Abstract
β-catenin contributes to the pathogenesis of lung fibrosis. However, the expression of β-catenin in fibroblasts under fibrotic conditions has not been studied. We investigated the expression of β-catenin in lung fibroblasts from bleomycin (BLM)-challenged mice and human lung fibroblasts treated with transforming growth factor β (TGF-β) or lysophosphatidic acid (LPA) by western blot analysis. The result showed that the expression of β-catenin was significantly increased in lung fibrotic foci and lung fibroblasts from bleomycin-challenged mice. TGF-β stimulated β-catenin expression and induced differentiation in human lung fibroblasts in vitro. Pretreatment of the NF-κB activation inhibitor attenuated the TGF-β-induced expression of β-catenin and differentiation in human lung fibroblasts. Similarly, LPA induced β-catenin expression in human lung fibroblasts, and pre-treatment of the neutralized anti-TGF-β antibody attenuated the LPA-induced expression of β-catenin and differentiation in human lung fibroblasts. The results suggested that β-catenin expression is upregulated in lung fibroblast during differentiation, and that TGF-β induced β-catenin expression in human lung fibroblasts through the activation of NF-κB.
Keywords: β-catenin, LPA, TGF-β, NF-κB, pulmonary fibrosis, fibroblast
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic and progressive interstitial lung disease characterized by distorted lung architecture and loss of respiratory function (1,2). Over five million individuals are afflicted with IPF in the USA, and the average survival time of IPF patients is only 2–5 years after initial diagnosis (3,4). Despite advances in understanding of the basic molecular pathways that drive this uncontrolled fibrotic process, lung transplantation remains the only effective cure for IPF (5). Thus, investigations to improve understanding of the pathological mechanisms and lead to the development of efficacious therapeutic approaches for IPF are necessary.
Fibroblast accumulation leads to excessive scarring of lung tissue, and progressive and irreversible destruction of lung architecture, leading to loss of lung function, disruption of gas exchange and fibrogenesis in lung (6). Investigations thus far have demonstrated that transforming growth factor-β (TGF-β), sphingosine 1 phospate (S1P) and lysophosphatidic acid (LPA) are involved in fibrogenesis in different organs, such as heart, liver and lung (4,6–9). Findings of recent studies suggest the crosstalk between TGF-β pathways and LPA or S1P pathways in lung fibroblasts (4,9). LPA also increases TGF-β expression and secretion in lung fibroblasts through the activation of its receptors (9). Additionally, in vitro investigations have shown that S1P induced the activation, differentiation and migration of lung fibroblasts (4). However, the crosstalk between the TGF-β pathway and other pathways on the effects in fibroblast differentiation remains to be determined.
Wnt/β-catenin signaling is a key regulator in tissue repair, fibrosis, remodeling or destruction (2,10,11). Immunohistochemical staining shows the increase of nuclear β-catenin staining in IPF tissue sections (12,13). Unbiased microarray screens have revealed an increased expression of Wnt/β-catenin target genes in lung tissue from IPF patients (12). Additionally, aberrant expression of extracellular matrix (ECM) proteins through epithelial-mesenchymal transition (EMT) and fibroblast differentiation contributes to pulmonary fibrosis (14–16). Mounting evidence suggest that the Wnt/β-catenin and TGF-β pathways contribute to the epithelial-mesenchymal transition (EMT) during pulmonary fibrogenesis (10,11,17,18). In vitro, upregulation of Wnt/β-catenin signaling promotes the proliferation of alveolar type II (AT2) cells and inhibits their ability to differentiate into alveolar type I (AT1 cells) cells (19). In lung fibroblasts, activation of Wnt/β-catenin signaling also stimulates ECM gene expression (20). However, the expression of β-catenin in lung fibroblasts has not been studied and the potential mechanism remains to be determined.
In the present study, we investigated β-catenin expression under fibrotic conditions by western blot analysis. The results showed that β-catenin expression was markedly increased in lung tissue and fibroblasts from bleomycin-challenged mice, and regulated by the activation of NF-κB.
Materials and methods
Reagents and kits
Bleomycin sulfate was obtained from Hospira Inc. (Lake Forest, IL, USA), and neutralizing chicken anti-TGF-β1 antibody and control chicken IgG were obtained from R&D Systems (Minneapolis, MN, USA). Oleoyl lysophosphatidic acid (18:1 LPA) was obtained from Avanti Polar Lipids (Alabaster, AL, USA), and the cell lysis buffer was purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Protease inhibitor cocktail tablets (EDTA-free Complete) were purchased from Roche Diagnostics (Indianapolis, IN, USA). Recombinant human TGF-β1 was obtained from Protech, Inc. (Rocky Hill, NJ, USA). Horseradish peroxidase-conjugated anti-mouse IgG and anti-rabbit IgG antibodies were obtained from Bio-Rad Laboratories, Inc. (Hercules, CA, USA). Rabbit anti-fibronectin and anti-β-catenin antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Mouse anti-α-smooth muscle actin (α-SMA) and anti-β actin antibodies and Bay 11-7082 (NF-κB inhibitor) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Experimental pulmonary fibrosis model
The animal experiment of pulmonary fibrosis was designed as previously described (4,9). Briefly, C57/BL6 mice (male, aged 8 weeks) purchased from Jackson Laboratory (Bar Harbor, ME, USA) were used for bleomycin-induced fibrosis. Briefly, C57/BL6 mice were anesthetized (with a 3 ml/kg mixture of 25 mg/kg of ketamine in 2.5 ml of xylazine), followed by treatment with saline or bleomycin sulfate (1.5 U/kg of body weight, ~0.03 units/animal) in saline by an intratracheal injection in a total volume of 50 μl. Twenty-one days post-bleomycin administration, the animals were sacrificed by cervical dislocation and the lungs were removed for histological staining and isolation of lung fibroblasts.
Immunohistochemical staining and Masson’s trichrome staining of mouse lung tissue
Lung tissues from mice with or without bleomycin challenge were embedded in paraffin and cut as 5-μm sections for staining. Following the removal of paraffin with xylene and clearing with alcohol, the slides were applied for immunohistochemical staining and Masson’s trichrome staining and examined as previously described (9).
Cell culture
Murine lung fibroblasts were isolated from mice with or without bleomycin (BLM) challenge as previously described (9,21). A human lung fibroblasts cell line (WI-38) was obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA) and cells were grown and maintained in 6-well dishes with Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS). Primary murine lung fibroblasts, isolated from C57/BL6 mice with or without bleomycin treatment, were also cultured in DMEM containing 10% FBS.
Treatment of neutralizing antibodies or NF-κB inhibitor
Serum-starved (for 24 h) human lung fibroblasts (WI-38, ~90% confluence) were pretreated with neutralized anti-TGF-β antibody or control IgG antibody (5 μg/ml, 1 h). For nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) inhibitor (Bay 11-7082), the compound was pretreated with a final concentration of 10 μM for 1 h (22). The cells were subsequently challenged with 18:1 LPA (10 μM) or TGF-β (5 ng/ml) for 48 h, and cell lysates (20 μg protein) were subjected to SDS-PAGE and western blotting.
SDS-PAGE and western blotting
SDS-PAGE and western blotting were performed as previously described (9). The integrated density of pixels in each membrane was quantified using ImageQuant 5.2 software (Molecular Dynamics, Sunnyvale, CA, USA).
Immunofluorescence staining
Immunofluorescence microscopy to determine protein expression was performed as previously described (9). Briefly, primary murine lung fibroblasts were grown on slide chambers for 24 h. The cells were fixed, incubated with primary antibodies (1:200 dilutions in blocking buffer) for 1 h and with Alexa Fluor secondary antibodies (1:200 dilutions in blocking buffer) for another 1 h, followed by mounting. The cells were then examined under a Nikon Eclipse TE 2000-S fluorescence microscope with a 60× oil immersion objective lens.
Statistical analysis
Data are expressed as means ± SEM from at least three independent sets of experiments. Results were subjected to statistical analysis using one-way ANOVA or a two-tailed Student’s t-test. P<0.05 was considered to indicate a significant result (23,24).
Results
Expression of β-catenin in lung fibrotic foci from mice with bleomycin challenge
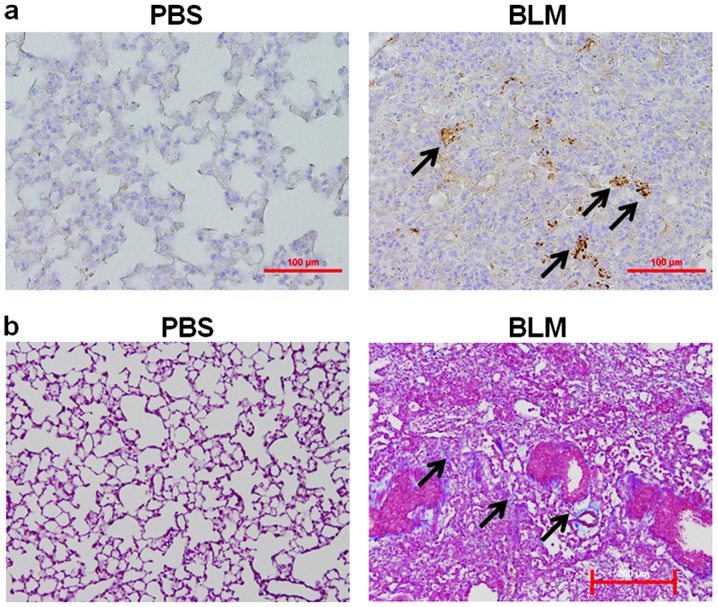
The expression of β-catenin in lung fibrotic foci from bleomycin-challenged mice was assessed. Immunohistochemical staining revealed that the expression of β-catenin was significantly increased in lung fibrotic foci from bleomycin-challenged mice (Fig. 1a). Trichrome staining revealed that the expression of collagen, bio-markers of pulmonary fibrosis, was markedly increased in these mice (Fig. 1b).
Figure 1.
Staining of β-catenin and collagen in lung tissue from mice with or without BLM challenge. Representative immunohistochemical staining for (a) β-catenin and (b) Masson’s trichrome staining for collagen in murine lung tissue isolated from mice with or without BLM challenge. Arrow shows the marked expression of (a) β-catenin (brown color) and (b) the deposition of collagen (blue color) in fibrotic foci. Scale bar, 200 μm.
Expression of β-catenin in lung fibroblasts from mice with or without bleomycin challenge
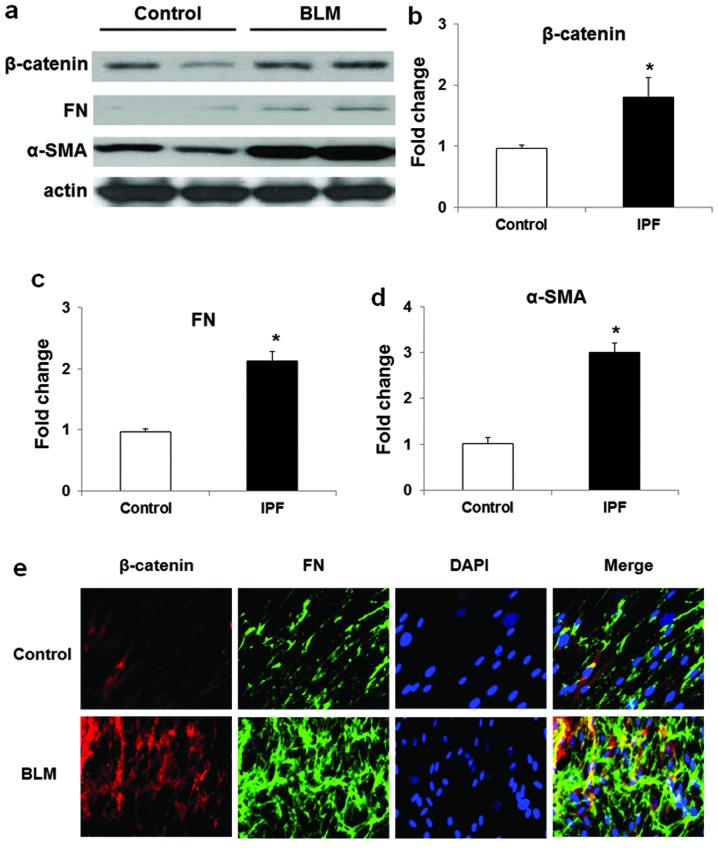
The expression of β-catenin in fibroblasts from control and fibrotic lungs was also examined. As shown in Fig. 2a–d, the expression of β-catenin, as well as α-SMA and FN, bio-markers of fibroblast differentiation, were markedly increased in lung fibroblasts from bleomycin-challenged mice as compared to that from the control mice. Immunofluorescence staining also showed that the expression of β-catenin was markedly increased in lung fibroblasts from bleomycin-challenged mice as compared to that from the control mice (Fig. 2e). These findings suggested that β-catenin expressed in lung fibroblasts may correlate to fibroblast differentiation under fibrotic conditions.
Figure 2.
Expression of β-catenin, α-SMA and FN in lung fibroblasts from mice with or without BLM challenge. (a) Representative western blot analysis (b–d) quantification of the expression of (b) β-catenin, (c) FN and (d) α-SMA) in lung fibroblasts from mice with or without BLM challenge. Data are expressed as means ± SEM of three independent experiments. *P<0.05 vs. cells from control mice. (e) Immunofloresent staining of β-catenin (red) and FN (green) in lung fibroblasts from mice with or without BLM challenge. Images were examined by immunofluorescence microscopy and recorded by using a 60× oil objective.
TGF-β induces β-catenin expression in human lung fibroblasts
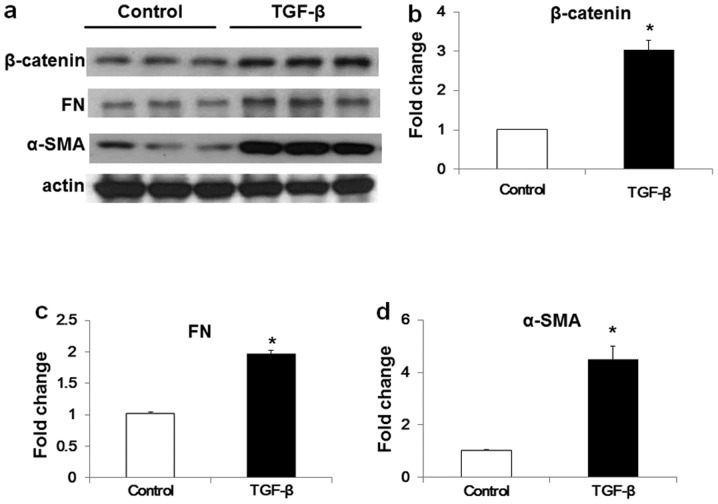
TGF-β is a key factor for fibroblast differentiation, and plays critical roles in fibroblast differentiation. To determine whether the expression of β-catenin is correlated with TGF-β-induced activation and differentiation of lung fibroblasts, we assessed the expression of β-catenin in human lung fibroblasts challenged by TGF-β. Western blot analysis revealed that TGF-β challenge (5 ng/ml, 48 h) markedly increased the expression of β-catenin and fibroblast differentiation (Fig. 3a and d).
Figure 3.
TGF-β induced the expression of β-catenin, FN and α-SMA in human lung fibroblasts. (a) Representative western blot and (b–d) quantification of the expression of (b) β-catenin, (c) FN and (d) α-SMA in human lung fibroblasts with or without TGF-β challenge. Data are expressed as means ± SEM of three independent experiments. *P<0.05 vs. cells without TGF-β challenge.
TGF-β induces β-catenin expression through NF-κB
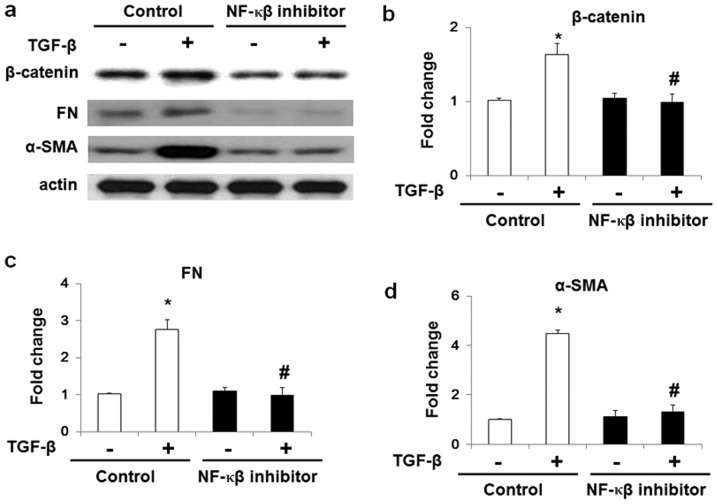
To investigate the molecular mechanisms of the TGF-β-induced expression of β-catenin expression in lung fibroblasts, we pretreated the WI-38 human lung fibroblast cell line with NF-κB inhibitor (10 μM) for 1 h prior to the TGF-β challenge. As shown in Fig. 4, TGF-β upregulated β-catenin, α-SMA and FN expression in human lung fibroblasts, whereas pretreatment of the NF-κB inhibitor markedly blocked the effects of TGF-β. These data showed that NF-κB signaling pathways were involved in TGF-β-induced β-catenin expression in human lung fibroblasts.
Figure 4.
NF-κB inhibitor attenuates TGF-β-induced expression of β-catenin, FN and α-SMA in human lung fibroblasts. (a) Representative and (b–d) quantification of the expression of (b) β-catenin, (c) FN and (d) α-SMA in human lung fibroblasts with or without TGF-β challenge. Data are expressed as means ± SEM of three independent experiments. *P<0.05 vs. cells without TGF-β challenge. #P<0.05 vs. cells without NF-κB inhibitor treatment and with TGF-β challenge.
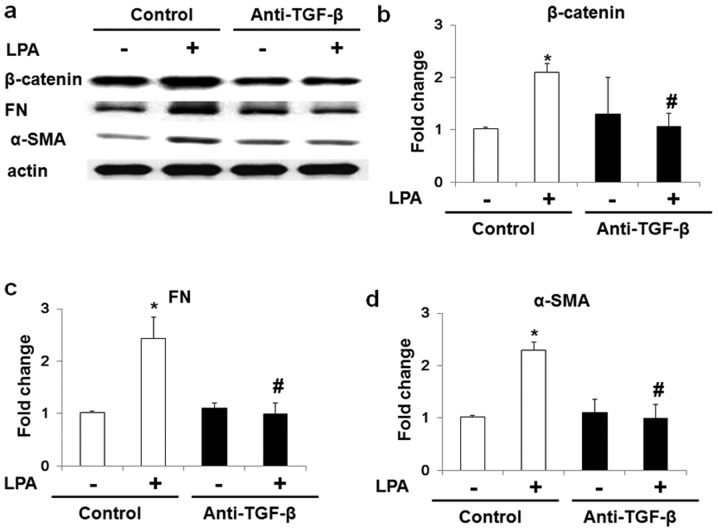
LPA-induced differentiation of fibroblasts and expression of β-catenin is attenuated by anti-TGF-β antibody
The LPA effects on the differentiation and expression of β-catenin in human lung fibroblasts were examined. As shown in Fig. 5, LPA treatment induced fibroblast differentiation, and increased β-catenin expression in human lung fibroblast. Additionally, to block the effect of secreted TGF-β under LPA challenge, the cells were pretreated with an anti-TGF-β antibody. Pretreatment of anti-TGF-β antibody markedly inhibited the LPA-induced differentiation of human lung fibroblasts and significantly blocked the LPA-induced expression of β-catenin (Fig. 5). These results suggested that LPA also induced the expression of β-catenin in human lung fibroblasts, and this effect at least, partly through LPA induced the expression and secretion of TGF-β.
Figure 5.
Lysophosphatidic acid (LPA) induced the differentiation and expression of β-catenin in human lung fibroblasts. (a) Representative western blot (b–d) quantification of the expression of (b) β-catenin, (c) FN and (d) α-SMA in LPA-challenged WI-38 cells. Data are expressed as means ± SEM of three independent experiments. *P<0.05 vs. cells with control antibody but without LPA treatment. #P<0.05 vs. LPA-challenged cells with pretreatment of control antibody.
Discussion
Idiopathic pulmonary fibrosis (IPF) is a fatal lung disease with no effective pharmacological treatment. Findings of previous studies have shown that following the progression of pulmonary fibrosis, various lipid ligands, cytokines and chemokines were altered (2,4,7,25). TGF-β has also been identified as a key factor for pulmonary fibrogenesis and differentiation in lung fibroblasts (2). Previous studies have proven crosstalk of the TGF-β and Wnt/β-catenin pathways during fibrogenesis (26). However, the role of TGF-β in the expression of β-catenin in fibroblasts remains to be investigated.
As an important component of Wnt/β-catenin pathway, β-catenin is involved in the regulation of branching morphogenesis, regional specialization of the epithelium and mesenchyme and establishment of progenitor cell pools. During pulmonary fibrogenesis, the Wnt/β-catenin signaling pathway contributes to proliferation, differentiation and migration in lung fibroblasts and EMT in lung epithelium cells. Immunohistochemical staining of lung tissue from IPF patients indicated that nuclear β-catenin accumulation was demonstrated in fibroblast foci. Aberrant activation of Wnt/β-catenin signaling and expression of the downstream genes were observed in bronchiolar lesions from IPF patients (27). Expression of β-catenin in nuclear also increased in fibrotic lesions and promoted the migration and proliferation in lung tissue from systemic sclerosis pulmonary fibrosis patients (12). Additional investigation by qPCR demonstrated that the Wnt/β-catenin pathway is expressed and operative in adult lung epithelium (10). In ventilator-induced pulmonary fibrosis, Wnt/β-catenin signaling was activated at an early stage by mechanical ventilation in lungs suggesting that modulation of the Wnt/β-catenin pathway may be a therapeutic option for the prevention of ventilator-induced pulmonary fibrosis (28). In bleomycin-induced pulmonary fibrosis, blockade of the Wnt/β-catenin pathway by β-catenin siRNA or ICG-001, a small molecule that specifically inhibits T-cell factor/β-catenin transcription in a cyclic AMP response-element binding protein binding protein (CBP)-dependent manner, inhibited bleomycin-induced pulmonary fibrosis in mouse (29,30). In lung fibroblasts from IPF patients, the lower expression of α2β1 integrin resulted in the activation of β-catenin pathway is critical for the proliferation of fibroblasts on collagen expression (20). The abovementioned studies suggested that targeting the expression and activation of β-catenin is a potential therapeutic strategy for IPF.
Recently, S1P and LPA, as well as other lipid ligands, were also proven to be involved in inflammatory reactions (4,9,31–35) and pulmonary fibrosis (4,9). In lung tissue from IPF patients, the level of S1P and LPA were markedly higher than that from control patients (4,7,36). In vitro studies also showed that S1P and LPA activated the recruitment, activation and differentiation of lung fibroblasts through their G protein-coupled receptors (4,7,9). LPA has also been found to induce the expression of TGF-β in human lung fibroblasts through the activation of LPA receptor type 2 in lung fibroblasts (9). The present study suggests that LPA induced expression of β-catenin in human lung fibroblasts at least partly through TGF-β.
In conclusion, results of the present study have shown that β-catenin expression increased in lung fibrotic foci and its expression was positively correlated with the activation of lung fibroblasts under pathological conditions in vitro and in vivo. Additionally, TGF-β increased β-catenin expression through the NF-κB signaling pathway.
Acknowledgements
This project was supported by the Science and Technology Department of Jiangsu Province (No. BK20140242).
References
- 1.King TE. Update in pulmonary medicine. Ann Intern Med. 1998;129:806–812. doi: 10.7326/0003-4819-129-10-199811150-00012. [DOI] [PubMed] [Google Scholar]
- 2.Wolters PJ, Collard HR, Jones KD. Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol. 2013;9:157–179. doi: 10.1146/annurev-pathol-012513-104706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raghu G, Freudenberger TD, Yang S, et al. High prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosis. Eur Respir J. 2006;27:136–142. doi: 10.1183/09031936.06.00037005. [DOI] [PubMed] [Google Scholar]
- 4.Huang LS, Berdyshev E, Mathew B, et al. Targeting sphingosine kinase 1 attenuates bleomycin-induced pulmonary fibrosis. FASEB J. 2013;27:1749–1760. doi: 10.1096/fj.12-219634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marks JH. Update in pulmonary medicine. Adolesc Med State Art Rev. 2013;24:307–329. xvi. [PubMed] [Google Scholar]
- 6.Wynn TA. Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011;208:1339–1350. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tager AM, LaCamera P, Shea BS, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14:45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 8.Funke M, Zhao ZW, Xu Y, Chun J, Tager AM. The lysophosphatidic acid receptor LPA1 promotes epithelial cell apoptosis after lung injury. Am J Resp Cell Mol. 2012;46:355–364. doi: 10.1165/rcmb.2010-0155OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang LS, Fu P, Patel P, et al. Lysophosphatidic acid receptor-2 deficiency confers protection against bleomycin-induced lung injury and fibrosis in mice. Am J Respir Cell Mol Biol. 2013;49:912–922. doi: 10.1165/rcmb.2013-0070OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konigshoff M, Balsara N, Pfaff EM, et al. Functional Wnt signaling is increased in idiopathic pulmonary fibrosis. PLoS One. 2008;3:e2142. doi: 10.1371/journal.pone.0002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo Y, Xiao L, Sun L, Liu F. Wnt/β-catenin signaling: a promising new target for fibrosis diseases. Physiol Res. 2012;61:337–346. doi: 10.33549/physiolres.932289. [DOI] [PubMed] [Google Scholar]
- 12.Lam AP, Flozak AS, Russell S, et al. Nuclear β-catenin is increased in systemic sclerosis pulmonary fibrosis and promotes lung fibroblast migration and proliferation. Am J Respir Cell Mol Biol. 2011;45:915–922. doi: 10.1165/rcmb.2010-0113OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konigshoff M, Kramer M, Balsara N, et al. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest. 2009;119:772–787. doi: 10.1172/JCI33950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosper NA, van den Berg PP, de Rond S, et al. Epithelial-to-mesenchymal transition in fibrosis: collagen type I expression is highly upregulated after EMT, but does not contribute to collagen deposition. Exp Cell Res. 2013;319:3000–3009. doi: 10.1016/j.yexcr.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Clarke DL, Carruthers AM, Mustelin T, Murray LA. Matrix regulation of idiopathic pulmonary fibrosis: the role of enzymes. Fibrogenesis Tissue Repair. 2013;6:20. doi: 10.1186/1755-1536-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim KK, Wei Y, Szekeres C, et al. Epithelial cell α3β1 integrin links β-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J Clin Invest. 2009;119:213–224. doi: 10.1172/JCI36940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milosevic J, Pandit K, Magister M, et al. Profibrotic role of miR-154 in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2012;47:879–887. doi: 10.1165/rcmb.2011-0377OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caraci F, Gili E, Calafiore M, et al. TGF-β1 targets the GSK-3β/β-catenin pathway via ERK activation in the transition of human lung fibroblasts into myofibroblasts. Pharmacol Res. 2008;57:274–282. doi: 10.1016/j.phrs.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Liu AR, Liu L, Chen S, et al. Activation of canonical wnt pathway promotes differentiation of mouse bone marrow-derived MSCs into type II alveolar epithelial cells, confers resistance to oxidative stress, and promotes their migration to injured lung tissue in vitro. J Cell Physiol. 2013;228:1270–1283. doi: 10.1002/jcp.24282. [DOI] [PubMed] [Google Scholar]
- 20.Xia H, Seeman J, Hong J, et al. Low α2β1 integrin function enhances the proliferation of fibroblasts from patients with idiopathic pulmonary fibrosis by activation of the β-catenin pathway. Am J Pathol. 2012;181:222–233. doi: 10.1016/j.ajpath.2012.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kono Y, Nishiuma T, Nishimura Y, et al. Sphingosine kinase 1 regulates differentiation of human and mouse lung fibroblasts mediated by TGF-β1. Am J Respir Cell Mol Biol. 2007;37:395–404. doi: 10.1165/rcmb.2007-0065OC. [DOI] [PubMed] [Google Scholar]
- 22.Chen L, Ruan Y, Wang X, et al. BAY 11-7082, a nuclear factor-κB inhibitor, induces apoptosis and S phase arrest in gastric cancer cells. J Gastroenterol. 2014;49:864–874. doi: 10.1007/s00535-013-0848-4. [DOI] [PubMed] [Google Scholar]
- 23.Huang LS, Kim MR, Sok DE. Enzymatic reduction of polyunsaturated lysophosphatidylcholine hydroperoxides by glutathione peroxidase-1. Eur J Lipid Sci Tech. 2009;111:584–592. [Google Scholar]
- 24.Huang LS, Kim MR, Sok DE. Oxygenation of 1-docosahexaenoyl lysophosphatidylcholine by lipoxygenases; conjugated hydroperoxydiene and dihydroxytriene derivatives. Lipids. 2007;42:981–990. doi: 10.1007/s11745-007-3112-y. [DOI] [PubMed] [Google Scholar]
- 25.Rancoule C, Pradere JP, Gonzalez J, et al. Lysophosphatidic acid-1-receptor targeting agents for fibrosis. Expert Opin Investig Drugs. 2011;20:657–667. doi: 10.1517/13543784.2011.566864. [DOI] [PubMed] [Google Scholar]
- 26.Scheraga RG, Thannickal VJ. Wnt/β-catenin and transforming growth factor-β signaling in pulmonary fibrosis. A case for antagonistic pleiotropy? Am J Respir Crit Care Med. 2014;190:129–131. doi: 10.1164/rccm.201406-1037ED. [DOI] [PubMed] [Google Scholar]
- 27.Chilosi M, Poletti V, Zamo A, et al. Aberrant Wnt/β-catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol. 2003;162:1495–1502. doi: 10.1016/s0002-9440(10)64282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villar J, Cabrera NE, Valladares F, et al. Activation of the Wnt/β-catenin signaling pathway by mechanical ventilation is associated with ventilator-induced pulmonary fibrosis in healthy lungs. PLoS One. 2011;6:e23914. doi: 10.1371/journal.pone.0023914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim TH, Kim SH, Seo JY, et al. Blockade of the Wnt/β-catenin pathway attenuates bleomycin-induced pulmonary fibrosis. Tohoku J Exp Med. 2011;223:45–54. doi: 10.1620/tjem.223.45. [DOI] [PubMed] [Google Scholar]
- 30.Henderson WR, Jr, Chi EY, Ye X, et al. Inhibition of Wnt/β-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc Natl Acad Sci USA. 2010;107:14309–14314. doi: 10.1073/pnas.1001520107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang LS, Hung ND, Sok DE, Kim MR. Lysophosphatidylcholine containing docosahexaenoic acid at the sn-1 position is anti-inflammatory. Lipids. 2010;45:225–236. doi: 10.1007/s11745-010-3392-5. [DOI] [PubMed] [Google Scholar]
- 32.Huang LS, Kim MR, Sok DE. Regulation of lipoxygenase activity by polyunsaturated lysophosphatidylcholines or their oxygenation derivatives. J Agric Food Chem. 2008;56:7808–7814. doi: 10.1021/jf801082x. [DOI] [PubMed] [Google Scholar]
- 33.Huang LS, Kang JS, Kim MR, Sok DE. Oxygenation of arachidonoyl lysophospholipids by lipoxygenases from soybean, porcine leukocyte, or rabbit reticulocyte. J Agric Food Chem. 2008;56:1224–1232. doi: 10.1021/jf073016i. [DOI] [PubMed] [Google Scholar]
- 34.Huang LS, Kim MR, Jeong TS, Sok DE. Linoleoyl lysophosphatidic acid and linoleoyl lysophosphatidylcholine are efficient substrates for mammalian lipoxygenases. Biochim Biophys Acta. 2007;1770:1062–1070. doi: 10.1016/j.bbagen.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Huang LS, Kim MR, Sok DE. Linoleoyl lysophosphatidylcholine is an efficient substrate for soybean lipoxygenase-1. Arch Biochem Biophys. 2006;455:119–126. doi: 10.1016/j.abb.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 36.Milara J, Navarro R, Juan G, et al. Sphingosine-1-phosphate is increased in patients with idiopathic pulmonary fibrosis and mediates epithelial to mesenchymal transition. Thorax. 2012;67:147–156. doi: 10.1136/thoraxjnl-2011-200026. [DOI] [PubMed] [Google Scholar]