Abstract
BclxL is a key prosurvival factor that in addition to controlling mitochondrial membrane permeability regulates mitochondrial network dynamics. The expression of BclxL is regulated at the level of transcription, splicing and selective translation. In this study, we show that the RNA-binding protein HuR, which is known to orchestrate an anti-apoptotic cellular program, functions as a translational repressor of BclxL. We show that HuR binds directly to the 5`UTR of BclxL, and represses BclxL translation through the inhibition of its internal ribosome entry site (IRES). Reduction of HuR levels leads to the derepression of BclxL translation and subsequent rearrangement of the mitochondrial network. Our results place BclxL into the HuR-regulated operon and provide further insight into the regulation of cellular stress response by HuR.
Keywords: translational control, ITAF, IRES, mitochondrial dynamics, RNA binding protein
Introduction
Mitochondrial dynamics has recently emerged as an important regulatory process in cellular bioenergetics, apoptosis and disease.1 Mitochondria exist as dynamic networks alternating between fusion and fission states that allow for the exchange and movement of cellular components such as mtDNA, proteins, ATP and lipids. This process is necessary for the proper maintenance of mitochondrial homeostasis and its misregulation is directly linked to a variety of human disease states.
One of the best studied regulatory mechanisms that is intimately linked to mitochondrial morphology is the cellular apoptotic machinery.2 Mitochondria serve as cellular sentinels that detect, integrate and propagate death signals. The key factors participating in this process are members of the Bcl-2 family of proteins which regulate membrane permeabilization and the release of pro-apoptotic factors such as cytochrome c and Smac/DIABLO, thus triggering cell death.3 Recently, studies have suggested an uncoupling between Bcl-2 family-mediated cell survival and mitochondrial dynamics.4 For example, different forms of Mcl-1 were shown to reside in distinct mitochondrial locations where they perform separate functions.5
BclxL is a key pro-survival member of the Bcl-2 family whose sole overexpression is sufficient to inhibit cell death.6 The role of BclxL in mitochondrial dynamics is not clear; overexpression of BclxL increased mitochondrial fission in ~50% of cells while the remaining cells exhibited increased mitochondrial fusion.7,8 Similarly, re-expression of BclxL in BclxL-null mice promoted both fusion and fission rates and mitochondrial mass in healthy neurons.9 The expression of BclxL is known to be regulated at the level of transcription and alternative splicing.10,11 In particular, Bcl-x pre-mRNA can be spliced into a long, anti-apoptotic isoform (xL) or a short, pro-apoptotic isoform (xS). Various stimuli, along with different splicing factors regulate the switch between these isoforms. For instance, mitogenic pathways promote the expression of the xL isoform whereas the sphingolipid ceramide stimulates expression of the xS isoform. The relative levels of these factors determine whether cells will be resistant to death triggers or engage in apoptosis.11 Additional point of BclxL regulation was identified recently. Yoon and colleagues have shown that translation of BclxL mRNA is specifically impaired in cells harbouring mutation of dyskerin, the gene mutated in patients suffering from Dyskeratosis congenita.12 They further demonstrated that the 5′ UTR of BclxL harbours an IRES element which drives BclxL translation under stress. Additional recent reports confirmed the 5′ UTR of BclxL as a key regulatory cis-element controlling expression of BclxL. Cytoplasmic accumulation of hnRNP A1 in cells undergoing osmotic shock was shown to inhibit BclxL mRNA translation by binding to the 5′ UTR and tipping the survival balance in favor of apoptosis.13 Similarly, binding of PDCD4 to the 5′ UTR of BclxL specifically repressed BclxL translation, and this repression was relieved in cells treated in FGF-2, resulting in the degradation of PDCD4 and de-repression of BclxL translation, thus contributing to enhanced chemoresistance.14
In the present study we demonstrate a link between the translational control of BclxL expression and mitochondrial morphology. We show that an RNA binding protein HuR specifically and directly binds to the 5′ UTR of BclxL and functions as a repressor of BclxL translation in cells. Reduction in HuR levels by siRNA results in marked increase in BclxL expression and subsequent fragmentation of the mitochondrial network. Our results suggest that HuR contributes to the maintenance of the mitochondrial network by controlling BclxL expression.
Results
HuR is a translational repressor of BclxL
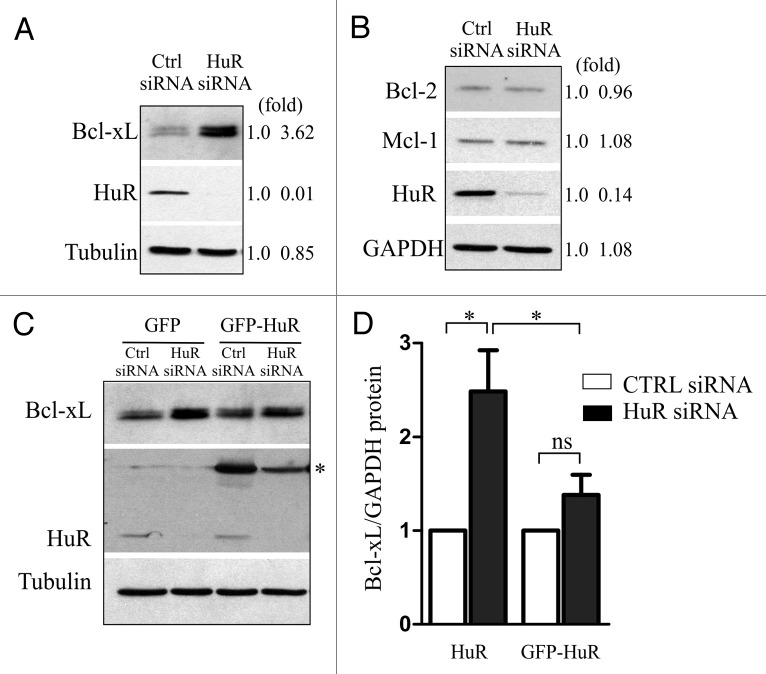
We have shown recently that HuR regulates translation of XIAP through the interaction with the XIAP 5′ UTR.15 Since XIAP and BclxL translation were shown to be coordinately regulated12-14 we hypothesized that HuR might also control translation of BclxL. We therefore examined whether modulating levels of HuR, either by knockdown with small interfering RNA (siRNA) or by overexpression, have any effect on the translation of endogenous BclxL mRNA. U2OS cells were transiently transfected with either HuR-targeting or non-silencing control siRNA and the levels of BclxL protein were determined 72 h later by western blot analysis. We found that reducing the levels of HuR by siRNA resulted in ~3.5 fold increase in BclxL protein levels compared with a non-silencing control (Fig. 1A). This increase in expression was specific to BclxL, since reducing the levels of HuR had no effect on the expression of closely related members of the Bcl-2 family of proteins, Bcl-2 and Mcl-1 (Fig. 1B). Importantly, the increase in BclxL protein levels caused by reduction in HuR could be significantly blunted by restoring the levels of HuR (Fig. 1C and D). These observations suggest that HuR is a negative regulator of BclxL expression.
Figure 1. Cellular levels of HuR control the expression of BclxL. U2OS cells were transiently transfected with HuR-targeting or non-silencing (Ctrl) siRNA and the levels of BclxL (A), or Bcl-2 and Mcl-1 (B) were determined 72 h after transfection by western blot analysis. Signal intensities were quantified by densitometry and are shown on the right (fold). (C) U2OS cells were transiently transfected with HuR-targeting or non-silencing (Ctrl) siRNA, and 48 h later transfected with GFP- or GFP-HuR expressing plasmid as indicated. The expression levels of BclxL were determined 24 h following the plasmid transfection by western blot analysis (* denotes GFP-HuR). Signal intensities were quantified by densitometry and are shown in (D). (* p < 0.05; n = 3).
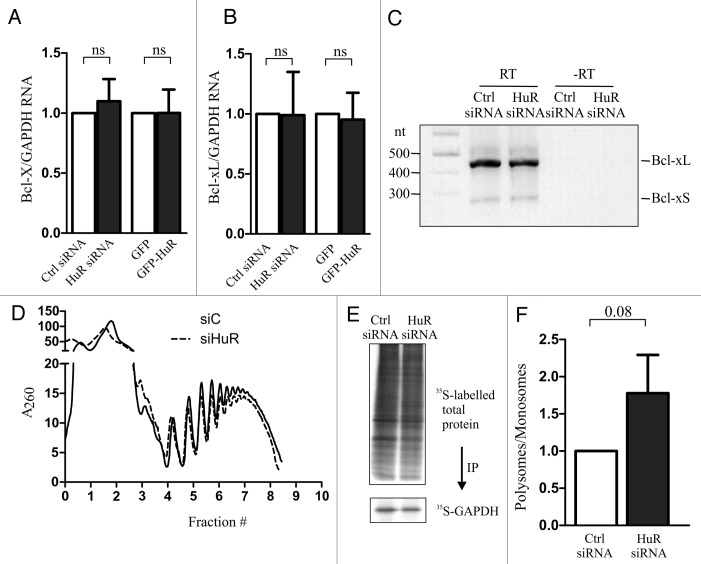
To further demonstrate that HuR controls the translation and not the steady-state levels of endogenous BclxL mRNA we examined the mRNA levels of BclxL by quantitative RT-PCR in cells in which the expression of HuR was either reduced by siRNA, or increased by ectopic expression. BclxL is one of the two alternatively spliced variants of the Bclx gene16; we therefore determined the steady-state levels of both BclxL and BclxS splice isoforms using a common primer set (Bclx; Fig. 2A) and specifically just that of BclxL (Fig. 2B). We found that modulating the levels of HuR had no effect on the abundance of BclxL mRNA. Furthermore, to eliminate the possibility that the increase in BclxL is due to the change in the splicing ratio between BclxL and BclxS we examined the abundance of each isoform by isoform specific RT-PCR. Again, we didn’t observe any differences in the ratio of the two isoforms in cells with reduced expression of HuR (Fig. 2C). To demonstrate that HuR indeed controls the translation of endogenous BclxL mRNA, we used polysome profiling to examine the association of BclxL mRNA with translating ribosomes in cells with reduced levels of HuR. Because we were unable to obtain sufficient amount of ribosomes-associated RNA from U2OS cells, we used HEK293 cells which recapitulate the HuR-mediated changes in BclxL expression seen in U2OS cells (data not shown). We observed that reducing HuR levels by siRNA had no measurable effect on the overall polysome profile (Fig. 2D), indicating that reduced levels of HuR do not affect global rate of protein synthesis. This was further corroborated by measuring 35S-Met incorporation which indicated no measurable difference in global de novo protein synthesis, nor a difference in de novo synthesis of GAPDH, a representative house-keeping gene, in cells with reduced levels of HuR (Fig. 2E). We could not test de novo synthesis of BclxL, as the anti-BclxL antibody did not precipitate sufficient amount of labeled BclxL for analysis. However, reverse transcriptase-PCR amplification of BclxL mRNA from individual polysome fractions showed increased polysome association in cells with reduced HuR levels (Fig. 2F), in keeping with the notion that HuR functions as a translation repressor of BclxL.
Figure 2. HuR regulates translation of BclxL. U2OS cells were transiently transfected with HuR-targeting or non-silencing (Ctrl) siRNA, or with GFP- or GFP-HuR expressing plasmid and the steady-state levels of Bclx (A) and BclxL (B) mRNAs were determined by quantitative RT-PCR. (C) Splice-variant specific RT-PCR was performed on RNA extracted from U2OS cells transiently transfected with HuR-targeting or non-silencing (Ctrl) siRNA. The position of BclxL and BclxS is indicated on the right. (D) Representative polysome profile trace of HEK293 cells transiently transfected with HuR-targeting or non-silencing (Ctrl) siRNA. Fractions (0 – top; 10 – bottom) are indicated below the graph. (E) Analysis of de novo proteins synthesis by L-[35S]methionine labeling of HEK293 cells after transfection with either HuR-targeting or non-silencing (Ctrl) siRNA. GAPDH was immunoprecipitated using anti-GAPDH antibodies. (F) The relative polysome abundance of BclxL mRNA calculated from polysome profiling (monosomes – fractions 1–4; polysomes – fractions 5–9).
HuR represses BclxL translation through interaction with its 5′ UTR
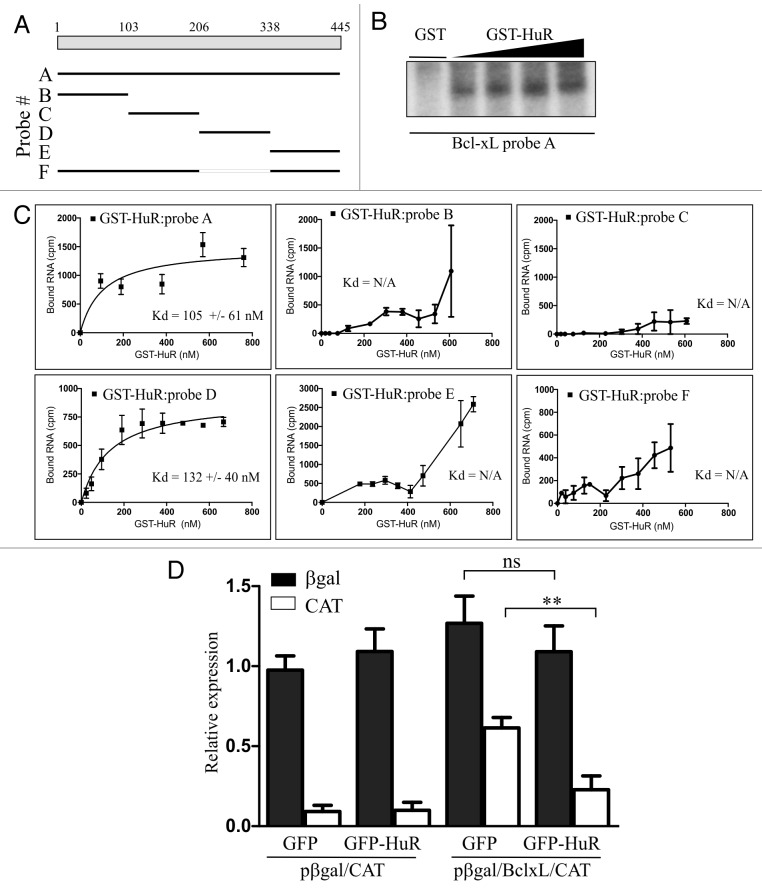
We wished to elucidate the molecular basis for the translational repression of BclxL by HuR. In previously published reports HuR was frequently found to interact with the 5′ UTR regions of mRNAs whose translation was regulated by HuR.15,17-19 We therefore questioned whether HuR interacts with the 5′ UTR of BclxL as well. To determine whether HuR binds directly to the BclxL 5′ UTR we performed a UV-crosslinking experiment using a radiolabelled BclxL 5′ UTR RNA probe and purified recombinant GST-HuR. Increasing amounts of GST-HuR were incubated with [32P]-labeled full length BclxL 5′ UTR RNA (Fig. 3A; probe A), followed by crosslinking and separation by SDS-PAGE. We found that BclxL 5′ UTR RNA is crosslinked to GST-HuR in a dose-dependent manner (Fig. 3B), indicating that HuR does indeed bind directly to BclxL 5′ UTR.
Figure 3. HuR binds directly to the 5′ UTR of BclxL and regulates its translation. (A) Schematic diagram of BclxL 5′ UTR and the deletion fragments used in the binding assays. (B) Recombinant GST-HuR was incubated in the presence of 32P-labeled, in vitro transcribed RNA (probe A) and subjected to UV cross-linking. RNA-protein complexes were separated by SDS-PAGE and analyzed by autoradiography. GST was used as a negative control. (C) Increasing concentrations of GST-HuR were incubated with indicated, 32P-labeled, in vitro transcribed RNAs and nitrocellulose filter binding assays were performed as described in Material and Methods. Levels of filter-bound RNA are plotted as a function of protein concentration. Apparent dissociation constants (Kd) are shown for each probe (mean +/− S.E.M., n = 3). (D) Bicistronic DNA construct containing BclxL 5′ UTR (pßgal/BclxL/CAT), or a parental vector (pßgal/CAT) were co-transfected into HEK293 cells along with GFP- or GFP-HuR expressing plasmids and the expression levels of each reporter gene were determined 24 h after transfection (** p < 0.01; n = 3).
We further assessed the interaction between HuR and the BclxL 5′ UTR by measuring the apparent equilibrium dissociation constant (Kd) of the recombinant GST-HuR and BclxL 5′ UTR RNA. To determine the Kd of this interaction we performed a nitrocellulose filter binding assay, in which varying amounts of GST-HuR are incubated with a constant amount of RNA. We found the Kd for the interaction between GST-HuR and BclxL 5′ UTR RNA is 105 ± 61 nM (Fig. 3C). In order to determine the region of HuR interaction with the BclxL 5′ UTR we generated a series of deletions (Fig. 3A) which were subsequently subjected to the filter binding assay with GST-HuR as described above. We found that GST-HuR does not effectively bind probes B, C, and E (Fig. 3C; we were unable to determine the actual Kd value because we were unable to saturate binding). In contrast, probe D of the BclxL 5′ UTR showed strong binding with the Kd similar to that of the full length RNA (132 ± 40 nM). Importantly, deletion of segment D from the full length 5′ UTR failed to bind GST-HuR (Fig. 3C; probe F). Therefore, HuR specifically associates with BclxL 5′ UTR RNA.
We have shown that cellular levels of HuR control translation of BclxL and that HuR binds to the 5′ UTR region of BclxL mRNA. The 5′ UTR of BclxL harbours an IRES element that controls translation of the BclxL mRNA under various physiological and pathophysiological conditions.12-14 We therefore examined whether modulating levels of HuR has any effect on the activity of BclxL IRES. To assess BclxL IRES activity, we used a previously characterized bicistronic reporter plasmid containing the BclxL IRES (pßgal/BclxL/CAT13,14). In this construct, expression of the first cistron (β-galactosidase, βgal) is cap dependent, whereas expression of the second cistron (chloramphenicol acetyl transferase, CAT) is driven by BclxL 5′ UTR. HEK293 cells were transiently co-transfected with pßgal/BclxL/CAT reporter plasmid and with either GFP- or a GFP-HuR expressing plasmid. We found that overexpression of GFP-HuR caused ~60% reduction in CAT expression compared with that of a GFP control (Fig. 3D). In contrast, overexpression of GFP-HuR had no effect on the expression of βgal. This observation was somewhat surprising since we have reported previously that overexpression of HuR caused a decrease in overall translation 15; however, the decrease in global translation was observed by polysome profile analysis whereas in the current study we only followed expression of a single, ectopically expressed transgene. Overexpression of GFP-HuR had no effect on the expression of either reporter protein from the control plasmid pßgal/CAT, suggesting that HuR specifically regulates translation controlled by the BclxL 5′ UTR. We wished to test the activity of BclxL 5′ UTR deleted of the HuR binding site (probe F). However, this deletion causes the loss of basal IRES activity12 (and data not shown) and we were therefore unable to test this construct in cells. Similarly, we were unable to perform the converse experiment, since downregulation of HuR by siRNA caused rapid degradation of the reporter RNA irrespective of which reporter plasmid was used in the assay.
HuR regulates mitochondrial morphology through BclxL
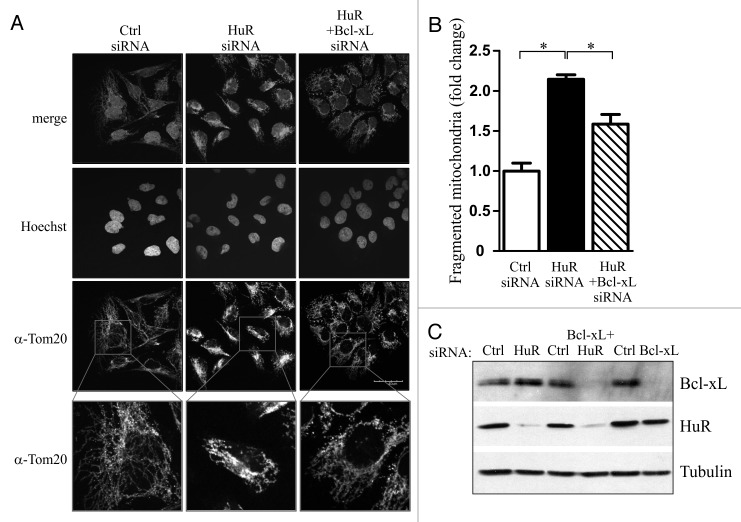
Members of the Bcl-2 family of proteins, including BclxL regulate the morphology of the mitochondrial network.4,20 We therefore wished to investigate if reducing the levels of HuR, and the consequent increase in BclxL expression, would impact mitochondrial morphology. U2OS cells were transiently transfected with siRNA targeting HuR or a non-silencing control, and the mitochondrial network was visualized 72 h later by staining the fixed cells for the outer mitochondrial marker Tom20. We observed that there was a 2.2-fold increase in fragmented mitochondria in HuR siRNA treated cells when compared with cells treated with non-silencing siRNA (Fig. 4A and B). Importantly, siRNA-mediated knockdown of BclxL significantly reduced the portion of fragmented mitochondria (Fig. 4A and B). These results indicate that the induction of BclxL expression through HuR downregulation contributes to the rearrangement of the mitochondrial network.
Figure 4. HuR regulates mitochondrial morphology through BclxL. (A) U2OS were transiently transfected with HuR-targeting, non-silencing (Ctrl), or a combination of HuR- and BclxL -targeting siRNA and the cells were prepared for immunofluorescent microscopy 72 h later. Mitochondrial morphology was visualized by staining with anti-Tom20 antibody. (B) The fraction of cells with fragmented mitochondria from (A) were determined by two blind observers and is plotted for each treatment (* p < 0.05; n = 83 cells for each treatment) (C) Expression levels of BclxL and HuR in lysates from cells treated as in (A) were determined by western blot analysis.
Discussion
BclxL is a key component of the cellular anti-apoptotic machinery. In addition to regulating the release of pro-apoptotic mitochondrial factors such as cytochrome c and Smac/DIABLO, recent data suggests that BclxL also actively participates in the control of mitochondrial dynamics that is independent of the regulation of apoptosis.4 We and others have shown that in addition to the regulation of BclxL splicing and transcription, control of BclxL translation is the key regulatory step, in particular in response to cellular stress.12-14 The 5′ UTR of BclxL was shown to harbour an IRES element that allows the BclxL mRNA to be translated in times of cellular stress when global translation is attenuated. Translation of the majority of cellular mRNAs under normal growth conditions occurs by a cap-dependent mechanism that requires interaction of specific initiation factors with the 5′ cap of the mRNA, followed by recruitment of ribosomal subunits, recognition of the AUG start codon and commencement of polypeptide chain elongation.21 In contrast, IRES-mediated translation initiation was identified as key mechanism which supports cap-independent translation under conditions of cellular stress such as nutrient deprivation, hypoxia or gamma irradiation.22 Although the precise mechanism of cellular IRES translation remains unclear, it has been postulated that cellular IRES require some of the canonical initiation factors as well as auxiliary proteins (termed IRES trans-acting factors, ITAFs) for their proper function.23,24 Two such factors were identified previously for the BclxL IRES. hnRNP A1, which accumulates in the cytoplasm in response to various forms of cellular stress, was shown to bind to the 5′ UTR of BclxL and to attenuate its translation during osmotic stress.13 Although the precise mechanism of this inhibition was not elucidated, it was suggested that hnRNP A1 specifically sequesters BclxL in stress granules in response to hypertonic stress, thus inhibiting its translation. The second protein that similarly inhibits BclxL translation is PDCD4.14 PDCD4 is a tumor suppressor which was shown to act as an inhibitor of eIF4A, an RNA helicase component of the eIF4F complex which is required for the recruitment of capped mRNA to the ribosome.25 Although PDCD4 is generally viewed as a general translation repressor, recent evidence suggests that PDCD4 preferentially represses mRNA with highly structured 5′ UTRs26,27 and those harbouring an IRES.14 We have shown previously that PDCD4 specifically interacts with the 5′ UTRs of at least two anti-apoptotic factors, XIAP and BclxL, and mediates repression of their translation by interfering with the assembly of the 48S initiation complex.14 In response to FGF-2 treatment of cells, PDCD4 is phosphorylated by S6K2 and targeted for proteosomal degradation, thus de-repressing translation of BclxL and resulting in enhanced chemoresistance.14,28 In the present study we have identified a third repressor of BclxL translation. We show that HuR binds directly and specifically to the 5′ UTR of BclxL. Importantly, we showed that HuR translationaly regulates BclxL through the IRES element located within the 5′ UTR. Using the 5′ UTR reporter construct we demonstrated that the IRES activity of BclxL decreases upon overexpression of HuR, while the loss of HuR results in enhanced recruitment of BclxL mRNA into polyribosomes and increase in BclxL protein levels. Interestingly, the same three BclxL ITAFs were also shown to regulate expression of another anti-apoptotic protein, XIAP14,15,29 suggesting an existence of a common regulatory network(s) that may control selective translation of mRNAs involved in the regulation of cell survival in response to stress. However, in contrast to hnRNP A1 and PDCD4, HuR functions as an enhancer of XIAP but a repressor of BclxL translation. Such dichotomy has been shown for HuR previously since it inhibits translation of some (e.g., p27;30) while enhancing activity of other (e.g., HCV;31) IRES. Although the basis for this dichotomy is not known, it is possible that conformational changes elicited by the binding of HuR to some IRES will render these elements non-functional, while binding to other IRES will induce conformation that is amenable to ribosome recruitment. In addition, the cohort of other ITAFs and possibly canonical initiation factors, which seems to be more or less distinct for various IRES elements, will play a significant role in rendering HuR a repressor or activator of the given IRES. Of note, translation of BclxL mRNA was shown previously to be significantly enhanced by overexpression of the cap-binding protein eIF4E 32 and this increase led to the inhibition of cytochrome c release from the mitochondria. Interestingly, the cohorts of HuR and eIF4E targets are at least partially overlapping 33 illustrating the interdependent regulation of critical genes involved in the regulation of cell growth, proliferation and apoptosis.
HuR is a multifunctional protein, a member of the ELAV family of RNA-binding proteins that has been implicated in a variety of RNA metabolism processes including mRNA stability and translational control.34 Interestingly, HuR appears to play a dual role in the regulation of cell death and survival. It has been shown to orchestrate both the pro- and anti-apoptotic cascades depending on the upstream pathways that are triggered. It is believed that activation of distinct signaling pathways triggers HuR-dependent control of specific groups of mRNAs (HuR regulons), thereby altering the cellular state.35 HuR prevents cell death by upregulating expression of inhibitors of apoptosis such as XIAP, ProT-α, p21, p53 and Survivin.15,36-38 Conversely, HuR suppresses pro-apoptotic factors such as Fas1.39 This HuR-dependent pro-survival regulation correlates well with the observations that HuR levels are elevated in numerous cancers and suggests that HuR could attenuate cell death from occurring in malignant cells and tumors, thereby promoting resistance to radiation and chemotherapies.40 However, other data suggests that when cellular stress is prolonged or lethal, a threshold is reached where HuR engages the apoptotic cascade. For example, upon staurosporine treatment, HuR is translocated to the cytoplasm in association with apoptosome activator pp32/PHAP-1 and is subsequently cleaved by caspases into a fragment (HuR-CP1) which was found to amplify the apoptotic response.41 Interestingly, it was observed that in muscle cells the HuR-CP1 fragment caused cytoplasmic accumulation of full length HuR and subsequent enhancement of myogenesis.42 Our data places BclxL within the HuR-regulated operon and shows that HuR, in addition to directly orchestrating cellular antiapoptotic program also contributes to the control of mitochondrial morphology.
Material and Methods
Cell culture, expression constructs and transfection
U2OS and HEK293cells were maintained at 37°C, 5% CO2 in complete Gibco®’s DMEM (1% FBS, 1% glutamine, 100,000 U/L penicillin and 100 μg/L streptomycin). The bicistronic reporter plasmid pßgal/BclxL/CAT has been described previously14 and contains the 5′UTR of BclxL.12 The GST-HuR and GFP-HuR expressing plasmids were described previously.41 For siRNA knockdown experiments, 1.5x105 cells were reverse transfected in a 6-well plate with 10 nM HuR siNRA, 30 nM BclxL siRNA, or corresponding non-silencing controls (Dharmacon) following the manufacturer’s protocol (Lipofectamine™ RNAiMAX, Invitrogen). Cells were harvested 72 h later. For overexpression experiments, 1.5x105 cells were seeded in a 6-well plate for 24 h, then 2 μg of GFP or GFP-HuR plasmid DNA was transfected following the manufacturer’s protocol (JetPrime™, Polyplus transfection). Cells were harvested 24 h later for RNA extraction or Western Blot analysis. For rescue experiments, 1.5x105 cells were first reverse transfected with 10 nM HuR siRNA or non-silencing control for 48 h, then 2 μg of GFP or GFP-HuR plasmid DNA was forward transfected for an additional 24 h and cells were harvested for further analysis.
UV-crosslinking and nitrocellulose filter binding assays of RNA-protein complexes
Radioactive BclxL 5′ UTR probes for UV-crosslinking and nitrocellulose filter binding assays were prepared by an in vitro transcription using Maxiscript® kit (Ambion) and gel purified. RNA-protein UV-crosslinking experiments and the nitrocellulose binding assay were conducted as previously described.14,15
β-galactosidase and CAT analysis
Transiently transfected cells were washed in 1ml of phosphate-buffered saline and harvested in 300 μl CAT ELISA kit lysis buffer according to the protocol provided by the manufacturer (Roche Molecular Biochemicals). β-galactosidase (β-gal) enzymatic activity was determined by spectrophotometric assay using o-nitrophenyl-β-D-galactopyranoside as previously described.43 CAT levels were determined using the CAT ELISA kit according to the protocol provided by the manufacturer (Roche Molecular Biochemicals).
Western blot analysis
Cells were washed in 1ml phosphate-buffered saline and lysed in 150 μl RIPA buffer (50 mM Tris base pH7.4, 1mM EDTA, 150 mM NaCl, 1% NP-40, 0.5% SDS, 0.25 g deoxycholate in 50 mL water; 1 mM PMSF and 10 μg/mL leupeptin were added fresh) for 30 min at 4°C, followed by centrifugation at 12 000 g for 10 min to pellet debris. Protein concentration was determined by the BCA Protein Assay Kit (Pierce Biotechnology) and equal amounts of protein extract were separated by 10% SDS–PAGE and transferred to nitrocellulose membrane by wet transfer. Samples were analyzed by western blotting using rabbit anti-BclxL (Cell Signaling Technologies), mouse monoclonal anti-HuR (Santa Cruz Biotechnology), mouse anti-GAPDH (Advanced Immunochemical Inc.), mouse anti-Tubulin (Abcam), rabbit anti-Mcl-1 (Santa Cruz Biotechnology), or rabbit anti-Bcl-2 (Cell Signaling Technologies) followed by secondary antibody (horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG; Cell Signaling Technologies). Antibody complexes were detected using the ECL or ECL Plus systems (GE Healthcare) and were quantified using Odyssey densitometry software (Li-Cor, Lincoln, NE, USA).
RNA extraction, RT-PCR and quantitative RT-PCR analysis
Total RNA was isolated from transfected cells using RNazol according to the manufacturer’s instructions (Molecular Research Center). cDNA was generated using an oligo dT18 primer and the qScriptTM cDNA Supermix according to the protocol provided by the manufacturer (Quanta Biosciences). The synthesized cDNA was used as the template for quantitative PCR using the QuantiTect SYBR Green PCR kit (Qiagen) along with gene specific primers for Bclx, BclxL (QuantiTect Primer Assay, Qiagen), and GAPDH,15 and analyzed on a Mastercycler realplex (Eppendorf) real-time thermocycler using the associated realplex software. Relative expression levels were determined using the standard curve method. Controls lacking RT demonstrated no significant genomic DNA amplification (> 10 cycle difference). For splice-specific variant RT-PCR, the cDNA was used as a template in standard PCR with KOD polymerase and primers Bcl-X2 and Bcl-X3 as described.44
Polysome profiling
HEK293T cells were reverse transfected with 10 nM HuR siRNA or non-silencing control siRNA for 72 h in 6-well plates (2 plates per condition) and polysome isolation was done as described.14 Gradient fractions were collected from the top using the ISCO Teledyne programmable gradient fractionation system, and RNA was monitored at 254 nm. RNA was isolated from individual fractions (1 ml) by proteinase K digestion followed by phenol-chloroform extraction and ethanol precipitation. Equal quantities of RNA from each fraction were used to generate cDNA as described above. Quantitative RT-PCR was used to determine the abundance of BclxL and GAPDH mRNA in each fraction as described above.
Immunofluorescent microscopy
U2OS cells were seeded at a density of 7.5 x 104 cells on coverslips in 6-well plates for 24 h before transfection with HuR siRNA, BclxL siRNA, or a non-silencing control siRNA for 72 h. Cells were rinsed 3 times in phosphate-buffered saline, fixed with 3.7% paraformaldehyde for 15 min and then permeabilized with 0.2% Triton-X100 for 15 min before blocking in 1% FBS for 15 min. Coverslips were incubated with α-Tom20 anti-rabbit antibody (Santa Cruz; 1: 2000 diluted in 0.2% Triton-X100/ 0.004% BSA) for 1 h, washed 3x for 5 min in Triton-X100/0.004% BSA buffer before incubation with secondary antibody for 1 h (Alex Fluor A594 goat anti-rabbit, Invitrogen, diluted in 0.2% Triton-X100/ 0.004% BSA). Hoechst dye was added (1 μg/mL) for 10 min to stain the nuclei and cells were washed 4x for 5 min in phosphate-buffered saline prior to mounting. Coverslips were mounted on slides using Dako Fluorescent Mouting Medium. Confocal microscopy was performed using the 60X objective with water (Olympus Fluoview FV1000).
Statistical analysis
All data are expressed as means ± standard error of the mean (SE), with a minimum of three independent experimental replicates unless otherwise noted. For reporter assays and for quantitative RT-PCR, independent replicates consisted of three biological triplicate experiments. A student t-test was performed to determine data significance using GraphPad Prism version 5.04 for Windows (GraphPad Software).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the members of the Apoptosis Research Centre and Drs. Martin Pelchat and Jocelyn Côté for critical discussion, and Dr. Imed Gallouzi for the generous gift of HuR-expression constructs. This work forms part of the M.Sc. dissertation of D.D. and was supported by an operating grant from the Canadian Institutes of Health Research (CIHR; MOP 89737) to M. H. and from the National Institutes of Health (NIH; DK060596 and DKO53307) to M. H. M. H. is the CHEO Volunteer Association Endowed Scholar.
References
- 1.Hyde BB, Twig G, Shirihai OS. . Organellar vs cellular control of mitochondrial dynamics. Semin Cell Dev Biol 2010; 21:575 - 81; http://dx.doi.org/ 10.1016/j.semcdb.2010.01.003; PMID: 20079451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galluzzi L, Kepp O, Kroemer G. . Mitochondria: master regulators of danger signalling. Nat Rev Mol Cell Biol 2012; 13:780 - 8; http://dx.doi.org/ 10.1038/nrm3479; PMID: 23175281 [DOI] [PubMed] [Google Scholar]
- 3.Youle RJ, Strasser A. . The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 2008; 9:47 - 59; http://dx.doi.org/ 10.1038/nrm2308; PMID: 18097445 [DOI] [PubMed] [Google Scholar]
- 4.Autret A, Martin SJ. . Emerging role for members of the Bcl-2 family in mitochondrial morphogenesis. Mol Cell 2009; 36:355 - 63; http://dx.doi.org/ 10.1016/j.molcel.2009.10.011; PMID: 19917245 [DOI] [PubMed] [Google Scholar]
- 5.Perciavalle RM, Stewart DP, Koss B, Lynch J, Milasta S, Bathina M, et al. . Anti-apoptotic MCL-1 localizes to the mitochondrial matrix and couples mitochondrial fusion to respiration. Nat Cell Biol 2012; 14:575 - 83; http://dx.doi.org/ 10.1038/ncb2488; PMID: 22544066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin XM, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, et al. . Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature 1999; 400:886 - 91; http://dx.doi.org/ 10.1038/23730; PMID: 10476969 [DOI] [PubMed] [Google Scholar]
- 7.Delivani P, Adrain C, Taylor RC, Duriez PJ, Martin SJ. . Role for CED-9 and Egl-1 as regulators of mitochondrial fission and fusion dynamics. Mol Cell 2006; 21:761 - 73; http://dx.doi.org/ 10.1016/j.molcel.2006.01.034; PMID: 16543146 [DOI] [PubMed] [Google Scholar]
- 8.Sheridan C, Delivani P, Cullen SP, Martin SJ. . Bax- or Bak-induced mitochondrial fission can be uncoupled from cytochrome C release. Mol Cell 2008; 31:570 - 85; http://dx.doi.org/ 10.1016/j.molcel.2008.08.002; PMID: 18722181 [DOI] [PubMed] [Google Scholar]
- 9.Berman SB, Chen YB, Qi B, McCaffery JM, Rucker EB 3rd, Goebbels S, et al. . Bcl-x L increases mitochondrial fission, fusion, and biomass in neurons. J Cell Biol 2009; 184:707 - 19; http://dx.doi.org/ 10.1083/jcb.200809060; PMID: 19255249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grad JM, Zeng XR, Boise LH. . Regulation of Bcl-xL: a little bit of this and a little bit of STAT. Curr Opin Oncol 2000; 12:543 - 9; http://dx.doi.org/ 10.1097/00001622-200011000-00006; PMID: 11085453 [DOI] [PubMed] [Google Scholar]
- 11.David CJ, Manley JL. . Alternative pre-mRNA splicing regulation in cancer: pathways and programs unhinged. Genes Dev 2010; 24:2343 - 64; http://dx.doi.org/ 10.1101/gad.1973010; PMID: 21041405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoon A, Peng G, Brandenburger Y, Zollo O, Xu W, Rego E, et al. . Impaired control of IRES-mediated translation in X-linked dyskeratosis congenita. Science 2006; 312:902 - 6; http://dx.doi.org/ 10.1126/science.1123835; PMID: 16690864 [DOI] [PubMed] [Google Scholar]
- 13.Bevilacqua E, Wang X, Majumder M, Gaccioli F, Yuan CL, Wang C, et al. . eIF2alpha phosphorylation tips the balance to apoptosis during osmotic stress. J Biol Chem 2010; 285:17098 - 111; http://dx.doi.org/ 10.1074/jbc.M110.109439; PMID: 20338999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liwak U, Thakor N, Jordan LE, Roy R, Lewis SM, Pardo OE, et al. . Tumor suppressor PDCD4 represses internal ribosome entry site-mediated translation of antiapoptotic proteins and is regulated by S6 kinase 2. Mol Cell Biol 2012; 32:1818 - 29; http://dx.doi.org/ 10.1128/MCB.06317-11; PMID: 22431522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durie D, Lewis SM, Liwak U, Kisilewicz M, Gorospe M, Holcik M. . RNA-binding protein HuR mediates cytoprotection through stimulation of XIAP translation. Oncogene 2011; 30:1460 - 9; http://dx.doi.org/ 10.1038/onc.2010.527; PMID: 21102524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sazani P, Kole R. . Therapeutic potential of antisense oligonucleotides as modulators of alternative splicing. J Clin Invest 2003; 112:481 - 6; PMID: 12925686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng Z, King PH, Nabors LB, Jackson NL, Chen CY, Emanuel PD, et al. The ELAV RNA-stability factor HuR binds the 5′-untranslated region of the human IGF-IR transcript and differentially represses cap-dependent and IRES-mediated translation. Nucleic Acids Res 2005; 33:2962-79. Print 005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kullmann M, Göpfert U, Siewe B, Hengst L. . ELAV/Hu proteins inhibit p27 translation via an IRES element in the p27 5’UTR. Genes Dev 2002; 16:3087 - 99; http://dx.doi.org/ 10.1101/gad.248902; PMID: 12464637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeh CH, Hung LY, Hsu C, Le SY, Lee PT, Liao WL, et al. . RNA-binding Protein HuR Interacts with Thrombomodulin 5′UTR and Represses IRES-mediated Translation under IL-1{beta} treatment. Mol Biol Cell 2008; 25:25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cleland MM, Norris KL, Karbowski M, Wang C, Suen DF, Jiao S, et al. . Bcl-2 family interaction with the mitochondrial morphogenesis machinery. Cell Death Differ 2011; 18:235 - 47; http://dx.doi.org/ 10.1038/cdd.2010.89; PMID: 20671748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holcik M, Sonenberg N. . Translational control in stress and apoptosis. Nat Rev Mol Cell Biol 2005; 6:318 - 27; http://dx.doi.org/ 10.1038/nrm1618; PMID: 15803138 [DOI] [PubMed] [Google Scholar]
- 22.Silvera D, Formenti SC, Schneider RJ. . Translational control in cancer. Nat Rev Cancer 2010; 10:254 - 66; http://dx.doi.org/ 10.1038/nrc2824; PMID: 20332778 [DOI] [PubMed] [Google Scholar]
- 23.Komar AA, Hatzoglou M. . Cellular IRES-mediated translation: the war of ITAFs in pathophysiological states. Cell Cycle 2011; 10:229 - 40; http://dx.doi.org/ 10.4161/cc.10.2.14472; PMID: 21220943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis SM, Holcik M. . For IRES trans-acting factors, it is all about location. Oncogene 2008; 27:1033 - 5; http://dx.doi.org/ 10.1038/sj.onc.1210777; PMID: 17767196 [DOI] [PubMed] [Google Scholar]
- 25.Suzuki C, Garces RG, Edmonds KA, Hiller S, Hyberts SG, Marintchev A, et al. . PDCD4 inhibits translation initiation by binding to eIF4A using both its MA3 domains. Proc Natl Acad Sci U S A 2008; 105:3274 - 9; http://dx.doi.org/ 10.1073/pnas.0712235105; PMID: 18296639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang H-S, Jansen AP, Komar AA, Zheng X, Merrick WC, Costes S, et al. . The transformation suppressor Pdcd4 is a novel eukaryotic translation initiation factor 4A binding protein that inhibits translation. Mol Cell Biol 2003; 23:26 - 37; http://dx.doi.org/ 10.1128/MCB.23.1.26-37.2003; PMID: 12482958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang HS, Cho MH, Zakowicz H, Hegamyer G, Sonenberg N, Colburn NH. . A novel function of the MA-3 domains in transformation and translation suppressor Pdcd4 is essential for its binding to eukaryotic translation initiation factor 4A. Mol Cell Biol 2004; 24:3894 - 906; http://dx.doi.org/ 10.1128/MCB.24.9.3894-3906.2004; PMID: 15082783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pardo OE, Wellbrock C, Khanzada UK, Aubert M, Arozarena I, Davidson S, et al. . FGF-2 protects small cell lung cancer cells from apoptosis through a complex involving PKCepsilon, B-Raf and S6K2. EMBO J 2006; 25:3078 - 88; http://dx.doi.org/ 10.1038/sj.emboj.7601198; PMID: 16810323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis SM, Veyrier A, Hosszu Ungureanu N, Bonnal S, Vagner S, Holcik M. . Subcellular relocalization of a trans-acting factor regulates XIAP IRES-dependent translation. Mol Biol Cell 2007; 18:1302 - 11; http://dx.doi.org/ 10.1091/mbc.E06-06-0515; PMID: 17287399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coleman J, Miskimins WK. . Structure and activity of the internal ribosome entry site within the human p27 Kip1 5′-untranslated region. RNA Biol 2009; 6:84 - 9; http://dx.doi.org/ 10.4161/rna.6.1.7572; PMID: 19106631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rivas-Aravena A, Ramdohr P, Vallejos M, Valiente-Echeverría F, Dormoy-Raclet V, Rodríguez F, et al. . The Elav-like protein HuR exerts translational control of viral internal ribosome entry sites. Virology 2009; 392:178 - 85; http://dx.doi.org/ 10.1016/j.virol.2009.06.050; PMID: 19647848 [DOI] [PubMed] [Google Scholar]
- 32.Li S, Takasu T, Perlman DM, Peterson MS, Burrichter D, Avdulov S, et al. . Translation factor eIF4E rescues cells from Myc-dependent apoptosis by inhibiting cytochrome c release. J Biol Chem 2003; 278:3015 - 22; http://dx.doi.org/ 10.1074/jbc.M208821200; PMID: 12441348 [DOI] [PubMed] [Google Scholar]
- 33.Topisirovic I, Siddiqui N, Borden KL. . The eukaryotic translation initiation factor 4E (eIF4E) and HuR RNA operons collaboratively regulate the expression of survival and proliferative genes. Cell Cycle 2009; 8:960 - 1; http://dx.doi.org/ 10.4161/cc.8.7.8093; PMID: 19287207 [DOI] [PubMed] [Google Scholar]
- 34.Hinman MN, Lou H. . Diverse molecular functions of Hu proteins. Cell Mol Life Sci 2008; 65:3168 - 81; http://dx.doi.org/ 10.1007/s00018-008-8252-6; PMID: 18581050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdelmohsen K, Gorospe M. . Posttranscriptional regulation of cancer traits by HuR. Wiley Interdiscip Rev RNA 2010; 1:214 - 29; http://dx.doi.org/ 10.1002/wrna.4; PMID: 21935886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazan-Mamczarz K, Galbán S, López de Silanes I, Martindale JL, Atasoy U, Keene JD, et al. . RNA-binding protein HuR enhances p53 translation in response to ultraviolet light irradiation. Proc Natl Acad Sci U S A 2003; 100:8354 - 9; http://dx.doi.org/ 10.1073/pnas.1432104100; PMID: 12821781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donahue JM, Chang ET, Xiao L, Wang PY, Rao JN, Turner DJ, et al. . The RNA-binding protein HuR stabilizes survivin mRNA in human oesophageal epithelial cells. Biochem J 2011; 437:89 - 96; http://dx.doi.org/ 10.1042/BJ20110028; PMID: 21443519 [DOI] [PubMed] [Google Scholar]
- 38.Lal A, Kawai T, Yang X, Mazan-Mamczarz K, Gorospe M. . Antiapoptotic function of RNA-binding protein HuR effected through prothymosin alpha. EMBO J 2005; 24:1852 - 62; http://dx.doi.org/ 10.1038/sj.emboj.7600661; PMID: 15861128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Izquierdo JM. . Hu antigen R (HuR) functions as an alternative pre-mRNA splicing regulator of Fas apoptosis-promoting receptor on exon definition. J Biol Chem 2008; 283:19077 - 84; http://dx.doi.org/ 10.1074/jbc.M800017200; PMID: 18463097 [DOI] [PubMed] [Google Scholar]
- 40.Abdelmohsen K, Gorospe M. . Posttranscriptional regulation of cancer traits by HuR. Wiley Interdiscip Rev RNA 2010; 1:214 - 29; http://dx.doi.org/ 10.1002/wrna.4; PMID: 21935886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mazroui R, Di Marco S, Clair E, von Roretz C, Tenenbaum SA, Keene JD, et al. . Caspase-mediated cleavage of HuR in the cytoplasm contributes to pp32/PHAP-I regulation of apoptosis. J Cell Biol 2008; 180:113 - 27; http://dx.doi.org/ 10.1083/jcb.200709030; PMID: 18180367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beauchamp P, Nassif C, Hillock S, van der Giessen K, von Roretz C, Jasmin BJ, et al. . The cleavage of HuR interferes with its transportin-2-mediated nuclear import and promotes muscle fiber formation. Cell Death Differ 2010; 17:1588 - 99; http://dx.doi.org/ 10.1038/cdd.2010.34; PMID: 20379198 [DOI] [PubMed] [Google Scholar]
- 43.MacGregor GR, Nolan GP, Fiering S, Roederer M, Herzenberg LA. Use of E. coli lacZ (β-Galactosidase) as a reporter gene. In: Murray EJ, Walker JM, eds. Methods in Molecular Biology. Clifton, N.J.: Humana Press Inc., 1991:217-35. [DOI] [PubMed] [Google Scholar]
- 44.Shkreta L, Michelle L, Toutant J, Tremblay ML, Chabot B. . The DNA damage response pathway regulates the alternative splicing of the apoptotic mediator Bcl-x. J Biol Chem 2011; 286:331 - 40; http://dx.doi.org/ 10.1074/jbc.M110.162644; PMID: 20980256 [DOI] [PMC free article] [PubMed] [Google Scholar]