Abstract
In the inner ear, Notch signaling has been shown to have two key developmental roles. The first occurs early in otic development and defines the prosensory domains that will develop into the six sensory organs of the inner ear. The second role occurs later in development and establishes the mosaic-like pattern of the mechanosensory hair cells and their surrounding support cells through the more well-characterized process of lateral inhibition. These dual developmental roles have inspired several different strategies to regenerate hair cells in the mature inner ear organs. These strategies include (1) modulation of Notch signaling in inner ear stem cells in order to increase hair cell yield, (2) activation of Notch signaling in order to promote the formation of ectopic sensory regions in normally non-sensory regions within the inner ear, and (3) inhibition of Notch signaling to disrupt lateral inhibition and allow support cells to transdifferentiate into hair cells. In this review, we summarize some of the promising studies that have used these various strategies for hair cell regeneration through modulation of Notch signaling and some of the challenges that remain in developing therapies based on hair cell regeneration.
Keywords: Delta, Jagged, sensory, inner ear, cochlea, vestibular, balance, hearing, γ-secretase
INTRODUCTION
The Notch signaling pathway was initially discovered in Drosophila where it earned its name from a mutation that caused notches in the wings of the flies [1]. From this humble beginning, Notch is now recognized as a highly evolutionarily conserved pathway important in the development of most organ systems in many species, including mammals [reviewed in 2]. In addition, Notch signaling is required for many regenerative processes, and is maintained in regions of ongoing adult neurogenesis, including the subventricular zone and the hippocampal subgranular layer [3–12].
In the inner ear, Notch signaling has been shown to be necessary for both the formation and the patterning of the various sensory organs. At specific points in inner ear development, Notch signaling is critical. These developmental processes, including Notch expression and activity, are recapitulated after various types of damage to mature sensory organs in non-mammalian vertebrates, leading to the regeneration of their structure and function. In the inner ear of people, however, a loss of the sensory hair cells can result in hearing loss and/or balance deficits and vertigo. These hair cell losses can occur in many ways, including from genetic disorders such as Usher’s syndrome, prolonged exposure to noise, drug-related ototoxicity, or from age. The critical roles for Notch have inspired multiple methods for regenerating hair cells through modulation of Notch signaling, including the differentiation of stem cells, the induction of new sensory patches, and the conversion of support cells into hair cells, which recapitulates the regenerative process found in non-mammalian vertebrates. In this review, we will summarize and discuss these various strategies and their limitations in the hopes of identifying some of the challenges we still face in inducing hair cell regeneration.
The dual roles of Notch signaling in inner ear development
The canonical Notch signaling pathway
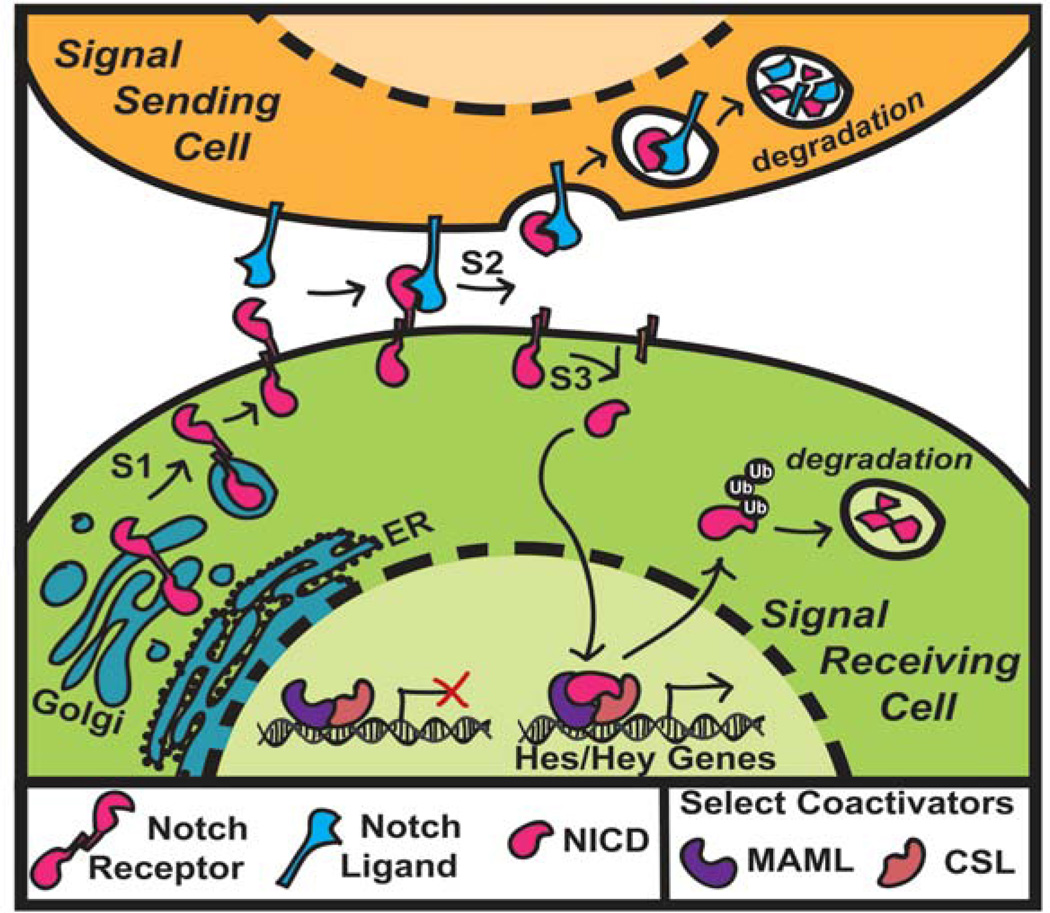
In mammals, there are four different Notch receptors, Notch1–4, and five canonical Notch ligands, Jagged1/2 and Dll1/3/4 (Figure 1) [2, 13, 14]. The Notch receptor is a transmembrane protein with an extracellular domain for ligand binding and an intracellular cytoplasmic domain. Activation of Notch signaling occurs through a series of proteolytic cleavages at different sites on the receptor, known as S1-S3. The S1 cleavage is mediated by furin-like convertases that generate the mature bipartite heterodimeric receptor that consists of an extracellular domain non-covalently bonded to a transmembrane and an intracellular domain. Upon binding of the receptor to a ligand, the receptor undergoes conformational changes that expose the S2 cleavage site on the extracellular domain to ADAM metalloproteases (A Disintegrin And Metalloproteinase). This cleavage results in the release of the extracellular domain, which is then endocytosed and degraded by the ligand-expressing cell along with the bound ligand. The remaining domain, known as the Notch extracellular truncation, then undergoes an intracellular S3 cleavage by the γ-secretase complex, which releases the Notch intracellular domain (NICD) from the membrane. Once released, the NICD translocates into the nucleus and forms an active transcriptional complex with CSL (RBP-Jκ) and Mastermind-like (MAML) that can recruit additional co-activators and drive transcription of target effector genes, such as the Hes and Hey genes.
Figure 1.
Canonical Notch signaling. In the canonical Notch signaling pathway, there are three main proteolytic cleavage events. The furin-mediated S1 cleavage is required to generate the mature form of the Notch receptor, which is then expressed on the cell membrane. Notch ligands expressed on neighboring cells bind to the receptor, which causes a conformational change in the extracellular domain of the receptor. This allows ADAM metalloproteases to perform the extracellular S2 cleavage. The freed extracellular domain bound to the ligand is endocytosed and ultimately degraded by the signal sending cell. The Notch extracellular truncation then undergoes a regulated cleavage at the S3 site by the γ-secretase complex. This cleavage releases the Notch intracellular domain (NICD), which then translocates to the nucleus and forms an active transcriptional complex with CSL and MAML. This leads to the transcription of various Notch effector genes such as the Hes/Hey genes.
There are many layers of regulation that can occur at each of these different steps along the pathway. Indeed, considering the breadth of function of Notch in different organs and different developmental stages, these would be required in order to generate such diversity from what appears to be a straightforward pathway. For example, in addition to regulation at each of the proteolytic cleavages, the pathway can be regulated through modification of the Notch receptors. Glycosylation by Pofut1 and Fringe proteins can change the responsiveness of the receptors to different ligands while the presence of Numb proteins can promote the degradation of the receptors through ubiquitination. Further, the NICD itself can also be regulated through modifications, including phosphorylation, hydroxylation, acetylation, and ubiquitination. Thus, this basic signaling pathway quickly becomes more complicated as the co-expression of specific components and regulators of the pathway in specific domains at different times can greatly change the cellular context of this signaling [2, 15].
Many of these regulatory mechanisms and how they function in different organs are still being elucidated and it will be interesting as this work unfolds to see specifically how such diverse functionality is generated. However, for this review, we will largely be dealing with a basic version of Notch signaling as outlined in Figure 1. It is important to keep in mind that many of these regulatory mechanisms are likely present, though it is currently unclear how they might be altering Notch signaling in these specific contexts.
Notch signaling in the inner ear
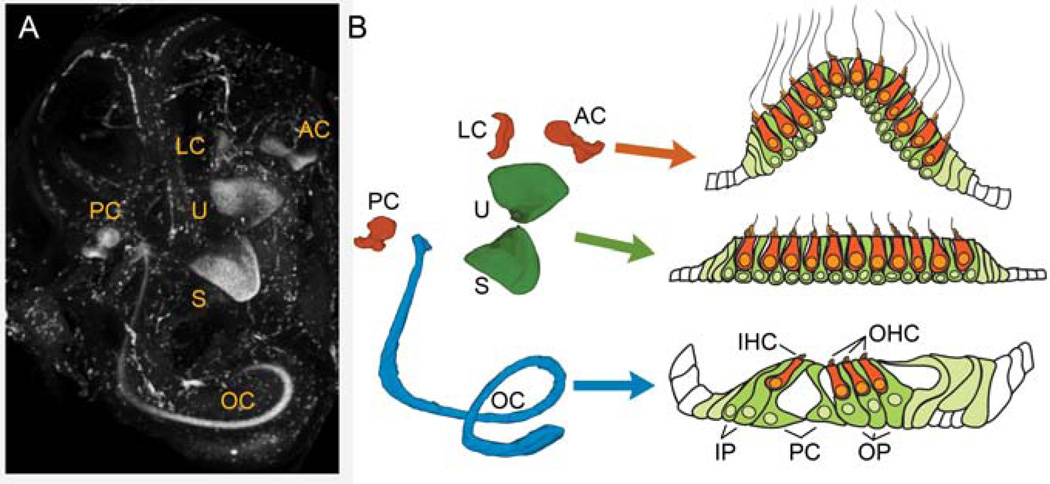
The mammalian inner ear is composed of six distinct sensory organs used to detect hearing and balance (Figure 2A). The first and only organ of the auditory system is the cochlea, containing the sensory organ of Corti (Figure 2B, blue). In the organ of Corti, hair cells are arranged into rows with one row of inner hair cells (IHCs) that detect sound and three rows of outer hair cells (OHCs) that function as the cochlear amplifier to increase amplitude and frequency sensitivity through a positive feedback mechanism. These rows of hair cells form a tonotopic map along the length of the spiraling cochlea such that higher frequencies are detected by the hair cells in the base of the cochlea and lower frequencies are detected by hair cells in the apex. The vestibular system of the inner ear contains two maculae, the saccule and the utricle, that use calcium carbonate crystals, or otoconia, resting on a membrane overlying the hair cells to detect linear acceleration such as gravity (Figure 2B, green). The vestibular system also contains the three canal ampulla, or cristae, that sit at the base of the semicircular canals and detect fluid motion through the canals caused by rotational head movements in the three cardinal planes, one for each canal ampullae (Figure 2B, orange). Together, the maculae and the cristae provide the precise information on the location and the movement of the head necessary for balance.
Figure 2.
The inner ear. A) Immunolabeling for Sox2 in an intact E15.5 inner ear shows the location of the sensory organs. The ear was cleared for confocal imaging using methyl salicylate and benzyl benzoate according to MacDonald and Rubel [81]. LC – lateral crista, AC– anterior crista, PC – posterior crista, U – utricle, S – saccule, OC – organ of Corti. B) A color coded model of the position of the Sox2-labeled sensory organs shown in A created by 3-dimensionally rendering tracings of the Sox2 regions in the individual confocal slices. In each of the inner ear organs, hair cells (orange) are arranged above the support cell layer (green). In the organ of Corti, the hair cells and support cells are highly specialized with obvious functional and morphological differences. In the vestibular system, these differences are not as pronounced. IHC – inner hair cell, OHC – outer hair cell, IP – inner phalangeal cell, PC – pillar cell, OP – outer phalangeal cell (Deiters’ cell).
Each of these sensory organs is comprised of two main cell types: the mechanosensory hair cells (Figure 2B, orange cells) and their underlying support cells (Figure 2B, green cells). In the cochlea, the support cells are highly differentiated and specialized with distinct morphologies and positions. Inner phalangeal cells surround the inner hair cells, while outer phalangeal cells, also known as Deiters’ cells, surround the outer hair cells. In addition, the tunnel of Corti, which is created by the highly specialized inner and outer pillar cells, separates the inner and outer hair cell rows. In the vestibular system, there are two types of hair cells classified as either type I or type II. While it is not currently clear how these two different hair cell types differ functionally, type I and type II hair cells differ in several ways, including in their locations, afferent nerve innervation, cell shape, stereocilia bundle properties, ultrastructural features, etc. [16]. The support cells of the vestibular system do not have any specific classifications, as they appear to have a more homogeneous appearance, lacking the obvious morphological specializations seen in the organ of Corti. However, different subpopulations of support cells can be identified by their expression of distinct markers and it is likely that they do have some specialized functions.
Lateral inhibition
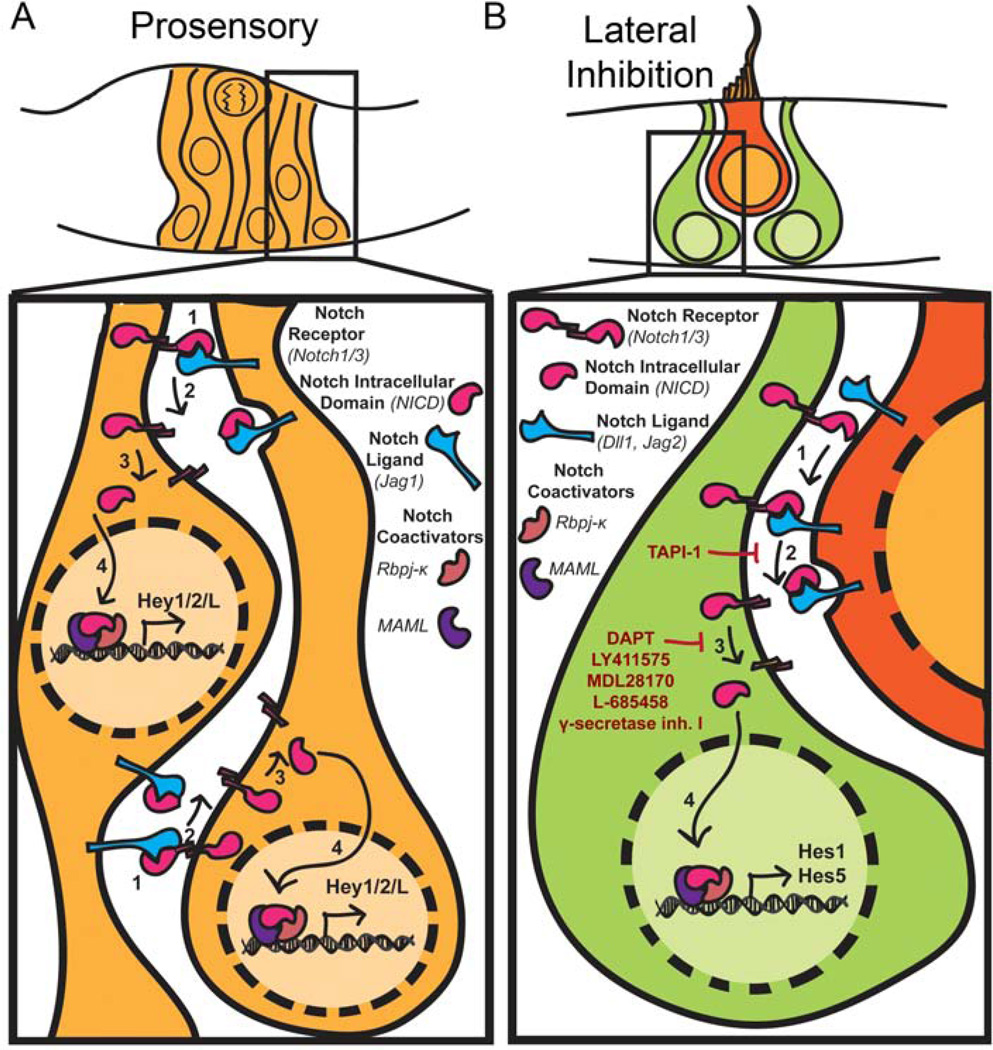
In the mature organs of both the auditory and vestibular system, the hair cells and support cells are arranged such that the support cell nuclei are located beneath the hair cells and extend processes up through the hair cell layer to the apical surface. The hair cells are surrounded by support cells and, in general, do not contact one another in the mature organs (Figure 2B). This mosaic-like pattern of hair cells and support cells is established through Notch signaling, via a process called lateral inhibition. This role has been well established in embryonic and early postnatal animals in both the auditory system [17–30] and the vestibular system [19, 29–31]. The details of this developmental role have been extensively described in multiple reviews [32, 33, 34] and so we will only briefly summarize them here. For lateral inhibition, Notch signaling acts through effectors to inhibit proneural basic helix-loop-helix (bHLHs) transcription factors. This in turn keeps Notch expressing cells from differentiating into neuronal cell types. In the inner ear, developing hair cells express the Notch ligands Delta1 (Dll1) and Jagged2 (Jag2) that bind to the Notch receptors on the surrounding cells (Figure 3B). The resulting release of the NICD in these cells ultimately leads to the up-regulation of the effectors Hes1 and Hes5, which results in the inhibition of the bHLH transcription factor Atoh1 (also known as Math1 in mice), which is essential for hair cell formation.
Figure 3.
Notch signaling in the inner ear. A) During the development of the prosensory domains, progenitors express the Notch ligand Jag1 and have reciprocal signaling such that Notch is broadly activated throughout the prosensory domain. In these progenitors, Notch signaling appears to act through the Hey family of Notch effectors as inhibition of Notch signaling during the prosensory phase results in a down-regulation of Hey1 and Hey2B) Later in development, as hair cells differentiate they begin to express the Notch ligands Dll1 and Jag2. These ligands then bind to the Notch receptors on the surrounding cells, where Notch signaling is then activated. Here Notch signaling appears to largely act through Hes1 and Hes5 in order to inhibit the proneural bHLH, Atoh1, and prevent the cells from differentiating into hair cells. Through this mechanism, hair cells, become surrounded by support cells, forming a mosaic-like pattern in each of the sensory organs. Common inhibitors of the S2 and S3 cleavages are depicted in red.
Atoh1 is the earliest known hair cell marker and its expression begins a transcriptional cascade necessary for hair cell differentiation [35]. Developmentally, most but not all inner ear cells that express Atoh1 will go on to become hair cells [36, 37]. This has been demonstrated using both Atoh1 lineage tracing and Atoh1 overexpression. Using an Atoh1-cre knock-in, Yang, et al. [37] showed that almost all of the hair cells in the inner ear of neonatal mice had undergone Atoh1-cre mediated recombination, as expected. Interestingly, a significant percentage of the support cells also underwent Atoh1-cre mediated recombination, showing that not all cells that express Atoh1, albeit even at low levels, become hair cells. In a similar study using an inducible Cre recombinase strategy to lineage trace Atoh1-expressing cells, Driver, et al. [36] found that in embryonic day 13 (E13) cochleae cultured for 1 day in vitro (DIV, E13 + 1DIV), 70% of lineage-traced cells that had expressed Atoh1 became hair cells. This number increased to 98.5% in E17 + 1DIV cochleae. Further, misexpression of Atoh1 into competent regions both in and near the developing sensory organs, such as the greater epithelial ridge (GER) and Kölliker’s organ can induce ectopic hair cells that mature and can even become innervated by nerve fibers [29, 38–40].
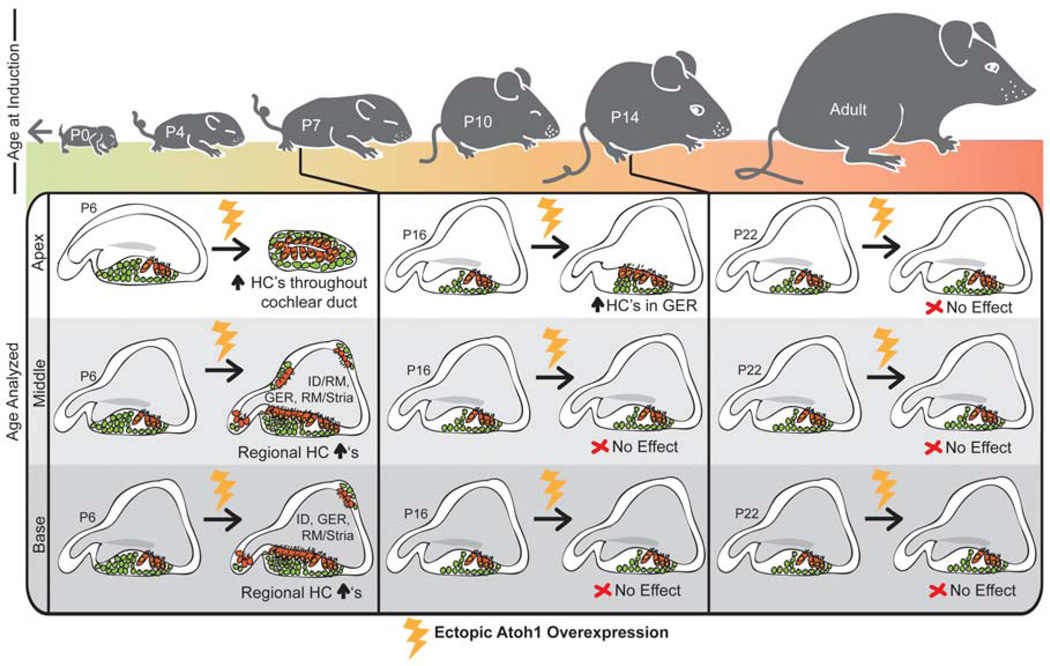
Modulation of Notch signaling can alter the fate of Atoh1-expressing cells. For example, in the study by Driver, et al. [36], inhibition of Notch signaling using the γ-secretase inhibitor, DAPT, resulted in more lineage-traced hair cells, while Notch activation using Dll1-Fc shifted the fate of the lineage-traced cells from that of hair cells to support cells. Further, overexpression of the Notch effector Hes1 with Atoh1 in postnatal cochleae reduces or abolishes the increase in ectopic hair cells seen with Atoh1 overexpression alone [29]. Conversely, DAPT treatment after Atoh1 overexpression in cultured cochleae from postnatal day 2 (P2) mice results in even more ectopic hair cells than Atoh1 or DAPT treatment alone [38]. These effects are also seen in the zebrafish inner ear and the chicken basilar papilla. In the developing zebrafish, overexpression of the NICD results in a loss of Atoh1a and Atoh1b expression, thereby blocking hair cell formation [41]. In the post-hatch chick, DAPT treatment increases the number of Atoh1-expressing cells and ultimately the number of hair cells expressing Myosin6 (Myo6) [42]. Since Atoh1 expression possesses the ability to induce hair cell differentiation even in non-sensory regions, many groups are investigating the use of Atoh1 overexpression to regenerate hair cells [43]. However, thus far there has been limited success using this method in mature organs since the ability to generate ectopic hair cells through Atoh1 overexpression appears to be limited not only regionally but also temporally; for example, Atoh1 overexpression in P14 mouse cochleae does not induce ectopic hair cells as it does in younger organs [38, 40].
Prosensory or lateral induction
In addition to a role for Notch in establishing the hair cell and support cell mosaic through lateral inhibition, Notch signaling also has an earlier role in the development of the regions that will become the various sensory organs, i.e. the prosensory domains. This phenomenon is generally referred to as prosensory specification in the inner ear [17, 20, 44–49, 32, 33, 34]. In brief, an early loss of Notch signaling either in Notch mutants or through pharmacological inhibition results in absent or smaller sensory domains with an overall decrease in both hair cells and support cells. Since initially the expression of markers for the prosensory domain appears normal, it seems that Notch signaling is required in these early stages to establish the proper domain size and/or to maintain the prosensory domains (Figure 3A). This could in part be mediated through Notch-induced proliferation of the sensory progenitors as was shown in a study generating ectopic sensory regions through transient NICD overexpression [50]. This related (and possibly identical) phenomenon has been called “lateral induction.” When NICD is experimentally activated in non-sensory regions of the inner ear, cells with the activated Notch, as well as their immediate neighbors, acquire a sensory identity. These neighboring cells are therefore thought to acquire a sensory identity via “lateral induction” [51].
The role of Notch signaling in regeneration of inner ear sensory tissue
The importance of Notch signaling in defining the development of the sensory epithelium and in determining the precise ratio of hair cells and support cells has naturally led to speculation that it may play a role during regeneration. Several labs have used different approaches to manipulate Notch signaling in order to regenerate hair cells [24, 31, 44, 48, 50–58]. The first approach uses modulation of Notch signaling in the culturing and differentiation of inner ear stem cells in order to increase hair cell yield. The second approach uses overexpression of active Notch (NICD) in order to specify sensory tissue in normally non-sensory regions of the inner ear. The last approach is to block Notch signaling in order to relieve the inhibition on Atoh1 by Notch effectors, allowing the normally Notch-expressing support cells to transdifferentiate into hair cells.
Notch signaling in inner ear stem cells
Since Notch signaling has several important developmental roles in vivo, it is not surprising that it would be important in the growth and differentiation of inner ear stem and progenitor cells. In the in vivo cochlea, Pan, et al. [50] showed that Notch signaling plays a role in the proliferation of sensory progenitors. By transiently overexpressing the NICD, they found that ectopic sensory regions formed in non-sensory areas of the cochlea. Using expression of eGFP to lineage trace the cells that had overexpressed NICD, they found that the eGFP-positive regions were larger in the NICD-expressing cochleae than the controls and that they also expressed pHistone-H3, a marker of mitosis. Overall, this suggested that the NICD-overexpressing cells were proliferating. A similar result was observed in progenitors isolated from early postnatal mouse cochleae and treated with Jagged1-Fc to activate Notch signaling. Treatment with Jagged1-Fc resulted in more secondary inner ear spheres and overall larger spheres than in controls. In addition, it increased the capacity for self-renewal of the progenitors and ultimately resulted in increased numbers of Myo7a–positive hair cells after differentiation [56]. Further, consistent with Notch signaling’s role in lateral inhibition, inhibition of Notch signaling with the γ-secretase inhibitor L-685458 in inner ear spheres created from early postnatal utricles increased the number of hair cells expressing Myo7a and the Atoh1-nGFP reporter. These hair cells were generated at the expense of support cells, identified by their expression of p27Kip1, Sox2, and Jag1 [53]. Therefore, it appears as if Notch may be able to increase hair cell yield in stem cell differentiation by 1) increasing the proliferation and self-renewal of the stem cells to increase overall yield and by 2) increasing the proportion of cells that differentiate as hair cells as opposed to support cells.
Regeneration through lateral induction
As noted above, the phenomenon of lateral induction and prosensory specification by Notch signaling has provided an additional approach to use this receptor to promote hair cell regeneration. This approach aims to regenerate hair cells by producing ectopic sensory regions complete with both hair cells and support cells. By overexpressing the NICD, ectopic sensory patches can be generated in both the developing cochlea [48, 50, 55] and vestibular system [51, 55]. The ectopic patches that form express normal markers for hair cells and support cells. Further, the hair cells in these ectopic patches can mature and develop polarized stereocilia bundles, acquire innervation by Tuj1-positive neurites, express synaptic markers, and develop Calretinin-positive calyces [50, 51, 55]. However, the ability to induce these ectopic sensory regions appears to be limited to a specific period in early development.
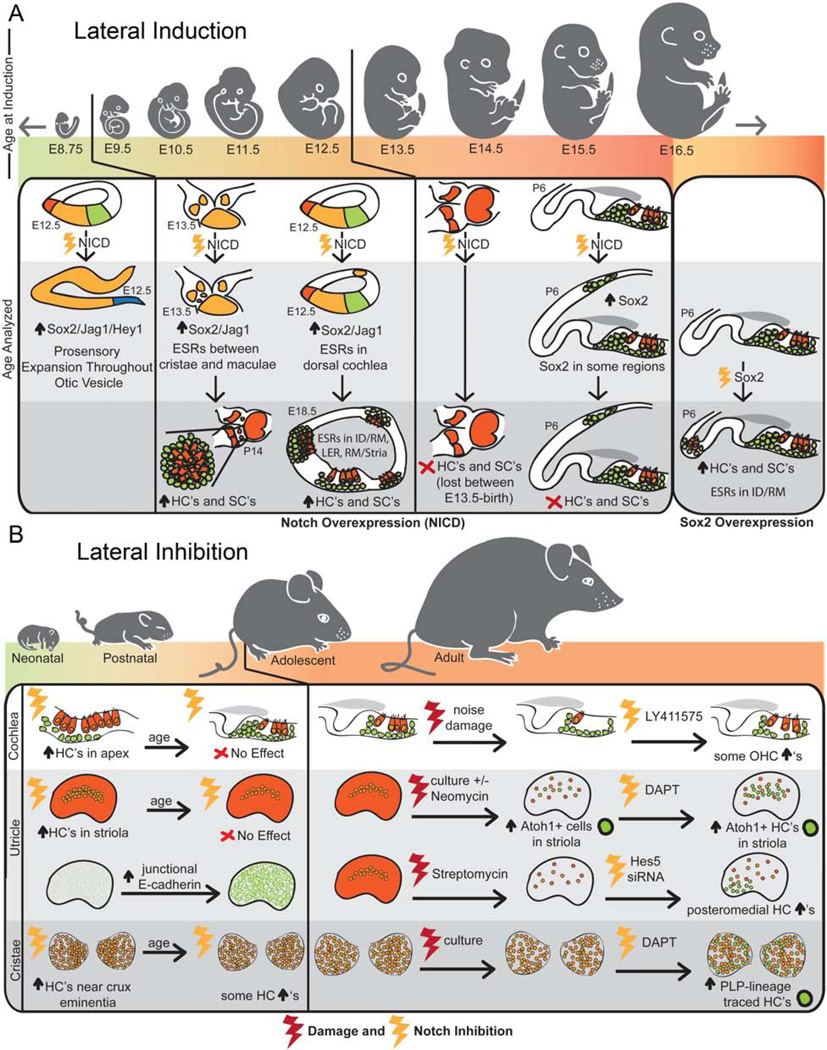
Using Notch overexpression, initially, every cell in the otic vesicle is competent to become prosensory in response to Notch. Constitutive overexpression of the NICD using Foxg1-Cre, which is expressed as early as E8.75, results in an expansion of the prosensory domains throughout the entire otic vesicle [48, 51]. Later in development, between E9.5 and E11.5, this competence is restricted regionally to specific zones near the normal sensory areas, including between the maculae and cristae in the vestibular system and along the entire length of the cochlea in the interdental region, Reissner’s membrane, the LER, and the region of the stria vascularis [48, 50, 55]. Even later in development, between E13.5 and E16.5, Notch overexpression can induce ectopic Sox2 expression, but does not result in the formation of ectopic sensory regions [44, 50, 55]. Interestingly, overexpression of Sox2 itself, which is directly downstream of Notch [8], is able to produce ectopic sensory regions at E16.5, past the limit for NICD overexpression [50]. However, ectopic sensory regions were only generated in specific areas, such as the interdental region, suggesting that the competence for Sox2-induction may also be decreasing with age.
The decrease in the ability to generate ectopic sensory regions through either NICD- or Sox2-overexpression may be a reflection of the changing role of Notch signaling in inner ear development during this same period. While it is not currently clear how this change is being mediated in the inner ear mechanistically, it does appear that different Notch ligands are involved in the two processes. Jag1 is expressed early in the prosensory domains and its loss results in a decrease in outer hair cells in the cochlea and in smaller or absent vestibular organs [17, 23, 34, 44–48, 59]. Later, as hair cells differentiate, Dll1 and Jag2 are expressed in hair cells and loss of these genes results in a lateral inhibition phenotype with supernumerary hair cells generated at the expense of support cells [17, 21, 23]. This is particularly interesting as it has been shown that glycosylation of the Notch receptor by Pofut1 and then subsequently by Fringe proteins can reduce the responsiveness of the Notch receptor to Jag1 while potentiating its interactions with Dll1 [60, 61]. In the cochlea, Lunatic fringe (Lfng) is present in the support cells at the appropriate time to increase their responsiveness to the Dll1-expressing hair cells [22, 27]. Further, Pofut1 conditional knockouts do not show a prosensory phenotype, but later exhibit supernumerary hair cells consistent with a loss of Notch signaling’s lateral inhibition role [44]. This is probably not the only regulatory change occurring as there also appears to be differences in the effectors transcribed in response to Notch signaling as well as specific expression of different effectors in different cell types and regions [18, 20, 22, 24–26, 31, 54, 58]. These types of transcriptional changes could be due to posttranslational modifications to the NICD, which we are only now beginning to understand. For example, it has been shown that phosphorylation of the ICD of the Notch2 receptor can inhibit its ability to induce specific effectors, such as Hes1 [62]. This type of mechanism could be partly responsible for the inability of the NICD-overexpression to induce sufficient Sox2 levels for full sensory specification.
While overall, this approach to generating hair cells has been very promising, in order for this strategy to be viable for hair cell regeneration in mature organs, it will be important to determine the mechanisms that limit the spatial and temporal competence of the inner ear regions, which is likely linked to the mechanisms mediating the change between prosensory specification and lateral inhibition.
Regeneration through lateral inhibition
The third approach that has been used to regenerate hair cells by modulating Notch signaling is in some ways analogous to the mechanism for hair cell regeneration and turnover that is found in non-mammalian vertebrates. For example, in the vestibular organs of the chick, hair cells are continuously being replaced, or turned over [63]. In this system, support cells require Notch signaling in order to maintain their support cell phenotype and Notch inhibition induces support cells to proliferate and to differentiate into hair cells. In other organs, such as the chick basilar papilla and the zebrafish lateral line, Notch signaling is not maintained in the healthy, mature organ and is therefore not required to maintain the support cell fate. In these healthy organs, Notch inhibition has no effect on hair cell or support cell numbers. Instead, Notch pathway genes are up-regulated in response to damage and then subsequent Notch inhibition leads to an overproduction of hair cells. In fish, this increase in hair cells is due to increased support cell proliferation [64], while in birds, the increase in hair cells after Notch inhibition occurs via transdifferentiation of the support cells into hair cells [65].
Organ of Corti
In the mammalian organ of Corti, Notch signaling is still active in newborn mice, and inhibition of Notch with γ-secretase inhibitors leads to an increase in hair cell numbers via transdifferentiation from support cells. However, Notch ligands are down-regulated within the first few days after birth [66] and the organ of Corti loses its ability to generate supernumerary hair cells in response to Notch inhibition [18, 20, 23, 26, 28, 30, 44, 59, 67]. Nevertheless, in more mature cochlea, some reports suggest that the Notch pathway can be up-regulated in response to damage. While Hartman, et al. [67] failed to find evidence for expression of a Notch pathway reporter (using transgenic mice expressing eGFP under the Hes5 promoter) after damage with a high dose of the ototoxic aminoglycoside antibiotic kanamycin (1 g/kg) followed by furosemide (400 mg/kg), Mizutari, et al. [24] reported that hair cell damage from exposure to 8–16 kHz octave band noise induced Hes5 expression as shown by RT-qPCR. Further, in guinea pig cochleae damaged with kanamycin (500 mg/kg) followed by ethacrynic acid, Hori, et al. [52] showed an increase in Jagged1 in support cells while both Jagged1 and Notch1 were up-regulated in inner sulcus cells.
If Notch signaling is re-established after certain types of damage in the cochlea, then the approaches to stimulate hair cell regeneration through Notch inhibition and transdifferentiation might be effective in this organ. Support for this possibility comes from two studies. Notch inhibition using the γ-secretase inhibitor LY411575 causes an increase in outer hair cells derived from support cells in the noise damaged cochlea, consistent with transdifferentiation [24]. In addition, in the guinea pig, occasional ectopic hair cells were found in the inner sulcus after 14 days of Notch inhibition with the γ-secretase inhibitor, MDL28170 [52]. Therefore, under the appropriate damage conditions, some mammalian species are able to express Notch signaling components after hair cell damage, and the modulation of Notch signaling could provide an approach to stimulate hair cell transdifferentiation leading to some functional recovery from the damage.
Utricle
While Notch signaling is not found in the mature undamaged cochlea, Hartman, et al. [67] found that Hes5-eGFP (from the transgenic mice) and Hes5 mRNA (by in situ hybridization) are expressed in the undamaged vestibular organs of adult mice. In the mature utricle, Hes5 is expressed in some of the support cells of the medial posterior region, suggesting that Notch inhibition may be able to induce these support cells to transdifferentiate. While this suggests that the mature utricle may possess the ability to generate supernumerary hair cells in response to Notch inhibition without damage, this remains untested since the studies to date have all included a damage component, either by design or due to the damage incurred during culture of the utricle in vitro. It is then also unclear whether Notch components are further up-regulated after hair cell damage, though it has been shown in vivo that Hes5 is spontaneously down-regulated 3 days after hair cell damage [68] and in cultured utricles that DAPT treatment results in decreased expression of Hes1, Hey1, and HeyL as compared to Dimethyl sulfoxide (DMSO) controls [58].
Despite agreement that Notch signaling continues in the vestibular system into adulthood, there are conflicting results as to whether inhibitors of Notch can stimulate hair cell transdifferentiation from support cells in the mouse utricle. Collado, et al. [31] reported no evidence for hair cell regeneration (Myosin7a–positive and E- cadherin-negative cells) in utricles explanted from P16 and older Swiss Webster mice cultured with 50 µM DAPT for up to 10 days in vitro. Conversely, Lin, et al. [58] found an increase in Atoh1-positive cells overall and in Atoh1-positive cells that also expressed Myo7a and phalloidin in the striolar and juxtastriolar regions of utricles explanted from 6–9 week old Swiss Webster mice cultured with 50 µM DAPT for up to 18 days in vitro. From these results, the authors infer that Notch inhibition is not only increasing the number of cells that express Atoh1, but is also promoting the differentiation of these cells into mature hair cells. This is similar to the result in the postnatal cochlea where overexpression of Atoh1 in conjunction with DAPT treatment generated more hair cells than either Atoh1-up-regulation or DAPT treatment alone [38]. Lastly, using a streptomycin lesion paradigm followed by treatment with Hes5 siRNA in utricles of 3–4 week old CD1 mice, Jung, et al. [54] found an increase in Myo7a–positive hair cells in the treated utricles in vivo 3 weeks after lesioning. These regenerated hair cells were located in the medial posterior utricle, which is appropriately where Hartman, et al. [67] showed that Hes5-GFP is expressed in the mature utricle.
While both of these studies demonstrate that the mature utricle has the capacity for modest hair cell regeneration after damage, these studies displayed stark differences in the regions of regeneration and the changes in Notch effectors. The study by Jung, et al. [54] is consistent with Notch signaling acting through Hes5 to maintain the support cell fate. Hes5 is normally expressed in the posteromedial utricle in the adult and therefore it is not surprising that treatment with Hes5 siRNA would result in the generation of hair cells in this same region. This raises the question of whether damage was necessary in order to induce generation of the hair cells through this method. In contrast, Lin, et al. [58] found hair cell increases in the striolar and juxtastriolar regions of the utricle. After damage, Atoh1 was spontaneously induced largely in these regions and subsequent DAPT treatment resulted in more differentiated hair cells in these regions as well. In addition, Lin, et al. [58] did not find significant levels of Hes5 expression in their damaged utricles, by RT-qPCR, but did see significant decreases in the expression of Hes1, Hey1, and HeyL after DAPT treatment. Therefore, it would be interesting to examine whether Hes1 and Hes5 are playing specific roles in the various regions of the undamaged and damaged mature utricle and to what extent varying degrees of hair cell damage are important. These and differences in methodology and the criteria for whether a hair cell was newly regenerated may account for the differences in results between studies including that of Collado, et al. [31] where no regeneration was found.
Cristae
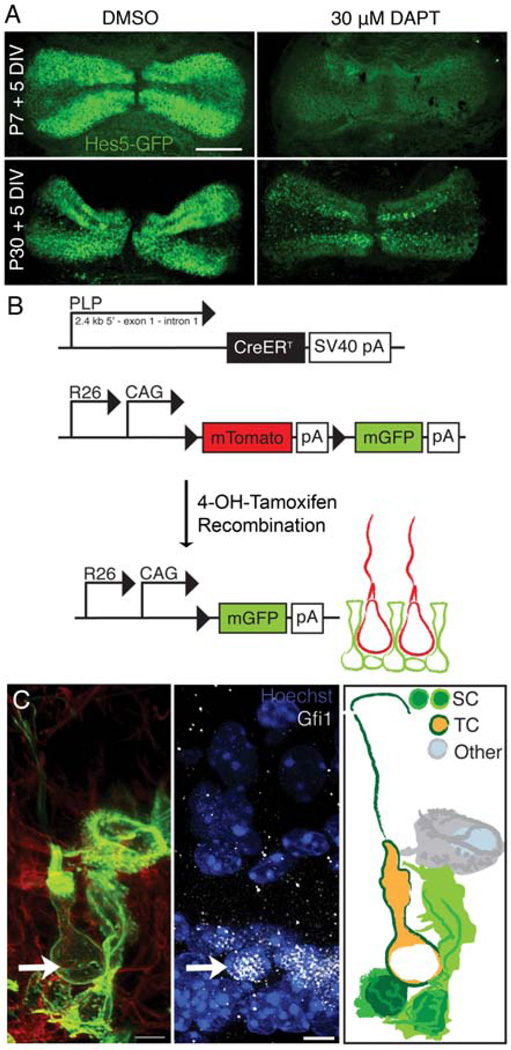
In the cristae, our lab previously demonstrated that there is robust Hes5 expression in the support cells of the peripheral region, higher than in any other organ of the mature inner ear (Figure 4A, DMSO) [67]. This suggested to us that Notch signaling may still be active in the cristae and that it may be required for maintaining the support cell phenotype through lateral inhibition. Therefore, we hypothesized that by inhibiting Notch signaling we would observe some support cells transdifferentiating into hair cells. Consistent with this hypothesis, we recently confirmed that Notch signaling is active in the cristae of postnatal and adult mice and that Notch inhibition using DAPT results in a down-regulation of Notch effector genes as seen by RT-qPCR. This down-regulation is specifically in support cells as seen using the Hes5-eGFP reporter mice (Figure 4A) [57]. DAPT-treatment also results in an increase in total hair cell number in both postnatal and adult mice. Since transdifferentiation is difficult to demonstrate directly, we used a cre-recombinase/reporter system to lineage trace support cells. Using proteolipid protein1 (PLP)-cre, which is expressed only in support cells in normal cristae, we found examples of lineage-traced hair cells in DAPT-treated cristae from mice up to 10 weeks of age (Figure 4B, C). This is the first evidence for direct transdifferentiation from support cells to hair cells in the mature mammalian vestibular system. Our results therefore show that Notch signaling is required in a subset of support cells in order to maintain the support cell phenotype and that inhibition of this pathway thus causes these support cells to adopt a hair cell fate, which could provide some potential for hair cell regeneration in this organ.
Figure 4.
Hair cell generation through lateral inhibition in the crista A) In cristae explanted from P7 and P30 mice cultured for 5 DIV, Hes5-eGFP is strongly expressed in the peripheral support cells. Upon Notch inhibition with DAPT, Hes5 is downregulated. B) The cre recombinase/reporter strategy used to lineage trace support cells in the mature cristae. Mice expressing PLP-creER, which is expressed only in peripheral support cells of the cristae, were crossed with R26-mTmG mice. Upon Tamoxifen treatment, PLP-expressing cells began expressing membrane-bound GFP (mGFP). C) An example of a lineage traced transitional cell (green) expressing the early hair cell marker Gfi1 (arrow) and possessing a normal hair cell morphology, including a kinocilium. [Reprinted with kind permission from Springer Science + Business Media: Slowik, A. D. and Bermingham-McDonogh, O. 2013, J. Assoc. Res. Otolaryngol., 14, 813.]
Although our experiments were performed in vitro and therefore involved some hair cell death due to the damage from culture, we believe that Notch signaling may be playing an ongoing role in maintenance of support cell fate in the mature cristae and that the hair cell regeneration we observe in response to Notch inhibition is not due to a damage-induced up-regulation of Notch signaling. We believe this is the case since Hes5 is expressed in the peripheral support cells of the mature uncultured cristae [67] and is down-regulated in these same cells in response to DAPT in vitro [57]. Further, we found that Hes5 mRNA, as assayed by RT-qPCR in uncultured cristae, is expressed at similar levels in late postnatal and mature cristae isolated from mice of up to 10 weeks of age. Expression of eGFP mRNA from the Hes5-eGFP reporter construct showed a similar trend with age. Therefore, while we cannot exclude the effect of damage, we believe that the ongoing presence and responsiveness of Hes5 to Notch inhibition suggests that Notch may be important in the quiescent, undamaged cristae.
Similar to the utricle, we did observe some regional differences in hair cell regeneration. Though our analysis was largely limited to the peripheral region of the crista, since both Hes5 and PLP are expressed only in peripheral support cells, we did not see any significant qualitative evidence of hair cell regeneration in the central zone. The central zone of the cristae shares many characteristics with the striola of the utricle and generally exhibited the highest degree of hair cell death in our cultures, with occasional loss of all of the hair cells in this region. There did not appear to be any increase in hair cells in this region in the DAPT-treated cultures as opposed to those treated with DMSO. This would be more similar to the study by Jung, et al. [54] where we observe most of our hair cell increases in the regions that maintain Hes5 expression in the mature organ. In addition, we found a larger decrease in Hes5 expression in response to Notch inhibition than of Hes1. It would be interesting to know in what cells Hes1 is being expressed in the mature cristae and, again, whether it and Hes5 have unique roles in both the undamaged and damaged cristae.
In addition to differences between the peripheral and central regions, we observed a gradient of regeneration along the horizontal axis of the postnatal crista, with more hair cell generation near the crux eminentia than at the edges of the crista near the planum semilunatum. This is similar to a result found by Lopez, et al. [69] studying spontaneous hair cell damage in the mature chinchilla crista, where hair cells regenerated along this same gradient. While we observed lineage-traced hair cells along the entire length of the mature cristae, we cannot definitively say whether more hair cells were generated near the crux eminentia from our current data. Such a finding would be particularly interesting, since the crux eminentia of the crista and the striola of the utricle are the only places where the zinc finger gene Gata-3 is expressed in the mature organs [70]. GATA proteins have been shown to act cooperatively with NICD-CSL at the promoter level [71] and in the inner ear, it has been suggested that Gata-3 expression could be important for hair cell regeneration through downstream signaling targets such as Wnt [72].
Conclusions and Ongoing Challenges
Taken together, these findings from a number of investigators demonstrate that manipulation of Notch signaling, particularly through γ-secretase inhibitors, can stimulate hair cell regeneration through support cell transdifferentiation in the mammalian inner ear. To date, only a single study has demonstrated functional recovery from Notch inhibition in vivo, and the effects were modest [24]. It is not known whether inhibition of Notch signaling in the vestibular organs would provide sufficient numbers of new hair cells to provide a functional benefit, but these are clearly the next steps towards a therapy based on this strategy. However, beyond this, we still have many challenges ahead of us as the current levels of regeneration in the mammal, even through modulation of Notch signaling, are far less than those found in non-mammalian vertebrates. In order to induce robust, sustainable hair cell regeneration we need to not only increase the degree of hair cell regeneration, but also ensure that these hair cells have the appropriate identity and characteristics, such as polarity, for their specific sensory organ and location within that organ. In addition, in order for hair cell regeneration to be sustainable, the “progenitorlike” pool of support cells from which the new hair cells are arising must be maintained. In the mammal, there appears to be a limited degree of support cell proliferation, but this is unlikely to be sufficient to maintain the level of hair cell regeneration that will ultimately be required for full functional recovery. Many groups are investigating how to induce more robust support cell proliferation in mammalian organs and have shown some success in mature organs through manipulations such as loss of c-Myc [73] and p27kip1 [74, 75] and through addition of TGF-α and EGF with insulin [76].
In addition, even if we can induce robust support cell proliferation, we must still be able to convert sufficient amounts of these support cells into hair cells. While the methods discussed here have shown promising results, the amount of hair cells generated is modest. With each of the regenerative methods used, there seems to be a specific competence window for hair cell regeneration (Figure 5 and Figure 6). Using Notch overexpression for lateral induction, the time window is very early in otic development and appears to follow the normal developmental timeline for prosensory formation (Figure 5A). It is likely that the decreasing competence is linked to the change between the prosensory and lateral inhibition roles that is occurring at this time. Since this change would be necessary for the continued development of the inner ear organs and appears to involve multiple complex regulatory elements, this method of hair cell regeneration may not be feasible or practical for use in mature organs.
Figure 5.
Summaries of the regenerative competence of the inner ear organs in response to Notch activation for lateral induction (A) and Notch inhibition for lateral inhibition (B). A) The ability to form ectopic sensory regions in response to Notch activation (NICD overexpression) is gradually lost embryonically and occurs in a similar development timeframe as the normal prosensory formation. With age, the competence of the otic cells is restricted to specific regions near the sensory organs until it is completely lost by E16.5. Overexpression of Sox2 at this same age, however, can induce ectopic sensory regions, though still only in specific areas. B) While supernumerary hair cells can be generated much later using Notch inhibition, each of the inner ear organs exhibits a declining competence for hair cell regeneration during postnatal maturation. In the cochlea and utricle, it appears that Notch signaling has no effect in adolescent mice without significant damage to the organs. However, with damage, each of the organs does appear to have a modest capacity for hair cell generation in the adult.
Figure 6.
The ability to generate hair cells through Atoh1 overexpression is gradually lost as the inner ear matures. Atoh1 overexpression in the very apical portion of neonatal animals can induce ectopic hair cells throughout the entire cochlear duct, while in the middle and basal regions ectopic hair cells are induced in specific areas near the sensory domain. After a week of age, ectopic hair cells are only generated in the GER of the apical portion of the cochlea and later, Atoh1 overexpression either ectopically or in support cells specifically has no effect.
The induction of robust hair cell generation from Notch inhibition currently seems to be a more feasible approach since all of the mature mammalian organs of the inner ear have some regenerative capacity in response to Notch inhibition (Figure 5B). While it is not clear whether Notch is required for the normal maintenance of the vestibular organs, Notch signaling does seem to play a role in each of the mouse inner ear organs following damage. More work, however, needs to be done in order to establish the conditions under which Notch signaling is induced after damage, as there is some controversy over whether only certain types or degrees of damage can induce a Notch-mediated regenerative response. Ideally, we would be able to regenerate hair cells under all damage conditions, including drug-induced ototoxicity, noise damage, and varying forms of degeneration.
Even with damage, inhibition of Notch signaling has a limited regenerative response. This was particularly apparent in our own data where almost all of the peripheral support cells down-regulated Notch signaling in response to DAPT treatment, but the majority of these cells did not undergo transdifferentiation. This is also true of the utricle and cochlea, where even though it is more difficult to determine how many of the support cells were expressing Notch components and down-regulated them in response to inhibition, it is clear that only a subset of them are transdifferentiating in response to Notch inhibition. What then is limiting support cell transdifferentiation in these mature organs? One possibility is that additional signaling factors might interact with the Notch pathway to regulate competence. For example, the expression of Hey2 in the pillar cells of the cochlea prevents their differentiation into hair cells, even after Notch inhibition. In fact, inhibition of both FGF signaling and Notch signaling is required for the down-regulation of Hey2 and subsequent pillar cell transdifferentiation [18]. Further, the Notch receptor is not the only target for γ-secretase, and therefore Notch might not be the only pathway whose inhibition is contributing to the regeneration observed through γ-secretase inhibition. The γ-secretase complex is involved in a process called regulated intramembrane proteolysis (RIP) that has over ninety known substrates including Notch receptors and ligands, amyloid precursor protein (APP), Ephs/Ephrins, Interleukin receptors, cadherins, and Erb/Neuregulins [reviewed in 77]. While Notch inhibition is sufficient for some hair cell regeneration, as shown by Jung, et al. [54] through specific knock down of Hes5, it is possible that inhibition of some of these other pathways might be modulating the regenerative response.
The regulation of Atoh1 downstream of Notch signaling is another possibility for limiting transdifferentiation as it has several known enhancer sites [78, 79] as well as multiple known repressors and activators under the control of multiple signaling pathways [reviewed in 43]. This is supported by the fact that overexpression of Atoh1 itself is not able to generate hair cells in the mature inner ear (Figure 6). Interestingly, Liu, et al. [40] found that the Atoh1-induced transdifferentiation of pillar cells and Deiters’ cells in the immature cochlea required activation of endogenous Atoh1, suggesting that a critical level of Atoh1 expression through activation of an autoregulatory feedback mechanism may be required [78].
Beyond these regulatory mechanisms, it is also possible that the specialized morphologies of the support cells are limiting their ability to transdifferentiate. This is easiest to imagine in the cochlea where these morphologies are the most extreme. However, even in the vestibular organs of mammals, E-cadherin accumulation in the junctions between support cells results in the thickening of the F-actin belts with age, which does not occur in non-mammalian vertebrates. Further, this thickening is inversely correlated with the decrease in regenerative potential with age as the organs mature [31, 80]. In postnatal cultured utricles, Collado, et al. [31] found that the support cells that were able to transdifferentiate, first down-regulated E-cadherin. Conversely, those support cells that did not transdifferentiate, which accounted for the majority of the support cells, maintained their E-cadherin expression. Therefore, if these specialized junctions are in fact limiting the regenerative potential of these organs, this will be another significant hurdle to inducing robust hair cell regeneration.
Overall, while the recent work investigating the role of Notch inhibition in hair cell regeneration has been very promising and has shown the potential for some therapeutic benefit, we still have many challenges that we must overcome. Ultimately, it appears that we need a better understanding of the different regulatory mechanisms involved in the maturation of the various sensory organs. For example, in order to understand what is limiting the competence of the inner ear for lateral induction, we must understand how the switch between the prosensory and lateral inhibition roles of Notch signaling is occurring. Further, to understand why Notch inhibition does not result in more hair cell transdifferentiation through lateral inhibition, we must understand how Atoh1 is being regulated and mechanistically how it is driving hair cell differentiation. Just as we gained many insights into different ways to use Notch signaling to induce regeneration from studying the role of Notch signaling in development, we must now go back to development to determine how Notch signaling and Atoh1 are being regulated there in order to develop strategies to induce more robust hair cell regeneration.
ACKNOWLEDGEMENTS
This work was supported by the following grants: PHS R21 DC010862 from NIDCD/NIH, PHS R01 DC009991 from NIDCD/NIH, PHS NRSA T32 GM007270 from NIGMS/NIH, and PHS NRSA T32 GM007108 from NIGMS/NIH. We thank Drs. Thomas Reh and Byron Hartman for critical comments on the manuscript and both past and present members of the Bermingham-McDonogh, Reh, and Chao labs for helpful discussions.
ABBREVIATIONS
- DAPT
N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester
- DMSO
Dimethyl sulfoxide
- NICD
Notch intracellular domain
- IHC
inner hair cell
- OHC
outer hair cell
- DIV
days in vitro
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare no competing financial interests.
REFERENCES
- 1.Wharton KA, Johansen KM, Xu T, Artavanis-Tsakonas S. Cell. 1985;43:567. doi: 10.1016/0092-8674(85)90229-6. [DOI] [PubMed] [Google Scholar]
- 2.Andersson ER, Sandberg R, Lendahl U. Development. 2011;138:3593. doi: 10.1242/dev.063610. [DOI] [PubMed] [Google Scholar]
- 3.Andreu-Agulló C, Morante-Redolat JM, Delgado AC, Fariñas I. Nat. Neurosci. 2009;12:1514. doi: 10.1038/nn.2437. [DOI] [PubMed] [Google Scholar]
- 4.Ables JL, Decarolis NA, Johnson MA, Rivera PD, Gao Z, Cooper DC, Radtke F, Hsieh J, Eisch AJ. J. Neurosci. 2010;30:10484. doi: 10.1523/JNEUROSCI.4721-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aguirre A, Rubio ME, Gallo V. Nature. 2010;467:323. doi: 10.1038/nature09347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breunig JJ, Silbereis J, Vaccarino FM, Sestan N, Rakic P. Proc. Natl. Acad. Sci. USA. 2007;104:20558. doi: 10.1073/pnas.0710156104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlen M, Meletis K, Goritz C, Darsalia V, Evergren E, Tanigaki K, Amendola M, Barnabe-Heider F, Yeung MS, Naldini L, Honjo T, Kokaia Z, Shupliakov O, Cassidy RM, Lindvall O, Frisen J. Nat. Neurosci. 2009;12:259. doi: 10.1038/nn.2268. [DOI] [PubMed] [Google Scholar]
- 8.Ehm O, Goritz C, Covic M, Schaffner I, Schwarz TJ, Karaca E, Kempkes B, Kremmer E, Pfrieger FW, Espinosa L, Bigas A, Giachino C, Taylor V, Frisen J, Lie DC. J. Neurosci. 2010;30:13794. doi: 10.1523/JNEUROSCI.1567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Givogri MI, de Planell M, Galbiati F, Superchi D, Gritti A, Vescovi A, de Vellis J, Bongarzone ER. Dev. Neurosci. 2006;28:81. doi: 10.1159/000090755. [DOI] [PubMed] [Google Scholar]
- 10.Imayoshi I, Sakamoto M, Yamaguchi M, Mori K, Kageyama R. J. Neurosci. 2010;30:3489. doi: 10.1523/JNEUROSCI.4987-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lugert S, Basak O, Knuckles P, Haussler U, Fabel K, Gotz M, Haas CA, Kempermann G, Taylor V, Giachino C. Cell Stem Cell. 2010;6:445. doi: 10.1016/j.stem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 12.Stump G, Durrer A, Klein AL, Lutolf S, Suter U, Taylor V. Mech. Dev. 2002;114:153. doi: 10.1016/s0925-4773(02)00043-6. [DOI] [PubMed] [Google Scholar]
- 13.D’Souza B, Meloty-Kapella L, Weinmaster G. Curr. Top. Dev. Biol. 2010;92:73. doi: 10.1016/S0070-2153(10)92003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kopan R, Ilagan MX. Cell. 2009;137:216. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moretti J, Brou C. Int. J. Mol. Sci. 2013;14:6359. doi: 10.3390/ijms14036359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eatock RA, Rusch A, Lysakowski A, Saeki M. Otolaryngol Head Neck Surg. 1998;119:172. doi: 10.1016/S0194-5998(98)70052-X. [DOI] [PubMed] [Google Scholar]
- 17.Brooker R, Hozumi K, Lewis J. Development. 2006;133:1277. doi: 10.1242/dev.02284. [DOI] [PubMed] [Google Scholar]
- 18.Doetzlhofer A, Basch ML, Ohyama T, Gessler M, Groves AK, Segil N. Dev. Cell. 2009;16:58. doi: 10.1016/j.devcel.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du X, Li W, Gao X, West MB, Saltzman WM, Cheng CJ, Stewart C, Zheng J, Cheng W, Kopke RD. Hear. Res. 2013;304:91. doi: 10.1016/j.heares.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayashi T, Kokubo H, Hartman BH, Ray CA, Reh TA, Bermingham-McDonogh O. Dev. Biol. 2008;316:87. doi: 10.1016/j.ydbio.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiernan AE, Cordes R, Kopan R, Gossler A, Gridley T. Development. 2005;132:4353. doi: 10.1242/dev.02002. [DOI] [PubMed] [Google Scholar]
- 22.Korrapati S, Roux I, Glowatzki E, Doetzlhofer A. PLoS One. 2013;8:e73276. doi: 10.1371/journal.pone.0073276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanford PJ, Lan Y, Jiang R, Lindsell C, Weinmaster G, Gridley T, Kelley MW. Nat. Genet. 1999;21:289. doi: 10.1038/6804. [DOI] [PubMed] [Google Scholar]
- 24.Mizutari K, Fujioka M, Hosoya M, Bramhall N, Okano HJ, Okano H, Edge AS. Neuron. 2013;77:58. doi: 10.1016/j.neuron.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takebayashi S, Yamamoto N, Yabe D, Fukuda H, Kojima K, Ito J, Honjo T. Dev. Biol. 2007;307:165. doi: 10.1016/j.ydbio.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto N, Tanigaki K, Tsuji M, Yabe D, Ito J, Honjo T. J. Mol. Med. (Berl) 2006;84:37. doi: 10.1007/s00109-005-0706-9. [DOI] [PubMed] [Google Scholar]
- 27.Zhang N, Martin GV, Kelley MW, Gridley T. Curr. Biol. 2000;10:659. doi: 10.1016/s0960-9822(00)00522-4. [DOI] [PubMed] [Google Scholar]
- 28.Zhao LD, Guo WW, Lin C, Li LX, Sun JH, Wu N, Ren LL, Li XX, Liu HZ, Young WY, Gao WQ, Yang SM. PLoS One. 2011;6:e23729. doi: 10.1371/journal.pone.0023729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng JL, Shou J, Guillemot F, Kageyama R, Gao WQ. Development. 2000;127:4551. doi: 10.1242/dev.127.21.4551. [DOI] [PubMed] [Google Scholar]
- 30.Zine A, Aubert A, Qiu J, Therianos S, Guillemot F, Kageyama R, de Ribaupierre F. J. Neurosci. 2001;21:4712. doi: 10.1523/JNEUROSCI.21-13-04712.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collado MS, Thiede BR, Baker W, Askew C, Igbani LM, Corwin JT. J. Neurosci. 2011;31:11855. doi: 10.1523/JNEUROSCI.2525-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cotanche DA, Kaiser CL. Hear. Res. 2010;266:18. doi: 10.1016/j.heares.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiernan AE. Semin. Cell Dev. Biol. 2013;24:470. doi: 10.1016/j.semcdb.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murata J, Ikeda K, Okano H. Adv. Exp. Med. Biol. 2012;727:161. doi: 10.1007/978-1-4614-0899-4_12. [DOI] [PubMed] [Google Scholar]
- 35.Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Science. 1999;284:1837. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- 36.Driver EC, Sillers L, Coate TM, Rose MF, Kelley MW. Dev. Biol. 2013;376:86. doi: 10.1016/j.ydbio.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang H, Xie X, Deng M, Chen X, Gan L. Genesis. 2010;48:407. doi: 10.1002/dvg.20633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelly MC, Chang Q, Pan A, Lin X, Chen P. J. Neurosci. 2012;32:6699. doi: 10.1523/JNEUROSCI.5420-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng JL, Gao WQ. Nat. Neurosci. 2000;3:580. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- 40.Liu Z, Dearman JA, Cox BC, Walters BJ, Zhang L, Ayrault O, Zindy F, Gan L, Roussel MF, Zuo J. J. Neurosci. 2012;32:6600. doi: 10.1523/JNEUROSCI.0818-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Millimaki BB, Sweet EM, Dhason MS, Riley BB. Development. 2007;134:295. doi: 10.1242/dev.02734. [DOI] [PubMed] [Google Scholar]
- 42.Lewis RM, Hume CR, Stone JS. Hear. Res. 2012;289:74. doi: 10.1016/j.heares.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mulvaney J, Dabdoub A. J. Assoc. Res. Otolaryngol. 2012;13:281. doi: 10.1007/s10162-012-0317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Basch ML, Ohyama T, Segil N, Groves AK. J. Neurosci. 2011;31:8046. doi: 10.1523/JNEUROSCI.6671-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hao J, Koesters R, Bouchard M, Gridley T, Pfannenstiel S, Plinkert PK, Zhang L, Praetorius M. Acta. Otolaryngol. 2012;132:1028. doi: 10.3109/00016489.2012.690533. [DOI] [PubMed] [Google Scholar]
- 46.Kiernan AE, Ahituv N, Fuchs H, Balling R, Avraham KB, Steel KP, Hrabe de Angelis M. Proc. Natl. Acad. Sci. USA. 2001;98:3873. doi: 10.1073/pnas.071496998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kiernan AE, Xu J, Gridley T. PLoS Genet. 2006;2:e4. doi: 10.1371/journal.pgen.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pan W, Jin Y, Stanger B, Kiernan AE. Proc. Natl. Acad. Sci. USA. 2010;107:15798. doi: 10.1073/pnas.1003089107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamamoto N, Chang W, Kelley MW. Dev. Biol. 2011;353:367. doi: 10.1016/j.ydbio.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 50.Pan W, Jin Y, Chen J, Rottier RJ, Steel KP, Kiernan AE. J. Neurosci. 2013;33:16146. doi: 10.1523/JNEUROSCI.3150-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hartman BH, Reh TA, Bermingham-McDonogh O. Proc. Natl. Acad. Sci. USA. 2010;107:15792. doi: 10.1073/pnas.1002827107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hori R, Nakagawa T, Sakamoto T, Matsuoka Y, Takebayashi S, Ito J. Neuroreport. 2007;18:1911. doi: 10.1097/WNR.0b013e3282f213e0. [DOI] [PubMed] [Google Scholar]
- 53.Jeon SJ, Fujioka M, Kim SC, Edge AS. J. Neurosci. 2011;31:8351. doi: 10.1523/JNEUROSCI.6366-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jung JY, Avenarius MR, Adamsky S, Alpert E, Feinstein E, Raphael Y. Mol. Ther. 2013;21:834. doi: 10.1038/mt.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Z, Owen T, Fang J, Zuo J. PLoS One. 2012;7:e34123. doi: 10.1371/journal.pone.0034123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Savary E, Sabourin JC, Santo J, Hugnot JP, Chabbert C, Van De Water T, Uziel A, Zine A. Mech. Dev. 2008;125:674. doi: 10.1016/j.mod.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 57.Slowik AD, Bermingham-McDonogh O. J. Assoc. Res. Otolaryngol. 2013;14:813. doi: 10.1007/s10162-013-0414-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin V, Golub JS, Nguyen TB, Hume CR, Oesterle EC, Stone JS. J. Neurosci. 2011;31:15329. doi: 10.1523/JNEUROSCI.2057-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murata J, Tokunaga A, Okano H, Kubo T. J. Comp. Neurol. 2006;497:502. doi: 10.1002/cne.20997. [DOI] [PubMed] [Google Scholar]
- 60.Hicks C, Johnston SH, diSibio G, Collazo A, Vogt TF, Weinmaster G. Nat. Cell Biol. 2000;2:515. doi: 10.1038/35019553. [DOI] [PubMed] [Google Scholar]
- 61.Kato TM, Kawaguchi A, Kosodo Y, Niwa H, Matsuzaki F. Mol. Cell Neurosci. 2010;45:12. doi: 10.1016/j.mcn.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 62.Espinosa L, Ingles-Esteve J, Aguilera C, Bigas A. J. Biol. Chem. 2003;278:32227. doi: 10.1074/jbc.M304001200. [DOI] [PubMed] [Google Scholar]
- 63.Roberson DF, Weisleder P, Bohrer PS, Rubel EW. Hear. Res. 1992;57:166. doi: 10.1016/0378-5955(92)90149-h. [DOI] [PubMed] [Google Scholar]
- 64.Ma EY, Rubel EW, Raible DW. J. Neurosci. 2008;28:2261. doi: 10.1523/JNEUROSCI.4372-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Daudet N, Gibson R, Shang J, Bernard A, Lewis J, Stone J. Dev. Biol. 2009;326:86. doi: 10.1016/j.ydbio.2008.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hartman BH, Hayashi T, Nelson BR, Bermingham-McDonogh O, Reh TA. Dev. Dyn. 2007;236:2875. doi: 10.1002/dvdy.21307. [DOI] [PubMed] [Google Scholar]
- 67.Hartman BH, Basak O, Nelson BR, Taylor V, Bermingham-McDonogh O, Reh TA. J. Assoc. Res. Otolaryngol. 2009;10:321. doi: 10.1007/s10162-009-0162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang GP, Chatterjee I, Batts SA, Wong HT, Gong TW, Gong SS, Raphael Y. Hear. Res. 2010;267:61. doi: 10.1016/j.heares.2010.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lopez I, Honrubia V, Lee SC, Schoeman G, Beykirch K. Int. J. Dev. Neurosci. 1997;15:447. doi: 10.1016/s0736-5748(96)00103-7. [DOI] [PubMed] [Google Scholar]
- 70.Karis A, Pata I, van Doorninck JH, Grosveld F, de Zeeuw CI, de Caprona D, Fritzsch B. J. Comp. Neurol. 2001;429:615. doi: 10.1002/1096-9861(20010122)429:4<615::aid-cne8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 71.Neves A, English K, Priess JR. Development. 2007;134:4459. doi: 10.1242/dev.008680. [DOI] [PubMed] [Google Scholar]
- 72.Alvarado DM, Veile R, Speck J, Warchol M, Lovett M. Dev. Dyn. 2009;238:3093. doi: 10.1002/dvdy.22149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Burns JC, Yoo JJ, Atala A, Jackson JD. PLoS One. 2012;7:e48704. doi: 10.1371/journal.pone.0048704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lowenheim H, Furness DN, Kil J, Zinn C, Gultig K, Fero ML, Frost D, Gummer AW, Roberts JM, Rubel EW, Hackney CM, Zenner HP. Proc. Natl. Acad. Sci. USA. 1999;96:4084. doi: 10.1073/pnas.96.7.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oesterle EC, Chien WM, Campbell S, Nellimarla P, Fero ML. Cell Cycle. 2011;10:1237. doi: 10.4161/cc.10.8.15301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yamashita H, Oesterle EC. Proc. Natl. Acad. Sci. USA. 1995;92:3152. doi: 10.1073/pnas.92.8.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haapasalo A, Kovacs DM. J. Alzheimers. Dis. 2011;25:3. doi: 10.3233/JAD-2011-101065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Helms AW, Abney AL, Ben-Arie N, Zoghbi HY, Johnson JE. Development. 2000;127:1185. doi: 10.1242/dev.127.6.1185. [DOI] [PubMed] [Google Scholar]
- 79.Helms AW, Johnson JE. Development. 1998;125:919. doi: 10.1242/dev.125.5.919. [DOI] [PubMed] [Google Scholar]
- 80.Burns JC, Christophel JJ, Collado MS, Magnus C, Carfrae M, Corwin JT. J. Comp. Neurol. 2008;511:396. doi: 10.1002/cne.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.MacDonald GH, Rubel EW. Hear. Res. 2008;243:1. doi: 10.1016/j.heares.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]