct example relevant to the treatment of lysosomal storage disorders, acidic hydrolases loaded onto small anti-ICAM carriers undergo fast lysosomal delivery and display quick and prolonged activity in this cellular compartment, thereby improving current approaches for enzyme replacement therapy of lysosomal storage diseases (Muro et al., 2008b).
CONCLUSION
Rational design of drug carriers, including optimization of their geometry and affinity to specific target epitopes, provides an unprecedented control of key parameters of drug delivery: pharmacokinetics, circulation in blood, binding to selected endothelial cell phenotypes, anchoring on the cell surface or internalization into the endothelium, subsequent intracellular addressing and duration of the drug effects. Current status of development of key biophysical and biomedical aspects of design of endothelium-targeted drug delivery systems supports optimistic views of avenues for translation of this strategy into the clinical domain (Ding, Dziubla, Shuvaev, Muro, & Muzykantov, 2006).
Acknowledgments
Authors express their gratitude to Drs. Eric Simone, Thomas Dziubla, Dennis Discher and Vladimir Shuvaev for numerous invaluable discussions, advice, contributions to the joint studies and publications, which materials have been used in preparation of this review. Funding for this work was provided by NIH RO1 HL087036.
NOMENCLATURE
- CAM
cell adhesion molecule
- CNT
carbon nanotube
- ICAM-1
intercellular adhesion molecule 1
- h
hour
- mAb
monoclonal antibody
- mole%
molecular percentage
- nm
nanometer
- PECAM-1
platelet endothelial cell adhesion molecule 1
- PEG
polyethylene glycol
- μm
micrometer
Contributor Information
Vladimir Muzykantov, Professor of Pharmacology, University of Pennsylvania, 3620 Hamilton Walk, 1 John Morgan Building, Philadelphia, PA 19104-6068, USA
Silvia Muro, Fischell Department of Bioengineering, University of Maryland, 5115 Plant Sciences Building, College Park, MD 20742.
References
- Bermudez H, Brannan AK, Hammer DA, Bates FS, Discher DE. Molecular weight dependence of polymersome membrane structure, elasticity, and stability. Macromolecules. 2002;35(21):8203–8208. [Google Scholar]
- Croce V, Cosgrove T, Dreiss CA, King S, Maitland G, Hughes T. Giant micellar worms under shear: a rheological study using SANS. Langmuir. 2005;21(15):6762–6768. doi: 10.1021/la0479410. [DOI] [PubMed] [Google Scholar]
- Dalhaimer P, Bates FS, Discher DE. Single molecule visualization of stable, stiffness-tunable, flow-conforming worm micelles. Macromolecules. 2003;36(18):6873–6877. [Google Scholar]
- Ding BS, Dziubla T, Shuvaev VV, Muro S, Muzykantov VR. Advanced drug delivery systems that target the vascular endothelium. Mol Interv. 2006;6(2):98–112. doi: 10.1124/mi.6.2.7. [DOI] [PubMed] [Google Scholar]
- Discher BM, Won YY, Ege DS, Lee JC, Bates FS, Discher DE, et al. Polymersomes: tough vesicles made from diblock copolymers. Science. 1999;284(5417):1143–1146. doi: 10.1126/science.284.5417.1143. [DOI] [PubMed] [Google Scholar]
- Discher DE, Eisenberg A. Polymer vesicles. Science. 2002;297(5583):967–973. doi: 10.1126/science.1074972. [DOI] [PubMed] [Google Scholar]
- Durr E, Yu J, Krasinska KM, Carver LA, Yates JR, Testa JE, et al. Direct proteomic mapping of the lung microvascular endothelial cell surface in vivo and in cell culture. Nat Biotechnol. 2004;22(8):985–992. doi: 10.1038/nbt993. [DOI] [PubMed] [Google Scholar]
- Dziubla TD, Karim A, Muzykantov VR. Polymer nanocarriers protecting active enzyme cargo against proteolysis. J Control Release. 2005;102(2):427–439. doi: 10.1016/j.jconrel.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Dziubla TD, Shuvaev VV, Hong NK, Hawkins BJ, Madesh M, Takano H, et al. Endothelial targeting of semi-permeable polymer nanocarriers for enzyme therapies. Biomaterials. 2008;29(2):215–227. doi: 10.1016/j.biomaterials.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnacho C, Albelda SM, Muzykantov VR, Muro S. Differential intra-endothelial delivery of polymer nanocarriers targeted to distinct PECAM-1 epitopes. J Control Release. 2008;130(3):226–233. doi: 10.1016/j.jconrel.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nano. 2007;2(4):249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajitou A, Trepel M, Lilley CE, Soghomonyan S, Alauddin MM, Marini FC, 3rd, et al. A hybrid vector for ligand-directed tumor targeting and molecular imaging. Cell. 2006;125(2):385–398. doi: 10.1016/j.cell.2006.02.042. [DOI] [PubMed] [Google Scholar]
- Klibanov AL, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990;268(1):235–237. doi: 10.1016/0014-5793(90)81016-h. [DOI] [PubMed] [Google Scholar]
- Kozower BD, Christofidou-Solomidou M, Sweitzer TD, Muro S, Buerk DG, Solomides CC, et al. Immunotargeting of catalase to the pulmonary endothelium alleviates oxidative stress and reduces acute lung transplantation injury. Nat Biotechnol. 2003;21(4):392–398. doi: 10.1038/nbt806. [DOI] [PubMed] [Google Scholar]
- Lasic DD. Product review: doxorubicin in sterically stabilized liposomes. Nature. 1996;380(6574):561. doi: 10.1038/380561a0. [DOI] [PubMed] [Google Scholar]
- Lee JC, Wong DT, Discher DE. Direct measures of large, anisotropic strains in deformation of the erythrocyte cytoskeleton. Biophys J. 1999;77(2):853–864. doi: 10.1016/S0006-3495(99)76937-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainardes RM, Silva LP. Drug delivery systems: past, present, and future. Curr Drug Targets. 2004;5(5):449–455. doi: 10.2174/1389450043345407. [DOI] [PubMed] [Google Scholar]
- Misra RD. Quantum dots for tumor-targeted drug delivery and cell imaging. Nanomed. 2008;3(3):271–274. doi: 10.2217/17435889.3.3.271. [DOI] [PubMed] [Google Scholar]
- Moghimi SM, Porter CJ, Muir IS, Illum L, Davis SS. Non-phagocytic uptake of intravenously injected microspheres in rat spleen: influence of particle size and hydrophilic coating. Biochem Biophys Res Commun. 1991;177(2):861–866. doi: 10.1016/0006-291x(91)91869-e. [DOI] [PubMed] [Google Scholar]
- Moghimi SM, Szebeni J. Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog Lipid Res. 2003;42(6):463–478. doi: 10.1016/s0163-7827(03)00033-x. [DOI] [PubMed] [Google Scholar]
- Muro S, Cui X, Gajewski C, Murciano JC, Muzykantov VR, Koval M. Slow intracellular trafficking of catalase nanoparticles targeted to ICAM-1 protects endothelial cells from oxidative stress. Am J Physiol Cell Physiol. 2003;285(5):C1339–1347. doi: 10.1152/ajpcell.00099.2003. [DOI] [PubMed] [Google Scholar]
- Muro S, Dziubla T, Qiu W, Leferovich J, Cui X, Berk E, et al. Endothelial targeting of high-affinity multivalent polymer nanocarriers directed to intercellular adhesion molecule 1. J Pharmacol Exp Ther. 2006;317(3):1161–1169. doi: 10.1124/jpet.105.098970. [DOI] [PubMed] [Google Scholar]
- Muro S, Gajewski C, Koval M, Muzykantov VR. ICAM-1 recycling in endothelial cells: a novel pathway for sustained intracellular delivery and prolonged effects of drugs. Blood. 2005;105(2):650–658. doi: 10.1182/blood-2004-05-1714. [DOI] [PubMed] [Google Scholar]
- Muro S, Garnacho C, Champion JA, Leferovich J, Gajewski C, Schuchman EH, et al. Control of Endothelial Targeting and Intracellular Delivery of Therapeutic Enzymes by Modulating the Size and Shape of ICAM-1-targeted Carriers. Mol Ther. 2008a doi: 10.1038/mt.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muro S, Garnacho C, Champion JA, Leferovich J, Gajewski C, Schuchman EH, et al. Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM-1-targeted carriers. Mol Ther. 2008b;16(8):1450–1458. doi: 10.1038/mt.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muro S, Koval M, Muzykantov V. Endothelial endocytic pathways: gates for vascular drug delivery. Curr Vasc Pharmacol. 2004;2(3):281–299. doi: 10.2174/1570161043385736. [DOI] [PubMed] [Google Scholar]
- Muro S, Muzykantov VR. Targeting of antioxidant and anti-thrombotic drugs to endothelial cell adhesion molecules. Curr Pharm Des. 2005;11(18):2383–2401. doi: 10.2174/1381612054367274. [DOI] [PubMed] [Google Scholar]
- Muro S, Schuchman EH, Muzykantov VR. Lysosomal enzyme delivery by ICAM-1-targeted nanocarriers bypassing glycosylation- and clathrin-dependent endocytosis. Mol Ther. 2006;13(1):135–141. doi: 10.1016/j.ymthe.2005.07.687. [DOI] [PubMed] [Google Scholar]
- Muro S, Wiewrodt R, Thomas A, Koniaris L, Albelda SM, Muzykantov VR, et al. A novel endocytic pathway induced by clustering endothelial ICAM-1 or PECAM-1. J Cell Sci. 2003;116(Pt 8):1599–1609. doi: 10.1242/jcs.00367. [DOI] [PubMed] [Google Scholar]
- Muzykantov VR. Biomedical aspects of targeted delivery of drugs to pulmonary endothelium. Expert Opin Drug Deliv. 2005;2(5):909–926. doi: 10.1517/17425247.2.5.909. [DOI] [PubMed] [Google Scholar]
- Muzykantov VR, Christofidou-Solomidou M, Balyasnikova I, Harshaw DW, Schultz L, Fisher AB, et al. Streptavidin facilitates internalization and pulmonary targeting of an anti-endothelial cell antibody (platelet-endothelial cell adhesion molecule 1): a strategy for vascular immunotargeting of drugs. Proc Natl Acad Sci U S A. 1999;96(5):2379–2384. doi: 10.1073/pnas.96.5.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumaier CE, Baio G, Ferrini S, Corte G, Daga A. MR and iron magnetic nanoparticles. Imaging opportunities in preclinical and translational research. Tumori. 2008;94(2):226–233. doi: 10.1177/030089160809400215. [DOI] [PubMed] [Google Scholar]
- Oh P, Li Y, Yu J, Durr E, Krasinska KM, Carver LA, et al. Subtractive proteomic mapping of the endothelial surface in lung and solid tumours for tissue-specific therapy. Nature. 2004;429(6992):629–635. doi: 10.1038/nature02580. [DOI] [PubMed] [Google Scholar]
- Photos PJ, Bacakova L, Discher B, Bates FS, Discher DE. Polymer vesicles in vivo: correlations with PEG molecular weight. J Control Release. 2003;90(3):323–334. doi: 10.1016/s0168-3659(03)00201-3. [DOI] [PubMed] [Google Scholar]
- Predescu D, Palade GE. Plasmalemmal vesicles represent the large pore system of continuous microvascular endothelium. Am J Physiol. 1993;265(2 Pt 2):H725–733. doi: 10.1152/ajpheart.1993.265.2.H725. [DOI] [PubMed] [Google Scholar]
- Rajotte D, Arap W, Hagedorn M, Koivunen E, Pasqualini R, Ruoslahti E. Molecular heterogeneity of the vascular endothelium revealed by in vivo phage display. J Clin Invest. 1998;102(2):430–437. doi: 10.1172/JCI3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad M, Garbuzenko OB, Ber E, Chandna P, Khandare JJ, Pozharov VP, et al. Receptor targeted polymers, dendrimers, liposomes: which nanocarrier is the most efficient for tumor-specific treatment and imaging? J Control Release. 2008;130(2):107–114. doi: 10.1016/j.jconrel.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuvaev VV, Tliba S, Nakada M, Albelda SM, Muzykantov VR. Platelet-endothelial cell adhesion molecule-1-directed endothelial targeting of superoxide dismutase alleviates oxidative stress caused by either extracellular or intracellular superoxide. J Pharmacol Exp Ther. 2007;323(2):450–457. doi: 10.1124/jpet.107.127126. [DOI] [PubMed] [Google Scholar]
- Simone E, Ding BS, Muzykantov V. Targeted delivery of therapeutics to endothelium. Cell Tissue Res. 2009;335(1):283–300. doi: 10.1007/s00441-008-0676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone EA, Dziubla TD, Muzykantov VR. Polymeric carriers: role of geometry in drug delivery. Expert Opin Drug Deliv. 2008;5(12):1283–1300. doi: 10.1517/17425240802567846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet-Regi M, Balas F, Arcos D. Mesoporous materials for drug delivery. Angew Chem Int Ed Engl. 2007;46(40):7548–7558. doi: 10.1002/anie.200604488. [DOI] [PubMed] [Google Scholar]