Abstract
Background
Although bone marrow-derived cells (BMCs) have shown great therapeutic potential in patients with chronic ischemic heart disease (CIHD), the exact efficacy and safety of BMCs therapy is still not completely defined.
Material/Methods
We searched PubMed, OVID, EMBASE, the Cochrane Library, and ClinicalTrials.gov and finally identified 20 qualified trials in this meta-analysis. Assessment of efficacy was based on left ventricular ejection fraction (LVEF), left ventricular end-systolic volume (LVESV), and left ventricular end-diastolic volume (LVEDV) improvement, by weighted mean difference (WMD) with 95% confidence intervals (CIs). Results of all-cause death, ventricular arrhythmia, recurrent myocardial infarction, and cerebrovascular accident were pooled to assess safety. Subgroup analysis was performed by stratifying RCTs into 2 subgroups of those with revascularization and without revascularization.
Results
BMC transplantation significantly improved LVEF in patients with revascularization (3.35%, 95% CI 0.72% to 5.97%, p=0.01; I2=85%) and without revascularization (3.05%, 95% CI 0.65% to 5.45%, p=0.01; I2=86%). In patients without revascularization, BMC transplantation was associated with significantly decreased LVESV (−11.75 ml, 95% CI −17.81 ml to −5.69 ml, p=0.0001; I2=81%), and LVEDV (−7.80 ml, 95% CI −15.31 ml to −0.29 ml, p=0.04; I2=39%). Subgroup analysis showed that the route of transplantation, baseline LVEF, and type of cells delivered could influence the efficacy of BMC transplantation.
Conclusions
Autologous transplantation of BMCs was safe and effective for patients who were candidates for revascularization with CABG/PCI and those who were not. However, large clinical trials and long-term follow-up are required to confirm these benefits.
MeSH Keywords: Mesenchymal Stromal Cells, Meta-Analysis, Myocardial Ischemia
Background
Chronic ischemic heart disease (CIHD) is characterized as reduced blood supply to the heart muscle due to plaque building up along the inner walls of the arteries of the heart. If not properly treated, it can lead to end-stage heart failure [1]. CIHD is a major cause of morbidity and mortality in developed countries and in some of the emerging countries. Although the application of medical therapy and coronary artery revascularization techniques (e.g., coronary artery bypass grafting [CABG] and percutaneous coronary intervention [PCI]) have improved clinical outcomes, CIHD is still a leading cause of angina and congestive heart failure refractory to traditional therapies [2]. Optimal therapies for this disease should achieve clinical improvement of cardiac function by realizing myocardial regeneration and revascularization, without severe adverse effects. Therefore, stem/progenitor cells-based cell therapy has great potential due to the ability to self-renew and to differentiate into specialized cells [3].
Since 2001, transplantation of bone marrow-derived cells (BMCs), either by intracoronary or intramyocardial route, has been tested as a therapy for myocardial infarction and other ischemic heart disease [4] due to their multipotent, autologous origin and readily usable features [5]. A number of studies were launched to evaluate the effect of this therapy and some reported improved left ventricular ejection fraction (LVEF) and exercise capacity in patients with myocardial infarction or other coronary artery diseases [3,6]. Initial meta-analysis also demonstrated the safety and beneficial effects of intracoronary BMC therapy [7,8]. However, due to small sample size of the trials, the exact efficacy of BMC therapy for patients with CIHD is still unclear. Some studies even reported no significant benefits of BMC therapy compared with controls [9,10]. In addition, previous meta-analyses also found significant heterogeneity of trials included and conflicting results [11–13]. Thus, it is necessary to integrate results of the latest clinical trials to perform an updated meta-analysis. The aim of this meta-analysis was to make an updated assessment of efficacy and safety of autologous transplantation of BMCs for patients with CIHD.
Material and Methods
Search strategy
This meta-analysis generally followed the Preferred Reporting Item for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Relevant studies focusing on BMC transplantation in patients with CIHD were retrieved from PubMed, OVID, EMBASE, the Cochrane Library and ClinicalTrials.gov from January 2000 to April 2014. The subject terms and free-text terms used for this study were: bone marrow cell, bone marrow mononuclear cell, stem cell, stromal cell, coronary artery disease, ischemic cardiomyopathy, ischemic heart disease, heart failure, myocardial ischemia, and myocardial infarction. Introduction and reference lists of included trials were manually searched to identify additional qualified studies. No language restriction was set when searching qualified studies.
Selection criteria
Studies included for meta-analysis had to meet all of the following criteria simultaneously: (1) randomized controlled trial (RCT) based on patients with chronic ischemic heart disease (CIHD); (2) cell therapy was autologous BMCs-based; (3) patients in the control arm received standard therapy; and (4) follow-up was at least 3 months. Exclusion criteria were: (1) studies provided patients cell precursors mobilized by cytokines due to the additional effect of cytokine myocardium and BMCs. In this study, CHID was defined as chronic coronary total occlusion, stable angina, refractory angina, myocardial ischemia, historical myocardial infarction, or patients with previous revascularization.
Data extraction
Two authors (CX and SZ) independently assessed eligibility of studies and extracted data from original studies. Disagreements and discrepancies were resolved through group discussion involving a third reviewer. The following information was extracted from original studies: first author, year of publication, number of patients recruited, basic information of patients (age, sex, and NYHA classification), clinical scenario, duration of follow-up, type and number of cells transplanted, route of delivery, and imaging modality. The outcomes of LV function and remodeling – primary endpoint-left ventricular ejection fraction (LVEF) and secondary endpoints-net changes in left ventricular end-systolic volume (LVESV), and left ventricular end-diastolic volume (LVEDV) – were extracted for further meta-analysis. For studies that reported primary or secondary endpoints measured by various imaging modalities, MRI data was preferentially used due to superior accuracy. Data on the major adverse effects were extracted for assessing the safety of BMCs transplantation for CIHD patients.
Quality assessment
The methodological quality of included studies was assessed according to the method proposed by Juni et al. [14], in combination with modified Jadad score (7 point). The major quality components were: adequate method to generate randomized sequence, adequate method of allocation concealment, blinding of outcome assessors, loss of participant follow-up (%), and all patients treated in assigned group.
Data analysis
RevMan 5.2 (Cochrane Collaboration) software was used for data analysis. To guarantee uniformity of outcomes, when multiple measurements of the change of primary and secondary endpoint from baseline to follow-up were available, 6-month data were obtained and used for further pooled analysis. If original data did not provide the changes in mean and SD form, the estimation method recommended by the Cochrane Handbook for Systematic Reviews of Interventions 16.1.3 was used (Version 5.1.0, the Cochrane Collaboration, 2011). Results are summarized in the form of weighted mean difference (WMD) with 95% confidence intervals (CIs). Heterogeneity between trials was assessed using chi-square test (χ2) and I2 statistics. P<0.1 or I2 >50% suggests a substantial level of inconsistency [15]. Primary assessment was performed with a fixed model. If significant heterogeneity was identified, the source of heterogeneity was further analyzed. If no significant clinical heterogeneity was observed, the random effects model based on Mantel-Haenszel method was used to make estimates. Otherwise, descriptive analysis was performed. Funnel plots were used to explore possible publication bias. Subgroup analysis was also performed according to type of cells (BMMNCs or MCSs), the route of cell delivery (IM or IC), and above or below average LVEF level (<35.5% or ≥35.5%). P<0.05 was considered as statistically significant.
Results
Search results
The search process is described in Supplement Figure 1. Initial retrieval yielded 1731 references, among which 1688 were excluded after reviewing the title and/or abstract. The remaining 43 trials were reviewed in detail for assessing eligibility. Among them, 11 were excluded due to non-RCT, 3 were excluded due to use of precursors mobilized by cytokine, 3 were excluded due to use of blood-derived stem cells, and 6 were excluded because LVEF data was not reported. Finally, a total of 20 trials were included for meta-analysis [6,9,10,16–32].
Characteristics of included studies
The key characteristics of trials included are summarized in Supplement Table 1. All of the trials had relatively small sample size, with number of patients ranging from 14 to 109. A total of 453 patients were included in the cell therapy groups and 322 patients were included in the control groups. All trials recruited patients with CIHD, including chronic coronary total occlusion, refractory angina, ischemic heart failure, and chronic myocardial infarction. Twelve studies used PCI or CABG as the control method for revascularization and 8 studies recruited patients unsuitable for revascularization. The autologous BMCs, including both BMMNCs and MCSs, were delivered either directly through intramyocardial or intracoronary approach. Mean patient age in the intervention groups was 61.0±3.3 and in the control groups was 61.2±3.3 (p=0.88), suggesting adequate match. Follow-up ranged from 3 to 12 months. The lowest and highest average number of cell delivered was 5×106 and 6.59×108. The median LVEF of patients at baseline in the intervention group was 35.5%. A funnel plot for LVEF outcome was used to assess publication bias. The roughly symmetrical distribution pattern suggested there was no significant publication bias (Supplement Figure 2).
Quality assessment
Methodological quality of the 20 studies included is summarized in Supplement Table 2. Generally, the quality of trials included was good. According to the modified Jadad score, 14 out 20 studies scored 5 or above, which suggests relatively high quality. There was no significant difference in patient characteristics between cell therapy and standard therapy groups. All of the trials were randomized, but 6 studies did not report the method of generating a randomized sequence and 6 did not give concealing allocation method. Eighteen studies had both blinded patients and practitioners and 2 studies had only blinded patients. All these studies had blinded assessors of outcome measurement. Eighteen studies had loss of follow-up rate under 15% and 2 were over this level.
Efficacy of BMC transplantation
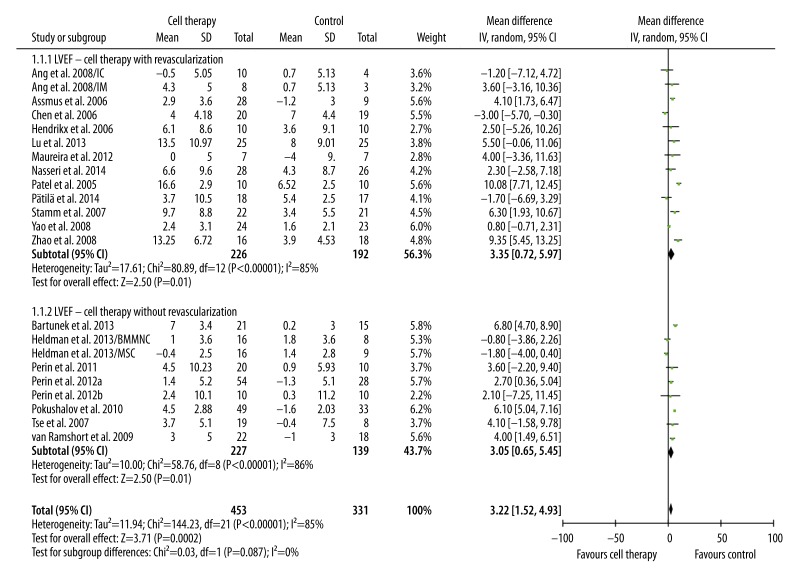
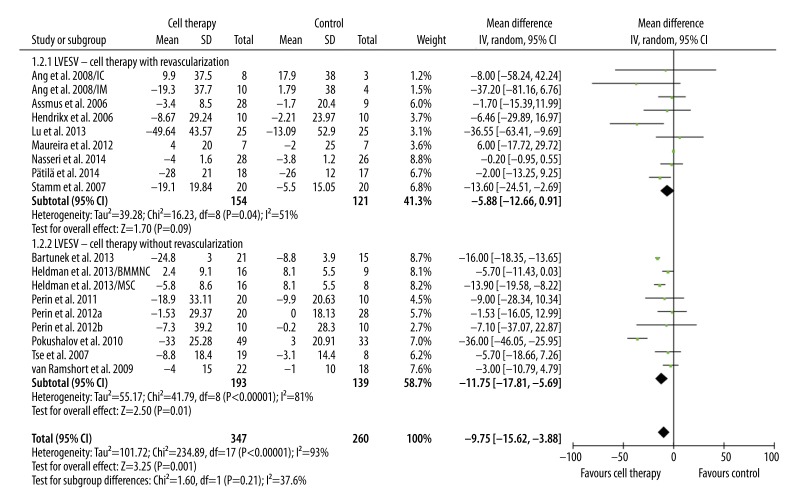
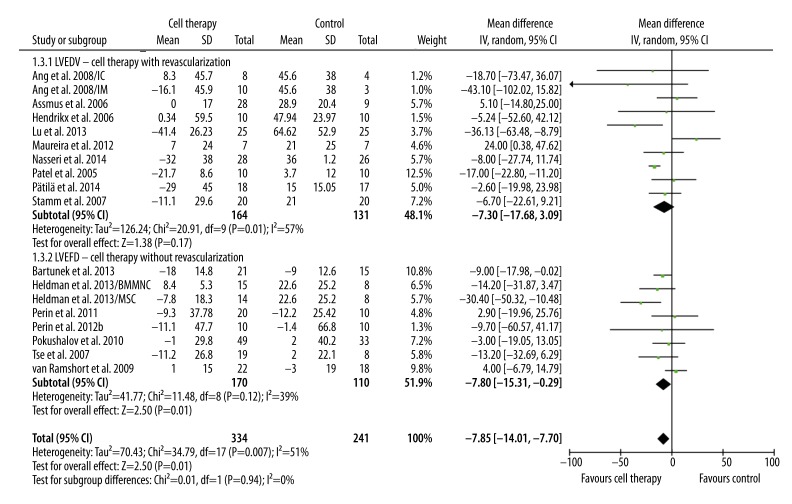
The primary and secondary outcome (LVEF, LVEDV, and LVESV) were pooled. Compared with standard treatment, BMC transplantation significantly improved LVEF in patients with revascularization (3.35%, 95%CI 0.72% to 5.97%, p=0.01; I2=85%) and in patients without revascularization (3.05%, 95% CI 0.65% to 5.45%, p=0.01; I2=86%). LVEF improvement was similar in these 2 groups (p=0.87) (Figure 1). LVESV and LVEDV are 2 important indicators of LV remodeling. Compared with control, BMC transplantation was associated with moderately decreased LVESV in patients with revascularization (−5.88 ml, 95% CI, −12.66 ml to 0.91 ml, p=0.09; I2=51%) and significantly decreased in patients without revascularization (−11.75 ml, 95% CI −17.81 ml to −5.69 ml, p=0.0001; I2=81%) (Figure 2). No significant difference in LVESV decrease was observed between these 2 groups (p=0.21) (Figure 2). In addition, a significant trend of decreased LVEDV was only observed in patients receiving only cell therapy (−7.80 ml, 95% CI −15.31 ml to −0.29 ml, p=0.04; I2=39%), but not in patients receiving combination of cell therapy and revascularization (−7.30, 95% CI −17.68 ml to 3.09 ml, p=0.17; I2=57%). However, the difference between these 2 groups was not significant (p=0.94) (Figure 3).
Figure 1.
Meta-analysis of LVEF improvement after therapy.
Figure 2.
Meta-analysis of LVESV improvement after therapy.
Figure 3.
Meta-analysis of LVEDV improvement after therapy.
Subgroup analysis
Due to significant heterogeneity observed within groups with or without revascularization, subgroup analysis was performed to explore whether the type of revascularization intervention (CABG or PCI), the type of cell delivered, and the level of baseline LVEF influenced the efficacy of cell therapy. Table 1 summarizes the results of subgroup analysis. Compared with LVEF improvement in intracoronary route (1.00%, 95% CI −0.11% to 2.10%), the improvement in intramyocardial delivery was significantly higher (4.77%, 95% CI 4.11% to 5.44%) (p<0.00001) (Table 1). However, no significant difference was observed in LVESV and LVEDV (p=0.68 and p=0.64, respectively). When comparing the effect of cell type transplanted on LV function improvement, it was observed LVEF improvement was significantly higher in the BMMNCs group (4.33%, 95% CI 3.69% to 4.96%) than in the MCSs group (1.32%, 95% CI −0.00% to 2.65%) (p<0.00001) (Table 1). However, LVESV decrease was more evident in the MCSs group (−15.69 ml, 95% CI −17.87 ml to −13.52 ml) than in the BMNNCs group (−8.51 ml, 95% CI −14.28 ml to −2.74 ml) (p=0.02). The median LVEF of patients in 20 trials was 35.5%. LVEF increase was significantly higher in the group with higher than average LVEF (5.11%, 95% CI 4.36% to 5.86%) than the group with lower than average LVEF (1.93%, 95% CI, 1.05% to 2.81%) (p<0.00001). Changes in LVESV and LVEDV were similar in these 2 groups (p=0.23 and p=0.32, respectively) (Table 1).
Table 1.
Subgroup analysis of factors influencing efficacy of cell therapy.
| Outcome | No. trials | Difference in mean [95% CI] | P | P-H | I2 | No. trials | Difference in mean (95% CI) | P | P-H | I2 | Group Comparison P value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| IM | IC | ||||||||||
| LVEF | 17 | 4.77 [4.11, 5.44] | <0.00001 | <0.00001 | 83% | 5 | 1.00 [−0.11, 2.10] | 0.08 | 0.001 | 78% | <0.00001 |
| LVESV | 13 | −9.38 [−15.60, −3.17] | 0.003 | <0.00001 | 94% | 3 | −14.74 [−39.79, 10.31] | 0.25 | 0.08 | 61% | 0.68 |
| LVEDV | 15 | −7.40 [−13.74, −1.06] | 0.02 | 0.01 | 52% | 3 | −14.86 [−45.13, 15.41] | 0.34 | 0.05 | 66% | 0.64 |
| BMMNC | MCS | ||||||||||
| LVEF | 18 | 4.33 [3.69, 4.96] | <0.00001 | <0.00001 | 79% | 3 | 1.32 [−0.00, 2.65] | 0.05 | <0.00001 | 95% | <0.0001 |
| LVESV | 15 | −8.51 [−14.28, −2.74] | 0.004 | <0.00001 | 78% | 2 | −15.69 [−17.87, −13.52] | <0.00001 | 0.5 | 0% | 0.02 |
| LVEDV | 15 | −9.40 [−13.30, −5.50] | <0.00001 | 0.01 | 51% | 2 | −12.62 [−20.81, −4.43] | 0.003 | 0.05 | 73% | 0.49 |
| Baseline LVEF <35.5% | Baseline LVEF ≥35.5% | ||||||||||
| LVEF | 9 | 5.11 [4.36, 5.86] | <0.00001 | <0.00001 | 88% | 11 | 1.93 [1.05, 2.81] | <0.00001 | <0.00001 | 73% | <0.00001 |
| LVESV | 7 | −14.34 [−24.60, −4.09] | 0.006 | <0.00001 | 97% | 9 | −7.89 [−11.23, −4.56] | 0.001 | <0.00001 | 93% | 0.24 |
| LVEDV | 8 | −12.96 [−17.24, −8.67] | 0.001 | 0.16 | 32% | 8 | −3.81 [−10.00, 2.39] | 0.34 | 0.03 | 54% | 0.32 |
P – p value; P-H – P value of Q for heterogeneity test; I2 >50%, high heterogeneity; Random effects model was used when P value of Q for heterogeneity test P-H >0.1 or I2 >50%; otherwise, fixed effect model was used.
Safety of BMC transplantation
Major adverse cardiovascular events reported in original studies were pooled to assess the safety of BMC transplantation (Table 2). Generally, compared with control, BMC transplantation had a similar risk ratio in ventricular arrhythmia, recurrent myocardial infarction, and cerebrovascular accident as control in subgroups both with and without revascularization. However, significantly lower risk of all-cause death was observed in the subgroup without revascularization (RR: 0.30, 95% CI: 0.12 to 0.77, P=0.01), but not in the subgroup with revascularization. No death directly related to BMC transplantation was reported in either subgroup.
Table 2.
Safety analysis of BMC transplantation.
| Safety outcome | No. of trials | Pooled risk ratio | 95%CI | P-H | I2 | P | |
|---|---|---|---|---|---|---|---|
| With revascularization | All-cause of death | 9 | 0.61 | 0.20–1.84 | 0.30 | 18% | 0.38 |
| Ventricular arrhythmia | 10 | 1.32 | 0.43–4.08 | 0.48 | 0% | 0.63 | |
| Recurrent myocardial infarction | 7 | 2.88 | 0.12–67.29 | – | – | 0.51 | |
| Cerebrovascular accident | 7 | 2.14 | 0.66–7.00 | 0.27 | 24% | 0.21 | |
| Without revascularization | All-cause of death | 8 | 0.30 | 0.12–0.77 | 0.43 | 0% | 0.01 |
| Ventricular arrhythmia | 7 | 0.45 | 0.10–1.98 | 0.51 | 0% | 0.29 | |
| Recurrent myocardial infarction | 4 | 0.33 | 0.06–1.80 | 0.19 | 41% | 0.20 | |
| Cerebrovascular accident | 2 | 0.30 | 0.03–2.74 | 0.93 | 0% | 0.29 |
P – p value; P-H – P value of Q for heterogeneity test; I2 >50%, high heterogeneity; Random effects model was used when P value of Q for heterogeneity test P-H >0.1 or I2 >50%; otherwise, fixed effect model was used; “–” – not applicable.
Discussion
For patients with CIHD, the goal of cell therapy is to achieve coronary neovascularization- and myocardial regeneration-based cardiac structural and functional improvement. LV function impairment is an important indicator of disease prognosis. Although previous meta-analyses explored the therapeutic effects and adverse events of BMCs compared with standard therapy, due to significant heterogeneity, small number of original trials and inconsistency of the findings, it is quite necessary to add outcomes new trials for better understanding of BMCs therapy.
To date, there is no available meta-analysis that explored the efficacy of BMCs-based cell therapy between patients with revascularizable and non-revascularizable CIHD. This meta-analysis of 20 RCTs showed that autologous transplantation of BMCs was safe and effective both for patients who were candidates for revascularization with CABG/PCI and those who were not. Findings of this study also indicated that for patients with no option for revascularization, single administration of BMCs could provide compatible effect in improving LV function, including increased LVEF and decreased LVEDV and LVESV compared with patients who received a combination of cell therapy and revascularization. Subgroup analysis found that the route of transplantation, baseline LVEF, and type of cells delivered could influence the efficacy of cell therapy. We found that IM injection was more beneficial to improve LVEF compared with IC injection (4.77% vs. 1.00%, p<0.00001). This finding is consistent that of Brunskill et al., who found that cell therapy through IM injection was associated with better effect [33]. This difference might be related to the higher proportion of cells retained in the heart through IM injection than through the IC route, which was confirmed by an animal study [34] and 1 small RCT [26]. Previous studies reported that cell therapy had better effect in patients with lower baseline LVEF [13,33]. Subgroup analysis of this study also found that patients with lower than average baseline LVEF (<35.5%) had better therapeutic outcome (more LVEF improvement) than those with higher than average baseline (5.11% vs. 1.93%, p<0.00001). Furthermore, this study found that transplantation of BMMNCs had better effect in improving LVEF than did MCSs. However, due to the small number of trials based on MCSs (n=3), the reliability of this finding should be confirmed in future RCTs. As to LVEDV and LVESV, no significant difference was observed between paired subgroups. Pooled safety assessment found that cell therapy significantly decreased all-cause death in the subgroup without revascularization. Other major adverse events were similar between cell therapy and control group in both subgroups.
The mechanisms by which BMCs contribute to cardiac repair and function improvement are still not fully understood. A previous study observed that BMCs could differentiate to cardiomyocytes in infarcted areas of the heart [35]. However, this was proven to be a minor mechanism because the process of transdifferentiation occurs very rarely in this situation [36]. The role of BMCs in impaired heart tissue could be multifunctional and multifactorial. Previous studies found that BMCs and bone marrow progenitor cells could secrete a series of cytokines that activate endogenous cardiac repair, such as activating resident cardiac progenitor cells and stimulating cardiomyocyte cell-cycle re-entry [37,38]. In addition, BMCs were involved in the process of neovascularization, inhibiting apoptosis of myocardial cells, and reducing inflammation through paracrine production of cytokines and growth factors [36,39,40]. However, how these mechanisms interact with each other and whether other mechanisms are involved is not clear. The exact and detailed mechanism should be explored in future research.
This study has several limitations. As observed in previous meta-analyses, this study also found a considerable degree of between-study heterogeneity. The high level of heterogeneity is closely related to patient baseline characteristics of each trial, route of transplantation, type and number of cell delivered, and methodology used to measure LV functional indicators. For example, a recent study indicated that use of low-dose mesenchymal stem cells (approximately 20×106 cells) was associated with the best effect in LV volume and LVEF increase [41]. However, the number of cells transplanted ranged from 106 to 108 in trials included in this meta-analysis. Secondly, in subgroup analysis, few trials assessed the effect of PCI and MCSs, which resulted in incompatible pooled sample size and thus might impair the reliability of observed phenomenon. Thirdly, most of the trials included had short-term follow-up (less than 1 year). The medium to long-term effect of cell therapy is still not quite clear.
Conclusions
In conclusion, the results of this meta-analysis suggest that transplantation of BMCs is a safe and effective therapy to improve left ventricular function both for patients who were candidates for revascularization with CABG/PCI and those who were not. Large clinical trials and long-term follow-up are required to confirm the benefits.
The search process.
Funnel plot of publication bias.
Supplement Table 1.
Characteristics of included studies.
| Study | Country | Sample size (I/C) | Average age (I/C) | NYHA classification | Duration (Mo) | Gender (male%) (I/C) | Revascularization | Baseline LVEF (mean SD) | Clinical scenario | Route of transplantation (no.) | No. of cells | Cell type | Imaging modality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ang et al., 2008 | UK | 42/20 | IM: 64.7/61.3; IC: 62.1/61.3 | N.A. | 6 | 86/14 | CABG | 25.4 (8.1) IM 28.5 (6.5) IC |
R-CIHD | IM(21)/IC(21) | 85±56×106 IM 115±73×106 IC |
BMMNC | MRI |
| Assmus et al., 2006 | Germany | 35/23 | 60/61 | 1–3 | 3 | 93/7 | PCI | 41 (11) | R-CIHD | IC | 205±110×106 | BMMNC | LVG |
| Chen et al., 2006 | China | 22/23 | N.A. | N.A. | 12 | N.A. | PCI | 26 (6) | R-CIHD | IC | 5×106 | MSC | Echo (EF) |
| Heldman et al., 2013 | US | MSC: 22/11 BMMNC: 22/10 |
MSC: 57.1/60.0 BMMNC: 61.1/61.3 |
1–3 | 12 | MSC: 94.7/90.9 BMMNC: 89.5/100 |
N.A. | 35.9 (8.2) BMMNC 35.7 (9.0) MSC |
N-CIHD | IM | ≥100×106 | BMMNC/MSC | MRI |
| Hendrikx et al., 2006 | Belgium | 10/10 | 63.2/66.8 | N.A. | 4 | 100/70 | CABG | 42.9 (10.3) | R-CIHD | IM | 60.25± 31.35×106 | BMMNC | MRI |
| Lu et al., 2013 | China | 25/25 | 57/58 | 2–3 | 12 | 96/88 | CABG | 23.4 (7.1) | R-CIHD | IC | 13.38±8.14×107 | BMMNC | MRI |
| Maureira et al., 2012 | France | 7/7 | 58/57 | I: 2.1 (mean) C: 1.9 (mean) |
6 | 100/86 | CABG | 41 (8) | R-CIHD | IM | ≥300×106 | BMMNC | MRI |
| Patel et al., 2005 | Argentina | 10/10 | 64.8/63.6 | I: 3.5 C: 3.4 | 6 | 80/80 | CABG | 29.4 (3.6) | R-CIHD | IM | 22×106 (median) | CD34+ BMC | Echo |
| Perin et al., 2011 | America | 20/10 | 60.5/56.3 | I: 2.3 C: 2.6 | 6 | 80/50 | N.A. | 37.5 (8.2) | N-CIHD | IM | 30×106 | BMMNC | LVG |
| Perin et al., 2012a | America | 61/31 | 63.95/62.32 | 1–3 | 6 | 92/98 | N.A. | 32.43 (9.23) | N-CIHD | IM | 100×106 | BMMNC | Echo |
| Perin et al., 2012b | America | 10/10 | 58.2/57.8 | I: 2.5 C: 2.6 | 6 | 90/80 | N.A. | 36.1(10.9) | N-CIHD | IM | 0.29×107 | ALDHbr BMC | Echo/LVG |
| Pokushalov et al., 2010 | Russia | 55/54 | 61/62 | I: 3.3 C: 3.5 | 12 | 48/46 | N.A. | 27.8 (3.4) | N-CIHD | IM | 41±16×106 | BMMNC | Echo |
| Stamm et al., 2007 | Germany | 22/21 | 62/63.5 | 2–3 | 6 | 75/80 | CABG | 37.4 (8.4) | R-CIHD | IM | 5.86×106 (median) | CD133+ BMC | Echo |
| Tse et al., 2007 | China & Australia | 19/9 | 65.2/68.9 | I: 2–4 C: 2–3 |
6 | 79/88 | N.A. | 45.7 (8.3) | N-CIHD | IM | 1.67±0.34×107 low 4.20±2.80×107 high |
BMMNC | MRI |
| van Ramshorst et al., 2009 | Netherlands | 25/25 | 64/62 | N.A. | 3 | 92/80 | N.A. | 56 (12) | N-CIHD | IM | 98±6×106 | BMMNC | MRI |
| Yao et al., 2008 | China | 24/23 | 54.8/56.3 | 1–3 | 6 | 96/96 | PCI | 44.3 (5.5) | R-CIHD | IC | 180×106 | BMMNC | MRI |
| Zhao et al., 2008 | China | 18/18 | 60.3/59.1 | 3–4 | 6 | 83.3/83.3 | CABG | 35.80 (7.28) | R-CIHD | IM | 6.59±5.12×108 | BMMNC | Echo |
| Bartunek et al., 2013 | Belgium/Serbia | 21/15 | 55.7/59.5 | 2–3 | 6 | 95.2/91.7 | N.A. | 27.5 (2) | N-CIHD | IM | >600×106 | MSC | Echo |
| Nasseri et al., 2014 | Germany | 30/30 | 61.9/62.7 | 1–4 | 6 | 93.3/96.7 | CABG | 27 (6) | R-CIHD | IM | 11.7×106 | CD133+ BMC | MRI |
| Pätilä et al., 2014 | Finland | 20/19 | 65/64 | 2–3 | 12 | 95/94.7 | CABG | 37.2 (4) | R-CIHD | IM | 8.4×106 (median) | BMMNC | MRI |
I – Intervention; C – control; NYHA – New York Heart Association; LVEF – left ventricular ejection fraction; N.A. – not available; R-CIHD – revascularizable chronic ischemic heart disease; IM – intramyocardial; IC – intracoronary; CABG – coronary artery bypass grafting; BMC – bone marrow cell; BMMNC – bone marrow mononuclear cell; ALDHbr – with a high level of aldehyde dehydrogenase activity; MRI – magnetic resonance imaging; Echo – echocardiography; LVG – left ventriculography.
Supplement Table 2.
Methodological quality of 20 studies included.
| Study | Adequate method to generate randomized sequence | Adequate method of allocation concealment | Blinding of outcome assessors | Loss of participant follow-up (%) | All patients treated in assigned group | Jadad Score |
|---|---|---|---|---|---|---|
| Ang et al., 2008 | N | N | Y | 3.3 | Y | 3 |
| Assmus et al., 2006 | N | N | Y | 12 | Y | 3 |
| Chen et al., 2006 | N | N | N | 13.3 | Y | 1 |
| Bartunek et al., 2013 | Y | Y | Y | 0 | Y | 7 |
| Hendrikx et al., 2006 | Y | Y | Y | 13 | Y | 6 |
| Heldman et al., 2013 | Y | Y | Y | 4.6 | Y | 6 |
| Lu et al., 2013 | N | N | Y | 16.7 | Y | 3 |
| Maureira et al., 2012 | Y | Y | N | 0 | Y | 5 |
| Nasseri et al., 2014 | Y | Y | Y | 10 | Y | 6 |
| Patel et al., 2005 | Y | Y | Y | 0 | Y | 7 |
| Pätilä et al., 2014 | Y | Y | Y | 0 | Y | 7 |
| Perin et al., 2011 | Y | Y | Y | 0 | Y | 7 |
| Perin et al., 2012a | Y | Y | Y | 4.7 | Y | 6 |
| Perin et al., 2012b | Y | Y | Y | 0 | Y | 6 |
| Pokushalov et al., 2010 | Y | Y | Y | 24.8 | Y | 6 |
| Stamm et al., 2007 | N | N | Y | 7 | Y | 3 |
| Tse et al., 2007 | Y | Y | Y | 3.6 | Y | 6 |
| van Ramshorst et al., 2009 | Y | Y | Y | 2 | Y | 6 |
| Yao et al., 2008 | N | N | Y | 0 | Y | 3 |
| Zhao et al., 2008 | Y | Y | Y | 5.6 | Y | 6 |
Footnotes
Source of support: Self financing
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics – 2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blecker S, Paul M, Taksler G, et al. Heart failure-associated hospitalizations in the United States. J Am Coll Cardiol. 2013;61:1259–67. doi: 10.1016/j.jacc.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burt RK, Loh Y, Pearce W, et al. Clinical applications of blood-derived and marrow-derived stem cells for nonmalignant diseases. JAMA. 2008;299:925–36. doi: 10.1001/jama.299.8.925. [DOI] [PubMed] [Google Scholar]
- 4.Strauer BE, Brehm M, Zeus T, et al. [Intracoronary, human autologous stem cell transplantation for myocardial regeneration following myocardial infarction]. Dtsch Med Wochenschr. 2001;126:932–38. doi: 10.1055/s-2001-16579-2. [in German] [DOI] [PubMed] [Google Scholar]
- 5.Dai W, Kloner RA. Bone marrow-derived cell transplantation therapy for myocardial infarction: lessons learned and future questions. Am J Transplant. 2011;11:2297–301. doi: 10.1111/j.1600-6143.2011.03750.x. [DOI] [PubMed] [Google Scholar]
- 6.Tse HF, Thambar S, Kwong YL, et al. Prospective randomized trial of direct endomyocardial implantation of bone marrow cells for treatment of severe coronary artery diseases (PROTECT-CAD trial) Eur Heart J. 2007;28:2998–3005. doi: 10.1093/eurheartj/ehm485. [DOI] [PubMed] [Google Scholar]
- 7.Lalu MM, McIntyre L, Pugliese C, et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PloS one. 2012;7:e47559. doi: 10.1371/journal.pone.0047559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun L, Zhang T, Lan X, et al. Effects of stem cell therapy on left ventricular remodeling after acute myocardial infarction: a meta-analysis. Clin Cardiol. 2010;33:296–302. doi: 10.1002/clc.20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perin EC, Silva GV, Henry TD, et al. A randomized study of transendocardial injection of autologous bone marrow mononuclear cells and cell function analysis in ischemic heart failure (FOCUS-HF) Am Heart J. 2011;161:1078–87 e3. doi: 10.1016/j.ahj.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 10.Hendrikx M, Hensen K, Clijsters C, et al. Recovery of regional but not global contractile function by the direct intramyocardial autologous bone marrow transplantation: results from a randomized controlled clinical trial. Circulation. 2006;114:I101–7. doi: 10.1161/CIRCULATIONAHA.105.000505. [DOI] [PubMed] [Google Scholar]
- 11.Fisher SA, Doree C, Brunskill SJ, et al. Bone Marrow Stem Cell Treatment for Ischemic Heart Disease in Patients with No Option of Revascularization: A Systematic Review and Meta-Analysis. PloS one. 2013;8:e64669. doi: 10.1371/journal.pone.0064669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu R, Ding S, Zhao Y, et al. Autologous Transplantation of Bone Marrow/Blood-Derived Cells for Chronic Ischemic Heart Disease: A Systematic Review and Meta-analysis. Can J Cardiol. 2014 doi: 10.1016/j.cjca.2014.01.013. pii: S0828-282X(14)00044-0. [DOI] [PubMed] [Google Scholar]
- 13.Jeevanantham V, Butler M, Saad A, et al. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circulation. 2012;126:551–68. doi: 10.1161/CIRCULATIONAHA.111.086074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ. 2001;323:42–46. doi: 10.1136/bmj.323.7303.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions Version 5.1: The Cochrane Collaboration. 2011. [Google Scholar]
- 16.Heldman AW, DiFede DL, Fishman JE, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311:62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu M, Liu S, Zheng Z, et al. A pilot trial of autologous bone marrow mononuclear cell transplantation through grafting artery: a sub-study focused on segmental left ventricular function recovery and scar reduction. Int J Cardiol. 2013;168:2221–27. doi: 10.1016/j.ijcard.2013.01.217. [DOI] [PubMed] [Google Scholar]
- 18.Maureira P, Tran N, Djaballah W, et al. Residual viability is a predictor of the perfusion enhancement obtained with the cell therapy of chronic myocardial infarction: a pilot multimodal imaging study. Clin Nucl Med. 2012;37:738–42. doi: 10.1097/RLU.0b013e318251e38a. [DOI] [PubMed] [Google Scholar]
- 19.Patel AN, Geffner L, Vina RF, et al. Surgical treatment for congestive heart failure with autologous adult stem cell transplantation: a prospective randomized study. J Thorac Cardiovasc Surg. 2005;130:1631–38. doi: 10.1016/j.jtcvs.2005.07.056. [DOI] [PubMed] [Google Scholar]
- 20.Perin EC, Willerson JT, Pepine CJ, et al. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. JAMA. 2012;307:1717–26. doi: 10.1001/jama.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pokushalov E, Romanov A, Chernyavsky A, et al. Efficiency of intramyocardial injections of autologous bone marrow mononuclear cells in patients with ischemic heart failure: a randomized study. J Cardiovasc Transl Res. 2010;3:160–68. doi: 10.1007/s12265-009-9123-8. [DOI] [PubMed] [Google Scholar]
- 22.Stamm C, Kleine HD, Choi YH, et al. Intramyocardial delivery of CD133+ bone marrow cells and coronary artery bypass grafting for chronic ischemic heart disease: safety and efficacy studies. J Thorac Cardiovasc Surg. 2007;133:717–25. doi: 10.1016/j.jtcvs.2006.08.077. [DOI] [PubMed] [Google Scholar]
- 23.van Ramshorst J, Bax JJ, Beeres SL, et al. Intramyocardial bone marrow cell injection for chronic myocardial ischemia: a randomized controlled trial. JAMA. 2009;301:1997–2004. doi: 10.1001/jama.2009.685. [DOI] [PubMed] [Google Scholar]
- 24.Yao K, Huang R, Qian J, et al. Administration of intracoronary bone marrow mononuclear cells on chronic myocardial infarction improves diastolic function. Heart. 2008;94:1147–53. doi: 10.1136/hrt.2007.137919. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Q, Sun Y, Xia L, et al. Randomized study of mononuclear bone marrow cell transplantation in patients with coronary surgery. Ann Thorac Surg. 2008;86:1833–40. doi: 10.1016/j.athoracsur.2008.08.068. [DOI] [PubMed] [Google Scholar]
- 26.Ang KL, Chin D, Leyva F, et al. Randomized, controlled trial of intramuscular or intracoronary injection of autologous bone marrow cells into scarred myocardium during CABG versus CABG alone. Nat Clin Pract Cardiovasc Med. 2008;5:663–70. doi: 10.1038/ncpcardio1321. [DOI] [PubMed] [Google Scholar]
- 27.Assmus B, Honold J, Schachinger V, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–32. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 28.Bartunek J, Behfar A, Dolatabadi D, et al. Cardiopoietic stem cell therapy in heart failure: the C-CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. J Am Coll Cardiol. 2013;61:2329–38. doi: 10.1016/j.jacc.2013.02.071. [DOI] [PubMed] [Google Scholar]
- 29.Perin EC, Silva GV, Zheng Y, et al. Randomized, double-blind pilot study of transendocardial injection of autologous aldehyde dehydrogenase-bright stem cells in patients with ischemic heart failure. Am Heart J. 2012;163:415–21. 21 e1. doi: 10.1016/j.ahj.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 30.Nasseri BA, Ebell W, Dandel M, et al. Autologous CD133+ bone marrow cells and bypass grafting for regeneration of ischaemic myocardium: the Cardio133 trialdagger. Eur HeartJ. 2014;35:1263–74. doi: 10.1093/eurheartj/ehu007. [DOI] [PubMed] [Google Scholar]
- 31.Patila T, Lehtinen M, Vento A, et al. Autologous bone marrow mononuclear cell transplantation in ischemic heart failure: A prospective, controlled, randomized, double-blind study of cell transplantation combined with coronary bypass. J Heart Lung Transplant. 2014;33:567–74. doi: 10.1016/j.healun.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Chen S, Liu Z, Tian N, et al. Intracoronary transplantation of autologous bone marrow mesenchymal stem cells for ischemic cardiomyopathy due to isolated chronic occluded left anterior descending artery. J Invasive Cardiol. 2006;18:552–56. [PubMed] [Google Scholar]
- 33.Brunskill SJ, Hyde CJ, Doree CJ, et al. Route of delivery and baseline left ventricular ejection fraction, key factors of bone-marrow-derived cell therapy for ischaemic heart disease. Eur J Heart Fail. 2009;11:887–96. doi: 10.1093/eurjhf/hfp101. [DOI] [PubMed] [Google Scholar]
- 34.Perin EC, Silva GV, Assad JA, et al. Comparison of intracoronary and transendocardial delivery of allogeneic mesenchymal cells in a canine model of acute myocardial infarction. J Mol Cell Cardiol. 2008;44:486–95. doi: 10.1016/j.yjmcc.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 35.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–5. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 36.Murry CE, Soonpaa MH, Reinecke H, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–68. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 37.Hatzistergos KE, Quevedo H, Oskouei BN, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–22. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang S, Piao J, Jin L, et al. Does pretreatment of bone marrow mesenchymal stem cells with 5-azacytidine or double intravenous infusion improve their therapeutic potential for dilated cardiomyopathy? Med Sci Monit Basic Res. 2013;19:20–31. doi: 10.12659/MSMBR.883737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow stem cells regenerate infarcted myocardium. Pediatr Transplant. 2003;7(Suppl 3):86–88. doi: 10.1034/j.1399-3046.7.s3.13.x. [DOI] [PubMed] [Google Scholar]
- 40.Kocher AA, Schuster MD, Szabolcs MJ, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–36. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 41.Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs. autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–79. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The search process.
Funnel plot of publication bias.
Supplement Table 1.
Characteristics of included studies.
| Study | Country | Sample size (I/C) | Average age (I/C) | NYHA classification | Duration (Mo) | Gender (male%) (I/C) | Revascularization | Baseline LVEF (mean SD) | Clinical scenario | Route of transplantation (no.) | No. of cells | Cell type | Imaging modality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ang et al., 2008 | UK | 42/20 | IM: 64.7/61.3; IC: 62.1/61.3 | N.A. | 6 | 86/14 | CABG | 25.4 (8.1) IM 28.5 (6.5) IC |
R-CIHD | IM(21)/IC(21) | 85±56×106 IM 115±73×106 IC |
BMMNC | MRI |
| Assmus et al., 2006 | Germany | 35/23 | 60/61 | 1–3 | 3 | 93/7 | PCI | 41 (11) | R-CIHD | IC | 205±110×106 | BMMNC | LVG |
| Chen et al., 2006 | China | 22/23 | N.A. | N.A. | 12 | N.A. | PCI | 26 (6) | R-CIHD | IC | 5×106 | MSC | Echo (EF) |
| Heldman et al., 2013 | US | MSC: 22/11 BMMNC: 22/10 |
MSC: 57.1/60.0 BMMNC: 61.1/61.3 |
1–3 | 12 | MSC: 94.7/90.9 BMMNC: 89.5/100 |
N.A. | 35.9 (8.2) BMMNC 35.7 (9.0) MSC |
N-CIHD | IM | ≥100×106 | BMMNC/MSC | MRI |
| Hendrikx et al., 2006 | Belgium | 10/10 | 63.2/66.8 | N.A. | 4 | 100/70 | CABG | 42.9 (10.3) | R-CIHD | IM | 60.25± 31.35×106 | BMMNC | MRI |
| Lu et al., 2013 | China | 25/25 | 57/58 | 2–3 | 12 | 96/88 | CABG | 23.4 (7.1) | R-CIHD | IC | 13.38±8.14×107 | BMMNC | MRI |
| Maureira et al., 2012 | France | 7/7 | 58/57 | I: 2.1 (mean) C: 1.9 (mean) |
6 | 100/86 | CABG | 41 (8) | R-CIHD | IM | ≥300×106 | BMMNC | MRI |
| Patel et al., 2005 | Argentina | 10/10 | 64.8/63.6 | I: 3.5 C: 3.4 | 6 | 80/80 | CABG | 29.4 (3.6) | R-CIHD | IM | 22×106 (median) | CD34+ BMC | Echo |
| Perin et al., 2011 | America | 20/10 | 60.5/56.3 | I: 2.3 C: 2.6 | 6 | 80/50 | N.A. | 37.5 (8.2) | N-CIHD | IM | 30×106 | BMMNC | LVG |
| Perin et al., 2012a | America | 61/31 | 63.95/62.32 | 1–3 | 6 | 92/98 | N.A. | 32.43 (9.23) | N-CIHD | IM | 100×106 | BMMNC | Echo |
| Perin et al., 2012b | America | 10/10 | 58.2/57.8 | I: 2.5 C: 2.6 | 6 | 90/80 | N.A. | 36.1(10.9) | N-CIHD | IM | 0.29×107 | ALDHbr BMC | Echo/LVG |
| Pokushalov et al., 2010 | Russia | 55/54 | 61/62 | I: 3.3 C: 3.5 | 12 | 48/46 | N.A. | 27.8 (3.4) | N-CIHD | IM | 41±16×106 | BMMNC | Echo |
| Stamm et al., 2007 | Germany | 22/21 | 62/63.5 | 2–3 | 6 | 75/80 | CABG | 37.4 (8.4) | R-CIHD | IM | 5.86×106 (median) | CD133+ BMC | Echo |
| Tse et al., 2007 | China & Australia | 19/9 | 65.2/68.9 | I: 2–4 C: 2–3 |
6 | 79/88 | N.A. | 45.7 (8.3) | N-CIHD | IM | 1.67±0.34×107 low 4.20±2.80×107 high |
BMMNC | MRI |
| van Ramshorst et al., 2009 | Netherlands | 25/25 | 64/62 | N.A. | 3 | 92/80 | N.A. | 56 (12) | N-CIHD | IM | 98±6×106 | BMMNC | MRI |
| Yao et al., 2008 | China | 24/23 | 54.8/56.3 | 1–3 | 6 | 96/96 | PCI | 44.3 (5.5) | R-CIHD | IC | 180×106 | BMMNC | MRI |
| Zhao et al., 2008 | China | 18/18 | 60.3/59.1 | 3–4 | 6 | 83.3/83.3 | CABG | 35.80 (7.28) | R-CIHD | IM | 6.59±5.12×108 | BMMNC | Echo |
| Bartunek et al., 2013 | Belgium/Serbia | 21/15 | 55.7/59.5 | 2–3 | 6 | 95.2/91.7 | N.A. | 27.5 (2) | N-CIHD | IM | >600×106 | MSC | Echo |
| Nasseri et al., 2014 | Germany | 30/30 | 61.9/62.7 | 1–4 | 6 | 93.3/96.7 | CABG | 27 (6) | R-CIHD | IM | 11.7×106 | CD133+ BMC | MRI |
| Pätilä et al., 2014 | Finland | 20/19 | 65/64 | 2–3 | 12 | 95/94.7 | CABG | 37.2 (4) | R-CIHD | IM | 8.4×106 (median) | BMMNC | MRI |
I – Intervention; C – control; NYHA – New York Heart Association; LVEF – left ventricular ejection fraction; N.A. – not available; R-CIHD – revascularizable chronic ischemic heart disease; IM – intramyocardial; IC – intracoronary; CABG – coronary artery bypass grafting; BMC – bone marrow cell; BMMNC – bone marrow mononuclear cell; ALDHbr – with a high level of aldehyde dehydrogenase activity; MRI – magnetic resonance imaging; Echo – echocardiography; LVG – left ventriculography.
Supplement Table 2.
Methodological quality of 20 studies included.
| Study | Adequate method to generate randomized sequence | Adequate method of allocation concealment | Blinding of outcome assessors | Loss of participant follow-up (%) | All patients treated in assigned group | Jadad Score |
|---|---|---|---|---|---|---|
| Ang et al., 2008 | N | N | Y | 3.3 | Y | 3 |
| Assmus et al., 2006 | N | N | Y | 12 | Y | 3 |
| Chen et al., 2006 | N | N | N | 13.3 | Y | 1 |
| Bartunek et al., 2013 | Y | Y | Y | 0 | Y | 7 |
| Hendrikx et al., 2006 | Y | Y | Y | 13 | Y | 6 |
| Heldman et al., 2013 | Y | Y | Y | 4.6 | Y | 6 |
| Lu et al., 2013 | N | N | Y | 16.7 | Y | 3 |
| Maureira et al., 2012 | Y | Y | N | 0 | Y | 5 |
| Nasseri et al., 2014 | Y | Y | Y | 10 | Y | 6 |
| Patel et al., 2005 | Y | Y | Y | 0 | Y | 7 |
| Pätilä et al., 2014 | Y | Y | Y | 0 | Y | 7 |
| Perin et al., 2011 | Y | Y | Y | 0 | Y | 7 |
| Perin et al., 2012a | Y | Y | Y | 4.7 | Y | 6 |
| Perin et al., 2012b | Y | Y | Y | 0 | Y | 6 |
| Pokushalov et al., 2010 | Y | Y | Y | 24.8 | Y | 6 |
| Stamm et al., 2007 | N | N | Y | 7 | Y | 3 |
| Tse et al., 2007 | Y | Y | Y | 3.6 | Y | 6 |
| van Ramshorst et al., 2009 | Y | Y | Y | 2 | Y | 6 |
| Yao et al., 2008 | N | N | Y | 0 | Y | 3 |
| Zhao et al., 2008 | Y | Y | Y | 5.6 | Y | 6 |