Abstract
Aberrant transcription and mRNA processing of multiple genes due to RNA-mediated toxic gain-of-function has been suggested to cause the complex phenotype in myotonic dystrophies type 1 and 2 (DM1 and DM2). However, the molecular basis of muscle weakness and wasting and the different pattern of muscle involvement in DM1 and DM2 are not well understood. We have analyzed the mRNA expression of genes encoding muscle-specific proteins and transcription factors by microarray profiling and studied selected genes for abnormal splicing. A subset of the abnormally regulated genes was further analyzed at the protein level. TNNT3 and LDB3 showed abnormal splicing with significant differences in proportions between DM2 and DM1. The differential abnormal splicing patterns for TNNT3 and LDB3 appeared more pronounced in DM2 relative to DM1 and are among the first molecular differences reported between the two diseases. In addition to these specific differences, the majority of the analyzed genes showed an overall increased expression at the mRNA level. In particular, there was a more global abnormality of all different myosin isoforms in both DM1 and DM2 with increased transcript levels and a differential pattern of protein expression. Atrophic fibers in DM2 patients expressed only the fast myosin isoform, while in DM1 patients they co-expressed fast and slow isoforms. However, there was no increase of total myosin protein levels, suggesting that aberrant protein translation and/or turnover may also be involved.
Keywords: Myotonic dystrophy type 1 (DM1), Myotonic dystrophy type 2 (DM2), Skeletal muscle, Aberrant splicing, Microarray expression profiling
Introduction
The myotonic dystrophies are dominantly inherited disorders with progressive myopathy, myotonia, and multi-organ involvement. Two genetically distinct types have been identified. Myotonic dystrophy type 1 [DM1; Steinert’s disease (OMIM #160900)] is caused by a (CTG)n expansion mutation in the 3′ untranslated region of dystrophia myotonica-protein kinase (DMPK) gene in chromosome 19q13.3 [5, 14, 18, 31]. The more recently identified myotonic dystrophy type 2 [DM2; proximal myotonic myopathy, PROMM (OMIM #602668)] is caused by a (CCTG)n repeat expansion mutation in the first intron of zinc finger protein 9 (ZNF9) gene [4, 29]. Although the two forms of myotonic dystrophy share many features, there are definite differences with respect to clinical, muscle biopsy, and genetic findings (for detailed comparison, see Table 1). Briefly, in DM2 the core symptoms include proximal muscle weakness, myotonia, cataracts, cardiac conduction defects, and endocrinological dysfunctions such as insulin resistance and male hypogonadism [9, 45, 62]. Myalgic pains may be the major complaint, while serum creatine kinase (CK) levels are normal or moderately elevated [3]. In DM1, the muscle weakness and wasting are more severe, preferentially distal and facial with ptosis, and with later evolving dysphagia, generalized weakness, and respiratory failure. A severe congenital form associated with DM1 has not been observed in DM2, and anticipation is the exception in DM2 [52, 62]. Moreover, clinical symptoms in DM2 vary widely, which makes its clinical diagnosis much more challenging than for DM1 [61, 63]. The basis for the differences between DM1 and DM2 has not been clarified at the molecular level.
Table 1.
Differences in clinical manifestations between DM1 and DM2
| DM1 | DM2 | |
|---|---|---|
| Core features | ||
| Clinical myotonia | Evident in adult-onset | Present in < 50% |
| EMG myotonia | Always present | Absent or variable in many |
| Muscle weakness | Disabling at age 50 | Onset may occur after age 60–70 |
| Cataracts | Always present | Present in minority |
| Localization of muscle weakness | ||
| Facial weakness, jaw muscles | Always | Usually absent |
| Bulbar weakness—dysphagia | Always later | Absent |
| Respiratory muscles | Always later | Exceptional cases |
| Distal limb muscle weakness | Always prominent | Only flexor digitorum profundus on testing, but only in some |
| Proximal limb muscle weakness | May be absent | Main disability in most patients, late |
| Sternocleidomastoid weakness | Always prominent | Prominent in few |
| Muscle symptoms | ||
| Myalgic pain | Absent or mild | Most disabling symptom in many |
| Muscle strength variations | No variations | Can be considerable |
| Visible muscle atrophy | Face, temporal, distal hands and legs | Usually absent |
| Calf hypertrophy | Absent | Present in ≥50% |
| Muscle biopsy | ||
| Fiber atrophy | Smallness of type-1 fibers, not always present | Subgroup of highly atrophic type-2 fibers always present |
| Nuclear clump fibers | In end stage only | Scattered early, before weakness |
| Sarcoplasmic masses | Very frequent in distal muscles | Extremely rare |
| Ring fibers | Frequent | May occur |
| Internal nuclei | Massive in distal muscle | Variable and mainly in type-2 fibers |
| Cardiac arrhythmias | Always present | From absent to severe |
| Brain | ||
| Tremors | Absent | Prominent in many |
| Behavioral change | Early in most | Not apparent |
| Hypersomnia | Prominent | Infrequent |
| Cognitive decline | Prominent | Not apparent |
| Manifest diabetes | Frequent | Infrequent |
| Male hypogonadism | Manifest | Subclinical in most |
| Frontal balding in males | Always present | Exceptionally |
| Other features | ||
| Anticipation | Always present | Exceptional |
| Childhood onset CNS-problems | Frequently present | Absent |
| Congenital Form | Present | Absent |
| Increased frequency of co-segregating CLCN1 mutation | Absent | Present |
| Incapacity (work and ADL) | Always after 30–35 | Rarely before 60 unless severe pains |
| Life expectancy | Reduced | Normal range |
ADL activities of daily life
Several lines of evidence, based on the study of both human patients and mouse models, indicate that the molecular pathomechanism underlying DM1 and DM2 is an RNA-mediated toxic gain-of-function [41]. Mutant transcripts containing (CUG)n or (CCUG)n expansions in DM1 and DM2, respectively, affect RNA-binding proteins leading to alterations in a variety of cellular functions, including the proper splicing of pre-mRNA for a number of downstream effector genes [12, 26, 29, 32, 33, 56]. Aberrant splicing in DM often favors the expression of protein isoforms expressed during development [41]. Many aberrantly spliced genes have been reported in DM1, including skeletal muscle chloride channel 1 (CLCN1) [34], insulin receptor (INSR) [50], fast skeletal muscle troponin T (TNNT3) [23], Z-disk alternatively spliced PDZ-motif containing protein, ZASP (LDB3) [28], myotubularin-related protein 1 (MTMR1) [7], skeletal muscle ryanodine receptor 1 (RYR1) [24], and sarcoplasmic/endoplasmic reticulum fast skeletal muscle Ca2+-ATPase, SERCA1 (ATP2A1) [24, 28]. In cardiac muscle, cardiac troponin T (TNNT2) [42] and ZASP [35] and in brain, microtubule-associated protein tau (MAPT) [27, 36] were shown to be abnormally spliced. In DM2, aberrant splicing has been confirmed for most but not all of the genes affected in DM1 [28], including CLCN1 [34], INSR [51], LDB3 [28], MAPT [36], and TNNT3 [49]. Some of the abnormal protein isoforms seen in DM patients have been related to specific clinical manifestations: for example, abnormal CLC1 leads to myotonia [8, 34], abnormal IR is associated with insulin resistance [50, 51], and abnormal tau isoforms appear to be involved in CNS changes [36]. Inappropriate redistribution or “leaching” of various transcription factors, both general and differentiation factors, such as Sp1 by mutant RNA species in DM1, has been suggested as another pathogenic mechanism [11]. The role of CUGBP1 in the regulation of translation and mRNA stability [47, 58, 59] has been shown in DM1 myoblasts. Recently, it was reported that the rate of protein translation is reduced in DM2 [21], and that a more global dysregulation of both translation and protein degradation is caused by the interaction of (CCUG)n-containing mutant transcripts with cytoplasmic multiprotein complexes, including translation factors [48].
The clinical differences between DM1 and DM2 likely arise from differences in the molecular pathophysiology. These may include distinct spatial and temporal expression patterns of the mutation harboring genes DMPK and ZNF9, and/or different affinities of (CTG)n/(CUG)n- and (CCTG)n/(CCUG)n-repeat containing sequences for specific transcription or splice factors. The presence of very atrophic type 2 fibers early in DM2 muscle pathogenesis, in contrast to DM1 [53, 65], prompted us to investigate the differences in muscle phenotypes at the molecular level. First, we characterized the expression of proteins preferentially expressed in type 2 fibers, and proteins involved in myogenic regeneration and denervation using immunohistochemistry, in order to identify specific abnormalities in the atrophic subpopulation of type 2 fibers in DM2. Second, we studied the expression of genes encoding sarcomeric structural proteins, including the MyHC family and the expression of muscle-specific transcription factors. Finally, we performed a detailed study of isoform expression of the sarcomeric proteins fTnT (TNNT3) and ZASP (LDB3) in DM1 and DM2 muscle, followed by splice variant analysis of the gene transcripts and the corresponding protein isoforms. Table 2 summarizes the genes and proteins analyzed in this study.
Table 2.
Summary of the genes analyzed in this study
| Gene | Full name of the protein | Synonyms | Site of expression/function | EP | SVA/WB | IHC |
|---|---|---|---|---|---|---|
| MYH1 | Myosin heavy chain (MHC) 1 | Myosin-1; MyHC-IIx | Adult skeletal fast type 2B | X | ||
| MYH2 | Myosin heavy chain 2 | Myosin-2, MyHC-IIa | Adult skeletal fast type 2A | X | X | |
| MYH3 | Myosin heavy chain 3 | Myosin-3; MyHC-emb | Embryonic/fetal and regenerating muscle | X | X | |
| MYH4 | Myosin heavy chain 4 | Myosin-4; MyHC-IIb | Adult skeletal muscle (masseter and abdominal external oblique only) | X | (X) | |
| MYH6 | Myosin heavy chain 6 | Myosin-6; MyHC-alpha | Heart | X | ||
| MYH7 | Myosin heavy chain 7 | Myosin-7; MyHC-beta/slow | Adult skeletal slow type 1 fibers; heart | X | X | |
| MYH7B | Myosin heavy chain 7B | Myosin-7B; myosin cardiac muscle beta chain | Heart | X | ||
| MYH8 | Myosin heavy chain 8 | Myosin-8; MyHC-pn | Perinatal/neonatal and reprogramed/regenerating muscle | X | X | |
| MYH10 | Myosin heavy chain 10 | Myosin-10; non-muscle II-b; NMMHC II-b | Brain | X | ||
| MYH13 | Myosin heavy chain 13 | Myosin-13; MyHC-eo | Skeletal muscle, extraocular | X | ||
| LDB3 | LIM domain-binding protein 3 | Z-disk alternatively spliced PDZ-motif protein, ZASP | Sarcomeric Z-disk | X | ||
| TNNT3 | Troponin T, fast skeletal muscle | fTnT | Preferentially adult skeletal fast type 2 fibers | X | X | X |
| CALML6 | Calmodulin-like protein 6 | Calglandulin-like protein | Ca2+ signalling | X | ||
| CAMKK2 | Ca2+/calmodulin dependent protein kinase kinase 2 | CaMKK beta | Ca2+ signalling | X | ||
| MEF2A | Myocyte-specific enhancer factor 2A | Serum response factor-like protein 1 | Transcription factor, muscle-specific | X | ||
| MEF2B | Myocyte-specific enhancer factor 2B | Serum response factor-like protein 2; XMEF2 | Transcription factor, muscle-specific | X | ||
| MEF2C | Myocyte-specific enhancer factor 2C | Transcription factor, muscle-specific | X | |||
| MYOD1 | Myoblast determination protein 1 | Myogenic factor 3, Myf-3 | Muscle differentiation | X | ||
| MYF5 | Myogenic factor 5 | Myf-5 | Muscle differentiation | X | ||
| NFATC4 | Nuclear factor of activated T-cells, cytoplasmic 4 | T-cell transcription factor NFAT3 | Transcription factor | X | ||
| MYF6 | Myogenic factor 6 | Muscle-specific regulatory factor 4, MRF4 | Muscle differentiation | X | ||
| SIX1 | Homeobox protein SIX1 | Sine oculis homeobox homolog 1 | Transcription factor, muscle-specific | X | ||
| TEAD4 | Transcriptional enhancer factor TEF-3 | TEA domain family member 4, TEAD-4; RTEF-1 | Transcription factor (preferentially in muscle) | X | ||
| NCAM1 | Neural cell adhesion molecule 1 | NCAM-1; CD56 | Membrane protein; (neural) adhesion | X | ||
| MYOG | Myogenin | Myogenic factor 4, Myf-4 | Muscle differentiation | X | X | |
| VIM | Vimentin | Class III intermediate filament; developing/regenerating muscle | X |
EP expression profiling; SVA splice variant analysis; WB western blotting; IHC immunohistochemistry
Materials and methods
Patients
Enrollment of patients was approved by the respective local institutional review boards. After obtaining informed consent from the patients, according to the Declaration of Helsinki, muscle biopsies were obtained. The patients and their biopsies used for the different analyses are summarized in Supplemental Table S1. All DM1 and DM2 diagnoses were based on DNA mutation testing [4, 46]. We used different sample sets for protein analyses, expression profiling and splice variant analyses. DM1 and DM2 patients of matched age, gender, skeletal muscle, and disease stage were used for mRNA expression studies. For immunohistochemical analysis of protein expression in highly atrophic fibers, we used more severely affected distal DM1 muscles, because highly atrophic fibers are not present at earlier stages of DM1 pathology.
Immunohistochemistry of protein expression in highly atrophic muscle fibers
Muscle biopsies from DM2 (n = 20) and DM1 (n = 5) patients were snap frozen in liquid nitrogen-cooled iso-pentane to make 6-μm cryosections on SuperFrost + slides (Kindler GmbH, Freiburg, Germany). We studied the expression of the following proteins in the highly atrophic fibers. Fast skeletal muscle troponin T (fTnT/TNNT3) is expressed preferentially in fast type 2 fibers [70]. Myosin heavy chain (MyHC)-encoding genes (MYH2, -3, -7, and -8) are differentially regulated in fast and slow fibers, and also during muscle development (Table 2). The transcription factor myogenin (MYOG) and the intermediate filament protein vimentin (VIM) appear early during myogenesis and serve as regeneration markers in muscular dystrophies [10, 40]. Nuclear clump fibers have been considered a hallmark of neurogenic atrophy; however, they are morphologically indistinguishable from the scattered nuclear clump fibers regularly seen in DM2. Therefore, the sections were also immunostained for the neural cell adhesion molecule (NCAM1).
Immunohistochemistry was performed with the following mouse monoclonal antibodies (mAb): MyHC-emb (MYH3), clone RNMy2/9D2, at 1:20; MyHC-pn (MYH8), clone WB-MHCn, at 1:25; fTnT (TNNT3), clone T1/61, at 1:1,000; clone WB-MHCf recognizing MyCH-IIa (MYH2) and possibly MyHC-IIb (MYH4) in humans, at 1:320; and MyHC-beta (MYH7), clone WB-MHCs, at 1:200 (from Novocastra Laboratories, Newcastle Upon Tyne, UK). To confirm the expression of fast MyCH-IIa in the highly atrophic fibers, we used two additional antibodies, clone MY-32 against human MyHC-IIa, at 1:40,000 (Sigma-Aldrich, St Louis, MO, USA), and clone A4.74, at 1:100 (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA, USA) [67] on five DM2 muscle biopsies (results not shown). Myogenin (MYOG) clone F12B was used at 1:100 (Sigma-Aldrich); vimentin (VIM) clone 3B4, at 1:300; NCAM1 (NCAM1) clone UJ13A, at 1:5. BenchMark (Ventana Medical Systems, Tucson, AZ, USA) or TechMate (DakoCytomation, Glostrup, Denmark) automated immunostainer were applied, with detection based on horseradish peroxidase (HRP)-conjugated secondary antibodies followed by diaminobenzidine (DAB) detection. Gill’s hematoxylin was used for counterstaining. Serial sections for identification of expression patterns of individual fibers were used. The number of highly atrophic fibers (diameter <7 μm) in the muscle biopsies studied varied between 20 and 200 per section, being highest in DM2 samples.
Western blotting
Muscle biopsies were treated as described before to prepare samples for SDS-PAGE and western blotting [17]. 12% SDS-PAGE gels were used for resolving fTnT (TNNT3) isoforms, and 8% gels for resolving ZASP (LDB3) isoforms, and ACTN2/3 (ACTN2/3) total expression. After SDS-PAGE, proteins were transferred onto PVDF membranes (Bio-Rad Laboratories, Hercules, CA, USA), and detected with anti-fTnT, mAb clone T1/61, at 1:2,000 (Novocastra Laboratories), anti-ZASP, mouse polyclonal Ab, at 1:20,000 [13], or anti-ACTN2/3, mAb clone EA-53, at 1:50,000 (Sigma-Aldrich). Following detection with HRP-conjugated secondary antibody at 1:1,000 (DAKO P260; Dako Cytomation), enhanced chemiluminescence (ECL) was performed using Bio-Rad Immun-Star™ kit (Bio-Rad Laboratories). MyHC expression was assessed by staining the 8% gels after SDS-PAGE with Bio-Rad Coo-massie Brilliant Blue R-250.
Microarray expression profiling
Skeletal muscle biopsies were homogenized using a shark-tooth pulveriser with TriZol (Invitrogen, Carlsbad, CA, USA), and total cellular RNA was extracted according to the manufacturer’s suggestions. RNA was further purified using the RNeasy kit (Qiagen, Valencia, CA, USA). The quality and integrity of the RNA was then analyzed on an Agilent BioAnalyzer using the RNA 6000 Nano LabChip (Agilent, Santa Clara, CA, USA); samples with a RIN (RNA integrity number) >7 were used. For RNA expression profiling on the U133Plus2 GeneChip (Affymetrix, Santa Clara, CA, USA), a total of 5 μg of total cellular RNA from each sample was used for cDNA synthesis according to the manufacturer’s protocol. Briefly, a mixture of in vitro transcribed cRNAs of cloned bacterial genes for lysA, pheB, thrB, and dap (American Type Culture Collection) was added as external controls to monitor the efficiency of cRNA synthesis. First-strand cDNA synthesis was performed at 42°C for 1 h with the Superscript II system (GIBCO/BRL) at a final concentration of 1× first-strand synthesis buffer, 10 mM DTT, 500 μM dNTPs, 100 pmol of T7-(T)24 primer, and 200 units of reverse transcriptase. Second-strand cDNA synthesis was performed at 16°C for 2 h at a final concentration of 1× second-strand buffer, 250 μM dNTP, 65 U/ml DNA ligase, 250 units/ml DNA polymerase I, 13 U/ml RNase H. Second-strand synthesis reaction mixtures were cleaned up with an Affymetrix cDNA purification column. In vitro transcription labeling with biotinylated UTP and CTP was performed according to the manufacturer’s recommendations (Enzo Diagnostics) for 16 h at 37°C. Amplified cRNA was purified on a cRNA purification column (RNeasy, Qiagen), and the quality of the amplification was verified by analysis on an Agilent Bio-Analyzer. Labeled cRNAs were fragmented for 35 min at 94 °C in 40 mM Tris–acetate, pH 8.1/100 mM KOAc/30 mM Mg(OAc)2. The hybridization cocktail consisted of 10 μg fragmented cRNA in 200 μl, containing 50 pM control oligonucleotide B2, 0.1 mg/ml herring sperm DNA, 0.5 mg/ml acetylated BSA, 100 mM Mes, 20 mM EDTA, 0.01% Tween 20 (total Na+ = 1 M), and bacterial sense cRNA controls for bioB, bioC, bioD, and cre at 1.5, 5.0, 25, and 100 pM, respectively. Fragmented cRNAs were then hybridized to Affymetrix U133Plus2 GeneChips and scanned according to the manufacturer’s protocol.
Data analysis
Expression data are available at GEO (www.ncbi.nlm.nih.gov/geo/) under GEO series number GSE7014. While we considered the expression profiles of the entire set of genes on the array, we decided to specifically focus on the subset of genes functionally associated with muscle a priori, in order to examine key muscle genes associated with the two types of DM. Using the DChip software package (DNA-Chip analyzer) annotation tools (February 2006 build, http://biosun1.harvard.edu/complab/dchip/) and a series of queries to gene ontology along with information from the literature, we generated a list of muscle-specific genes, including transcription factors whose function is related to muscle development and activity. To this list we added all of the genes tested by immunohistochemistry, as well as additional members of the respective gene families (e.g., MYH) for a total of 327 probe set IDs (Supplemental Table S2). Using U133Plus2 data from DM1 (n = 10), DM2 (n = 20), and normal adult controls (n = 6), we performed global gene expression analysis using the 327 probe set list as a filter to identify genes of interest. It is important to note, however, that the genes were not selected on the basis of a contrast between the DM groups, or on the basis of any particular direction to these contrasts. Normalization was performed with the Invariant Set Normalization method (PM-only model with DChip default settings) on a normal sample as the reference; DChip model-based expression was applied to calculate the expression values for each probe set. Comparisons between groups were performed in DChip, using the described list of 327 probe set IDs with a fold-change (FC) cut-off FC ≥ 1.2, a lower bound (lb) limit lb = 90% (default), e–b, b–e difference thresholds of 100 (e experiment; b baseline), and a 50-permutations false discovery rate (FDR) calculation for each comparison. Clustering on samples and differentially regulated genes was performed using the Eucledian Distance metric in DChip with default settings.
Splice variant analysis
cDNA from total muscle RNA was generated using standard methods. Briefly, for each sample 5 μg of total RNA were DNaseI-treated (Ambion, Austin, TX, USA) according to the manufacturer’s suggestions. cDNA synthesis (SuperScript™ III First-strand cDNA Synthesis Kit, Invitrogen, Carlsbad, CA, USA) was performed on half and half of the DnaseI-treated RNA in separate reactions using random hexamer and oligo-(dT) priming according to the manufacturer’s protocol. All cDNAs were then Rnase H-treated. Equal amounts of random hexamer and oligo-(dT) primed cDNA were pooled and diluted to 200 μl with molecular grade RNAse-free H2O.
To estimate the amount of aberrant isoform present in samples, we performed RT-PCR with a FAM-labeled universal primer to incorporate a fluorescent label for quantification [4]. For each RT-PCR in log-linear range (23–28 cycles) 2 μl of cDNA were used. Capillary electrophoresis on an ABI3100 sequencer allowed peak heights for the isoforms to be measured. All peak heights were added together to determine the total signal. Each individual peak was then expressed as a percent of the total signal. To test for statistical significance, we used the Student’s t test applied to the mean percentage of the aberrant isoforms in each group. For TNNT3 we conducted PCR between exons 2 and 9b using published primers [23]. Primers for LDB3 are described in Supplementary Table S3, and the assay design is shown in Fig. 4. RT-PCR for both genes was conducted as previously described [4] employing a three primer reaction to incorporate the fluorescent label. Appropriate RT controls were included in all experiments.
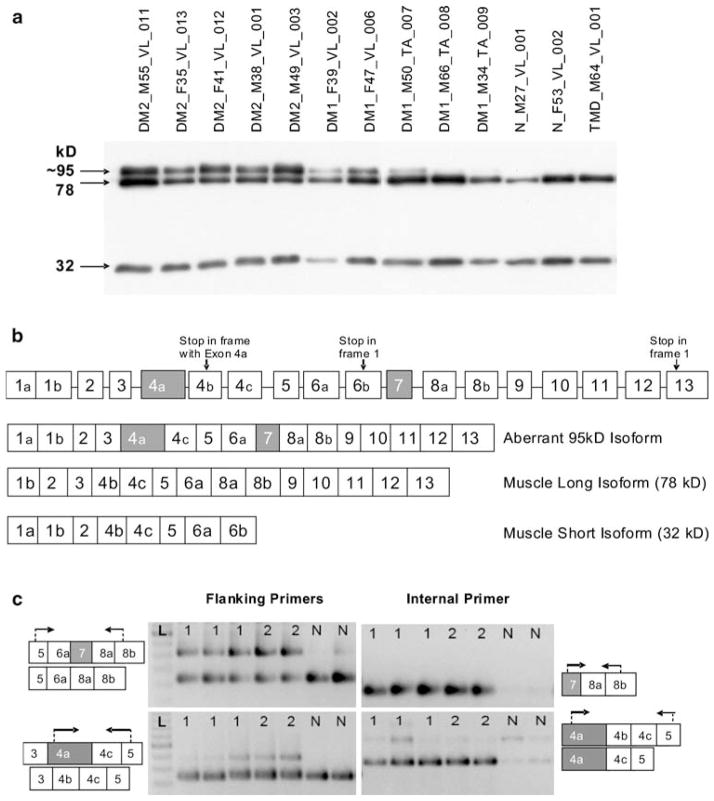
Fig. 4.
LBD3/ZASP expression. a Western blot of ZASP using samples identical to Fig. 3d. ZASP isoforms of 78 and 32 kD are present in all samples, while a strong band of ~95 kD is seen in all DM2 samples and three DM1 samples but not in the controls. The size of this band is consistent with the simultaneous inclusion of both cardiac exons in the protein (see Fig. 4b). Ten microliters of protein lysate was loaded per well. b Intron–exon structure of LDB3/ZASP. Exons 4a and 7 (shown in gray) are cardiac-specific and normally not present in skeletal muscle isoforms. Inclusion of these two exons was predicted to produce a protein of about 95 kD, as observed by western blot (Fig. 4a). Exons are not drawn to scale. c Representative RT-PCR assays to detect aberrant inclusion of exons 4a and 7. Arrows indicate the location of the primers for RT-PCR. Lanes are labeled 1 (DM1), 2 (DM2), N (normal), or L (100-bp ladder). Each predicted event is interrogated by two assays: one with primers flanking the aberrant exon, the other with the forward primer within the aberrantly spliced exon. The upper left panel shows the strong presence of cardiac exon 7 in DM but not in normal individuals. The presence of this exon is confirmed in the upper right panel. The lower left panel shows the presence of an isoform containing cardiac exon 4a in DM but not in normal individuals. This isoform is present more strongly in DM2 than DM1 patients. Exon 4a inclusion is confirmed in the lower right panel. The weak band (isoform containing 4a-4b-4c-5) represents a transcript which is not translated due to presence of a premature termination codon
Results
Protein expression in highly atrophic muscle fibers
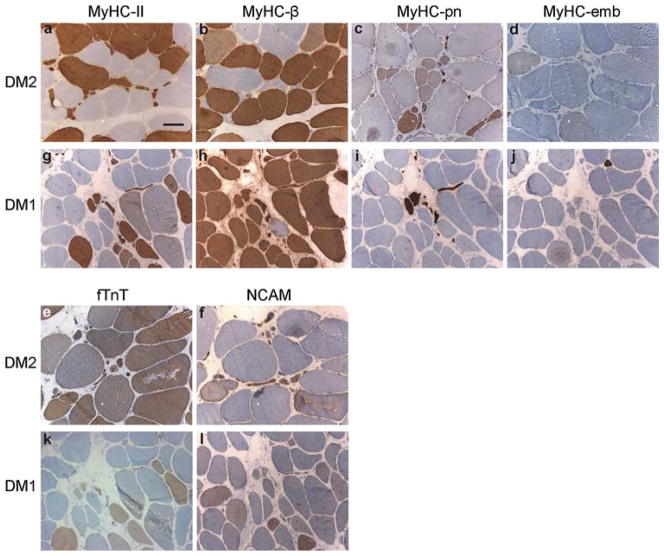
The results of immunohistochemical analysis of DM1 and DM2 muscle biopsies are shown in Fig. 1 and summarized in Table 3. Almost all nuclear clump fibers and other scattered highly atrophic fibers in both DM2 and DM1 expressed fast fiber-specific MyHC-IIa (MYH2), previously only reported in DM2 [53, 65]. In DM2 these fibers occur early in large numbers without other major myopathology, whereas in DM1 they appear at a very late stage together with marked myopathology. Co-expression of slow MyHC-beta (MYH7) in these atrophic fibers was evident in DM1 and absent in DM2. In both DM1 and DM2, the perinatal MyHC-pn showed markedly increased expression in the highly atrophic fibers, whereas only a few fibers in the most severely affected muscles expressed the embryonic MyHC-emb. NCAM expression was detected in both groups, more prominently in DM2. Very few nuclei showed myogenin labeling in both diseases, and vimentin was expressed only in some DM1 muscle fibers with severe muscle pathology (Table 3).
Fig. 1.
Immunohistochemistry of representative DM2 and DM1 muscle biopsies (for details see Supplemental Table 1). a–l DM type and investigated protein are indicated; a, b DM2_M38_VL_001; c–f DM2_M55_VL_011; g–l DM1_M48_TA_004. In DM2, essentially all highly atrophic fibers were identified as fast fibers by positivity for MyHC-II, with no expression of slow MyHC-beta. In contrast, in DM1 the majority of them co-expressed both fast and slow myosin isoforms. fTnT was expressed in nearly all large fast fibers and in about 40% of highly atrophic fibers in both DM2 and DM1. Most of the very atrophic fibers in both DM1 and DM2 expressed perinatal MyHC-pn. Only a few fibers, in the most severely affected muscles, expressed embryonic MyHC-emb. NCAM1 showed increased expression in ~35% of DM2 highly atrophic fibers, whereas only one of five (20%) DM1 muscle samples showed this high number of NCAM1 positive atrophic fibers. Scale bar 100 μm
Table 3.
Immunohistochemistry results of highly atrophic fibers
| Protein | Gene | DM2 | DM1 |
|---|---|---|---|
| MyHC-IIa | MYH2 | +++ | +++ |
| MyHC-beta | MYH7 | (+) | +++ |
| MyHC-pn | MYH8 | +++ | +++ |
| MyHC-emb | MYH3 | (+) | (+) |
| fTnT | TNNT3 | ++ | ++ |
| NCAM | NCAM1 | ++ | + |
| Myogenin | MYOG | (+) | (+) |
| Vimentin | VIM | (+) | + |
Protein expression: (+), in <1% of highly atrophic fibers; +, in 1–10%; ++, in 30–50%; +++, in >75%. The results indicate how many fibers of the highly atrophic fibers pool expressed each given antigen in DM2 (n = 20) and DM1 (n = 5) muscle biopsies
mRNA expression of sarcomeric proteins and muscle-specific transcription factors
To identify dysregulated genes in DM1 and DM2 and to determine their expression differences at the mRNA level, we performed microarray expression profiling of skeletal muscle biopsies of DM1 and DM2 patients and unaffected controls (U133Plus2, Affymetrix). Summary of the number of probe sets/genes showing differential expression from the list of 327 under the different comparisons are given in Table 4 (for a complete listing and analysis expression values, see Supplemental Table S2).
Table 4.
Summary of microarray (U133Plus2, Affymetrix) expression profiling experiments for skeletal muscle biopsies of DM patients (DM1, n = 10; DM2, n = 20) and normal controls (n = 6)
| Comparisona | No. probe sets/unique genes dysregulated | DChip-calculated FDR with 50 permutations (%)b |
|---|---|---|
| DM1 versus N | 27/21 | 3.7 |
| DM2 versus N | 37/27 | 2.7 |
| (DM1 + DM2) versus N | 36/28 | 2.8 |
| DM1 versus DM2 | 3/3 | 0 |
Analysis focused on muscle-specific genes using an annotated list of 327 probe sets. For a complete listing and analysis expression values, see Supplemental Table S2
N normal controls
Using the default number of permutations, DChip automatically calculates a false-discovery rate (FDR)
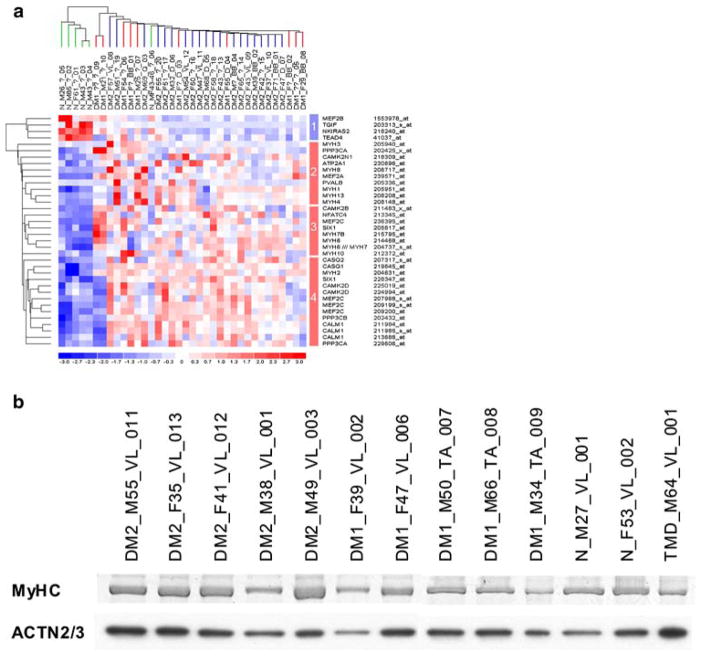
Comparing DM1 and DM2 patients to normal controls, 36 of the total of 327 probe sets (11%) interrogating 28 unique genes showed differential regulation. Unsupervised two-way hierarchical cluster analysis identified distinct sample clusters of healthy controls and DM patients, with DM1 and DM2 patients clustering together (Fig. 2a). Four (14%) genes showed decreased expression in DM1 and DM2 (Group 1 in Fig. 2a). TEAD4 and MEF2B are directly involved in muscle-specific gene regulation, the latter being one of the earliest markers of muscle differentiation and being expressed as early as myogenin [38, 55].
Fig. 2.
Analysis of dysregulated genes encoding sarcomeric structural proteins and myogenic transcription factors. a Two-way hierarchical cluster analysis of microarray expression profiling data of skeletal muscle biopsies from DM1 (n = 10, red lines) and DM2 (n = 20, blue lines) patients and unaffected controls (n = 6, green lines), using an annotation filter of 327 probe set IDs for muscle-specific genes involved in myogenic regeneration, denervation, and apoptosis including those analyzed by immunohistochemistry and muscle-specific transcription factors regulating their expression, identified 28 dysregulated genes represented by 36 unique probe sets in four groups (indicated by colored bar on the right; blue, down-regulated in DM; red, up-regulated in DM; for a complete listing and analysis expression values, see Supplemental Table S2). b Total MyHC protein on SDS-PAGE gel, stained with Coomassie Brilliant Blue, showed no significant difference between the DM2 (n = 5), DM1 (n = 5), and control (n = 3) groups, when compared to ACTN2/3 expression detected by ECL on a PVDF membrane
The majority of the dysregulated genes (24 of 28, 86%) showed increased mRNA expression in DM1 and DM2 samples compared to healthy controls. Among these, hierarchical cluster analysis identified three major gene groups (Groups 2–4 in Fig. 2a): group 2 including the adult myosin heavy chain genes (MYH1, MYH4, and MYH13) and the early development skeletal muscle genes (MYH3 and MYH8), group 3 with the skeletal slow and cardiac muscle gene (MYH7) and cardiac muscle genes (MYH6 and MYH7B), and group 4 with the adult skeletal muscle fast MYH2 gene. The majority of the genes in group 4 (9 of 14 probe sets, 64%) consisted of genes involved in the calcium signalling pathway. This group also contained the myogenic transcription factors SIX1 and MEF2C. For most of the genes, DM2 patient samples showed overall higher levels of upregulation than DM1 patients.
A direct comparison of the mRNA expression profiles between the DM2 and DM1 patient groups identified three of 327 probe sets (1%, interrogating three distinct genes) as differentially regulated (Table 4, Supplemental Table S2). Two genes, CAMKK2 (downregulated in DM2) and CALML6 (upregulated in DM2), are involved in calcium signal transduction, and one is a myogenic transcription factor, MEF2C (upregulated in DM2). MEF2C was represented by four probe sets, of which only one (Affymetrix probe set 207968_s_at) showed upregulation in DM2 relative to DM1 patients. This probe set interrogates a different mRNA splice variant compared to the others.
Compared to healthy controls, the mRNA expression levels of the myosin genes MYH1, -2, -4, -6, -7, -7B, -8, -10, and -13 were approximately twofold higher in both DM2 and DM1 patients when assessed by microarray expression profiling (Fig. 2a). However, the total amount of MyHC protein isoforms of MW ~200 kD was not increased in DM2 and DM1 samples, when normalized with the sarcomeric α-actinin (ACTN2/3) protein expression (Fig. 2b).
Aberrant splicing and expression of sarcomeric proteins
TNNT3/fTnT
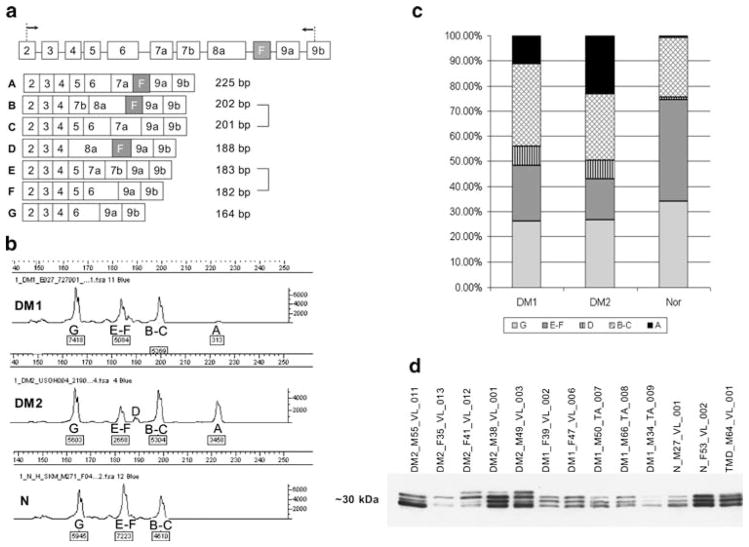
RT-PCR designed to amplify the TNNT3 fragment between exons 2 and 9b (Fig. 3a) identified three different products representing isoforms of G = 164, E/F = 182–183, and B/C = 201–202 bp in all samples, in both patients and controls. Furthermore, two aberrant TNNT3 isoforms (D = 188 bp and A = 225 bp) predicted to contain the fetal exon (F) were identified only in DM1 and DM2 patients (Fig. 3; DM1 vs. N p value = 0.0045 and DM2 vs. N p value = 1.732 × 10−6). The average percentage of isoform A in the DM2 group was nearly double that of the DM1 group (Fig. 3a–c). However, this difference did not quite reach statistical significance (p = 0.059) because of extreme sample variability within both groups: one DM1 patient had an isoform profile similar to DM2; likewise one DM2 patient had a profile more similar to DM1.
Fig. 3.
TNNT3/fTnT expression. a TNNT3 intron–exon structure and RT-PCR assay to detect expression of alternatively spliced exons. The fetal exon (F) is indicated (black; exons not drawn to scale). Arrows indicate RT-PCR primers. Observed isoforms (A–G) are shown with predicted sizes. It is impossible to distinguish the isoforms of 201/202 bp or 182/183 bp. b Typical electropherograms of TNNT3 RT-PCR. Isoforms (upper label) and peak intensity (lower labels) are indicated. Signal intensity is proportional to the amount of RT-PCR product. The peak corresponding to the aberrant large isoform was much stronger in DM2 than DM1 patients, and was completely absent in normals. c Stacked histogram showing percentages of each splice isoform in DM1 (n = 3), DM2 (n = 6), and normals (n = 5) averaged across each group. The 188- and 225-bp isoforms contain the fetal exon and are seen only in DM patients. The 225-bp isoform represents a larger percentage of total in DM2 than DM1 patients. d Western blot of fTnT isoforms in DM2 (n = 5), DM1 (n = 5), and control (n = 3) muscle samples. Three isoforms of approximate MW of 30 kD (located between MW markers of 25 and 37 kD; not shown), differing only by ~1 kD from each other, are seen in all samples. An additional large isoform is present only in DM2 (3 of 5, 60%) and not in DM1 samples. The different quantities of fTnT are caused by different proportions of fast muscle fibers in the biopsies. Fifteen microliters of protein lysate was loaded per well
fTnT protein expression by western blot analysis (Fig. 3d) confirmed three bands of approximate MW of 30 kD in controls, consistent with the mRNA results. A fourth, slightly larger protein isoform, corresponding to the 225-bp cDNA isoform A seen by RT-PCR, was present in three of five (60%) DM2 patients and was completely absent in DM1 patients and controls (Fig. 3d).
LDB3/ZASP
Western blot analysis of ZASP protein expression showed expected bands of 32 and 78 kD in all samples, and an additional strong band of 95–100 kD in all DM2 specimens, while weaker expression of this band was observed in three of five DM1 specimens (Fig. 4a). In order to account for this larger band, we examined the intron/exon structure of the LDB3 gene (Fig. 4b) and estimated that the aberrant inclusion of the two cardiac-specific exons (4a and 7) in the muscle isoform would be expected to produce a protein of about the correct size [20]. To determine if this event had indeed occurred, we designed a series of four RT-PCR assays. One pair of assays interrogated the presence of exon 4a, and the second pair interrogated exon 7. For each event we conducted RT-PCR using primers flanking the test exon; we also paired the flanking reverse with a forward primer located within the predicted exon. The results of these assays, shown in Fig. 4c, are consistent with the simultaneous inclusion of both exons 4a and 7 in DM but not in control samples. Aberrant bands were stronger in DM2 than in DM1 patients. Quantification using fluorescent RT-PCR in log-linear range and capillary electrophoresis showed that on average 6.3% of all isoforms in DM1 patients and 9.8% in DM2 contain exon 7, while normal samples contained only 0.8% of this exon. Differences between DM1, DM2, and control were significant (DM1 vs. N p value = 0.0357 and DM2 vs. N p value = 0.0079). Notably, the difference between DM1 and DM2 for exon 7 inclusion was also significant (DM1 vs. DM2 p value = 0.0357). On average, exon 4a comprised 8.6% of all DM1 isoforms and 23.2% of DM2. This exon represented only 0.8% of all isoforms in control samples. For exon 4a, DM1 and DM2 were significantly different from normal (DM1 vs. N p value = 0.0357 and DM2 vs. N p value = 0.0079) but not from each other (DM1 vs. DM2 p value = 0.0714). Again, inter-sample variability within the DM1 group appeared to account for the lack of statistical significance. One DM1 sample had an isoform ratio similar to the DM2 group. The aberrant bands were excised from gels, sequenced, and the presence of the predicted exons was confirmed.
Discussion
Despite considerable clinical similarities and overlap between DM2 and DM1, there are definite differences with respect to the severity and spectrum of symptoms, particularly in the patterns of muscle and fiber type involvement (Table 1). Overall, the current consensus is that interaction of expanded CUG or CCUG RNA with binding proteins leads to abnormal regulation of alternative splicing for a selected group of pre-mRNAs [41]. Sequestration of muscleblind (MBNL) RNA binding proteins by expanded mutant repeat transcripts and concomitant upregulation of CUG binding protein 1 CUGBP1, possibly due to its stabilization within cytoplasmic RNA–protein complexes [41, 57], have been reported as the mechanisms of the spliceopathy in DM. Together, imbalances in these two antagonistic splice factors can cause aberrant splicing of their target genes. CUGBP1 has also been shown to affect translation and RNA stability [44, 59] and to have a role in mRNA decay [39, 66]. In addition, the possibility of epigenetic regulation was recently suggested for DM2 [22]. Whether additional splicing changes occur as a secondary result of muscle regeneration/remodeling is a matter of ongoing investigation. Expression differences may also occur as a direct result of the expansion mutations, or as changes secondary to the disease process. Apart from the repeat expansion mutations and their genomic context, no differences at the molecular level have been reported to date to account for the differences in clinical presentation between DM1 and DM2. The major cause of disability in both disorders, muscle weakness, has remained elusive in terms of the underlying molecular pathophysiology.
Our expression profiling data of muscle-specific genes for DM1, DM2, and normal skeletal muscle indicated, in DM patients, significant upregulation of genes encoding structural sarcomeric proteins (MYH family), as well as genes encoding proteins involved in the calcium (Ca2+) signalling pathway and myogenic transcription factors (SIX1, MEF2A, and MEF2C). Significantly more genes showed over- than under-expression (86 vs. 14%). The list of upregulated genes included numerous genes usually expressed during earlier stages of development (MYH3 and -8, encoding embryonic and perinatal MyHC) or predominantly in heart (MYH6, -7 and -7B, CASQ2).
Myosin heavy chain genes
Different fiber types express different myosin heavy chains, myofibrillar proteins, and metabolic enzymes in different proportions, resulting in adaptable physical properties. MyHC isoforms, the major structural constituents of the sarcomeric thick filament, are encoded by distinct genes. In adult human skeletal muscle, one slow MyHC isoform, MyHC-beta (MYH7) is expressed in slow type 1 fibers, and two fast MyHC isoforms, MyHC-IIa (MYH2) and MyHC-IIx (MYH1), are expressed in 2A fibers and 2B fibers, respectively, of which 2A fibers predominate [68]. Hybrid fibers expressing two or more isoforms occur more frequently in pathologic states [10]. The elevated MYH2 expression in both DM1 and DM2 compared to normal controls detected by microarray expression profiling is intriguing, because the highly atrophic fibers are of type 2 in DM2; however, this might be due to the bimodal size distribution of type 2 fibers. Besides the atrophic subpopulation, there are also hypertrophic type 2 fibers [65], which may, in part, account for the high MYH2 transcription levels in the DMs. However, this explanation by itself is likely insufficient, since the hypertrophic fibers are present in variable amounts and are not prevalent in all muscles [65]. While no significant mRNA expression differences in total muscle tissue for MYH1, -2, -3, -7, and -8 were detected between DM1 and DM2, we saw some differences in myosin protein expression when studying the atrophic fibers by immunohistochemistry. In DM2, the highly atrophic fibers express, of the adult myosins, only fast myosin (MYH2), whereas the atrophic fibers in DM1 usually co-express both fast and slow (MYH7) isoforms, or the slow isoform alone. In both DM1 and DM2 the atrophic fibers co-express the perinatal myosin isoform MyHC-pn, which may be directly correlated to the observed increase in mRNA expression of MYH8 or it may reflect more complex myosin expression regulation in the myotonic dystrophies. The significance of MYH4 mRNA expression in muscle biopsies is not clear, because its expression has not been reported before in other human muscles except masseter and abdominal external oblique [19], but it could be part of the abnormal transcriptional activation of the whole set of myosin genes, possibly due to the observed aberrant regulation of their transcription factors. Despite the increased transcription of all MYH genes, the total amount of MyHC protein, however, was not significantly increased based on total protein of ~200 kD MW on SDS-PAGE. Together with the differential fiber atrophy and the differential MyHC-IIa/MyHC-beta expression in very atrophic fibers in DM1 and DM2, this may be a reflection of more complex translational regulatory mechanisms in the DM pathology as recently reported, including the reduction of the rate of protein synthesis in DM2 [21].
Dysregulation of myogenic transcription factors
We found significant dysregulation of the myogenic transcription factors MEF2B (one isoform down), MEF2A and -C, and SIX1 (up) in both DM1 and DM2 muscle, whereas MEF2D and the myogenic regulatory factors, including MYOD/Myf-3 (MYOD1), myogenin/Myf-4 (MYOG), Myf-5 (MYF5), and Myf-6 (MYF6) were not significantly altered. These transcription factors regulate most muscle-specific genes during both development and adult stages in a complex transcriptional network [2]. Several other factors, including nerve stimuli and intracellular Ca2+ concentration, modify their activity. For example, high cytoplasmic Ca2+ increases the activity of the MYH2 promoter through MEF2A [1]. The homeobox protein SIX1, which is active during earlier stages of embryogenesis, is enriched in the nuclei of fast muscle fibers and is also involved in the establishment and maintenance of the fast fiber phenotype [15]. In mouse, MEF2C promotes the slow fiber phenotype [43]. The higher upregulation of one distinct MEF2C isoform (Affymetrix probe set 207968_s_at) in DM2 relative to DM1 muscle could also be of importance for fiber type specific differences between the two forms of DM.
In the context of myogenic transcription factor upregulation, the concomitant overexpression of genes that encode proteins involved in the Ca2+ signalling pathway is noteworthy. Several of the dysregulated myogenic transcription factors (MEF2A-C and NFATC4) respond to multiple Ca2+-regulated signals in skeletal muscle differentiation, fiber type specificity and fiber size determination [37]. Increased intracellular Ca2+ is known to increase the activity of early myogenic transcription factors (MEF2 family) during myogenesis either through the Ca2+/cal-modulin-dependent kinase (CaMK) pathway [37] or the calcineurin (CaN) pathway [25]. It is possible that the combined dysregulation of the myogenic transcriptional network and the Ca2+ signaling pathway contribute to the observed increase in overall expression of MYH genes, or it may reflect a feedback response due to translational disturbance.
Immunohistochemical characterization of very atrophic muscle fibers
In addition to the observed differences in adult MyHC-IIa and -beta isoform expression, immunohistochemical analysis detected other differences in protein expression in the highly atrophic muscle fibers of DM1 and DM2 biopsies. NCAM1 was analyzed because of its reported expression transiently after denervation [69], and because the abundant nuclear clump fibers in DM2 are morphologically indistinguishable from those seen in neurogenic atrophy. Interestingly, NCAM1 labeling was more frequent in nuclear clump fibers and other highly atrophic fibers in DM2 than in DM1. In neurogenic atrophy, also large, likely recently denervated fibers express NCAM1 [10], while in this study large fibers in DM2 never expressed NCAM1. This difference suggests distinct mechanisms in fiber atrophy in DM2 compared to fiber atrophy in neurogenic disorders, even though the end product, the nuclear clump fibers, are morphologically similar. Our results with MyHC-emb (MYH3), myogenin (MYOG), and vimentin (VIM) did not show significantly increased expression in any of the DM1 or DM2 specimens studied, suggesting that necrotizing processes are not a major part of the muscle pathogenesis. However, re-programing involving MyHC-pn (MYH8) expression was present in highly atrophic fibers in both DM2 and DM1.
Aberrant splicing of TNNT3 and LDB3
TNNT3 is preferentially expressed in fast skeletal muscle fibers and extensively alternatively spliced [70]. Aberrant splicing of TNNT3 was previously reported in DM1 [23] and DM2 [49]. Our results show that the relative proportion of the aberrant transcript is different, being twice as frequent in DM2 compared to DM1. Importantly, the difference is also present at the protein level; in western blotting the aberrant large fast troponin T isoform was present in most DM2 samples but not in DM1 samples. For the function of the muscle fiber, the observed protein changes are more significant than the mRNA expression differences. Recently, the functional outcome of cardiac TNNT2 mutations was shown to be modified by MyHC isoforms [60], and in rat skeletal muscle there is coordinated regulation of troponin and myosin isoform expression [6]. Since the expression of genes for structural sarcomeric proteins is controlled by complex regulatory networks, abnormal expression of one structural gene can have far reaching effects on many other genes.
The functions of the Z-disk interaction protein ZASP (encoded by LDB3) are not fully understood. Mutations in LDB3 have been associated with both cardiac and skeletal myopathies [16, 54, 64]. Abnormal splicing of the cardiac-specific exon 7 was previously described in skeletal muscle of both DM1 and DM2 (previously reported as exon 11 [28] and exon 6a [30]), but was not confirmed at the protein level. As a novel finding, we show here that also exon 4a, normally present only in the cardiac isoform, is aberrantly spliced in DM1 and DM2 skeletal muscle transcripts, a finding which we confirmed at the protein level. Moreover, we observed varying levels of the aberrantly spliced LDB3 transcript in different muscles: vastus lateralis showed higher levels than other skeletal muscles. Together with the significantly higher level of the abnormal large ZASP isoform in DM2 muscle compared to DM1, it provides the first direct finding of divergent aberrant splicing in proximal versus distal muscles. How important is this for different distal versus proximal muscle involvement in DM1 and DM2 remains to be clarified in animal models.
In summary, we have identified molecular differences in muscle-gene expression and splicing between DM1 and DM2 patients. The difference in proportions of TNNT3/fTnT aberrantly spliced isoforms, at both the mRNA and protein levels, is especially interesting, since TNNT3 is preferentially expressed in fast type 2 fibers. Our findings are one of the first to provide a potential molecular explanation for the differential muscle and fiber type involvement in the two myotonic dystrophies. In addition, we demonstrated partially different protein expression patterns by immunohistochemistry in the highly atrophic fibers in DM2 and DM1, which suggest divergent molecular pathomechanisms underlying the different muscle and muscle fiber type involvement in these two diseases.
Supplementary Material
Acknowledgments
We are grateful to the participating patients for their cooperation. This study has been accomplished through the active collaboration and sharing of patient samples within the European Neuromuscular Centre (ENMC) consortium on DM2 and Other Myotonic Dystrophies by the following members: Josep Gamez, Jerry Mendell, Guillaume Bassez, Bruno Eymard, Tetsuo Ashizawa, and Lubov Timchenko. We thank Valerie L. Neubauer and Tamara J. Nixon for expert assistance with the generation of microarray expression data, and Georgine Faulkner, Trieste, Italy for the ZASP antibody. The mAb clone A4.74 developed by Helen M. Blau was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA. RK was supported by grants from the National Institutes of Health, NIH (AR48171), Muscular Dystrophy Association USA and the Kleberg Foundation. BU was supported by funding from the Folkhälsan Research Foundation, and grants from the Liv & Hälsa Foundation, the Vasa Central Hospital District Medical Research funds and Kung Gustav V Adolfs och Drottning Victorias minnesfond Foundation.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00401-010-0637-6) contains supplementary material, which is available to authorized users.
Conflict of interest statement The authors declare that they have no conflict of interest.
Contributor Information
Anna Vihola, Department of Medical Genetics, Folkhälsan Institute of Genetics, University of Helsinki, 00014 Helsinki, Finland.
Linda L. Bachinski, Department of Genetics, University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA
Mario Sirito, Department of Genetics, University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA.
Shodimu-Emmanuel Olufemi, Department of Genetics, University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA.
Shohrae Hajibashi, Department of Genetics, University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA. University of Texas Graduate School of Biomedical Sciences, Houston, TX 77030, USA.
Keith A. Baggerly, Department of Bioinformatics and Computational Biology, University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA. Graduate Program in Human and Molecular Genetics, University of Texas at Houston Graduate School of Biomedical Sciences, Houston, TX 77030, USA
Olayinka Raheem, Neuromuscular Research, Department of Neurology, University Hospital of Tampere, 33520 Tampere, Finland.
Hannu Haapasalo, Department of Pathology, Center for Laboratory Medicine, Pirkanmaa Hospital District, 33520 Tampere, Finland.
Tiina Suominen, Neuromuscular Research, Department of Neurology, University Hospital of Tampere, 33520 Tampere, Finland.
Jeanette Holmlund-Hampf, Department of Medical Genetics, Folkhälsan Institute of Genetics, University of Helsinki, 00014 Helsinki, Finland.
Anders Paetau, Department of Pathology, University of Helsinki and University Hospital of Helsinki, 00014 Helsinki, Finland.
Rosanna Cardani, Department of Molecular Biology and Biotechnologies, University of Milan, 20133 Milan, Italy.
Giovanni Meola, Department of Neurology, IRCCS Policlinico San Donato, University of Milan, San Donato Milanese, 20097 Milan, Italy.
Hannu Kalimo, Department of Pathology, University of Helsinki and University Hospital of Helsinki, 00014 Helsinki, Finland.
Lars Edström, Department of Clinical Neuroscience, Karolinska Institute, 17176 Stockholm, Sweden.
Ralf Krahe, Email: rkrahe@mdanderson.org, Department of Genetics, University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA. Graduate Program in Human and Molecular Genetics, University of Texas at Houston Graduate School of Biomedical Sciences, Houston, TX 77030, USA. Graduate Program in Genes and Development, University of Texas at Houston Graduate School of Biomedical Sciences, Houston, TX 77030, USA. Department of Genetics, University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA.
Bjarne Udd, Email: bjarne.udd@netikka.fi, Department of Medical Genetics, Folkhälsan Institute of Genetics, University of Helsinki, 00014 Helsinki, Finland. Neuromuscular Research, Department of Neurology, University Hospital of Tampere, 33520 Tampere, Finland. Department of Neurology, Vaasa Central Hospital, 65100 Vaasa, Finland. Biomedicum Helsinki, Folkhälsan Institute of Genetics, Helsinki University, C304B, P.O. Box 63, 00014 Helsinki, Finland.
References
- 1.Allen DL, Leinwand LA. Intracellular calcium and myosin isoform transitions. Calcineurin and calcium-calmodulin kinase pathways regulate preferential activation of the IIa myosin heavy chain promoter. J Biol Chem. 2002;277(47):45323–45330. doi: 10.1074/jbc.M208302200. [DOI] [PubMed] [Google Scholar]
- 2.Allen DL, Weber JN, Sycuro LK, Leinwand LA. Myocyte enhancer factor-2 and serum response factor binding elements regulate fast myosin heavy chain transcription in vivo. J Biol Chem. 2005;280(17):17126–17134. doi: 10.1074/jbc.M501207200. [DOI] [PubMed] [Google Scholar]
- 3.Auvinen S, Suominen T, Hannonen P, Bachinski LL, Krahe R, Udd B. Myotonic dystrophy type 2 found in two of sixty-three persons diagnosed as having fibromyalgia. Arthritis Rheum. 2008;58(11):3627–3631. doi: 10.1002/art.24037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachinski LL, Udd B, Meola G, et al. Confirmation of the type 2 myotonic dystrophy (CCTG)n expansion mutation in patients with proximal myotonic myopathy/proximal myotonic dystrophy of different european origins: a single shared haplotype indicates an ancestral founder effect. Am J Hum Genet. 2003;73(4):835–8485. doi: 10.1086/378566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brook JD, McCurrach ME, Harley HG, et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell. 1992;68(4):799–808. doi: 10.1016/0092-8674(92)90154-5. [DOI] [PubMed] [Google Scholar]
- 6.Brotto MA, Biesiadecki BJ, Brotto LS, Nosek TM, Jin JP. Coupled expression of troponin T and troponin I isoforms in single skeletal muscle fibers correlates with contractility. Am J Physiol Cell Physiol. 2006;290(2):567–576. doi: 10.1152/ajpcell.00422.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buj-Bello A, Furling D, Tronchere H, et al. Muscle-specific alternative splicing of myotubularin-related 1 gene is impaired in DM1 muscle cells. Hum Mol Genet. 2002;11(19):2297–2307. doi: 10.1093/hmg/11.19.2297. [DOI] [PubMed] [Google Scholar]
- 8.Charlet-Berguerand N, Savkur RS, Singh G, Philips AV, Grice EA, Cooper TA. Loss of the muscle-specific chloride channel in type 1 myotonic dystrophy due to misregulated alternative splicing. Mol Cell. 2002;10(1):45–53. doi: 10.1016/s1097-2765(02)00572-5. [DOI] [PubMed] [Google Scholar]
- 9.Day JW, Ricker K, Jacobsen JF, et al. Myotonic dystrophy type 2: molecular, diagnostic and clinical spectrum. Neurology. 2003;60(4):657–664. doi: 10.1212/01.wnl.0000054481.84978.f9. [DOI] [PubMed] [Google Scholar]
- 10.Dubowitz V, Sewry CA. muscle biopsy—a practical approach. 3. Saunders Elsevier; UK: 2007. Immunohistochemistry; pp. 195–245. [Google Scholar]
- 11.Ebralidze A, Wang Y, Petkova V, Ebralidse K, Junghans RP. RNA leaching of transcription factors disrupts transcription in myotonic dystrophy. Science. 2004;303(5656):383–387. doi: 10.1126/science.1088679. [DOI] [PubMed] [Google Scholar]
- 12.Fardaei M, Rogers MT, Thorpe HM, et al. Three proteins, MBNL, MBLL and MBXL, co-localize in vivo with nuclear foci of expanded-repeat transcripts in DM1 and DM2 cells. Hum Mol Genet. 2002;11(7):805–814. doi: 10.1093/hmg/11.7.805. [DOI] [PubMed] [Google Scholar]
- 13.Faulkner G, Pallavicini A, Formentin E, et al. ZASP: a new Z-band alternatively spliced PDZ-motif protein. J Cell Biol. 1999;146(2):465–475. doi: 10.1083/jcb.146.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu YH, Pizzuti A, Fenwick RG, Jr, et al. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science. 1992;255(5049):1256–1258. doi: 10.1126/science.1546326. [DOI] [PubMed] [Google Scholar]
- 15.Grifone R, Laclef C, Spitz F, et al. Six1 and Eya1 expression can reprogram adult muscle from the slow-twitch phenotype into the fast-twitch phenotype. Mol Cell Biol. 2004;24(14):6253–6267. doi: 10.1128/MCB.24.14.6253-6267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griggs R, Vihola A, Hackman P, et al. Zaspopathy in a large classic late-onset distal myopathy family. Brain. 2007;130(Pt 6):1477–1484. doi: 10.1093/brain/awm006. [DOI] [PubMed] [Google Scholar]
- 17.Haravuori H, Vihola A, Straub V, et al. Secondary calpain3 deficiency in 2q-linked muscular dystrophy: titin is the candidate gene. Neurology. 2001;56(7):869–877. doi: 10.1212/wnl.56.7.869. [DOI] [PubMed] [Google Scholar]
- 18.Harper PS. Myotonic dystrophy. Saunders; London: 2001. [Google Scholar]
- 19.Horton MJ, Brandon CA, Morris TJ, Braun TW, Yaw KM, Sciote JJ. Abundant expression of myosin heavy-chain IIB RNA in a subset of human masseter muscle fibres. Arch Oral Biol. 2001;46(11):1039–1050. doi: 10.1016/s0003-9969(01)00066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang C, Zhou Q, Liang P, et al. Characterization and in vivo functional analysis of splice variants of cypher. J Biol Chem. 2003;278(9):7360–7365. doi: 10.1074/jbc.M211875200. [DOI] [PubMed] [Google Scholar]
- 21.Huichalaf C, Schoser B, Schneider-Gold C, Jin B, Sarkar P, Timchenko L. Reduction of the rate of protein translation in patients with myotonic dystrophy 2. J Neurosci. 2009;29(28):9042–9049. doi: 10.1523/JNEUROSCI.1983-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin J, Wang G-L, Salisbury E, Timchenko L, Timchenko NA. GSK3β-cyclin D3-CUGBP1-eIF2 pathway in aging and in myotonic dystrophy. Cell Cycle. 2009;8(15):2356–2360. doi: 10.4161/cc.8.15.9248. [DOI] [PubMed] [Google Scholar]
- 23.Kanadia RN, Johnstone KA, Mankodi A, et al. A muscle-blind knockout model for myotonic dystrophy. Science. 2003;302(5652):1978–1980. doi: 10.1126/science.1088583. [DOI] [PubMed] [Google Scholar]
- 24.Kimura T, Nakamori M, Lueck JD, et al. Altered mRNA splicing of the skeletal muscle ryanodine receptor and sarcoplasmic/endoplasmic reticulum Ca2+-ATPase in myotonic dystrophy type 1. Hum Mol Genet. 2005;14(15):2189–2200. doi: 10.1093/hmg/ddi223. [DOI] [PubMed] [Google Scholar]
- 25.Konig S, Beguet A, Bader CR, Bernheim L. The calci-neurin pathway links hyperpolarization (Kir2.1)-induced Ca2+ signals to human myoblast differentiation and fusion. Development. 2006;133(16):3107–3114. doi: 10.1242/dev.02479. [DOI] [PubMed] [Google Scholar]
- 26.Krahe R, Bachinski LL, Udd B. Myotonic dystrophy type 2: clinical and genetic aspects. In: Wells RD, Ashizawa T, editors. Genetic instabilities and neurological diseases. 2. Academic Press; Boston: 2006. pp. 131–150. [Google Scholar]
- 27.Leroy O, Wang J, Maurage CA, et al. Brain-specific change in alternative splicing of tau exon 6 in myotonic dystrophy type 1. Biochim Biophys Acta. 2006;1762(4):460–467. doi: 10.1016/j.bbadis.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Lin X, Miller JW, Mankodi A, et al. Failure of MBNL1-dependent post-natal splicing transitions in myotonic dystrophy. Hum Mol Genet. 2006;15(13):2087–2097. doi: 10.1093/hmg/ddl132. [DOI] [PubMed] [Google Scholar]
- 29.Liquori CL, Ricker K, Moseley ML, et al. Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9. Science. 2001;293(5531):864–867. doi: 10.1126/science.1062125. [DOI] [PubMed] [Google Scholar]
- 30.Machuca-Tzili L, Thorpe H, Robinson TE, Sewry C, Brook JD. Flies deficient in muscleblind protein model features of myotonic dystrophy with altered splice forms of Z-band associated transcripts. Hum Genet. 2006;120(4):487–499. doi: 10.1007/s00439-006-0228-8. [DOI] [PubMed] [Google Scholar]
- 31.Mahadevan M, Tsilfidis C, Sabourin L, et al. Myotonic dystrophy mutation: an unstable CTG repeat in the 3′ untranslated region of the gene. Science. 1992;255(5049):1253–1255. doi: 10.1126/science.1546325. [DOI] [PubMed] [Google Scholar]
- 32.Mahadevan MS, Yadava RS, Yu Q, et al. Reversible model of RNA toxicity and cardiac conduction defects in myotonic dystrophy. Nat Genet. 2006;38(9):1066–1070. doi: 10.1038/ng1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mankodi A, Logigian E, Callahan L, et al. Myotonic dystrophy in transgenic mice expressing an expanded CUG repeat. Science. 2000;289(5485):1769–1773. doi: 10.1126/science.289.5485.1769. [DOI] [PubMed] [Google Scholar]
- 34.Mankodi A, Takahashi MP, Jiang H, et al. Expanded CUG repeats trigger aberrant splicing of ClC-1 chloride channel pre-mRNA and hyperexcitability of skeletal muscle in myotonic dystrophy. Mol Cell. 2002;100(1):35–44. doi: 10.1016/s1097-2765(02)00563-4. [DOI] [PubMed] [Google Scholar]
- 35.Mankodi A, Lin X, Blaxall BC, Swanson MS, Thornton CA. Nuclear RNA foci in the heart in myotonic dystrophy. Circ Res. 2005;97(11):1152–1155. doi: 10.1161/01.RES.0000193598.89753.e3. [DOI] [PubMed] [Google Scholar]
- 36.Maurage CA, Udd B, Ruchoux MM, et al. Similar brain tau pathology in DM2/PROMM and DM1/Steinert disease. Neurology. 2005;65(10):1636–1638. doi: 10.1212/01.wnl.0000184585.93864.4e. [DOI] [PubMed] [Google Scholar]
- 37.McKinsey TA, Zhang CL, Olson EN. MEF2: a calcium-dependent regulator of cell division, differentiation and death. Trends Biochem Sci. 2002;27(1):40–47. doi: 10.1016/s0968-0004(01)02031-x. [DOI] [PubMed] [Google Scholar]
- 38.Molkentin JD, Firulli AB, Black BL, et al. MEF2B is a potent transactivator expressed in early myogenic lineages. Mol Cell Biol. 1996;16(7):3814–3824. doi: 10.1128/mcb.16.7.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moraes KCM, Wilusz CJ, Wilusz J. CUG-BP binds to RNA substrates and recruits PARN deadenylase. RNA. 2006;12:1084–1091. doi: 10.1261/rna.59606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olive M, Martinez-Matos JA, Pirretas P, Povedano M, Navarro C, Ferrer I. Expression of myogenic regulatory factors (MRFs) in human neuromuscular disorders. Neuropathol Appl Neurobiol. 1997;23(6):475–482. doi: 10.1111/j.1365-2990.1997.tb01324.x. [DOI] [PubMed] [Google Scholar]
- 41.Osborne RJ, Thornton CA. RNA-dominant diseases. Hum Mol Genet. 2006;15(Spec2):R162–R169. doi: 10.1093/hmg/ddl181. [DOI] [PubMed] [Google Scholar]
- 42.Philips AV, Timchenko LT, Cooper TA. Disruption of splicing regulated by a CUG-binding protein in myotonic dystrophy. Science. 1998;280(5364):737–741. doi: 10.1126/science.280.5364.737. [DOI] [PubMed] [Google Scholar]
- 43.Potthoff MJ, Wu H, Arnold MA, et al. Histone deacetylase degradation and MEF2 activation promote the formation of slow-twitch myofibers. J Clin Investig. 2007;117:2459–2467. doi: 10.1172/JCI31960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ranum LP, Cooper TA. RNA-mediated neuromuscular disorders. Annu Rev Neurosci. 2006;29:259–277. doi: 10.1146/annurev.neuro.29.051605.113014. [DOI] [PubMed] [Google Scholar]
- 45.Ricker K, Koch MC, Lehmann-Horn F, et al. Proximal myotonic myopathy. Clinical features of a multisystemic disorder similar to myotonic dystrophy. Arch Neurol. 1995;52(1):25–31. doi: 10.1001/archneur.1995.00540250029009. [DOI] [PubMed] [Google Scholar]
- 46.Sallinen R, Vihola A, Bachinski LL, et al. New methods for molecular diagnosis and demonstration of the (CCTG)n mutation in myotonic dystrophy type 2 (DM2) Neuromuscul Disord. 2004;14(4):274–283. doi: 10.1016/j.nmd.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 47.Salisbury E, Sakai K, Schoser B, et al. Ectopic expression of cyclin D3 corrects differentiation of DM1 myoblasts through activation of RNA CUG-binding protein, CUGBP1. Exp Cell Res. 2008;314:2266–2278. doi: 10.1016/j.yexcr.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salisbury E, Schoser B, Schneider-Gold C, et al. Expression of RNA CCUG repeats dysregulates translation and degradation of proteins in myotonic dystrophy 2 patients. Am J Pathol. 2009;175(2):748–762. doi: 10.2353/ajpath.2009.090047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salvatori S, Furlan S, Fanin M, et al. Comparative transcriptional and biochemical studies in muscle of myotonic dystrophies (DM1 and DM2) Neurol Sci. 2009;30(3):185–192. doi: 10.1007/s10072-009-0048-4. [DOI] [PubMed] [Google Scholar]
- 50.Savkur RS, Philips AV, Cooper TA. Aberrant regulation of insulin receptor alternative splicing is associated with insulin resistance in myotonic dystrophy. Nat Genet. 2001;29(1):40–47. doi: 10.1038/ng704. [DOI] [PubMed] [Google Scholar]
- 51.Savkur RS, Philips AV, Cooper TA, et al. Insulin receptor splicing alteration in myotonic dystrophy type 2. Am J Hum Genet. 2004;74(6):1309–1313. doi: 10.1086/421528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schneider C, Ziegler A, Ricker K, et al. Proximal myotonic myopathy: evidence for anticipation in families with linkage to chromosome 3q. Neurology. 2000;55(3):383–388. doi: 10.1212/wnl.55.3.383. [DOI] [PubMed] [Google Scholar]
- 53.Schoser BG, Schneider-Gold C, Kress W, et al. Muscle pathology in 57 patients with myotonic dystrophy type 2. Muscle Nerve. 2004;29(2):275–281. doi: 10.1002/mus.10545. [DOI] [PubMed] [Google Scholar]
- 54.Selcen D, Engel AG. Mutations in ZASP define a novel form of muscular dystrophy in humans. Ann Neurol. 2005;57(2):269–276. doi: 10.1002/ana.20376. [DOI] [PubMed] [Google Scholar]
- 55.Stewart AF, Richard CW, 3rd, Suzow J, et al. Cloning of human RTEF-1, a transcriptional enhancer factor-1-related gene preferentially expressed in skeletal muscle: evidence for an ancient multigene family. Genomics. 1996;37(1):68–76. doi: 10.1006/geno.1996.0522. [DOI] [PubMed] [Google Scholar]
- 56.Taneja KL, McCurrach M, Schalling M, Housman D, Singer RH. Foci of trinucleotide repeat transcripts in nuclei of myotonic dystrophy cells and tissues. J Cell Biol. 1995;128(6):995–1002. doi: 10.1083/jcb.128.6.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Timchenko NA, Cai ZJ, Welm AL, Reddy S, Ashizawa T, Timchenko LT. RNA CUG repeats sequester CUGBP1 and alter protein levels and activity of CUGBP1. J Biol Chem. 2001;276(11):7820–7826. doi: 10.1074/jbc.M005960200. [DOI] [PubMed] [Google Scholar]
- 58.Timchenko NA, Iakova P, Cai Z-J, Smith JR, Timchenko LT. Molecular basis for impaired muscle differentiation in myotonic dystrophy. Mol Cell Biol. 2001;21:6927–6938. doi: 10.1128/MCB.21.20.6927-6938.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Timchenko NA, Patel R, Iakova P, Cai ZJ, Quan L, Timchenko LT. Overexpression of CUG triplet repeat-binding protein, CUGBP1, in mice inhibits myogenesis. J Biol Chem. 2004;279(13):13129–13139. doi: 10.1074/jbc.M312923200. [DOI] [PubMed] [Google Scholar]
- 60.Tschirgi ML, Rajapakse I, Chandra M. Functional consequence of mutation in rat cardiac troponin T is affected differently by myosin heavy chain isoforms. J Physiol. 2006;574(Pt 1):263–273. doi: 10.1113/jphysiol.2006.107417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Udd B, Krahe R, Wallgren-Pettersson C, Falck B, Kalimo H. Proximal myotonic dystrophy—a family with autosomal dominant muscular dystrophy, cataracts, hearing loss and hypogonadism: heterogeneity of proximal myotonic syndromes? Neuromuscul Disord. 1997;7(4):217–228. doi: 10.1016/s0960-8966(97)00041-2. [DOI] [PubMed] [Google Scholar]
- 62.Udd B, Meola G, Krahe R, et al. Report of the 115th ENMC workshop: DM2/PROMM and other myotonic dystrophies. Neuromuscul Disord; 3rd workshop; 14–16 February 2003; Naarden, the Netherlands. 2003. pp. 589–596. [DOI] [PubMed] [Google Scholar]
- 63.Udd B, Meola G, Krahe R, et al. 140th ENMC international workshop: myotonic dystrophy DM2/PROMM and other myotonic dystrophies with guidelines on management. Neuromuscul Disord. 2006;16(6):403–413. doi: 10.1016/j.nmd.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 64.Vatta M, Mohapatra B, Jimenez S, et al. Mutations in Cypher/ZASP in patients with dilated cardiomyopathy and left ventricular non-compaction. J Am Coll Cardiol. 2003;42(11):2014–2027. doi: 10.1016/j.jacc.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 65.Vihola A, Bassez G, Meola G, et al. Histopathological differences of myotonic dystrophy type 1 (DM1) and PROMM/DM2. Neurology. 2003;60(11):1854–1857. doi: 10.1212/01.wnl.0000065898.61358.09. [DOI] [PubMed] [Google Scholar]
- 66.Vlasova IA, Tahoe NM, Fan D, et al. Conserved GU-rich elements mediate RNA decay by binding to CUG-binding protein 1. Mol Cell. 2008;29:263–270. doi: 10.1016/j.molcel.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Webster C, Silberstein L, Hays AP, Blau HM. Fast muscle fibers are preferentially affected in duchenne muscular dystrophy. Cell. 1988;52(4):503–513. doi: 10.1016/0092-8674(88)90463-1. [DOI] [PubMed] [Google Scholar]
- 68.Weiss A, Schiaffino S, Leinwand LA. Comparative sequence analysis of the complete human sarcomeric myosin heavy chain family: implications for functional diversity. J Mol Biol. 1999;290(1):61–75. doi: 10.1006/jmbi.1999.2865. [DOI] [PubMed] [Google Scholar]
- 69.Winter A, Bornemann A. NCAM, vimentin and neonatal myosin heavy chain expression in human muscle diseases. Neuropathol Appl Neurobiol. 1999;25(5):417–424. doi: 10.1046/j.1365-2990.1999.00178.x. [DOI] [PubMed] [Google Scholar]
- 70.Wu QL, Jha PK, Raychowdhury MK, Du Y, Leavis PC, Sarkar S. Isolation and characterization of human fast skeletal beta troponin T cDNA: comparative sequence analysis of isoforms and insight into the evolution of members of a multigene family. DNA Cell Biol. 1994;13(3):217–233. doi: 10.1089/dna.1994.13.217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.