Abstract
Tooth development has attracted the attention of researchers since the 19th century. It became obvious even then that morphogenesis could not fully be appreciated from two-dimensional histological sections. Therefore, methods of three-dimensional (3D) reconstructions were employed to visualize the surface morphology of developing structures and to help appreciate the complexity of early tooth morphogenesis. The present review surveys the data provided by computer-aided 3D analyses to update classical knowledge of early odontogenesis in the laboratory mouse and in humans. 3D reconstructions have demonstrated that odontogenesis in the early stages is a complex process which also includes the development of rudimentary odontogenic structures with different fates. Their developmental, evolutionary, and pathological aspects are discussed. The combination of in situ hybridization and 3D reconstruction have demonstrated the temporo-spatial dynamics of the signalling centres that reflect transient existence of rudimentary tooth primordia at loci where teeth were present in ancestors. The rudiments can rescue their suppressed development and revitalize, and then their subsequent autonomous development can give rise to oral pathologies. This shows that tooth-forming potential in mammals can be greater than that observed from their functional dentitions. From this perspective, the mouse rudimentary tooth primordia represent a natural model to test possibilities of tooth regeneration.
Keywords: Tooth, development, 3D reconstruction, mouse, human, odontogenesis
Introduction
Teeth first appeared as skin appendages in the vertebrate fossil record and so may be considered as the earliest expression of a fundamental epithelial-mesenchymal interaction.1 The dentition characterizes species and, until recently, it was the main criterion used by paleontologists to classify fossil vertebrate lineages and mammalian species.
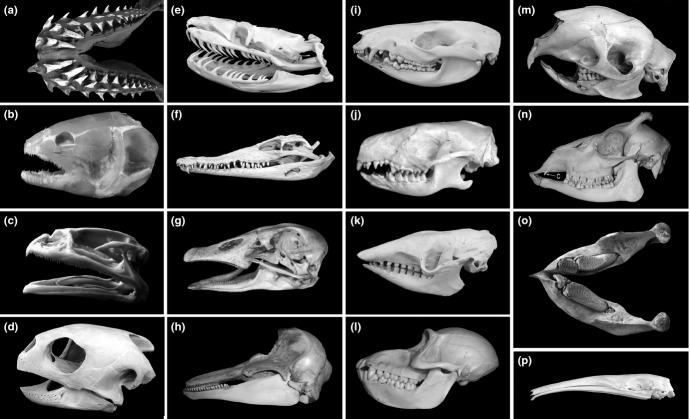
Tooth number, shape and size vary considerably among species (Figs. 1 and 2). This indicates that different geometric designs arose during evolution to enable specific adaptations to different types of food and thus tooth functions. Depending on their position in the mouth, different dental crown morphologies can be specified (heterodonty). This aspect is best manifested in mammals, where different tooth classes occur (incisor, canine, premolar or molar). However, even teeth of the same class can differ highly between species (Fig. 1).
Figure 1.
Variability of teeth in vertebrates. (a) cartilaginous fish – shark; (b) an example of a bony fish; (c) amphibian – a frog (teeth are absent in the lower jaw); (d) reptile – turtle (teeth are absent); (e) reptile – python; (f) reptile – crocodile; (g) bird – goose (teeth are absent); (h) mammal – dolphin; (i) opossum; (j) hedgehog; (k) armadillo; (l) baboon; (m) porcupine; (n) deer (note the atavistic canine – c); (o) mandible of elephant (an example of horizontal replacement of teeth: the molars are permanently shifting anteriorly, where they are finally shed, while a new molar emerges posteriorly); (p) toothless ant-eater (tamanoir) (from the ref.,160 with permission).
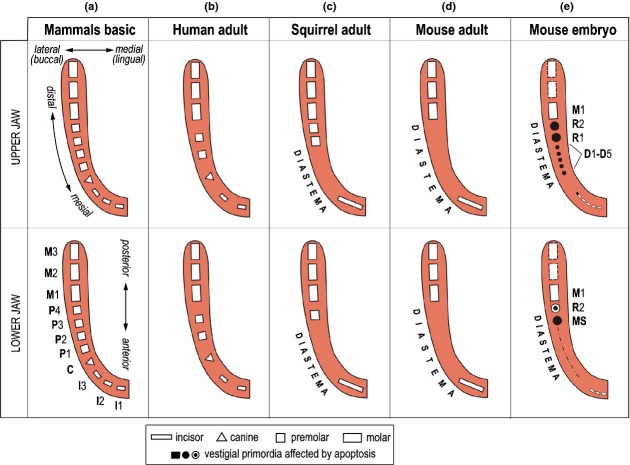
Figure 2.
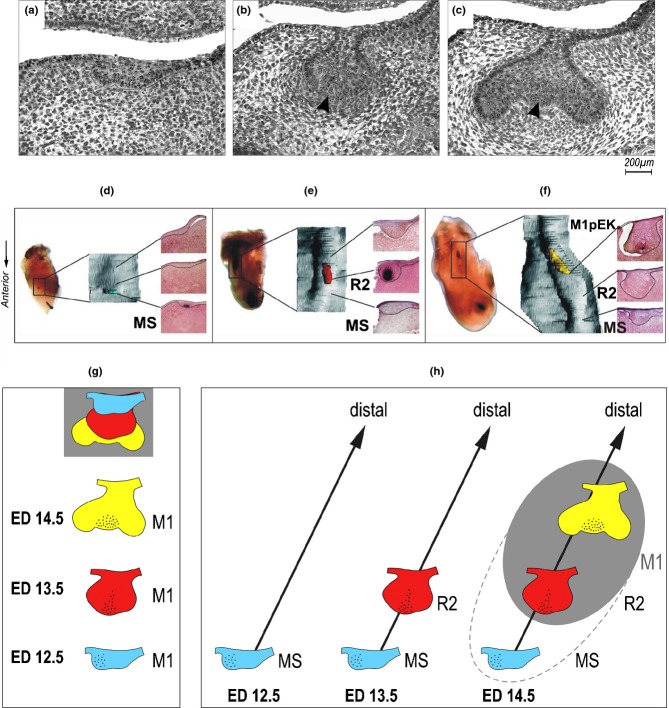
A schematic comparison of various tooth patterns in mammals. (a) The basic tooth pattern in mammals; (b, c, d) the tooth pattern in adult humans, squirrel and mouse, respectively; (e) mouse embryo. In the mouse embryonic upper jaw, the primordium of the functional incisor originates from joint development of several epithelial prominences (for details see Fig. 12). In the embryonic mandible, two to three epithelial prominences (bridges) come before the origin of the functional incisor primordium (for details see Fig. 17). I1, I2, I3 – the first, second and third incisor, respectively; C – canine; P1, P2, P3, P4 – the first, second, third and fourth premolar, respectively. M1, M2, M3 – the first, second and third molar, respectively. D1–D5, the small rudimental tooth primordia in the mouse diastema; MS, R1, R2 – the large rudimental tooth primordia in mouse diastema. Apoptosis accumulation – black.
A majority of fish, amphibians and reptiles have teeth (Fig. 1a–f). They generally have a large number of teeth (polyodontia) with similar shape (homodontia), and undergo continuous replacement due to the formation of many tooth generations (polyphyodontia). The teeth are usually comprised of dentine, enameloid or enamel. They are attached by bony ankylosis or fibrous tissue.2 However, the teeth in many species show different crown size and/or shape, depending on their position in the oral cavity (heterodontia). The posterior teeth can even be multicusped (molariform). In crocodiles, teeth are attached by a periodontal ligament to a bony socket (for review see3). Heterodontia, multicusped teeth and a periodontal ligament are typical characters of the dentition in mammals.
Birds lost teeth during evolution. In some species, horny structures may occur along the beak margin (Fig. 1g). On first examination of their external morphology and pattern, these horny structures are reminiscent of teeth in a homodont dentition (compare with Fig. 1h).
In monotremes (e.g. echidna), which are considered as the most primitive among living mammals, many reptilian characters are maintained (e.g. egg laying). The very young animals also possess a rudimentary unpaired egg tooth, similar to reptiles and birds. The egg tooth is a small hard protuberance at the tip of the beak or nose which assists during hatching. In the mouths of adult monotremes, there are horny plates.2 Although jaws are toothless in adults, rudimentary minute teeth may occur during development.4
In therian mammals (all living mammals except monotremes), a typical dentition comprises teeth differentiated into tooth types. Tooth number is reduced in comparison with what is generally observed in non-mammalian species (Fig. 1a–c, e–f), and some of the teeth appear in two generations (diphyodontia). Such a dentition already occurs in marsupial (pouched) mammals (Fig. 1i). They can show a maximum of 52 teeth: five upper or four lower incisors, one canine, three premolars and four molars in a jaw quadrant. Since the rudimental anlage of a successional dentition occurs in the ante-molar teeth, the latter teeth have been interpreted as a persisting deciduous dentition. Only one ‘milk’ or primary molar is replaced.5 A set of minute rudimental (so-called prelacteal) teeth can occur externally to the primary dentition.6
The dentition of the actual eutherian (placental) mammals is presumed to originate from the basic tooth formula comprising three incisors, one canine, four premolars and three molars (Fig. 2a). Such a number of teeth is present, for example, in some insectivores (Fig. 1j). The posterior teeth (cheek teeth) in mammals are typically multicusped – premolars and molars. Exceptionally, a fourth molar can also occur. Usually, two generations of incisors, canines and premolars develop. However, tooth number is reduced in the majority of mammalian species. Tooth number, size and shape of tooth crowns show high variability (Fig. 1k–p) in accordance with food requirements. The dentition in placental aquatic mammals, cetaceans, is completely different from the typical dentition of eutherian mammals (e.g. Fig. 1h). A functional unreduced heterodont dentition (Fig. 2a) was still preserved in some fossil whales.7 However, in the course of adaptation to aquatic life and feeding, functional teeth changed substantially. In toothed whales, the typical dentition shows a reptile-like appearance (Fig. 1h), with teeth of a simple shape that occur in large numbers (some dolphins may have more than 250 teeth). There is only one set of teeth that are interpreted as lacteal ones. In baleen whales (e.g. fin whales), the functional dentition has been lost. Although teeth are absent postnatally, a set of rudimental teeth develops prenatally, being shed before birth.4,5 The simple teeth in whales have been reported to arise by segregation from complex tooth germs during development.5 Both the presence of the reptile-like dentition in adult toothed whales or of a rudimentary dentition in embryos of baleen whales can be presented as two of many examples documenting the existence of a ‘phylogenetic memory’ during tooth development in mammals.8
Thus, during the evolution of the synapsid lineage of amniotes, including mammals, the dentition with a high number of simple teeth that appeared in many generations transformed into a dentition with a reduced number of teeth that typically exhibit a multicusped shape in the posterior jaw region, and appear in two generations as a maximum. About 100 years ago, two main and rather contradictory theories were formulated to explain the origin of multicusped teeth in mammals (Fig. 3a–b).
Figure 3.
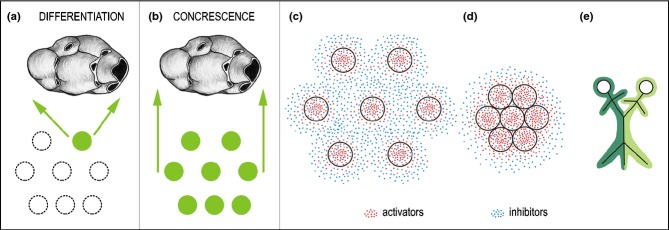
A scheme presenting the ‘differentiation’ and ‘concrescence’ theories on the origin of a multicusped tooth in mammals. Tooth primordia of mammalian ancestors are represented by rings. (a, b) Green rings – tooth primordia involved in the evolution of a multicusped tooth in mammals. Dashed-line rings – tooth primordia that are not involved in the evolution of a multicusped mammalian tooth. (c, d) A hypothetical model of the regulation of tooth concrescence. (c) The individual tooth buds (rings) are induced and maintained by growth activators, while growth inhibitors regulate origin of inter-bud domains (zones where further budding is inhibited – inhibition zones). The final distance between bud primordia results from the interaction between the activating and inhibiting signals that co-express in the originating buds. (d) Tooth concrescence results from the deficient separation of tooth primordia due to deficient formation of the gaps (inhibition zones).14 The further joined development can be compared to that seen in Siamese twins (e).
The differentiation theory (also called the Cope-Osborn or trituberculy theory) was supported by paleontological and comparative-anatomical investigations of adult teeth. According to this theory, the complex multicusped teeth in mammals evolved by differentiation from one simple-shaped tooth of mammalian ancestors (Fig. 3a). A triangle of cusps has been determined as the basic arrangement of molars in early mammals.9,10 Since tooth number decreased during evolution of mammals, most simple shaped teeth would have had to be suppressed, while the remaining teeth would have had to differentiate and produce completely new material for multicusped crowns (Fig. 3a). However, such a process seems to be rather wasteful. Regardless of later criticisms, the differentiation theory and its related terminology are generally accepted at present.10,11
The concrescence (integrated development) theory (Fig. 3b) is supported by embryological data on tooth development in actual species. This theory proposes that multicusped teeth in mammals evolved by joined development (integration) of several primordia of simple teeth inherited from mammalian ancestors; tooth concrescence has been accompanied by a shortening of jaws.5,12 By a fusion of two generations of reptilian-like tooth primordia, the origin of the lateral (buccal) and medial (lingual) row of cusps in multicusped molars of mammals has been explained (so-called ‘dimer theory’).13 Joined development (concrescence) of ancestral tooth primordia would explain the developmental potentiality of tooth anlagen from ancestors and rationalize them when integrating into a more complex, multicusped tooth (Fig. 3b).12
Authors of the concrescence theory were embryologists. By the term ‘concrescence’, they meant integrated (joined) development of tooth primordia, i.e. the mechanism acting during ontogenesis. However, since then, the theory of concrescence has often been misinterpreted in the literature as a fusion of teeth in mammalian ancestors. This might invoke the incorrect idea that the concrescence implies a fusion of adult teeth formed by mineralized tissues. Such a misunderstanding might be at the origin of the persisting general refusal to accept the concrescence theory.
It is possible that what the authors of the differentiation theory observed as an increase of cusp number in adult teeth (see above) had resulted from a concrescence of tooth primordia during ontogenesis of corresponding animals.14 In this respect, the concrescence theory explains the developmental process, while the differentiation theory depicts its outcome in adult tooth morphology.
There are examples showing that the integration of several tooth primordia into a more complex single tooth operates quite commonly during ontogenetic development in actual species. For example, there are composed ‘tooth plates’ in lower vertebrates15,16 that develop during ontogeny from unicusped teeth that fuse at their bases (for a review see the ref.15).Very large and ‘double’ teeth are uncommon developmental anomalies that may arise from either the fusion of two tooth germs or the partial division of one germ.17–19 The integrated development (concrescence) of presumable ancestral tooth primordia has been observed during the development of the mouse incisor and molar (see below).14,20,21 Recently, the existence of fusion of tooth primordia to form a more complex tooth has been experimentally proven during development of the first molar (M1) in the mouse mandible.22 A tentative model of the regulation of the concrescence of tooth primordia has been proposed (Fig. 3c–e).
In conclusion, a general trend during evolution of the mammalian dentition was a reduction of tooth number and diversification of tooth shapes with increased tooth complexity in the cheek region. At the positions of suppressed teeth, vestigial teeth or tooth primordia may occur during ontogeny in extant animals – as the remaining manifestation of tooth reduction.4 The latent odontogenic potential of rudimentary tissues can be evoked (e.g. under pathological conditions), to give rise to an extra tooth or to pathological development at the position where a tooth was regularly present in ancestors.8,14,23–27 From this aspect, it is important to also take into account developing rudimentary structures in odontogenetic research to better understand the control at a molecular level, which physiologically leads to structure suppression; failure of this process may have pathological consequences not only in experimental models but also in humans.
Tooth development has been a focus of attention by researchers since the 19th century. Not only were two-dimensional (2D) sections used to study odontogenesis, but handmade models of 3D reconstructions were also constructed to help understand such a dynamic and complex process. Recently, computer aided 3D reconstructions have been employed to visualize the morphological aspects of developing structures.
The application of 3D reconstructions in odontogenetic research has a long history. Firstly, construction of solid models from serial sections was used. The contours of dental epithelium have been transferred onto plates of different materials: wax,28–34 glass,30,35 gelatine,36 synthetic resin37 or paper.38–40 The end of the 20th century brought new innovative trends in 3D modelling using computerization and graphical visualization of surfaces of developing teeth and adjacent structures. This has allowed increased effectiveness and precision of 3D studies. However, the principle of making 3D models remains similar to former manual techniques (Fig. 4d). The contours of structures are digitalized and further processed by computer, using special software to obtain virtual 3D models.
Figure 4.
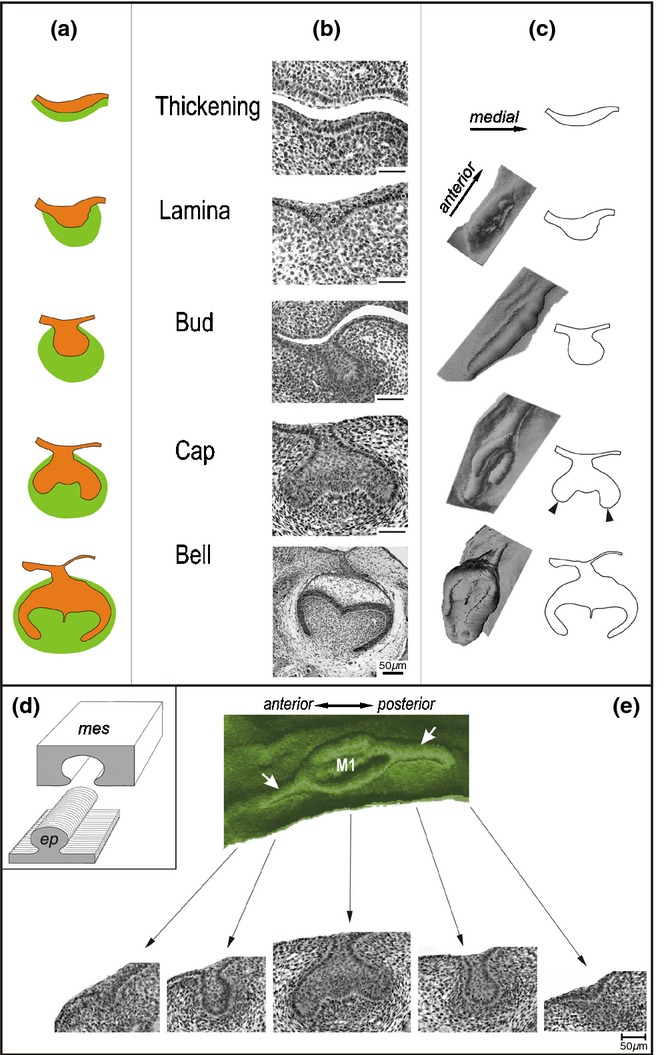
Stages of tooth crown development in mammals. The shape of the dental epithelium is shown in schemes (a), frontal histological sections (b) and computer aided 3D reconstructions (c – left column) based on drawings of the epithelium on sections (c – right column). Dental epithelium – orange, dental mesenchyme – green. The first morphological sign of tooth formation is a thickening of the oral epithelium, which later forms a dental lamina. The dental lamina gives rise to epithelial tooth buds with surrounding condensed mesenchyme. Then the cervical loop (arrowhead) starts to grow at the cap stage, while a dental papilla fills in a cap activity. At the early bell stage, cusp formation is initiated, and the cervical loop progressively elongates. For later development see Lesot et al.54 in this supplement. (d) 3D reconstruction of an epithelial structure (ep) is based on the epithelium contours on sections. It makes visible the epithelium surface adjacent to the mesenchyme (mes). (e) Changes in the shape of dental epithelium along the antero-posterior jaw axes. Green – 3D reconstruction shows the mesenchymal aspect of the dental and adjacent oral epithelium in the cheek region of the mandible in a mouse embryo at embryonic day 14.5. M1 – the cap of the first lower molar. Bottom, frontal histological sections show the shape of the reconstructed dental epithelium at corresponding locations along the antero-posterior sequence: lamina – bud – cap – bud – lamina. White arrows point to the dental mound.
During the past 20 years, computer assisted 3D reconstruction methods have helped greatly to reappreciate the complexity of early tooth morphogenesis in humans.25,41–44 As well, the classical, 40–50 year old descriptive data on mouse odontogenesis,45–48 have been revisited and updated due to combined analysis of sections and 3D reconstructions in detailed staged49 series of wild type mouse embryos.20,21,50–58 The 3D representations have also shown the co-existence of different morphologies of dental epithelium along the antero-posterior jaw axis (Fig. 4), as well as the dynamics in spatial distribution of mitoses/apoptoses,24,26,51,52,55,56,59–63 or the exact location of expression domains of signalling molecules in the developing tissues.22,58,59,64 Knowledge about mouse odontogenesis is currently being expanded by 3D representations of developing tooth anomalies in mutant mice.23,24,26,62,63,65–67 (For the outcome of 3D methods at later stages of odontogenesis, see the ref.54)
Here, data provided by computer aided 3D analyses of the development of the dentition will be reviewed with the aim of updating classical knowledge on early odontogenesis in laboratory mice and humans. Existence of rudimentary tooth primordia will be considered, including their developmental, evolutionary, and pathological aspects. Emphasis will be placed on morphological aspects of early development of the dentition, since the cellular and molecular aspects of odontogenesis are reviewed in other parts of this special issue. (For 3D aspects of tooth development at later stages see the ref.54)
Early Tooth Development
General aspects of tooth development
Developing teeth (tooth germs) in mammals pass through classical developmental stages named according to the shape of the dental epithelium on frontal sections: epithelial thickening, dental lamina, tooth bud, cap and bell stages (Fig. 4a–c). The epithelial thickening and dental lamina represent the anlage of the dentition (organ system) having developmental potential for giving rise to several primordia of individual teeth (organs). Then, individual tooth primordia develop from this common anlage by sequential morphogenesis through bud, cap and bell stages. The cap and bell staged epithelium is also called the enamel organ, since enamel-producing cells (ameloblasts) finally differentiate there. The mesenchyme is progressively specified to give rise to the dental papilla and dental sac, from which dentine-producing odontoblasts and cement-producing cementoblasts arise, respectively.
There are sometimes problems in distinguishing particular stages of early tooth development on frontal sections. In the present text, the early stages of tooth development are classified according to the criteria introduced in Fig. 5. The criteria have been formulated on the basis of correlation between frontal histological sections and corresponding 3D reconstructions.
Figure 5.
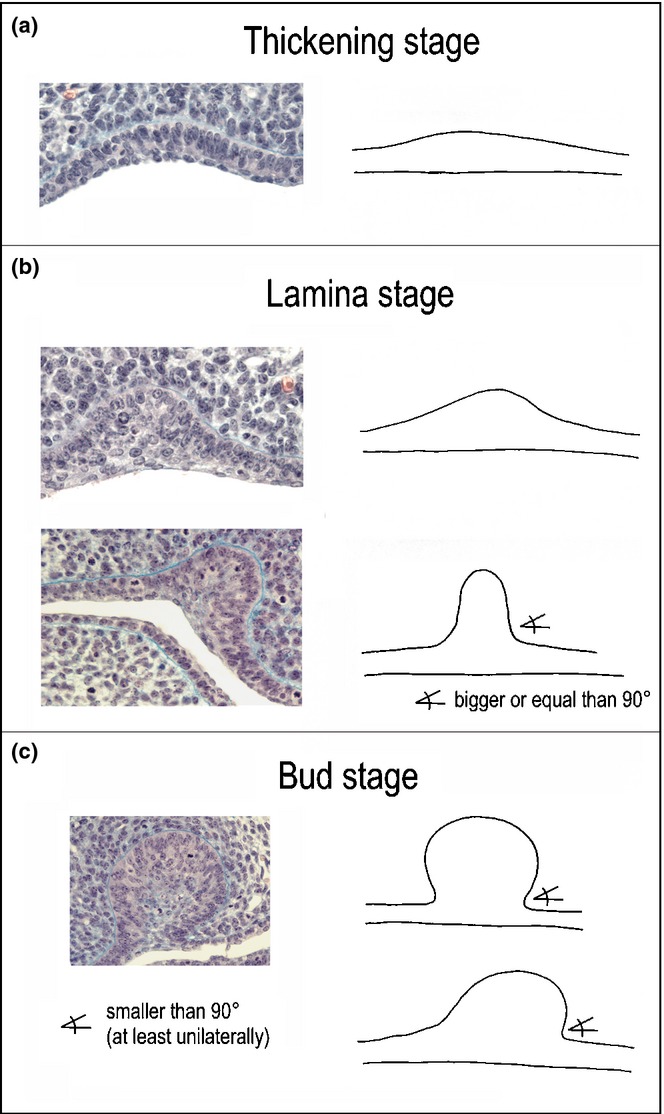
Distinction of early stages of odontogenesis on frontal sections in mouse embryos. The epithelial thickening differs from the adjacent oral epithelium by comprising a higher stratum of basal cells with prevalent orientation of the long axes of their nuclei perpendicular to the basement membrane. At the oral surface, one to two layers of flat cells are present, as elsewhere. The dental lamina is formed by folding of a thick stratum of columnar cells, and by the accumulation of smaller internal cells. Few layers of flat cells cover the oral surface. The angle included between the slope of the lamina and oral epithelium is larger than or equal to 90°. The tooth bud has a similar cell arrangement. At least one of the medial and lateral sides of the bud is vaulted, so that the angle between the bud and oral epithelium is smaller than 90°.
The terms ‘bud’, ‘cap’ and ‘bell’ fit with the ‘2D’ shape of dental epithelium on sections. However, 3D reconstructions have revealed that the 2D terms do not always express the real spatial shape of the dental epithelium (Fig. 4b–c). Among these stages, the identification of a tooth primordium at a bud stage appears to be the most difficult (compare to Fig. 10) – not only in the mouse8,50 but also in humans25,44 or field voles68 – see below the section under ‘Dental mound’.
Figure 10.
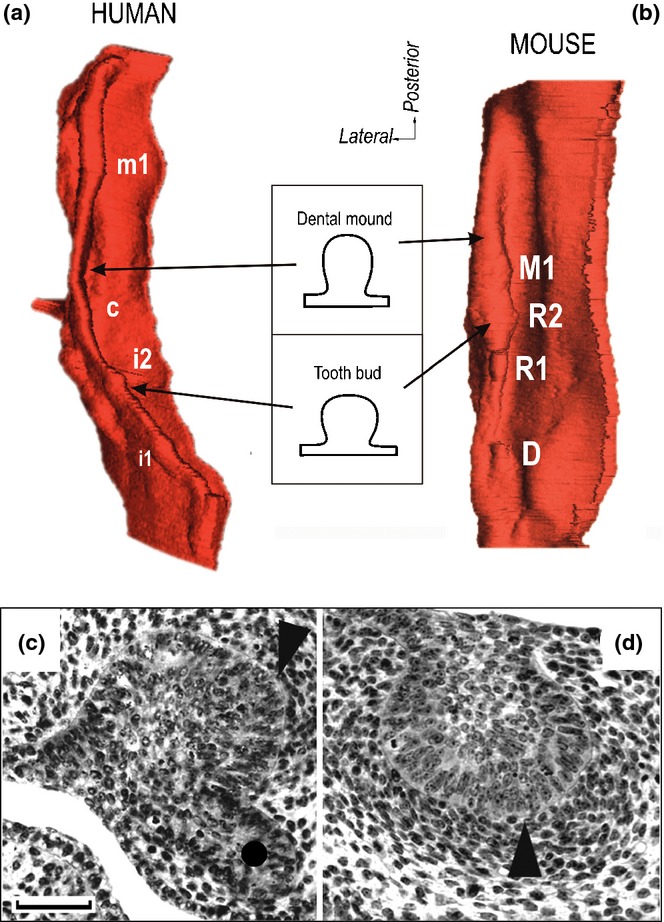
The tooth buds as swellings on a mound of dental epithelium. 3D reconstructions show. Mesenchyme-facing aspect of the dental and adjacent oral epithelium in the upper jaw in a 44–46 day old human embryo (a), and 13.5 day-old mouse embryo (b). The i1, i2, c and m1 – the swellings corresponding to the bud of the human deciduous upper first incisor, second incisor, canine and first molar, respectively. R1, R2 – the swellings corresponding to the rudimentary large buds in the posterior part of upper diastema. D – remnant of small diastemal buds in the anterior part of the upper diastema (compare to Fig. 16d). M1 – bud of the mouse upper first molar. (c, d) Frontal histological sections show the enamel knot (arrowhead) at the tip of the large diastemal bud R2 in mouse maxilla (c) and mandible (d) at ED13.5. A circle indicates the epithelium budding suggesting development of a successive tooth generation.14 Bar = 50 μm.
Moreover, the antero-posterior growth gradient leads to distinct changes in the shape of the dental epithelium along the antero-posterior axis in the cheek region. This implies that dental epithelium can exhibit different ‘stages’ of tooth development when its shape is followed on an antero-posterior series of frontal sections (Fig. 4e). This may lead to misinterpretation of the results of odontogenetic studies employing individual sections, if data on a position of the sections are not available. Appropriate positioning of molecular data regarding the whole tooth-forming region is very important, since they should be very specifically localized if related to the regulation of morphogenetic events.
Mouse odontogenesis as an experimental model in tooth developmental studies
Tooth development is usually investigated in rodents, and the mouse model has been selected by most laboratories. This model allows standardization of studies in animals with a defined genetic background. Furthermore, the quite short generation time of mice, and the small size of their teeth facilitate further analyses. Quite a large amount of data has already been accumulated, not only on tooth development in wild type mice, but also in mice with defined genetic alterations.
However, the mouse dentition is highly functionally and morphologically specialized (Fig. 2d) in comparison to the basic tooth formula in mammals, or when compared to the human dentition (Fig. 2). It comprises a continuously growing incisor, which is separated by a toothless diastema from a segment of three molars (Fig. 6a–b). This high specialization of the mouse dentition has been pointed out by critics of the rodent model. Surprisingly, these specificities of the mouse dentition appear to be a benefit for experimental studies on odontogenesis: it is very advantageous that the jaw of a mouse embryo allows studying and comparing the formation of a tooth with continuous/unlimited growth (incisor), the origin of a toothless diastema, and successive development of molars with limited growth (Fig. 6c). The continuously-growing teeth are now being studied closely for the presence of stem cells that support permanent cell renewal.69–71
Figure 6.
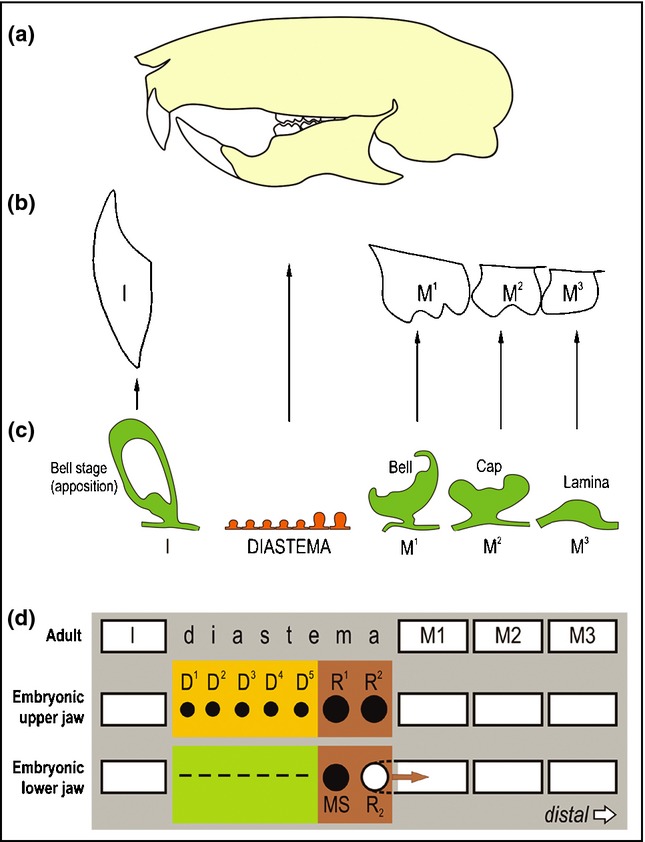
Three segments of the dentition in the mouse. I – incisor; M1, M2, M3 – the first, second and third molar, respectively. (a) A scheme of the skull of an adult mouse. The gnawing incisor and the 3 molars are separated by a toothless gap called a diastema in place of missing incisors, canine and premolars. (b) Scheme of the upper jaw dentition in an adult mouse. (c) The model of mouse odontogenesis provides an opportunity to investigate development of three different segments of the dentition, where different tooth primordia occur: a large incisor, prospective toothless diastema, and the three successively developing molars. The incisor is covered by enamel only on the anterior (labial) face (crown-like analogue), while cement covers the other side (root-like analogue). Due to the asymmetrical distribution of enamel, tooth abrasion is asymmetrical resulting in a sharp incisor margin. (d) Two parts of the mouse embryonic diastema according to the stage of odontogenesis arrest. Odontogenesis is blocked most strongly and/or early in the anterior part of the lower diastema (green), where it arrests at the epithelial thickening stage (dashed line). In the anterior part of the upper diastema (yellow), odontogenesis progresses to the stage of the dental lamina or small buds (D1–D5) before these structures are completely eliminated. The most advanced stage of tooth development is achieved in the posterior part of the upper or lower diastema (brown). Two large rudimentary buds develop there in each jaw (R1, R2 or MS, R2), before their development is stopped. The R1, R2 in the maxilla and MS in the mandible are transformed into epithelial ridges that are presumably later incorporated into the expanding enamel organ of the first molar (for a detailed review see the ref.14). The R2 bud in the mandible is incorporated in the M1 cap.22
Despite the presence of a toothless diastema in adult mice, rudimentary tooth primordia of the suppressed teeth are transiently distinct in the diastema region of mouse embryos (Fig. 6c–d). Comparison of odontogenesis in prospective toothless and tooth-bearing areas in the same jaw makes it possible to identify and compare control mechanisms essential for tooth growth, suppression, or revitalisation.24,27,60,72–81 The rudimental tooth primordia provide a natural tool to understand the mechanisms regulating tooth suppression in the course of ontogeny that can be extrapolated to phylogeny as well, and to test the possibilities of revival of rudimentary tooth primordia and controlled tooth regeneration.8,24,27
Temporo-spatial dynamics of tooth suppression in mouse diastema
Based on the displayed level of tooth development, the mouse embryonic diastema (the space between developing incisor and first molar) can be distinguished as having anterior and posterior parts.
In the anterior part of the lower diastema, odontogenesis has already stopped at the epithelial thickening stage (Figs. 2e and 6d). Similarly, the developing dentition in the chick embryonic mandible is aborted at the epithelial thickening stage.82 In the anterior part of the upper diastema, tooth development is stopped later – at a minute bud stage. These structures have been compared to tooth placodes in reptiles, and interpreted as rudimentary primordia of teeth inherited from the ancestors of mammals.14
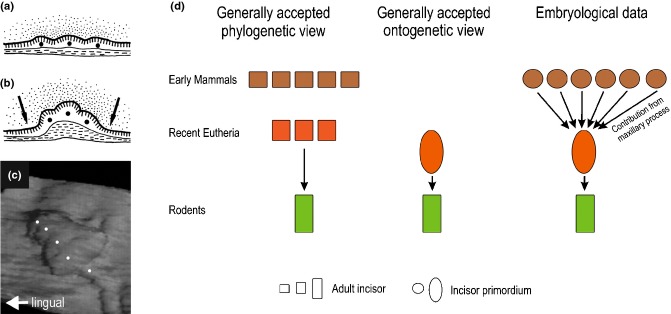
In contrast to the anterior part of diastema, development achieves a more advanced stage in the posterior part of both the upper and lower diastema. Two large vestigial tooth primordia consequently develop there at prenatal days 12 and 13, respectively, but their growth is stopped after they achieve a well-formed bud stage, and apoptosis concentrates in their epithelium. Then, both large rudiments (R1 and R2) in the upper jaw and the more anterior rudiment (MS) in the lower jaw transform into epithelial ridges connected with the anterior limit of the M1 cap. The more posterior rudiment (R2) in the lower jaw becomes incorporated into the anterior part of the arising M1 cap (Fig. 6d) (see also below). The large diastemal rudiments have been interpreted as rudimentary primordia of the premolars lost during mouse evolution (compare to Figs. 2 and 5).14,22,50,83
The large rudiments in the posterior part of the diastema can lead to a problem with interpretation of data from studies on mouse odontogenesis. The reason is that their development is anticipated in comparison to the M1 in both maxilla and mandible.50,57,60,84 This implies that the rudiments represent the most conspicuous structures in the cheek region during early stages (before ED14), while the M1 is delayed in development. That is why the two large diastemal rudiments at day 12 and 13, respectively, have been generally considered to be the M1 itself, and molecular data on early mouse odontogenesis have been automatically related to the regulation of the M1 formation instead of rudiment progression, arrest or incorporation into M1 (see below).
Chronological and biological staging of embryos
Staging of embryos is very important for designing experiments and obtaining reliable and standardized results. A detailed staging of individual embryos allows composition of a homogenous sample of embryos at a particular stage of developmental progress (e.g. for quantitative or comparative studies in wild type and mutant mice), or of ranking the embryos in a series of detailed step-by-step developmental stages to trace the dynamics of a specific event. There are methods for decreasing natural developmental variability of embryos between and within littermates (e.g. short-termed mating of mice), or ensuring a more detailed staging than the chronological one (e.g. by taking into account various morphological criteria of embryos).85–87 The wet body weight of mouse embryos can easily be used as a biological parameter to specify chronological stage in more detail. Moreover, embryonic body weight is a reliable indicator of prenatal ‘tooth age’, i.e. the stage of tooth development (Fig. 7).49
Figure 7.
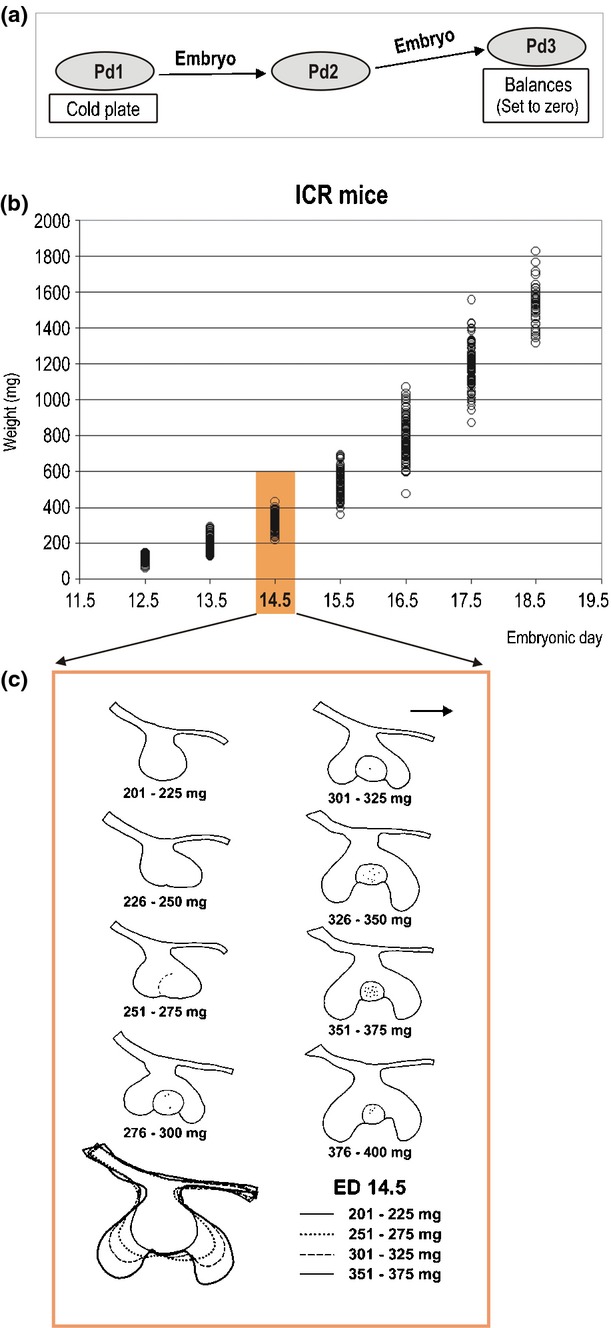
Determination of embryonic body weight and its correlation with tooth age in mouse embryos. (a) The whole mouse uterus is put in a Petri dish (Pd1) on a cold plate. Immediately after each individual embryo is dissected from the uterus, it is picked up at the waist, and its inferior part is gently touched several times on the bottom of the dry Petri dish (Pd2), to remove any excess of amniotic fluid on the body surface. (For the embryos after ED 14.4, a filter paper is put on the bottom of Pd2). Then the embryo is put on the Petri dish (Pd3) located on a balance set to zero. This weighing procedure takes just a few seconds. (b) The graph shows the distribution of the wet body weight in prenatal ICR mice during embryonic day (ED) 11.5–19.5. Each ring corresponds to one animal. (c) The correlation between body weight in milligrams (mg) and developmental stage (tooth age) of the first lower molar (M1) at ED 14.5. Contour drawings of dental epithelium on frontal sections document the central part of the M1 in different weight classes. The embryos have been randomly selected from each corresponding weight class. Note the step-by-step progress in tooth development according to increasing body weight. The margin of the enamel knot structure is indicated by a solid or dashed line. Black dots – apoptosis (modified according to the ref.49).
Morphological stages of early tooth development
Early tooth development is considered, in the present text, as the period from the initiation of tooth development up to the cap-staged enamel organ. This developmental process is regulated by continuous and reciprocal interactions between the dental epithelium (ectoderm origin) and mesenchyme (neural crest origin) in mammals. These interactions are mediated by the interposed basement membrane and involve diffusible signalling molecules that are linked to complex regulatory pathways.27,88–94
Previous experiments have shown that the neural crest cells migrating into the mandibular arch are tooth-unspecified before and during migration, and that the oral epithelium determines the site of prospective teeth.95 The epithelium from the mouse mandibular arch (at ED 9–11) can induce teeth in non-dental ectomesenchyme in both mice and birds.96,97 However, this odontogenic potential of the oral epithelium might be achieved, from the ectomesenchymal cells via migrating neural crest.98 Tooth development is initiated at specific positions, and the types of developing teeth are determined. A wide range of molecular factors is implicated in tooth initiation in the incisor and cheek region.27,91,99–101 Since a rudimentary anlage of the dentition is also present in the prospective mouse diastema, tooth development has to also be initiated there, before it becomes arrested (Figs. 2e and 6). It is possible that some published data on the initiation of tooth development may not concern the molar but rudimentary tooth primordia in the diastema (yellow and green segments on Fig. 6d).
During further tooth development, morphogenesis and histogenesis progress in parallel. All morphogenetic mechanisms are involved – differential mitotic activities, apoptosis, cell adhesion and cell migration (segregation), regulated in time and space. Tooth morphogenesis (morphodifferentiation) is a very dynamic process that progressively gives rise to a tooth primordium at bud, cap and bell stages. Histodifferentiation of the dental epithelium ends up with the formation of the inner dental epithelium (IDE), outer dental epithelium (ODE), and a stellate reticulum (SR) at the cap stage (compare to Fig. 16c). The stratum intermedium (SI) becomes distinct at the beginning of the bell stage.54 The histo-differentiation of dental mesenchyme includes several steps (Fig. 4b). Mesenchymal cells condense in proximity to the epithelial thickening and dental lamina. Then, the condensed cells become arranged concentrically around the tooth bud, cap or bell (Fig. 4b). The dental papilla does not arise from a part of condensed mesenchyme which is passively enveloped by the extending margins of epithelial tooth cap. Instead, the papilla exhibits different compartments depending on cell proliferation and migration. The majority of the dental papilla arises from a small population of highly proliferative mesenchymal cells adjacent to the dental epithelium at the cap centre.102 Specific arrangement of the mesenchyme surrounding the enamel organ results in formation of the dental sac with distinct inner and outer layer. Blood vessels start to enter the papilla mesenchyme of the mouse M1 at the cap stage,103–105 then nerves appear about three days post-natally.106–108 Nerve fibres have not been found to grow towards the small (‘D’) rudiments (Fig. 6) in the maxillary diastema.109
Figure 16.
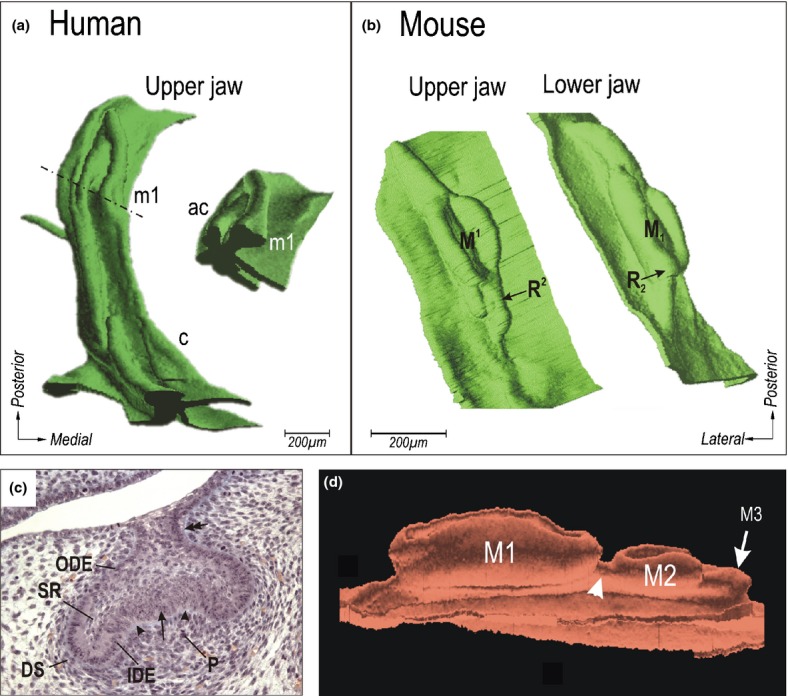
Cap stage of tooth development. (a) Human embryo (prenatal week 8) in 3D reconstruction. The dashed-dotted line indicates the position of the section shown in the insert; c and m1, cap-staged upper deciduous canine and first molar; ac, accessory budding of the vestibular epithelium. (b) The cap of the upper or lower first molar cap (M1 or M1 respectively) of the mouse at ED14.5. The arrow indicates the former posterior large diastemal rudiment in maxilla (R2) or mandible (R2). (c) Lower first molar in a mouse embryo at ED14.5 on a frontal histological section. Histo-differentiation results in the appearance of the inner dental epithelium (IDE), outer dental epithelium (ODE), stellate reticulum (SR), dental sac (DS) and dental papilla (P). The arrow points to the enamel knot, the arrowheads to the enamel grooves; the double arrow indicates the stalk of the enamel organ. (d) The 3D reconstruction shows the lower molar germs in a mouse at ED 17.5. The first (M1), second (M2) and third (M3) molar are at the bell, cap and bud stages, respectively. Note the enamel organs of the molars are not separated by a low oral epithelium, but are attached on and interconnected by the mound of dental epithelium (arrowhead).
Epithelial thickening
The first morphological evidence of development of the dentition is a thickening of the oral epithelium (Figs. 4a–b and 5a) which occurs on the oral surface of the facial outgrowths. Dental epithelial thickening differs from the adjacent oral epithelium by comprising more layers of taller columnar cells with the prevalent orientation of the long axes of their nuclei perpendicular to the basement membrane. At the oral surface, 1–2 layers of flat cells are present, as elsewhere.50
Epithelial thickening in human embryos
Epithelial thickenings already exist on not yet fused facial processes in five-week-old human embryos.110 The thickenings fuse as a result of fusion of the facial processes.111 3D reconstructions have revealed that the upper lateral incisor has a double origin, since it takes a contribution from two facial processes (medial nasal and maxillary). Such a composite origin can explain the high developmental vulnerability of the upper lateral incisor (absence, duplication, hypoplasia) in human populations, and particularly in cleft patients.43 A contribution of the maxillary outgrowth to incisor development has also been found in the mouse21 and the rat.112,113
Epithelial thickening in mouse embryos
In mouse embryos, a thickened epithelium occurs along the oral surface of the developing jaws. It is also present in the prospective diastema in the lower jaw.21 In the upper jaw, the thickened epithelium even covers the whole oral surface of the maxilla in the early stages and gives rise, not only to the dental lamina, but also to palatal ridges and oral vestibule. Based on this common origin during ontogenesis, teeth, palatal ridges and oral vestibule have been proposed to have a common precursor not only during ontogeny, but also during phylogeny – simple teeth in sub-mammalian vertebrates. This may explain why the metameric patterns of the palatal ridges and tooth cusps show close similarity along the antero-posterior axis of the oral cavity in some species (e.g. armadillo, horse).14,114
Dental placode
At the general embryological level, the term ‘placode’ refers to a morphological structure representing initial stage of development of sense organs (e.g. auditory placode) and sensory ganglia (e.g. trigeminal). The placodes give rise to various non-epidermal cell types.115 A placode is a specialized area of the cranial non-neural ectoderm; its cells undergo changes in shape resulting in thickening, invagination, and/or cell delamination.116
In odontogenetic research, the term placode has been used to refer to the initial stage of development of skin appendages such as teeth, hair, feathers, and scales.117 A dental placode has been described as a localized epithelial thickening from which a simple tooth develops in some non-mammalian species.118,119
Recently, the original morphological term ‘placode’ started to be identified with a functional aspect – the expression domain (signalling centre) of early tooth primordia in mice on whole mounts.120,121 However, combined analysis of whole mount in situ hybridization on sections and 3D reconstructions has shown that the corresponding structures at the place of expression ‘placode’ do not always exhibit a placode structure on sections (see the last section of this paper on reappraisal of the signalling centres during early mouse odontogenesis and related Fig. 19).
Figure 19.
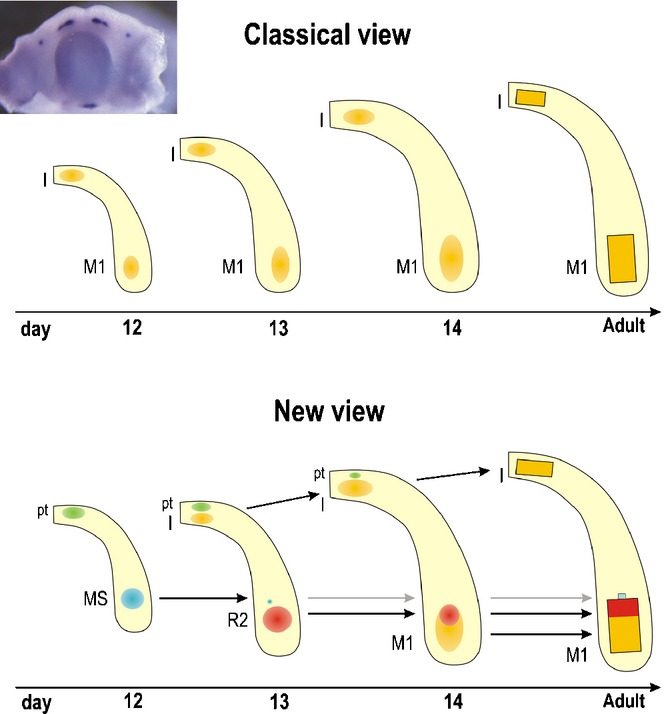
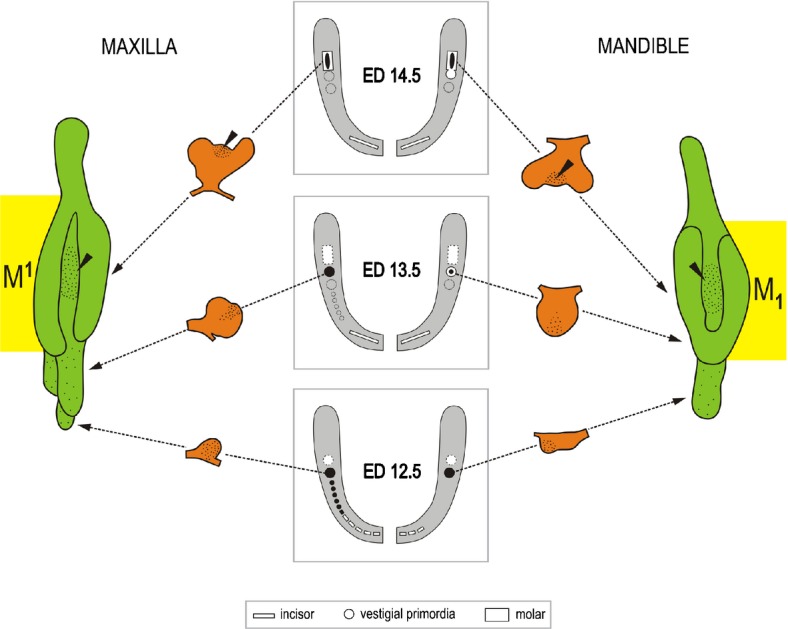
A scheme summarizing the correlation between the signalling centres and developing teeth in a mandible of WT mice. Insert: mouse mandible at ED12.5 – whole mount Shh in situ hybridization. Rectangles – functional teeth; round spots – Shh expression domains. The yellow symbols indicate functional teeth; green, blue and red round symbols indicate the rudiments. Classical view: a single Shh expression domain is generally presented in each incisor and cheek area of the mandible during early development and interpreted as a signalling centre of the developing functional incisor or M1. New view: Recent studies have documented sequential appearance of several temporo-spatial distinct Shh expression domains along the antero-posterior jaw axes. The earlier-appearing domains correspond to rudimentary tooth primordia in both incisor and cheek regions. In the incisor region, Shh is expressed in two temporo-spatially distinct areas. The earlier, anterior and superficial Shh expression (green) reflects the development of rudimentary pre-lacteal teeth (pt). The later, posterior and deeper Shh expression (yellow domain) is located in the germ of the functional incisor (I). A similar situation is also found in the upper incisor region (compare to Fig. 17).58,138 In the cheek region of mandible, three Shh signalling centres are sequentially patterned during early stages of development: the blue, red and yellow Shh domains indicate the signalling centre of the MS rudiment, R2 rudiment and M1, respectively (compare to Fig. 18). The adult M1 develops with the participation of the R2 diastemal rudiment (red rectangle);22 a minor contribution by the residuum of the rudiment MS (blue rectangle) is not excluded. The variable participation of the rudiments during M1 development has been proposed to result in variability of the anterior part of the adult M1.144 In the molar region, only the M1 is considered. That is why the region of the second and third molar is not presented in the scheme. These data supporting the existence of rudimentary structures are very helpful in searching for homologies of structures.164
To prevent misunderstanding, the term dental ‘placode’ will only be used in the present paper in its original morphological sense – to indicate an early tooth primordium which is formed by a local thickening of oral epithelium in sub-mammalian vertebrates.
Dental lamina
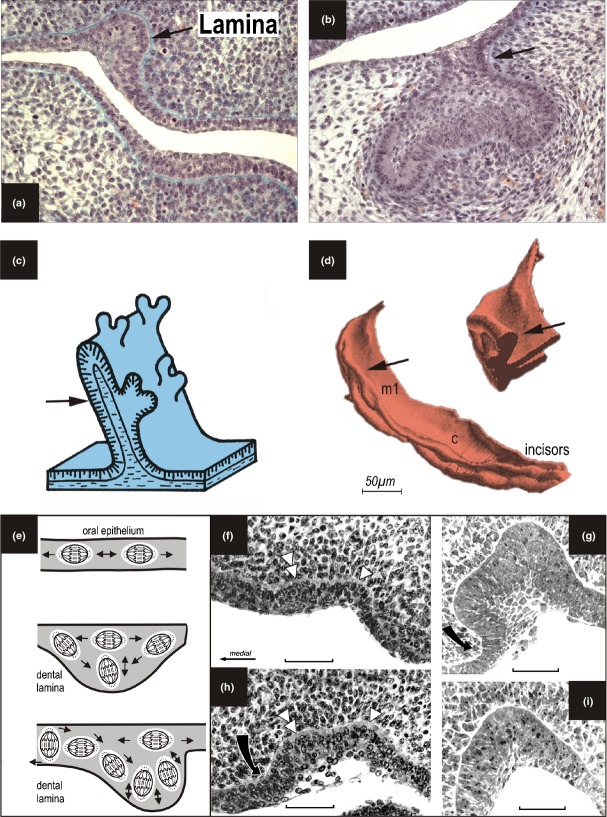
In the literature, the term ‘dental lamina’ may refer to different structures (Fig. 8a–d). In this text, a ‘dental lamina’ indicates a structure in mammals represented by a folding of dental epithelium. The folding is formed by a thick stratum of columnar cells; the prevalent orientation of the long axes of their nuclei is perpendicular to the basement membrane (Figs. 5b and 8a). The rising groove is filled by an accumulation of smaller cells (Fig. 8f–i).14,50 On the mesenchymal face, the medial and lateral slopes of the dental lamina show an angle equal to or larger than 90° with the adjacent oral epithelium (Fig. 5b).
Figure 8.
Different structures are called a ‘dental lamina’ in the literature. This term is used to refer to the structures indicated by an arrow: (a) early stage of tooth development; (b) the stalk of an enamel organ during later development; (c, d) the whole formation of the dental epithelium which is submerged into the mesenchyme and which bears developing tooth primordia in e.g. reptiles (c) or humans (d); (e) the proposed role of reorientation of mitotic spindles during origin of dental lamina:126 When the long axes of mitotic spindles are oriented in parallel to the basement membrane, the epithelium extends its surface. The long axes of mitotic spindles oriented perpendicular to the basement membrane result in an increase of epithelium thickness and dental lamina formation. (modified according to the ref.126) (f–i). Frontal sections of the maxilla in mouse embryos at ED 12.5. A small primordium in the anterior part of the upper diastema (f), the large posterior diastemal rudiment R1 at the dental lamina stage (h), R2 at an early bud stage (g), dental lamina formation in the molar area (i). The black arrow indicates the bulging of dental epithelium by the mejenchyme expanding medially to the forming dental anlage. Bar = 100 μm.
During dental lamina formation, the thickened epithelium invaginates into the adjacent mesenchyme and this involves multiple signalling networks.122 The boundary between the oral epithelium and the dental epithelium is set up through an interaction between SHH and members of the WNT family.123 Sonic hedgehog (Shh) expression is restricted to the localized thickenings of the oral epithelium that grow into the mesenchyme.124,125 In general, increase in mitotic activity is assumed to give rise to the dental lamina. However, measurement of mitotic activity during the formation of the dental lamina could not show any local increase in mitotic activity, but a preferential orientation of the mitotic spindles – perpendicular to the basement membrane126 (Fig. 8e).
Besides the above-mentioned mechanisms referring to an active ingrowth of the dental epithelium into the mesenchyme, another mechanism might also be implicated. Morphological data on mouse odontogenesis in the upper jaw suggest an active expansion (bulging) of the adjacent non-dental mesenchyme towards the oral cavity that can end up with a passive infolding of the whole sheet of the thickened dental epithelium14,114 (Fig. 8g–h; compare with Fig. 12a–b). During infolding of the sheet of dental epithelium into the mesenchyme, surface epithelial cells slide to fill in the forming groove47 (Fig. 8f–i), and may later be involved in the origin of the stellate reticulum.14 Local variations in the physical or mechanical stimuli from tissue microenvironment127 can also be implicated in the origin of dental lamina.
Figure 12.
The upper mouse incisor – phylogenetic and ontogenetic aspects. (a, b) Schemes show integration of several epithelial structures (black spots) during formation of the early bud of the functional mouse incisor. The morphology of these structures on sections is similar to the tooth placodes in reptiles, as described by Westergaard.118 (a) Dental epithelium at ED12.0. (b) Folding of the dental epithelial sheet forms a complex incisor primordium by an integrated (conjoint) development of original placodes at ED12.5. The arrows show the growth direction of the adjacent non-dental mesenchyme. Vertical or horizontal dashes indicate the layer of basal or superficial cells, respectively. (c) The placodes (white spots) give rise commonly to the incisor early bud in a 3D reconstruction at ED 13.5. (d) According to the generally accepted view, the number of incisors was progressively reduced during rodent evolution; the single incisor in rodents should correspond to the second incisor of placental mammals with unreduced incisor number (left scheme). Therefore, it is taken for granted that only one incisor develops in mice (middle scheme). However, embryological data document integrated development (concrescence) of 5–6 placodes that commonly give rise to the early bud of the upper incisor in mouse embryos; the most lateral placode takes its origin in the maxillary facial process (right scheme).20,21
Dental lamina in humans
According to embryological textbooks, the dental lamina is a U-shaped epithelial ridge in the embryonic upper or lower jaws arches; another U-shaped ridge, called vestibular lamina, runs in parallel and externally to the dental lamina (Fig. 9a). The vestibular lamina (the ‘lip-furrow band’ or ‘labio-gingival ridge’) is regarded as the origin of the oral vestibule, a free space between jaws bearing teeth on one side and the lips or cheeks on the other side.
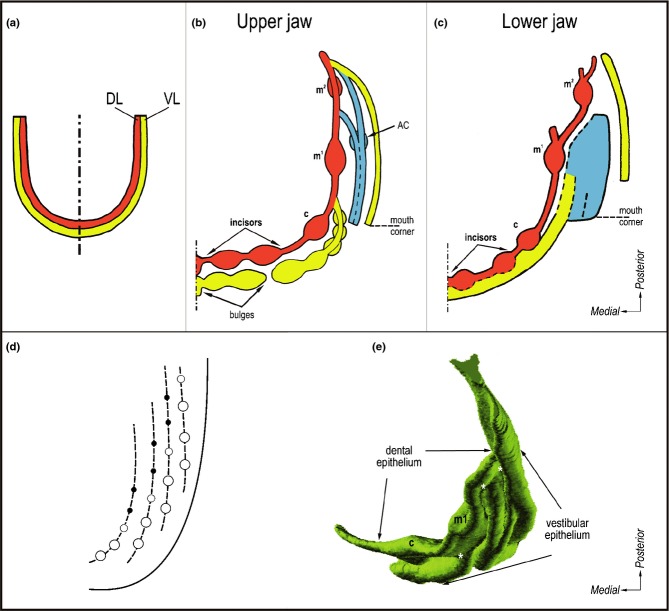
Figure 9.
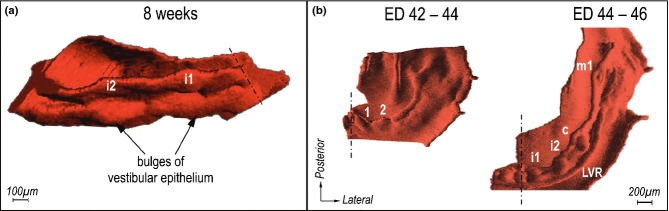
Developing dentition and oral vestibule in humans and their comparison with developing teeth in fish. (a) Embryological textbooks present two parallel U-shaped ridges in human embryos: DL – dental lamina (giving rise to the primary dentition) and VL – vestibular lamina or labio-gingival band (where the oral vestibule will form). (b-c) Summarization of data by 3D reconstructions showing that no continuous vestibular lamina exists. Instead, a set of discontinuous epithelial structures (ridges and bulges) transiently occurs externally to the dental epithelium. Red – dental epithelium. Yellow or blue – vestibular epithelium. c, m1, m2 – the deciduous canine, first and second molar, respectively. AC – the accessory cap-shaped structure (modified according to the refs25,44). (d) The schematic pattern of tooth rows (‘Zahnreihen’) in fishes. The empty rings and black spots indicate the older and younger teeth respectively, new teeth are formed at the posterior end of each Zahnreihen (modified according to the ref.161). (e) Dental and vestibular epithelia in an 8-week-old human embryonic maxilla in a 3D reconstruction viewed from the mesenchymal aspect. Note the reiterative fusions (white asterisks) between the dental epithelium and particular ridges of the vestibular epithelium. c, m1 – the deciduous canine and the first molar, respectively.
In embryological textbooks, three concepts can be found concerning the developmental relationship between the dental and vestibular lamina: (1) separate origin; (2) common origin; or (3) common origin anteriorly and separate origin posteriorly. These different concepts might result from observations made by different authors on different jaws (upper or lower), different jaw regions (lip or cheek region), and in human embryos at different developmental stages. Recent combined analysis of histological sections and 3D reconstructions has shown that the situation is more complex.25,44 Firstly, there is no continuous U-shaped dental or vestibular lamina in the early stages of odontogenesis in human embryos. Furthermore, there is no general scheme for early development of the dentition and oral vestibule which would contemporaneously fit with all the upper and lower jaws, and their anterior and posterior regions (Fig. 9b–c). The dental and vestibular epithelia have a common origin in the lip region of the mandible. In the upper jaw, the dental and vestibular epithelium originate separately, but there are multiple fusions between them. The situation is less complex in the cheek region of the oral cavity, where the oral vestibule arises laterally to the dental epithelium in both jaws (Fig. 9b–c, e).
The 3D data have shown that the epithelium referred to as the vestibular lamina in embryological textbooks is, in fact, represented by a complicated arrangement of epithelial structures, including bulges and ridges (Fig. 9b–c, e).25,44 On initial examination, the embryonic pattern of the dental and vestibular epithelia in human embryos resembles the pattern of rows of primitive teeth seen in lower vertebrates (Fig. 9d).8
The complex development of the vestibular epithelium might help to explain some pathologies (e.g. cysts, odontomes) located externally to the dentition. Part of the alveolar cysts that are seen in newborns128 can take their origin in the residua of the epithelial structures that occur during oral vestibule development. The existence of a latent odontogenic potential of the tissues of the oral vestibule is supported by the finding that a tumour of dental epithelium origin develops in oral vestibule in about 20% of ameloblastin-null mice; the tumour cells express specific enamel matrix proteins (amelogenin, enamelin, tuftelin).129
Dental lamina in the mouse
In the mouse, the dental epithelium does not form a continuous dental lamina. A separate infolding of the thickened dental epithelium occurs in the incisor region and in the cheek region. In the cheek region, a dental lamina is present in the posterior part of the diastema (large rudiments) and in the molar region. The dental epithelium is lower in the anterior part of diastema. In this area, the small D1-D5 rudiments or epithelial thickenings appear in the upper or lower jaw, respectively (Fig. 6c). In the posterior direction, the height of the dental lamina gradually decreases so that an epithelial thickening is only present in the very posterior part of the oral cavity on both jaws.
The phenomena observed in humans – fusion between the dental and vestibular epithelium and different origins of the oral vestibule between the lip and cheek region have also been reported in mice.14,114
All the above data indicate a close developmental relationship between the vestibular and dental epithelium during ontogenesis. Moreover, an evolutionary relationship might exist between the formations of the vestibular epithelium in mammals and the earliest generations of teeth in reptiles.8
Tooth bud
A tooth bud is characterized on sections by a stratum consisting of larger (mostly columnar) cells at the basement membrane and smaller cells in the centre (Figs. 5c and 10). These two cell types express different cadherins.130 At least one of its medial and lateral mesenchymal faces exhibits a protrusion showing an angle smaller than 90° with the mesenchymal face of the adjacent epithelium (Fig. 5c).
Epithelial derived organs (epithelial appendages) arise from buds by budding morphogenesis.131 The bud patterning and further growth are regulated by reaction-diffusion processes that produce spatial variation in growth-activators and growth-inhibitors. The branching, which appears during further development of more complex structures (e.g. lungs, glands), only represents a variant of budding.131–133
Tooth buds are assumed to result from a local increase in mitotic activity of epithelial cells. In vitro experiments have shown that SHH acts as a mitogen (growth activator) when tooth buds arise.27,123,125,134 During odontogenesis, the interaction between growth activators and inhibitors can control the patterning and development of the rudimentary and regular tooth primordia in the mouse14,84 and spacing and growth of cusps in mouse molars.135,136 A reaction-diffusion model has been proposed recently, in which WNT, SHH and Sostdc1 act as activator, mediator and inhibitor, respectively, and their interaction can generate the tooth pattern of a wild-type mouse and explain the various tooth patterns produced experimentally.137
Dental mound
When considering a tooth bud, one would expect it would emerge in 3D as an isolated bulbous structure protruding (like a mushroom) from a flat ground of oral epithelium. However, only the incisors in the mouse are reminiscent of this shape (compare to Fig. 17). The other embryonic teeth in mice and humans that show a bud shape on sections appear as local swellings on a mound of dental epithelium in 3D (Fig. 10a–b). The dental mound is quite elongated along the antero-posterior course of the jaw (Figs. 4 and 10). The problem is that the epithelial mound, as well as tooth swellings on it, all exhibit a bud-shape on frontal sections; they only differ by size on sections.14,25,50 For these reasons, the anterior and posterior boundaries of a tooth germ are not yet distinct at the bud stage, and only start to be clearly apparent after the tooth reaches the cap stage.
Figure 17.
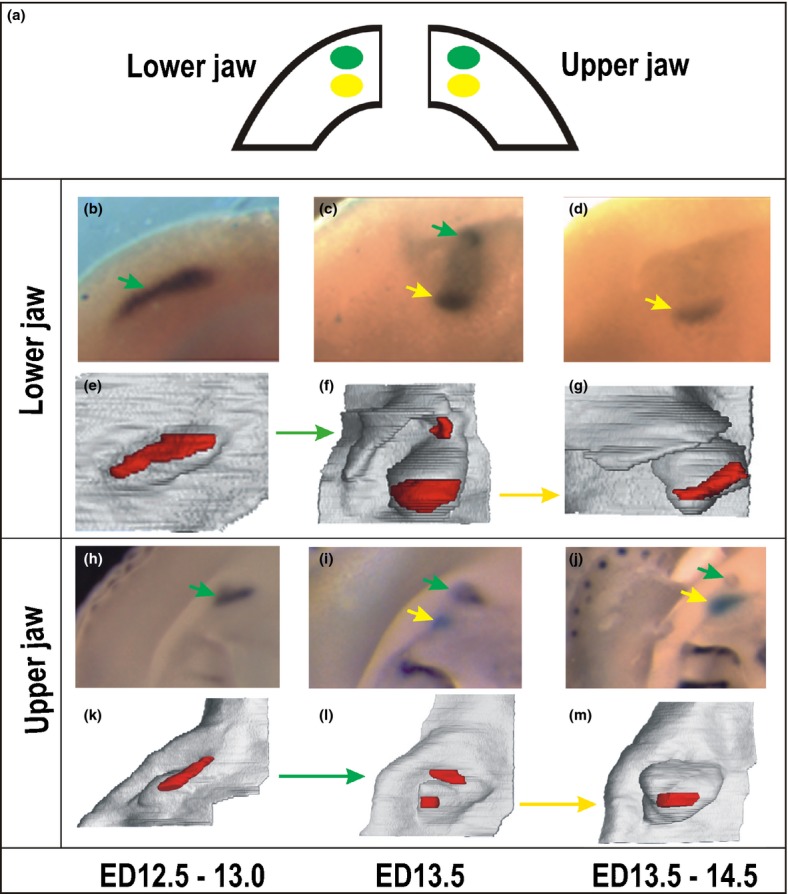
The tooth primordia in the prospective incisor region of wild type mice. Two generations of the Shh expression domains (a). Their sequential development is documented on the hybridized lower (b–d) and upper (h–j) jaws and corresponding 3D reconstructions (e–g and k–m). Two regions of the Shh expression located antero-posteriorly develop sequentially in each quadrant of the upper and lower jaws in mice. These domains reflect the sequential development of two generations of tooth primordia. The earlier-appearing anterior and more superficially located expression (green arrow) corresponds to the rudimentary prelacteal tooth development. The later-appearing, deeper and more posterior Shh expression domain (yellow arrow) is located in the central part of the germ of the functional incisor.58,138
The term ‘dental mound’ has been introduced to solve a terminological discrepancy at the early stages when referring to the epithelial ridge, which exhibits a bud shape on sections.8,25 Such a structure is usually also called a ‘dental lamina’ in papers. In the present text, the term ‘dental lamina’ is best used to indicate a stage of tooth development in mammals, which has not yet exhibited a bud shape on sections (Figs. 4 and 5).
Tooth bud in humans
The buds of developing teeth of the primary dentition start as swellings on a mound of dental epithelium (Figs. 10a and 11). An interesting aspect can be observed in the lip region of the upper jaw: in parallel to the tooth buds, bulges of the vestibular epithelium emerge suggesting a relationship between the regionalization (segmentation) of the dental epithelium and the vestibular epithelium.25 A parallel segmentation of the dental and vestibular epithelium also exists in the lower lip region in human embryos44 (Fig. 11).
Figure 11.
Incisor region of human embryos in 3D reconstructions. (a) A parallel regionalization of the dental and vestibular epithelium in the upper incisor region. On a mound of dental epithelium, there are distinct swellings corresponding to the first (i1) and second (i2) deciduous incisor at the bud stage. Externally to each swelling, a bulge of vestibular epithelium protrudes against the mesenchyme.25 (b) Differentiation of the incisor primordia and labial vestibular ridge in the lower incisor region. The bulges (1, 2) represent a common origin of the dental and vestibular epithelium. From the lingual and labial part of each bulge, the deciduous incisor and vestibular epithelium differentiate, respectively. The vestibular epithelium finally forms a labial vestibular ridge (LVR).44 c, m1 – the deciduous canine and the first molar. The middle line is dot-dashed.
Tooth bud in the mouse
The bud of the lower incisor starts to be distinct at embryonic day (ED) 12.5 and becomes clearly apparent at ED13.5 (compare to Fig. 17). The incisor bud develops posteriorly to the area, where a thickened epithelium and its Shh expression domain start to be present about ED11.5. This thickening gives rise to 2–3 distinct epithelial bridges that enter the posteriorly-adjacent incisor bud and interconnect it with the vestibular lamina.58 In contrast, the vestibular lamina does not develop as a prominent structure in the upper incisor region. There is also an area of thickened epithelium where six epithelial anlagen have been documented at about ED12.0, and hypothetically related to the primordia of teeth inherited from the ancestors of mammals. This whole epithelial area folds into the mesenchyme and takes part in the formation of the early bud of the upper functional incisor (Fig. 12a–c). From this bud, epithelial projections extend anteriorly20,21 that are reminiscent of the epithelial bridges in the mandible. Both the epithelial projections in the upper jaw and the epithelial bridges in the lower jaw accommodate a developing rudimentary tooth.58,138 This minute tooth has been described as a rudimentary ‘milk’ or ‘deciduous’ incisor in the upper and lower jaw in mice139,140 and rats.4 This minute tooth is similar, by its structure, origin (directly from the epithelial thickening) and location (externally to functional teeth), to the first-formed primitive teeth in sub-mammalian species (for a review see the ref.8), and so it has been classified as a ‘prelacteal tooth’.14 The term ‘prelacteal’ (pre-milk) dentition has been introduced to indicate rudimentary tooth primordia or minute teeth located externally (labially/buccally) to the primary dentition in mammals.6,141–143
In the mouse embryonic diastema corresponding to the region between the incisor and M1 germs, the temporo-spatial dynamics of tooth bud development differs depending on their location (Fig. 6d). Once the small ‘D’ diastemal buds appear in the anterior part of the upper diastema, they become immediately affected by epithelial apoptosis and soon they are eliminated entirely. In the anterior part of the lower diastema, tooth development is stopped at the epithelial thickening stage.
In the posterior part of the embryonic diastema and in the molar region (Fig. 6d), the temporo-spatial dynamics of bud formation is rather complex. The tooth buds appear in three consecutive periods in an antero-posterior sequence: the first large diastemal bud, the second large diastemal bud, and the M1 itself (Fig. 13).50,84 This has been experimentally proven in the lower jaw22 (compare to Fig. 8d–f). The emergence of tooth primordia is followed by three consecutive waves of apoptosis affecting the first diastemal bud (ED12.5), the second diastemal bud (ED13.5), and the central part (EK) of the M1 cap (ED14.5), respectively (Fig. 14). However, this apoptosis does not totally eliminate the respective tooth primordia, but just plays a part in the growth arrest (see below). It implies that as the growth of the dental epithelium progresses sequentially in the posterior direction, it is stopped sequentially from the anterior side.74
Figure 13.
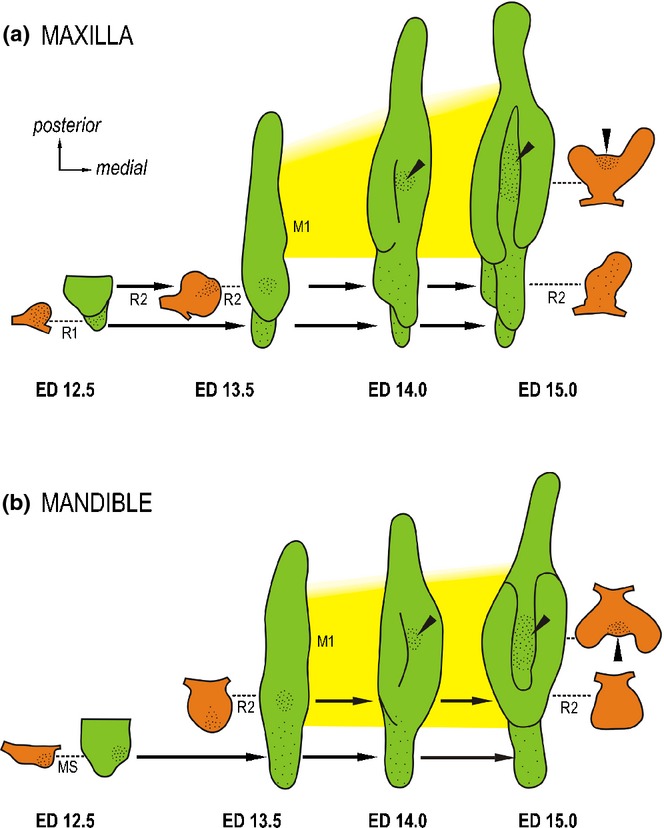
Dental epithelium of the cheek region of mouse embryos. Dental epithelium in projection drawings of 3D reconstructions (green) and of frontal sections (orange) localized according to dashed lines in the appropriate 3D reconstruction. Note the sequential progress of growth of dental epithelium in a posterior direction. Consecutive appearance of two large rudimentary diastemal buds R1/MS and R2 is followed by the first molar (M1 – yellow field). The large rudimentary buds represent the most conspicuous structures in the cheek region before embryonic day (ED)14.0. Epithelial apoptosis – black dots. Black arrows suggest the final location of the rudimentary buds. (a) While the posteriorly situated M1 is still at the lamina stage, diastemal R1 and R2 buds reach maximum development at ED12.5 and ED 13.5 respectively, which is followed by growth arrest and apoptosis accumulation in the dental epithelium. Then the former buds become transformed into epithelial ridges fusing posteriorly with the first molar.50 (b) The anterior rudiment (MS) is the most conspicuous structure until ED12.5. Then it becomes affected by apoptosis and is transformed into the epithelial ridge. A large rudimentary bud R2 appears as a swelling in 3D at ED13.5. Apoptosis only transiently affects the tip of the R2, which becomes incorporated into the anterior end of the M1 cap.57 The growth of the upper and lower first molar is delayed compared with the diastemal rudiments. As the M1 cap develops, the third episodic concentration of cell death appears in its enamel knot (arrowhead) (modified according to the ref.84).
Figure 14.
Three episodes of apoptosis in the dental epithelium in the mouse cheek region. Programmed cell death by apoptosis plays a part during formation of the mouse upper diastema and in the development of the cap of the first molar. The dashed line interconnects the most prominent tooth primordium (on a scheme of a jaw arch at each ED12.5, 13.5 and 14.5 with its projection drawing on a frontal section). The dashed arrow shows the final location of the structure in the 3D reconstruction (the dental epithelium in the cheek region of mouse embryos at ED 15.0). The disappearing or emerging tooth primordia are delineated by dotted or dashed lines, respectively. The rudimentary tooth primordia develop and then regress sequentially along the antero-posterior (bottom-up) direction. The third concentration of apoptosis appears in the enamel knot (arrowhead). (modified according to the ref.74).
This dynamic brings a problem: in the early stages, the large diastemal (premolar) buds in the upper and lower diastema are the most conspicuous structures in the cheek region of the jaw (Figs. 13 and 14). However, their growth is soon stopped as a result of decreased proliferation and increased apoptosis, when compared to the M1 epithelium.24,144 Then the molar region starts to predominate. This explains why the large rudimentary buds have been erroneously considered to be the M1 itself at early stages (ED 12.5 and 13.5). Consequently, the molecular data co-localized with the rudiments have solely been related to the progressive development of M1, despite the fact that some of them might be implicated in the control of growth arrest and onset of apoptosis in the rudiments.22
The fate of the large diastemal rudiments varies depending on their position. The posterior diastemal bud in the mandible stops its autonomous development and becomes incorporated into the M1 arising cap; the kinetic of this process has been followed by time lapse after DiI micro-injection and has documented the integration and common development (concrescence) of the diastemal rudiment (called R2) and M1.22 In contrast, the two rudimentary buds in the maxilla and the anterior diastemal bud in the mandible are transformed to epithelial ridges after their growth stops and apoptosis accumulates in their epithelium (Figs. 13 and 14). The ridges, representing residual rudiments, fuse posteriorly with the M1 cap.50,51,57,77 They can be implicated in the growth of the M1 enamel organ at later stages.23,51
The large rudimentary diastemal buds possess their own signalling centre.22 Very transiently, a rudimentary enamel knot (rEK) structure is present at the tip of the posterior diastemal bud R2 (Fig. 10c–d). However, the rEK is a specific structure of the rudiment and it does not represent a precursor of the so-called primary enamel knot of the M1 (pEK), see below and compare to Fig. 6c.
From the early bud stage, the control of tooth development shifts to the mesenchyme which can induce the dental epithelium to form an enamel organ.95,145,146 Several groups have investigated the genetic pathways that may intervene in the control of tooth bud development.27,88–94 The main difficulty in interpretation is due to the interplay of different pathways and also to the existence of functional redundancies between signalling molecules or/and between their membrane receptors. It is also possible that part of the molecular data in the literature on M1 at dental lamina and bud stages might concern the large diastemal buds (see above), where not only progressive development, but also growth arrest and programmed cell death by apoptosis, should be regulated at molecular level.14,22,74
The development of functional teeth in mouse mutants also often arrests at the bud stage.88,100,147 It is not known why such a developmental arrest is so stage-specific. Perhaps this problem can be solved with the help of a natural model of physiological blockage of tooth development in the diastemal tooth primordia at a bud stage.
Supernumerary tooth can develop from diastemal buds in premolar region
A supernumerary tooth develops in some mutant mice in front of the first molar (for recent survey see27,78). Origin of this extra tooth in mouse mutants has been explained by a revitalization and autonomous development of a rudimentary diastemal primordium inherited from ancestors, which manifests its latent odontogenic potential (Fig. 15).14,23,24,27,65,66,77,78,148,149 A similar mechanism might explain the origin of an extra incisor in Sprouty mutant mice.26 The ‘revitalization’ is characterized by changes in cell proliferation and apoptosis. In wild type mice, the growth of the rudiments stops and proliferation is decreased and apoptosis increased in their epithelium, when compared to the M1.144 In comparison to wild type mice, revitalization of diastemal buds in Spry2 or Spry4 null mice, where the supernumerary tooth develops, is accompanied by higher proliferation and lower apoptosis in the epithelium, which is similar to that seen in the M1.24,144
Figure 15.
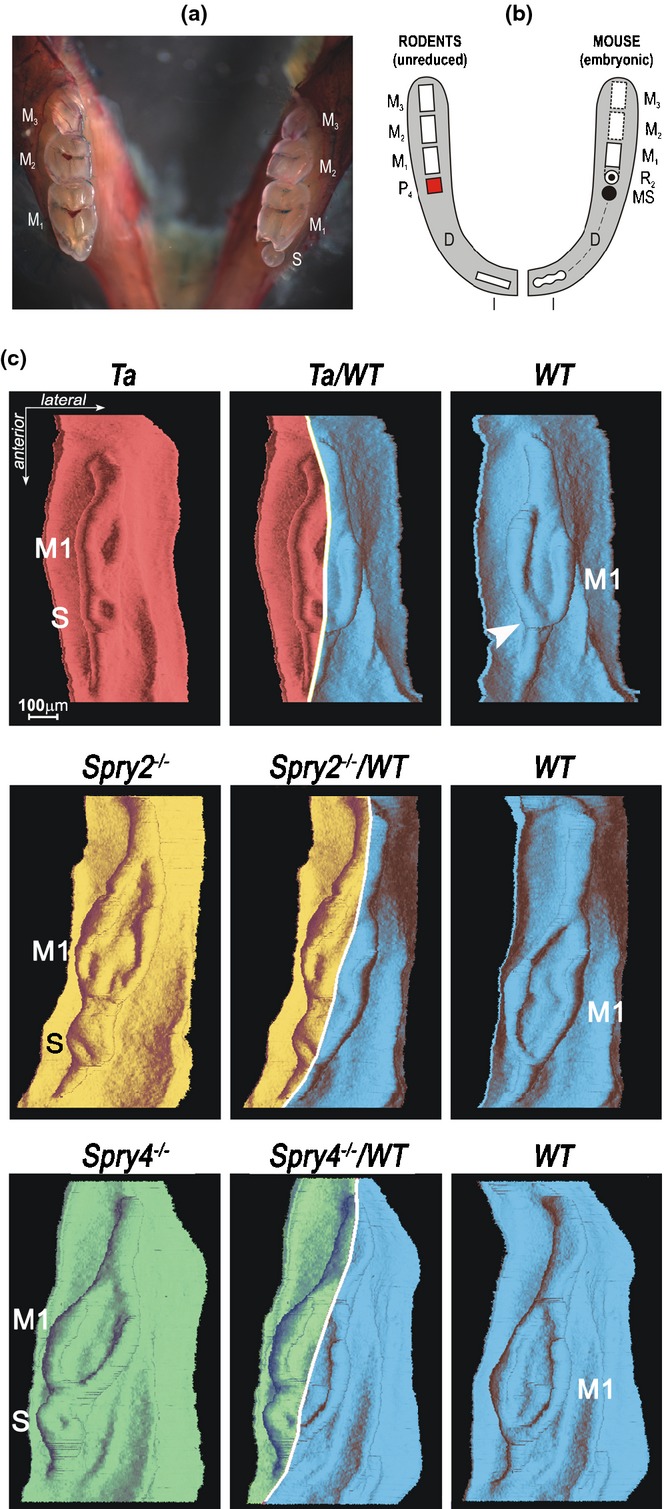
A supernumerary cheek tooth in a mouse mandible. (a) Unilateral occurrence of a small supernumerary tooth (S) in the mandible of a Tabby heterozygous mouse. The presence of S is accompanied by a reduction of the anterior part of the adjacent first molar (M1), when compared to the normal situation on the contra-lateral side of the same animal.23 (b) It has been hypothesized that the S is homologous to a premolar (P) lost during mouse evolution, and can be considered as an atavistic tooth.8, 65,148, Occurrence of atavistic teeth at the place of suppressed teeth of remote ancestors is also known in other species of mammals (e.g. compare to Fig. 1n).162,163 (c) 3D reconstructions of the dental and adjacent oral epithelium in the cheek region of the mandible of mutant Tabby (Ta), Spry2-/-, Spry4-/-, and wild type (WT) mouse fetuses at ED15.5. The mutant and the corresponding WT specimen have been coupled to exhibit not only a similar age (in ED) but also a similar body weight (in mg). Such detailed stage matching shows that the total length of dental epithelium is similar in the mutant and corresponding WT mouse. The graphic chimera (middle column) demonstrates the supernumerary tooth develops at the place corresponding to the anterior part of the M1 cap in WT embryo, where the large diastemal rudiment (R2) has been incorporated at an earlier stage (arrowhead).24,65,144
Mice developing a supernumerary tooth are very useful in helping to solve questions about the molecular control of tooth suppression/revitalisation. Several pathways are involved in the specification of dentate versus toothless areas, and the control mechanisms might vary along the antero-posterior axis of the diastema.24,27,72,73,75–79,81,149 Restriction of Shh signalling appears to be a key event implicated in the rudiments’ regression,27,75 and SHH and FGF4 production maintained in a diastema bud in Spry null mouse embryos enable it to develop into a supernumerary tooth.149
The possibility of reactivating the latent tooth-forming potential of odontogenic tissues raises exciting possibilities for controlled tooth regeneration.8,24,27,74,78,150
Tooth cap
At the morphogenesis level, transition from bud to cap is characterized by growth of the cervical loops and by specific differentiation of the dental epithelium where the enamel knot (EK) appears (Fig. 16c). This structure has been known for more than 100 years (for review see Butler151). The EK has been suggested to play a role in tooth cusp formation151 which has been reconsidered more recently with different interpretations.51,53,61,152,153 For further details concerning specific morphogenesis and histogenesis and their cellular and molecular mechanisms at the cap stage of tooth development see Lesot et al.54 in this supplement.
The EK is formed by a clump of epithelial cells at the centre of the epithelial cap (Fig. 16c). In the EK of human and mouse teeth, cell death has been reported154,155 and later specified as apoptosis in the mouse.51,156 According to previous authors, the EK arises at the place of junction (fusion) between two rapidly ensuing tooth primordia in the medio-lateral (bucco-lingual) plane. Such a junction has been documented by the formation of specific structures (the enamel knot, navel and septum) in mammals,13,151 and can also explain the concentration of apoptosis in the EK arising at the junction place.51
At the molecular level, the EK at a cap stage of M1 (the so-called primary EK – pEK) is presented as a signalling centre regulating elongation of the cervical loop and cap formation.59,136,153,157 According to the concept of budding morphogenesis of epithelial appendages,131 the development of the cervical loop is reminiscent of the branching morphogenesis of the epithelial bud in lungs.14 Growth inhibition at the bud tip precedes the epithelial budding adjacent to this inhibition area. Similarly, a growth arrest at the tooth bud tip, where the pEK will appear at a cap stage (Fig. 7c), anticipates the epithelium budding resulting in cervical loop formation.14,84
However, the cervical loop can also form in the absence of the adjacent EK, as it does in the posterior (talonid) part of the mouse lower M1.53 On the other hand, the formation of the EK is not always followed by development of the cervical loop. This can be observed at the tip of a bud of the posterior large diastemal rudiment (R2) in both the upper and lower jaws: although the EK is present, a typical cervical loop does not develop there (Fig. 10c–d). Despite these differences in tooth morphogenesis, a similar set of genes is expressed in the EK of the rudiments (rEK) as in the cap of the lower M1 (pEK).22 These data suggest that the first role of the EK signalling might not be to govern growth of the cervical loop (i.e. tooth cap formation), but rather to regulate growth at the tip of a tooth bud that should be stopped before epithelial branching and cervical loop development.14
In humans, the caps of deciduous teeth start to develop during prenatal week 7, being distinct one week later (Fig. 16a). In the mouse, the cap of the first molar (M1) is initiated at ED14.0–14.5 (depending on the mouse strain) (Fig. 16b). However, there is a difference in the manner in which the upper or lower M1 cap arises in mice (Fig. 16b). While the upper M1 cap is firstly closed at the posterior side and remains open anteriorly51; the opposite occurs in the lower M1.56 The difference at the anterior limit of the M1 between the upper and lower jaws may result from different interaction between the M1 and the most posterior diastemal rudiment (R2). In the upper jaw, the R2 rudiment becomes a component of the epithelial ridge in front of the M1 cap, which remains widely opened anteriorly50 because its lateral and medial cervical loop are not interconnected51 (Figs. 13a and 16b). In contrast, the R2 is incorporated anteriorly into the M1 cap in the mandible22 and so is involved in its anterior enclosure (Figs. 13b and 16b). It is still unclear to what extent the differences in development and fate of tooth primordia between the maxilla and mandible might be influenced by the differences in molecular control of tooth patterning between the upper and lower jaws that have been observed at earlier stages.99,158,159
The tooth caps originate from swellings (buds) on the epithelial mound. This implies that the enamel organs of developing teeth are not separated by gaps of low oral epithelium, but interconnected by a mound of dental epithelium protruding against mesenchyme (Figs. 10, 11 and 16d).
Reappraisal of the Shh signalling during early stages
3D reconstructions have enabled updating of our knowledge on the spatial morphology of developing teeth, and they have become a standard tool for investigating morphogenesis and the temporo-spatial dynamics of morphogenetic processes (proliferation, apoptosis). The combination of molecular and 3D methods applied in the last decades22,58,59,138 represents a new and very efficient tool for understanding developmental processes. This combination provides for spatial positioning of molecular events in developing structures and their correlation with the temporo-spatial dynamics of morphogenesis. Recently, such an approach has allowed reappraisal of early development of the incisor58,138 as well as molar22 in the mouse model of odontogenesis (Figs. 17–19).
Figure 18.
The tooth primordia in a cheek region of the mouse embryonic mandible. Frontal histological sections show the three sequentially signalling structures: (a) the first diastemal rudiment (MS) at ED12.5; (b) the second diastemal rudiment (R2) at ED13.5; and (c) the first molar (M1) at ED14.5. Arrowheads point to the enamel knot of R2 and M1. (d–f) The three Shh expression domains are sequentially patterned along the antero-posterior axes in the cheek region of the mandible. The mandibles at ED12.7 (d), 13.3 (e) and 14.3 (f) have been hybridized with Shh anti-sense probe (left) and then sectioned frontally (right) to show the localization of the Shh expression domain in the dental epithelium using 3D reconstruction technique (middle). The Shh signalling centres (blue in MS, red in R2 and yellow in M1) correspond to the respective morphological structures (a, b, c).22 (g, h) A scheme compares the classical (g) and new (h) interpretations of the signalling structures during ED12.5–14.5. (g) According to the classical view, the characteristic structures in the cheek region of the mandible at respective days 12, 13 and 14 were generally assumed to correspond to various developmental stages of the M1 development (frame on the top). In these structures, similar signalling22 as well as concentration of apoptosis (black dots)74 have been found (compare to Fig. 14). (h) The 3D reconstructions have shown that the characteristic structures (a–c), in fact, are localized in different segments of the dental epithelium that appear sequentially in the posterior direction (arrow). The R2 becomes incorporated anteriorly into the M1 cap (grey area), as shown experimentally.22 A residuum of the MS might be implicated during the growth of the enamel organ at later stages (dashed line). The presence of the specific Shh expression domains along the antero-posterior jaw axes can be related to the transient manifestation of the tooth pattern in remote ancestors, where the premolars were still present (modified according to the refs.22,23).
It has been generally assumed that the functional tooth in wild type mice develops from a tooth germ which exhibits either a single Shh expression domain (incisor) or reiterative expression (first molar) during embryonic days (ED) 12, 13 and 14. The combination of whole mount Shh in situ hybridization and computer aided 3D reconstructions has revealed a much more complex situation (Figs. 17–19).22,58
Instead of a single expression domain in the incisor region, two Shh expression areas appear in an antero-posterior sequence in each right and left incisor region during early development.58 The first anterior domain is located more superficially – in the epithelial thickening. This thickening gives rise to two to three epithelial bridges in the lower jaw,58 and to two epithelial projections in the upper jaw20; in both these locations, a rudimentary tooth develops. In the upper jaw, the posterior part of the thickening infolds in the mesenchyme to form the early bud of the functional incisor (Fig. 12a–c). The anterior Shh expression domain and the related rudimentary tooth have been interpreted as the anlage of a rudimentary (prelacteal8,14) dentition, transiently apparent in mouse embryos. Only the second, later appearing, more posteriorly and deeply localized expression domain corresponds to the signalling centre of the proper bud of the functional incisor58,138 (Fig. 17).
Instead of only one expressing area attributed to the developing M1 in the lower cheek region, a series of three signalling centres sequentially appear belonging to the diastemal rudiment MS, rudiment R2, and M1 during ED12.5–14.5 (Fig. 19). The incorporation of the diastemal (premolar) rudiment R2 during morphogenesis of the functional M1 has been proven by the tracing of the Shh signalling centres, and can explain the anterior enlargement of the M1 during murine evolution.22
Conclusions and Clinical Significance
The combined analysis of sections and 3D reconstructions has allowed updating of the classical data on early odontogenesis in humans and mice. In humans, the classical data refer to a continuous horseshoe-shaped anlage of the dentition (dental lamina), which runs internally and parallel to the anlage of the oral vestibule (vestibular lamina). Instead of a continuous vestibular lamina, a complex of epithelial ridges and bulges exists transiently. Along the jaw axis, the dental and vestibular epithelia are regionalized in parallel and exhibit multiple fusions. Moreover, there is no general scheme for the early development of the dental and vestibular epithelia that fits both the upper and lower jaws, or their anterior and posterior regions. In mice, the classical concept assumes the pattern of embryonic tooth primordia and their signalling centres correspond to the pattern of adult teeth. Instead, there is a sequential appearance of several distinct tooth primordia with specific Shh signalling along the antero-posterior axes in both incisor and cheek region of the mandible. The first-appearing primordia/signalling centres are rudimentary. They have been related to teeth of remote ancestors. The rudiments can rescue their suppressed development and revitalize. The following autonomous development can give rise to oral pathological entities.
In summary, different rudimentary odontogenic structures with different fates represent an integral component of early odontogenesis in humans and mice. Their existence and ability to revitalize indicate that the tooth-forming potential in mammals is greater than what is evident in their functional dentitions. From this perspective and of considerable importance in terms of clinical application, the rudimentary tooth primordia in mice represent a natural model to elucidate the mechanisms that support or inhibit tooth development and to test possibilities of tooth regeneration. Given that missing teeth represent a common dental problem, the benefits of being able to generate new teeth are enormous.
Acknowledgments
The authors thank Brona Rokytova for her technical assistance. Support was obtained from the Grant Agency of the Czech Republic (CZ:GA ČR:GAP305/12/1766) and from the Université de Strasbourg, Faculté de Chirurgie Dentaire.
Glossary
- ED
embryonic day
- EK
enamel knot
- IDE
inner dental epithelium
- M1, M2, M3
the first, second, third molar, respectively
- ODE
outer dental epithelium
- pEK
primay enamel knot
- rEK
rudimentary enamel knot
- Shh
Sonic hedgehog
- WT
wild type
Disclosure
The authors have no conflicts of interest to declare.
References
- Moss ML. Phylogeny and comparative anatomy of oral ectodermal ectomesenchymal inductive interactions. J Dent Res. 1969;48:732–737. doi: 10.1177/00220345690480052101. [DOI] [PubMed] [Google Scholar]
- Miles AEW, Poole DGF. The history and general organization of dentitions. In: Miles AEW, editor. Structural and chemical organization of teeth. New York, London: Academic Press; 1967. pp. 3–44. [Google Scholar]
- McIntosh JE, Anderton X, Flores-De-Jacoby L, Carlson DS, Shuler CF, Diekwisch TG. Caiman periodontium as an intermediate between basal vertebrate ankylosis-type attachment and mammalian ‘true’ periodontium. Microsc Res Tech. 2002;59:449–459. doi: 10.1002/jemt.10222. [DOI] [PubMed] [Google Scholar]
- Moss-Salentijn L. Vestigial teeth in rabbit, rat and mouse: their relationship to the problem of lacteal dentitions. In: Butler PM, Joysey KA, editors. Development, function and evolution of teeth. London: Academic Press; 1978. pp. 13–29. [Google Scholar]
- Kükenthal W. Über den Ursprung und die Entwickelung der Säugertierzähne. Jenaer Zeitsch Naturwiss. 1892;26:469–489. [Google Scholar]
- Röse C. Über die Zahnentwickelung der Bauteltiere. Anat Anz. 1892;7:693–707. [Google Scholar]
- Peyer B. In: Comparative odontology. Zangler R, editor. Chicago: The University of Chicago Press; 1968. p. 269. [Google Scholar]
- Peterkova R, Lesot H, Peterka M. Phylogenetic memory of developing mammalian dentition. J Exp Zool. 2006;306B:234–250. doi: 10.1002/jez.b.21093. [DOI] [PubMed] [Google Scholar]
- Osborn HF. The evolution of mammalian molars to and from the tritubercular type. Am Naturalist. 1888;22:1067–1079. [Google Scholar]
- Ungar PS. Mammal teeth: origin, evolution, and diversity. Baltimore: The Johns Hopkins University Press; 2010. [Google Scholar]
- Young JZ. The life of mammals. Oxford: Clarendon Press; 1957. [Google Scholar]
- Adloff P. Die Entwicklung des Zahnsystems der Säugetiere und des Menschen. Berlin: Verlag von Hermann Meusser; 1916. [Google Scholar]
- Bolk L. Odontological essays. On the relation between reptilian and mammalian teeth. J Anat. 1922;56:107–136. [PMC free article] [PubMed] [Google Scholar]
- Peterkova R, Peterka M, Viriot L, Lesot H. Development of the vestigial tooth primordia as part of mouse odontogenesis. Connect Tissue Res. 2002;43:120–128. doi: 10.1080/03008200290000745. [DOI] [PubMed] [Google Scholar]
- Reiff WE. Evolution of dermal skeleton and dentition in vertebrates. The odontode regulation theory. Evol Biol. 1982;15:287–368. [Google Scholar]
- Huysseune A, Sire JY. Evolution of patterns and processes in teeth and tooth-related tissues in non-mammalian vertebrates. Eur J Oral Sci. 1998;106(Suppl 1):437–481. doi: 10.1111/j.1600-0722.1998.tb02211.x. [DOI] [PubMed] [Google Scholar]
- Hitchin AD, Morris I. Geminated odontome – connation of the incisors in the dog – its etiology and ontogeny. J Dent Res. 1966;45:575–583. [Google Scholar]
- Guttal KS, Venkatesh G, Naikmasur VG, Bhargava P, Bathi RJ. Frequency of developmental dental anomalies in the Indian population. Eur J Dent. 2010;4:263–269. [PMC free article] [PubMed] [Google Scholar]
- Marinelli A, Giuntini V, Franchi L, Tollaro I, Baccetti T, Defraia E. Dental anomalies in the primary dentition and their repetition in the permanent dentition: a diagnostic performance study. Odontology. 2012;100:22–27. doi: 10.1007/s10266-011-0021-6. [DOI] [PubMed] [Google Scholar]
- Peterkova R, Peterka M, Vonesch JL, Ruch JV. Multiple developmental origin of the upper incisor in mouse: histological and computer assisted 3D reconstruction studies. Int J Dev Biol. 1993;37:581–588. [PubMed] [Google Scholar]
- Peterkova R, Peterka M, Vonesch JL, Ruch JV. Contribution of 3D computer-assisted reconstructions to the study of the initial steps of mouse odontogenesis. Int J Dev Biol. 1995;39:239–247. [PubMed] [Google Scholar]
- Prochazka J, Pantalacci S, Churava S, et al. Patterning by heritage in mouse molar row development. Proc Natl Acad Sci U S A. 2010;107:15497–15502. doi: 10.1073/pnas.1002784107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkova R, Lesot H, Viriot L, Peterka M. The supernumerary cheek tooth in tabby/EDA mice–a reminiscence of the premolar in mouse ancestors. Arch Oral Biol. 2005;50:219–225. doi: 10.1016/j.archoralbio.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Peterkova R, Churava S, Lesot H, et al. Revitalization of a diastemal tooth primordium in Spry2 null mice results from increased proliferation and decreased apoptosis. J Exp Zool. 2009;312B:292–308. doi: 10.1002/jez.b.21266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovorakova M, Lesot H, Peterka M, Peterkova R. The developmental relationship between the deciduous dentition and the oral vestibule in human embryos. Anat Embryol (Berl) 2005;209:303–313. doi: 10.1007/s00429-004-0441-y. [DOI] [PubMed] [Google Scholar]
- Charles C, Hovorakova M, Ahn Y, et al. Regulation of tooth number by fine-tuning levels of receptor-tyrosine kinase signaling. Development. 2011;138:4063–4073. doi: 10.1242/dev.069195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobourne MT, Sharpe PT. Making up the numbers: the molecular control of mammalian dental formula. Semin Cell Dev Biol. 2010;21:314–324. doi: 10.1016/j.semcdb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Born G. Die Plattenmodelirmethode. Arch Mikr Anat. 1883;22:584–599. [Google Scholar]
- Born G. Noch einmal die Plattenmodelirmethode. Z Wiss Mikr. 1888;5:433–455. [Google Scholar]
- Ahrens H. Die Entwicklung der menschlichen Zähne. Anat Hefte. 1913;48:169–257. [Google Scholar]
- Knudsen PA. Congenital malformations of upper incisors in exencephalic mouse embryos, induced by hypervitaminosis A. II. Morphology of fused upper incisors. Acta Odontol Scand. 1965;23:391–409. doi: 10.3109/00016356509007521. [DOI] [PubMed] [Google Scholar]
- Knudsen PA. Fusion of upper incisors at bud or cap stage in mouse embryos with exencephaly induced by hypervitaminosis A. Acta Odontol Scand. 1965;23:549–565. doi: 10.3109/00016356509021769. [DOI] [PubMed] [Google Scholar]
- Knudsen PA. Malformations of upper incisors in mouse embryos with exencephaly, induced by trypan blue. Acta Odontol Scand. 1966;24:647–675. doi: 10.3109/00016356609028734. [DOI] [PubMed] [Google Scholar]
- Knudsen PA. Dental anomalies in mouse embryos with hydrocephalus induced by hypervitaminosis. Acta Odontol Scand. 1967;25:677–691. doi: 10.3109/00016356709019784. [DOI] [PubMed] [Google Scholar]
- Thomee S. Über Glasrekonstruktion. Z Wiss Mikr. 1928;45:356. [Google Scholar]
- Rolshoven E. Rekonstruktion histologischer Objekte auf durchsichtigen Wrkstoffen. Z Wiss Mikr. 1937;54:328. [Google Scholar]
- Blechschmidt E Rekonstruktionsverfahren mit Verwendung von Kunststoffen. Ein Verfahren zur Ermittlung und Rekonstruktion von Entwicklungsbewegungen. Z Anat Entwickl-Gesch. 1954;118:170–174. [PubMed] [Google Scholar]
- Ooë T. On the development of position of the tooth germs in the human deciduous front teeth. Okajimas Folia Anat Jpn. 1956;28:317–340. doi: 10.2535/ofaj1936.28.1-6_317. [DOI] [PubMed] [Google Scholar]
- Ooë T. Three instances of supernumerary tooth germs observed with serial sections of human foetal jaws. Z Anat Entwicklungsgesch. 1971;135:202–209. doi: 10.1007/BF00521110. [DOI] [PubMed] [Google Scholar]
- Ooë T. Development of human first and second permanent molar, with special reference to the distal portmon of the dental lamina. Anat Embryol. 1979;155:221–240. doi: 10.1007/BF00305754. [DOI] [PubMed] [Google Scholar]
- Radlanski RJ. Contributions to the development of human deciduous primordia. Illinois: Quintessence Publishing Co; 1993. pp. 5–87. [Google Scholar]
- Radlanski RJ. Morphogenesis of human tooth primordia: the importance of 3D computer-assisted reconstruction. Int J Dev Biol. 1995;39:249–256. [PubMed] [Google Scholar]
- Hovorakova M, Lesot H, Peterkova R, Peterka M. Origin of the deciduous upper lateral incisor and its clinical aspects. J Dent Res. 2006;85:167–171. doi: 10.1177/154405910608500210. [DOI] [PubMed] [Google Scholar]
- Hovorakova M, Lesot H, Vonesch JL, Peterka M, Peterkova R. Early development of the lower deciduous dentition and oral vestibule in human embryos. Eur J Oral Sci. 2007;115:280–287. doi: 10.1111/j.1600-0722.2007.00464.x. [DOI] [PubMed] [Google Scholar]
- Gaunt WA. The development of the molar pattern of the mouse (Mus musculus) Acta Anat. 1955;24:249–268. doi: 10.1159/000141046. [DOI] [PubMed] [Google Scholar]
- Hay MF. The development in vivo and in vitro of the lower incisor and molars of the mouse. Arch Oral Biol. 1961;3:86–109. doi: 10.1016/0003-9969(61)90142-x. [DOI] [PubMed] [Google Scholar]
- Pourtois M. Contribution to the study of tooth buds in the mouse. I. Periods of induction and morphodifferentiation. Arch. Biol. 1961;72:17–95. [PubMed] [Google Scholar]
- Cohn SA. Development of the molar teeth in the albino mouse. Am J Anat. 1957;101:295–319. doi: 10.1002/aja.1001010205. [DOI] [PubMed] [Google Scholar]
- Peterka M, Lesot H, Peterkova R. Body weight in mouse embryos specifies staging of tooth development. Connect Tissue Res. 2002;43:186–190. doi: 10.1080/03008200290000673. [DOI] [PubMed] [Google Scholar]
- Peterkova R, Lesot H, Vonesch JL, Peterka M, Ruch JV. Mouse molar morphogenesis revisited by three-dimensional reconstruction. I. Analysis of initial stages of the first upper molar development revealed two transient buds. Int J Dev Biol. 1996;40:1009–1016. [PubMed] [Google Scholar]
- Lesot H, Vonesch JL, Peterka M, Tureckova J, Peterkova R, Ruch JV. Mouse molar morphogenesis revisited by three-dimensional reconstruction. II. Spatial distribution of mitoses and apoptosis in cap to bell staged first and second upper molar teeth. Int J Dev Biol. 1996;40:1017–1031. [PubMed] [Google Scholar]
- Lesot H, Peterkova R, Viriot L, et al. Early stages of tooth morphogenesis in mouse analyzed by 3D reconstructions. Eur J Oral Sci. 1998;106(Suppl 1):64–70. doi: 10.1111/j.1600-0722.1998.tb02155.x. [DOI] [PubMed] [Google Scholar]
- Lesot H, Peterkova R, Schmitt R, et al. Initial features of the inner dental epithelium histo-morphogenesis in the first lower molar in mouse. Int J Dev Biol. 1999;43:245–254. [PubMed] [Google Scholar]
- Lesot H, Hovorakova M, Peterka M, Peterkova R. Three-dimensional analysis of molar development in the mouse from the cap to bell stage. Aust Dent J. 2014 doi: 10.1111/adj.12132. doi: 10.1111/adj.12132 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Kieffer S, Peterkova R, Vonesch JL, Ruch JV, Peterka M, Lesot H. Morphogenesis of the lower incisor in the mouse from the bud to early bell stage. Int J Dev Biol. 1999;43:531–539. [PubMed] [Google Scholar]
- Viriot L, Peterkova R, Vonesch JL, Peterka M, Ruch JV, Lesot H. Mouse molar morphogenesis revisited by three-dimensional reconstruction. III. Spatial distribution of mitoses and apoptoses up to bell-staged first lower molar teeth. Int J Dev Biol. 1997;41:679–690. [PubMed] [Google Scholar]
- Viriot L, Lesot H, Vonesch JL, Ruch JV, Peterka M, Peterkova R. The presence of rudimentary odontogenic structures in the mouse embryonic mandible requires reinterpretation of developmental control of first lower molar histomorphogenesis. Int J Dev Biol. 2000;44:233–240. [PubMed] [Google Scholar]
- Hovorakova M, Prochazka J, Lesot H, et al. Shh expression in a rudimentary tooth offers new insights into development of the mouse incisor. J Exp Zool. 2011;316B:347–358. doi: 10.1002/jez.b.21408. [DOI] [PubMed] [Google Scholar]
- Vaahtokari A, Aberg T, Jernvall J, Keränen S, Thesleff I. The enamel knot as a signaling center in the developing mouse tooth. Mech Dev. 1996;54:39–43. doi: 10.1016/0925-4773(95)00459-9. [DOI] [PubMed] [Google Scholar]
- Peterkova R, Peterka M, Vonesch JL, et al. Correlation between apoptosis distribution and BMP-2 and BMP-4 expression in vestigial tooth primordia in mice. Eur J Oral Sci. 1998;106:667–670. doi: 10.1046/j.0909-8836..t01-5-.x. [DOI] [PubMed] [Google Scholar]
- Coin R, Lesot H, Vonesch JL, Haikel Y, Ruch JV. Aspects of cell proliferation kinetics of the inner dental epithelium during mouse molar and incisor morphogenesis: a reappraisal of the role of the enamel knot area. Int J Dev Biol. 1999;43:261–267. [PubMed] [Google Scholar]
- Miard S, Peterkova R, Vonesch JL, Peterka M, Ruch JV, Lesot H. Alterations in the incisor development in the Tabby mouse. Int J Dev Biol. 1999;43:517–529. [PubMed] [Google Scholar]
- Lisi S, Peterkova R, Peterka M, Vonesch JL, Ruch JV, Lesot H. Tooth morphogenesis and pattern of odontoblast differentiation. Connect Tissue Res. 2003;44(Suppl 1):167–170. [PubMed] [Google Scholar]
- Lesot H, Kieffer-Combeau S, Fausser JL, et al. Cell-cell and cell-matrix interactions during initial enamel organ histomorphogenesis in the mouse. Connect Tissue Res. 2002;43:191–200. doi: 10.1080/03008200290000529. [DOI] [PubMed] [Google Scholar]
- Peterkova R, Kristenova P, Lesot H, et al. Different morphotypes of the tabby (EDA) dentition in the mouse mandible result from a defect in the mesio-distal segmentation of dental epithelium. Orthod Craniofac Res. 2002;5:215–226. doi: 10.1034/j.1600-0544.2002.02226.x. [DOI] [PubMed] [Google Scholar]
- Ohazama A, Haycraft CJ, Seppala M, et al. Primary cilia regulate Shh activity in the control of molar tooth number. Development. 2009;136:897–903. doi: 10.1242/dev.027979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatomi M, Hovorakova M, Gritli-Linde A, et al. Evc regulates a symmetrical response to Shh signaling in molar development. J Dent Res. 2013;92:222–228. doi: 10.1177/0022034512471826. [DOI] [PubMed] [Google Scholar]
- Witter K, Lesot H, Peterka M, Vonesch JL, Mísek I, Peterkova R. Origin and developmental fate of vestigial tooth primordia in the upper diastema of the field vole (Microtus agrestis, Rodentia) Arch Oral Biol. 2005;50:401–409. doi: 10.1016/j.archoralbio.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Harada H, Toyono T, Toyoshima K, et al. FGF10 maintains stem cell compartment in developing mouse incisors. Development. 2002;129:1533–1541. doi: 10.1242/dev.129.6.1533. [DOI] [PubMed] [Google Scholar]
- Balic A, Mina M. Characterization of progenitor cells in pulps of murine incisors. J Dent Res. 2010;89:1287–1292. doi: 10.1177/0022034510375828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapthanasupkul P, Feng J, Mantesso A, et al. Ring1a/b polycomb proteins regulate the mesenchymal stem cell niche in continuously growing incisors. Dev Biol. 2012;367:140–153. doi: 10.1016/j.ydbio.2012.04.029. [DOI] [PubMed] [Google Scholar]
- Tureckova J, Sahlberg C, Aberg T, Ruch JV, Thesleff I, Peterkova R. Comparison of expression of the msx-1, msx-2, BMP-2 and BMP-4 genes in the mouse upper diastemal and molar tooth primordia. Int J Dev Biol. 1995;39:459–468. [PubMed] [Google Scholar]
- Keränen SV, Kettunen P, Aberg T, Thesleff I, Jernvall J. Gene expression patterns associated with suppression of odontogenesis in mouse and vole diastema regions. Dev Genes Evol. 1999;209:495–506. doi: 10.1007/s004270050282. [DOI] [PubMed] [Google Scholar]
- Peterkova R, Peterka M, Lesot H. The developing mouse dentition: a new tool for apoptosis study. Ann N Y Acad Sci. 2003;1010:453–466. doi: 10.1196/annals.1299.083. [DOI] [PubMed] [Google Scholar]
- Cobourne MT, Miletich I, Sharpe PT. Restriction of sonic hedgehog signalling during early tooth development. Development. 2004;131:2875–2885. doi: 10.1242/dev.01163. [DOI] [PubMed] [Google Scholar]
- Yuan GH, Zhang L, Zhang YD, Fan MW, Bian Z, Chen Z. Mesenchyme is responsible for tooth suppression in the mouse lower diastema. J Dent Res. 2008;87:386–390. doi: 10.1177/154405910808700412. [DOI] [PubMed] [Google Scholar]
- Ahn Y, Sanderson BW, Klein OD, Krumlauf R. Inhibition of Wnt signaling by Wise (Sostdc1) and negative feedback from Shh controls tooth number and patterning. Development. 2010;137:3221–3231. doi: 10.1242/dev.054668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porntaveetus T, Ohazama A, Choi HY, Herz J, Sharpe PT. Wnt signaling in the murine diastema. Eur J Orthod. 2012;34:518–524. doi: 10.1093/ejo/cjr049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Yuan G, Liu C, et al. Exogenous fibroblast growth factor 8 rescues development of mouse diastemal vestigial tooth ex vivo. Dev Dyn. 2011;240:1344–1353. doi: 10.1002/dvdy.22596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miletich I, Yu WY, Zhang R, et al. Developmental stalling and organ-autonomous regulation of morphogenesis. Proc Natl Acad Sci U S A. 2011;108:19270–19275. doi: 10.1073/pnas.1112801108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae YM, Jin YJ, Kim HS, et al. Proteome analysis of developing mice diastema region. BMB Rep. 2012;45:337–341. doi: 10.5483/bmbrep.2012.45.6.025. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhang Y, Jiang TX, et al. Conservation of early odontogenic signaling pathways in Aves. Proc Natl Acad Sci USA. 2000;97:10044–10049. doi: 10.1073/pnas.160245097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viriot L, Peterkova R, Peterka M, Lesot H. Evolutionary implications of the occurrence of two vestigial tooth germs during early odontogenesis in the mouse lower jaw. Connect Tissue Res. 2002;43:129–133. doi: 10.1080/03008200290001168. [DOI] [PubMed] [Google Scholar]
- Peterkova R, Peterka M, Viriot L, Lesot H. Dentition development and budding morphogenesis. J Craniofac Genet Dev Biol. 2000;20:158–172. [PubMed] [Google Scholar]
- Theiler K. The House Mouse. Berlin, Heidelberg, New York: Springer-Verlag; 1972. pp. 1–162. [Google Scholar]
- Kaufman MH. The atlas of mouse development. London: Academic Press; 1992. [Google Scholar]
- Miyake T, Cameron AM, Hall BK. Detailed staging of inbred C57BL/6 mice between Theiler’s [1972] stages 18 and 21 (11–13 days of gestation) based on craniofacial development. J Craniofac Genet Dev Biol. 1996;16:1–31. [PubMed] [Google Scholar]
- Bei M. Molecular genetics of tooth development. Curr Opin Genet Dev. 2009;19:504–510. doi: 10.1016/j.gde.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook AH. Multilevel complex interactions between genetic, epigenetic and environmental factors in the aetiology of anomalies of dental development. Arch Oral Biol. 2009;54:S3–S17. doi: 10.1016/j.archoralbio.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchtova M, Handrigan GR, Tucker AS, et al. Initiation and patterning of the snake dentition are dependent on Sonic hedgehog signaling. Dev Biol. 2008;319:132–145. doi: 10.1016/j.ydbio.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Catón J, Tucker AS. Current knowledge of tooth development: patterning and mineralization of the murine dentition. J Anat. 2009;214:502–515. doi: 10.1111/j.1469-7580.2008.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesot H, Brook AH. Epithelial histogenesis during tooth development. Arch Oral Biol. 2009;54(Suppl 1):S25–S33. doi: 10.1016/j.archoralbio.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Jernvall J, Thesleff I. Tooth shape formation and tooth renewal: evolving with the same signals. Development. 2012;139:3487–3497. doi: 10.1242/dev.085084. [DOI] [PubMed] [Google Scholar]
- Townsend G, Bockmann M, Hughes T, Brook A. Genetic, environmental and epigenetic influences on variation in human tooth number, size and shape. Odontology. 2012;100:1–9. doi: 10.1007/s10266-011-0052-z. [DOI] [PubMed] [Google Scholar]
- Lumsden AG. Spatial organization of the epithelium and the role of neural crest cells in the initiation of the mammalian tooth germ. Development. 1988;103(Suppl):155–169. doi: 10.1242/dev.103.Supplement.155. [DOI] [PubMed] [Google Scholar]
- Kollar EJ, Mina M. Role of the early epithelium in the patterning of the teeth and Meckel’s cartilage. J Craniofac Genet Dev Biol. 1991;11:223–228. [PubMed] [Google Scholar]
- Ohazama A, Modino SA, Miletich I, Sharpe PT. Stem-cell-based tissue engineering of murine teeth. J Dent Res. 2004;83:518–522. doi: 10.1177/154405910408300702. [DOI] [PubMed] [Google Scholar]
- Cobourne MT, Mitsiadis T. Neural crest cells and patterning of the mammalian dentition. J Exp Zool. 2006;306B:251–260. doi: 10.1002/jez.b.21084. [DOI] [PubMed] [Google Scholar]
- Thomas BL, Tucker AS, Qui M, et al. Role of Dlx-1 and Dlx-2 genes in patterning of the murine dentition. Development. 1997;124:4811–4818. doi: 10.1242/dev.124.23.4811. [DOI] [PubMed] [Google Scholar]
- Peters H, Balling R. Teeth. Where and how to make them. Trends Genet. 1999;15:59–65. doi: 10.1016/s0168-9525(98)01662-x. [DOI] [PubMed] [Google Scholar]
- Tucker AS, Sharpe PT. Molecular genetics of tooth morphogenesis and patterning: the right shape in the right place. J Dent Res. 1999;78:826–834. doi: 10.1177/00220345990780040201. [DOI] [PubMed] [Google Scholar]
- Rothova M, Peterkova R, Tucker AS. Fate map of the dental mesenchyme: dynamic development of the dental papilla and follicle. Dev Biol. 2012;366:244–254. doi: 10.1016/j.ydbio.2012.03.018. [DOI] [PubMed] [Google Scholar]
- Nait LechguerA, Kuchler-Bopp S, Hu B, Haïkel Y, Lesot H. Vascularization of engineered teeth. J Dent Res. 2008;87:1138–1143. doi: 10.1177/154405910808701216. [DOI] [PubMed] [Google Scholar]
- Rothova M, Feng J, Sharpe PT, Peterkova R, Tucker AS. Contribution of mesoderm to the developing dental papilla. Int J Dev Biol. 2011;55:59–64. doi: 10.1387/ijdb.103083mr. [DOI] [PubMed] [Google Scholar]
- Keller LV, Kuchler-Bopp S, Lesot H. Restoring physiological cell heterogeneity in the mesenchyme during tooth engineering. Int J Dev Biol. 2012;56:737–746. doi: 10.1387/ijdb.120076hl. [DOI] [PubMed] [Google Scholar]
- Mohamed SS, Atkinson ME. A histological study of the innervation of developing mouse teeth. J Anat. 1983;136:735–749. [PMC free article] [PubMed] [Google Scholar]
- Fried K, Nosrat C, Lillesaar C, Hildebrand C. Molecular signaling and pulpal nerve development. Crit Rev Oral Biol Med. 2000;11:318–332. doi: 10.1177/10454411000110030301. [DOI] [PubMed] [Google Scholar]
- Luukko K, Kvinnsland IH, Kettunen P. Tissue interactions in the regulation of axon pathfinding during tooth morphogenesis. Dev Dyn. 2005;234:482–488. doi: 10.1002/dvdy.20586. [DOI] [PubMed] [Google Scholar]
- Løes S, Kettunen P, Kvinnsland H, Luukko K. Mouse rudimentary diastema tooth primordia are devoid of peripheral nerve fibers. Anat Embryol. 2002;205:187–191. doi: 10.1007/s00429-002-0247-8. [DOI] [PubMed] [Google Scholar]
- Ooë T. On the early development of human dental lamina. Okajimas Folia Anat Jpn. 1957;30:198–210. [PubMed] [Google Scholar]
- Nery EB, Kraus BS, Croup M. Timing and topography of early human tooth development. Arch Oral Biol. 1970;15:1315–1326. doi: 10.1016/0003-9969(70)90020-8. [DOI] [PubMed] [Google Scholar]
- Kriangkrai R, Chareonvit S, Yahagi K, Fujiwara M, Eto K, Iseki S. Study of Pax6 mutant rat revealed the association between upper incisor formation and midface formation. Dev Dyn. 2006;235:2134–2143. doi: 10.1002/dvdy.20875. [DOI] [PubMed] [Google Scholar]
- Kriangkrai R, Iseki S, Eto K, Chareonvit S. Dual odontogenic origins develop at the early stage of rat maxillary incisor development. Anat Embryol. 2006;211:101–108. doi: 10.1007/s00429-005-0068-7. [DOI] [PubMed] [Google Scholar]
- Peterkova R. The common developmental origin and phylogenetic aspects of teeth, rugae palatinae, and fornix vestibuli oris in the mouse. J Craniofac Genet Dev Biol. 1985;5:89–104. [PubMed] [Google Scholar]
- Schlosser G. Evolutionary origins of vertebrate placodes: insights from developmental studies and from comparisons with other deuterostomes. J Exp Zool. 2005;304B:347–399. doi: 10.1002/jez.b.21055. [DOI] [PubMed] [Google Scholar]
- Schlosser G. Development and evolution of lateral line placodes in amphibians. I. Development. Zoology. 2002;105:119–146. doi: 10.1078/0944-2006-00058. [DOI] [PubMed] [Google Scholar]
- Mikkola ML. Genetic basis of skin appendage development. Semin Cell Dev Biol. 2007;18:225–236. doi: 10.1016/j.semcdb.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Westergaard B. Early dentition development in the lower jaws of Anguis fragilis and Lacerta agilis. Mem Soc Fauna Flora Fenn. 1988;64:148–151. [Google Scholar]
- Huysseune A, Witten PE. Developmental mechanisms underlying tooth patterning in continuously replacing osteichthyan dentitions. J Exp Zool. 2006;306B:204–215. doi: 10.1002/jez.b.21091. [DOI] [PubMed] [Google Scholar]
- Pispa J, Thesleff I. Mechanisms of ectodermal organogenesis. Dev Biol. 2003;262:195–205. doi: 10.1016/s0012-1606(03)00325-7. [DOI] [PubMed] [Google Scholar]
- Munne PM, Felszeghy S, Jussila M, Suomalainen M, Thesleff I, Jernvall J. Splitting placodes: effects of bone morphogenetic protein and Activin on the patterning and identity of mouse incisors. Evol Dev. 2010;12:383–392. doi: 10.1111/j.1525-142X.2010.00425.x. [DOI] [PubMed] [Google Scholar]
- Blackburn J, Ohazama A, Kawasaki K, et al. The role of Irf6 in tooth epithelial invagination. Dev Biol. 2012;365:61–70. doi: 10.1016/j.ydbio.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar L, Cobourne M, Naylor S, Smalley M, Dale T, Sharpe PT. Wnt/Shh interactions regulate ectodermal boundary formation during mammalian tooth development. Proc Natl Acad Sci U S A. 2000;97:4520–4524. doi: 10.1073/pnas.97.9.4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol. 1995;172:126–138. doi: 10.1006/dbio.1995.0010. [DOI] [PubMed] [Google Scholar]
- Hardcastle Z, Mo R, Hui CC, Sharpe PT. The Shh signalling pathway in tooth development: defects in Gli2 and Gli3 mutants. Development. 1998;125:2803–2811. doi: 10.1242/dev.125.15.2803. [DOI] [PubMed] [Google Scholar]
- Ruch JV. Tooth morphogenesis and differentiation. In: Linde A, editor. Dentin and dentinogenesis. I. Boca Raton, FL, USA: CRC Press; 1984. pp. 47–79. [Google Scholar]
- Ingber DE. The mechanochemical basis of cell and tissue regulation. Mech Chem Biosyst. 2004;1:53–68. [PubMed] [Google Scholar]
- Donley CL, Nelson LP. Comparison of palatal and alveolar cysts of the newborn in premature and full-term infants. Pediatr Dent. 2000;22:321–324. [PubMed] [Google Scholar]
- Fukumoto S, Kiba T, Hall B, et al. Ameloblastin is a cell adhesion molecule required for maintaining the differentiation state of ameloblasts. J Cell Biol. 2004;167:973–983. doi: 10.1083/jcb.200409077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara N, Suzuki Y, Nagai Y, Takeda M. Expression of E- and P-cadherin during tooth morphogenesis and cytodifferentiation of ameloblasts. Anat Embryol. 1998;197:469–475. doi: 10.1007/s004290050157. [DOI] [PubMed] [Google Scholar]
- Hogan BL. Morphogenesis. Cell. 1999;96:225–233. doi: 10.1016/s0092-8674(00)80562-0. [DOI] [PubMed] [Google Scholar]
- Jung HS, Francis-West PH, Widelitz RB, et al. Local inhibitory action of BMPs and their relationships with activators in feather formation: implications for periodic patterning. Dev Biol. 1998;196:11–23. doi: 10.1006/dbio.1998.8850. [DOI] [PubMed] [Google Scholar]
- Widelitz RB, Chuong CM. Early events in skin appendage formation: induction of epithelial placodes and condensation of dermal mesenchyme. J Investig Dermatol Symp Proc. 1999;4:302–306. doi: 10.1038/sj.jidsp.5640234. [DOI] [PubMed] [Google Scholar]
- Cobourne MT, Hardcastle Z, Sharpe PT. Sonic hedgehog regulates epithelial proliferation and cell survival in the developing tooth germ. J Dent Res. 2001;80:1974–1979. doi: 10.1177/00220345010800110501. [DOI] [PubMed] [Google Scholar]
- Weiss KM, Stock DW, Zhao Z. Dynamic interactions and the evolutionary genetics of dental patterning. Crit Rev Oral Biol Med. 1998;9:369–398. doi: 10.1177/10454411980090040101. [DOI] [PubMed] [Google Scholar]
- Jernvall J, Thesleff I. Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech Dev. 2000;92:19–29. doi: 10.1016/s0925-4773(99)00322-6. [DOI] [PubMed] [Google Scholar]
- Cho SW, Kwak S, Woolley TE, et al. Interactions between Shh, Sostdc1 and Wnt signaling and a new feedback loop for spatial patterning of the teeth. Development. 2011;138:1807–1816. doi: 10.1242/dev.056051. [DOI] [PubMed] [Google Scholar]
- Hovorakova M, Smrckova L, Lesot H, Lochovska K, Peterka M, Peterkova R. Sequential Shh expression in the development of the mouse upper functional incisor. J Exp Zool B Mol Dev Evol. 2013 doi: 10.1002/jez.b.22522. Aug 1. doi: 10.1002/jez.b.22522 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Woodward MF. On the milk dentition of the rodentia, with a description of a vestigial milk incisor in the mouse (Mus musculus) Anat Anz. 1894;9:619–631. [Google Scholar]
- Fitzgerald LR. Deciduous incisor teeth of the mouse (Mus musculus) Arch Oral Biol. 1973;18:381–389. doi: 10.1016/0003-9969(73)90162-3. [DOI] [PubMed] [Google Scholar]
- Leche W. Nachträge zu Studien über die Entwickelung der Zahnsystems bei Säugertieren. Morph Jahrb. 1893;20:113–142. [Google Scholar]
- Röse C. Überreste einer vorzeitigen prälactealen un einer vierten Zahnreihe beim Menschen. Österreichisch-Ungarische Vierteljahrschr Zahnheilk. 1895;2:45–50. [Google Scholar]
- Adloff P. Überreste einer prälactealen Zahnreihe beim Menschen. Deut Monatschr Zahnheilk. 1909;11:828–832. [Google Scholar]
- Lagronova-Churava S, Spoutil F, Vojtechova S, et al. The dynamics of supernumerary tooth development are differentially regulated by Sprouty genes. J Exp Zool. 2013;320B:307–320. doi: 10.1002/jez.b.22502. [DOI] [PubMed] [Google Scholar]
- Kollar EJ, Baird GR. The influence of the dental papilla on the development of tooth shape in embryonic mouse tooth germs. J Embryol Exp Morphol. 1969;21:131–148. [PubMed] [Google Scholar]
- Mina M, Kollar EJ. The induction of odontogenesis in non-dental mesenchyme combined with early murine mandibular arch epithelium. Arch Oral Biol. 1987;32:123–127. doi: 10.1016/0003-9969(87)90055-0. [DOI] [PubMed] [Google Scholar]
- Fleischmannova J, Matalova E, Tucker AS, Sharpe PT. Mouse models of tooth abnormalities. Eur J Oral Sci. 2008;116:1–10. doi: 10.1111/j.1600-0722.2007.00504.x. [DOI] [PubMed] [Google Scholar]
- Peterkova R. Dental lamina develops even within the mouse diastema. J Craniofac Genet Dev Biol. 1983;3:133–142. [PubMed] [Google Scholar]
- Klein OD, Minowada G, Peterkova R, et al. Sprouty genes control diastema tooth development via bidirectional antagonism of epithelial-mesenchymal FGF signaling. Dev Cell. 2006;11:181–190. doi: 10.1016/j.devcel.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza RN, Klein OD. Unraveling the molecular mechanisms that lead to supernumerary teeth in mice and men: current concepts and novel approaches. Cells Tissues Organs. 2007;186:60–69. doi: 10.1159/000102681. [DOI] [PubMed] [Google Scholar]
- Butler PM. The ontogeny of molar teeth. Biol Rev. 1956;31:30–70. [Google Scholar]
- MacKenzie A, Ferguson MW, Sharpe PT. Expression patterns of the homeobox gene, Hox-8, in the mouse embryo suggest a role in specifying tooth initiation and shape. Development. 1992;115:403–420. doi: 10.1242/dev.115.2.403. [DOI] [PubMed] [Google Scholar]
- Jernvall J, Kettunen P, Karavanova I, Martin LB, Thesleff I. Evidence for the role of the enamel knot as a control center in mammalian tooth cusp formation: non-dividing cells express growth stimulating Fgf-4 gene. Int J Dev Biol. 1994;38:463–469. [PubMed] [Google Scholar]
- Nozue T. Specific spindle cells and globular substances in enamel knot. Okajimas Folia Anat Jpn. 1971;48:139–151. doi: 10.2535/ofaj1936.48.2-3_139. [DOI] [PubMed] [Google Scholar]
- Kindaichi K. An electron microscopic study of cell death in molar tooth germ epithelia of mouse embryos. Arch Histol Jpn. 1980;43:289–304. doi: 10.1679/aohc1950.43.289. [DOI] [PubMed] [Google Scholar]
- Vaahtokari A, Aberg T, Thesleff I. Apoptosis in the developing tooth: association with an embryonic signaling center and suppression by EGF and FGF-4. Development. 1996b;122:121–129. doi: 10.1242/dev.122.1.121. [DOI] [PubMed] [Google Scholar]
- Jernvall J, Aberg T, Kettunen P, Keränen S, Thesleff I. The life history of an embryonic signaling center: BMP-4 induces p21 and is associated with apoptosis in the mouse tooth enamel knot. Development. 1998;125:161–169. doi: 10.1242/dev.125.2.161. [DOI] [PubMed] [Google Scholar]
- Sharpe PT. Homeobox genes and orofacial development. Connect Tissue Res. 1995;32:17–25. doi: 10.3109/03008209509013701. [DOI] [PubMed] [Google Scholar]
- Qiu M, Bulfone A, Ghattas I, et al. Role of the Dlx homeobox genes in proximodistal patterning of the branchial arches: mutations of Dlx-1, Dlx-2, and Dlx-1 and -2 alter morphogenesis of proximal skeletal and soft tissue structures derived from the first and second arches. Dev Biol. 1997;185:165–184. doi: 10.1006/dbio.1997.8556. [DOI] [PubMed] [Google Scholar]
- Seichert V, Čihák R, Naňka O. The Guide of the Collections of the Institute of Anatomy, First Faculty of Medicine, Charles University in Prague. Prague: Charles University in Prague, Karolinum Publisher; 2006. pp. 7–99. [Google Scholar]
- Edmund AG. Tooth replacement phenomena in the lower vertebrates. R Ont Mus Life Sci. 1960;52:1–190. [Google Scholar]
- Wolsan M. The origin of extra teeth in mammals. Acta Theriol. 1984;29:128–133. [Google Scholar]
- Drehmer CJ, Fabián ME, Meneghetiet JO, et al. Dental anomalies in the Atlantic population of South American sea lion, Otaria byronia (Pinnipedia, Otariidae): evolutionary implications and ecological approach. LAJAM. 2004;3:7–18. [Google Scholar]
- Darwin C. On the Origin of Species. A facsimile of the First Edition. Cambridge, Massachusetts: Harvard University Press. Sixteenth printing. 1859;2000:411–458. [Google Scholar]