Introduction
Necrotizing soft tissue infections (NSTIs) rank among the more difficult disease processes encountered by physicians and surgeons. NSTIs can arise primarily in the dermis and epidermis, but they more commonly affect the deeper layers of adipose tissue, fascia, or muscle. NSTIs are typically caused by toxin-producing bacteria and are characterized clinically by very rapid progression of disease with significant local tissue destruction. Varying amounts of early or late systemic toxicity depend on the strain of bacteria and toxins produced. Once symptomatic, the progression of disease is typically measured in hours; early diagnosis and treatment are crucial to survival. Diagnosis is hindered by the fact that the disease progresses below the surface, and the cutaneous manifestations belie the severity of disease. As the infection disseminates, patients develop pain and signs of systemic toxicity disproportionate to the findings of skin examination, and physicians must maintain a high index of suspicion to rapidly diagnose NSTIs. The standard treatment consists of broad-spectrum antibiotics, wide surgical debridement, and supportive care. Most patients require multiple surgical debridements, and survivors often have large and complex wounds requiring soft tissue coverage and prolonged hospitalizations. Even with optimal treatment, NSTIs portend significant morbidity and have mortality rates of 25%-35% in recent series.
In this review, we provide historical context; discuss the epidemiology, etiology, and pathogenesis of disease; summarize the major microbiology; and elaborate the clinical presentation and diagnosis of disease with a special discussion of NSTIs in immune-compromised patients. We then outline the major and novel treatment strategies, organized by phase of treatment and with special emphasis on the importance of multidisciplinary care teams. Finally, we summarize the information on in-hospital outcomes and close with a brief discussion of posthospital and developing information on long-term quality of life and functional outcomes.
Scope of the problem, diagnostic considerations, and short-term treatment of NSTI
Historical background
NSTI was first described by Hippocrates in the 5th century BC. He described it as a complication of acute streptococcal infection, writing “[m]any were attacked by the erysipelas all over the body when the exciting cause was a trivial accident...flesh, sinews, and bones fell away in large quantities...there were many deaths.”1
The first English-language descriptions of NSTIs were by the British surgeon Leonard Gillespie and British physicians Gilbert Blaine and Thomas Trotter in the late 18th century. The disease was known as phagedaenic ulcer, phagedena gangrenous, gangrenous ulcer, malignant ulcer, putrid ulcer, or hospital gangrene.2
The term hospital gangrene became the dominant term and is how the disease was first described in the United States by Confederate Army surgeon Joseph Jones in 1871, when he reported on 2642 cases with a mortality rate of 46%.3 In 1883, Dr Jean-Alfred Fournier described a necrotizing infection of the perineum and scrotum, and the term Fournier gangrene is still in common usage.4 Necrotizing fasciitis, the most commonly used term to describe NSTI, was first coined by Wilson.5 Recently, the term NSTI is used to encompass infections not only of the fascia, as in necrotizing fasciitis, but also of the other soft tissues affected. The taxonomy is further complicated by the use of other terms, such as “complicated skin and skin structure infections” and “acute bacterial skin and skin structure infections.” Several highly publicized cases of “flesh-eating bacteria” across the United States, most recently of a male patient dying of an NSTI of the lower extremity caused by a virulent strain of the marine bacterium Vibrio vulnificus, have once again piqued public interest in this rare, but highly morbid disease.
Epidemiology
NSTIs are an exceedingly rare clinical entity, with an estimated 1000 cases annually in the United States; however it appears that this incidence has been increasing.6,7 The cause for this increase is unclear, but it may be a result of greater awareness of the problem leading to higher rates of reporting, increasing bacterial virulence, increased antimicrobial resistance, or all 3. Most physicians will see only 1 case of NSTI throughout their career. This rarity prevents most physicians from maintaining sufficient clinical suspicion important for establishing the diagnosis early and initiating appropriate treatment.
There is no age or gender predilection, but higher rates of NSTIs are seen in obese, diabetic, and immunocompromised patients, as well as alcoholics and patients with peripheral vascular disease. However, NSTIs can (and do) occur in young, otherwise healthy patients with none of these predisposing factors. Geographic differences in etiology and microbiology have been shown on the national and regional level.8 Variance in patient risk factors, environmental levels and types of bacteria, and exposures related to factors such as the quality and purity of intravenous (IV) drugs may contribute to regional differences.
There have been reports in the literature suggesting a link between nonsteroidal anti-inflammatory drug (NSAID) usage and NSTIs. However, given the widespread usage of NSAIDs overall, and the use of NSAIDs for pain relief during the early phases of the disease process, this association does not necessarily represent a causal pathway. NSAIDs inhibit the conversion of arachadonic acid via cyclooxegenase-1 (COX1) or COX2 into thromboxane or other prostaglandins, respectively. COX1 is constitutively expressed at significantly higher levels than COX2, but in times of inflammation, expression of COX2 is increased to levels significantly higher than COX1. Therapeutically, NSAIDs inhibit conversion of arachadonic acid by COX2 into prostaglandin E2, which is thought to be responsible for pain and fever associated with inflammation. Various other prostaglandins produced via COX2 pathway have been shown to be involved in gastrointestinal epithelial cell migration in animal models, modulate activity of myofibroblasts and collagen deposition in wounds, and have immunomodulatory effects on leukocyte adhesion and granulocyte functions, including chemotaxis, oxidative burst, and bacterial killing.9-16 Therefore, it is possible that use of NSAIDs in the setting of NSTI may be associated with a worsened clinical course owing to alterations in the immune response.
Etiology
NSTIs can occur in any area of the body but are most commonly seen in the extremities, perineum, and genitalia, with fewer arising on the chest or the abdomen. Infection requires inoculation of the pathogen into the subcutaneous tissue. This can occur via any break in an epithelial or mucosal surface. Trauma, IV drug and insulin injection, skin infections and ulcers, animal and insect bites, visceral-cutaneous fistulas, surgical complications, percutaneous catheter insertion, abscesses, and idiopathic etiologies have been reported. Inoculation may also occur via hematogenous spread from distant sites; this has been suggested as the etiology among patients with streptococcal NSTIs with a history of suspected streptococcal pharyngitis.17 NSTIs of the perineum and genitalia, also known as Fournier gangrene, can occur as a result of trauma, urinary tract infections or stones, Bartholin gland abscesses, and surgery or other instrumentation. NSTIs of the extremities typically occur secondary to extrinsic compromise of the skin, such as from trauma or IV drug injection.
Classification
NSTIs have been described according to their anatomical locations (ie, Fournier gangrene), the depth of infections (ie, cellulitis, adipositis, fasciitis, and myositis), and the associated microbiological organisms. The various methods of classification are not clinically useful in that the diagnostic maneuvers and treatment modalities are not different. The bacterial etiology of NSTIs is important clinically, as it does alter clinical presentation and some adjunctive treatments discussed later may be more or less effective in subsets of patients.
Most commonly, NSTIs are described as 1 of 3 bacteriologic classes. This classification system was first described by Giuliano and colleagues.18 Each class does have different microbial etiologies, as well as potential differences in patient populations and typical presentation. No differences in clinical course, morbidity, or mortality have been demonstrated between groups. It is important to note that the different types of NSTIs are not limited to the patient populations described later, and the clinician should base treatment decisions on the overall clinical condition of the patient, regardless of demographics.
Type I NSTIs
Type I infections are classically polymicrobial with various species of gram-positive cocci, gram-negative rods, and anaerobes—including clostridial species—typically being isolated.7,19-21 The affected anatomical location is the trunk and the perineum. Patients with type I NSTIs are typically older, with more medical comorbidities such as diabetes, and often have no history of trauma. Although frank trauma is often absent, it is believed that some breakdown in tissue integrity occurs from antecedent abscesses, perforations, or bacterial translocation.
Clostridial infections, traditionally known as gas gangrene likely represent the subtype of NSTI first described and most strongly associated with the historical disease and deserve special attention within type I NSTIs. Clostridial infections are relatively rare; this is thought to be because of improvements in sanitation and hygiene. However, at our institution in the Pacific Northwest, where clostridial species are endemic to the soil, Clostridia are still responsible for approximately 10% of NSTIs.19 Various clostridial species, such as Clostridium perfringens, C. septicum, and C. sordellii, have been associated with NSTIs among IV drug users injecting “black tar” heroin subcutaneously.22 Two primary toxins are thought to be most responsible for their lethality: α-toxin and θ-toxin. α-Toxin has several effects throughout the progression of disease. Early on, it acts as a potent platelet agonist, leading to platelet aggregation and thrombi formation.22,23 This leads to local ischemic conditions at the site of infection, which reduces the tissue pH and establishes a favorable local environment for bacterial proliferation. As α-toxin is absorbed locally, it also leads to neutrophil dysfunction by interfering with diapedesis both directly and also via platelet adherence to neutrophils.23 This accounts for the paucity or total lack of neutrophils on Gram stain of fluid that is obtained from clostridial wounds. As the infection progresses and both α- and θ-toxins are absorbed systemically, they directly impair phagocyte function, cause intravascular hemolysis, decrease endothelial cell integrity, suppress cardiac function, and markedly decrease vascular tone.23
Clostridial infections present typically with the rapid onset of severe pain, often out of proportion to initial clinical examination. The wound often drains a foul-smelling, thin serosanguinous fluid. Woody induration of nearby tissue with associated tissue crepitus gas formation is also classically described but is not always present. Bacteremia and sepsis typically occur late and portend poor outcomes with significant mortality. It should be noted that clostridial species are notoriously difficult to grow and isolate in culture, and thus blood cultures negative for clostridial species should not be relied on to rule out systemic infection. Once patients manifest shock, mortality exceeds 50%.23 It is important to note that clostridial infections can manifest extremely quickly and can become symptomatic only hours after initial injury and inoculation, whereas most bacterial species (with the notable additional exception of group A streptococcus) require 2 or more days to become symptomatic. Thus, anytime a patient presents with rapidly presenting signs of infection from a wound, whether traumatic or surgical, clostridial and streptococcal infections must be considered.
Type II NSTIs
Type II infections involve group A β-hemolytic streptococci (GAS), either alone or in combination with staphylococcal species. GAS infections of soft tissue have a significant potential for aggressive local spread, as well as systemic toxicity including toxic shock syndrome. When compared with type I NSTIs, patients with type II infections tend to be younger, healthier, and more commonly have a history of trauma, surgery, or IV drug use.
Several microbiologic mechanisms potentiate the toxicity of GAS infections. Streptococci elaborate multiple M proteins that manifest superantigen activity. The M proteins allow the bacteria to adhere to tissue and evade phagocytosis, but probably more importantly, they allow streptococcal species to bypass the typical antigen presentation pathway. Normally, bacterial antigens are presented by antigen-presenting cells via the major histocompatibility complex-II molecule and activate only a very small percentage of T cells, typically approximately 0.1%. M proteins can bind directly to and activate a common structural aspect of T-cell receptors, present on up to 20% of T cells. This leads to inflammatory response that is orders of magnitude larger owing to a massive release of cytokines IL-1, IL-6, and tumor necrosis factor-α.24 Activation of this inflammatory cascade leads to the catastrophic shock often seen with GAS NSTIs. GAS species also elaborate several potent exotoxins that damage neutrophils, prevent phagocytosis and bacterial clearance by fluid secretions, and break down hyaluronic acid in connective tissues.7,24,25
Type III NSTIs
Type III NSTIs are caused by gram-negative marine organisms, most commonly V. vulnificus. Although not as widely accepted as a class of NSTI compared with types I and II, type III infections have been reported along warm-water coastal regions in the southeastern United States, Central and South America, and Asia. Infection can occur via exposure through an open wound or other break in the skin, but infection has also been reported via ingestion of colonized oysters by patients with cirrhosis. Type III infections clinically present and manifest progression of disease similar to that of type II infections in that there is early evidence of significant systemic toxicity. Multisystem organ failure and cardiovascular collapse occur very early and have been observed without any localized cutaneous evidence of infection.26,27
Clinical presentation and diagnosis
Signs and symptoms
Early signs and symptoms of NSTI are identical to those seen with cellulitis or abscesses potentially making the correct diagnosis difficult. The clinical presentation will vary depending on the microbiologic pathogen responsible, as well as the anatomical region and depth of infection. In general, erythema, pain beyond the margins of obvious infection, swelling, and fever are the most common findings on physical examination. In our experience, induration and edema beyond the area of skin changes are commonly present. Pain that is disproportionate to examination findings should alert the clinician to the possibility of NSTI. Several “hard” clinical signs are more suggestive of NSTI but occur late in the course of the disease. These include (1) the presence of bullae, (2) skin ecchymosis that precedes skin necrosis, (3) presence of gas in the tissues by examination or radiographic evaluation, and (4) cutaneous anesthesia. These signs are present in the minority of cases (7%-44%) but should prompt immediate surgical exploration. Other clinical signs that are often seen in NSTI, but are less specific, include (5) pain out of proportion to examination, (6) edema that extends beyond the skin erythema, (7) systemic toxicity, and (8) progression of infection despite antibiotic therapy. Subcutaneous emphysema, which has been traditionally stressed as a classic finding of NSTI, is rarely seen. More importantly, its absence is not sufficient to rule out NSTI.
Perhaps one of the most important pieces of evidence to support the diagnosis of NSTI is the chronology of disease ascertained on history. In contrast to patients with severe cellulitis or abscesses or both, the symptoms and signs of NSTI usually progress very rapidly. This makes early diagnosis critical and becomes vitally important to clinicians making treatment decisions. Patients who present with signs and symptoms of systemic toxicity or shock already have very advanced disease, and appropriate surgical evaluation must happen quickly.
Laboratory evaluation
There have been multiple scoring systems developed to facilitate the diagnosis of NSTI. Wall and colleagues28,29 found that a white blood cell (WBC) count <15,000 cells/mm3 and a serum sodium level greater than 135 mmol/L had a negative predictive value of 99% and a 90% sensitivity for detecting NSTIs. The most widely adopted score is the laboratory risk indicator for necrotizing fasciitis (LRINEC) developed by Wong and colleagues.30 The LRINEC, developed based on data from 89 consecutive patients with NSTI compared with 314 patients with severe cellulitis or abscess or both, stratifies patients based on level of C-reactive protein, WBC count, hemoglobin level, serum sodium level, serum creatinine level, and serum glucose level at admission (Table 1). Scores ≥6 were found to have a 92% positive predictive value and a 96% negative predictive value. Although this score is widely used, it has never been validated and the authors themselves noted that many other conditions might cause similar laboratory derangements. Furthermore, its use is limited when competing inflammatory states are present. Thus, it is our opinion that it should only be used within the context of the broader clinical presentation and must be interpreted with caution; treatment decisions should not be based solely on the LRINEC score.
Table 1.
Elements of the laboratory risk indicator for necrotizing fasciitis (LRINEC) score
| Laboratory marker | Scoring evaluation |
|---|---|
| CRP (mg/dL) | < 150 = 0 points > 150 = 4 points |
| WBC (per mm3) | < 15 = 0 points 15-25 = 1 point > 25 = 2 points |
| Hgb (g/dL) | > 13.5 = 0 points 11.6-13.5 = 1 point < 11.5 = 2 points |
| Serum sodium | > 135 = 0 points < 135 = 2 points |
| Serum creatinine | ≤ 1.6 = 0 points > 1.6 = 2 points |
| Serum glucose | < 180 = 0 points > 180 = 1 point |
CRP, C-reactive protein; Hgb, hemoglobin.
Imaging evaluation
There is a limited role for imaging in the evaluation of a suspected NSTI. It is our opinion that the delay caused by imaging outweighs its potential benefit in helping to diagnose deeper infections. There have been no well-designed or powered studies to compare the various imaging modalities. Plain film radiography can show subcutaneous emphysema, which is considered a fairly specific finding for clostridial NSTI. However, it is not sensitive and nonclostridial NSTIs only very rarely demonstrate this finding. Computed tomography (CT) is more sensitive than plain radiography; however, findings are again nonspecific. Images obtained via CT scan may demonstrate fascial thickening, edema, subcutaneous gas, and abscess formation. Magnetic resonance imaging has similarly been shown to be fairly sensitive but lacks specificity as the tissue enhancement on T2-weighted imaging is frequently seen after trauma and other noninfectious inflammatory processes.31 Finally, ultrasonography can be used to detect superficial abscesses but lacks sensitivity or specificity for NSTIs.32
Operative exploration
The gold standard for diagnosis of NSTI remains operative exploration in the setting of high clinical suspicion. In questionable cases, a small skin incision can be made with dissection carried down to the fascia allowing the surgeon to assess the adherence of the fascia to other soft tissue layers. In classic necrotizing fasciitis, the diseased fascia is no longer adherent to the adjoining layers, allowing the surgeon to easily slide his or her finger along the fascial plane. This test is not applicable to cases of necrotizing adipositis or deep necrotizing myositis; however, both of these conditions can be diagnosed through similarly small incisions with local exploration for necrotic tissue.
Special issues: Immunocompromised patients
Because of the very limited experience most clinicians will have in their careers with NSTIs, a high index of suspicion must be maintained to facilitate early diagnosis. Immunocompromised patients can pose a diagnostic challenge in this regard, as they may not manifest symptoms the same or as severely as immune-competent patients can. In a 2010 study using the National Surgical Quality Improvement Program registry, both malignancy and treatment with corticosteroids were found to be associated with increased mortality among 668 cases of NSTI. Immunocompromised patients accounted for less than 10% of the overall cases, and their clinical characteristics were poorly described. A more recent study by Keung and colleagues33 found that immunocompromised patients (defined as active corticosteroid usage, active malignancy, receipt of chemotherapy or radiation therapy, diagnosis of human immunodeficiency virus or AIDS, or prior solid organ or bone marrow transplantation with long-term immunosuppression) presented differently than immune-competent patients did and had significant delays in diagnosis. Immunocompromised patients presented with lower systolic blood pressures, lower serum glucose levels, and lower WBC counts. These patients were also less likely to be transferred, less likely to be admitted to a surgical service, and far less likely to undergo surgical debridement at the time of admission. Immunocompromised patients also had a 2-fold higher NSTI-related mortality. The authors suggested that immunocompromised patients with NSTIs present atypically and feel that clinicians caring for immunocompromised patients must maintain an even higher index of suspicion for NSTIs to facilitate early diagnosis and avoid delays in treatment.
Short-term treatment of NSTIs
The primary treatment modality for patients with NSTI is radical surgical debridement to remove infected and necrotic tissue. However, surgical debridement must be undertaken in the context of a larger system of care and involve a truly multidisciplinary team to care for the patient through the continuum of treatment from initial resuscitation and debridement to permanent wound management and rehabilitation.
Resuscitation, initial debridements, and antimicrobial therapy
Initial treatment of patients with NSTI requires coordination between the surgical and intensive care team. Treatment consists of radical debridement, broad-spectrum antimicrobial therapy, and hemodynamic support. The issues related to critical care necessary to support these patients is beyond the scope of this review, but the fundamental aspects of surgical treatment and antimicrobial therapy are discussed.
The cornerstone of appropriate treatment for NSTIs is early and wide surgical debridement. Increased time from admission to debridement and inadequacy of initial debridement have repeatedly been associated with increased mortality.20,27,29,34-38 The surgical incisions should initially extend at least beyond the area of induration, when present. The presence of significant induration suggests that dermal lymphatics are blocked and postcapillary venules are thrombosed, which lead to tissue necrosis. Some have suggested that the initial incision begin beyond any area of cellulitic changes, but in our experience we find that skin with cellulitic changes but no apparent necrosis can often be safely spared as long as the subcutaneous debridement is sufficient and a thick tissue flap can be left. The cellulitis often retracts significantly following excision of necrotic tissue and antibiotic treatment, and this skin-sparing approach simplifies later attempts at wound coverage. Excision should extend to healthy, bleeding tissue at all margins, and often needs to be carried significantly beyond the area of skin changes because subcutaneous disease is usually much greater than can be appreciated on the skin examination. The extent of necrotic tissue can be impressive, and surgeons must be willing to aggressively debride en mass organs, tissues, and structures. Surgeons who do not regularly see and treat patients with NSTIs are often concerned about the size of the incision. In our experience, patients with NSTIs have poor outcomes when their debridements are too small and conservative compared with when their debridements are too wide and aggressive. NSTIs of the extremities often require amputation, and these must be considered when a joint is involved or if the area of infection is rapidly spreading. Necrotizing infections have the potential for rapid progression despite surgical debridement. A planned second look operation within 24 hours is recommended and the average number of operative procedures is 3-4 per patient.
NSTIs of the perineum (Fournier gangrene) are also unique because of their proximity to the anus. Debridement in this area and subsequent wound care can be extremely difficult owing to soilage from stool. For this reason, patients with perineal NSTIs have often undergone diverting colostomy. This has been done in the name of decreasing soilage, decreasing the number of dressing changes required, and protecting the perineal skin and soft tissue, which may be needed for eventual coverage and closure. However, we do not routinely perform diverting colostomy on patients with perineal NSTIs. It has been our experience that the wound bed can be kept sufficiently clean with regular dressing changes. Although the patient remains intubated, typically between the first and final debridements, stooling can be managed by the use of a fecal management system and maintaining liquid stools. Once the patient is extubated, alert, and continent, regular dressing changes typically suffice.
Broad-spectrum antimicrobial therapy should be initiated in all cases of suspected NSTI. Initial empiric therapy should include gram-positive, gram-negative, and anaerobic coverage. Local incidence of methicillin-resistant Staphylococcus aureus (MRSA) infection should be considered and anti-MRSA coverage based on locoregional resistance patterns may be indicated. Because of a significant local incidence of MRSA infection in our institution's referral region, we routinely include vancomycin for empiric coverage with NSTIs. Our empiric therapy regimen consists of 4 antimicrobials: penicillin G, clindamycin, vancomycin, and gentamicin. Penicillin G has significant activity against streptococcal and clostridial species. As stated, vancomycin provided empiric coverage for MRSA infection. Clindamycin has relatively broad activity, but specifically it has been shown to decrease α-toxin production by clostridial species, reduce superantigen M protein by streptococcal species, and suppress lipopolysaccharide–induced tumor necrosis factor-α production by monocytes.39-41 Gentamicin provides broad coverage for many gram-negative rods and anaerobic species. At our institution, if patients are found on initial evaluation to have significantly elevated serum creatinine levels concerning for acute kidney injury, we employ fluoroquinolones—instead of gentamicin to avoid the nephrotoxicity of aminoglycosides. We continue this regimen until microbial culture allows appropriate narrowing of coverage, or until operative debridement is complete and the patients become hemodynamically stable with a normal or near-normal WBC count. We feel that protracted courses of antibiotics are not indicated after surgical source control is achieved, unless there is concern for separate infectious disease processes that would independently dictate ongoing antimicrobial therapy.
Adjunctive treatment
Hyperbaric oxygen treatment
Hyperbaric oxygen (HBO) therapy is a treatment modality in which a patient is placed in a high-pressure chamber, resulting in delivery of oxygen at 2-3 times typical atmospheric pressure. This leads to arterial oxygen tension as high as 2000 mm Hg with resulting tissue oxygen tension of 300 mm Hg. This compares with arterial oxygen tension of 300 mm Hg and tissue oxygen tension of 75 mm Hg when breathing 100% oxygen at normal atmospheric pressure. The use of HBO is based on animal and human studies showing that elevated levels of oxygen at the tissue level reduce edema, stimulate fibroblast growth, increase the killing ability of leukocytes by augmenting the oxidative burst, have independent cytotoxic effects on some anaerobes, inhibit bacterial toxin elaboration and release, and enhance antibiotic efficacy.42-46
Multiple studies have examined the use of HBO in the treatment of NSTIs with mixed results. Early single-institution and small retrospective studies suggested a mortality benefit for patients with NSTI who were treated with HBO.47-50 However, several other more contemporary studies with larger sample sizes have found no differences in mortality associated with HBO therapy.51-55 With the lack of proven benefit from HBO, and significant limitations on care delivery possible in hyperbaric chambers, we do not recommend routine consideration of HBO therapy for patients with NSTI and feel its use should only be considered in hemodynamically stable patients in whom HBO therapy will not delay surgical debridement.
Intravenous immunoglobulin
Intravenous immunoglobulin (IVIg) therapy involves the administration of pooled IVIg from human donors. Its use in the treatment of NSTI is based on the theory that it binds exotoxins produced by staphylococcal and streptococcal bacterial infections and subsequently limits systemic inflammatory response.56,57 Although there is weak evidence suggesting benefit, the studies to date have been underpowered and nonrandomized.58-60 We suggest that consideration of IVIg therapy be limited to critically ill patients with only staphylococcal or streptococcal NSTIs or both.
Management of complications
Concurrent with and following completion of surgical debridements, patients with NSTI are at a high risk of complications stemming from critical illness. These complications include, but are not limited to, ventilator-associated pneumonia, myocardial infarctions, cardiac arrhythmias, acute kidney injury, and line or in-dwelling catheter-associated infections or both. These entities contribute significantly to the morbidity and mortality associated with NSTIs, and their prevention and management are vitally important to the overall treatment of patients with NSTIs. Keys for systemic stabilization include tight glucose level control, early enteral nutrition, and many of the interventions that have improved outcomes in the trauma patient. Care should be closely coordinated between the surgical and critical care teams.
Wound management, rehabilitation, and the importance of multidisciplinary systems of care
Most physicians and surgeons would be quick to describe the initial treatments needed for NSTIs, but those experienced with the disease know that the definitive management extends far beyond the operative debridement and period of critical care. Similar to severe burn injuries, the magnitude and scope of the wounds necessitated by radical debridement for NSTI, although required to save the patient's life, often create unique and difficult challenges in terms of wound care, preservation of function, and cosmesis. These problems require time and a truly multidisciplinary approach. But, we feel this aspect of care, which extends from early wound care through reconstruction and long-term rehabilitation, is of paramount importance to attaining the best long-term functional and quality-of-life outcomes for patients.
Wound care and pain control
The initial stages of the wound management begin during the surgical debridement. The room temperature must be kept warm to prevent hypothermia, and the dressing supplies must be available to promote efficiency in the operating room. Dressings must be tailored to avoid exposure of tendons, bone, or cartilage to prevent these vital tissues from drying out. The dressings must also allow access to reassess secondary problems such as secondary infections or overgrowth of yeast. At the time of the initial debridement, it is important to document the location of the wound, the dimensions of the wound, the appearance of the wound base and edges along with the surrounding skin, and the presence of any undermining or tracts. This is important for further wound staging and subsequent assessments.
The goals of treatment are to achieve a clean wound, to protect it from dessication, to assess the need for further surgical debridement, and to promote wound healing. Many articles have been published that focus on aiding healing in acute wounds. Franz and colleagues61 published guidelines to address impediments to healing of the acute wound. The expert panel identified both systemic and local factors that contribute to poor wound healing. Wound healing obstacles that often complicate but are not unique to NSTIs include local factors such as wound infection, wound necrosis, repeated trauma, and tissue hypoperfusion. Systemic factors such as chronic illnesses and malnutrition must also be addressed. Table 2 highlights major obstacles to wound healing in patients with NSTI and some options for therapy.
Table 2.
Obstacles to wound healing and treatment options for NSTI
| Systemic factors | Treatment options |
|---|---|
| Age | Not modifiable, quality-of-life discussion, and palliative care involvement |
| Coagulopathies | Reversal agents |
| Malnutrition | Early provision and optimization of enteral nutrition |
| Immune suppression | Stop steroids if possible, vitamin A, and continue antiretovirals if HIV patients |
| Diabetes and hyperglycemia | Insulin infusion and control postprandial glucose elevations |
| Jaundice and liver failure | Provide enteral nutrition and treat high ammonia levels |
| Local factors | Treatment options |
| Bleeding | Correct coagulopathy, meticulous hemostasis, and transfusions as needed |
| Tissue hypoperfusion | Defer coverage procedures until patient is hemodynamically stable, is off vasopressors, and optimal wound base is achieved |
| Wound infection and tissue necrosis | Additional excisional debridement and adjunctive antibiotic use |
| Wound soilage (feces) | Harden stools, fecal management systems, prevent or treat antibiotic-associated diarrhea, and fecal diversion if all else fails |
| Repeat trauma to tissues | Use tangential excision techniques and avoid aggressive use of electrocautery |
| Heavy wound contamination or colonization | Use temporary skin substitutes |
HIV, human immunodeficiency virus.
Progressive skin necrosis may occur from polymicrobial synergy, infectious spread, or hypotension, which require the patient to undergo further excisional debridements as often as necessary, resulting in larger soft tissue defects. Adjuncts to reduce bacterial contamination after surgical debridement to these large wounds include the use of mafenide or Dakin solution soaks, pulse lavage, and negative pressure wound therapy. Mafenide is prepared as a 2.5% or 5% dilution and is used in those wounds that have positive cultures for infection or clinical signs of infection. Our group also uses a modified Dakin solution prepared as a 0.025% concentration in those wounds, where infection has been controlled but difinitive coverage has not been achieved. Mechanical debridement can also be accomplished by forceful saline irrigation such as pulse lavage. Pulse lavage can be performed in the operating room and at the bedside. It provides wound irrigation under pressure of 10-15 psi, therefore removing fibrinous debris and decreasing bacterial load.
A negative pressure device can promote enhanced vascularization of the wound bed and eliminates the need for daily dressing changes in adequately debrided wounds. Subsequent dressing changes allow for removal of debris and reduction of bacterial load. Employing a clean technique during negative pressure wound therapy dressing change is essential to avoid cross-contamination. Our surgical group favors negative pressure wound therapy for deep recessed wounds such as those in the perineum, groin, axilla, and pannus of individuals who are morbidly obese. Once the wound has been stabilized and no signs of infection are present, the wound may be assessed for the possibility of a more definitive closure.
Wound dressings should promote mobility of the patient to prevent nosocomial complications such as pneumonia, urinary tract infections, and venous thrombotic events to prevent drying of the wound and to minimize the health care costs. Attention should be paid to ensure that the dressing does not impede the range of motion of the joint or extremity and that it does not pull the tissue into a position of deformity. Having a large wound should not be a contraindication to mobilization. Similar to a patient with extensive burn injuries, we favor early mobilization of the patient with NSTI and involve physical and occupational therapy teams as soon as the resuscitation phase is complete.
One of the main deterrents of optimal wound care is pain. Patients often undergo multiple, painful, and anxiety-provoking procedures during wound care and rehabilitation for NSTIs. Achieving the best results requires frequent evaluation and assessment of pain and anxiety along with the administration of appropriate pharmacologic and nonpharmacologic therapies. Pharmacologic therapies are the backbone of analgesia and sedation, whereas nonpharmaco-logic therapies such as virtual reality, relaxation techniques, distraction interventions, music, massage, and hypnosis are adjuncts to achieving adequate pain relief. Clinicians must assess pain intensity frequently with an age-appropriate validated pain measurement tool. The tools used for the assessment of severity of pain, self-report systems such as the visual analog scale, Faces Pain Scale, or numeric rating scale, should be matched to the patients' age and sociocultural and educational status (Fig). Repeated assessments help in formulating an effective treatment plan for the different types of pain.
Fig.
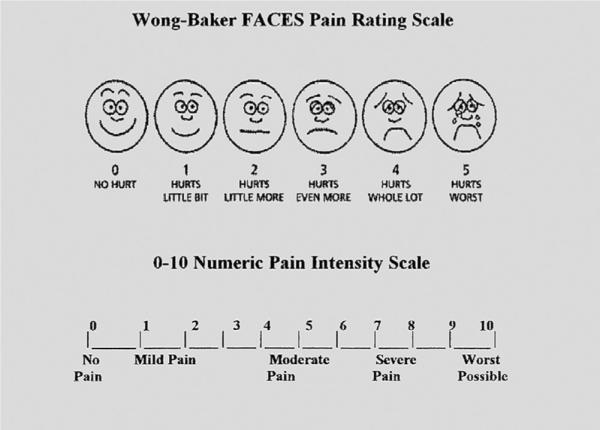
Visual analog scale and numeric pain rating systems.
Pain can be divided into 3 types including background pain, breakthrough pain, and procedural pain. Each type of pain must be addressed separately. Background pain is relatively constant and may start even before diagnosis of NSTI. The pain is mild to moderate and is best treated with a long-acting narcotic such as methadone to maintain a sustained systemic concentration. Breakthrough pain is a transient worsening of background pain often provoked by a movement or by emotions. It should be treated with the addition of short-acting narcotics, as a rescue-dose, an example being morphine or oxycodone. It is our preference to avoid combination narcotics that incorporate acetaminophen or NSAIDs into the same pill. We can then increase the dose of narcotics without being limited by acetaminophen or NSAIDs. We often add acetaminophen or NSAIDs as an adjunct owing to their synergistic effects with opiates. Procedural pain is the most intense pain often described as burning and stinging in character. Wound debridements, dressing changes, and physiotherapy often provoke this intense pain, leading to significant anxiety and distress. Fentanyl in combination with morphine or oxycodone can be used to relieve procedural pain along with a short-acting anxiolytic such as midazolam or lorazepam. A key point is separating a patient's pain from the anxiety of wound care while providing medication to relieve both. Patients may be discharged home on a combination of narcotics to address each type of pain. A thorough weaning protocol is provided for each patient upon discharge, remembering that these patients often require much higher doses than the typical surgical patient.
Gabapentin has been identified in small, randomized clinical studies to decrease acute pain and is noted to have an opioid-sparing effect.62 Gabapentin is an anticonvulsant and is used to relieve pain in postherpetic neuralgia. It has recently established its efficacy in the reduction of burn-induced hyperalgesia and allodynia. Our institution uses gabapentin as an adjunct to opioids in the management of neuropathic pain following major surgical debridements or amputation in both patients with burn injuries and patients with NSTI.
Wound coverage and reconstruction
Definitive coverage of the soft tissue defects focuses on achieving wound closure while minimizing donor site morbidity. The decision-making process is based on the anatomical location of the defect and donor site availability. Traditionally, reconstructive options have included primary closure, split-thickness skin grafts, full-thickness skin grafts, delayed primary closure vs healing by secondary intention, tissue expansion, and pedicled or free flaps. Fortunately, most NSTI cases do not require the surgeon to reach the upper rungs of the reconstructive ladder. In many instances, skin grafting and local tissue rearrangements are sufficient to achieve wound closure. As such, we believe that general surgeons can play an important role in the reconstruction for patients with NSTI, while collaborating with plastic surgery colleagues in cases where more advanced reconstructive techniques are required. It is also important to remember that a well-designed amputation often achieves a faster recovery and return to function than hopeless serial attempts at limb salvage where debridements to achieve source control exposes extensive neurovascular and bony structures. However, each option in the ultimate decision for coverage must be weighed based on the anatomical constraints, the patient's preferences, and the local resources and skill sets available.
As the excised defects become larger, temporary skin substitutes such as porcine skin xenografts and cadaveric skin allografts provide an important role in wound control. Use of temporary skin substitutes decreases wound colonization and prepares the wound bed for definitive closure.63 They allow for immediate tissue coverage to reduce fluid, protein, and electrolyte loss, protection from microbial invasion, less painful wound care, and permit early mobilization. Temporary coverage is also prudent as many of these devastating soft tissue defects leave exposed neurovascular and vital structures that are prone to desiccation and rupture. If the patient is unstable, the wound is heavily contaminated, or the wound bed is questionable for viability, we favor porcine xenograft placement as a temporary measure. Porcine skin xenografts provide a biological coverage to the wound that is cheap and readily available. After pigskin placement, nurses are asked to soak the outer dressings with Dakin solution every 3-4 hours. If there is a question of continued infection, mafenide soaks may be substituted or used on an alternating basis for antimicrobial coverage. The temporary skin substitute may be left in place until the patient is stabilized and further surgical interventions can be scheduled. If the pigskin xenograft is left in place for more than 7-10 days, it tends to incorporate into the underlying granulation tissues and further excision may be required to achieve a wound bed that is free of xenograft tissue. AlloDerm and Integra are sometimes used as well. These dermal substitutes may play an adjunctive role in optimizing the wound surface for eventual skin grafting. AlloDerm can also be used as a temporary dressing to protect exposed neurovascular structures before definitive reconstruction. The underlying wound bed is allowed to granulate and before the definitive closure, the AlloDerm is completely removed or used to bridge the large tissue defects. Integra is a bilayer matrix wound dressing composed of a silicone layer over a collagen-based dermal analogue. It mimics the structure of normal dermis and acts as a template for fibroblasts to lay down collagen. The thin sheet of silicone provides a temporary fluid barrier and is meshed 1:1 to allow for the exudate to escape. After the application of Integra, the patient's native fibroblasts, macrophages, and lymphocytes infiltrate, and there is new capillary growth in the matrix of the inner layer. The inner layer becomes degraded, and an endogenous collagen matrix is deposited by the patient's fibroblasts, forming a “neodermis.” The Integra is evaluated for engraftment and after 10-14 days, the silicone layer is removed and a split-thickness skin graft may be placed over the neodermis. Integra usage allows for placement of an ultrathin skin graft over it, reducing the chance of scarring and promotes rapid healing in the donor sites. It also gives a more cosmetically pleasing appearance to the grafted skin, while providing pliability to the overlying epidermis.
The functional goal of the reconstructed tissue is critical in choosing the correct tissue coverage. Skin grafts are often the most convenient option but may be limited by donor site availability or questionable underlying wound bed viability. Choosing the right skin graft can also be a challenge for surgeons inexperienced with skin grafting. Thinner skin grafts have a higher take rate, have a faster donor site healing, but are more fragile in the immediate period, whereas a thicker skin graft often results in a better color match and contracts less over time. A meshed skin graft conforms to the wound bed, allows exudates to escape from the tissue, and covers a large area of defect while a sheet graft may be more cosmetically pleasing. Full-thickness skin grafts must be defatted and can only be used for small tissue defects, as donor sites are limited. Partial graft failure can have significant deleterious effects and result in further grafting or healing by secondary intention. These may result in additional scarring and deformity. Genitourinary defects constitute a special circumstance that is best addressed in a multidisciplinary manner. Unique decisions such as whether to perform skin grafting on denuded testicles vs relocating them into the thigh, long-term follow-up to assure best outcomes in voiding, and sexual function require the expertise of urologic surgeons. When possible, primary closure for small- to moderate-size wounds often results in the most cosmetically pleasing appearance with the fastest coverage. Rearrangement of local skin or soft tissue flaps for coverage is also possible if a well-vascularized flap has been preserved during the debridement procedures. Flap preservation can minimize the extent of skin grafting necessary, or sometimes avoid skin grafting altogether. We have found that this is at times possible for NSTI cases involving the trunk, groins, and perineum. In cases in which extremity amputation is necessary, preserving a myofascial cutaneous skin flap from healthy soft tissue at the time of amputation will allow for pedicle flap coverage soon after wound control is achieved. Techniques in the higher rungs of the reconstruction ladder include tissue expansion and free flaps. Tissue expansion is a valuable reconstruction technique, although seldom used in the acute setting because of the concern for infection in wounds already colonized with bacteria. Free tissue transfers may sometimes become necessary and their need should be identified in conjunction with plastic surgeons. Complex defects that involve bony and neurovascular structures also require the expertise of a multidisciplinary surgical team.
Proposal for a system of care to optimize outcomes
The creation of integrated and regionalized trauma systems has increased access and improved survival for all trauma victims, yet there has been no research to specifically address the subset of patients with NSTI. Recent literature has shown that most (87.1%) patients with NSTI receive their definitive care at nonreferral centers.64 Survival is frequently the primary outcome of interest but it may no longer be a sufficient indicator of quality care. Current research indicates that care processes and posthospital outcomes, including health-related quality of life (HRQOL), long-term effects on family and care-takers, and psychosocial recovery may be better overall quality care measures for patients with NSTI. A multidisciplinary team approach such as those modeled after the burn center model of care focuses not only on survival but also on long-term functional outcomes.
Critical care expertise is mandatory in the management of patients with NSTI along with access to resources for proper rehabilitation after recovery from their acute illness. The multidisciplinary team includes providers, such as critical care specialists, surgeons, and rehabilitation medicine physicians. Nurse educators oversee nurses with specialized training in critical care and wound care needs, along with pharmacists, dieticians, and therapist or counselors. Individual needs are addressed from a physical and occupational therapy, speech, and child life standpoint, whereas social workers, discharge nurses, and vocational rehabilitation counselors help reintegrate patients into society. Reintegration into society can be a challenge after a long hospitalization and recovery for patients with NSTI. Motor, cognitive, and sometimes behavioral functions can be impaired owing to extensive soft tissue defects. Inpatient rehabilitation evaluation and treatment focus on long-term outcomes as indicators of recovery, rehabilitation, and reintegration. Spiritual guidance is also made available for patients and family. Based on the specific needs of this subset of patients with traumatic injuries, a multidisciplinary approach to patient care should be adopted to address not only the medical needs, but also the quality of life in survivors of NSTI.
Rehabilitation
After an extended hospitalization, multiple dressing changes and surgical procedures, the survivor of NSTI faces months of continued physical therapy to regain functional independence. Strong similarities exist between the rehabilitative needs of the patients with NSTI and the patients with burn injuries. The term “burn rehabilitation” incorporates the physical, psychological, and social aspects of care for the patient with burn injuries. This approach can be broadened to the patient with NSTI as rehabilitation is an essential and integral component of recovery. The team must begin planning therapeutic interventions during the initial stabilization phase to maximize functional recovery. For instance, limb-threatening NSTIs present patients and clinicians with a difficult decision: whether to pursue primary amputation or limb salvage. If the infection is severe, life over limb prevails. Busse and colleagues65 published a meta-analysis of observational studies comparing complex limb salvage or early amputation and found that the functional outcomes at 7 years were not significantly different. Length of hospital stay was similar between the 2 groups, whereas limb-salvage patients had longer lengths of rehabilitation and higher total costs. Stineman and colleagues recently published their findings comparing the timing of rehabilitation services after major lower limb amputations.66 Using the Functional Independence Measure gain scores, the authors reported that patients who received specialized rehabilitation had better recovery of activities of daily living and mobility compared with amputees who received only consultative rehabilitation. As such, rehabilitation specialists can play an important role in coordinating support and specialist services required to permit community reintegration. The aims of the rehabilitation team were to minimize the adverse effects caused by the injury in terms of healing the wound, minimizing the development and effect of scarring, maximizing functional outcomes, and providing support to maximize psychological well-being and reintegration into society. Table 3 summarizes important conditions and sequelae of NSTI that must be addressed during the rehabilitation phase.
Table 3.
Important factors contributing to rehabilitation needs and treatment options
| Condition | Complications and rehabilitation needs | Treatment options |
|---|---|---|
| Impaired or delayed wound healing | Chronic open wounds | Wound care Pain control |
| Scarring | Contractures Disfigurement |
Scar massages Exercises or outpatient therapy splinting Surgical release Cosmetic consultation |
| Lymphatic interruption | Lymphedema | Compression therapy Elevation |
| Amputation | Functional impairment Residual limb problems Phantom limb pain |
Adaptation or assist devices Occupational or physical therapy Prosthesis training Surgical revision Long-term pain management |
| Nerve damage | Pruritus Spasticity Chronic pain |
Medications Massage or desensitization Stretching and splinting |
| Prolonged critical illness | Post-intensive care unit syndrome Loss of lean body mass Weakness Fatigue or lack of endurance |
Screening for cognitive deficits Resistance and aerobic exercise Nutrition consultation Anabolic agents (potential role) |
| Prolonged immobility | Deep venous thrombosis Pressure sores Edema Disuse Heterotopic ossification |
Early and aggressive mobilization Thromboprophylaxis Nonsteroidal anti-inflammatory and bisphosphonate agents Surgical excision or radiation therapy |
| Pain | Chronic pain Neuropathic pain |
Long-term multimodality pain management |
| Distress | Depression Sleep disturbances Sexual dysfunction Posttraumatic stress Lack of psychosocial support Alcohol and drug abuse |
Routine screening Medications Counseling Family or spousal training Support group(s) |
| Loss of work | Chronic unemployment | Vocational rehabilitation |
Challenges to implementation
Even from the earliest reports of the disease, it has been repeatedly observed that early surgical fasciotomy and debridement is essential to an efficient, effective treatment.20,67 Delay of more than 12 hours to initial debridement has been associated with increased number of surgical debridements and a higher incidence of septic shock and acute renal failure.68 Wong and colleagues, who developed the LRINEC score, reported a 9-fold increased risk of mortality if treatment was initiated more than 24 hours after diagnosis.37 But lack of experience may lead to failure to recognize the severity of the disease, or to influence a surgeon's willingness to perform radical debridement, leading to transfer of the patient to a referral center.
Although transfer seems like the most appropriate course of action, particularly if a surgeon is not available at the initial evaluation, the influence on the timing of debridement is notable. Wall at Harbor-UCLA, Los Angeles reported a 21 matched consecutive case series of necrotizing fasciitis vs cellulitis or subcutaneous abscess controls and found a 29% vs 0% mortality, respectively.29 Among patients transferred from other hospitals there was a 10-fold risk of mortality, compared with those treated initially at University of California, Los Angeles. Transfers had an average delay of 5.8 hours, primarily driven by initial misdiagnosis of cellulitis. A follow-up study comparing survivors with nonsurvivors of NSTI treated at the same institution revealed that the median time to surgical excision was 8 hours from emergency department arrival.69 The authors noted that there was a higher mortality rate among patients who were transferred in from other institutions, and they cited the role of the misdiagnosed “shooter's abscess” as the reason. An analysis of burn vs nonburn center NSTI outcomes using the National Inpatient Sample from 2001 and 2004 established that most patients with NSTI were treated at a nonburn center. Those treated at burn centers were twice as likely to have been transferred in from another hospital and that transfer independently predicted a 28% increased risk of mortality. In 2011, Holena and colleagues used the National Inpatient Sample to examine NSTIs debrided within 72 hours, comparing patients with NSTI who underwent surgery after transfer with those debrided at the index admission hospital. Controlling for comorbidities, but not acute physiological derangement, the authors found that transfer independently predicted mortality.70 Large, teaching hospitals, with high surgical volume were also found to be associated with increased morality. Significant variation in the management of NSTI and mortality was demonstrated across 6 academic centers in Texas, where mortality rate ranged from 9%-25%. Nonprofit hospitals had higher rate of transfers patients and higher mortality rates.
Although the data makes it clear that transfer—when it delays surgical debridement—is strongly associated with increased mortality, it must be pointed out that many centers are not equipped to provide the level of care that patients with NSTIs require. Thus, transfer to referral centers is likely necessary and prudent following initial debridement. The complex requirements of intensive care early in the disease process, and the coordination requirements between surgical, nursing, and rehabilitative therapy services during the recovery process are best met by larger tertiary care centers. We feel strongly that this multidisciplinary approach maximizes the potential for early survival and good functional long-term outcomes.
In-hospital and long-term outcomes
Historically, in-hospital mortality rates have been the dominant outcome measure assessed not only for patients with NSTI. It has been said that the goal of a mature trauma system—in our opinion the goal of any mature system of care—should not simply be to achieve survival upon discharge, but rather the reintegration of patients into their respective communities with successful return to work and activities of daily life.71
Only modest improvements have been made in the overall mortality rate since Dr Jones reported a 46% mortality rate associated with hospital gangrene in 1871. Most modern series report in-hospital or 30-day mortality rates between 9% and 76%, with average rates between 20% and 40%.8,19-21,53,67,72
Several studies have assessed patient- and treatment-related risk factors for NSTI-associated mortality. Important risk factors described have been time to antimicrobial therapy, time to debridement, hospital transfer status, treatment setting, patients' age, comorbidities, socioeconomic status, microbiologic etiology, and extent of disease.19,30,53,67-70,72-77 Early diagnosis and early complete surgical debridement have repeatedly been shown to improve outcomes.53,67,70,78
Anaya and colleagues79 reported the multicenter development of a clinical score, based on factors known at time of admission, and predicts risk of death. They stratified patients into 3 groups based on the presence of the following criteria: heart rate >110 bpm, temperature <36°C, serum creatinine level >1.5 mg/dL, older than 50 years, WBC >40,000 per μL, and hematocrit >50%. Patients with few of these findings had a 6% risk of death; patients with several findings had as high as an 88% risk of death. This simple tool helps to assess prognosis and can be used in decision making surrounding transfer of patients and aggressiveness of surgical debridement, the inclusion and use of adjunctive treatment modalities, and in family discussions to help better describe likely outcomes.
Posthospital outcomes
Little is known about posthospital outcomes for survivors of NSTIs. To date, only a single published study analyzed long-term mortality rates for survivors of NSTI. Comparing survivors of NSTI from a single large burn and trauma center with age- and gender-matched controls from the same region, Light and colleagues80 found that survivors of NSTI had markedly higher rates of death. Using information from death certificates, they also found that a significantly higher proportion of deaths in the cohort with NSTI were due to infectious causes.
Just as survivors of NSTI appear to be at an increased risk for long-term mortality, because of their often prolonged hospitalization and propensity to have complex wounds or amputations or both, their physical status and limitations are likely important for long-term functional outcomes. Because of similarities between survivors of severe burns and survivors of NSTI, Pham and colleagues81 adapted the impairment rating scale, which had been validated among survivors of burn injuries, to quantify the physical limitations of survivors of NSTI. They found that 30% of survivors had mild to severe functional limitation at time of discharge. They also found that extremity involvement was significantly associated with increased functional impairment, but that extremity amputation was not, suggesting that factors such as pain or decreased range of motion (ROM) either from wound or graft retraction that lead to functional impairment. The study was limited in that it only assessed impairment at time of discharge but did not include long-term follow-up and assessment. This study population was also limited to survivors of NSTI who were managed in the recovery phase by a plastic surgery service, most of whom had some type of wound coverage procedure. Although this limits the generalizability of their findings, the importance of assessing long-term functional status is important to understanding long-term outcomes for survivors of NSTIs.
We have begun a multiphase study with long-term follow-up of survivors of NSTI from our institution. Qualitative interviews with survivors identified several themes important to the individual's perceptions of recovery. These included areas including relationship stresses, physical functioning, depression, posttraumatic stress disorder (PTSD), and employment concerns, with the latter 3 themes being important in the patient but also in the spouse or partner when applicable. Information gained from these in-depth interviews allowed us to survey survivors of NSTIs and assess domains identified as important by patients themselves. A pilot study was performed in 2012-2013 with 20 consecutive survivors of NSTI from our institution. Follow-up was obtained at 6-, 12-, and 18-month intervals. We used the Short Form 36 (SF-36) instrument because it addresses many of the domains of physical, mental, and social function identified by survivors of NSTI. We augmented the SF-36 with questions regarding depressive and posttraumatic symptoms as well as questions about return to previous level of employment. These additional questions were asked not only of the survivor, but also of their spouse or partner when applicable. Early data showed that survivors of NSTI had decreased HRQOL and significant impairments in physical, emotional, and social functioning.82,84 There is also an increased prevalence of PTSD in both the patient and their partners. A large proportion of survivors are unable to return to previous employment. Decreases in HRQOL appear to stabilize by 12 months but persist out to 18 months following admission.
We are currently completing follow-up with a significantly larger cohort of survivors. Surveys of 202 survivors between 3 and 7 years after discharge have demonstrated lower summary scores for physical and mental domains compared with the general population.83 Subscores were lower in all 8 domains of the SF36v2. There is a high incidence of depression and PTSD symptoms. Overall, 48% (95% CI: 37%-59%) of patients were unable to return to previous level of employment. Upper extremity amputation, greater than 5 debridements, greater than 10 intensive care unit days, renal failure without return of function before discharge, and involvement of the hand or face were independently associated with poor HRQOL. Wound coverage procedure, less than 3 debridements, and involvement of the trunk or perineum were independently associated with better HRQOL.
Conclusion
NSTI is an increasingly common problem, often characterized by progressive and potentially fatal soft tissue infection that requires prompt, radical, and often multiple surgical debridement of all involved tissue. Early diagnosis of the presence of a NSTI is critical if optimal outcomes are to be achieved. Treating physicians must be familiar with the sign and symptoms of the disease and maintain a high index of suspicion. Any delay in diagnosis is potentially catastrophic, as the concomitant delay in surgical therapy significantly increases mortality. Patient care requires close coordination between surgical and critical care teams. Radical surgical debridement of all necrotic tissue remains the mainstay of treatment, and further research is required before any strong recommendations can be made about advanced adjunctive therapies. The resultant massive soft tissue defect requires special attention and can present significant challenges. Early principles of reconstruction after NSTI focuses on preservation of the skin, local wound control, and preparation for anatomically based reconstruction. This may require assistance from plastic and reconstructive surgeons in concert with strong support from rehabilitative services in the hospital and posthospital setting. Despite strong advances in critical care medicine over the last few decades, NSTIs still portend a very high case mortality rate and survivors are at a significant risk of long-term morbidity, which we are only now beginning to understand.
Contributor Information
Timo W. Hakkarainen, University of Washington Seattle, Washington.
Nicole M. Kopari, Department of Surgery University of Washington Seattle, Washington.
Tarn N. Pham, UW Burn Center University of Washington Seattle, Washington.
Heather L. Evans, Director of Surgical Infectious Desease Harborview Medical Center University of Washington Seattle, Washington.
References
- 1.Descamps V, Aitken J, Lee M. Hippocrates on necrotizing fasciitis. Lancet. 1994;344:556. doi: 10.1016/s0140-6736(94)91956-9. [DOI] [PubMed] [Google Scholar]
- 2.Loudon I. Necrotizing fasciitis, hospital gangrene, and phagedena. Lancet. 1994;344:1416–1419. doi: 10.1016/s0140-6736(94)90574-6. [DOI] [PubMed] [Google Scholar]
- 3.Jones J. Surgical Memoirs of the War of the Rebellion. Investigation Upon the Nature, Causes, and Treatment of Hospital Gangrene as Prevailed in the Confederate Armies 1861-1865. United States Sanitary Commission; New York, NY: p. 1871. [Google Scholar]
- 4.Sarani B, Strong M, Pascual J, Schwab CW. Necrotizing fasciitis: current concepts and review of the literature. J Am Coll Surg. 2009;208(2):279–288. doi: 10.1016/j.jamcollsurg.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 5.Wilson B. Necrotising fasciitis. Am Surg. 1952;18:416–431. [PubMed] [Google Scholar]
- 6.Simonsen E, Orman E, van Hatch B, et al. Cellulitis incidence in a defined population. Epidemiol Infect. 2006;134(2):293–299. doi: 10.1017/S095026880500484X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salcido R. Necrotizing fasciitis: reviewing the causes and treatment strategies. Adv Skin Wound Care. 2007;20(5):288–293. doi: 10.1097/01.ASW.0000269317.76380.3b. [DOI] [PubMed] [Google Scholar]
- 8.Kao LS, Lew DF, Arab SN, et al. Local variations in the epidemiology, microbiology, and outcome of necrotizing soft-tissue infections: a multicenter study. Am J Surg. 2011;202(2):139–145. doi: 10.1016/j.amjsurg.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simon L, Mills J. Non-steroidal antiinflammatory drugs. N Engl J Med. 1980;302:1179–1185. doi: 10.1056/NEJM198005223022105. [DOI] [PubMed] [Google Scholar]
- 10.Horan T, Noujaim A, McPherson T. Effecto findomethacin on human neutrophil chemiluminescence and microbicidal activity. Immunopharmacology. 1983;6:97–106. doi: 10.1016/0162-3109(83)90003-6. [DOI] [PubMed] [Google Scholar]
- 11.Abramson S, Edelson H, Kaplan Z, Ludewig R, Weissmann G. Inhibition of neutrophil activation by nonsteroidal antiinflammatory drugs. Am J Med. 1984;77:3–6. doi: 10.1016/s0002-9343(84)80085-6. [DOI] [PubMed] [Google Scholar]
- 12.Stevens DL. Could nonsteroidal antiinflammatory drugs (NSAIDs) enhance the progression of bacterial infections to toxic shock syndrome?. Shock. 2013;21(4):977–980. doi: 10.1093/clinids/21.4.977. [DOI] [PubMed] [Google Scholar]
- 13.Busti AJ, Hooper JS, Amaya CJ, Kazi S. Effects of perioperative antiinflammatory and immunomodulating therapy on surgical wound healing [internet]. Pharmacotherapy. 2005;25(11):1566–1591. doi: 10.1592/phco.2005.25.11.1566. [DOI] [PubMed] [Google Scholar]
- 14.Haws M, Kucan J, Roth A, Suchy H, Brown R. The effects of chronic ketorolac tromathamine (Toradol) on wound healing. Ann Plast Surg. 1996;37:147–151. doi: 10.1097/00000637-199608000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Dong Z, Declan F, Yan T, Herndon D, Waymack J. Effect of ibuprofen on the inflammatory response to surgical wounds. J Trauma. 1993;35:340–343. doi: 10.1097/00005373-199309000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Riley G, Cox M, Harrall R. Inhibition of tendon cell proliferation and matrix glycosaminoglycan synthesis by nonsteroidal anti-inflammatory drugs in vitro. J Hand Surg Br. 2001;26:224–228. doi: 10.1054/jhsb.2001.0560. [DOI] [PubMed] [Google Scholar]
- 17.Stevens D. Streptococcal toxic-shock syndrome: spectrum of disease, pathogenesis, and new concepts in treatment. Emerg Infect Dis. 1995;1(3):69. doi: 10.3201/eid0103.950301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giuliano A, Lewis F, Hadley K, Blaisdell F. Bacteriology of necrotizing fasciitis. Am J Surg. 1977;134(1):52–57. doi: 10.1016/0002-9610(77)90283-5. [DOI] [PubMed] [Google Scholar]
- 19.Anaya DA, Sullivan SR, Foy H, Bulger E. Predictors of mortality and limb loss in necrotizing soft tissue infections. Arch Surg. 2005;140:151–157. doi: 10.1001/archsurg.140.2.151. [DOI] [PubMed] [Google Scholar]
- 20.Freischlag J, Ajalat G, Busuttil R. Treatment of necrotizing soft-tissue infections. Am J Surg. 1985;149:751–755. doi: 10.1016/s0002-9610(85)80180-x. [DOI] [PubMed] [Google Scholar]
- 21.Miller L, Carrick M, Scott B, Hodges J, Pham H. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcal aureus in Los Angeles. N Engl J Med. 2005;352(14):1445–1453. doi: 10.1056/NEJMoa042683. [DOI] [PubMed] [Google Scholar]
- 22.Bryant AE, Stevens DL. Clostridial myonecrosis: new insights in pathogenesis and management. Curr Infect Dis Rep. 2010;12(5):383–391. doi: 10.1007/s11908-010-0127-y. [DOI] [PubMed] [Google Scholar]
- 23.Stevens DL, Aldape MJ, Bryant AE. Life-threatening clostridial infections. Anaerobe. 2011;18(2):254–259. doi: 10.1016/j.anaerobe.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Shiroff A, Herlitz G, Gracias V. Necrotizing soft tissue infections. J Intensive Care Med. 2014;29(3):138–144. doi: 10.1177/0885066612463680. [DOI] [PubMed] [Google Scholar]
- 25.Umbert I, Oliver G, Winkelmann R, Peters M. Necrotizing fasciitis: a clinical, microbiological and histopathologic study of 14 patients. J Am Acad Dermatol. 1989;20(5):774–781. doi: 10.1016/s0190-9622(89)70089-x. [DOI] [PubMed] [Google Scholar]
- 26.Howard R, Pessa M, Brennaman B, Ramphal R. Necrotizing soft-tissue infections caused by marine vibrios. Surgery. 1985;98(1):126–130. [PubMed] [Google Scholar]
- 27.Goodell K, Jordan M, Graham R, Cassidy C, Nasraway S. Rapidly advancing necrotizing fasciitis caused by Phytobacterium (Vibrio) damsela: a hyperaggressive variant. Crit Care Med. 2004;32(1):278–281. doi: 10.1097/01.CCM.0000104920.01254.82. [DOI] [PubMed] [Google Scholar]
- 28.Wall D, Kleain S, Black S, de Virgilio C. A simple model to help distinguish necrotizing from non-necrotizing soft-tissue infections. J Am Coll Surg. 2000;191(3):227–231. doi: 10.1016/s1072-7515(00)00318-5. [DOI] [PubMed] [Google Scholar]
- 29.Wall D, de Virgilio C, Black S, Klein S. Objective criteria may assist in distinguishing necrotizing fasciitis from nonnecrotizing sift-tissue infections. Am J Surg. 2000;179(1):17–21. doi: 10.1016/s0002-9610(99)00259-7. [DOI] [PubMed] [Google Scholar]
- 30.Wong C, Khin L, Heng K, Tan K, Low C. The LRINEC (laboratory risk indicator for necrotising fasciitis) score: a tool for distinguishing necrotising fasciitis from other soft-tissue infections. Crit Care Med. 2004;32(7):1535–1541. doi: 10.1097/01.ccm.0000129486.35458.7d. [DOI] [PubMed] [Google Scholar]
- 31.Arslan A, Pierre-Jerome C, Borthne A. Necrotising fasciitis: unreliabile MRI findings in the preoperative diagnosis. Eur J Radiol. 2000;36:139–143. doi: 10.1016/s0720-048x(00)00164-9. [DOI] [PubMed] [Google Scholar]
- 32.Loyer E, DuBrow R, David C. Imaging of superficial soft-tissue infections: sonographic findings in cases of cellulitis and abscess. Am J Roentgenol. 1996;166:149–152. doi: 10.2214/ajr.166.1.8571865. [DOI] [PubMed] [Google Scholar]
- 33.Keung E, Liu X, Nuzhad A, Adams C, Ashley S, Askari R. Immunocompromised status in patients with necrotizing soft-tissue infections. JAMA Surg. 2013;148(5):419–426. doi: 10.1001/jamasurg.2013.173. [DOI] [PubMed] [Google Scholar]
- 34.Childers B, Potyondy L, Nachreiner R. Necrotizing fasciitis: a fourteen-year retrospective study of 163 consecutive patients. Am Surg. 2002;68(2):109–116. [PubMed] [Google Scholar]
- 35.Voros D, Pissiotis C, Georgantas D, Al E. Role of early and extensive surgery in the treatment of severe necrotizing soft tissue infection. Br J Surg. 1993;80:1190–1191. doi: 10.1002/bjs.1800800943. [DOI] [PubMed] [Google Scholar]
- 36.Bilton B, Zibari B, McMillan R, Al E. Aggressive surgical management of necrotizing fasciitis serves to decrease mortality: a retrospective study. Am Surg. 1998;64:397–400. [PubMed] [Google Scholar]
- 37.Wong C, Haw-Chong C, Shanker P. Necrotizing fasciitis: clinical presentation, microbiology, and determinants of mortality. J Bone Joint Surg Am. 2003;85:1454–1460. [PubMed] [Google Scholar]
- 38.Mok M, Wong S, Chan T. Necrotizing fasciitis in rheumatic diseases. Lupus. 2006;15:380–383. doi: 10.1191/0961203306lu2314cr. [DOI] [PubMed] [Google Scholar]
- 39.Gemmell C, Peterson P, Schmeling D. Potentiation of opsonization and phagocytosis of Streptococcus Pyogenes following growth in the presence of clindamycin. J Clin Invest. 1981;67(5):1249–1256. doi: 10.1172/JCI110152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stevens D, Meier K, Mitten J. Effect of antibiotics on toxin production and viability of Clostridium perfringens. Antimicrob Agents Chemother. 1987;31(2):213–218. doi: 10.1128/aac.31.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stevens D, Bryant A, Hackett S. Antiobiotic effects on bacterial viability, toxin production, and host response. Clin Infect Dis. 1995;20(suppl 2):S154–S157. doi: 10.1093/clinids/20.supplement_2.s154. [DOI] [PubMed] [Google Scholar]
- 42.Kaye D. Effect of hyperbaric oxygen on aerobic bacteria in vitro and in vivo. Proc Soc Exp Biol Med. 1967;124(4):1090–1093. doi: 10.3181/00379727-124-31932. [DOI] [PubMed] [Google Scholar]
- 43.Park MK, Muhvich KH, Myers RA, Marzella L. Hyperoxia prolongs the aminoglycoside-induced postantibiotic effect in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1991;35(4):691–695. doi: 10.1128/aac.35.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mader JT, Brown GL, Guckian JC, Wells CH, Reinarz JA. A mechanism for the amelioration by hyperbaric oxygen of experimental staphylococcal osteomyelitis in rabbits. J Infect Dis. 1980;142(6):915–922. doi: 10.1093/infdis/142.6.915. [DOI] [PubMed] [Google Scholar]
- 45.Knighton DR, Halliday B, Hunt TK. Oxygen as an antibiotic. A comparison of the effects of inspired oxygen concentration and antibiotic administration on in vivo bacterial clearance. Arch Surg. 1986;121(2):191–195. doi: 10.1001/archsurg.1986.01400020077009. [DOI] [PubMed] [Google Scholar]
- 46.Korhonen K, Kuttila K, Niinikoski J. Tissue gas tensions in patients with necrotising fasciitis and healthy controls during treatment with hyperbaric oxygen: a clinical study. Eur J Surg. 2000;166(7):530–534. doi: 10.1080/110241500750008583. [DOI] [PubMed] [Google Scholar]
- 47.Wilkinson D, Doolette D. Hyperbaric oxygen treatment and survival from necrotizing soft tissue infection. Arch Surg. 2004;139:1339. doi: 10.1001/archsurg.139.12.1339. [DOI] [PubMed] [Google Scholar]
- 48.Pizzorno R, Bonini F, Donelli A. Hyperbaric oxygen therapy in the treatment of Fournier's disease in 11 male patients. J Urol. 1997;158:837. doi: 10.1097/00005392-199709000-00039. [DOI] [PubMed] [Google Scholar]
- 49.Escobar S, Slade J, Jr, Hunt T. Adjuvant hyperbaric oxygen therapy (HBO2) for treatment of necrotizing fasciitis reduces mortality and amputation rate. Undersea Hyperb Med. 2005;32:437. [PubMed] [Google Scholar]
- 50.Riseman J, Zamboni W, Curtis A. Hyperbaric oxygen therapy for necrotizing fasciitis reduces mortality and the need for debridements. Surgery. 1990;108:847. [PubMed] [Google Scholar]
- 51.George ME, Rueth NM, Skarda DE, Chipman JG, Quickel RR, Beilman GJ. Hyperbaric oxygen does not improve outcome in patients with necrotizing soft tissue infection. Surg Infect. 2009;10:21–28. doi: 10.1089/sur.2007.085. [DOI] [PubMed] [Google Scholar]
- 52.Brown DR, Davis NL, Lepawsky LM, Cunningham J, Kortbeek J. A multicenter review of the treatment of major truncal necrotizing infections with and without hyperbaric oxygen therapy. Am J Surg. 1994;167:485. doi: 10.1016/0002-9610(94)90240-2. [DOI] [PubMed] [Google Scholar]
- 53.Elliot D, Kufera J, Myers R. Necrotizing soft tissue infections: risk factors for mortality and strategies for management. Ann Surg. 1996;224:672. doi: 10.1097/00000658-199611000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shupak A, Shoshani O, Goldenberg I. Necrotizing fasciitis: an indication for hyperbaric oxygenation therapy? Surgery. 1995;118:873. doi: 10.1016/s0039-6060(05)80278-8. [DOI] [PubMed] [Google Scholar]
- 55.Massey PR, Sakran JV, Mills AM, et al. Hyperbaric oxygen therapy in necrotizing soft tissue infections. J Surg Res. 2012;177(1):146–151. doi: 10.1016/j.jss.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 56.Takei S, Arora Y, Walker S. Intravenous immunoglobulin contains specific antibodies inhibitory to activation of T-cells by staphylococcal toxin superantigens. J Clin Invest. 1993;91:602–607. doi: 10.1172/JCI116240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Norrby-Teglund A, Kaul R, Low D. Plasma from patients with severe invasive group A streptococcal infections treated with normal polyspecific IgG inhibits stretococcal superantigen-induced T cell proliferation and cytokine production. J Immunol. 1996;156:3057–3064. [PubMed] [Google Scholar]
- 58.Alejandria MM, Lansang MA, Dans LF, Mantaring JB. Intravenous immunoglobulin for treating sepsis and septic shock. Cochrane Database Syst Rev. 2002:1. doi: 10.1002/14651858.CD001090. [DOI] [PubMed] [Google Scholar]
- 59.Kaul R, McGeer A, Norrby-Teglund A, et al. Clin Infect Dis. 4. Vol. 28. The Canadian Streptococcal Study Group; 1999. Intravenous immunoglobulin therapy for streptococcal toxic shock syndrome—a comparative observational study. pp. 800–807. [DOI] [PubMed] [Google Scholar]
- 60.Lamothe F, D'Amico P, Ghosn P, Tremblay C, Braidy J, Patenaude JV. Clinical usefulness of intravenous human immunoglobulins in invasive group A streptococcal infections: case report and review. Clin Infect Dis. 1995;21(6):1469–1470. doi: 10.1093/clinids/21.6.1469. [DOI] [PubMed] [Google Scholar]
- 61.Franz MG, Robson MC, Steed DL, et al. Guidelines to aid healing of acute wounds by decreasing impediments of healing. Wound Repair Regen. 2007;16(6):723–748. doi: 10.1111/j.1524-475X.2008.00427.x. [DOI] [PubMed] [Google Scholar]
- 62.Tiippana EM, Hamunen K, Kontinen VK, Kalso E. Do surgical patients benefit from perioperative gabapentin/ pregabalin? A systematic review of efficacy and safety. Anesth Analg. 2007;104(6):1545–1556. doi: 10.1213/01.ane.0000261517.27532.80. [DOI] [PubMed] [Google Scholar]
- 63.Saymen DG, Nathan P, Holder IA, Hill EO, Macmillan BG. Control of surface wound infection: skin versus synthetic grafts. Appl Microbiol. 1973;25(6):921–934. doi: 10.1128/am.25.6.921-934.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Endorf FW, Klein MB, Mack CD, Jurkovich GJ, Rivara FP. Necrotizing soft-tissue infections: differences in patients treated at burn centers and non-burn centers. J Burn Care Res. 2004;29(6):933–938. doi: 10.1097/BCR.0b013e31818ba112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Busse JW, Jacobs CL, Swiontkowski MF, Bosse MJ, Bhandari M. Complex limb salvage or early amputation for severe lower-limb injury: a meta-analysis of observational studies. J Orthop Trauma. 2007;21(1):70–76. doi: 10.1097/BOT.0b013e31802cbc43. [DOI] [PubMed] [Google Scholar]
- 66.Stineman MG, Kwong PL, Xie D, et al. Prognostic differences for functional recovery after major lower limb amputation: effects of the timing and type of inpatient rehabilitation services in the Veterans Health Administration. PM R. 2010;2(4):232–243. doi: 10.1016/j.pmrj.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McHenry C, Piotrowski J, Petrinic D, Malangoni M. Determinants of mortality for necrotizing soft-tissue infections. Ann Surg. 1995;221:558–565. doi: 10.1097/00000658-199505000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kobayashi L, Konstantinidis A, Shackelford S, et al. Necrotizing soft tissue infections: delayed surgical treatment is associated with increased number of surgical debridements and morbidity [internet]. J Trauma. 2011;71(5):1400–1405. doi: 10.1097/TA.0b013e31820db8fd. [DOI] [PubMed] [Google Scholar]
- 69.Yaghoubian A, de Virgilio C, Dauphine C, Lewis RJ, Lin M. Use of admission serum lactate and sodium levels to predict mortality in necrotizing soft-tissue infections. Arch Surg. 2007;142(9):840–846. doi: 10.1001/archsurg.142.9.840. [DOI] [PubMed] [Google Scholar]
- 70.Holena DN, Mills AM, Carr BG, et al. Transfer status: a risk factor for mortality in patients with necrotizing fasciitis. Surgery. 2011;150(3):363–370. doi: 10.1016/j.surg.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 71.Livingston DH, Tripp T, Biggs C, Lavery RF. A fate worse than death? Long-term outcome of trauma patients admitted to the surgical intensive care unit. J Trauma. 2009;67(2):341–348. doi: 10.1097/TA.0b013e3181a5cc34. [DOI] [PubMed] [Google Scholar]
- 72.Bosshardt T, Henderson V, Organ C. Necrotizing soft-tissue infections. Arch Surg. 1996;131:846–854. doi: 10.1001/archsurg.1996.01430200056011. [DOI] [PubMed] [Google Scholar]
- 73.Phan HH, Cocanour CS. Necrotizing soft tissue infections in the intensive care unit. Crit Care Med. 2010;38(suppl 9):460–468. doi: 10.1097/CCM.0b013e3181ec667f. [DOI] [PubMed] [Google Scholar]
- 74.Mills MK, Faraklas I, Davis C, Stoddard GJ, Saffle J. Outcomes from treatment of necrotizing soft-tissue infections: results from the National Surgical Quality Improvement Program database. Am J Surg. 2010;200(6):790–796. doi: 10.1016/j.amjsurg.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 75.Chan T, Yaghoubian A, Rosing D, Kaji A, de Virgilio C. Low sensitivity of physical examination findings in necrotizing soft tissue infection is improved with laboratory values: a prospective study. Am J Surg. 2008;196(6):926–930. doi: 10.1016/j.amjsurg.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 76.Frazee BW, Fee C, Lynn J, et al. Community-acquired necrotizing soft tissue infections: a review of 122 cases presenting to a single emergency department over 12 years. J Emerg Med. 2008;34(2):139–146. doi: 10.1016/j.jemermed.2007.03.041. [DOI] [PubMed] [Google Scholar]
- 77.Huang K-F, Hung M-H, Lin Y-S, et al. Independent predictors of mortality for necrotizing fasciitis: a retrospective analysis in a single institution. J Trauma. 2011;71(2):467–473. doi: 10.1097/TA.0b013e318220d7fa. [DOI] [PubMed] [Google Scholar]
- 78.Boyer A, Vargas F, Coste F, et al. Influence of surgical treatment timing on mortality from necrotizing soft tissue infections requiring intensive care management. Intensive Care Med. 2009;35(5):847–853. doi: 10.1007/s00134-008-1373-4. [DOI] [PubMed] [Google Scholar]
- 79.Anaya DA, Bulger EM, Kwon YS, Kao LS, Evans H, Nathens AB. Predicting death in necrotizing soft tissue infections: a clinical score. Surg Infect. 2009;10(6):517–522. doi: 10.1089/sur.2008.112. [DOI] [PubMed] [Google Scholar]
- 80.Light TD, Choi KC, Thomsen TA, et al. Long-term outcomes of patients with necrotizing fasciitis. J Burn Care Res. 2010;31(1):93–99. doi: 10.1097/BCR.0b013e3181cb8cea. [DOI] [PubMed] [Google Scholar]
- 81.Pham TN, Moore ML, Costa BA, Cuschieri J, Klein MB. Assessment of functional limitation after necrotizing soft tissue infection. J Burn Care Res. 2006;30(2):301–306. doi: 10.1097/BCR.0b013e318198a241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hakkarainen T, Burkette Ikebata N, Evans H, Bulger EM. Survivors of necrotizing soft-tissue infections have impaired health-related quality of life. Surg infect. 2013;14(S1):S25–S26. [Google Scholar]
- 83.Hakkarainen T, Tran T, Burkette Ikebata N, Bulger E, Evans H. Long-term health-related quality of life among necrotizing soft tissue infection survivors and clinical factors important to outcomes. Surg infect. 2014;15(S1):S17–S18. [Google Scholar]
- 84.Hakkarainen TW, Burkette Ikebata N, Bulger E, Evans HL. J Surg Res. 2014 May 9;:S0022–4804(14)00448-X. doi: 10.1016/j.jss.2014.05.006. doi: 10.1016/j.jss.2014.05.006. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


