SUMMARY
Alzheimer’s disease (AD) is an age-related neurological disorder characterized by synaptic loss and dementia. The low-density lipoprotein receptor-related protein 6 (LRP6) is an essential co-receptor for Wnt signaling and its genetic variants have been linked to AD risk. Here we report that neuronal LRP6-mediated Wnt signaling is critical for synaptic function and cognition. Conditional deletion of Lrp6 gene in mouse forebrain neurons leads to age-dependent deficits in synaptic integrity and memory. Neuronal LRP6 deficiency in an amyloid mouse model also leads to exacerbated amyloid pathology due to increased APP processing to amyloid-β. In humans, LRP6 and Wnt signaling are significantly down-regulated in AD brains, likely by a mechanism that depends on amyloid-β. Our results define a critical pathway in which decreased LRP6-mediated Wnt signaling, synaptic dysfunction and elevated Aβ synergistically accelerate AD progression, and suggest that restoring LRP6-mediated Wnt signaling can be explored as a novel strategy for AD therapy.
INTRODUCTION
The low-density lipoprotein receptor-related protein 6 (LRP6) is an essential co-receptor for the canonical Wnt pathway. Wnt ligands activate the pathway by binding to LRP6 cooperatively with Frizzled receptors, and transduce signal through the stabilization of β-catenin. The stabilized β-catenin in turn translocates to the nucleus, where it activates Wnt target genes (Niehrs, 2012). Wnt signaling, which regulates diverse developmental processes in the nervous system (Budnik and Salinas, 2011), has been implicated in the modulation of neurogenesis, dendritic morphogenesis, and synaptic function (Inestrosa and Arenas, 2010; Park and Shen, 2012). Synapses and dendritic spines are dynamic structures whose plasticity underlies learning and memory (Bourne and Harris, 2008). Wnt ligands have also been shown to modulate neurotransmitter release at the presynaptic terminal (Cerpa et al., 2008). In the postsynaptic regions, Wnt signaling regulates the trafficking of glutamate receptors and their interactions with postsynaptic density protein 95 (PSD-95) (Cerpa et al., 2011). These findings suggest that misregulation of this pathway likely contributes to synaptic dysfunction in neurodegenerative diseases, including Alzheimer’s disease (AD) (Inestrosa et al., 2007).
AD is characterized by synaptic loss and progressive cognitive deficits and is the most common cause of dementia affecting a growing population of elderly individuals (Thies and Bleiler, 2013). The neuropathological hallmarks of AD are the presence of amyloid-β (Aβ) plaques and neurofibrillary tangles, along with dystrophic neurites, and gliosis (Holtzman et al., 2011). Aβ, generated from sequential proteolytic processing of β-amyloid precursor protein (APP) through β- and γ-secretases (Chami and Checler, 2012; Cole and Vassar, 2007; Steiner and Haass, 2000), has been shown to disrupt synapses and initiate a cascade of toxic events that lead to eventual neuronal loss (Shankar et al., 2008). In addition to amyloid pathogenesis, synaptic dysfunction is an early feature of AD, perhaps even prior to Aβ deposition (Arendt, 2009; Selkoe, 2002). Consistent with this notion, disturbance in synaptic integrity is detected in patients with mild cognitive impairment, a prodromal state of AD (Scheff et al., 2006). Indeed, loss of synaptic markers is a strong predictor of clinical symptoms and disease progression in AD (Selkoe, 2002). Thus, understanding molecular pathways underlying synaptic dysfunction in AD will help to define specific molecular targets for therapy. Genome-wide association studies have defined a broad susceptibility region for late-onset AD on chromosome 12, which includes the region encoding LRP6 (De Ferrari et al., 2007). Indeed, two LRP6 SNPs and an alternative splice variant were found to be associated with increased risk of developing AD, which is most likely due to a suppression of Wnt signaling activity (Alarcon et al., 2013; De Ferrari et al., 2007). Despite these implications, the molecular mechanism by which LRP6 regulates AD pathogenesis is poorly understood. We thus focused on testing how an impairment of LRP6 function impacts AD pathogenesis and examining potential changes in LRP6-mediated Wnt signaling in human AD brains.
Herein, we show that deletion of neuronal Lrp6 in mice is sufficient to cause age-dependent synaptic loss and memory impairments. In addition, LRP6 deficiency in neurons exacerbates amyloid pathology and cognitive deficits in an amyloid mouse model. Furthermore, LRP6-mediated Wnt signaling is down-regulated in postmortem AD brains and negatively correlates with Aβ levels. Our findings define a critical role of LRP6-mediated Wnt signaling in synaptic integrity and establish that an impairment of this pathway contributes to AD pathogenesis.
RESULTS
Neuronal LRP6 Deficiency Leads to Cognitive Impairment, Synaptic Deficits and Neuroinflammation in Aged Mice
Emerging studies have shown that Wnt signaling regulates synaptic function and plasticity (Inestrosa and Varela-Nallar, 2013; Jensen et al., 2012). To investigate the potential role of neuronal LRP6, we conditionally deleted the Lrp6 gene in neurons by crossing Lrp6flox/flox mice (Joeng et al., 2011; Zhong et al., 2012) with calcium/calmodulin-dependent protein kinase II alpha (CaMKII-Cre) mice (Tsien et al., 1996), generating Lrp6 conditional KO (Lrp6 cKO) mice and their corresponding littermate control (Ctrl) mice. As the Cre recombinase is expressed in postnatal forebrain (Tsien et al., 1996), LRP6 levels in the cortex of Lrp6 cKO mice were significantly decreased at 3 months of age, which progressively reached maximal deletion at 12 months of age (Figure 1A). The residual LRP6 observed at older ages likely represents the expression within glial cells and/or brain vasculature (Ren et al., 2013; Wang et al., 2004). Consistent with a neuronal deletion of LRP6, free β-catenin levels were significantly decreased in the cortex of Lrp6 cKO mice at different ages (Figures 1B and 1C).
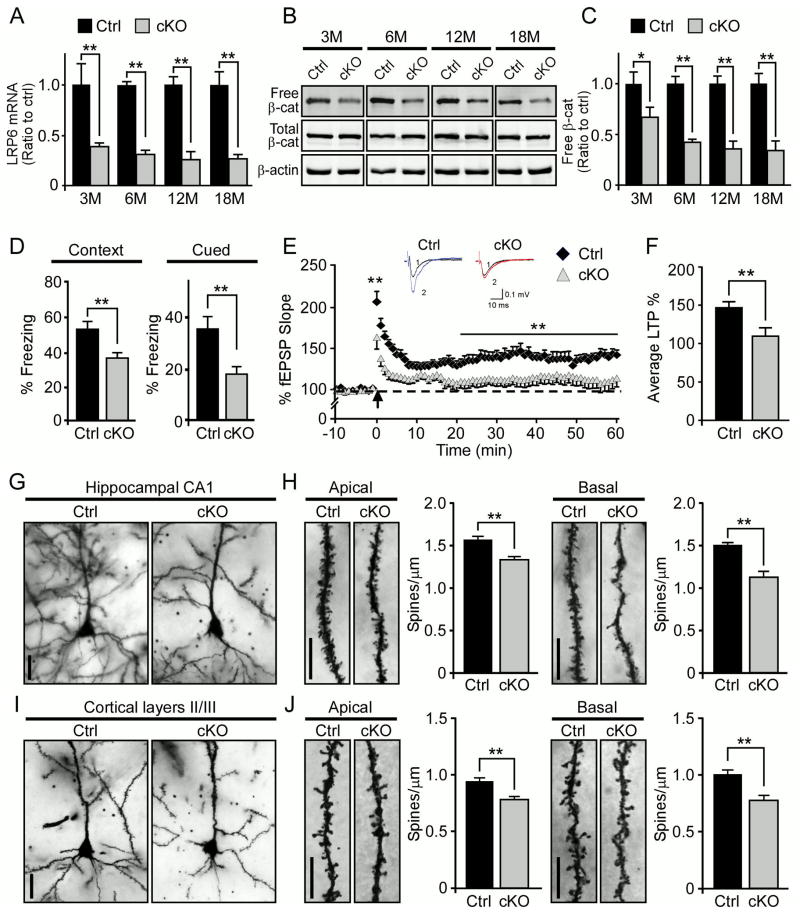
Figure 1. Neuronal LRP6 Deficiency Leads to Synaptic Dysfunction, Memory Impairments, and Dendritic Spine Loss in Aged Mice.
(A) LRP6 expression levels in the cortex of control (Ctrl) and Lrp6 cKO (cKO) mice (n=3, per genotype) examined at 3, 6, 12 and 18 months of age (n=3, each age) by real-time PCR. Results were normalized to β-actin levels. In the following figures, data are normalized to those of Ctrl mice for comparison. Values are mean ± SEM. **p < 0.01.
(B and C) Total and free β-catenin (β-cat) levels in the cortex of Ctrl and Lrp6 cKO mice at indicated ages. Free β-catenin levels were evaluated by GST-E-Cadherin pull-down, followed by Western blot analysis. Data represent mean ± SEM. *p < 0.05; **p < 0.01.
(D)The cognitive function of Ctrl (n=15) and Lrp6 cKO (n=18) mice at 22 months of age examined by fear conditioning tests. The percentage of time spent showing freezing behavior in response to stimulus during contextual or cued memory tests is shown. Data represent mean ±SEM. **p < 0.01.
(E) Impaired hippocampal LTP in the CA1 region of Lrp6 cKO mice compared to Ctrl mice. Normalized fEPSP responses to field stimulation before and after tetanus (at 100 Hz for 1 sec, repeated three times) were summarized during recording from CA1 region of Ctrl (n=13) and Lrp6 cKO (n=12) hippocampal slices. Data represent mean ± SEM. **p < 0.01. Representative traces before (1) and after (2) tetanic stimulation from the recordings of Ctrl and Lrp6 cKO mice are shown above fEPSP recording. Scale bars depict 10 ms and 0.1 mV.
(F) Averages of the last 5 min of fEPSP recording. Data represent mean ± SEM. **p < 0.01.
(G and I) Representative Golgi-impregnated neurons in hippocampal CA1 region (G) and cortical layer II/III (I) from Ctrl and Lrp6 cKO mice at 18 months of age. Scale bars, 10 μm.
(H and J) Representative apical oblique and basal shaft dendrites from hippocampal CA1 region (H) and cortical layers II/III (J). Scale bars, 10 μm. Spine numbers of apical oblique (n=100 dendritic segments on 50 neurons from 5 mice of each genotype) and basal shaft (n=100 dendritic segments on 50 neurons from 5 mice of each genotype) dendrites (70 μm segments) were counted. The spine densities are expressed as mean spine number per 1 μm dendrite segment ± SEM. **p < 0.01.
See also Figures S1 and S2.
To examine the potential roles of neuronal LRP6 in cognition, the memory function of Lrp6 cKO mice and their age-matched controls was analyzed by contextual and cued fear conditioning tests. In this behavioral paradigm, animals were exposed to an auditory stimulus followed by a mild electric foot shock. Upon re-exposure to the same environment (contextual test) 24 hours later, animals with intact cognition will freeze more. Likewise, animals with intact cognition placed in an altered environment will display freezing behavior upon hearing the auditory stimulus (cued test). These two tasks test associative memory, which depends on the function of hippocampus, or in case of cued test also amygdala. We found that LRP6 deficiency resulted in significant memory deficits in Lrp6 cKO mice at 22 months of age as assessed by contextual and cued fear conditioning tests (Figure 1D). Interestingly, we did not observe significant differences in memory- and anxiety-related behaviors between Ctrl and Lrp6 cKO mice at 6 months of age (Figures S1A–C), suggesting that memory impairments associated with neuronal LRP6 deletion is age-dependent. We also examined the effects of LRP6 deficiency on general behaviors including those that measure locomotor activity and anxiety levels by open field, light/dark exploration and elevated plus maze paradigms. We found that the locomotor activity and anxiety behaviors were not affected in Lrp6 cKO mice at old ages (Figures S1D–F), indicating that the observed cognitive impairment in Lrp6 cKO mice was not due to anxiety. Furthermore, to investigate whether LRP6 regulates motor learning and coordination, Ctrl and Lrp6 cKO mice were subjected to the rotarod test but no significant differences were detected between genotypes (Figure S1G). These results indicate that LRP6 deficiency in neurons selectively impairs memory performance in aged animals.
The plasticity of synapses and dendritic spines is critical for learning and memory. Given that Lrp6 cKO mice exhibited significant cognitive deficits at old ages, we next investigated the effect of neuronal LRP6 deficiency on long-term potentiation (LTP), a neurophysiological measure that correlates with learning and memory. The synaptic plasticity was evaluated after tetanic stimulation and upon recording of field excitatory postsynaptic potentials (fEPSPs) in Ctrl and Lrp6 cKO mice at 18 months of age. Importantly, we found that LRP6 deficiency in neurons leads to impaired LTP induction and maintenance (Figure 1E). Hippocampal LTP in the Schaffer collaterals of Lrp6 cKO mice decayed sharply within 25 minutes compared with Ctrl mice, suggesting that Lrp6 cKO mice failed to maintain the potentiated state (Figures 1E and 1F). LRP6 deletion did not lead to significant changes in LTP in young mice (Figures S2A and S2B), suggesting that this defect in synaptic plasticity of Lrp6 cKO mice is age-dependent.
Dendritic spines serve as the postsynaptic platform for most excitatory synapses in the brain, and their morphological changes are tightly correlated with synaptic strength (Yuste and Bonhoeffer, 2001). As aged Lrp6 cKO mice exhibit abnormal synaptic plasticity, we next examined the dendritic spine structure in Ctrl and Lrp6 cKO mice by Golgi staining. We observed a significant dendritic spine loss in hippocampal CA1 and cortical regions in Lrp6 cKO mice (Figures 1G and 1I). Moreover, the spine density of both the pyramidal neurons from apical oblique and basal shaft dendrites in the CA1 region of the hippocampus and layer II/III of the cortex were significantly decreased in Lrp6 cKO mice at 18 months of age (Figures 1H and 1J), but unaltered at 6 months of age (Figures S2C–F). Together, these results indicate that LRP6 plays critical roles in maintaining synaptic functions and dendritic spine integrity in an age-dependent manner.
We next analyzed the levels of synaptophysin and PSD-95, presynaptic and postsynaptic markers, respectively, in the cortex and hippocampus of Ctrl and Lrp6 cKO mice. There was no significant difference in the levels of either synaptic marker between the two genotypes at 6 months of age (Figures 2A and S2G). Importantly, we found a significant decrease in PSD-95 levels in both the cortex and hippocampus of Lrp6 cKO mice compared with Ctrl mice at 18 months of age, while the levels of synaptophysin were unchanged (Figure 2B). PSD-95 is a core postsynaptic scaffold protein in mature excitatory glutamatergic synapses, where two subtypes of glutamate receptors (i.e., N-methyl-D-aspartate receptor (NMDAR) and α-amino-3-hydroxy-5-methyl-4-isoxazole -propionic acid receptor (AMPAR)) mediate synaptic transmission and plasticity. In addition to PSD-95, the levels of NMDAR1 and AMPAR subunit GluR1 were also reduced in the cortex of Lrp6 cKO mice compared with Ctrl mice (Figure S2H). As a control, the levels of synaptic markers were unaltered in the cerebellum where CaMKII-Cre is not active (Figure S2I). These results indicate that LRP6 plays an important role in maintaining synaptic integrity in aged mice.
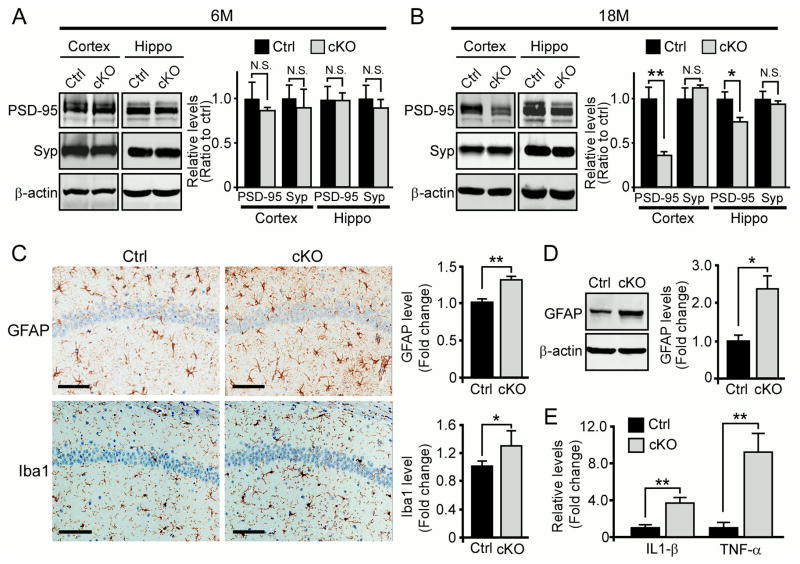
Figure 2. Compromised Synaptic Integrity and Enhanced Neuroinflammation in Neuronal LRP6 Deficient Mice.
(A and B) The levels of synaptic markers in cortex and hippocampus (Hippo) of control (Ctrl) and Lrp6 cKO (cKO) mice at 6 months (n=4–5 per genotype) and 18 months of age (n=5–7 per genotype). Densitometric quantification is expressed as mean ± SEM. *p < 0.05; **p < 0.01; N.S., not significant.
(C) Representative images and quantification (n=5–7 mice per genotype) of GFAP and Iba1 staining in the hippocampal CA1 region of Ctrl and Lrp6 cKO mice at 22 months of age. Stained sections were scanned on the Aperio ScanScope slide scanner and analyzed using the ImageScope software. Scale Bar, 100 μm. Data represent mean ± SEM. *p < 0.05; **p < 0.01.
(D) The levels of GFAP in the hippocampus of Ctrl and Lrp6 cKO mice (n= 4–5 per genotype) examined by Western blot. Data represent mean ± SEM. *p < 0.05.
(E) The levels of IL-1β and TNF-α in the hippocampus of Ctrl and Lrp6 cKO mice (n=4) evaluated by ELISA. Data represent mean ± SEM. **p < 0.01.
See also Figure S2.
The loss of synapses and dendritic spines in Lrp6 cKO mice led us to hypothesize that LRP6 deficiency might lead to neuroinflammation. Using antibodies to glial fibrillary acidic protein (GFAP) and ionized calcium-binding adapter molecule 1 (Iba1), which are markers for activation of astrocytes and microglia, respectively, we found that GFAP-positive astrocytes and Iba1-positive microglia in the hippocampus were significantly increased in aged Lrp6 cKO mice (Figure 2C). The increase of GFAP in Lrp6 cKO mice was also confirmed by Western blot (Figure 2D). To further confirm microglial activation, we measured the expression levels of proinflammatory cytokines interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) by enzyme-linked immunosorbent assay (ELISA), and found that they were significantly increased in the hippocampus of aged Lrp6 cKO mice (Figure 2E). These results indicate that loss of neuronal LRP6 leads to neuroinflammation, which might further damage synapses and contribute to cognitive dysfunction.
LRP6-mediated Wnt Signaling is Critical for Synaptic Integrity and Neuronal Viability
To further investigate whether LRP6 is required for maintaining synaptic integrity, we established experimental conditions under which LRP6 was efficiently knocked down in primary neurons using lentivirus carrying LRP6 shRNA. As expected, upon LRP6 knockdown in mouse primary neurons (Figure 3A), the levels of both total and free β-catenin were significantly decreased (Figure 3B), indicating a suppression of Wnt signaling. Consistent with the effects of LRP6 deficiency in vivo, we observed a significant reduction in the levels of PSD-95, but not synaptophysin, in LRP6-knockdown (LRP6-KD) neurons (Figure 3C). Furthermore, LRP6 knockdown significantly suppressed the levels of NMDAR1 and GluR1 (Figure 3C). Effects of LRP6 knockdown on synapses were further confirmed by immunofluorescence staining. We found that PSD-95 positive, but not synaptophysin positive puncta, were significantly reduced in LRP6-KD neurons (Figure 3D). These results suggest that LRP6-mediated Wnt signaling plays an important role in maintaining postsynaptic integrity.
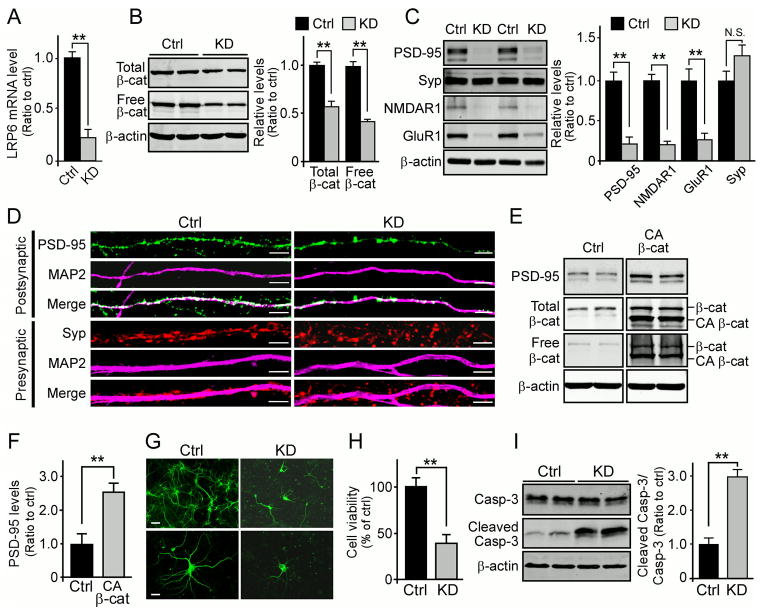
Figure 3. LRP6-mediated Wnt Signaling Regulates Synaptic Integrity and Cell Survival in Primary Neurons.
(A) LRP6 levels in control and LRP6-KD neurons examined by real-time PCR. **p < 0.01.
(B) Reduction in the levels of total and free β-catenin in LRP6-KD neurons compared with control neurons. Free β-catenin levels were evaluated by GST-E-Cadherin pull-down, followed by Western blot analysis. Densitometric data are expressed as mean ± SEM. **p < 0.01.
(C) Western blot analyses of synaptic proteins (PSD-95, synaptophysin (Syp), NMDAR1 and GluR1) in control and LRP6-KD neurons. Densitometric data are expressed as mean ± SEM. **p < 0.01; N.S., not significant.
(D) Representative confocal immunofluorescence images of presynaptic (synaptophysin, red) and postsynaptic protein clusters (PSD-95, green) in control and LRP6-KD primary neurons. The specific dendritic marker, microtubule-associated protein (MAP2), is shown in magenta. Scale bar, 5 μm.
(E and F) The levels of PSD-95, total and free β-catenin in LRP6-KD primary neurons treated with control vector or CA β-catenin (deletion of amino acids 1–90) examined by Western blot. Densitometric analysis of PSD-95 level is shown as mean ± SEM. **p < 0.01.
(G and H) Cell morphology and viability in control or LRP6-KD primary neurons (4 d knockdown) examined by MAP2 staining (G) and MTT assay (H). Scale bars, 50 μm (top panel); 10 μm (bottom panel). MTT quantification data represent mean ± SEM. **p < 0.01.
(I) Levels of caspase-3 (Casp-3) and cleaved caspase-3 in control or LRP6-KD neurons assessed by Western blot. Data represent mean ± SEM. **p < 0.01.
To examine whether effects of LRP6 knockdown on synaptic integrity can be rescued by restoring downstream Wnt signaling, LRP6-KD neurons were infected with lentivirus expressing a constitutively active form of β-catenin (CA β-catenin) (Guo et al., 2012). We found that forced expression of CA β-catenin significantly enhanced Wnt signaling activation and partially rescued the level of PSD-95 (Figures 3E and 3F). This result further suggests that LRP6 and LRP6-dependent Wnt signaling are important for synaptic integrity, particularly at the postsynaptic sites.
Next, to determine whether down-regulation of LRP6 induces neuronal death, we evaluated changes in neuronal morphology and cell viability following LRP6 shRNA infection. Prolonged LRP6 knockdown induced pronounced neuritic dystrophy and neuronal loss compared with control treatments (Figures 3G and 3H). Furthermore, LRP6 down-regulation resulted in activation of caspase-3 in LRP6-KD neurons (Figure 3I), indicating that the decreased cell survival was due to an induction of apoptosis. These results support a role of LRP6-mediated Wnt signaling in regulating postsynaptic integrity and neuronal survival.
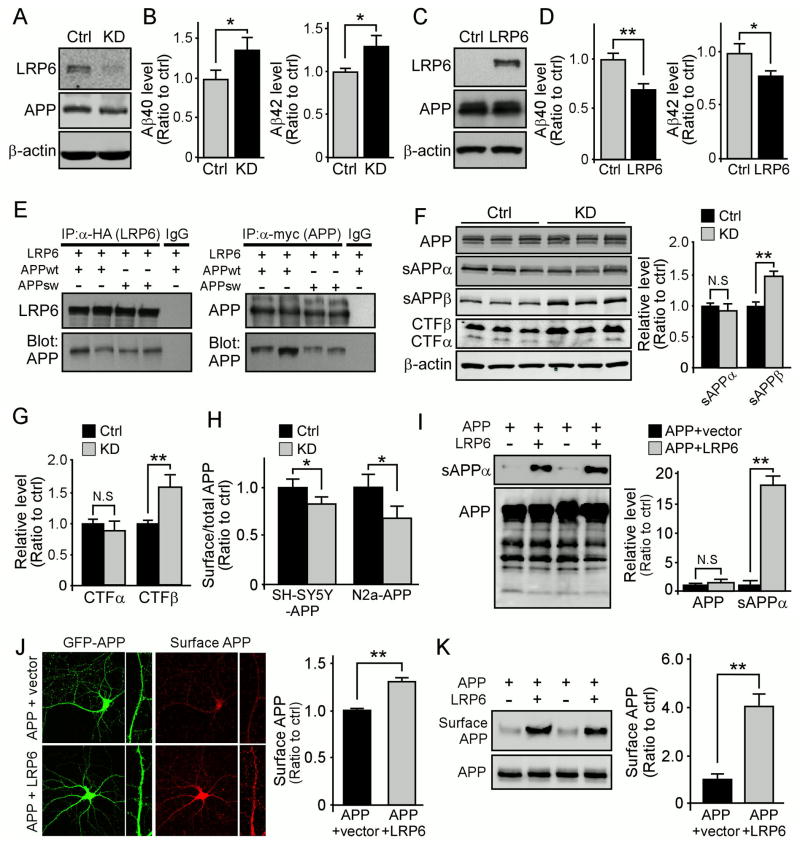
Neuronal LRP6 Deficiency Increases Aβ Levels and Exacerbates Amyloid Pathology in an Amyloid Mouse Model
To examine whether LRP6 modulates amyloid pathology, we next examined endogenous Aβ levels in the brain of Lrp6 cKO mice. We found that neuronal LRP6 deficiency significantly increased mouse endogenous Aβ40 and Aβ42 levels in the brain compared with Ctrl mice (Figure 4A). To assess the roles of LRP6 in APP processing, we investigated the effects of LRP6 deletion on various APP processing products, including soluble APP ectodomains (sAPPα and sAPPβ) and C-terminal fragments (CTFα and CTFβ). The levels of sAPPβ and CTFβ were slightly yet significantly increased in the cortex of Lrp6 cKO mice, while total APP remains unchanged (Figure 4B).
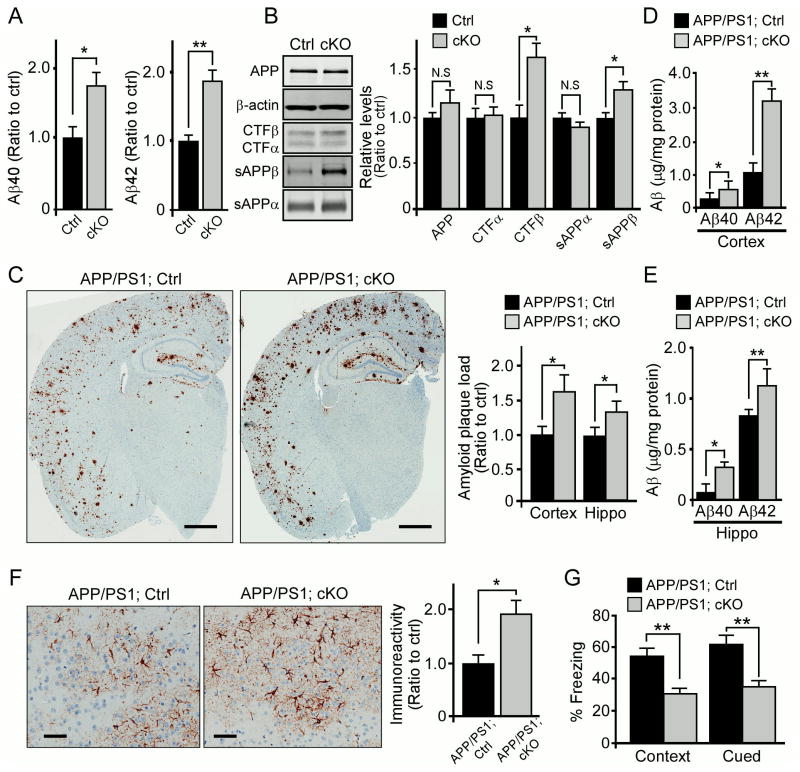
Figure 4. LRP6 Deficiency in Neurons Exacerbates Amyloid Pathology, Cognitive Impairment and Neuroinflammation.
(A) The endogenous Aβ levels in the cortex of 12-month-old control (Ctrl) and Lrp6 cKO (cKO) mice (n=5/per group). *p < 0.05; **p < 0.01.
(B) Levels of full-length APP and various APP processing fragments evaluated in Ctrl and Lrp6 cKO mice (n=5/per group) by Western blot analysis and densitometric analyses. *p < 0.05; N.S., not significant.
(C) Brain sections from APP/PS1; Ctrl and APP/PS1; cKO mice (n=5 per group) at 9 months of age immunostained for Aβ. Scale bar, 100 μm. The percentage of area covered by plaques was quantified, and the plaque load was normalized to that of APP/PS1; Ctrl mice. Values are mean ± SEM. *p < 0.05.
(D and E) Aβ40 and Aβ42 levels in the GDN fraction of APP/PS1; Ctrl and APP/PS1; cKO mice (n=5 per group) at 9 months of age. Values are mean ± SEM. *p < 0.05; **p < 0.01.
(F) Cortical sections from APP/PS1; Ctrl and APP/PS1; cKO mice at 9 months of age immunostained with GFAP antibody. Scale bar, 50 μm. The percentage of area covered by GFAP staining was quantified (n=5 per group). Values are mean ±SEM. *p < 0.05.
(G) The cognitive function of APP/PS1; Ctrl and APP/PS1; cKO mice (n=14 per group) at 12 months of age examined by fear conditioning tests. The percentage of time spent showing freezing behavior in response to stimulus during contextual or cued memory tests is shown. Data represent mean ±SEM. **p < 0.01.
See also Figure S3.
To further examine whether LRP6 deficiency in neurons influences amyloid pathology, we bred Lrp6 cKO mice into the background of APPswe/PS1ΔE9 (APP/PS1) amyloid mouse model, which exhibit accelerated Aβ amyloidosis (Jankowsky et al., 2004). When the plaque burdens in APP/PS1; Lrp6 cKO (APP/PS1; cKO) mice and littermate control (APP/PS1; Ctrl) mice were compared by immunohistochemical staining, amyloid plaque loads in the cortex and hippocampus were markedly higher in APP/PS1; Lrp6 cKO mice compared with APP/PS1; Ctrl mice at 9 months of age (Figure 4C). We also found corresponding increases in the levels of insoluble Aβ40 and Aβ42 in APP/PS1; Lrp6 cKO mice (Figures 4D and 4E). When amyloid plaque loads were assessed in mice at 6 months of age, APP/PS1; Lrp6 cKO mice also had more Aβ deposition in the cortex than APP/PS1; Ctrl mice (Figures S3A and S3B). These results indicate that loss of neuronal LRP6 leads to increased amyloid pathology.
We next compared the extent of neuroinflammation in APP/PS1; Lrp6 cKO and APP/PS1; Ctrl mice by immunohistochemical staining using GFAP and Iba1 antibodies, and observed a profound increase in astrocyte and microglia activation in APP/PS1; Lrp6 cKO mice (Figures 4F and S3C). To examine whether these pathological events correlate with cognitive deficits, we next evaluated the memory performance of APP/PS1; Lrp6 cKO and their littermate APP/PS1; Ctrl mice at 12 months of age by fear conditioning tests. We found that APP/PS1; Lrp6 cKO mice exhibited significant deficits in both contextual and cued fear conditioning compared to APP/PS1; Ctrl mice (Figure 4G). To minimize potentially confounding factors, such as anxiety, we also examined general behaviors including locomotor activity and anxiety levels by open field and light/dark exploration paradigms. No significant deficits were observed in these non-memory-related tests in APP/PS1; Lrp6 cKO mice (Figures S3D and S3E). Together, these results indicate that impairment of LRP6-mediated Wnt signaling directly leads to increased amyloid pathology and cognitive deficits.
LRP6 Regulates APP Trafficking and Processing to Aβ
To investigate the potential mechanism by which LRP6 regulates Aβ levels, we knocked down LRP6 expression in human neuroblastoma SH-SY5Y cells overexpressing human APP (SH-SY5Y-APP) (Kounnas et al., 2010) with lentivirus carrying LRP6 shRNA (Figure 5A). Consistent with our in vivo findings, LRP6 knockdown significantly increased the levels of both Aβ40 and Aβ42 (Figure 5B). Similar effects were observed in mouse neuroblastoma N2a cells overexpressing human APP (N2a-APP) treated with LRP6 shRNA (Figures S4A–D). Although LRP5 and LRP6 are homologous, the expression of LRP5 in neurons and brain is very low compared to LRP6 (Figure S5A) (Dong et al., 1998). As an alternative control, we tested the effect of knocking down the expression of low density lipoprotein receptor (LDLR), another member of the LDLR family that is abundantly expressed in the brain but does not bind to APP, in N2a-APP cells on Aβ production. As expected, we did not detect any significant effects of knocking down LDLR on Aβ production (Figures S5B and S5C), suggesting that the observed effects of LRP6 knockdown on Aβ is specific. Conversely, overexpression of LRP6 together with Mesd, a specialized chaperone for the proper folding of LRP6, resulted in a significant decrease of both Aβ40 and Aβ42 levels (Figures 5C and 5D). While exogenously administration of Mesd is known to moderately suppress Wnt signaling (Liu et al., 2010a), Mesd co-transfection with LRP6 as an ER chaperone did not result in Mesd secretion to the medium (Figure S5D), thus it should not affect LRP6-mediated Wnt signaling. As a negative control for overexpression, co-transfection of LDLR and APP in HEK293 cells did not lead to changes in Aβ production (Figure S5E). Together, these results indicate that LRP6 suppresses Aβ production.
Figure 5. LRP6 Regulates APP Trafficking and Processing to Aβ.
(A) The levels of LRP6, APP and β-actin in SH-SY5Y-APP cells expressing control and LRP6 shRNA examined by Western blot analysis.
(B) The levels of Aβ40 or Aβ42 in the media of SH-SY5Y-APP cells expressing control and LRP6 shRNA evaluated by ELISA. Values are mean ± SD. *p < 0.05.
(C) The levels of LRP6, APP and β-actin in HEK293 cells overexpressing APP with either vector or LRP6/Mesd analyzed by Western blot.
(D) The levels of Aβ40 or Aβ42 in the media of HEK293 cells overexpressing APP with either vector or LRP6/Mesd analyzed by ELISA. Data represent mean ± SD; *p < 0.05; **p < 0.01.
(E) Co-immunoprecipitation of LRP6 and APP in HEK293 cells co-transfected with myc-APP (wild-type or Swedish mutant) and HA-LRP6/Mesd. Normal mouse IgG was used as a negative control. IP, immunoprecipitation.
(F and G) The levels of APP, APP-CTFs, soluble APP fragments and β-actin in N2a-APP cells expressing control and LRP6 shRNA examined by Western blot. Data represent mean ± SD; **p < 0.01; N.S., not significant.
(H) The levels of total and cell surface LRP6 in SH-SY5Y-APP or N2a-APP cells expressing control and LRP6 shRNA analyzed by FACS. Data represent mean ± SD; *p < 0.05.
(I) The levels of sAPPα and full-length APP in HEK293 cells overexpressing APP with either vector or LRP6/Mesd examined by Western blot. Data represent mean ± SD; **p < 0.01.
(J) Primary neurons transfected with GFP-APP together with vector or LRP6/Mesd for 24 h. Cell surface APP was examined by immunofluorescence microscopy and quantified (n = 8–10). Data represent mean ± SD; **p < 0.01.
(K) The levels of cell surface and total APP in COS7 cells overexpressing APP together with vector or LRP6/Mesd examined by cell surface biotinylation assay. Data represent mean ± SD; **p < 0.01.
See also Figures S4 and S5.
To examine whether there is specific interaction between LRP6 and APP, we performed co-immunoprecipitation assays in HEK293 cells co-transfected with APP and LRP6/Mesd. We found that APP was significantly co-immunoprecipitated with LRP6, correspondingly, immunoprecipitation of APP pulled down LRP6 (Figure 5E). To further identify the interacting domains in LRP6 and APP, HEK293 cells were transfected with HA-tagged LRP6 and myc-tagged APP constructs with various deletions (Figures S5F and S5G). All APP truncated proteins were expressed at the expected sizes as determined by Western blotting (Figure S5H). We then immunoprecipitated APP (anti-myc) and probed for LRP6 (anti-HA), and found that LRP6 was co-immunoprecipitated with full-length APP, APP-E2 and APP-ΔE1 (Figure S5I), but not with APP-E1, APP-ΔE1E2 and C99 (Figure S5J). These results indicate that APP-E2 domain is necessary and sufficient for APP-LRP6 interaction. To determine the LRP6 domain required for the APP-LRP6 interaction, we co-transfected cells with APP together with LRP6 constructs with either cytoplasmic domain deletion (LRP6ΔC) or extracellular deletion (LRP6ΔN) (Figure S5G). The LRP6 truncated plasmids were expressed at the expected sizes (Figure S5K) and we found that LRP6ΔC, but not LRP6ΔN, co-immunoprecipitates with APP (Figure S5L), suggesting that LRP6 extracellular region is required for this interaction.
To examine whether LRP6 regulates APP processing, we knocked down LRP6 expression in N2a-APP cells and analyzed potential effects on the levels of APP and its processing products. We found that LRP6 knockdown significantly increased the levels of sAPPβ and CTFβ without affecting full-length APP (Figures 5F and 5G). Current models of Aβ generation suggest that APP is cleaved by β-secretases in early endosomes (Vetrivel and Thinakaran, 2006), whereas retention of APP at the cell surface increases sAPPα levels and decreases Aβ production (Carey et al., 2005; Haass et al., 1993). Interestingly, we found that cell surface APP was significantly decreased in LRP6-KD cells (Figure 5H). In addition, overexpression of LRP6 with Mesd significantly increased Wnt signaling and the levels of sAPPα without affecting the level of full-length APP (Figures 5I and S4E–G). To examine whether increased sAPPα is due to increased cell surface APP, primary neurons were co-transfected with GFP-APP together with either control vector or LRP6/Mesd and the level of cell surface APP was quantified as previously described (Hoe et al., 2009; Megill et al., 2013). We found that overexpression of LRP6 significantly enhanced cell surface APP level (Figure 5J). The increased cell surface APP upon LRP6 overexpression was also confirmed by cell surface biotinylation assay (Figure 5K). These results suggest that LRP6 interaction with APP leads to increased cell surface APP, which favors non-amyloidogenic processing by α secretase and a concomitant reduction of Aβ production.
LRP6 Levels and Wnt Signaling are Down-regulated in AD Brains
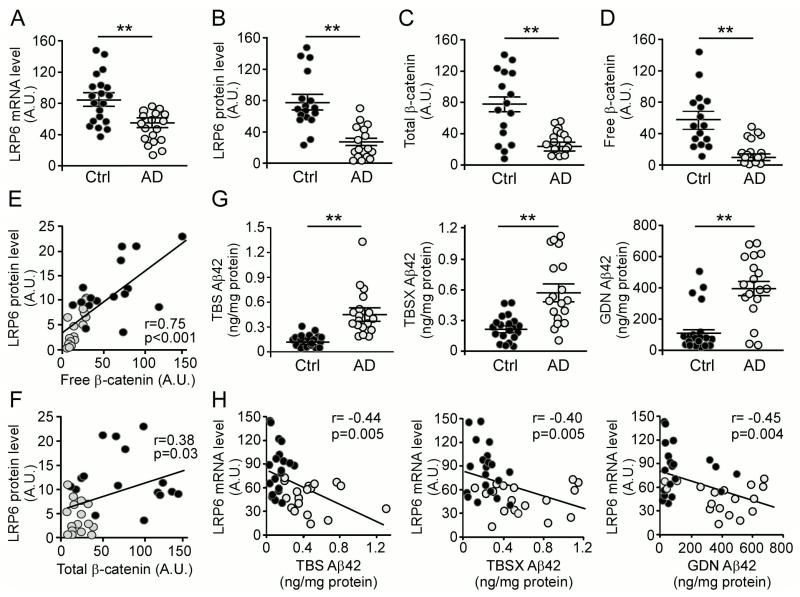
To address the relevance of LRP6-mediated Wnt signaling pathway in human AD, we analyzed LRP6 levels in the temporal cortex from postmortem brains of AD patients (n = 18, average age 84.2 ± 4.1 years old) and age-matched controls (n = 20, average age 85.1 ± 5.7 years old) (Table S1). LRP6 mRNA and protein levels were significantly lower in AD cases compared to age-matched controls, as assessed by quantitative RT-PCR and Western blotting (Figures 6A and 6B; Figure S6A). As comparison, the levels of LRP5 (Figure S6B) and a membrane protein Na+/K+ ATPase (Figures S6D and S6E) were not changed in AD brains compared to those of controls. In addition, there was no significant difference in the levels of neuronal β-tubulin and synaptophysin, although as expected PSD-95 level was decreased in AD patients compared with control individuals (Figures S6D and S6E). As LRP6 is an essential Wnt signaling receptor, we found that the levels of both total β-catenin and free β-catenin, which translocates to the nucleus to activate Wnt signaling (Nelson and Nusse, 2004), were lower among AD cases than controls (Figures 6C and 6D; Figure S6F). Interestingly, the levels of LRP6 positively correlated with those of free β-catenin (r=0.75, p<0.001), and to a lesser extent with that of total β-catenin (r=0.38, p=0.03) (Figures 6E and 6F). These results indicate that the decreased levels of LRP6 likely contribute to compromised Wnt signaling in AD brains.
Figure 6. Decreased LRP6 and Wnt Signaling in Human AD Brains.
(A) LRP6 mRNA levels in human control (n=20) and AD (n=18) brain tissues quantified by real-time PCR. Results are normalized to β-actin levels and presented in relative units. In the following figures, data are shown as scatterplots in which each symbol represents an individual case. A.U., arbitrary unit. Data represent mean ± SEM. **p < 0.01.
(B) LRP6 protein levels in human control (n=17) and AD (n=17) brain tissues quantified by Western blot analysis. Data represent mean ± SEM. **p < 0.01.
(C and D) The total and free β-catenin levels in human control (n=16) and AD (n=16) brain tissues examined by Western blot analysis. Free β-catenin levels were analyzed by GST-E-Cadherin pull-down assay. Data represent mean ± SEM. **p < 0.01.
(E) Correlation between LRP6 protein levels and free β-catenin levels in human control (dark circles) and AD (light circles) brain tissues (r represents the correlation coefficient; p is significance).
(F) Correlation between LRP6 protein levels and total β-catenin levels in human control (dark circles) and AD (light circles) brain tissues.
(G) Aβ42 levels in TBS, TBSX, and GDN fractions of human control (n=20) and AD (n=18) brain tissues. Data represent mean ± SEM. **p < 0.01.
(H) Correlation between LRP6 mRNA levels in (A) and those of Aβ42 in various extraction fractions (TBS, TBSX or GDN) in (G) in human control (dark circles) and AD (light circles) brain tissues.
To analyze the relationship between LRP6 level and the dynamic pools of Aβ, we fractionated human brain samples into TBS-soluble, detergent-soluble (TBSX), and insoluble (guanidine-HCl, GDN) fractions (Youmans et al., 2011), and quantified Aβ levels by ELISA. As expected, Aβ42 (Figure 6G) and Aβ40 (Figure S6G) levels were significantly higher in the brains of AD cases compared with controls in all fractions. We also found that LRP6 at both mRNA level (Figure 6H) and protein level (Figure S6H) inversely correlate with those of Aβ42 in the TBS, TBSX and GDN fractions, suggesting that accumulation of Aβ might be associated with down-regulation of LRP6 expression. Similar negative correlations were observed between LRP6 and Aβ40 in all fractions (Figures S6I and S6J). Furthermore, LRP6 at the protein level was positively correlated with mini-mental state examination (MMSE) score (r=−0.57, p=0.001) (Figure S6C), a widely used tool for assessing cognitive function (Folstein et al., 1975). We also observed slight decrease in free β-catenin and LRP6 levels in aged APP/PS1 mice (Figures S7A and S7B), consistent with a previous finding (Toledo and Inestrosa, 2010). Together, these results imply that suppression of LRP6-mediated Wnt signaling likely contribute to AD pathogenesis.
Aβ Down-regulates LRP6-mediated Wnt Signaling
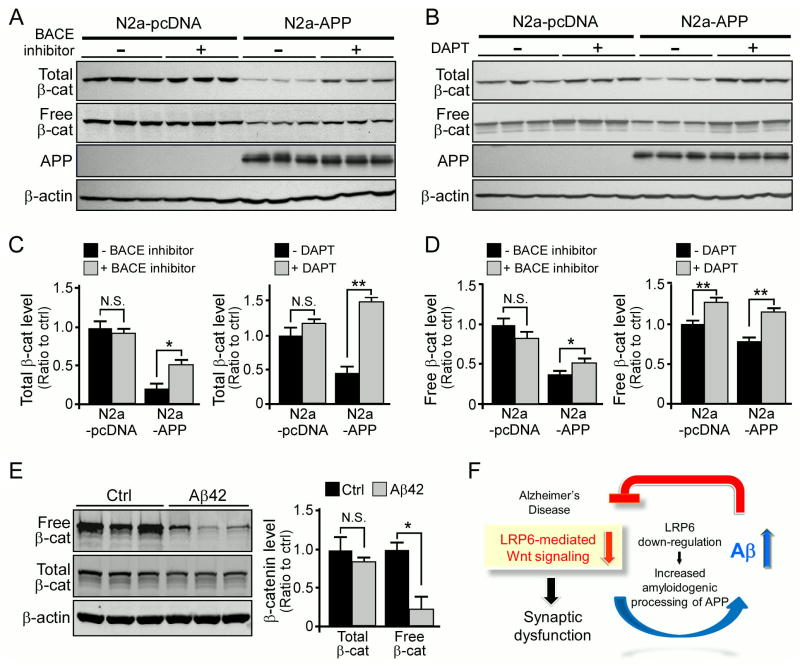
Our data indicated that LRP6-mediated Wnt signaling is compromised in AD brain tissues compared to controls (Figures 6C and 6D). To investigate the potential mechanism underlying this observation, we examined the levels of Wnt signaling in N2a-pcDNA control and N2a-APP cells. While total β-catenin and free β-catenin levels were significantly down-regulated in N2a-APP cells compared to control cells, this suppression was partially restored upon administration of a β-secretase or γ-secretase inhibitor (Figures 7A–D). Since treatments of these inhibitors significantly suppressed Aβ production and altered the levels of APP fragments (Figures S7C–F), these results suggest that APP processing mediated by these secretases is required for this event. To examine if the observed effect is mediated by Aβ, we treated primary neurons with Aβ42 oligomers and found that the levels of free β-catenin were down-regulated (Figure 7E). We also found that Aβ42 oligomers down-regulated LRP6 levels and suppressed Wnt signaling in SH-SY5Y cells (Figure S7G). Interestingly, treatment with exogenous Wnt3a ligands mitigated the Aβ-induced suppression of Wnt signaling (Figure S7G), suggesting that up-regulation of Wnt signaling might ameliorate Aβ toxicity. Together, our results suggest that Aβ likely down-regulates LRP6 expression and its associated Wnt signaling, thus initiating a vicious cycle in which decreased LRP6 and increased Aβ levels in AD brains synergistically promote AD pathogenesis.
Figure 7. Aβ Suppresses LRP6 Expression and Wnt Signaling Activation.
(A–D) The levels of total β-catenin, free β-catenin, APP and β-actin in N2a-pcDNA and N2a-APP cells in the presence or absence of a BACE inhibitor (β-secretase inhibitor IV, 2μM) or a γ-secretase inhibitor (DAPT, 10 μM) examined by Western blot. Free β-catenin levels were analyzed by GST-E-Cadherin pull-down assay. Data represent mean ± SEM. *p < 0.05; N.S., not significant.
(E) The levels of total and free β-catenin in primary neurons treated with 5 μM Aβ42 oligomers examined by Western blot. Data represent mean ± SEM. *p < 0.05; N.S., not significant.
(F) Illustrative diagram depicting a potential vicious cycle between increased Aβ and decreased LRP6-mediated Wnt signaling in AD pathogenesis. See text for details.
See also Figure S7.
DISCUSSION
Mounting evidence demonstrates that synaptic dysfunction and substantial amyloid buildup are present in AD brains long before the clinical onset of the disease (Huang and Mucke, 2012; Tarawneh and Holtzman, 2010). Identifying the molecular mechanisms that contribute to these pathogenic processes may help to develop new therapeutic approaches for the prevention and treatment of AD. Towards this effort, we sought to define critical pathways regulating AD pathogenesis. Genetic evidence has suggested a role of LRP6-mediated Wnt signaling in AD pathogenesis; however, biological and pathological evidences are lacking. In this report, using in vivo animal and in vitro cellular models, we demonstrated a critical role for LRP6 and its associated Wnt signaling in maintaining synaptic integrity and functions. We also found that LRP6 deficiency in neurons leads to increased Aβ accumulation and amyloid pathology by accelerating APP processing to Aβ, which in turn down-regulates LRP6-mediated Wnt signaling. Although we cannot rule out the possibility that APP intracellular domain (AICD) or soluble APP fragments also have inhibitory effects on Wnt signaling (Zhou et al., 2012), our evidence points to a direct effect of oligomer Aβ on down-regulating LRP6-mediated Wnt signaling. We further showed that LRP6-mediated Wnt signaling is compromised in AD brains. Thus, down-regulated LRP6/Wnt signaling and increased Aβ pathology in AD brains constitute a vicious cycle in which the two events synergistically promote synaptic dysfunction, leading to eventual neurodegeneration in AD (Figure 7F). Although LRP5 is a homologous receptor of LRP6, these two receptors likely mediate distinct actions through the differences in tissue distribution and affinity for individual Wnt ligands (Mi and Johnson, 2005). Global deletion of the Lrp6 gene in mice results in significant brain abnormalities, while deletion of the Lrp5 gene leads to abnormality primarily in bone density (Castelo-Branco et al., 2010; Gong et al., 2001). A previous study has also shown that LRP5 is expressed at a very low level in the nervous system (Dong et al., 1998) consistent with our current findings. Thus, LRP6, rather than LRP5, is likely the primary canonical Wnt signaling receptor in the brain under both physiological and pathological conditions.
Wnt signaling plays a critical role in spine morphogenesis and neurotransmission during development and in mature neurons (Inestrosa and Arenas, 2010). During development, several Frizzled Wnt receptors are known to be involved in synaptogenesis and synaptic connectivity (Sahores et al., 2010). Moreover, different ligands have been found to regulate synaptic structure and function in both pre- and postsynaptic regions (Inestrosa and Varela-Nallar, 2013). In addition, the release of Wnt ligands and the activated signaling cascade are modulated by neuronal activity, suggesting the importance of this pathway in synaptic plasticity (Ataman et al., 2008). In the adult brain, our current results show that neuronal LRP6 regulates synaptic plasticity likely through the modulation of components at postsynaptic sites. However, we cannot exclude the possibility that Wnt-independent functions might also play a role in LRP6-regulated pathways in synapses. LRP6 is required for efficient activation of Gαs-mediated cyclic adenosine monophosphate (cAMP) signaling by various guanine nucleotide-binding protein (G protein)-coupled receptors (Wan et al., 2011). It is possible that loss of LRP6 in neurons attenuates cAMP-response element-binding protein (CREB) signaling essential for synaptic plasticity, resulting in synaptic abnormalities and cognitive decline (Bourtchuladze et al., 1994). In our studies, Lrp6 cKO mice display marked hippocampal LTP impairment in the Schaffer collateral CA1 synapses. These results suggest that LRP6 might mediate the proper maintenance of glutamate receptors in dendritic spines where CA1 LTP is an NMDA-dependent post-synaptic phenomenon controlling AMPA receptor function (Deák and Sonntag, 2012). Thus, determining whether LRP6 alters the trafficking and stability of glutamate receptors and whether such events are dependent upon Wnt signaling warrants further investigation.
A common variant of LRP6 (Ile-1062→Val), which suppresses Wnt signaling, has been shown to be a genetic risk factor for late-onset AD (De Ferrari et al., 2007). LRP6 genetic variants have also been linked to other disorders frequently seen in the elderly (Ding et al., 2011); single-nucleotide polymorphisms of the LRP6 gene significantly increases the risk of osteoporosis (van Meurs et al., 2006) and macular degeneration (Haines et al., 2006). In addition, LRP6 regulates glucose and lipid metabolism; a rare loss-of-function mutation in LRP6 (Arg-611→Cys) is linked to early-onset coronary artery disease, metabolic syndrome and insulin resistance (Mani et al., 2007; Singh et al., 2013), conditions that are known to increase the risk for AD (Razay et al., 2007; Sims-Robinson et al., 2010). Therefore, it is possible that suppression of LRP6-mediated Wnt signaling increases the risks of dyslipidemia and insulin resistance, which might further exacerbate AD pathogenesis (Sims-Robinson et al., 2010). Importantly, we demonstrated that LRP6 levels and Wnt signaling are significantly reduced in postmortem brains from late-onset AD patients. Consistent with our findings, altered expression of several Wnt signaling components has been implicated in the pathogenesis of AD (Caricasole et al., 2004; He and Shen, 2009; Wan et al., 2014). In addition, the levels of β-catenin are significantly reduced in AD individuals bearing presenilin-1 mutations (Nishimura et al., 1999). Conversely, Dickkopf-1 (Dkk1), a well-known antagonist of the Wnt pathway, is increased in AD brains, up-regulated by Aβ treatment, and is associated with Aβ-mediated synaptic loss (Caricasole et al., 2004; Purro et al., 2012). Glycogen synthase kinase 3 (GSK-3), which is negatively regulated by LRP6-mediated Wnt signaling, plays a central role in AD and its misregulation accounts for several pathological hallmarks of the disease, including hyperphosphorylation of tau and amyloid plaque formation (Hooper et al., 2008). Such evidence in combination with our findings indicate that LRP6 perturbation is critically involved in AD pathogenesis and demonstrate the need for future studies aimed at uncovering the mechanisms through which LRP6 is down-regulated in AD.
Our results also suggest that deficiency in LRP6-mediated Wnt signaling might exacerbate neuronal vulnerability to Aβ toxicity in AD brains, in addition to their direct effects on synapses. Emerging evidence supports a neuroprotective role for Wnt signaling in neurodegenerative disorders, including AD (Inestrosa and Toledo, 2008). Activation of Wnt signaling protects hippocampal neurons from Aβ toxicity (Alvarez et al., 2004). Treatment with lithium, which activates Wnt signaling by inhibition of GSK3β, was shown to reduce amyloid burden and ameliorate neurodegeneration in AD mouse models (Noble et al., 2005; Rockenstein et al., 2007) though its use is limited by several adverse effects (Pachet and Wisniewski, 2003). Our results further indicate that LRP6-mediated Wnt signaling plays critical roles in regulating both synaptic functions and amyloid pathology and suggest that deficiency of this pathway contributes to AD pathogenesis.
In summary, we have demonstrated that neuronal LRP6-mediated Wnt signaling is critical for synaptic maintenance, cognitive function and amyloidogenesis. Our work identifies a novel pathway that contributes to AD pathogenesis and suggests that restoring LRP6-mediated Wnt signaling in AD may be a promising therapeutic strategy.
EXPERIMENTAL PROCEDURES
Human Postmortem Brain Tissues
Temporal lobe cortex samples from neurologically unimpaired subjects (n = 20) and from subjects with AD (n = 18) were obtained from the University of Kentucky Alzheimer’s Disease Center (Lexington, Kentucky, USA). Diagnosis of AD was confirmed by pathological and clinical criteria. The average age of subjects was 84.2 ± 4.1 years in the AD group and 85.1 ± 5.7 years in the control group (p = 0.61). Average postmortem interval was 3.2 h and was not significantly different between the two groups (p = 0.28).
Animals
All animal procedures were approved by the Mayo Clinic Institutional Animal Care and Use Committee (IACUC) and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Lrp6flox/flox mice were a generous gift from Dr. Bart Williams (Van Andel Research Institute, Grand Rapids, MI). Double transgenic APPswe/PS1ΔE9 (APP/PS1) mice were purchased from Jackson Laboratory. See Supplemental Experimental Procedures for details.
Golgi Staining and Dendritic Spine Analysis
Golgi staining was performed using the FD Rapid Golgi Stain kit (FD Neuro Technologies) as described (Liu et al., 2010b). See Supplemental Experimental Procedures for details.
Extracellular Field Recording from Acute Hippocampal Slices
Extracellular recordings were performed from acute hippocampal slices with an adopted protocol as originally described (Oka et al., 1999). See Supplemental Experimental Procedures for details.
Behavioral Analysis
Learning and memory performance was assessed using a fear conditioning paradigm. Freezing behavior was measured with an overhead camera and FreezeFrame software (Actimetrics). Open field, elevated plus maze, and light/dark exploration tests were used for the assessment of anxiety behaviors which were tracked with an overhead camera and AnyMaze software. The rotarod test was used to examine the motor coordination and motor learning in mice. See Supplemental Experimental Procedures for details.
Statistical Analysis
Comparisons between two groups were performed with Student’s t tests, and those among more than two groups were performed with ANOVA. A p value of < 0.05 was considered statistically significant. Correlations were analyzed by Pearson correlation and regression tests.
Supplementary Material
Figure S1. Analyses of Neurobehavior in Control and LRP6 Neuronal Knockout Mice at 6 and 22 Months of Age, Related to Figure 1
(A) Analyses of anxiety-related behaviors in control (Ctrl) and Lrp6 cKO (cKO) mice at 6 months of age (n=13–15 mice per genotype) assessed by open field tests. The total distance traveled, average speed, time spent mobile, and ratio of time spent in the center quadrants to total distance traveled in the open field apparatus are shown. In this figure, data are presented as mean ±SEM. N.S., not significant.
(B) Analyses of anxiety-related behaviors in Ctrl and Lrp6 cKO mice at 6 months of age (n=13–15 mice per genotype) assessed by light/dark exploration tests. The time spent in the light chamber, time spent in the dark chamber, and ratio of time spent in light to dark chambers are shown. N.S., not significant.
(C) The cognitive function of Ctrl and Lrp6 cKO mice (n=13–15 mice per genotype) at 6 months of age examined by fear conditioning tests. The percentage of the time with freezing behavior in response to stimulus during contextual or cued memory tests is shown. N.S., not significant.
(D) Analyses of anxiety-related behaviors in Ctrl and Lrp6 cKO mice at 22 months of age (n=10–11 mice per group) assessed by open field tests. The average speed, time spent mobile, total distance traveled, and ratio of time spent in the center quadrants to total distance traveled in the open field apparatus are shown.. N.S., not significant.
(E) Analyses of anxiety-related behaviors in Ctrl and Lrp6 cKO mice at 22 months of age (n=10–11 mice per group) assessed by light/dark exploration tests. The total distance traveled, time spent in the light chamber, time spent in the dark chamber, and ratios of time spent in light to dark chambers are shown. N.S., not significant.
(F) Analyses of anxiety-related behaviors in Ctrl and Lrp6 cKO mice at 22 months of age (n=10–11 mice per group) assessed by elevated plus maze test. Total time in the open arms and ratio of time spent in open to closed arms are shown. N.S., not significant.
(G) The locomotor activity and motor learning in Ctrl and Lrp6 cKO mice at 22 months of age (n=10–11 mice per group) examined by rotarod test. N.S., not significant.
Figure S2. Analyses of Long-term Plasticity, Dendritic Spine Density, and Synaptic Integrity in Control and LRP6 Neuronal Knockout Mice, Related to Figures 1 and 2
(A) Normal hippocampal LTP in the CA1 region of Lrp6 cKO (cKO) (n=12) mice at 6 months of age compared to control (Ctrl) (n=16) mice. Changes in fEPSP slope were expressed as a percentage of baseline. Data represent mean ± SEM.
(B) Representative traces before (black) and after (red) high frequency stimulation from the recordings of Ctrl and Lrp6 cKO mice. Averages of the last 5 min of recording.
(C and D) Representative Golgi-impregnated neurons in hippocampal CA1 region (C) and cortical layer II/III (D) from Ctrl and Lrp6 cKO mice at 6 months of age. Scale bars, 10 μm.
(E and F) Representative apical oblique and basal shaft dendrites from hippocampal CA1 region (E) and cortical layers II/III (F) of Ctrl and Lrp6 cKO mice at 6 months of age. Scale bars, 10 μm.
(G) NMDAR1 and GluR1 levels in the cortex of Ctrl and Lrp6 cKO mice at 6 months of age (n=4–5 per genotype) examined by Western blot analysis. The densitometric quantification of Western blot is shown as mean ± SEM.
(H) NMDAR1 and GluR1 levels in the cortex of Ctrl and Lrp6 cKO mice at 18 months of age (n=5–7 per genotype) examined by Western blot analysis. The densitometric quantification of Western blot is shown as mean ± SEM. *p < 0.05.
(I) The levels of synaptic markers in the cerebellum of Ctrl and Lrp6 cKO mice at 18 months of age (n=6 per genotype) examined by Western blot analysis. Syp, Synaptophysin. The densitometric quantification of Western blot is shown as mean ± SEM. N.S., not significant.
Figure S3. Amyloid Deposition, Neuroinflammatory Response and Anxiety Behaviors in APP/PS1 Mice with or without Neuronal LRP6 Deletion, Related to Figure 4
(A and B) Brain sections from APP/PS1; Ctrl and APP/PS1; cKO mice (n=5 per group) at 6 months of age immunostained for Aβ. Scale bar, 100 μm. (B) The percentage of area covered by plaques in cortex was quantified, and the plaque load was normalized to that of APP/PS1; Ctrl mice. Values are mean ± SEM. *p < 0.05.
(C) Hemi-brain sections from APP/PS1; Ctrl and APP/PS1; cKO mice at 9 months of age immunostained with Iba1 antibody, a marker of microgliosis. Representative pictures of hippocampus are shown. Scale bar, 100 μm.
(D) The analyses of anxiety-related behaviors in APP/PS1; Ctrl and APP/PS1; cKO mice (n=14 mice per group) at 12 months of age assessed by open field tests. Total distance traveled, average speed, time spent mobile, and ratio of time spent in the center quadrants to total distance traveled in the open field are shown. N.S., not significant.
(E) Anxiety-related behaviors in APP/PS1; Ctrl and APP/PS1; cKO mice at 12 months of age assessed by light/dark exploration tests. The time spent in the light chamber and ratio of time spent in light to dark chambers (n=14 mice per group) are shown. N.S., not significant.
Figure S4. Modulation of LRP6 Affects Wnt Signaling, Related to Figure 5
(A) The mRNA levels of LRP6 in N2a-APP cells expressing control shRNA (Ctrl) or LRP6 shRNA (KD) examined by real-time PCR analysis. Results were normalized to β-actin levels. Values are mean ± SD. *p < 0.05.
(B and C) The APP levels in N2a-APP cells expressing control shRNA (Ctrl) or LRP6 shRNA (KD) examined by Western blot analysis. Densitometric analyses of Western blots are shown in (C). N.S., not significant.
(D) The Aβ40 or Aβ42 levels in the medium of N2a-APP cells expressing control shRNA (Ctrl) or LRP6 shRNA (KD) examined by ELISA. All measurements were normalized to protein concentrations in the cell lysates. Values are mean ± SD. *p < 0.05.
(E and F) The levels of APP, LRP6 and free β-catenin in HEK293 cells co-transfected with APP together with pcDNA or LRP6/Mesd examined by Western blot analysis. Free β-catenin levels were evaluated by GST-E-Cadherin pull-down, followed by Western blot analysis. Densitometric analyses of Western blots are shown in (F). Values are mean ± SD. **p < 0.01; N.S., not significant.
(G) The levels of Wnt signaling in HEK293 cells co-expressing TOPFlash reporter and APP together with pcDNA or LRP6/Mesd examined by TOPFlash luciferase assay. Values are mean ± SD. **p < 0.01.
Figure S5. The APP E2 Domain Interacts with LRP6 Extracellular Domain, Related to Figure 5
(A) LRP5 and LRP6 mRNA levels in human brain tissue and SH-SY5Y-APP cells quantified by real-time PCR. The standard curves for human LRP5 and LRP6 was derived from the serial dilutions of plasmids with known concentration. The logarithms (base 10) of concentrations were plotted against threshold cycle (Ct), and the levels of LRP5 and LRP6 were calculated using its corresponding standard curves. Values are mean ± SD. **p < 0.01.
(B and C) The levels of LDLR and β-actin in N2a-APP cells expressing control and LDLR siRNA examined by Western blot analysis. (C) The level of Aβ40 in the medium was evaluated by ELISA. N2a-APP cells were transfected with control or LDLR siRNA. 48 h post co-transfection, cell medium were replaced with Opti-MEN medium and incubated for 12 h. The Aβ40 level in the medium was normalized to total protein levels in cell lysates. N.S., not significant.
(D) The levels of Mesd in HEK293 cells with or without overexpressing flag-tagged Mesd and in the concentrated medium analyzed by Western blot.
(E) The level of Aβ40 in the medium of HEK293 cells overexpressing APP with either vector or HA-tagged LDLR was evaluated by ELISA. The levels of LDLR, APP and β-actin in the cell lysates were analyzed by Western blot. HEK293 cells were co-transfected with APP together with pcDNA or LDLR. 24 h post co-transfection, cell medium were replaced with DMEM medium containing 1% FBS and incubated for 12 h. The Aβ40 level in the concentrated medium was quantified after normalization with total protein levels in the cell lysates. Values are mean ± SD. *p < 0.05.
(F and G) Schematic diagrams of APP and LRP6 constructs used in this study.
(H) Western blot of LRP6 and different APP fragments used as inputs of immunoprecipitation.
(I and J) LRP6 co-immunoprecipitated with full-length APP, APP-E2 and APP-ΔE1, but not C99, APP-E1 and APP-ΔE1E2. HEK293 cells were co-transfected with plasmids expressing various myc-APP and HA-LRP6 together with Mesd. Cell lysates were immunoprecipitated with an anti-myc antibody for APP, and probed with an anti-HA antibody for LRP6.
(K and L) LRP6 extracellular domain is required for the APP-LRP6 interaction. HEK293 cells were co-transfected with plasmids expressing HA-APP, LRP6ΔC-VSVG or myc-LRP6ΔN together with Mesd. Cell lysates were subjected to immunoprecipitation with anti-HA antibody against APP, and probed with anti-LRP6 or anti-myc antibodies.
Figure S6. The Levels of LRP6-mediated Wnt Signaling and its Correlation with Aβ Levels in Human Control and AD Brain Tissues, Related to Figure 6
(A) LRP6 protein levels in human control (n=17) and AD (n=17) brain tissues examined by Western blot analysis.
(B) LRP5 mRNA levels in human control (n=20) and AD (n=18) brain tissues quantified by real-time PCR. Results were normalized to β-actin levels and presented in relative units. In the following figures, data are shown as scatterplots in which each symbol represents an individual case. A.U., arbitrary unit. Data represent mean ± SEM. **p < 0.01.
(C) Correlation between LRP6 protein levels (Figures 6B and S6A) and mini-mental state examination (MMSE) score of human control (dark circles) and AD (light circles) brain tissues (r represents the correlation coefficient; p is significance).
(D and E) Synaptic markers (PSD-95 and Synaptophysin), sodium/potassium ATPase, a critical membrane marker and neuron-specific β-tubulin in control (n=17) and AD (n=17) brain tissues examined by Western blot analysis and quantified.
(F) The levels of total and free β-catenin in human control (n=16) and AD (n=16) brain tissues examined by Western blot analysis. Free β-catenin levels were evaluated by GST-E-Cadherin pull-down, followed by Western blot analysis.
(G) Aβ40 levels in TBS, TBSX, and GDN fractions of human control (n=20) and AD (n=18) brain tissues. Data are shown as scatterplots in which each symbol represents an individual case. Data represent mean ± SEM. **p < 0.01.
(H) Correlation between LRP6 protein levels (Figures 6B and S6A) and Aβ42 levels (Figure 6G) in various extraction fractions (TBS, TBSX or GDN) in human control (dark circles) and AD (light circles) brain tissues (r represents the correlation coefficient; p is significance). In this and following figure, LRP6 protein levels are presented in relative units. A.U., arbitrary unit.
(I) Correlation between LRP6 mRNA levels (Figure 6A) and Aβ40 levels (Figure S6G) in various extraction fractions (TBS, TBSX or GDN) in human control (dark circles) and AD (light circles) brain tissues(r represents the correlation coefficient; p is significance).
(J) Correlation between LRP6 protein levels (Figures 6B and S6A) and Aβ40 levels (Figure S6G) in various extraction fractions (TBS, TBSX or GDN) in human control (dark circles) and AD (light circles) brain tissues(r represents the correlation coefficient; p is significance).
Figure S7. The Levels of LRP6-mediated Wnt Signaling is Down-regulated in APP/PS1 Mouse Brains and in Neuronal Cells upon Aβ Treatment, Related to Figure 7
(A and B) The levels of total and free β-catenin (β-cat) and LRP6 in the hippocampus of wild-type (WT) mice and APP/PS1 mice at 12 months of age (n=4–5/per genotype) examined by Western blot analysis. Free β-catenin levels were analyzed by GST-E-Cadherin pull-down assay. Data represent mean ± SEM. N.S., not significant; *p < 0.05.
(C–F) The levels of soluble APP fragments (sAPPα and sAPPβ) or APP-CTFs (CTFα and CTFβ) in N2a-APP cells treated with or without BACE inhibitor (β-secretase inhibitor IV, 2 μM) or γ-secretase inhibitor (DAPT, 10 μM) examined by Western blot analysis. The levels of Aβ40 in the media were evaluated by ELISA (C and E). Data represent mean ± SD. **p < 0.01.
(G) The levels of LRP6, total and free β-catenin in SH-SY5Y cells treated with 5 μM Aβ42 oligomers in the presence or absence of Wnt3a ligands examined by Western blot analysis. L CM, L cell conditioned medium; Wnt3a, Wnt3a conditioned medium.
Table S1: Characteristics of Examined Individuals, Related to Figure 6
Highlights.
LRP6-mediated Wnt signaling is critical for synaptic integrity and cognition.
Neuronal LRP6 deficiency in an AD mouse model exacerbates amyloid pathology.
LRP6-mediated Wnt signaling is compromised in AD brains.
LRP6 deficits, synapse loss and Aβ toxicity synergistically accelerate AD progression.
Acknowledgments
This work was supported by NIH grants R01AG027924, R01AG035355, R01AG046205 P01AG030128, P01NS074969, and grants from the Alzheimer’s Association (to G.B.), from Mayo Clinic CRM Career Development Award and NIRG from the Alzheimer’s Association (to T.K.) and from the GHR Foundation (to F.D). We thank Dr. Bart Williams (Van Andel Research Institute) for providing Lrp6flox/flox mice; Dr. Mingjie Li and Nada Husic from the Viral Vectors Core (Washington University) for producing the lentiviruses, Lester Manly for assistance of dendritic spine counting, Dr. Jaekwang Kim for providing LDLR siRNA, and Dr. Bob Weinberg (Whitehead Institute, Cambridge) for providing β-catenin deltaN90 plasmid. We are grateful to Dr. Dennis Dickson, Monica Castanedes Casey, Linda Rousseau, and Virginia Phillips for histology and immunohistochemical analyses, Dr. Terrone Rosenberry and Dr. William Tay for advice and assistance with Aβ oligomer preparation, and Dr. Melissa Murray for developing algorithms in ImageScope software to quantify immunohistochemical staining. We also thank Caroline Stetler, Melissa Wren and Caroline Casey for careful reading of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alarcon MA, Medina MA, Hu Q, Avila ME, Bustos BI, Perez-Palma E, Peralta A, Salazar P, Ugarte GD, Reyes AE, et al. A novel functional low-density lipoprotein receptor-related protein 6 gene alternative splice variant is associated with Alzheimer’s disease. Neurobiol Aging. 2013;34:1709, e1709–1718. doi: 10.1016/j.neurobiolaging.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Alvarez AR, Godoy JA, Mullendorff K, Olivares GH, Bronfman M, Inestrosa NC. Wnt-3a overcomes beta-amyloid toxicity in rat hippocampal neurons. Exp Cell Res. 2004;297:186–196. doi: 10.1016/j.yexcr.2004.02.028. [DOI] [PubMed] [Google Scholar]
- Arendt T. Synaptic degeneration in Alzheimer’s disease. Acta Neuropathol. 2009;118:167–179. doi: 10.1007/s00401-009-0536-x. [DOI] [PubMed] [Google Scholar]
- Ataman B, Ashley J, Gorczyca M, Ramachandran P, Fouquet W, Sigrist SJ, Budnik V. Rapid activity-dependent modifications in synaptic structure and function require bidirectional Wnt signaling. Neuron. 2008;57:705–718. doi: 10.1016/j.neuron.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Ann Rev Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Budnik V, Salinas PC. Wnt signaling during synaptic development and plasticity. Curr Opin Neurobiol. 2011;21:151–159. doi: 10.1016/j.conb.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey RM, Balcz BA, Lopez-Coviella I, Slack BE. Inhibition of dynamin-dependent endocytosis increases shedding of the amyloid precursor protein ectodomain and reduces generation of amyloid beta protein. BMC Cell Biol. 2005;6:30. doi: 10.1186/1471-2121-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caricasole A, Copani A, Caraci F, Aronica E, Rozemuller AJ, Caruso A, Storto M, Gaviraghi G, Terstappen GC, Nicoletti F. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is associated with neuronal degeneration in Alzheimer’s brain. J Neurosci. 2004;24:6021–6027. doi: 10.1523/JNEUROSCI.1381-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelo-Branco G, Andersson ER, Minina E, Sousa KM, Ribeiro D, Kokubu C, Imai K, Prakash N, Wurst W, Arenas E. Delayed dopaminergic neuron differentiation in Lrp6 mutant mice. Dev Dyn. 2010;239:211–221. doi: 10.1002/dvdy.22094. [DOI] [PubMed] [Google Scholar]
- Cerpa W, Gambrill A, Inestrosa NC, Barria A. Regulation of NMDA-receptor synaptic transmission by Wnt signaling. J Neurosci. 2011;31:9466–9471. doi: 10.1523/JNEUROSCI.6311-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerpa W, Godoy JA, Alfaro I, Farias GG, Metcalfe MJ, Fuentealba R, Bonansco C, Inestrosa NC. Wnt-7a modulates the synaptic vesicle cycle and synaptic transmission in hippocampal neurons. J Biol Chem. 2008;283:5918–5927. doi: 10.1074/jbc.M705943200. [DOI] [PubMed] [Google Scholar]
- Chami L, Checler F. BACE1 is at the crossroad of a toxic vicious cycle involving cellular stress and beta-amyloid production in Alzheimer’s disease. Mol Neurodegener. 2012;7:52. doi: 10.1186/1750-1326-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SL, Vassar R. The Alzheimer’s disease beta-secretase enzyme, BACE1. Mol Neurodegener. 2007;2:22. doi: 10.1186/1750-1326-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ferrari GV, Papassotiropoulos A, Biechele T, Wavrant De-Vrieze F, Avila ME, Major MB, Myers A, Saez K, Henriquez JP, Zhao A, et al. Common genetic variation within the low-density lipoprotein receptor-related protein 6 and late-onset Alzheimer’s disease. Proc Natl Acad Sci USA. 2007;104:9434–9439. doi: 10.1073/pnas.0603523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deák F, Sonntag WE. Aging, synaptic dysfunction, and insulin-like growth factor (IGF)-1. The journals of gerontology. J Gerontol A Biol Sci Med Sci. 2012;67:611–625. doi: 10.1093/gerona/gls118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding JD, Johnson LV, Herrmann R, Farsiu S, Smith SG, Groelle M, Mace BE, Sullivan P, Jamison JA, Kelly U, et al. Anti-amyloid therapy protects against retinal pigmented epithelium damage and vision loss in a model of age-related macular degeneration. Proc Natl Acad Sci USA. 2011;108:E279–287. doi: 10.1073/pnas.1100901108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Lathrop W, Weaver D, Qiu Q, Cini J, Bertolini D, Chen D. Molecular cloning and characterization of LR3, a novel LDL receptor family protein with mitogenic activity. Biochem Biophys Res Commun. 1998;251:784–790. doi: 10.1006/bbrc.1998.9545. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- Guo W, Keckesova Z, Donaher JL, Shibue T, Tischler V, Reinhardt F, Itzkovitz S, Noske A, Zurrer-Hardi U, Bell G, et al. Slug and Sox9 cooperatively determine the mammary stem cell state. Cell. 2012;148:1015–1028. doi: 10.1016/j.cell.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Hung AY, Schlossmacher MG, Teplow DB, Selkoe DJ. beta-Amyloid peptide and a 3-kDa fragment are derived by distinct cellular mechanisms. J Biol Chem. 1993;268:3021–3024. [PubMed] [Google Scholar]
- Haines JL, Schnetz-Boutaud N, Schmidt S, Scott WK, Agarwal A, Postel EA, Olson L, Kenealy SJ, Hauser M, Gilbert JR, Pericak-Vance MA. Functional candidate genes in age-related macular degeneration: significant association with VEGF, VLDLR, and LRP6. Invest Ophthalmol Vis Sci. 2006;47:329–335. doi: 10.1167/iovs.05-0116. [DOI] [PubMed] [Google Scholar]
- He P, Shen Y. Interruption of beta-catenin signaling reduces neurogenesis in Alzheimer’s disease. J Neurosci. 2009;29:6545–6557. doi: 10.1523/JNEUROSCI.0421-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoe HS, Lee KJ, Carney RS, Lee J, Markova A, Lee JY, Howell BW, Hyman BT, Pak DT, Bu G, Rebeck GW. Interaction of reelin with amyloid precursor protein promotes neurite outgrowth. J Neurosci. 2009;29:7459–7473. doi: 10.1523/JNEUROSCI.4872-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Morris JC, Goate AM. Alzheimer’s disease: the challenge of the second century. Sci Transl Med. 2011;3:77sr71. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper C, Killick R, Lovestone S. The GSK3 hypothesis of Alzheimer’s disease. J Neurochem. 2008;104:1433–1439. doi: 10.1111/j.1471-4159.2007.05194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148:1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inestrosa NC, Arenas E. Emerging roles of Wnts in the adult nervous system. Nat Rev Neurosci. 2010;11:77–86. doi: 10.1038/nrn2755. [DOI] [PubMed] [Google Scholar]
- Inestrosa NC, Toledo EM. The role of Wnt signaling in neuronal dysfunction in Alzheimer’s Disease. Mol Neurodegener. 2008;3:9. doi: 10.1186/1750-1326-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inestrosa NC, Varela-Nallar L. Wnt Signaling Roles on the Structure and Function of the Central Synapses: Involvement in Alzheimer’s Disease 2013 [Google Scholar]
- Inestrosa NC, Varela-Nallar L, Grabowski CP, Colombres M. Synaptotoxicity in Alzheimer’s disease: the Wnt signaling pathway as a molecular target. IUBMB Life. 2007;59:316–321. doi: 10.1080/15216540701242490. [DOI] [PubMed] [Google Scholar]
- Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, Copeland NG, Lee MK, Younkin LH, Wagner SL, et al. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet. 2004;13:159–170. doi: 10.1093/hmg/ddh019. [DOI] [PubMed] [Google Scholar]
- Jensen M, Hoerndli FJ, Brockie PJ, Wang R, Johnson E, Maxfield D, Francis MM, Madsen DM, Maricq AV. Wnt signaling regulates acetylcholine receptor translocation and synaptic plasticity in the adult nervous system. Cell. 2012;149:173–187. doi: 10.1016/j.cell.2011.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joeng KS, Schumacher CA, Zylstra-Diegel CR, Long F, Williams BO. Lrp5 and Lrp6 redundantly control skeletal development in the mouse embryo. Dev Biol. 2011;359:222–229. doi: 10.1016/j.ydbio.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kounnas MZ, Danks AM, Cheng S, Tyree C, Ackerman E, Zhang X, Ahn K, Nguyen P, Comer D, Mao L, et al. Modulation of gamma-secretase reduces beta-amyloid deposition in a transgenic mouse model of Alzheimer’s disease. Neuron. 2010;67:769–780. doi: 10.1016/j.neuron.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Prior J, Piwnica-Worms D, Bu G. LRP6 overexpression defines a class of breast cancer subtype and is a target for therapy. Proc Natl Acad Sci USA. 2010a;107:5136–5141. doi: 10.1073/pnas.0911220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Trotter J, Zhang J, Peters MM, Cheng H, Bao J, Han X, Weeber EJ, Bu G. Neuronal LRP1 knockout in adult mice leads to impaired brain lipid metabolism and progressive, age-dependent synapse loss and neurodegeneration. J Neurosci. 2010b;30:17068–17078. doi: 10.1523/JNEUROSCI.4067-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani A, Radhakrishnan J, Wang H, Mani MA, Nelson-Williams C, Carew KS, Mane S, Najmabadi H, Wu D, Lifton RP. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007;315:1278–1282. doi: 10.1126/science.1136370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megill A, Lee T, DiBattista AM, Song JM, Spitzer MH, Rubinshtein M, Habib LK, Capule CC, Mayer M, Turner RS, et al. A tetra(ethylene glycol) derivative of benzothiazole aniline enhances Ras-mediated spinogenesis. J Neurosci. 2013;33:9306–9318. doi: 10.1523/JNEUROSCI.1615-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi K, Johnson GV. Role of the intracellular domains of LRP5 and LRP6 in activating the Wnt canonical pathway. J Cell Biochem. 2005;95:328–338. doi: 10.1002/jcb.20400. [DOI] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehrs C. The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol. 2012;13:767–779. doi: 10.1038/nrm3470. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Yu G, Levesque G, Zhang DM, Ruel L, Chen F, Milman P, Holmes E, Liang Y, Kawarai T, et al. Presenilin mutations associated with Alzheimer disease cause defective intracellular trafficking of beta-catenin, a component of the presenilin protein complex. Nat Med. 1999;5:164–169. doi: 10.1038/5526. [DOI] [PubMed] [Google Scholar]
- Noble W, Planel E, Zehr C, Olm V, Meyerson J, Suleman F, Gaynor K, Wang L, LaFrancois J, Feinstein B, et al. Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc Natl Acad Sci USA. 2005;102:6990–6995. doi: 10.1073/pnas.0500466102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka H, Shimono K, Ogawa R, Sugihara H, Taketani M. A new planar multielectrode array for extracellular recording: application to hippocampal acute slice. J Neurosci Methods. 1999;93:61–67. doi: 10.1016/s0165-0270(99)00113-2. [DOI] [PubMed] [Google Scholar]
- Pachet A, Wisniewski A. The effects of lithium on cognition: an updated review. Psychopharmacology. 2003;170:225–234. doi: 10.1007/s00213-003-1592-x. [DOI] [PubMed] [Google Scholar]
- Park M, Shen K. WNTs in synapse formation and neuronal circuitry. EMBO J. 2012;31:2697–2704. doi: 10.1038/emboj.2012.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purro SA, Dickins EM, Salinas PC. The secreted Wnt antagonist Dickkopf-1 is required for amyloid beta-mediated synaptic loss. J Neurosci. 2012;32:3492–3498. doi: 10.1523/JNEUROSCI.4562-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razay G, Vreugdenhil A, Wilcock G. The metabolic syndrome and Alzheimer disease. Arch Neurol. 2007;64:93–96. doi: 10.1001/archneur.64.1.93. [DOI] [PubMed] [Google Scholar]
- Ren S, Johnson BG, Kida Y, Ip C, Davidson KC, Lin SL, Kobayashi A, Lang RA, Hadjantonakis AK, Moon RT, Duffield JS. LRP-6 is a coreceptor for multiple fibrogenic signaling pathways in pericytes and myofibroblasts that are inhibited by DKK-1. Proc Natl Acad Sci USA. 2013;110:1440–1445. doi: 10.1073/pnas.1211179110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockenstein E, Torrance M, Adame A, Mante M, Bar-on P, Rose JB, Crews L, Masliah E. Neuroprotective effects of regulators of the glycogen synthase kinase-3beta signaling pathway in a transgenic model of Alzheimer’s disease are associated with reduced amyloid precursor protein phosphorylation. J Neurosci. 2007;27:1981–1991. doi: 10.1523/JNEUROSCI.4321-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahores M, Gibb A, Salinas PC. Frizzled-5, a receptor for the synaptic organizer Wnt7a, regulates activity-mediated synaptogenesis. Development. 2010;137:2215–2225. doi: 10.1242/dev.046722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2006;27:1372–1384. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims-Robinson C, Kim B, Rosko A, Feldman EL. How does diabetes accelerate Alzheimer disease pathology? Nat Rev Neurol. 2010;6:551–559. doi: 10.1038/nrneurol.2010.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, De Aguiar RB, Naik S, Mani S, Ostadsharif K, Wencker D, Sotoudeh M, Malekzadeh R, Sherwin RS, Mani A. LRP6 enhances glucose metabolism by promoting TCF7L2-dependent insulin receptor expression and IGF receptor stabilization in humans. Cell Metab. 2013;17:197–209. doi: 10.1016/j.cmet.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H, Haass C. Intramembrane proteolysis by presenilins. Nat Rev Mol Cell Biol. 2000;1:217–224. doi: 10.1038/35043065. [DOI] [PubMed] [Google Scholar]
- Tarawneh R, Holtzman DM. Biomarkers in translational research of Alzheimer’s disease. Neuropharmacology. 2010;59:310–322. doi: 10.1016/j.neuropharm.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thies W, Bleiler L. 2013 Alzheimer’s disease facts and figures. Alzheimers Dement. 2013;9:208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Toledo EM, Inestrosa NC. Activation of Wnt signaling by lithium and rosiglitazone reduced spatial memory impairment and neurodegeneration in brains of an APPswe/PSEN1DeltaE9 mouse model of Alzheimer’s disease. Mol Psychiatry. 2010;15:272–285. 228. doi: 10.1038/mp.2009.72. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Chen DF, Gerber D, Tom C, Mercer EH, Anderson DJ, Mayford M, Kandel ER, Tonegawa S. Subregion- and cell type-restricted gene knockout in mouse brain. Cell. 1996;87:1317–1326. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]
- van Meurs JB, Rivadeneira F, Jhamai M, Hugens W, Hofman A, van Leeuwen JP, Pols HA, Uitterlinden AG. Common genetic variation of the low-density lipoprotein receptor-related protein 5 and 6 genes determines fracture risk in elderly white men. J Bone Miner Res. 2006;21:141–150. doi: 10.1359/JBMR.050904. [DOI] [PubMed] [Google Scholar]
- Vetrivel KS, Thinakaran G. Amyloidogenic processing of β-amyloid precursor protein in intracellular compartments. Neurology. 2006;66:S69–S73. doi: 10.1212/01.wnl.0000192107.17175.39. [DOI] [PubMed] [Google Scholar]
- Wan M, Li J, Herbst K, Zhang J, Yu B, Wu X, Qiu T, Lei W, Lindvall C, Williams BO, et al. LRP6 mediates cAMP generation by G protein-coupled receptors through regulating the membrane targeting of Galpha(s) Sci Signal. 2011;4:ra15. doi: 10.1126/scisignal.2001464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan W, Xia S, Kalionis B, Liu L, Li Y. The Role of Wnt Signaling in the Development of Alzheimer’s Disease: A Potential Therapeutic Target? Biomed Res Int. 2014;2014:301575. doi: 10.1155/2014/301575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Adhikari N, Li Q, Hall JL. LDL receptor-related protein LRP6 regulates proliferation and survival through the Wnt cascade in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2004;287:H2376–2383. doi: 10.1152/ajpheart.01173.2003. [DOI] [PubMed] [Google Scholar]
- Youmans KL, Leung S, Zhang J, Maus E, Baysac K, Bu G, Vassar R, Yu C, LaDu MJ. Amyloid-beta42 alters apolipoprotein E solubility in brains of mice with five familial AD mutations. J Neurosci Methods. 2011;196:51–59. doi: 10.1016/j.jneumeth.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Ann Rev Neurosci. 2001;24:1071–1089. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Baker JJ, Zylstra-Diegel CR, Williams BO. Lrp5 and Lrp6 play compensatory roles in mouse intestinal development. J Cell Biochem. 2012;113:31–38. doi: 10.1002/jcb.23324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Gong K, Song B, Ma T, van Laar T, Gong Y, Zhang L. The APP intracellular domain (AICD) inhibits Wnt signalling and promotes neurite outgrowth. Biochim Biophys Acta. 2012;1823:1233–1241. doi: 10.1016/j.bbamcr.2012.05.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Analyses of Neurobehavior in Control and LRP6 Neuronal Knockout Mice at 6 and 22 Months of Age, Related to Figure 1
(A) Analyses of anxiety-related behaviors in control (Ctrl) and Lrp6 cKO (cKO) mice at 6 months of age (n=13–15 mice per genotype) assessed by open field tests. The total distance traveled, average speed, time spent mobile, and ratio of time spent in the center quadrants to total distance traveled in the open field apparatus are shown. In this figure, data are presented as mean ±SEM. N.S., not significant.
(B) Analyses of anxiety-related behaviors in Ctrl and Lrp6 cKO mice at 6 months of age (n=13–15 mice per genotype) assessed by light/dark exploration tests. The time spent in the light chamber, time spent in the dark chamber, and ratio of time spent in light to dark chambers are shown. N.S., not significant.
(C) The cognitive function of Ctrl and Lrp6 cKO mice (n=13–15 mice per genotype) at 6 months of age examined by fear conditioning tests. The percentage of the time with freezing behavior in response to stimulus during contextual or cued memory tests is shown. N.S., not significant.
(D) Analyses of anxiety-related behaviors in Ctrl and Lrp6 cKO mice at 22 months of age (n=10–11 mice per group) assessed by open field tests. The average speed, time spent mobile, total distance traveled, and ratio of time spent in the center quadrants to total distance traveled in the open field apparatus are shown.. N.S., not significant.
(E) Analyses of anxiety-related behaviors in Ctrl and Lrp6 cKO mice at 22 months of age (n=10–11 mice per group) assessed by light/dark exploration tests. The total distance traveled, time spent in the light chamber, time spent in the dark chamber, and ratios of time spent in light to dark chambers are shown. N.S., not significant.
(F) Analyses of anxiety-related behaviors in Ctrl and Lrp6 cKO mice at 22 months of age (n=10–11 mice per group) assessed by elevated plus maze test. Total time in the open arms and ratio of time spent in open to closed arms are shown. N.S., not significant.
(G) The locomotor activity and motor learning in Ctrl and Lrp6 cKO mice at 22 months of age (n=10–11 mice per group) examined by rotarod test. N.S., not significant.
Figure S2. Analyses of Long-term Plasticity, Dendritic Spine Density, and Synaptic Integrity in Control and LRP6 Neuronal Knockout Mice, Related to Figures 1 and 2
(A) Normal hippocampal LTP in the CA1 region of Lrp6 cKO (cKO) (n=12) mice at 6 months of age compared to control (Ctrl) (n=16) mice. Changes in fEPSP slope were expressed as a percentage of baseline. Data represent mean ± SEM.
(B) Representative traces before (black) and after (red) high frequency stimulation from the recordings of Ctrl and Lrp6 cKO mice. Averages of the last 5 min of recording.
(C and D) Representative Golgi-impregnated neurons in hippocampal CA1 region (C) and cortical layer II/III (D) from Ctrl and Lrp6 cKO mice at 6 months of age. Scale bars, 10 μm.
(E and F) Representative apical oblique and basal shaft dendrites from hippocampal CA1 region (E) and cortical layers II/III (F) of Ctrl and Lrp6 cKO mice at 6 months of age. Scale bars, 10 μm.
(G) NMDAR1 and GluR1 levels in the cortex of Ctrl and Lrp6 cKO mice at 6 months of age (n=4–5 per genotype) examined by Western blot analysis. The densitometric quantification of Western blot is shown as mean ± SEM.
(H) NMDAR1 and GluR1 levels in the cortex of Ctrl and Lrp6 cKO mice at 18 months of age (n=5–7 per genotype) examined by Western blot analysis. The densitometric quantification of Western blot is shown as mean ± SEM. *p < 0.05.
(I) The levels of synaptic markers in the cerebellum of Ctrl and Lrp6 cKO mice at 18 months of age (n=6 per genotype) examined by Western blot analysis. Syp, Synaptophysin. The densitometric quantification of Western blot is shown as mean ± SEM. N.S., not significant.
Figure S3. Amyloid Deposition, Neuroinflammatory Response and Anxiety Behaviors in APP/PS1 Mice with or without Neuronal LRP6 Deletion, Related to Figure 4
(A and B) Brain sections from APP/PS1; Ctrl and APP/PS1; cKO mice (n=5 per group) at 6 months of age immunostained for Aβ. Scale bar, 100 μm. (B) The percentage of area covered by plaques in cortex was quantified, and the plaque load was normalized to that of APP/PS1; Ctrl mice. Values are mean ± SEM. *p < 0.05.
(C) Hemi-brain sections from APP/PS1; Ctrl and APP/PS1; cKO mice at 9 months of age immunostained with Iba1 antibody, a marker of microgliosis. Representative pictures of hippocampus are shown. Scale bar, 100 μm.
(D) The analyses of anxiety-related behaviors in APP/PS1; Ctrl and APP/PS1; cKO mice (n=14 mice per group) at 12 months of age assessed by open field tests. Total distance traveled, average speed, time spent mobile, and ratio of time spent in the center quadrants to total distance traveled in the open field are shown. N.S., not significant.
(E) Anxiety-related behaviors in APP/PS1; Ctrl and APP/PS1; cKO mice at 12 months of age assessed by light/dark exploration tests. The time spent in the light chamber and ratio of time spent in light to dark chambers (n=14 mice per group) are shown. N.S., not significant.
Figure S4. Modulation of LRP6 Affects Wnt Signaling, Related to Figure 5
(A) The mRNA levels of LRP6 in N2a-APP cells expressing control shRNA (Ctrl) or LRP6 shRNA (KD) examined by real-time PCR analysis. Results were normalized to β-actin levels. Values are mean ± SD. *p < 0.05.
(B and C) The APP levels in N2a-APP cells expressing control shRNA (Ctrl) or LRP6 shRNA (KD) examined by Western blot analysis. Densitometric analyses of Western blots are shown in (C). N.S., not significant.
(D) The Aβ40 or Aβ42 levels in the medium of N2a-APP cells expressing control shRNA (Ctrl) or LRP6 shRNA (KD) examined by ELISA. All measurements were normalized to protein concentrations in the cell lysates. Values are mean ± SD. *p < 0.05.
(E and F) The levels of APP, LRP6 and free β-catenin in HEK293 cells co-transfected with APP together with pcDNA or LRP6/Mesd examined by Western blot analysis. Free β-catenin levels were evaluated by GST-E-Cadherin pull-down, followed by Western blot analysis. Densitometric analyses of Western blots are shown in (F). Values are mean ± SD. **p < 0.01; N.S., not significant.
(G) The levels of Wnt signaling in HEK293 cells co-expressing TOPFlash reporter and APP together with pcDNA or LRP6/Mesd examined by TOPFlash luciferase assay. Values are mean ± SD. **p < 0.01.
Figure S5. The APP E2 Domain Interacts with LRP6 Extracellular Domain, Related to Figure 5
(A) LRP5 and LRP6 mRNA levels in human brain tissue and SH-SY5Y-APP cells quantified by real-time PCR. The standard curves for human LRP5 and LRP6 was derived from the serial dilutions of plasmids with known concentration. The logarithms (base 10) of concentrations were plotted against threshold cycle (Ct), and the levels of LRP5 and LRP6 were calculated using its corresponding standard curves. Values are mean ± SD. **p < 0.01.
(B and C) The levels of LDLR and β-actin in N2a-APP cells expressing control and LDLR siRNA examined by Western blot analysis. (C) The level of Aβ40 in the medium was evaluated by ELISA. N2a-APP cells were transfected with control or LDLR siRNA. 48 h post co-transfection, cell medium were replaced with Opti-MEN medium and incubated for 12 h. The Aβ40 level in the medium was normalized to total protein levels in cell lysates. N.S., not significant.
(D) The levels of Mesd in HEK293 cells with or without overexpressing flag-tagged Mesd and in the concentrated medium analyzed by Western blot.
(E) The level of Aβ40 in the medium of HEK293 cells overexpressing APP with either vector or HA-tagged LDLR was evaluated by ELISA. The levels of LDLR, APP and β-actin in the cell lysates were analyzed by Western blot. HEK293 cells were co-transfected with APP together with pcDNA or LDLR. 24 h post co-transfection, cell medium were replaced with DMEM medium containing 1% FBS and incubated for 12 h. The Aβ40 level in the concentrated medium was quantified after normalization with total protein levels in the cell lysates. Values are mean ± SD. *p < 0.05.
(F and G) Schematic diagrams of APP and LRP6 constructs used in this study.
(H) Western blot of LRP6 and different APP fragments used as inputs of immunoprecipitation.
(I and J) LRP6 co-immunoprecipitated with full-length APP, APP-E2 and APP-ΔE1, but not C99, APP-E1 and APP-ΔE1E2. HEK293 cells were co-transfected with plasmids expressing various myc-APP and HA-LRP6 together with Mesd. Cell lysates were immunoprecipitated with an anti-myc antibody for APP, and probed with an anti-HA antibody for LRP6.
(K and L) LRP6 extracellular domain is required for the APP-LRP6 interaction. HEK293 cells were co-transfected with plasmids expressing HA-APP, LRP6ΔC-VSVG or myc-LRP6ΔN together with Mesd. Cell lysates were subjected to immunoprecipitation with anti-HA antibody against APP, and probed with anti-LRP6 or anti-myc antibodies.
Figure S6. The Levels of LRP6-mediated Wnt Signaling and its Correlation with Aβ Levels in Human Control and AD Brain Tissues, Related to Figure 6
(A) LRP6 protein levels in human control (n=17) and AD (n=17) brain tissues examined by Western blot analysis.
(B) LRP5 mRNA levels in human control (n=20) and AD (n=18) brain tissues quantified by real-time PCR. Results were normalized to β-actin levels and presented in relative units. In the following figures, data are shown as scatterplots in which each symbol represents an individual case. A.U., arbitrary unit. Data represent mean ± SEM. **p < 0.01.
(C) Correlation between LRP6 protein levels (Figures 6B and S6A) and mini-mental state examination (MMSE) score of human control (dark circles) and AD (light circles) brain tissues (r represents the correlation coefficient; p is significance).
(D and E) Synaptic markers (PSD-95 and Synaptophysin), sodium/potassium ATPase, a critical membrane marker and neuron-specific β-tubulin in control (n=17) and AD (n=17) brain tissues examined by Western blot analysis and quantified.
(F) The levels of total and free β-catenin in human control (n=16) and AD (n=16) brain tissues examined by Western blot analysis. Free β-catenin levels were evaluated by GST-E-Cadherin pull-down, followed by Western blot analysis.
(G) Aβ40 levels in TBS, TBSX, and GDN fractions of human control (n=20) and AD (n=18) brain tissues. Data are shown as scatterplots in which each symbol represents an individual case. Data represent mean ± SEM. **p < 0.01.
(H) Correlation between LRP6 protein levels (Figures 6B and S6A) and Aβ42 levels (Figure 6G) in various extraction fractions (TBS, TBSX or GDN) in human control (dark circles) and AD (light circles) brain tissues (r represents the correlation coefficient; p is significance). In this and following figure, LRP6 protein levels are presented in relative units. A.U., arbitrary unit.
(I) Correlation between LRP6 mRNA levels (Figure 6A) and Aβ40 levels (Figure S6G) in various extraction fractions (TBS, TBSX or GDN) in human control (dark circles) and AD (light circles) brain tissues(r represents the correlation coefficient; p is significance).
(J) Correlation between LRP6 protein levels (Figures 6B and S6A) and Aβ40 levels (Figure S6G) in various extraction fractions (TBS, TBSX or GDN) in human control (dark circles) and AD (light circles) brain tissues(r represents the correlation coefficient; p is significance).
Figure S7. The Levels of LRP6-mediated Wnt Signaling is Down-regulated in APP/PS1 Mouse Brains and in Neuronal Cells upon Aβ Treatment, Related to Figure 7
(A and B) The levels of total and free β-catenin (β-cat) and LRP6 in the hippocampus of wild-type (WT) mice and APP/PS1 mice at 12 months of age (n=4–5/per genotype) examined by Western blot analysis. Free β-catenin levels were analyzed by GST-E-Cadherin pull-down assay. Data represent mean ± SEM. N.S., not significant; *p < 0.05.
(C–F) The levels of soluble APP fragments (sAPPα and sAPPβ) or APP-CTFs (CTFα and CTFβ) in N2a-APP cells treated with or without BACE inhibitor (β-secretase inhibitor IV, 2 μM) or γ-secretase inhibitor (DAPT, 10 μM) examined by Western blot analysis. The levels of Aβ40 in the media were evaluated by ELISA (C and E). Data represent mean ± SD. **p < 0.01.
(G) The levels of LRP6, total and free β-catenin in SH-SY5Y cells treated with 5 μM Aβ42 oligomers in the presence or absence of Wnt3a ligands examined by Western blot analysis. L CM, L cell conditioned medium; Wnt3a, Wnt3a conditioned medium.
Table S1: Characteristics of Examined Individuals, Related to Figure 6