Abstract
Micro- and nanotechnologies have emerged as potentially effective fabrication tools for addressing the challenges faced in tissue engineering and drug delivery. The ability to control and manipulate polymeric biomaterials on the micron and nanometer scale with these fabrication techniques has allowed for the creation of controlled cellular environments, engineering of functional tissues, and development of better drug delivery systems. In tissue engineering, micro- and nanotechnologies have enabled the recapitulating of the micro- and nanoscale detail of cell’s environment through controlling surface chemistry and topography of materials, generating 3D cellular scaffolds, and regulating cell-cell interactions. Furthermore, these technologies have led to advances in high-throughput screening (HTS), enabling rapid and efficient discovery of a library of materials and screening of drugs that induce cell-specific responses. In drug delivery, controlling the size and geometry of drug carriers with micro- and nanotechnologies have allowed for modulation of parameters such as bioavailability, pharmacodynamics, and cell-specific targeting. In this review, we introduce recent developments in micro- and nanoscale engineering of polymeric biomaterials with an emphasis on lithographic techniques, and present an overview of their applications in tissue engineering, HTS, and drug delivery.
Keywords: Biomaterials, Microtechnology, Nanotechnology, Tissue Engineering, High-throughput screening, Drug delivery
1. Introduction
Tissue engineering and drug delivery are promising approaches to address many current therapeutic shortcomings in the treatment of diseased or damaged tissues and organs.(Langer and Vacanti 1993) However, the clinical applicability of tissue engineering has been limited by a number of challenges including the inability to accurately control the spatial and temporal components of the cell’s microenvironment and to recreate biomimetic three-dimensional (3D) cell culture platforms.(Naderi et al. 2011) Furthermore, in the pharmaceutical industry, new and existing drugs continue to be scrutinized for their poor specificity, solubility, therapeutic index, and immunogenicity.(Petros and DeSimone 2010) One area of research that has gained traction in terms of addressing these needs has been through the development of polymeric biomaterials.(Peppas et al. 2006) With advances in biology, chemistry, and material science, polymeric materials can now be synthetized from a combinatorial array of monomers, oligomers, and polymers with tunable chemical, mechanical, and geometrical properties to create new, biocompatible substances.(Slaughter et al. 2009) In the early days of tissue engineering, it was believed that biomaterials simply function as scaffolds for cells; hence, the majority of the emphasis at the time was placed on biocompatibility and mass transport. However, it is now known that the in vivo cellular microenvironment contains critical information-rich cues embedded in the extracellular matrix (ECM),(Hynes 2009) neighboring cells, soluble and tethered cytokines, and metabolites that regulate cell survival, adhesion,(Geiger et al. 2009) migration,(Petrie et al. 2009) and differentiation.(Dolatshahi-Pirouz et al. 2011; Edalat et al.) Therefore, fabricating biomimetic cell culture systems that resemble the microenvironment of native tissues requires greater control over the micro- and nanometer features of biomaterials.(Ma 2008) In the field of drug delivery, it has been shown that the size and shape—on the order of nano- and micrometers—of drug carriers can affect a drug’s circulation time, distribution, cellular internalization.(Petros and DeSimone 2010) Hence, it is not surprising that micro- and nanoscale technologies have emerged as powerful tools for addressing the existing challenges in tissue engineering and drug delivery given their ability to control material properties at the cellular and subcellular length-scales.(Khademhosseini et al. 2006; Shi et al. 2010) These technologies have been increasingly used to fabricate functional polymeric materials to control cellular behaviors, serve as tools for tissue engineers to develop improved scaffolds, and enhance a drug’s pharmacodynamics parameters. In addition, microfabrication has accelerated advances in tissue engineering and drug delivery via the generation of high-throughput assays to facilitate simultaneous screening of thousands of materials,(Hook et al. 2010) cytokines, and drugs(Fernandes et al. 2009), which has led to miniaturization, cost reduction, and automated analysis.
This paper reviews recent works in micro- and nanoscale technologies that have had significant contribution towards the development of functional biomaterials. In particular, we will review a variety of micro- and nanoscale fabrication techniques that have been applied to the biomedical field, followed by a discussion of their impact on studying cell-material and cell-cell interactions, development of HTS microarrays, and fabrication of drug carriers of specific sizes and shapes for drug delivery. The prospective contributions of these techniques to future biomedical and pharmaceutical applications will also be discussed.
2. Micro- and nanotechnologies: a preamble
Micro- and nanotechnology refers to a set of techniques used for the fabrication of materials with micron and sub-micron scale features, respectively (Fig. 1).(Gates et al. 2005) Recently, the critical threshold for nanotechnological approaches has been redefined to sub-100nm. Although, these technologies were first developed by the electronics industry as a means to increase the density of transistors in integrated circuits, in the past few decades, they have been adapted and expanded for biomedical applications. There remains many newly developed micro- and nanotechnologies, whose potential are yet to be realized in the biomedical field. In this section, we discuss a few conventional and emerging micro- and nanotechnologies that have been widely used or we predict will be utilized in tissue engineering and drug delivery.
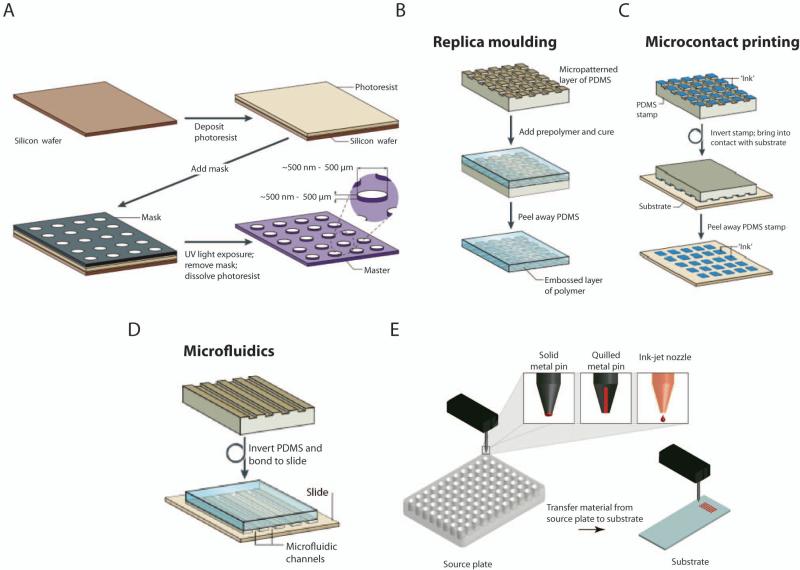
Figure 1.
Schematics of common micro- and nanotechnologies. (A) Photolithography. (B) Replica molding. (C) Microcontact printing. (D) Microfluidics. (E) Inkjet Printing and Robotic Deposition. (A-D): (Weibel et al. 2007) Adapted with permission from Macmillan Publishers Ltd: [Nature Reviews Microbiology], copyright (2007). (E): (Hook et al. 2010) Adapted by permission from Elsevier: [Biomaterials], copyright (2010).
2.1. Photolithography
Photolithography is a widely used and well-studied technique for microfabrication, having initially been developed in the semiconductor industry.(Ito and Okazaki 2000) In this technique, a photoreactive material, typically a monomer, oligomer, or polymer, is coated onto a substrate such as a silicon wafer (Fig. 1A). The photoreactive material polymerizes, crosslinks, or degrades upon ultraviolet (UV) light exposure. Selective areas of the material may be exposed to UV via using a mask with micron-scale features designed on a computer-aided design (CAD) software.(del Campo and Arzt 2008) Moreover, mask-less, selective exposure can also be achieved with optical interference techniques such as two-photon absorption(Hahn et al. 2006) or stereolithography.(Lee et al. 2008) Thereafter, unwanted areas may be dissolved by development in an organic solvent. The resulting pattern can be used on its own or it can act as a bas-relief master. The resolution achieved by photolithography depends primarily on the wavelength of light and the type of mask used, and range from micrometers to 45nm.(Rothschild 2005) Photolithography has been used to pattern a wide range of synthetic and natural polymers for use as 2-dimensional (2D)(Song et al. 2011) or cell-encapsulating scaffolds.(Bae et al. 2011)
2.2. Soft lithography
Soft lithography is a set of microfabrication techniques that utilizes a soft, flexible material, called an elastomer, to generate micron and sub-micron scale structures or molecules on a surface.(Xia and Whitesides 1998) A master mold fabricated via other lithographic techniques is used to emboss structures onto the elastomer, commonly made from poly(dimethylsiloxane) (PDMS). The elastomer can then be used for molding, printing, or embossing. The most commonly used soft lithography techniques include replica molding, nano- and microcontact printing (μCP),(Li et al. 2003) and microfluidics (Fig. 1B-D). In replica molding, a patterned elastomer is used to emboss structures onto other polymers or soft materials. This technique can be used to generate stencils, which are polymeric membranes containing micron-scale holes of specified geometry and dimension, and have been used to study heterotopic cell-cell interactions.(Folch et al. 2000) In μCP, a patterned elastomer is used to transfer “ink” onto a surface via adsorption.(Kaufmann and Ravoo 2010) The choice of “ink” includes proteins, nucleic acids, and cell suspensions.(Perl et al. 2009) Finally, microfluidic devices are generated by placing PDMS embossed with channels against a glass substrate to form closed channels.(Whitesides 2006) Microfluidics is characterized by laminar flow and diffusive mixing, and require only pico- to nanoliter volume of reagents.(Burdick et al. 2004)
The extension of soft lithography to the third dimension has been achieved via multilayer soft lithographic approaches in which separate structures are assembled on each other on a chip.(Unger et al. 2000) These chips can be used to generate robust micromechanical valves and microfluidic channels that minimize cross contamination or leakage between the processes(Hong et al. 2004), and have been used for protein crystallization,(Hansen et al. 2002) nanoliter-volume polymerase chain reaction,(Liu et al. 2002) microfabricated fluorescence activated cell sorting,(Fu et al. 2002) and single-cell enzyme screening.(Thorsen et al. 2002) Moreover, these techniques are capable of controlling the topography and spatial allotment of molecules on a surface, as well as the subsequent deposition of cells.
2.3. Electron beam lithography
Instead of using photons as in photolithography, electron beam lithography (EBL) uses electron beams to pattern electron-sensitive resists.(Norman and Desai 2006) Due to the low diffraction of electrons, significantly smaller features (3-5nm resolution) can be achieved.(Vieu et al. 2000) EBL can be used to fabricate nanopatterns composed of inorganic materials,(Werts et al. 2002; Das et al. 2009) synthetic polymers,(Peng et al. 2003; Idota et al. 2009), proteins,(Pesen et al. 2007; Christman et al. 2009) and self-assembled monolayers. However, one major disadvantage of EBL is the high cost of the equipment and the length of time required to generate a patterned surface. Other weaknesses, such as electrostatic charging, which reduces the smallest feature size, must also be considered.(Egerton et al. 2004)
2.4. Nanoimprint lithography
Nanoimprint lithography (NIL) is another high-resolution technique for the fabrication of nanoscale features onto a substrate.(Chou et al. 1996) Depending on the type of substrate, NIL is categorized as either a thermal- or light-based process; however, in both cases, a rigid mold is used to transfer patterns onto a material. Thermal NIL begins with the pressing of a mold against a thermoplastic polymer whose temperature is above its glass transition temperature, followed by a cooling process that returns the polymer to a glassy state. In contrast, UV-NIL, otherwise known as step-and-flash imprint lithography, uses UV light and a transparent mold to pattern a photoreactive polymer precursor.(Guo 2007; Schift 2008) NIL has been used to generate structures with resolutions as high as 2nm,(Hua et al. 2004) and has been applied for protein patterning,(Hoff et al. 2004) nucleic acid manipulation,(Guo et al. 2004) and cell alignment.(Subramani et al. 2011)
2.5. Direct-write techniques
Direct-write or ejecting technologies include inkjet printing and robotic deposition, and use a nozzle or a printing head to spatially deposit “ink” on a surface (Fig. 1E). Inorganic and organic small molecules, synthetic polymers, proteins, nucleic acids, and cells may be deposited at addressable locations on a surface.(Kim et al. 2010; Ker et al. 2011) Given the automated nature of these technologies, thousands of different combinations of molecules may be used, which have been utilized to fabricate microarrays for HTS. While in 2D patterning, materials are simply deposited onto a substrate, 3D structures can be formed by a layer-by-layer approach.(Mironov et al. 2011) The resolution of inkjet printing is down to 10μm, whereas robotic deposition can achieve resolutions as low as hundreds of nanometers.(Nie and Kumacheva 2008)
3. Functionalizing materials using micro- and nanotechnologies for tissue engineering applications
3.1. Control over cell-material interactions
Mimicking the complexity of the cellular microenvironment, from the structure of ECM to the presentation of cytokines and intracellular signaling, is an essential component of constructing biologically functioning tissues.(Lutolf 2009) For instance, the extracellular milieu contains ECM molecules with nanoscale dimensions (ten to hundreds of nanometers) that act as substrates for cell attachment, and present a host of biochemical and mechanical signals to cells.(Murtuza et al. 2009) The latest developments in micro- and nanoscale technologies have focused on modification of biomaterial surfaces, fabrication of substrates with 3D micron- or nanoscale geometric features, and organization of cells in 3D matrices to engineer functional tissues.(Gauvin and Khademhosseini 2011; Gauvin et al. 2011)
3.1.1. 2-Dimensional control of materials
Current cell culture platforms use glass or polystyrene surfaces coated with ECM-derived proteins. However, these platforms do not recapitulate the biochemical signals present in the cell’s microenvironment. Hence, microtechnological approaches have been used to fabricate natural and synthetic matrices, with tunable chemical properties to more closely resemble in vivo conditions. One class of material that closely resembles the structure of ECM is hydrogels, consisting of a network of a crosslinked polymer containing 95-99% water.(Slaughter et al. 2009) Hydrogel and other classes of materials are amenable to chemical modification via conjugating or adsorbing cell-adhesion molecules such as arginine-glycine-aspartate (RGD) or growth factors.(Lutolf and Hubbell 2005; Place et al. 2009) A substrate can be biochemically altered in a selective fashion to constrain cell adhesion and control cell morphology. The importance of cell morphology is inherent in its role as a regulator of cell processes such as apoptosis(Chen et al. 1997) and differentiation.(Kilian et al. 2010) For example, the effect of interligand spacing ranging from 55-100nm was studied by patterning a surface with cyclic RGD ligands via micelle lithography.(Huang et al. 2009) A critical interligand spacing value of 70nm was found, below which cell adhesion, through integrin clustering and focal adhesion formation, was favored. To impart geometric features onto 2D surfaces, microscale techniques such as photolithography,(Khademhosseini A et al. 2006; Karp et al. 2007) stencils,(Moeller et al. 2008) and μCP have been developed.(Bauwens et al. 2008) These techniques have enabled researchers to pattern cells on 2D substrates to investigate the effect of morphology on cell or tissue function.(Khademhosseini et al. 2007) For example, Karp et al. fabricated chitosan hydrogels in various geometrical forms—such as squares, circles, triangles, and lanes—using photolithography, as substrates for patterning cardiac fibroblasts, cardiomyocytes, and osteoblasts.(Karp et al. 2006). In another example, Yamazoe et al. created micropatterned cell adhesive albumin surfaces for fibroblast patterning.(Yamazoe et al. 2008) Although albumin in its native form is not conducive to cell attachment, exposure to UV light renders it cell-adhesive. Selective UV irradiation of an albumin-coated surface through a photomask led to the formation of cell-adhesive patterns. Cell sheet engineering is another area where microtechnology has been influential. Cell sheet engineering relies on the formation of cell monolayers and their subsequent manipulation, such as stacking or rolling, for assembly of mechanically robust tissues. However, in this technique, unlike their in vivo counterparts, cells lack orientation. μCP has been used to align cellular sheets.(Williams et al. 2009; Williams et al. 2011) Briefly, fibronectin was selectively stamped onto a poly(N-isopropylacrylamide) (PNIPAAm) substrate, forming cell-adhesive lanes. Cells, seeded in serum-free medium on these substrates, attached and elongated on the lanes only. After the addition of serum-containing media, cells grew to confluence in all areas of the substrate, but retained their orientation. The oriented, confluent cellular sheets could then be released from their substrate by lowering of temperature and be transferred to another substrate. While the aforementioned examples demonstrate the benefits of using micro- and nanotechnologies to modulate cell morphology, the potential of these studies are limited given their 2D nature and inadequate representation of in vivo conditions.
3.1.2. Topography
ECM is an information-rich scaffold, containing many biological cues such as cell adhesion sites as well as tethered growth factors.(Hynes 2009) In addition to these biochemical cues, ECM presents, through the shape of its structure (i.e. topography), physical and geometrical cues that influence many different types of cell behaviors.(Stevens and George 2005) Micro- and nanofabrication techniques have enabled the generation of micro- and nanoscale topographies, mimicking those of ECM.(Lim and Donahue 2007; Dvir et al. 2011) Topography can be fabricated in an ordered, symmetrical fashion with techniques such as photolithography, soft lithography, EBL, and NIL, or in a disordered manner with methods such as polymer demixing, phase separation, and electrospinning.(Norman and Desai 2006; Sill and von Recum 2008) Modulating surface roughness, defined as the average distance from the peaks to the troughs of the surface, is one way of introducing topography onto a substrate’s surface, and can be achieved with sandblasting, anodic oxidation, and acid-etching.(Sugita et al. 2011) One area where surface roughness has been used to promote favorable cell-biomaterial interactions has been in titanium implants for orthopedic applications. For instance, in one study, roughened titanium substrates, compared with smooth titanium surfaces, promoted greater osteoblastic differentiation, alkaline phosphatase activity, and calcium deposition in preosteoblastic cells.(Zhuang et al. 2012) Whereas roughened surfaces embody a disordered morphology, nanoscale, geometrically-defined structures, such as grooves, pits, and pillars, can be created (Fig. 2A). In a study by McMurray et al., 120nm-diameter polycaprolactone pillars of variable offset spacing, but with a constant average center-to-center spacing, were fabricated by EBL and used to maintain multipotency of mesenchymal stem cells (MSCs). As the level of offset was reduced, MSCs grown on these nanotopographies were less prone to osteogenic differentiation and retained their MSC markers.(McMurray et al. 2011) While the mechanism behind the effect of topography on cell function is not clearly understood, it is believed that it modulates cell attachment through contact-guidance, and produce anisotropic stresses in the cell’s cytoskeleton.(Bettinger et al. 2009) Control over the nanotopography of scaffolds has been shown to influence cell shape,(Kim et al. 2010) adhesion, migration, proliferation,(Ranzinger et al. 2009) and differentiation,(Yang et al. 2011) and hence provides an additional degree of control in the design of biomaterials used to engineer functioning tissues.
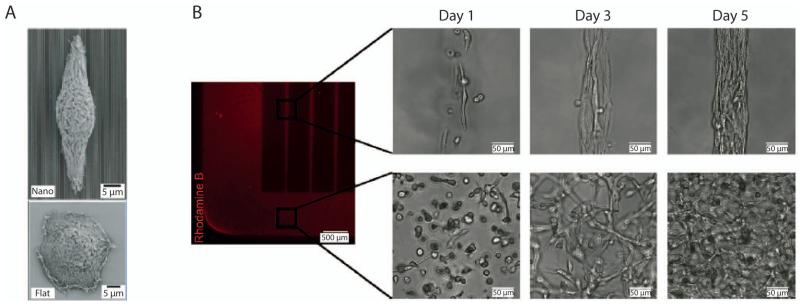
Figure 2.
Cell-Material Interactions. (A) Scanning electron microscopy images of a corneal epithelial cell on a nanograting topography (top) and flat surface (bottom).(Teixeira et al. 2003) Adapted with permission from Company of Biologists Ltd: [Journal of Cell Science], copyright (2003). (B) Fibroblast morphology and organization in patterned, 50μm-width rectangular (top) and unpatterned (bottom) gelatin methacrylate constructs.(Aubin et al. 2010) Adapted with permission from Elsevier: [Biomaterials], copyright (2010).
3.1.3. 3-Dimensional cell cultures
In native tissues, cells are exposed to a multitude of biological signals that surround them in a 3D fashion.(Cukierman et al. 2001; Doyle et al. 2009) Attempts to more precisely mimic the in vivo environment have been the driving force behind creating 3D engineered tissues. Our group has demonstrated the feasibility of using gelatin methacrylate (GelMA)(Nichol et al. 2010) as a cell-responsive hydrogel for directing 3D cellular behavior (Fig. 2B).(Aubin et al. 2010) Nuclear alignment and elongation was demonstrated for cells encapsulated in microfabricated 3D GelMA hydrogel channels. The results demonstrated that cells, which natively elongate and align in vivo, will self-organize in vitro when confined in these 3D microarchitecture. The versatility of this technique was validated by using a number of different cell types including fibroblasts, myoblasts, cardiac stem cells, and endothelial cells. While in the previous example, a substrate of constant stiffness was used for different cell types, there is evidence that cell function is enhanced when a material with elasticity similar to the cell’s in vivo substrate is used as a scaffold.(Engler et al. 2008) Even though increasing the crosslinking density or the concentration of polymers are often done to increase the stiffness of hydrogels, these methods often compromise other bulk mechanical properties of the material such as porosity, or cell growth and migration. One way of circumventing this problem is to reinforce the hydrogel with carbon nanotubes (CNT). Shin et al. showed that CNT-GelMA hybrid hydrogels maintained their porosity and cell growth capacity, while increasing the elastic modulus.(Shin et al. 2011) The composite hydrogel was amenable to photopatterning, and showed favorable fibroblast and human MSC proliferation.
While there continues to be intense research invested in the development of new biomaterials, the existing, developed polymers are being used in a variety of applications. Cell-based actuators is one such application; these actuators contain living biological components that help power synthetic components by the conversion of chemical to mechanical energy.(Chan et al. 2012) For instance, a cardiomyocyte-driven actuator was constructed by cardiac cells seeded on a poly(ethylene glycol) (PEG) diacrylate and acrylic-PEG-collagen composite hydrogel. With the aid of stereolithography, a micron-scale cantilever, embedded with cardiomyocytes, was fabricated and powered by the cells. With the rapid pace of progress in using materials as 3D cellular scaffolds, future challenges that needs to be addressed include appropriate crosslinking conditions as to not harm encapsulated cells, adequate gas and nutrient exchange, and control over mechanical properties approximately those of cell’s nature environment.(Lutolf et al. 2009)
3.2 Controlling cell-cell interactions
Cells are in contact, or in close proximity, with many neighboring cells of the same or different type in a highly organized manner in vivo, and the cross-talk between these adjacent cells governs many important biological processes.(Engler et al. 2009; Huh et al. 2010) Therefore, controlling cell-cell interactions can improve the proper functioning of tissue-engineered constructs by mimicking the architecture and geometry of native tissues. Microscale technologies that have been used to investigate and characterize cell-cell interactions include micromolding, μCP,(Nelson and Chen 2003) stencils,(Wright et al. 2007) interdigitating micromachined plates,(Hui and Bhatia 2007) stereolithography,(Zorlutuna et al. 2011) robotic deposition, and dielectrophoresis.(Albrecht et al. 2006)
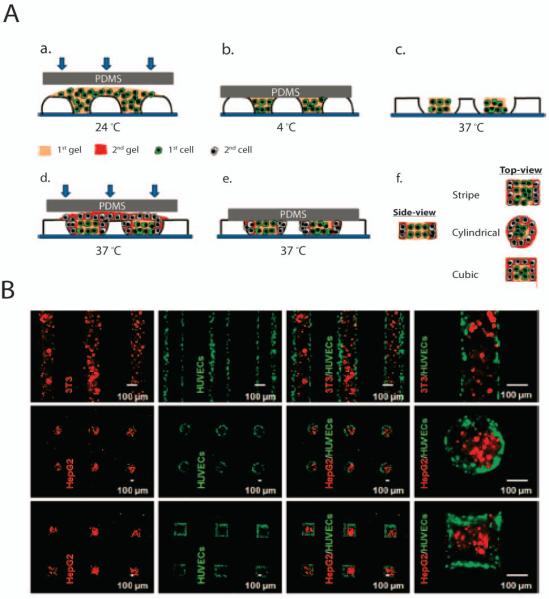
Patterning of different cell types at addressable locations on a substrate has been used to generate patterned co-culture systems to investigate cell-cell interactions. One method of fabricating such systems is to use stimuli-responsive polymers. These polymers are a class of materials that respond to external stimuli via conformational or chemical changes.(Stuart et al. 2010) These stimuli may include temperature, chemical, mechanical, radiation, electrical, or magnetic field changes. PNIPAAm is a temperature-responsive hydrogel with a lower critical solution temperature of 32 °C, above which it shrinks and below which it swells. Using PNIPAAm as a bas-relief master, Tekin et al. were able to generate patterned hydrogel microstructures containing different cell types (Fig. 3). Briefly, the PNIPAAm master was filled with agarose gel at room temperature and crosslinked at 4 °C. The master mold was then incubated at 37 °C to shrink the PNIPAAm molds, creating space between the molds and the agarose gel. A second gel precursor was used to fill the newly created space, and upon further incubation at 37 °C, crossliking of the second precursor occurred. Patterened co-cultures of 3T3/human umbilical vein endothelial cells (HUVECs) and HepG2/HUVECs were created using the abovementioned technique. Microfabricated stencils have also been used to pattern cells in a co-culture system. For example, micropatterns of hepatocytes, embryonic stem cells (ESC), and fibroblasts were generated by using a parylene-C stencils.(Wright et al. 2008)
Figure 3.
Generation of organized heterotopic cell co-cultures. The sequential patterning of hydrogels is illustrated in the schematic (A). Patterning of differerent cell types encapsulated in microgels (B). Adapted with permission from (Tekin et al. 2010). Copyright (2011) American Chemical Society.
A disadvantage of the aforementioned works on cell-cell communication is the static nature of the culture platforms. However, it is well known that dynamic cell-cell communication are important for understanding a number of biological phenomena, such as wound healing and morphogenesis.(Kaji et al. 2011) To recreate a dynamic cellular environment, a silicon platform consisting of two interdigitating pieces was fabricated by micromachining, enabling the adjustment of the distance between the interdigitating plates—containing different cell types—and facilitating dynamic manipulation of the cell-cell interactions.(Hui and Bhatia 2007) Using this device, the dynamics of intercellular communication between hepatocytes and stromal cells was assessed, revealing that short distances between cells (<400μm) are likely to be required for the maintenance of hepatocytes. As mentioned above, a variety of microscale technologies have been introduced to regulate the degree of cell-cell contacts, allowing greater control over generation of spatially organized tissue constructs.
4. High-throughput screening microarrays
Despite significant efforts made by the pharmaceutical industry towards drug discovery, a handful of drugs get approved annually.(Chung et al. 2007) Each year, only a few of the thousands of developed or discovered compounds proceed to human clinical trials, which then takes years to complete. Therefore, HTS systems using microscale technologies have been developed to miniaturize the drug discovery process, enabling a dramatic increase in the number of screenable drug candidates while reducing reagent consumption and cost.(Fernandes et al. 2009) The HTS traditionally used in the pharmaceutical industry has been expanded to other applications such as testing of cellular responses to various biomolecules. Moreover, as mentioned previously, cells grown in 3D culture more closely resemble their in vivo counterparts than traditional 2D systems. Such an implication—demonstrated in gene expression, cell adhesion and migration,(Cukierman et al. 2001) epithelial morphogenesis,(Grant et al. 2006) tumor biology,(Mueller-Klieser 2000) and developmental biology,(Hove et al. 2003)—could mean that more effective material and drug screening needs to take place in 3D platforms. In this regard as well, micro- and nanoscale technologies have provided powerful tools to generate miniaturized HTS systems through techniques such as soft lithography, robotic spotting,(Kwon et al. 2011) and inkjet printing(Sele et al. 2005; Park et al. 2007). These cell-based assays can be used to perform thousands of tests in parallel and are valuable tools to analyze cell-material and cell-cell interactions in a rapid and reproducible manner, both in 2D and 3D.
2D monolayers of a broad range of molecules can be printed on a glass surface using robotic spotting technology(Mei et al. 2010). In the case of polymeric materials, the polymers can either be synthesized prior to their deposition or the polymerization may be initiated on the substrate. Subsequently, cells can be seeded across the array and their behavior analyzed using various detection methods. For example, Mei et al. fabricated a combinatorial synthetic material microarray for testing of the self-renewal capability of human pluripotent stem cells.(Mei et al. 2010) Their array contained 496 different combinations of 22 acrylate monomers that were robotically deposited and polymerized via UV light. The material properties of each substrate, such as elastic modulus, topography, surface chemistry, and wettability were quantified in a high-throughput manner. Substrates with high acrylate content favored maintenance of pluripotency. Other studies have generated combinatorial libraries of synthetic materials,(Anderson et al. 2005) ECM proteins,(Flaim et al. 2005) and ECM/growth factors.(Flaim et al. 2008) One of the disadvantages of these systems are susceptibility to region-to-region contamination, caused by the lateral diffusion of molecules between test spots.(Fernandes et al. 2009) To overcome this problem, Wu et al. developed a sandwich HTS platform in which cells were seeded in a microwell array and separately, chemical compounds were printed on microposts. Finally, the posts and wells were aligned leading to the formation of isolated reaction chambers where the effect of a test compound on cells could be studied without risk of cross-contamination.(Wu et al. 2010)
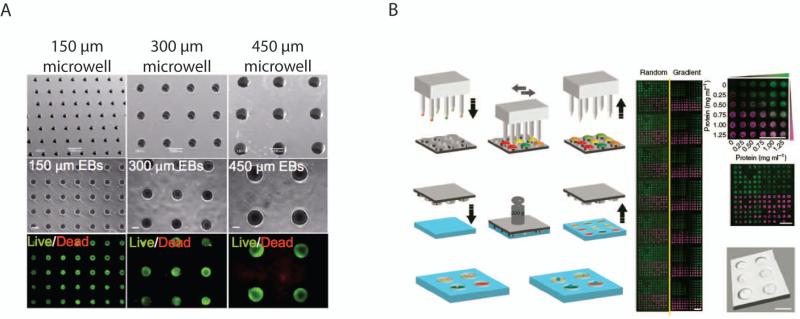
To investigate biomimetic 3D microenvironments, a number of HTS technologies have been developed for creating 3D cell-laden microgel arrays.(Fernandes et al. 2010) In this approach, arrays of murine ESC-laden alginate hydrogels were created to study the interactions between cells and soluble factors in a 3D environment. Such an array demonstrated an efficient method of studying the expansion or neural commitment of ESCs, and the effects of fibroblast growth factor-4 (FGF-4) on pluripotency. Microtechnological approaches can also be used to fabricate polymeric microwell arrays with defined dimensions for controlling supracellular interactions and cell aggregation.(Khademhosseini et al. 2006; Moeller et al. 2008) For instance, soft lithography and laser micromachining have been used to generate an array of PEG(Moeller et al. 2008), PNIPAAm,(Tekin et al. 2010) and polyester microwells,(Selimovic et al. 2011). These microwell arrays exhibit low shear stress inside the wells, which allows for cell docking and positioning. This method of cell seeding is a useful research tool for generating uniform ESC aggregates, called embryoid bodies (EBs), by controlling the size of the microwells (Fig. 4A).(Hwang et al. 2009) In one study, modulating the EB size via control of microwell size (150, 300, and 450μm) led to size-dependent endothelial and cardiac cell differentiation in the EBs. In smaller EBs endothelial cell differentiation was enhancd, while cardiogenesis was favored in larger EBs. Furthermore, non-canonical Wnt molecules that were differentially expressed as a function of EB size were identified. While the abovementioned microwells provide a high-throughput platform, they do not allow for rapid screening of cues that affect cells. To overcome this limitation, Gobaa et al. designed a microwell array with each well having its own unique biochemical properties.(Gobaa et al. 2011) A microfabricated silicon stamp, onto which different proteins at various concentrations had been deposited with a DNA spotter, was pressed against an incompletely cross-linked PEG hydrogel to make microwells with unique biochemical cues (Fig. 4B). By changing the concentration of the PEG prepolymer, varying degrees of substrate stiffness in the range of 1-50 kPa was obtained. This microwell array platform showed that adipogenic differentiation is favored in microwells containing a greater number of MSCs; further, osteogenesis occurred to a greater extend in microwells with higher elastic moduli.
Figure 4.
High-throughput systems. (A) A poly(ethylene glycol) microwell array for generating uniformly sized embryoid bodies.(Hwang et al. 2009) Copyright (2009) National Academy of Sciences, USA. (B) A method for creating a high-throughput microarray with different biochemical signals. Different proteins (represented by the different colors) are deposited onto a microfabricated stamp via a DNA spotter (left). The stamp is then pressed against a partially cross-linked hydrogel to transfer the proteins and generate microwells. A microarray of a combinatorial gradients of two fluorescently labeled proteins is shown (right).(Gobaa et al. 2011) Adapted with permission from Macmillan Publishers Ltd: [Nature Methods], copyright (2011).
5. Micro- and nanotechnologies in drug delivery
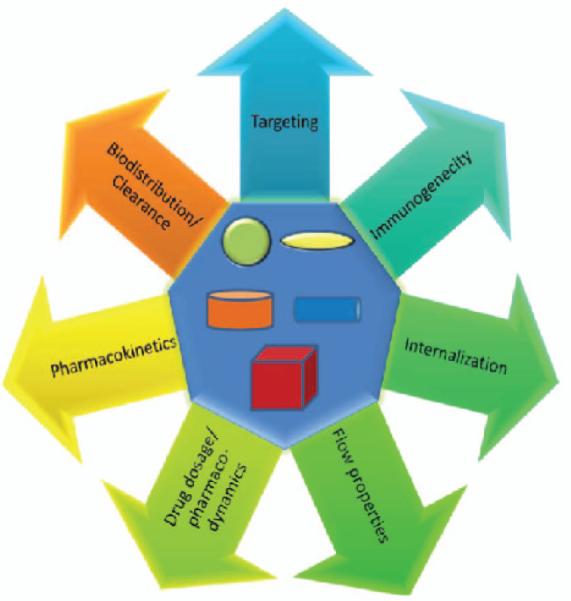
From the structural simplicity of a virus to the complexity conferred by a bacteria or a eukaryotic cell, the size and shape of these species partly dictate the nature of their interactions with other biological entities.(Young 2010) For example, the discoid shape of inactivated platelets allows them to adhere or roll on the vascular endothelium, and the biconcave disk-shape and elasticity of erythrocytes enables them to squeeze through capillaries, avoid filtration in the spleen, and maximize surface area for gas exchange. Thus, in biology, size and shape are essential determinants of functionality within the body. In the field of drug delivery, the size and shape of drug carriers have emerged as important design criteria in the pursuit of the next generation of therapeutic delivery systems. Significant research in the area of drug delivery is focused on discovering new chemical and molecular recognition patterns for improved control over pharmacokinetic and pharmacodynamic properties of drugs such as half-life, solubility, release rates, and toxicity.(Mitragotri 2009; Mitragotri and Lahann 2009) A major focus in this area has been on size, material chemistry, and particle surface characteristics of drug carriers. Gaining micro- and nanoscale control over particle size has helped researchers study the effects of size on various in vivo functions such as immunogenicity,(Champion et al. 2008) circulation times,(Decuzzi et al. 2009) uptake, intracellular trafficking,(Rejman et al. 2004; Gao et al. 2005; Sant et al. 2008) extravasation,(Stolnik et al. 1995) targeting, degradation,(Glangchai et al. 2008) and blood flow (Fig. 5).(Goldsmith and Turitto 1986; Lamprecht et al. 2001; Patil et al. 2001) For instance, tumors are known to accumulate nanometer-scale particles such as liposomes and nanoparticles (NPs), due to their leaky vasculature and undeveloped lymphatic drainage, a phenomenon known as the enhanced permeability and retention (EPR) effect.(Matsumura and Maeda 1986; Yuan et al. 1995; Hobbs et al. 1998) Hence, drug carriers for cancer therapeutics have been designed to be in the range of 10-100nm which demonstrate the EPR effect.(Moghimi et al. 2005)
Figure 5.
Schematic illustration of some of the parameters of drug delivery that may be affected by shape and size of particulate drug delivery agents.
Apart from size, particle geometry has been shown to be an important parameter in the biodistribution, phagocytosis, and intracellular trafficking of NPs.(Gratton et al. 2008) In particular, developing methods to simultaneously control shape and size have been challenging. Traditional particle synthesis methods vary from emulsion polymerization(Clark et al. 1999), self-assembly,(Moghimi et al. 2005) and jet breaking,(Berkland et al. 2001) while more recently developed methods include soft lithography,(Rolland et al. 2005) microfluidics,(Dendukuri et al. 2006) self-assembly,(Manoharan et al. 2003) and electrospinning.(Bhaskar et al. 2010) Despite decades of experience with these techniques, emulsion and nanoprecipitation methods for particle synthesis can produce only spherical particles with little control over their shape and size. Direct extension of microfluidic and lithographic techniques to drug delivery has enabled researchers to precisely control the size, shape, particle rigidity, biological cargo, and surface properties of these nanocarriers. Using these methods, distributions obtained are highly homogenous and allow more complex study of shape-specific interactions. In this section, we will highlight the applications of micro- and nanofabrication approaches to the control of the size and shape of polymeric drug delivery systems along with brief descriptions of the fabrication processes.
Researchers have found that the shape of particles influence their biodistribution, as well as their pharmacokinetics and pharmacodynamics.(Champion et al. 2007; Mitragotri 2009) Mathematical models have described receptor-mediated endocytosis,(Decuzzi and Ferrari 2008) adhesive behaviour,(Decuzzi and Ferrari 2006) and margination dynamics of non-spherical particles,(Gentile et al. 2008; Decuzzi et al. 2009) allowing the study of the transport, internalization and vascular dynamics of these particles. Theoretical studies using these models have predicted that oblate particles will result in more efficient adherence to the vascular endothelium compared to spherical particles of comparable volume. Particle geometry has been shown to be one of the crucial parameters in cell internalization pathways as well. It has been experimentally shown that oblate particles with their high aspect ratio have the ability to induce internalization when they contact macrophages along their length.(Champion and Mitragotri 2006) Despite evidence demonstrating the need to control geometry for drug delivery applications, progress in control of shape has been limited by product yield and non-homogeneity.
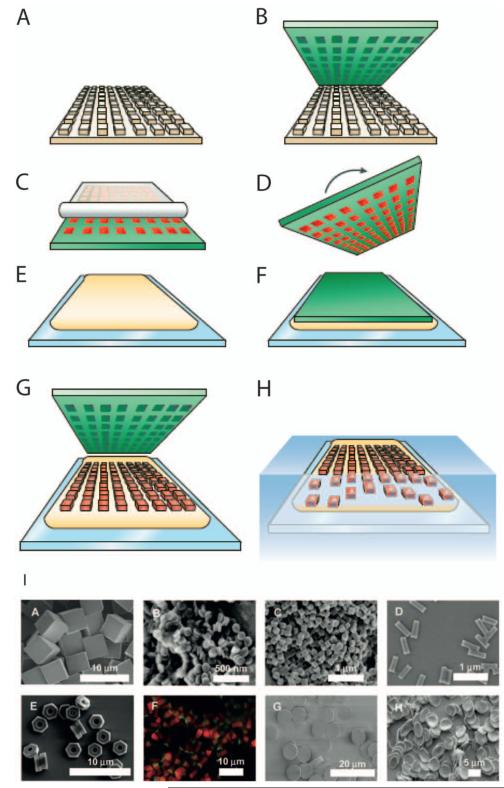
A production method combining photolithography and soft lithography, called Particle Replication In Non-wetting Templates (PRINT), was developed by DeSimone and colleagues representing a major step towards improved control of particle geometry (Fig. 6).(Gratton et al. 2008) This method is used to obtain monodispersed particles of controlled shape and size by means of creating patterns on a silicon master template, which is subsequently used in creating cavities on a fluorinated mold. The particle pre-polymer is then used to fill these cavities by means of capillary filling favoured by the fluorinated polymer’s higher surface energy. These molds have been used with different substrate materials to make particles of specific geometries.(Rolland et al. 2005) The PRINT technology is capable of controlling particle size (20nm to >100μm), shape (spheres, discs, cylinders, toroidal), composition (solid/porous, organic/inorganic), mechanical properties (deformable, stiff,), cargo (hydrophilic or hydrophobic compounds, oligonucleotides, siRNA, imaging agents), surface properties (cationic/anion charges, targeting peptides, aptamers, antibodies, stealth PEG chains), and in a simultaneous and independent manner.(Gratton et al. 2008; Gratton et al. 2008) The difference between PRINT and traditional soft lithography is that instead of using silicone-based polymers, PRINT uses low surface energy, non-wetting perfluoropolyethers, which overcomes scum layer formation.(Rolland et al. 2005) By using this robust method, studies were carried out on the biodistribution of particles (Gratton et al. 2007; Gratton et al. 2008); also, it was observed that particles with higher aspect ratio were internalized more readily.(Gratton et al. 2008) It was also possible to modulate the surface charge of shape-controlled particles to study the effect on cellular internalization mechanisms.(Gratton et al. 2008) It was observed that positively charged particles were internalized more efficiently than negatively charged ones, which could be used to improve the targeting function of such particles. Furthermore, the mechanism of the cellular uptake of positively-charge 1μm cylindrical particles was predominantly clathrin-mediated endocytosis and macropinocytosis. More recently, this technology has been applied in colloidal chemistry giving anisotropic chemical properties to the particle.(Bhaskar et al. 2010) While microfabrication techniques like PRINT can be used to control various parameters such as shape and size, greater targeting specificity and understanding of the biological mechanism behind shape-specific uptake of drug carriers are needed.
Figure 6.
Diagram of the Particle Replication In Non-wetting Templates (PRINT) process: A silicon master (A) is used as a master template to make perfluoropolyether molds (green) (B); capillary filling of the molds with liquid precursors (red), followed by their solidification (C) generates particles that can be harvested with an adhesive film. Alternatively, the solidified particles can be obtained by turning over the mold (D) onto a liquid harvesting layer (yellow) (E,F); the harvesting layer is then cured, trapping the particles, and the mold is peeled away (G). Finally, the harvesting layer is dissolved and individual particles are generated (H).(Petros and DeSimone 2010) Adapted with permission from Macmillan Publishers Ltd: [Nature Reviews Drug Discovery], copyright (2010). (I) PRINT Particles varying in size and shape (A-H), surface chemistry (F), and deformability (G,H). Adapted with permission from (Gratton et al. 2008). Copyright (2008) American Chemical Society.
6. Conclusions and future perspectives
In the past, developments in the biomedical and pharmaceutical fields was hindered by limitations of traditional methodologies such as inaccurate, macroscopic control of cellular behaviors and labor intensive, expensive testing of cellular responses to pharmaceutical agents in low-throughput systems. Currently, due to the rapid growth of micro- and nanoscale technologies combined with advances of biomaterials, new solutions have been proposed. As discussed in this review, micro- and nanoscale technologies demonstrate the feasibility to regulate the spatial and temporal aspects of the cell microenvironment in biomimetic scaffolds by precisely controlling cell-material and cell-cell interactions; these advances will pave the road for fabrication of functional cellular tissue constructs for regenerative medicine purposes. In addition, the development of HTS systems using microfabrication techniques demonstrates the ability to dramatically enhance screening efficiencies in drug target validation and preclinical toxicology processes at considerably lower cost. Furthermore, the control of size and shape of drug carriers with technologies such as PRINT has allowed for a modulating of pharmacological properties. In conclusion, current and future biotechnologies will be further advanced by the continued development of micro- and nanoscale technologies presenting a bright future for tissue engineering and drug delivery.
Footnotes
Author Contributions
H.B., H.C., and F.E. contributed equally to this work. H.B., H.C., F.E., A.F.A, S.S., Y.W, and A.K. generated idea and designed the manuscript; H.B., H.C., F.E., A.F.A., J.M.C., S.S., A.K., C.H.K., B.Z., Y.W., and A.K. wrote the manuscript; H.B., H.C., F.E., A.F.A., J.M.C., J.W.N., S.M., Y.W., and A.K. revised the manuscript.
References
- Albrecht DR, Underhill GH, Wassermann TB, Sah RL, Bhatia SN. Probing the role of multicellular organization in three-dimensional microenvironments. Nat Methods. 2006;3:369–375. doi: 10.1038/nmeth873. [DOI] [PubMed] [Google Scholar]
- Anderson DG, Putnam D, Lavik EB, Mahmood TA, Langer R. Biomaterial microarrays: Rapid, microscale screening of polymer-cell interaction. Biomaterials. 2005;26:4892–4897. doi: 10.1016/j.biomaterials.2004.11.052. [DOI] [PubMed] [Google Scholar]
- Aubin H, Nichol JW, Hutson CB, Bae H, Sieminski AL, Cropek DM, Akhyari P, Khademhosseini A. Directed 3D cell alignment and elongation in microengineered hydrogels. Biomaterials. 2010;31:6941–6951. doi: 10.1016/j.biomaterials.2010.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae H, Ahari AF, Shin H, Nichol JW, Hutson CB, Masaeli M, Kim SH, Aubin H, Yamanlar S, Khademhosseini A. Cell-laden microengineered pullulan methacrylate hydrogels promote cell proliferation and 3D cluster formation. Soft Matter. 2011;7:1903–1911. doi: 10.1039/C0SM00697A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauwens CL, Peerani R, Niebruegge S, Woodhouse KA, Kumacheva E, Husain M, Zandstra PW. Control of human embryonic stem cell colony and aggregate size heterogeneity influences differentiation trajectories. Stem Cells. 2008;26:2300–2310. doi: 10.1634/stemcells.2008-0183. [DOI] [PubMed] [Google Scholar]
- Berkland C, Kim K, Pack DW. Fabrication of PLG microspheres with precisely controlled and monodisperse size distributions. J Control Release. 2001;73:59–74. doi: 10.1016/s0168-3659(01)00289-9. [DOI] [PubMed] [Google Scholar]
- Bettinger C, Langer R, Borenstein J. Engineering substrate topography at the micro- and nanoscale to control cell function. Angew Chem Int Ed Engl. 2009;48:5406–5415. doi: 10.1002/anie.200805179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar S, Pollock KM, Yoshida M, Lahann J. Towards designer microparticles: Simultaneous control of anisotropy, shape, and size, Small. 2010;6:404–411. doi: 10.1002/smll.200901306. [DOI] [PubMed] [Google Scholar]
- Burdick JA, Khademhosseini A, Langer R. Fabrication of gradient hydrogels using a microfluidics/photopolymerization process. Langmuir. 2004;20:5153–5156. doi: 10.1021/la049298n. [DOI] [PubMed] [Google Scholar]
- Champion JA, Katare YK, Mitragotri S. Particle shape: A new design parameter for micro- and nanoscale drug delivery carriers. J Control Release. 2007;121:3–9. doi: 10.1016/j.jconrel.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci U S A. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion JA, Walker A, Mitragotri S. Role of particle size in phagocytosis of polymeric microspheres. Pharm Res. 2008;25:1815–1821. doi: 10.1007/s11095-008-9562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan V, Jeong JH, Bajaj P, Collens M, Saif T, Kong H, Bashir R. Multi-material bio-fabrication of hydrogel cantilevers and actuators with stereolithography. Lab Chip. 2012;12:88–98. doi: 10.1039/c1lc20688e. [DOI] [PubMed] [Google Scholar]
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- Chou SY, Krauss PR, Renstrom PJ. Imprint lithography with 25-nanometer resolution. Science. 1996;272:85–87. [Google Scholar]
- Christman KL, Schopf E, Broyer RM, Li RC, Chen Y, Maynard HD. Positioning multiple proteins at the nanoscale with electron beam cross-linked functional polymers. J Am Chem Soc. 2009;131:521–527. doi: 10.1021/ja804767j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung BG, Kang L, Khademhosseini A. Micro- and nanoscale approaches for tissue engineering and drug discovery. Expt Opin Drug Dis. 2007;2:1653–1668. doi: 10.1517/17460441.2.12.1653. [DOI] [PubMed] [Google Scholar]
- Clark HA, Kopelman R, Tjalkens R, Philbert MA. Optical nanosensors for chemical analysis inside single living cells. 2 Sensors for pH and calcium and the intracellular application of PEBBLE sensors. Anal Chem. 1999;71:4837–4843. doi: 10.1021/ac990630n. [DOI] [PubMed] [Google Scholar]
- Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- Das G, Mecarini F, Gentile F, De Angelis F, Kumar HGM, Candeloro P, Liberale C, Cuda G, Di Fabrizio E. Nano-patterned SERS substrate: Application for protein analysis vs. temperature. Biosens Bioelectron. 2009;24:1693–1699. doi: 10.1016/j.bios.2008.08.050. [DOI] [PubMed] [Google Scholar]
- Decuzzi P, Ferrari M. The adhesive strength of non-spherical particles mediated by specific interactions. Biomaterials. 2006;27:5307–5314. doi: 10.1016/j.biomaterials.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Decuzzi P, Ferrari M. The receptor-mediated endocytosis of nonspherical particles. Biophys J. 2008;94:3790–3797. doi: 10.1529/biophysj.107.120238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decuzzi P, Pasqualini R, Arap W, Ferrari M. Intravascular delivery of particulate systems: Does geometry really matter? Pharm Res. 2009;26:235–243. doi: 10.1007/s11095-008-9697-x. [DOI] [PubMed] [Google Scholar]
- del Campo A, Arzt E. Fabrication approaches for generating complex micro-and nanopatterns on polymeric surfaces. Chem Rev. 2008;108:911–945. doi: 10.1021/cr050018y. [DOI] [PubMed] [Google Scholar]
- Dendukuri D, Pregibon DC, Collins J, Hatton TA, Doyle PS. Continuous-flow lithography for high-throughput microparticle synthesis. Nat Mater. 2006;5:365–369. doi: 10.1038/nmat1617. [DOI] [PubMed] [Google Scholar]
- Dolatshahi-Pirouz A, Nikkhah M, Kolind K, Dokmeci MR, Khademhosseini A. Micro- and nanoengineering approaches to control stem cell-biomaterial interactions. J Funct Biomater. 2011;2:88–106. doi: 10.3390/jfb2030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle AD, Wang FW, Matsumoto K, Yamada KM. One-dimensional topography underlies three-dimensional fibrillar cell migration. J Cell Biol. 2009;184:481–490. doi: 10.1083/jcb.200810041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvir T, Timko BP, Kohane DS, Langer R. Nanotechnological strategies for engineering complex tissues. Nat Nanotechnol. 2011;6:13–22. doi: 10.1038/nnano.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edalat F, Bae H, Manoucheri S, Cha JM, Khademhosseini A. Engineering approaches toward deconstructing and controlling the stem cell environment. Ann Biomed Eng. 2011 doi: 10.1007/s10439-011-0452-9. 10.1007/s10439-011-0452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton RF, Li P, Malac M. Radiation damage in the TEM and SEM. Micron. 2004;35:399–409. doi: 10.1016/j.micron.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang H-Y, Speicher DW, Sanger JW, Sanger JM, Discher DE. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: Scar-like rigidity inhibits beating. J Cell Sci. 2008;121:3794–3802. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Humbert PO, Wehrle-Haller B, Weaver VM. Multiscale modeling of form and function. Science. 2009;324:208–212. doi: 10.1126/science.1170107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes TG, Diogo MM, Clark DS, Dordick JS, Cabral JM. High-throughput cellular microarray platforms: Applications in drug discovery, toxicology and stem cell research. Trends Biotechnol. 2009;27:342–349. doi: 10.1016/j.tibtech.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes TG, Kwon SJ, Bale SS, Lee MY, Diogo MM, Clark DS, Cabral JM, Dordick JS. Three-dimensional cell culture microarray for high-throughput studies of stem cell fate. Biotechnol Bioeng. 2010;106:106–118. doi: 10.1002/bit.22661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaim CJ, Chien S, Bhatia SN. An extracellular matrix microarray for probing cellular differentiation. Nat Methods. 2005;2:119–125. doi: 10.1038/nmeth736. [DOI] [PubMed] [Google Scholar]
- Flaim CJ, Teng D, Chien S, Bhatia SN. Combinatorial signaling microenvironments for studying stem cell fate. Stem Cells Dev. 2008;17:29–39. doi: 10.1089/scd.2007.0085. [DOI] [PubMed] [Google Scholar]
- Folch A, Jo BH, Hurtado O, Beebe DJ, Toner M. Microfabricated elastomeric stencils for micropatterning cell cultures. J Biomed Mater Res. 2000;52:346–353. doi: 10.1002/1097-4636(200011)52:2<346::aid-jbm14>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Fu AY, Chou HP, Spence C, Arnold FH, Quake SR. An integrated microfabricated cell sorter. Anal Chem. 2002;74:2451–2457. doi: 10.1021/ac0255330. [DOI] [PubMed] [Google Scholar]
- Gao H, Shi W, Freund LB. Mechanics of receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 2005;102:9469–9474. doi: 10.1073/pnas.0503879102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates BD, Xu Q, Stewart M, Ryan D, Willson CG, Whitesides GM. New approaches to nanofabrication: molding, printing, and other techniques. Chem Rev. 2005;105:1171–1196. doi: 10.1021/cr030076o. [DOI] [PubMed] [Google Scholar]
- Gauvin R, Khademhosseini A. Microscale technologies and modular approaches for tissue engineering: Moving toward the fabrication of complex functional structures. ACS Nano. 2011;5:4258–4264. doi: 10.1021/nn201826d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvin R, Parenteau-Bareil R, Dokmeci MR, Merryman WD, Khademhosseini A. Hydrogels and microtechnologies for engineering the cellular microenvironment. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011 doi: 10.1002/wnan.171. 10.1002/wnan.171. [DOI] [PubMed] [Google Scholar]
- Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Bio. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- Gentile F, Chiappini C, Fine D, Bhavane RC, Peluccio MS, Cheng MM-C, Liu X, Ferrari M, Decuzzi P. The effect of shape on the margination dynamics of non-neutrally buoyant particles in two-dimensional shear flows. J Biomech. 2008;41:2312–2318. doi: 10.1016/j.jbiomech.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Glangchai LC, Caldorera-Moore M, Shi L, Roy K. Nanoimprint lithography based fabrication of shape-specific, enzymatically-triggered smart nanoparticles. J Control Release. 2008;125:263–272. doi: 10.1016/j.jconrel.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Gobaa S, Hoehnel S, Roccio M, Negro A, Kobel S, Lutolf MP. Artificial niche microarrays for probing single stem cell fate in high throughput. Nat Methods. 2011;8:949–955. doi: 10.1038/nmeth.1732. [DOI] [PubMed] [Google Scholar]
- Goldsmith HL, Turitto VT. Rheological aspects of thrombosis and haemostasis: Basic principles and applications. ICTH-Report--Subcommittee on Rheology of the International Committee on Thrombosis and Haemostasis. Thromb Haemost. 1986;55:415–435. [PubMed] [Google Scholar]
- Grant MR, Kim SH, Hunt CA. Simulating in vitro epithelial morphogenesis in multiple environments. Comput Syst Bioinformatics Conf. 2006:381–384. [PubMed] [Google Scholar]
- Gratton SE, Napier ME, Ropp PA, Tian S, DeSimone JM. Microfabricated particles for engineered drug therapies: Elucidation into the mechanisms of cellular internalization of PRINT particles. Pharm Res. 2008;25:2845–2852. doi: 10.1007/s11095-008-9654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton SE, Pohlhaus PD, Lee J, Guo J, Cho MJ, Desimone JM. Nanofabricated particles for engineered drug therapies: A preliminary biodistribution study of PRINT nanoparticles. J Control Release. 2007;121:10–18. doi: 10.1016/j.jconrel.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton SE, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, DeSimone JM. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci U S A. 2008;105:11613–11618. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton SE, Williams SS, Napier ME, Pohlhaus PD, Zhou Z, Wiles KB, Maynor BW, Shen C, Olafsen T, Samulski ET, Desimone JM. The pursuit of a scalable nanofabrication platform for use in material and life science applications. Acc Chem Res. 2008;41:1685–1695. doi: 10.1021/ar8000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo LJ. Nanoimprint lithography: Methods and material requirements. Adv Mater. 2007;19:495–513. [Google Scholar]
- Guo LJ, Cheng X, Chou CF. Fabrication of size-controllable nanofluidic channels by nanoimprinting and its application for DNA stretching. Nano Lett. 2004;4:69–73. [Google Scholar]
- Hahn MS, Miller JS, West JL. Three dimensional biochemical and biomechanical patterning of hydrogels for guiding cell behavior. Adv Mater. 2006;18:2679–2684. [Google Scholar]
- Hansen CL, Skordalakes E, Berger JM, Quake SR. A robust and scalable microfluidic metering method that allows protein crystal growth by free interface diffusion. Proc Natl Acad Sci U S A. 2002;99:16531–16536. doi: 10.1073/pnas.262485199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc Natl Acad Sci U S A. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff JD, Cheng LJ, Meyhöfer E, Guo LJ, Hunt AJ. Nanoscale protein patterning by imprint lithography. Nano Lett. 2004;4:853–857. [Google Scholar]
- Hong JW, Studer V, Hang G, Anderson WF, Quake SR. A nanoliter-scale nucleic acid processor with parallel architecture. Nat Biotechnol. 2004;22:435–439. doi: 10.1038/nbt951. [DOI] [PubMed] [Google Scholar]
- Hook AL, Anderson DG, Langer R, Williams P, Davies MC, Alexander MR. High throughput methods applied in biomaterial development and discovery. Biomaterials. 2010;31:187–198. doi: 10.1016/j.biomaterials.2009.09.037. [DOI] [PubMed] [Google Scholar]
- Hove JR, Koster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421:172–177. doi: 10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- Hua F, Sun Y, Gaur A, Meitl MA, Bilhaut L, Rotkina L, Wang J, Geil P, Shim M, Rogers JA. Polymer imprint lithography with molecular-scale resolution. Nano Lett. 2004;4:2467–2471. [Google Scholar]
- Huang J, Gräter SV, Corbellini F, Rinck S, Bock E, Kemkemer R, Kessler H, Ding J, Spatz JP. Impact of order and disorder in RGD nanopatterns on cell adhesion. Nano Lett. 2009;9:1111–1116. doi: 10.1021/nl803548b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science. 2010;328:1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui EE, Bhatia SN. Micromechanical control of cell-cell interactions. Proc Natl Acad Sci U S A. 2007;104:5722–5726. doi: 10.1073/pnas.0608660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang YS, Chung BG, Ortmann D, Hattori N, Moeller HC, Khademhosseini A. Microwell-mediated control of embryoid body size regulates embryonic stem cell fate via differential expression of WNT5a and WNT11. Proc Natl Acad Sci U S A. 2009;106:16978–16983. doi: 10.1073/pnas.0905550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. The Extracellular matrix: Not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idota N, Tsukahara T, Sato K, Okano T, Kitamori T. The use of electron beam lithographic graft-polymerization on thermoresponsive polymers for regulating the directionality of cell attachment and detachment. Biomaterials. 2009;30:2095–2101. doi: 10.1016/j.biomaterials.2008.12.058. [DOI] [PubMed] [Google Scholar]
- Ito T, Okazaki S. Pushing the limits of lithography. Nature. 2000;406:1027–1031. doi: 10.1038/35023233. [DOI] [PubMed] [Google Scholar]
- Kaji H, Camci-Unal G, Langer R, Khademhosseini A. Engineering systems for the generation of patterned co-cultures for controlling cell-cell interactions. Biochim Biophys Acta. 2011;1810:239–250. doi: 10.1016/j.bbagen.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp J, Yeo Y, Geng W, Cannizarro C, Yan K, Kohane D, Vunjak-Novakovic G, Langer R, Radisic M. A photolithographic method to create cellular micropatterns. Biomaterials. 2006;27:4755–4764. doi: 10.1016/j.biomaterials.2006.04.028. [DOI] [PubMed] [Google Scholar]
- Karp JM, Yeh J, Eng G, Fukuda J, Blumling J, Suh K-Y, Cheng J, Mahdavi A, Borenstein J, Langer R, Khademhosseini A. Controlling size, shape and homogeneity of embryoid bodies using poly(ethylene glycol) microwells. Lab Chip. 2007;7:786–794. doi: 10.1039/b705085m. [DOI] [PubMed] [Google Scholar]
- Kaufmann T, Ravoo BJ. Stamps, inks and substrates: Polymers in microcontact printing. Polym Chem. 2010;1:371–387. [Google Scholar]
- Ker EDF, Chu B, Phillippi JA, Gharaibeh B, Huard J, Weiss LE, Campbell PG. Engineering spatial control of multiple differentiation fates within a stem cell population. Biomaterials. 2011;32:3413–3422. doi: 10.1016/j.biomaterials.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khademhosseini A, Eng G, Yeh J, Fukuda JBJ, 3rd, Langer R, et al. Micromolding of photocrosslinkable hyaluronic acid for cell encapsulation and entrapment. J Biomed Mater Res A. 2006;79:522–532. doi: 10.1002/jbm.a.30821. [DOI] [PubMed] [Google Scholar]
- Khademhosseini A, Eng G, Yeh J, Kucharczyk PA, Langer R, Vunjak-Novakovic G, Radisic M. Microfluidic patterning for fabrication of contractile cardiac organoids. Biomed Microdevices. 2007;9:149–157. doi: 10.1007/s10544-006-9013-7. [DOI] [PubMed] [Google Scholar]
- Khademhosseini A, Ferreira L, Blumling J, 3rd, Yeh J, Karp JM, Fukuda J, Langer R. Co-culture of human embryonic stem cells with murine embryonic fibroblasts on microwell-patterned substrates. Biomaterials. 2006;27:5968–5977. doi: 10.1016/j.biomaterials.2006.06.035. [DOI] [PubMed] [Google Scholar]
- Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci U S A. 2006;103:2480–2487. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian KA, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci U S A. 2010;107:4872–4877. doi: 10.1073/pnas.0903269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D-H, Lipke EA, Kim P, Cheong R, Thompson S, Delannoy M, Suh K-Y, Tung L, Levchenko A. Nanoscale cues regulate the structure and function of macroscopic cardiac tissue constructs. Proc Natl Acad Sci. 2010;107:565–570. doi: 10.1073/pnas.0906504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JD, Choi JS, Kim BS, Chan YC, Cho YW. Piezoelectric inkjet printing of polymers: Stem cell patterning on polymer substrates. Polymer. 2010;51:2147–2154. [Google Scholar]
- Kwon CH, Wheeldon I, Kachouie NN, Lee SH, Bae H, Sant S, Fukuda J, Kang JW, Khademhosseini A. Drug-eluting microarrays for cell-based screening of chemical-induced apoptosis. Anal Chem. 2011;83:4118–4125. doi: 10.1021/ac200267t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht A, Schafer U, Lehr CM. Size-dependent bioadhesion of micro- and nanoparticulate carriers to the inflamed colonic mucosa. Pharm Res. 2001;18:788–793. doi: 10.1023/a:1011032328064. [DOI] [PubMed] [Google Scholar]
- Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- Lee K-S, Kim RH, Yang D-Y, Park SH. Advances in 3D nano/microfabrication using two-photon initiated polymerization. Prog Polym Sci. 2008;33:631–681. [Google Scholar]
- Li HW, Muir BVO, Fichet G, Huck WTS. Nanocontact printing: A route to sub-50-nm-scale chemical and biological patterning. Langmuir. 2003;19:1963–1965. [Google Scholar]
- Lim JY, Donahue HJ. Cell sensing and response to micro- and nanostructured surfaces produced by chemical and topographic patterning. Tissue Eng. 2007;13:1879–1891. doi: 10.1089/ten.2006.0154. [DOI] [PubMed] [Google Scholar]
- Liu J, Enzelberger M, Quake S. A nanoliter rotary device for polymerase chain reaction. Electrophoresis. 2002;23:1531–1536. doi: 10.1002/1522-2683(200205)23:10<1531::AID-ELPS1531>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Lutolf MP. Integration column: Artificial ECM: Expanding the cell biology toolbox in 3D. Integr Biol. 2009;1:235–241. doi: 10.1039/b902243k. [DOI] [PubMed] [Google Scholar]
- Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462:433–441. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- Ma PX. Biomimetic materials for tissue engineering. Adv Drug Deliv Rev. 2008;60:184–198. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoharan VN, Elsesser MT, Pine DJ. Dense packing and symmetry in small clusters of microspheres. Science. 2003;301:483–487. doi: 10.1126/science.1086189. [DOI] [PubMed] [Google Scholar]
- Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- McMurray RJ, Gadegaard N, Tsimbouri PM, Burgess KV, McNamara LE, Tare R, Murawski K, Kingham E, Oreffo ROC, Dalby MJ. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat Mater. 2011;10:637–644. doi: 10.1038/nmat3058. [DOI] [PubMed] [Google Scholar]
- Mei Y, Saha K, Bogatyrev SR, Yang J, Hook AL, Kalcioglu ZI, Cho S-W, Mitalipova M, Pyzocha N, Rojas F, Van Vliet KJ, Davies MC, Alexander MR, Langer R, Jaenisch R, Anderson DG. Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells. Nat Mater. 2010;9:768–778. doi: 10.1038/nmat2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov V, Kasyanov V, Markwald RR. Organ printing: From bioprinter to organ biofabrication line. Curr Opin Biotech. 2011;22:1–7. doi: 10.1016/j.copbio.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Mitragotri S. In drug delivery, shape does matter. Pharm Res. 2009;26:232–234. doi: 10.1007/s11095-008-9740-y. [DOI] [PubMed] [Google Scholar]
- Mitragotri S, Lahann J. Physical approaches to biomaterial design. Nat Mater. 2009;8:15–23. doi: 10.1038/nmat2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller HC, Mian MK, Shrivastava S, Chung BG, Khademhosseini A. A microwell array system for stem cell culture. Biomaterials. 2008;29:752–763. doi: 10.1016/j.biomaterials.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghimi SM, Hunter AC, Murray JC. Nanomedicine: Current status and future prospects. FASEB J. 2005;19:311–330. doi: 10.1096/fj.04-2747rev. [DOI] [PubMed] [Google Scholar]
- Mueller-Klieser W. Tumor biology and experimental therapeutics. Crit Rev Oncol Hematol. 2000;36:123–139. doi: 10.1016/s1040-8428(00)00082-2. [DOI] [PubMed] [Google Scholar]
- Murtuza B, Nichol JW, Khademhosseini A. Micro- and nanoscale control of the cardiac stem cell niche for tissue fabrication. Tissue Eng Part B Rev. 2009;15:443–454. doi: 10.1089/ten.teb.2009.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naderi H, Matin MM, Bahrami AR. Review paper: Critical issues in tissue engineering: Biomaterials, cell sources, angiogenesis, and drug delivery systems. J Biomater Appl. 2011;26:383–417. doi: 10.1177/0885328211408946. [DOI] [PubMed] [Google Scholar]
- Nelson CM, Chen CS. VE-cadherin simultaneously stimulates and inhibits cell proliferation by altering cytoskeletal structure and tension. J Cell Sci. 2003;116:3571–3581. doi: 10.1242/jcs.00680. [DOI] [PubMed] [Google Scholar]
- Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 2010;31:5536–5544. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z, Kumacheva E. Patterning surfaces with functional polymers. Nat Mater. 2008;7:277–290. doi: 10.1038/nmat2109. [DOI] [PubMed] [Google Scholar]
- Norman JJ, Desai TA. Methods for fabrication of nanoscale topography for tissue engineering scaffolds. Ann Biomed Eng. 2006;34:89–101. doi: 10.1007/s10439-005-9005-4. [DOI] [PubMed] [Google Scholar]
- Park J-U, Hardy M, Kang SJ, Barton K, Adair K, Mukhopadhyay Dk, Lee CY, Strano MS, Alleyne AG, Georgiadis JG, Ferreira PM, Rogers JA. High-resolution electrohydrodynamic jet printing. Nat Mater. 2007;6:782–789. doi: 10.1038/nmat1974. [DOI] [PubMed] [Google Scholar]
- Patil VRS, Campbell CJ, Yun YH, Slack SM, Goetz DJ. Particle diameter influences adhesion under flow. Biophys J. 2001;80:1733–1743. doi: 10.1016/s0006-3495(01)76144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng CY, Nam WJ, Fonash SJ, Gu B, Sen A, Strawhecker K, Natarajan S, Foley HC, Kim SH. Formation of nanostructured polymer filaments in nanochannels. J Am Chem Soc. 2003;125:9298–9299. doi: 10.1021/ja0345423. [DOI] [PubMed] [Google Scholar]
- Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv Mater. 2006;18:1345–1360. [Google Scholar]
- Perl A, Reinhoudt DN, Huskens J. Microcontact printing: Limitations and achievements. Adv Mater. 2009;21:2257–2268. [Google Scholar]
- Pesen D, Heinz WF, Werbin JL, Hoh JH, Haviland DB. Electron beam patterning of fibronectin nanodots that support focal adhesion formation. Soft Matter. 2007;3:1280–1284. doi: 10.1039/b710659a. [DOI] [PubMed] [Google Scholar]
- Petrie RJ, Doyle AD, Yamada KM. Random versus directionally persistent cell migration. Nat Rev Mol Cell Biol. 2009;10:538–549. doi: 10.1038/nrm2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater. 2009;8:457–470. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- Ranzinger J, Krippner-Heidenreich A, Haraszti T, Bock E, Tepperink J, Spatz JP, Scheurich P. Nanoscale arrangement of apoptotic ligands reveals a demand for a minimal lateral distance for efficient death receptor activation. Nano Lett. 2009;9:4240–4245. doi: 10.1021/nl902429b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem J. 2004;377:159–169. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland JP, Maynor BW, Euliss LE, Exner AE, Denison GM, DeSimone JM. Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. J Am Chem Soc. 2005;127:10096–10100. doi: 10.1021/ja051977c. [DOI] [PubMed] [Google Scholar]
- Rothschild M. Projection optical lithography. Mater Today. 2005;8:18–24. [Google Scholar]
- Sant S, Poulin S, Hildgen P. Effect of polymer architecture on surface properties, plasma protein adsorption, and cellular interactions of pegylated nanoparticles. J Biomed Mater Res A. 2008;87:885–895. doi: 10.1002/jbm.a.31800. [DOI] [PubMed] [Google Scholar]
- Schift H. Nanoimprint lithography: An old story in modern times? A review. J Vac Sci Technol B. 2008;26:458–480. [Google Scholar]
- Sele CW, von’Werne T, Friend RH, Sirringhaus H. Lithography-free, self-aligned inkjet printing with sub-hundred-nanometer resolution. Adv Mater. 2005;17:997–1001. [Google Scholar]
- Selimovic S, Piraino F, Bae H, Rasponi M, Redaelli A, Khademhosseini A. Microfabricated polyester conical microwells for cell culture applications. Lab Chip. 2011;11:2325–2332. doi: 10.1039/c1lc20213h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Votruba AR, Farokhzad OC, Langer R. Nanotechnology in drug delivery and tissue engineering: From discovery to applications. Nano Lett. 2010;10:3223–3230. doi: 10.1021/nl102184c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SR, Bae H, Cha JM, Mun JY, Chen Y-C, Tekin H, Shin H, Farshchi S, Dokmeci MR, Tang S, Khademhosseini A. Carbon nanotube reinforced hybrid microgels as scaffold materials for cell encapsulation. ACS Nano. 2011 doi: 10.1021/nn203711s. 10.1021/nn203711s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sill TJ, von Recum HA. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials. 2008;29:1989–2006. doi: 10.1016/j.biomaterials.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA. Hydrogels in regenerative medicine. Adv Mater. 2009;21:3307–3329. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Lu H, Kawazoe N, Chen G. Adipogenic differentiation of individual mesenchymal stem cell on different geometric micropatterns. Langmuir. 2011;27:6155–6162. doi: 10.1021/la200487w. [DOI] [PubMed] [Google Scholar]
- Stevens MM, George JH. Exploring and engineering the cell surface interface. Science. 2005;310:1135–1138. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- Stolnik S, Illum L, Davis SS. Long circulating microparticulate drug carriers. Adv Drug Deliv Rev. 1995;16:195–214. [Google Scholar]
- Stuart MAC, Huck WTS, Genzer J, Muller M, Ober C, Stamm M, Sukhorukov GB, Szleifer I, Tsukruk VV, Urban M, Winnik F, Zauscher S, Luzinov I, Minko S. Emerging applications of stimuli-responsive polymer materials. Nat Mater. 2010;9:101–113. doi: 10.1038/nmat2614. [DOI] [PubMed] [Google Scholar]
- Subramani C, Cengiz N, Saha K, Gevrek TN, Yu X, Jeong Y, Bajaj A, Sanyal A, Rotello VM. Direct fabrication of functional and biofunctional nanostructures through reactive imprinting. Adv Mater. 2011;23:3165–3169. doi: 10.1002/adma.201101292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita Y, Ishizaki K, Iwasa F, Ueno T, Minamikawa H, Yamada M, Suzuki T, Ogawa T. Effects of pico-to-nanometer-thin TiO2 coating on the biological properties of microroughened titanium. Biomaterials. 2011;32:8374–8384. doi: 10.1016/j.biomaterials.2011.07.077. [DOI] [PubMed] [Google Scholar]
- Takayama S, Ostuni E, LeDuc P, Naruse K, Ingber DE, Whitesides GM. Subcellular positioning of small molecules. Nature. 2001;411:1016. doi: 10.1038/35082637. [DOI] [PubMed] [Google Scholar]
- Teixeira AI, Abrams GA, Bertics PJ, Murphy CJ, Nealey PF. Epithelial contact guidance on well-defined micro- and nanostructured substrates. J Cell Sci. 2003;116:1881–1892. doi: 10.1242/jcs.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekin H, Anaya M, Brigham MD, Nauman C, Langer R, Khademhosseini A. Stimuli-responsive microwells for formation and retrieval of cell aggregates. Lab Chip. 2010;10:2411–2418. doi: 10.1039/c004732e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsen T, Maerkl SJ, Quake SR. Microfluidic large-scale integration. Science. 2002;298:580–584. doi: 10.1126/science.1076996. [DOI] [PubMed] [Google Scholar]
- Unger MA, Chou HP, Thorsen T, Scherer A, Quake SR. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science. 2000;288:113–116. doi: 10.1126/science.288.5463.113. [DOI] [PubMed] [Google Scholar]
- Vieu C, Carcenac F, Pepin A, Chen Y, Mejias M, Lebib A, Manin-Ferlazzo L, Couraud L, Launois H. Electron beam lithography: Resolution limits and applications. Appl Surf Sci. 2000;164:111–117. [Google Scholar]
- Weibel DB, DiLuzio WR, Whitesides GM. Microfabrication meets microbiology. Nat Rev Micro. 2007;5:209–218. doi: 10.1038/nrmicro1616. [DOI] [PubMed] [Google Scholar]
- Werts MHV, Lambert M, Bourgoin JP, Brust M. Nanometer scale patterning of Langmuir-Blodgett films of gold nanoparticles by electron beam lithography. Nano Lett. 2002;2:43–47. [Google Scholar]
- Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442:368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- Williams C, Tsuda Y, Isenberg BC, Yamato M, Shimizu T, Okano T, Wong JY. Aligned cell sheets grown on thermo-responsive substrates with microcontact printed protein patterns. Adv Mater. 2009;21:2161–2164. [Google Scholar]
- Williams C, Xie AW, Yamato M, Okano T, Wong JY. Stacking of aligned cell sheets for layer-by-layer control of complex tissue structure. Biomaterials. 2011;32:5625–5632. doi: 10.1016/j.biomaterials.2011.04.050. [DOI] [PubMed] [Google Scholar]
- Wright D, Rajalingam B, Karp JM, Selvarasah S, Ling Y, Yeh J, Langer R, Dokmeci MR, Khademhosseini A. Reusable, reversibly sealable parylene membranes for cell and protein patterning. J Biomed Mater Res A. 2008;85:530–538. doi: 10.1002/jbm.a.31281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright D, Rajalingam B, Selvarasah S, Dokmeci MR, Khademhosseini A. Generation of static and dynamic patterned co-cultures using microfabricated parylene-C stencils. Lab Chip. 2007;7:1272–1279. doi: 10.1039/b706081e. [DOI] [PubMed] [Google Scholar]
- Wu J, Wheeldon I, Guo Y, Lu T, Du Y, Wang B, He J, Hu Y, Khademhosseini A. Sandwiched microarray for bench-top cell-based high throughput screening. Biomaterials. 2010 doi: 10.1016/j.biomaterials.2010.09.026. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Whitesides GM. Soft lithography. Annu Rev Mater Sci. 1998;28:153–184. [Google Scholar]
- Yamazoe H, Uemura T, Tanabe T. Facile cell patterning on an albumin-coated surface. Langmuir. 2008;24:8402–8404. doi: 10.1021/la801221r. [DOI] [PubMed] [Google Scholar]
- Yang MT, Fu J, Wang Y-K, Desai RA, Chen CS. Assaying stem cell mechanobiology on microfabricated elastomeric substrates with geometrically modulated rigidity. Nat Protoc. 2011;6:187–213. doi: 10.1038/nprot.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KD. Bacterial shape: Two-dimensional questions and possibilities. Annu Rev Microbiol. 2010;64:223–240. doi: 10.1146/annurev.micro.112408.134102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, Jain RK. Vascular-permeability in a human tumor xenograft - molecular-size dependence and cutoff Size. Cancer Res. 1995;55:3752–3756. [PubMed] [Google Scholar]
- Zhuang L-F, Jiang H-H, Qiao S-C, Appert C, Si M-s, Gu Y-X, Lai H-C. The roles of extracellular signal-regulated kinase 1/2 pathway in regulating osteogenic differentiation of murine preosteoblasts MC3T3-E1 cells on roughened titanium surfaces. Journal of Biomedical Materials Research Part A. 2012;100A:125–133. doi: 10.1002/jbm.a.33247. [DOI] [PubMed] [Google Scholar]
- Zorlutuna P, Jeong JH, Kong H, Bashir R. Stereolithography-based hydrogel microenvironments to examine cellular interactions. Adv Funct Mater. 2011;21:3642–3651. [Google Scholar]