Abstract
Burkholderia pseudomallei is the etiologic agent of the disease melioidosis and is a category B biological threat agent. The genomic sequence of B. pseudomallei K96243 was recently determined, but little is known about the overall genetic diversity of this species. Suppression subtractive hybridization was employed to assess the genetic variability between two distinct clinical isolates of B. pseudomallei, 1026b and K96243. Numerous mobile genetic elements, including a temperate bacteriophage designated φ1026b, were identified among the 1026b-specific suppression subtractive hybridization products. Bacteriophage φ1026b was spontaneously produced by 1026b, and it had a restricted host range, infecting only Burkholderia mallei. It possessed a noncontractile tail, an isometric head, and a linear 54,865-bp genome. The mosaic nature of the φ1026b genome was revealed by comparison with bacteriophage φE125, a B. mallei-specific bacteriophage produced by Burkholderia thailandensis. The φ1026b genes for DNA packaging, tail morphogenesis, host lysis, integration, and DNA replication were nearly identical to the corresponding genes in φE125. On the other hand, φ1026b genes involved in head morphogenesis were similar to head morphogenesis genes encoded by Pseudomonas putida and Pseudomonas aeruginosa bacteriophages. Consistent with this observation, immunogold electron microscopy demonstrated that polyclonal antiserum against φE125 reacted with the tail of φ1026b but not with the head. The results presented here suggest that B. pseudomallei strains are genetically heterogeneous and that bacteriophages are major contributors to the genomic diversity of this species. The bacteriophage characterized in this study may be a useful diagnostic tool for differentiating B. pseudomallei and B. mallei, two closely related biological threat agents.
Burkholderia pseudomallei is the causative agent of the glanders-like disease melioidosis (21, 22, 67). This organism is endemic in Southeast Asia and northern Australia, where it can be isolated from moist soil and surface water. Humans and animals can be infected by B. pseudomallei by direct inoculation of soil or water into skin abrasions or by inhalation of contaminated material. Underlying diseases such as diabetes mellitus and chronic renal failure are risk factors for melioidosis, but apparently healthy individuals can also develop clinical melioidosis (18). The clinical manifestations of melioidosis are protean and often include fever and abscess formation. The clinical spectra of melioidosis in endemic regions are similar, but brainstem encephalitis and genitourinary infections are more common in northern Australia while suppurative parotitis is more common in Southeast Asia (21, 22, 67). The basis for geographic differences in disease presentation is currently unknown, but the differences may be due to genetic differences in patients and/or in the B. pseudomallei strains present in the different regions.
Capsular polysaccharide and lipopolysaccharide (LPS) O antigen are important for B. pseudomallei virulence in animal models of melioidosis (4, 24, 55). The recently completed genome sequence of B. pseudomallei K96243 (http://www.sanger.ac.uk/) has facilitated identification of several new virulence gene candidates. In particular, K96243 harbors multiple genomic islands with relatively low G+C contents, suggesting that there was recent acquisition by lateral gene transfer (34, 35, 49, 50). Lateral gene transfer is a process in which genetic material is transferred from a donor to a recipient via mobile genetic elements, such as plasmids, transposons, integrons, or bacteriophages. The laterally acquired genetic material can alter the phenotype of the recipient and promote adaptation to its environment. Further studies are required to elucidate the biology of B. pseudomallei mobile genetic elements and to examine their contribution to genomic diversity, niche adaptation, and virulence.
The goal of this study was to examine the genomic diversity of B. pseudomallei clinical isolates by performing subtractive hybridization between B. pseudomallei 1026b (tester) and K96243 (driver). B. pseudomallei 1026b was isolated in Thailand from a human case of septicemic melioidosis with skin, soft tissue, and spleen involvement and has been studied extensively in the laboratory (26). In this study, numerous mobile genetic elements in 1026b that were not present in K96243 were identified. One of the 1026b-specific mobile genetic elements was a temperate bacteriophage (φ1026b) that was spontaneously produced during growth in liquid broth. The morphology, host range, genomic sequence, and immunological reactivity of bacteriophage φ1026b are reported here.
MATERIALS AND METHODS
Bacterial plasmids, strains, and growth conditions.
The plasmids used in this study are described in Tables 1 and 2. The Burkholderia mallei strains used in this study are listed in Table 3. The following B. pseudomallei strains were used in this study: 316c, NCTC 4845, 1026b, WRAIR 1188, USAMRU Malaysia 32, Pasteur 52237, STW 199-2, STW 176, STW 115-2, STW 152, STW 102-3, STW 35-1, K96243, 576a, 295, 296, 503, 506, 112c, 238, 423, 465a, 776, 439a, 487, 644, 713, 730, E8, E12, E13, E24, E25, E40, E203, E210, E214, E215, E250, E272, E277, E279, E280, E283, E284, E300, E301, E302, and E304 (3, 21, 23, 29, 31, 61, 70). Burkholderia thailandensis strains E27, E30, E32, E96, E100, E105, E111, E120, E125, E132, E135, E202, E251, E253, E254, E255, E256, E257, E258, E260, E261, E263, E264, E266, E267, E275, E285, E286, E290, E293, E295, and E299 (7, 61, 71) were also utilized in this study. Other Burkholderia strains used in this study included Burkholderia cepacia LMG 1222 (44), Burkholderia multivorans C5568, B. multivorans LMG 18823 (44), Burkholderia cenocepacia LMG 18863 (44), B. cenocepacia 715j (46), Burkholderia stabilis LMG 07000, Burkholderia vietnamiensis LMG 16232 (44), B. vietnamiensis LMG 10929 (44), Burkholderia gladioli 2-72 (58), B. gladioli 2-75 (58), B. gladioli 4-54 (58), B. gladioli 5-62 (58), Burkholderia uboniae EY 3383 (73), Burkholderia cocovenans ATCC 33664, Burkholderia pyrrocinia ATCC 15958, Burkholderia glathei ATCC 29195, Burkholderia caryophylli Pc 102, Burkholderia andropogonis PA-133, Burkholderia kururiensis KP23 (74), Burkholderia sacchari IPT101 (6), Burkholderia sp. strain 2.2N (13), and Burkholderia sp. strain T-22-8A. Ralstonia solanacearum FC228, FC229, and FC230, Pandoraea apista LMG 16407 (19), Pandoraea norimbergensis LMG 18379 (19), Pandoraea pnomenusa LMG 18087 (19), Pandoraea pulmonicola LMG 18106 (19), Stenotrophomonas maltophilia XM16 (43), S. maltophilia XM47 (43), Pseudomonas aeruginosa PAO (38), P. aeruginosa PA14 (54), Pseudomonas syringae DC3000 (66), Salmonella enterica serovar Typhimurium SL1344 (37), Serratia marcescens H11, Escherichia coli TOP10 (Invitrogen), and E. coli S17-1λpir (59) were also used in this study. E. coli was grown at 37°C on Luria-Bertani (LB) agar (Lennox) or in LB broth (Lennox). P. syringae, B. andropogonis, Burkholderia sp. strain 2.2N, Burkholderia sp. strain T-22-8A, B. glathei, and B. caryophylli were grown at 25°C on LB agar or in LB broth containing 4% glycerol. All other bacterial strains were grown at 37°C on LB agar or in LB broth containing 4% glycerol. When appropriate, antibiotics were added at the following concentrations: 100 μg of ampicillin per ml, 25 μg of kanamycin per ml, and 15 μg of tetracycline per ml for E. coli; and 100 μg of streptomycin per ml and 50 μg of tetracycline per ml for B. pseudomallei DD5025. In addition, B. mallei DD3008 was grown in the presence of 5 μg of gentamicin per ml, and B. mallei NCTC 120(pBHR1-wbiE) was grown in the presence of 15 μg of polymyxin B per ml and 5 μg of kanamycin per ml.
TABLE 1.
Plasmids used in this study
| Plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| pCR2.1-TOPO | 3.9-kb TA cloning vector; pMB1 ori; Kmr Apr | Invitrogen |
| pSKM11 | Positive selection cloning and suicide vector; IncP oriT; ColE1 ori; Apr Tcs | 47 |
| pGEM-7zf(+) | Standard cloning vector; pMB1 ori; Apr | Promega |
| pMOLUC | Vector for cloning large DNA fragments; pBR322 ori; Apr | 28 |
| pDD80 | pGEM-7zf(+) containing 3,625-bp HindIII fragment from φ1026b | This study |
| pDD81 | pGEM-7zf(+) containing 3,105-bp HindIII fragment from φ1026b | This study |
| pDD82 | pGEM-7zf(+) containing 1,068-bp HindIII fragment from φ1026b | This study |
| pDD83 | pGEM-7zf(+) containing 602-bp HindIII fragment from φ1026b | This study |
| pDD84 | pGEM-7zf(+) containing 3,791-bp HindIII fragment from φ1026b | This study |
| pDD85 | pGEM-7zf(+) containing 2,515-bp HindIII fragment from φ1026b | This study |
| pDD86 | pGEM-7zf(+) containing 8,411-bp HindIII fragment from φ1026b | This study |
| pDD87 | pGEM-7zf(+) containing 9,355-bp HindIII fragment from φ1026b | This study |
| pDD88 | pMOLUC containing 12,775-bp HindIII fragment from φ1026b | This study |
| pDD94 | pSKM11 containing 930-bp internal fragment of φ1026b gene 59 generated by PCR with primers MFS-2 and MFS-3; Apr Tcr | This study |
| pDD101 | pCR2.1-TOPO containing 126-bp HindIII fragment from φ1026b | This study |
| pDD5025B | pSKM11 containing 9,492-bp HindIII fragment from φ1026b; Apr Tcr | This study |
Kmr, kanamycin resistant; Apr, ampicillin resistant; Tcs, tetracycline sensitive; Tcr, tetracycline resistant.
TABLE 2.
Subtractive hybridization products present in B. pseudomallei 1026b but not in B. pseudomallei K96243
| Plasmid | Insert size (bp) | G+C content (%) | Protein function | Best BLASTP hit | E value | Accession no. |
|---|---|---|---|---|---|---|
| pSH3 | 756 | 53.6 | Hypothetical protein | Helicobacter pylori J99 | 1e-04 | NP_224110 |
| Helicobacter pylori 26695 | 3e-04 | NP_208290 | ||||
| pSH4 | 235 | 55.7 | Phage-related protein | Phage φE125 | 7e-05 | NP_536410 |
| pSH5 | 1,113 | 57.8 | Transposase B of insertion sequence ISBp1 | Burkholderia pseudomallei | 4e-77 | AAG39072 |
| pSH6 | 326 | 50.6 | Putative Rossmann fold nucleotide-binding protein involved in DNA uptake | Novosphingobium aromaticivorans Thermoanaerobacter tengcongensis | 2e-35 2e-14 | ZP_00093056 NP_623068 |
| pSH7 | 350 | 50.3 | Putative ATP binding protein | Ralstonia solanacearum | 2e-26 | NP_521299 |
| pSH8 | 321 | 52.0 | ||||
| pSH10 | 1,217 | 47.6 | ||||
| pSH11 | 517 | 52.2 | ||||
| pSH13 | 432 | 44.2 | Hypothetical protein | Ralstonia metallidurans | 9e-64 | ZP_00022203 |
| Nostoc sp. strain PCC 7120 | 3e-34 | NP_487474 | ||||
| pSH18 | 1,440 | 49.2 | Phage-related and hypothetical proteins | Magnetococcus sp. strain MC-1 | 3e-13 | ZP_00042491 |
| Nostoc punctiforme | 1e-42 | ZP_00112321 | ||||
| Phage φE125 | NP_536385 | |||||
| pSH19 | 478 | 48.9 | ||||
| pSH21 | 379 | 43.5 | ||||
| pSH23 | 270 | 53.7 | Putative ATP binding protein | Ralstonia solanacearum | 2e-13 | NP_521299 |
| pSH25 | 434 | 49.5 | Phage-related integrase | Xanthomonas axonopodis | 5e-05 | NP_642500 |
| Ralstonia metallidurans | 8e-05 | ZP_00023697 | ||||
| pSH26 | 419 | 42.7 | ||||
| pSH29 | 167 | 50.3 | ||||
| pSH31 | 490 | 53.8 | Putative acetyltransferase | Methanosarcina mazei Goe1 | 0.008 | NP_632505 |
| Pseudomonas aeruginosa PAO1 | 0.019 | NP_253367 | ||||
| pSH35 | 268 | 54.5 | ||||
| pSH36 | 321 | 52.0 | Putative site-specific DNA methyltransferase | Cenarchaeum symbiosum Pseudomonas syringae | 5e-04 6e-04 | T31327 ZP_00127033 |
| pSH37 | 888 | 59.7 | Phage-related and hypothetical proteins | Phage φE125 | 3e-52 | NP_536387 |
| Bordetella parapertussis | 0.25 | NP_884214 | ||||
| pSH38 | 741 | 46.8 | ||||
| pSH40 | 441 | 50.3 | Putative AraC family transcriptional regulator | Vibrio vulnificus Bradyrhizobium japonicum | 2e-06 4e-06 | NP_935090 NP_773722 |
| pSH42 | 389 | 49.9 | Putative ATP binding protein | Ralstonia solanacearum | 1e-05 | NP_521299 |
| pSH47 | 790 | 45.1 | Phage-related integrase | Xanthomonas axonopodis | 7e-15 | NP_643606 |
| Vibrio parahaemolyticus | 3e-14 | NP_797022 | ||||
| pSH49 | 235 | 55.7 | ||||
| pSH51 | 531 | 46.1 | Putative plasmid mobilization protein | Zymomonas mobilis plasmid ZM2 | 1.0 | P15255 |
| Treponema denticola plasmid pTS1 | 2.9 | NP_073756 | ||||
| Bartonella grahamii plasmid pBGR1 | 3.4 | NP_696963 | ||||
| pSH53 | 335 | 61.5 |
TABLE 3.
Bacteriophage φ1026b plaque formation on B. mallei strains
| Strain | Relevant characteristics | Plaque formation | Reference or source |
|---|---|---|---|
| NCTC 120 | LPS O-antigen mutant; wbiE::IS407A | − | 70 |
| NCTC 120 (pBHR1-wbiE) | LPS O antigen positive; wbiE provided in trans on pBHR1 | + | 70 |
| NCTC 10248 | + | 70 | |
| NCTC 10229 | + | 70 | |
| NCTC 10260 | + | 70 | |
| NCTC 10247 | + | 70 | |
| NCTC 3708 | + | 70 | |
| NCTC 3709 | + | 70 | |
| ATCC 23344 | Type strain; genomic sequence completeda | + | 70 |
| ATCC 10399 | + | 70 | |
| ATCC 15310 | Produces LPS O antigen | + | 70 |
| DB110795 | Laboratory-passaged ATCC 15310; LPS O-antigen mutant; wbiG::IS407A | − | 70 |
| BML1 | Lysogen; ATCC 23344 (φ1026b) | − | This study |
| BML10 | Lysogen; ATCC 23344 (φE125) | − | 70 |
| DD3008 | ATCC 23344 derivative; capsule mutant | + | 27 |
| 2002721273 | + | 30 | |
| 2002721274 | + | 30 | |
| 2002721276 | + | 30 | |
| 2002721277 | + | 30 | |
| 2002721278 | + | 30 | |
| 2002721279 | + | 30 | |
| 2002721280 | + | 30 | |
| 2000031064 | + | 30 | |
| 2000031065 | + | 30 | |
| 2000031066 | + | 30 | |
| Turkey 1 | + | USDAb | |
| Turkey 2 | + | USDA | |
| Turkey 3 | + | USDA | |
| Turkey 4 | Produces LPS O antigen | − | USDA |
| Turkey 5 | Produces LPS O antigen | − | USDA |
| Turkey 6 | + | USDA | |
| Turkey 7 | + | USDA | |
| Turkey 8 | + | USDA | |
| Turkey 9 | + | USDA | |
| Turkey 10 | + | USDA | |
| ISU | LPS O-antigen mutant; genetic mutation unknown | − | USDA |
http://www.tigr.org/.
USDA, United States Department of Agriculture.
MLST of B. pseudomallei 1026b.
The pairs of primers used for PCR amplification and sequencing of the seven housekeeping gene fragments have been described previously (32). The multilocus sequence typing (MLST) database (www.mlst.net) identification number for B. pseudomallei 1026b is 208.
Subtractive hybridization.
Subtractive hybridization was performed by using B. pseudomallei 1026b genomic DNA as the tester and B. pseudomallei K96243 genomic DNA as the driver. The protocol described in the CLONTECH PCR-Select bacterial genome subtraction kit user manual was followed, except that the hybridization temperature was 73°C instead of 63°C. The subtractive hybridization products were cloned into pCR2.1-TOPO and transformed into chemically competent E. coli TOP10 cells.
Bacteriophage production, propagation, and DNA purification.
The procedures used for bacteriophage production, propagation, and DNA purification have been described previously (70).
Enzyme-linked immunosorbent assay.
The wells of a round-bottom microtiter plate were coated with approximately 5 × 106 bacteria in 100 μl of 0.05 M carbonate buffer (pH 9.6), and the plate was incubated for 1 h at 37°C. The wells were washed with phosphate-buffered saline containing 0.05% Tween 20 and blocked with a 3% solution of skim milk in phosphate-buffered saline-Tween 20 for 1 h at 37°C. The wells were washed, a 1:1,000 dilution of monoclonal antibody 3D11 (Research Diagnostics, Inc.) was added, and the plate was incubated at 37°C for 1 h. Monoclonal antibody 3D11 is specific for the LPS O antigen of B. mallei. The wells were washed, and a 1:1,000 dilution of a peroxidase-labeled goat anti-mouse immunoglobulin G(H+L) [IgG(H+ L)] antibody (KPL) was added to each well. The plate was incubated for 1 h at 37°C, washed, and developed with the 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS) peroxidase substrate system (KPL) for 10 min. The optical density at 410 nm was determined.
φ1026b sensitivity testing.
Approximately 102 PFU of φ1026b was added to a saturated bacterial culture and incubated at 25°C for 20 min, and 4.8 ml of molten LB top agar (0.7%) containing 4% glycerol was added. The mixture was immediately poured onto an LB agar plate containing 4% glycerol and incubated overnight at 25 or 37°C, depending on the bacterial species being tested. Bacteria were considered to be sensitive to φ1026b if they formed plaques under these conditions and resistant if they did not. The positive control, B. mallei ATCC 23344, formed plaques in the presence of φ1026b after incubation at 25 and 37°C. No bacterial species tested formed plaques in the absence of φ1026b.
Negative staining of φ1026b.
The procedure used for negatively staining bacteriophage φ1026b with 1% phosphotungstic acid (PTA) (pH 6.6) has been described previously (70).
DNA manipulation and plasmid conjugation.
Restriction enzymes and T4 DNA ligase were purchased from Roche Molecular Biochemicals and were used according to the manufacturer's instructions. DNA fragments used in cloning procedures were excised from agarose gels and purified with a GeneClean III kit (Q · BIOgene). Bacterial genomic DNA was prepared by a previously described protocol (68). Plasmids were purified from overnight cultures by using Wizard Plus SV Minipreps (Promega). The suicide vector pDD94 was electroporated into E. coli S17-1λpir (12.25 kV/cm) and conjugated with B. pseudomallei 1026b for 8 h, as described elsewhere (23). The resulting strain, B. pseudomallei DD5025, contained pDD94 integrated into gene 59 of the φ1026b prophage. Chromosomal DNA was isolated from DD5025 and digested with restriction endonuclease BamHI, and the bacteriophage attachment site and flanking bacterial DNA were obtained by self-cloning (23).
DNA sequencing and analysis.
DNA sequencing was performed at ACGT, Inc. (Wheeling, Ill.) and at the LMT Sequencing Lab (Frederick, Md.). Most φ1026b genes were identified by using GeneMark.hmm (http://opal.biology.gatech.edu/GeneMark/gmhmm2_prok.cgi); other genes were identified by visual inspection, guided by BLAST (2) results. DNA and protein sequences were analyzed with GeneJockeyII and MacVector 7.2 software for the Macintosh computer. The gapped BLASTX and BLASTP programs were used to search the nonredundant sequence database for homologous proteins (2). The φ1026b and φE125 genomes were aligned by using BLAST 2 SEQUENCES (http://www.ncbi.nlm.nih.gov/BLAST/bl2seq/bl2.html) with the Mega BLAST option selected.
Animal studies.
Syrian hamsters (five animals per group) were infected intraperitoneally with 102, 103, and 104 1026b cells and 102, 103, and 104 DD5025 cells. The deaths in each group were monitored for 2 days, and the 50% lethal doses (LD50) were determined. All of the animals died within 48 h of infection. This research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adhered to principles stated in the Guide for the Care and Use of Laboratory Animals (http://oacu.od.nih.gov/regs/guide/guidex.htm). The facility where this research was conducted is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Phenotype microarray studies.
PM1 and PM2 MicroPlates were purchased from BIOLOG (www.biolog.com) and were used according to the instructions supplied by the manufacturer.
Production of polyclonal antiserum against φE125.
One New Zealand rabbit was immunized with 1 ml of a 1:1 mixture of bacteriophage φE125 (∼105 PFU) and the RIBI R-700 adjuvant system (Corixa). Five hundred microliters of the antigen-adjuvant mixture was injected intramuscularly into each hind leg on days 1 and 28. Antiserum was obtained by cardiac puncture on day 39 and was stored at −20°C until it was used.
Immunogold electron microscopy.
The methods used for immunogold electron microscopy have been described previously (24). Briefly, φE125 and φ1026b were reacted with polyclonal rabbit antiserum directed against φE125, washed, and reacted with goat anti-rabbit IgG gold conjugate (Sigma).
PCR amplifications.
The sizes of PCR products were determined by agarose electrophoresis, and the products were cloned by using a pCR2.1 TOPO TA cloning kit (Invitrogen) and chemically competent E. coli TOP10 (Invitrogen). PCR amplifications were performed in 100-μl (final volume) mixtures containing 1× Taq PCR master mix (QIAGEN), each oligodeoxyribonucleotide primer at a concentration of 1 μM, and approximately 200 ng of genomic DNA. PCR mixtures were transferred to a PTC-150 MiniCycler with a Hot Bonnet accessory (MJ Research) and heated to 97°C for 5 min. This was followed by 30 cycles of a three-temperature cycling protocol (97°C for 30 s, 55°C for 30 s, and 72°C for 2 min) and one cycle at 72°C for 10 min. Genomic DNA from φ1026b was used to PCR amplify an internal fragment of gene 59 with the following oligodeoxyribonucleotide primer pair: MFS-2 (5′-ACAACCTGTCTCTGTTGCTG-3′) and MFS-3 (5′-CTGGAAACATGTCGCTAAGC-3′).
In order to determine the order and orientation of the HindIII fragments in the intact φ1026b genome, outward-oriented primers specific for the ends of each HindIII fragment (except the 126-, 602-, and 1,068-bp fragments) were synthesized, and PCRs were performed with φ1026b genomic DNA and all possible primer combinations. It was hypothesized that two HindIII fragments were adjacent if a PCR product was obtained with primer pairs specific for the corresponding ends of those fragments. All PCR products were cloned and sequenced to confirm the PCR results. The sequences of the 16 oligodeoxyribonucleotide primers used in this analysis were as follows: 8.4R, 5′-GTGCTGTCGCACTAATCATG-3′; 3.6L, 5′-CAACGGAAGAGTCGCGATTG-3′; 3.6R, 5′-CCGACGATCTGATCAAGATC-3′; 3.1L, 5′-TGCTGCTGAAACGATATTGC-3′; 3.1R, 5′-ATCGTGAAACTCGGCGTGTC-3′; 12.8L, 5′-AACGCGCTTTGTCGATCGTG-3′; 12.8R, 5′-ACCATCTCGAAGAGTTCGTG-3′; 9.3L, 5′-TCAAGGTAGAACAGCGTGTG-3′; 9.3R, 5′-CAGCGCTCACGTAGTTCAAG-3′; 98A, 5′-TCTGACAATTCGATACGCGTG-3′; 96B, 5′-AAGCTCGAGACGTTTCTTGG-3′; 2.5L-2, 5′-TAGCCACTCGCGAAACATCG-3′; 2.5R, 5′-TGGTTTATCGTTCGCGCATG-3′; 3.8L, 5′-GCCCCTTACTTCATTGAACC-3′; 3.8R, 5′-AAGAGGACTCGCCGATCAAC-3′; and 8.4L, 5′-ATCGCAGTTCGCCATGCAAC-3′.
PCRs were performed with genomic DNAs from B. pseudomallei K96243 and 1026b, B. mallei ATCC 23344 and BML1, and φ1026b and with primers Int2 (5′-CACCGACGAGAAGATGACTG-3′) and Int5 (5′-TTGAATCGCACCGTTTGGTG-3′) to determine if φ1026b integrated into the tRNAPro-3 gene. A single PCR product of the expected size (447 bp) was obtained with B. mallei BML1 DNA and B. pseudomallei 1026b DNA. This product was cloned, and its nucleotide sequence was determined. As expected, no PCR products were obtained when genomic DNAs from B. pseudomallei K96243, B. mallei ATCC 23344, and φ1026b were used in the PCR.
Nucleotide sequence accession numbers.
The nucleotide sequences reported in this paper have been deposited in the GenBank database under accession numbers AY471580 to AY471606 (1026b-K96243 subtractive hybridization products) and AY453853 (bacteriophage φ1026b).
RESULTS
MLST of B. pseudomallei 1026b.
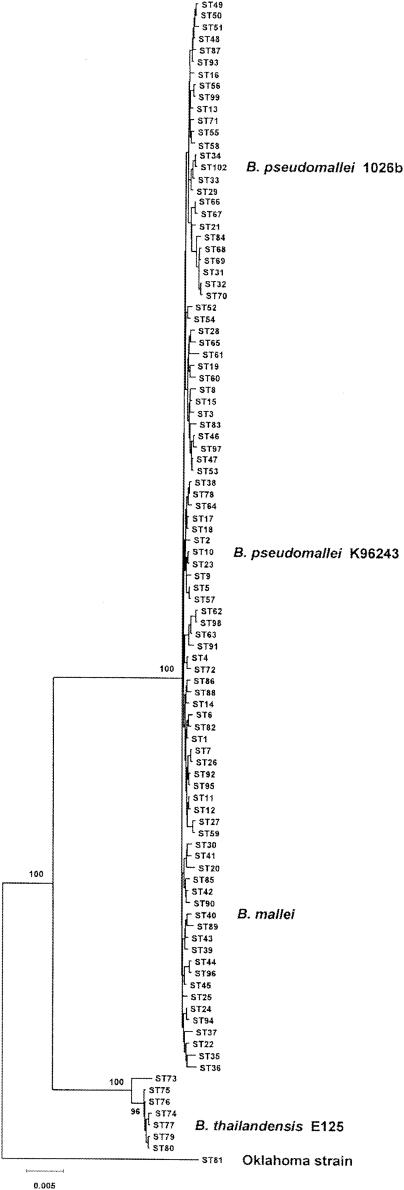
Godoy et al. developed an MLST scheme for B. pseudomallei, B. mallei, and B. thailandensis based on sequence variations in seven housekeeping genes, but B. pseudomallei 1026b was not one of the isolates examined (32). In this study, the allelic profile of 1026b was determined to be 3-4-12-1-1-4-1, which corresponds to a new sequence type (ST102). Figure 1 shows a minimum-evolution tree based on the concatenated sequences of the seven MLST loci for 92 sequence types of B. pseudomallei. Note that B. mallei isolates (ST40) cluster with B. pseudomallei isolates on the minimum-evolution tree and are considered to be a distinct clone of B. pseudomallei (32). Clinical isolates 1026b and K96243 were resolved into two genetically distinct clones, ST102 and ST10, based on the MLST analysis (Fig. 1). The MLST results suggest that 1026b and K96243 are excellent candidates for examining the genomic diversity of B. pseudomallei.
FIG.1.
Minimum-evolution tree constructed from the concatenated sequences of seven MLST loci. The seven housekeeping genes used for the MLST scheme are ace, gltB, gmhD, lepA, lipA, narK, and ndh (32). The concatenated sequences from 100 sequence types, representing isolates of B. pseudomallei, B. mallei, and B. thailandensis and the Oklahoma strain (72), were used to construct the minimum-evolution tree. The positions of the B. mallei clone (ST40), B. pseudomallei 1026b (ST102), B. pseudomallei K96243 (ST10), B. thailandensis E125 (ST77), and the Oklahoma strain (ST81) are indicated. The levels of recovery of the major nodes in 1,000 bootstrap replicates (expressed as percentages) are also indicated. Bar = differences at 0.5% of the nucleotide sites.
B. pseudomallei 1026b-specific subtractive hybridization library contains multiple mobile genetic elements.
The goal of this study was to identify genetic determinants present in B. pseudomallei 1026b but not in B. pseudomallei K96243 by subtractive hybridization. Forty plasmid inserts from a 1026b-K96243 subtractive hybridization library were identified and used to perform BLASTN searches with the completed K96243 genome (http://www.sanger.ac.uk/). Twenty-seven of the subtractive hybridization products were not present in K96243 (Table 2). The sizes of the 1026b-specific subtractive hybridization products ranged from 167 to 1,440 bp, and these products had relatively low G+C contents compared to the G+C content of the K96243 genome (68.1%). The putative functions of proteins encoded by genes in the subtractive hybridization library included several mobile genetic elements (51), including phage-related proteins, an insertion sequence element, and a plasmid-like mobilization protein (Table 2). Plasmids pSH4, pSH18, pSH25, pSH37, and pSH47 encode phage-related proteins. These proteins include phage-related integrases (pSH25 and pSH47) and several proteins that are similar to bacteriophage φE125 proteins (pSH4, pSH18, and pSH37). The IS3 family insertion sequence ISBp1 (69) was present in plasmid pSH5 (Table 2). Woo et al. previously demonstrated that ISBp1 was present in ∼65% of B. pseudomallei strains but was absent from K96243 (69). The nucleotide sequence of the pSH5 DNA insert suggests that ISBp1 is also present in the genome of B. pseudomallei 1026b. The 531-bp subtractive hybridization insert of plasmid pSH51 encodes a putative plasmid mobilization protein that was also isolated from a B. pseudomallei 1026b-B. thailandensis E264 subtractive library (55). No plasmids have been described in B. pseudomallei 1026b, and the pSH51 insert may represent an integrated plasmid that is not present in B. pseudomallei K96243 or B. thailandensis E264. Three plasmid inserts, pSH7, pSH23, and pSH42, encoded distinct regions of a putative ATP binding protein (Table 2). Finally, 13 subtractive hybridization products encoded hypothetical proteins (pSH3 and pSH13) or novel proteins (pSH8, pSH10, pSH11, pSH19, pSH21, pSH26, pSH29, pSH35, pSH38, pSH49, and pSH53) (Table 2). Taken together, the results demonstrate the genomic diversity of these two clinical isolates of B. pseudomallei, especially with respect to mobile genetic elements and novel gene sequences.
B. pseudomallei 1026b spontaneously produces a bacteriophage that is specific for B. mallei.
The subtractive hybridization product library contained multiple 1026b-specific bacteriophage sequences, and it was of interest to see if this strain actually produced a bacteriophage. B. mallei was chosen as a host because previous studies demonstrated that it is susceptible to infection with B. pseudomallei and B. thailandensis bacteriophages (45, 62, 70). 1026b spontaneously produced a bacteriophage, designated φ1026b, that formed turbid plaques with a diameter of 1.5 to 2.0 mm on B. mallei ATCC 23344. No other plaque types were identified, which suggests that 1026b produces only one bacteriophage under the growth conditions used. However, it is possible that 1026b produces additional bacteriophages that cannot use B. mallei as a host. Bacteriophage production was only slightly increased by brief exposure to UV light (470 versus 540 PFU/ml). After infection, the φ1026b genome integrated into the B. mallei chromosome at a specific site and became a prophage (see below). B. mallei ATCC 23344 was infected with φ1026b, and a lysogenic derivative was isolated and designated BML1 (Table 3).
Bacteriophage φ1026b formed plaques on 29 of 36 B. mallei strains used in this study (Table 3). Bacteriophages initiate infection by specifically binding to a surface receptor on the bacterial host, such as LPS O antigen and capsular polysaccharide. LPS O-antigen production by B. mallei strains was examined by an enzyme-linked immunosorbent assay by using monoclonal antibody 3D11 (Table 3). Three of the φ1026b-resistant B. mallei strains, NCTC 120, DB110795, and ISU, did not produce LPS O antigen. When LPS O-antigen production in B. mallei NCTC 120 was complemented by providing pBHR1-wbiE in trans, the resulting strain was susceptible to infection with bacteriophage φ1026b (Table 3). Surprisingly, B. mallei strains Turkey 4 and Turkey 5 were resistant to infection with φ1026b even though they produced LPS O antigen (Table 3). The B. mallei lysogens BML1 and BML10 produced LPS O antigen and were resistant to infection with φ1026b, presumably due to immunity or superinfection exclusion proteins encoded by the prophages that they harbor. Capsular polysaccharide was not required for plaque formation as φ1026b formed plaques on DD3008, a capsule-deficient mutant derived from ATCC 23344 (Table 3). The host range of φ1026b was further examined by using B. pseudomallei, B. thailandensis, B. cepacia, B. multivorans, B. cenocepacia, B. stabilis, B. vietnamiensis, B. gladioli, B. uboniae, B. cocovenans, B. pyrrocinia, B. glathei, B. caryophylli, B. andropogonis, B. kururiensis, Burkholderia sp. strain 2.2N, Burkholderia sp. strain T-22-8A, P. apista, P. norimbergensis, P. pnomenusa, P. pulmonicola, P. aeruginosa, P. syringae, R. solanacearum, S. maltophilia, S. enterica serovar Typhimurium, S. marcescens, and E. coli. Bacteriophage φ1026b formed plaques with none of these bacteria. These results demonstrate that bacteriophage φ1026b forms plaques only on B. mallei strains and that LPS O antigen is required but is not sufficient for plaque formation by φ1026b. Note that the host range of φ1026b is identical to the host range of bacteriophage φE125 (70). These results suggest that bacteriophage φ1026b may be a useful diagnostic tool for differentiating B. pseudomallei and B. mallei, two closely related biological threat agents (56). However, there is no advantage to using φ1026b rather than φE125 for this application (70).
Bacteriophage φ1026b has an isometric head and a long, noncontractile tail.
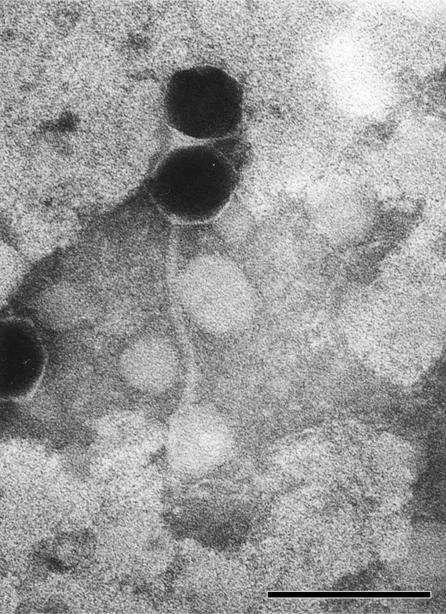
Bacteriophages may be tailed, polyhedral, filamentous, or pleomorphic and can be classified by morphotype and by the nature of the nucleic acid (1). Numerous negatively stained bacteriophages were examined, and a representative image of φ1026b is shown in Fig. 2. φ1026b possessed an isometric head that was 56 nm in diameter and a long, noncontractile tail that was approximately 200 nm long and 8 nm in diameter. Based on its morphotype, φ1026b can be classified as a member of the order Caudovirales and the family Siphoviridae (1).
FIG. 2.
Transmission electron micrograph of bacteriophage φ1026b negatively stained with 1% PTA. One intact bacteriophage (head and tail) and one bacteriophage head without an attached tail are shown. Scale bar = 100 nm.
Molecular characterization of the bacteriophage φ1026b genome.
The φ1026b genome was digested with HindIII, and 11 fragments were generated; these fragments were 0.1, 0.6, 1.1, 2.5, 3.1, 3.6, 3.8, 8.4, 9.4, 9.5, and 12.7 kb long. The fragments were heated to 80°C, and the 8.4-kb fragment dissociated into two fragments (2.3 and 6.1 kb), suggesting that a cohesive (cos) site was present (data not shown). The 11 HindIII fragments were cloned, and their nucleotide sequences were determined. The nucleotide sequencing results are shown schematically in Fig. 3. The subtractive hybridization DNA insert of pSH4 was identical to bacteriophage φ1026b from position 44417 to position 44651, which included the 3′ ends of gene 63 and gene 64 (Table 2 and Fig. 3).
FIG. 3.
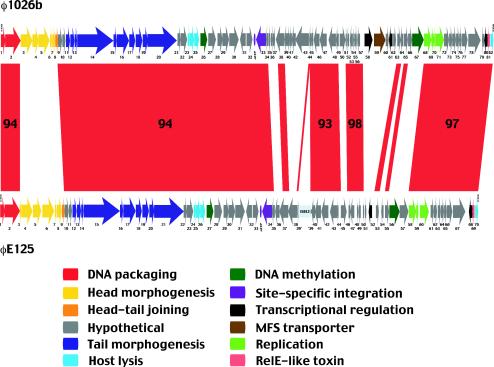
Comparative analysis of the genomes of Burkholderia bacteriophages φ1026b and φE125. The genomes of temperate bacteriophages φ1026b and φE125 are depicted schematically at the top and bottom, respectively. Red indicates DNA sequences that are present in both bacteriophages, and the numbers in the red areas indicate the percentages of nucleotide identity in conserved regions that are 1 kb long or longer. The putative functions of proteins encoded by φ1026b genes are color coded, and insertion sequence ISBt3 in gene 39 of φE125 is indicated by pale blue. Gene 25a (φ1026b) and gene 26a (φE125) are not shown for clarity.
The φ1026b genome is a linear molecule that is 54,865 bp long, and it contains 10-base 3′ single-stranded extensions on the left (3′-GCGGGCGAAG-5′) and right (5′-CGCCCGCTTC-3′), as shown in Fig. 3. The cos site of φ1026b is identical to the cos site of bacteriophage φE125 (70). The G+C content of the φ1026b genome is 60.7%, which is lower than the G+C content of the B. pseudomallei K96243 genome (68.1%) (http://www.sanger.ac.uk/). The φ1026b genome encodes 83 proteins, and 58 of these proteins generated best hits to bacteriophage φE125 proteins when the BLASTP search algorithm was used.
Bacteriophage genomes are composed of a mosaic of multigene modules, each of which encodes a group of proteins involved in a common function, such as DNA packaging, head biosynthesis, tail biosynthesis, host lysis, lysogeny, or replication (10, 36, 40). The φ1026b genome contains multigene modules involved in DNA packaging, head morphogenesis, tail morphogenesis, host lysis, and DNA replication (Fig. 3). The relative order of these modules in the φ1026b genome is similar to the order in other Siphoviridae genomes (10, 40, 70). φ1026b also encodes a LysR family transcriptional regulator (57) and a major facilitator superfamily (MFS) transporter (52), encoded by gene 58 and gene 59 (Fig. 3). It is interesting that these genes have been found in tandem in several recently completed bacterial genomes, including those of R. solanacearum, B. fungorum, and P. syringae. gp59 is a member of the metabolite:H+ symporter family of MFS proteins which function by proton symport and allow the uptake of a wide variety of metabolites (52).
Temperate bacteriophage genomes often contain an attachment site (attP) utilized for integration into a homologous region within the bacterial genome (attB) via site-specific recombination (20). The attP site of φ1026b was adjacent to the site encoding gp33, a site-specific integrase (Fig. 3). The nucleotide sequence of attP contained a 49-bp sequence that was identical to attB sequences present in the genomes of B. mallei ATCC 23344 and B. pseudomallei K96243. This sequence corresponded to the 3′ end of the tRNAPro-3 gene on chromosome 1 of B. mallei (positions 830691 to 830615) and chromosome 1 of B. pseudomallei (positions 1604091 to 1604043). tRNA genes often serve as target sequences for site-specific integration of temperate bacteriophages, plasmids, and pathogenicity islands (14, 34). The attP site of φ1026b was identical to the attP site of φE125 (70). It is worth emphasizing that B. pseudomallei 1026b and B. thailandensis E125 both contain bacteriophages integrated at tRNAPro-3, while B. pseudomallei K96243 does not (Fig. 1). However, B. pseudomallei K96243 does have a prophage-like region on chromosome 2 that is 98% identical to φ1026b gene 48 to gene 52 and 97% identical to φ1026b gene 55 to gene 57 (http://www.sanger.ac.uk/). It is not known if this is a functional or defective prophage.
Comparative analysis of the genomes of temperate bacteriophages φ1026b and φE125.
The host range and morphology of φ1026b are remarkably similar to the host range and morphology of φE125 (70), a temperate bacteriophage harbored by B. thailandensis E125 (Fig. 1). As mentioned above, the two genomes contain identical cos and attP sites, and 70% of the φ1026b proteins generate best hits to φE125 proteins when the BLASTP search algorithm is used. The genome of φ1026b is marginally larger (1.5 kb) than the genome of φE125. Figure 3 shows a comparative analysis of the genomes of φ1026b and φE125 generated by using the BLAST 2 SEQUENCES program (63). Large segments of DNA are shared by the two genomes, and the levels of nucleotide identity are 93 to 98%. These conserved regions are interspersed with DNA segments that exhibit little or no sequence similarity (Fig. 3). The mosaic nature of the genomes is illustrated by the head morphogenesis and head-tail joining genes in φ1026b (gene 3 to gene 8) and φE125 (gene 3 to gene 9). The φ1026b genes more closely resemble head morphogenesis and head-tail joining genes of P. aeruginosa and Pseudomonas putida bacteriophages than the corresponding genes in φE125. However, the DNA packaging and tail morphogenesis genes flanking this region in φ1026b and φE125 are 94% identical (Fig. 3). The most likely explanation for this finding is that recombination between one of these bacteriophages and an unrelated bacteriophage (or prophage) resulted in acquisition of a different set of head morphogenesis and head-tail joining genes (11, 36). Because the proteins involved in head morphogenesis interact with one another, it is not surprising that the genes encoding them are laterally acquired as a group. The putative crossover points for this recombination event and those described below occur at or near gene boundaries. The modular exchange of head morphogenesis genes suggests that DNA packaging proteins (gp1 and gp2) can associate with two distinct head protein sets, while head-to-tail association seems to require the mediation of a specific head-tail joining protein (gp8 in φ1026b and gp9 in φE125).
Genetic mosaicism was readily evident in the region spanning the site-specific integrase and DNA replication genes of φ1026b and φE125 (Fig. 3). This large mosaic region includes five modules of conserved genes and six modules of genes with no sequence similarity. Note that one of the conserved modules in φE125 is disrupted by an ISBt3 insertion in gene 39 (70), which corresponds to gene 44 in φ1026b (Fig. 3). The biological function(s) of this large mosaic region probably includes lysogeny, lysogenic conversion, and superinfection immunity (10, 39). As mentioned above, bacteriophage φ1026b cannot form plaques on the lysogens BML1 and BML10 (Table 3). In comparison, bacteriophage φE125 can form plaques on BML1 but not on BML10. This indicates that the φE125 lysogen (BML10) can prevent superinfection with both φE125 and φ1026b but that the φ1026b lysogen (BML1) can prevent superinfection only with φ1026b. It seems likely that one or more of the novel gene modules in the mosaic region are responsible for the differences in superinfection immunity, but further studies are required to prove this.
Several additional features of the large mosaic region should be mentioned here. First, φ1026b gene 66 and gene 67 were replaced in φE125 by gene 56 and gene 57 (Fig. 3). This modular replacement occurred precisely at the gene boundaries, suggesting that these gene pairs perform analogous functions. The biological function likely involves DNA methylation because both gene 67 (φ1026b) and gene 56 (φE125) encode DNA methyltransferases. Interestingly, DNA methyltransferases are relatively common in bacterioprophages from gram-positive bacteria but not in bacteriophages from gram-negative bacteria. Second, single-gene modular replacement between φ1026b gene 50 and φE125 gene 45 also occurred, but the biological importance of this exchange is not known because it involved genes with no known functions (Fig. 3). Finally, the large mosaic region of φ1026b includes gene 58 and gene 59, genes that encode a LysR family transcriptional regulator and an MFS transporter (Fig. 3). These genes were not present in the φE125 genome, supporting the notion that they were acquired by lateral gene transfer from a bacterial genome (see above).
Phenotypic analysis of B. pseudomallei DD5025.
The prophage-encoded MFS transporter (gp59) may provide B. pseudomallei 1026b with a selective advantage over other B. pseudomallei strains by allowing the uptake of a nutrient(s) from the environment (36, 52). In order to examine the function of gene 59, a strain harboring a mutation in this gene was constructed. An internal gene fragment of gene 59 was PCR amplified and cloned into the suicide vector pSKM11 (Table 1). Plasmid pDD94 was mobilized into B. pseudomallei 1026b, and the resulting merodiploid strain was designated DD5025. There were no detectable differences between the growth of 1026b and the growth of DD5025 in complex or defined media (data not shown). Both strains were examined to determine their abilities to metabolize 190 different carbon sources by using PM1 and PM2 phenotype microarrays (www.biolog.com), but no differences were observed. Prophage-encoded virulence factors in other bacterial species have been described (5), and it was of interest to see if gene 59 provided a selective benefit to 1026b in an animal model of melioidosis (25). Syrian hamsters were infected intraperitoneally with 102, 103, and 104 cells of 1026b and DD5025, and the LD50s were determined 2 days postinfection. The LD50 for both strains was <102 cells, suggesting that gene 59 is not important for the pathogenesis of 1026b in this animal model of melioidosis.
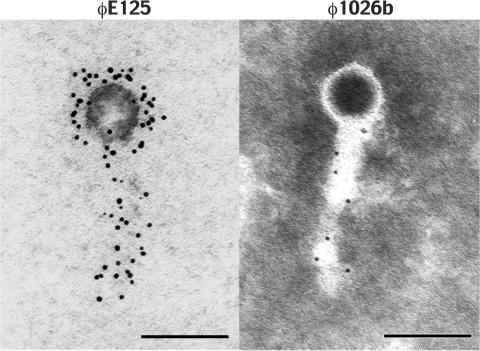
Immunogold electron microscopy of φE125 and φ1026b.
The comparative genomics analysis of φE125 and φ1026b predicted that these phages contain antigenically related tails but antigenically distinct heads (Fig. 3). Immunogold electron microscopy was performed to see if polyclonal antiserum against φE125 reacted with φ1026b (Fig. 4). The bacteriophages were reacted with polyclonal rabbit antiserum directed against φE125, washed, and reacted with a goat anti-rabbit IgG gold conjugate. As expected, the antibodies reacted with the head and tail of bacteriophage φE125 (Fig. 4). The anti-φE125 antibodies did not react with the head of φ1026b but did react with the tail (Fig. 4). These results corroborate the comparative genomics results and demonstrate that the tails of bacteriophages φE125 and φ1026b are antigenically related but the heads are antigenically distinct. Tailed bacteriophages bind to the surfaces of their bacterial hosts by using their tails, and the genetic and antigenic relatedness of the tails of φE125 and φ1026b probably accounts for their specificity for B. mallei.
FIG. 4.
Immunogold electron microscopy of bacteriophages φE125 and φ1026b. The bacteriophages were reacted with polyclonal rabbit antiserum directed against φE125, washed, and reacted with goat anti-rabbit IgG gold conjugate (5 nm). Bacteriophage φ1026b was subsequently negatively stained with 1% PTA. Scale bar = 100 nm.
DISCUSSION
The results presented here demonstrate that clinical isolates of B. pseudomallei exhibit genetic diversity, especially with regard to the mobile genetic elements that they harbor. It should be emphasized that only two clinical strains were compared in this study, but it is likely that future B. pseudomallei genome sequencing and comparative genome hybridization projects will yield similar results. At least five prophages (or prophage-like elements) were identified in B. pseudomallei 1026b but not in B. pseudomallei K96243 (http://www.sanger.ac.uk/). Prophages are a major source of strain-specific differences in several pathogenic bacteria, including Shiga toxin-producing E. coli, Streptococcus pyogenes, Staphylococcus aureus, S. enterica, and Xylella fastidiosa (11, 16, 17). The genomic sequencing results for multiple strains of Streptococcus and Xylella suggest that different disease pathologies may be due to differences in the prophage contents of the infecting strains (48, 64). Prophages are responsible for much of the laterally transferred DNA in bacteria, and they play a major role in the evolution of bacterial pathogens by providing new virulence determinants (5, 15). Manzeniuk et al. found that 92% of B. pseudomallei strains produced temperate bacteriophages, demonstrating that prophages are relatively common in this bacterial species (45). Brown and Beacham performed subtractive hybridization between B. pseudomallei and B. thailandensis and identified multiple B. pseudomallei-specific mobile genetic elements, including a P2-like prophage (9). Taken together, the results demonstrate that there is considerable diversity in the mobile genetic elements that B. pseudomallei strains harbor. It is tempting to speculate that the variable clinical presentation of melioidosis is due, at least in part, to the prophage contents of the infecting B. pseudomallei strains. The genomic sequences of additional B. pseudomallei strains isolated from melioidosis patients with defined clinical manifestations are needed to further explore this possibility.
It is widely accepted that tailed bacteriophage genomes are a mosaic collection of genetic material resulting from recombination between bacteriophages (or prophages) (11, 16, 17, 36). A comparative genome analysis of φ1026b and φE125 revealed regions with high sequence similarity interspersed with regions displaying no sequence similarity (Fig. 3). This mosaic genetic relationship indicates that recombination between φ1026b or φE125 and an unrelated bacteriophage(s) occurred during the evolution of these Burkholderia bacteriophages, which resulted in acquisition of new head and lysogeny genes. The φ1026b head morphogenesis genes more closely resemble the head morphogenesis genes of P. aeruginosa and P. putida bacteriophages than the corresponding genes in φE125. In addition, the host lysis cassettes of φ1026b (genes 23 to 25) and φE125 (genes 24 to 26) are located directly downstream of the putative tail fiber module, which is similar to the genetic organization of P. aeruginosa bacteriophage D3 (42). This genetic organization is commonly found in Siphoviridae from low-G+C-content gram-positive bacteria (10) but not in Siphoviridae from gram-negative bacteria. The tail fiber module-host lysis cassette module organization seems to be an ancestral trait in at least a subgroup of Burkholderia and Pseudomonas Siphoviridae.
It is curious that bacteriophages φ1026b and φE125 specifically infect B. mallei but are harbored by B. pseudomallei and B. thailandensis. What is the mechanism by which B. pseudomallei and B. thailandensis strains are resistant to infections with φ1026b and φE125? First, B. pseudomallei and B. thailandensis strains may be immune to superinfection with these bacteriophages because they harbor similar prophages. The genomic sequence of B. pseudomallei K96243 contains eight genes that are nearly identical to φ1026b genes (http://www.sanger.ac.uk/), and Woods et al. (70) found that 31% of B. thailandensis strains harbor a φE125-like prophage. Thus, it is clear that some B. pseudomallei and B. thailandensis strains are lysogenic and may be immune to superinfection with φ1026b and φE125. However, 69% of B. thailandensis strains did not possess an φE125-like prophage, suggesting that superinfection immunity alone is not responsible for their resistance to infection with φE125 (70). Second, there may be differences in the bacteriophage receptors present on B. mallei and on B. pseudomallei and B. thailandensis. LPS O antigen is required for plaque formation on B. mallei, indicating that this is the surface-exposed bacteriophage receptor (Table 3). B. mallei LPS O antigen is similar to the antigen previously described for B. pseudomallei and B. thailandensis except that it is devoid of an O-acetyl group at the 4′ position of the l-talose residue (7, 12, 41, 53). B. pseudomallei and B. thailandensis strains may be resistant to infection with φ1026b and φE125 because the O-acetyl group at the 4′ position of the l-talose residue alters the conformation of the LPS O antigen and/or blocks the bacteriophage binding site. Finally, B. pseudomallei and B. thailandensis may be resistant to infection with these bacteriophages because they do not produce a coreceptor. φ1026b and φE125 do not form plaques on B. mallei strains Turkey 4 and Turkey 5, two strains that produce LPS O antigen (Table 3). Taken together, the results indicate that LPS O antigen is required, but is not sufficient, for infection with these bacteriophages. It is possible that B. mallei strains Turkey 4 and Turkey 5 do not produce a coreceptor that participates with LPS O antigen in the initial interaction with φ1026b and φE125. Further studies are required to identify and characterize this putative coreceptor and examine if it is present in B. pseudomallei and B. thailandensis.
φ1026b gene 58 and gene 59 encode a LysR family transcriptional regulator (57) and an MFS transporter (52), respectively. These genes are not present in the φE125 genome, but similar gene pairs are present in several bacterial genomes (Fig. 3). Given this information, it is feasible that φ1026b gene 58 and gene 59 were acquired together by lateral transfer from a bacterial genome. The tandem arrangement of these genes in diverse genomes suggests that they may function together. One obvious possibility is that expression of the MFS transporter is regulated by the LysR family transcriptional regulator. It is hypothesized that gene 58 and gene 59 provide a selective advantage to B. pseudomallei 1026b by allowing it to take up a solute(s) from the environment that may not be accessible to other bacteria, including other strains of B. pseudomallei. In addition, the genes may also benefit the prophage by ensuring that it is maintained in the chromosome of its host. Unfortunately, no phenotype was observed for a strain (DD5025) harboring a mutation in gene 59. There was no difference in the growth, virulence, or catabolism of 190 carbon sources between 1026b and DD5025. Preliminary studies have indicated that while these two strains have similar growth rates in brain heart infusion broth, DD5025 grows noticeably slower in brain heart infusion broth containing 3.5% NaCl. One of the strategies used by bacteria to cope with environments with elevated osmolarity is to take up osmoprotective compounds, termed compatible solutes (60). gp59 is a member of the metabolite:H+ symporter family of transporters, and the metabolites transported by this family include compatible solutes (52). Thus, the biological function of gp59 may be to transport a compatible solute into the cell and allow B. pseudomallei 1026b to overcome environmental salt stress (60).
φ1026b and φE125 encode a RelE-like toxin that is flanked by a transcriptional regulator and a class I holin (Fig. 3). The presence of both a class I holin (gp82 in φ1026b, gp70 in φE125) and a class II holin (gp23 in φ1026b, gp24 in φE125) in these bacteriophages is unusual, and it is not known if one or both of these holins are required for the programmed release of lysozyme from the cytoplasm prior to the bacteriophage burst (65). RelE toxin and RelB antitoxin are members of an E. coli toxin-antitoxin protein system that reversibly inhibits protein synthesis in response to nutrient limitation (33). The genes encoding toxin-antitoxin systems are widespread in bacteria and are typically adjacent to one another on plasmids or chromosomes (8). The antitoxin binds the toxin and prevents it from killing the bacterial host by binding to essential enzymes or disrupting important cellular functions. The antitoxin component is typically less stabile than the toxin component, and decreased transcription or translation of the antitoxin results in death of the bacterial host. Toxin-antitoxin systems were first identified on plasmids, where they play an important role in plasmid stabilization (8). The presence of toxin-antitoxin genes in bacteriophage genomes is uncommon and may be a mechanism by which prophages maintain their genomes in their bacterial hosts. However, φ1026b and φE125 do not harbor an obvious antitoxin gene, and future experiments should explore if there is a novel antitoxin gene and what, if any, function the putative toxin-antitoxin system plays in these bacteriophages.
In conclusion, bacteriophages are significant contributors to the genomic diversity of B. pseudomallei isolates. The bacteriophage described in this study was specific for B. mallei, and it exhibited a mosaic genetic relationship with bacteriophage φE125, another B. mallei-specific bacteriophage produced by B. thailandensis (70). Thus, it appears that B. mallei may be an ideal host for the study of additional bacteriophages produced by B. pseudomallei and B. thailandensis. Direct comparisons of the bacteriophages produced by these species may reveal virulence genes that are present in B. pseudomallei bacteriophages but not in B. thailandensis bacteriophages.
Acknowledgments
This research was sponsored by the Medical Biological Defense Research Program, U.S. Army Medical Research and Materiel Command (project 02-4-5X-026).
I thank Brain G. Spratt and Daniel Godoy for help with the MLST analysis of B. pseudomallei 1026b, Kathy Kuehl for assistance with electron microscopy, David M. Waag for assistance with producing polyclonal φE125 antiserum, and Ricky L. Ulrich for critically reading the manuscript.
The opinions, interpretations, conclusions, and recommendations expressed here are those of the author and are not necessarily endorsed by the U.S. Army in accordance with AR 70-31.
REFERENCES
- 1.Ackermann, H.-W. 2003. Bacteriophage observations and evolution. Res. Microbiol. 154:245-251. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anuntagool, N., P. Aramsri, T. Panichakul, V. Wuthiekanun, R. Kinoshita, N. J. White, and S. Sirisinha. 2000. Antigenic heterogeneity of lipopolysaccharide among Burkholderia pseudomallei clinical isolates. Southeast Asian J. Trop. Med. Public Health 31:146-152. [PubMed] [Google Scholar]
- 4.Atkins, T., R. Prior, K. Mack, P. Russell, M. Nelson, J. Prior, J. Ellis, P. C. Oyston, G. Dougan, and R. W. Titball. 2002. Characterization of an acapsular mutant of Burkholderia pseudomallei identified by signature tagged mutagenesis. J. Med. Microbiol. 51:539-547. [DOI] [PubMed] [Google Scholar]
- 5.Boyd, E. F., and H. Brussow. 2002. Common themes among bacteriophage-encoded virulence factors and diversity among the bacteriophages involved. Trends Microbiol. 10:521-529. [DOI] [PubMed] [Google Scholar]
- 6.Brämer, C. O., P. Vandamme, L. F. da Silva, J. G. C. Gomez, and A. Steinbüchel. 2001. Burkholderia sacchari sp. nov., a polyhydroxyalkanoate-accumulating bacterium isolated from soil of a sugar-cane plantation in Brazil. Int. J. Syst. Bacteriol. E vol. Microbiol. 51:1709-1713. [DOI] [PubMed] [Google Scholar]
- 7.Brett, P. J., D. DeShazer, and D. E. Woods. 1998. Burkholderia thailandensis sp. nov., description of a Burkholderia pseudomallei-like species. Int. J. Syst. Bacteriol. 48:317-320. [DOI] [PubMed] [Google Scholar]
- 8.Brown, J. M., and K. J. Shaw. 2003. A novel family of Escherichia coli toxin-antitoxin gene pairs. J. Bacteriol. 185:6600-6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown, N. F., and I. R. Beacham. 2000. Cloning and analysis of genomic differences unique to Burkholderia pseudomallei by comparison with B. thailandensis. J. Med. Microbiol. 49:993-1001. [DOI] [PubMed] [Google Scholar]
- 10.Brussow, H., and F. Desiere. 2001. Comparative phage genomics and the evolution of Siphoviridae: insights from dairy phages. Mol. Microbiol. 39:213-222. [DOI] [PubMed] [Google Scholar]
- 11.Brussow, H., and R. W. Hendrix. 2002. Phage genomics: small is beautiful. Cell 108:13-16. [DOI] [PubMed] [Google Scholar]
- 12.Burtnick, M. N., P. J. Brett, and D. E. Woods. 2002. Molecular and physical characterization of Burkholderia mallei O antigens. J. Bacteriol. 184:849-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cain, C. C., A. T. Henry, R. H. Waldo III, L. J. Casida, Jr., and J. O. Falkinham III. 2000. Identification and characteristics of a novel Burkholderia strain with broad-spectrum antimicrobial activity. Appl. Environ. Microbiol. 66:4139-4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell, A. 2003. Prophage insertion sites. Res. Microbiol. 154:277-282. [DOI] [PubMed] [Google Scholar]
- 15.Canchaya, C., G. Fournous, S. Chibani-Chennoufi, M.-L. Dillmann, and H. Brussow. 2003. Phage as agents of lateral gene transfer. Curr. Opin. Microbiol. 6:417-424. [DOI] [PubMed] [Google Scholar]
- 16.Canchaya, C., C. Proux, G. Fournous, A. Bruttin, and H. Brussow. 2003. Prophage genomics. Microbiol. Mol. Biol. Rev. 67:238-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casjens, S. 2003. Prophages and bacterial genomics: what have we learned so far? Mol. Microbiol. 49:277-300. [DOI] [PubMed] [Google Scholar]
- 18.Chaowagul, W., N. J. White, D. A. Dance, Y. Wattanagoon, P. Naigowit, T. M. Davis, S. Looareesuwan, and N. Pitakwatchara. 1989. Melioidosis: a major cause of community-acquired septicemia in northeastern Thailand. J. Infect. Dis. 159:890-899. [DOI] [PubMed] [Google Scholar]
- 19.Coenye, T., E. Falsen, B. Hoste, M. Ohlen, J. Goris, J. R. W. Govan, M. Gillis, and P. Vandamme. 2000. Description of Pandoraea gen. nov. with Pandoraea apista sp. nov., Pandoraea pulmonicola sp. nov., Pandoraea pnomenusa sp. nov., Pandoraea sputorum sp. nov. and Pandoraea norimbergensis comb. nov. Int. J. Syst. E vol. Microbiol. 50:887-899. [DOI] [PubMed] [Google Scholar]
- 20.Craig, N. L. 1988. The mechanism of conservative site-specific recombination. Annu. Rev. Genet. 22:77-105. [DOI] [PubMed] [Google Scholar]
- 21.Currie, B. J., D. A. Fisher, D. M. Howard, J. N. Burrow, D. Lo, S. Selva-Nayagam, N. M. Anstey, S. E. Huffam, P. L. Snelling, P. J. Marks, D. P. Stephens, G. D. Lum, S. P. Jacups, and V. L. Krause. 2000. Endemic melioidosis in tropical northern Australia: a 10-year prospective study and review of the literature. Clin. Infect. Dis. 31:981-986. [DOI] [PubMed] [Google Scholar]
- 22.Dance, D. A. B. 2002. Melioidosis. Curr. Opin. Infect. Dis. 15:127-132. [DOI] [PubMed] [Google Scholar]
- 23.DeShazer, D., P. J. Brett, R. Carlyon, and D. E. Woods. 1997. Mutagenesis of Burkholderia pseudomallei with Tn5-OT182: isolation of motility mutants and molecular characterization of the flagellin structural gene. J. Bacteriol. 179:2116-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeShazer, D., P. J. Brett, and D. E. Woods. 1998. The type II O-antigenic polysaccharide moiety of Burkholderia pseudomallei lipopolysaccharide is required for serum resistance and virulence. Mol. Microbiol. 30:1081-1100. [DOI] [PubMed] [Google Scholar]
- 25.DeShazer, D., and D. E. Woods. 1999. Animal models of melioidosis, p. 199-203. In O. Zak and M. Sande (ed.), Handbook of animal models of infection. Academic Press Ltd., London, United Kingdom.
- 26.DeShazer, D., and D. E. Woods. 1999. Pathogenesis of melioidosis: use of Tn5-OT182 to study the molecular basis of Burkholderia pseudomallei virulence. J. Infect. Dis. Antimicrob. Agents 16:91-96. [Google Scholar]
- 27.DeShazer, D., D. M. Waag, D. L. Fritz, and D. E. Woods. 2001. Identification of a Burkholderia mallei polysaccharide gene cluster by subtractive hybridization and demonstration that the encoded capsule is an essential virulence determinant. Microb. Pathog. 30:253-269. [DOI] [PubMed] [Google Scholar]
- 28.Feng, T., Z. Li, W. Jiang, B. Breyer, L. Zhou, H. Cheng, R. C. Haydon, A. Ishikawa, M. A. Joudeh, and T.-C. He. 2002. Increased efficiency of cloning large DNA fragments using a lower copy number plasmid. BioTechniques 32:992-998. [DOI] [PubMed] [Google Scholar]
- 29.Finkelstein, R. A., P. Atthasampunna, and M. Chulasamaya. 2000. Pseudomonas (Burkholderia) pseudomallei in Thailand, 1964-1967; geographic distribution of the organism, attempts to identify cases of active infection, and presence of antibody in representative sera. Am. J. Trop. Med. Hyg. 62:232-239. [DOI] [PubMed] [Google Scholar]
- 30.Gee, J. E., C. T. Sacchi, M. B. Glass, B. K. De, R. S. Weyant, P. N. Levett, A. M. Whitney, A. R. Hoffmaster, and T. Popovic. 2003. Use of 16S rRNA gene sequencing for rapid identification and differentiation of Burkholderia pseudomallei and B. mallei. J. Clin. Microbiol. 41:4647-4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Godfrey, A. J., S. Wong, D. A. Dance, W. Chaowagul, and L. E. Bryan. Pseudomonas pseudomallei resistance to beta-lactam antibiotics due to alterations in the chromosomally encoded beta-lactamase. Antimicrob. Agents Chemother. 35:1635-1640. [DOI] [PMC free article] [PubMed]
- 32.Godoy, D., G. Randle, A. J. Simpson, D. M. Aanensen, T. L. Pitt, R. Kinoshita, and B. G. Spratt. 2003. Multilocus sequence typing and evolutionary relationships among the causative agents of melioidosis and glanders, Burkholderia pseudomallei and B. mallei. J. Clin. Microbiol. 41:2068-2079. (Erratum, 41:4913.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gotfredsen, M., and K. Gerdes. 1998. The Escherichia coli relBE genes belong to a new toxin-antitoxin gene family. Mol. Microbiol. 29:1065-1076. [DOI] [PubMed] [Google Scholar]
- 34.Hacker, J., G. Blum-Oehler, I. Muldorfer, and H. Tschape. 1997. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol. Microbiol. 23:1089-1097. [DOI] [PubMed] [Google Scholar]
- 35.Hacker, J., and E. Carniel. 2001. Ecological fitness, genomic islands and bacterial pathogenicity. EMBO Rep. 2:376-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hendrix, R. W., G. F. Hatfull, and M. C. M. Smith. 2003. Bacteriophage with tails: chasing their origins and evolution. Res. Microbiol. 154:253-257. [DOI] [PubMed] [Google Scholar]
- 37.Hoiseth, S. K., and B. A. D. Stocker. 1981. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 291:238-239. [DOI] [PubMed] [Google Scholar]
- 38.Holloway, B. W., U. Romling, and B. Tummler. 1994. Genomic mapping of Pseudomonas aeruginosa PAO. Microbiology 140:2907-2929. [DOI] [PubMed] [Google Scholar]
- 39.Iandolo, J. J., V. Worrell, K. H. Groicher, Y. Qian, R. Tian, S. Kenton, A. Dorman, H. Ji, S. Lin, P. Loh, S. Qi, H. Zhu, and B. A. Roe. 2002. Comparative analysis of the genomes of the temperate bacteriophages phi 11, phi 12 and phi 13 of Staphylococcus aureus 8325. Gene 289:109-118. [DOI] [PubMed] [Google Scholar]
- 40.Juhala, R. J., M. E. Ford, R. L. Duda, A. Youlton, G. F. Hatfull, and R. W. Hendrix. 2000. Genomic sequences of bacteriophages HK97 and HK022: pervasive genetic mosaicism in the lambdoid bacteriophages. J. Mol. Biol. 299:27-51. [DOI] [PubMed] [Google Scholar]
- 41.Knirel, Y. A., N. A. Paramonov, A. S. Shashkov, N. K. Kochetkov, R. G. Yarullin, S. M. Farber, and V. I. Efremenko. 1992. Structure of the polysaccharide chains of Pseudomonas pseudomallei lipopolysaccharides. Carbohydr. Res. 233:185-193. [DOI] [PubMed] [Google Scholar]
- 42.Kropinski, A. M. 2000. Sequence of the genome of the temperate, serotype-converting, Pseudomonas aeruginosa bacteriophage D3. J. Bacteriol. 182:6066-6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laing, F. P. Y., K. Ramotar, R. R. Read, N. Alfieri, A. Kureishi, E. A. Henderson, and T. J. Louie. 1995. Molecular epidemiology of Xanthomonas maltophilia colonization and infection in the hospital environment. J. Clin. Microbiol. 33:513-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahenthiralingam, E., T. Coenye, J. W. Chung, D. P. Speert, J. R. W. Govan, P. Taylor, and P. Vandamme. 2000. Diagnostically and experimentally useful panel of strains from the Burkholderia cepacia complex. J. Clin. Microbiol. 38:910-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manzeniuk, O. I., N. V. Volozhantsev, and E. A. Svetoch. 1994. Identification of Pseudomonas mallei bacteria with the help of Pseudomonas pseudomallei bacteriophages Mikrobiologiya 63:537-544. (In Russian.) [PubMed] [Google Scholar]
- 46.McKevitt, A. I., S. Bajaksouzian, J. D. Klinger, and D. E. Woods. 1989. Purification and characterization of an extracellular protease from Pseudomonas cepacia. Infect. Immun. 57:771-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mongkolsuk, S., S. Rabibhadana, P. Vattanaviboon, and S. Loprasert. 1994. Generalized and mobilizabile positive-selection cloning vectors. Gene 143:145-146. [DOI] [PubMed] [Google Scholar]
- 48.Nakagawa, I., K. Kurokawa, A. Yamashita, M. Nakata, Y. Tomiyasu, N. Okahashi, S. Kawabata, K. Yamazaki, T. Shiba, T. Yasunaga, H. Hayashi, M. Hattori, and S. Hamada. 2003. Genomic sequencing of an M3 strain of Streptococcus pyogenes reveals a large-scale genomic rearrangement in invasive strains and new insights into phage evolution. Genome Res. 13:1042-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ochman, H., J. G. Lawrence, and E. A. Groisman. 2000. Lateral gene transfer and the nature of bacterial innovation. Nature 405:299-304. [DOI] [PubMed] [Google Scholar]
- 50.Ochman, H., and N. A. Moran. 2001. Genes lost and genes found: evolution of bacterial pathogenesis and symbiosis. Science 292:1096-1098. [DOI] [PubMed] [Google Scholar]
- 51.Osborn, A. M., and D. Boltner. 2002. When phage, plasmids, and transposons collide: genomic islands, and conjugative- and mobilizable-transposons as a mosaic continuum. Plasmid 48:202-212. [DOI] [PubMed] [Google Scholar]
- 52.Pao, S. S., I. T. Paulsen, and M. H. Saier, Jr. 1998. Major facilitator superfamily. Microbiol. Mol. Biol. Rev. 62:1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perry, M. B., L. L. MacLean, T. Schollaardt, L. E. Bryan, and M. Ho. 1995. Structural characterization of the lipopolysaccharide O antigens of Burkholderia pseudomallei. Infect. Immun. 63:3348-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rahme, L. G., E. J. Stevens, S. F. Wolfort, J. Shao, R. G. Tompkins, and F. M. Ausubel. 1995. Common virulence factors for bacterial pathogenicity in plants and animals. Science 268:1899-1902. [DOI] [PubMed] [Google Scholar]
- 55.Reckseidler, S. L., D. DeShazer, P. A. Sokol, and D. E. Woods. 2001. Detection of bacterial virulence genes by subtractive hybridization: identification of capsular polysaccharide of Burkholderia pseudomallei as a major virulence determinant. Infect. Immun. 69:34-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rotz, L. D., A. S. Khan, S. R. Lillibridge, S. M. Ostroff, and J. M. Hughes. 2002. Public health assessment of potential biological terrorism agents. Emerg. Infect. Dis. 8:225-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schell, M. A. 1993. Molecular biology of the LysR family of transcriptional regulators. Annu. Rev. Microbiol. 47:597-626. [DOI] [PubMed] [Google Scholar]
- 58.Segonds, C., T. Heulin, N. Marty, and G. Chabanon. 1999. Differentiation of Burkholderia species by PCR-restriction fragment length polymorphism analysis of the 16S rRNA gene and application to cystic fibrosis isolates. J. Clin. Microbiol. 37:2201-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 60.Sleator, R. D., and C. Hill. 2002. Bacterial osmoadaptation: the role of osmolytes in bacterial stress and virulence. FEMS Microbiol. Rev. 26:49-71. [DOI] [PubMed] [Google Scholar]
- 61.Smith, M. D., V. Wuthiekanun, A. L. Walsh, and N. J. White. 1995. Quantitative recovery of Burkholderia pseudomallei from soil in Thailand. Trans. R. Soc. Trop. Med. Hyg. 89:488-490. [DOI] [PubMed] [Google Scholar]
- 62.Smith, P. B., and W. B. Cherry. 1957. Identification of Malleomyces by specific bacteriophages. J. Bacteriol. 74:668-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tatusova, T. A., and T. L. Madden. 1999. BLAST 2 Sequences, a new tool for comparing protein and nucleotide sequences. FEMS Microbiol. Lett. 174:247-250. [DOI] [PubMed] [Google Scholar]
- 64.Van Sluys, M. A., M. C. de Oliveira, C. B. Monteiro-Vitorello, C. Y. Miyaki, L. R. Furlan, L. E. Camargo, A. C. da Silva, D. H. Moon, M. A. Takita, E. G. Lemos, M. A. Machado, M. I. Ferro, F. R. da Silva, M. H. Goldman, G. H. Goldman, M. V. Lemos, H. El-Dorry, S. M. Tsai, H. Carrer, D. M. Carraro, R. C. de Oliveira, L. R. Nunes, W. J. Siqueira, L. L. Coutinho, E. T. Kimura, E. S. Ferro, R. Harakava, E. E. Kuramae, C. L. Marino, E. Giglioti, I. L. Abreu, L. M. Alves, A. M. do Amaral, G. S. Baia, S. R. Blanco, M. S. Brito, F. S. Cannavan, A. V. Celestino, A. F. da Cunha, R. C. Fenille, J. A. Ferro, E. F. Formighieri, L. T. Kishi, S. G. Leoni, A. R. Oliveira, V. E. Rosa, Jr., F. T. Sassaki, J. A. Sena, A. A. de Souza, D. Truffi, F. Tsukumo, G. M. Yanai, L. G. Zaros, E. L. Civerolo, A. J. Simpson, N. F. Almeida, Jr., J. C. Setubal, and J. P. Kitajima. 2003. Comparative analysis of the complete genome sequence of Pierce's disease and citrus varigated chlorosis strains of Xylella fastidiosa. J. Bacteriol 185:1018-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang, I.-N., D. L. Smith, and R. Young. 2000. Holins: the protein clocks of bacteriophage infections. Annu. Rev. Microbiol. 54:799-825. [DOI] [PubMed] [Google Scholar]
- 66.Whalen, M. C., R. W. Innes, A. F. Bent, and B. J. Staskawicz. 1991. Identification of Pseudomonas syringae pathogens of Arabidopsis and a bacterial locus determining avirulence on both Arabidoposis and soybean. Plant Cell 3:49-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.White, N. J. 2003. Melioidosis. Lancet 361:1715-1722. [DOI] [PubMed] [Google Scholar]
- 68.Wilson, K. 1987. Preparation of genomic DNA from bacteria, p. 2.4.1-2.4.5. In F. M. Ausubel, R. Brent, R. E. Kingston, et al. (ed.), Current protocols in molecular biology. John Wiley & Sons, New York, N.Y. [DOI] [PubMed]
- 69.Woo, P. C. Y., P. K. L. Leung, H.-W. Tsoi, B. Y. L. Chan, T.-L. Que, and K.-Y. Yuen. 2002. Characterization of a novel insertion sequence, ISBp1, in Burkholderia pseudomallei. Arch. Microbiol. 177:267-273. [DOI] [PubMed] [Google Scholar]
- 70.Woods, D. E., J. A. Jeddeloh, D. F. Fritz, and D. DeShazer. 2002. Burkholderia thailandensis E125 harbors a temperate bacteriophage specific for Burkholderia mallei. J. Bacteriol. 184:4003-4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wuthiekanun, V., M. D. Smith, D. A. Dance, and N. J. White. 1995. Isolation of Pseudomonas pseudomallei from soil in north-eastern Thailand. Trans. R. Soc. Trop. Med. Hyg. 89:41-43. [DOI] [PubMed] [Google Scholar]
- 72.Yabuuchi, E., Y. Kosako, M. Arakawa, H. Hotta, and I. Yano. 1992. Identification of Oklahoma isolate as a strain of Pseudomonas pseudomallei. Microbiol. Immunol. 36:1239-1249. [DOI] [PubMed] [Google Scholar]
- 73.Yabuuchi, E., Y. Kawamura, T. Ezaki, M. Ikedo, S. Dejsirilert, N. Fujiwara, T. Naka, and K. Kobayashi. 2000. Burkholderia uboniae sp. nov., l-arabinose-assimilating but different from Burkholderia thailandensis and Burkholderia vietnamiensis. Microbiol. Immunol. 44:307-317. [DOI] [PubMed] [Google Scholar]
- 74.Zhang, H., S. Hanada, T. Shigematsu, K. Shibuya, Y. Kamagata, T. Kanagawa, and R. Kurane. 2000. Burkholderia kururiensis sp. nov., a trichloroethylene (TCE)-degrading bacterium isolated from an aquifer polluted with TCE. Int. J. Syst. Evol. Microbiol. 50:743-749. [DOI] [PubMed] [Google Scholar]