Abstract
The epidemiology, phylogeny, and biology of nonencapsulated Streptococcus pneumoniae are largely unknown. Increased colonization capacity and transformability are, however, intriguing features of these pneumococci and play an important role. Twenty-seven nonencapsulated pneumococci were identified in a nationwide collection of 1,980 nasopharyngeal samples and 215 blood samples obtained between 1998 and 2002. On the basis of multilocus sequence typing and capsule region analysis we divided the nonencapsulated pneumococci into two groups. Group I was closely related to encapsulated strains. Group II had a clonal population structure, including two geographically widespread clones able to cause epidemic conjunctivitis and invasive diseases. Group II strains also carried a 1,959-bp homologue of aliB (aliB-like ORF 2) in the capsule region, which was highly homologous to a sequence in the capsule region of Streptococcus mitis. In addition, strains of the two major clones in group II had an additional sequence, aliB-like ORF 1 (1,968 to 2,004 bp), upstream of aliB-like ORF 2. Expression of aliB-like ORF 1 was detected by reverse transcription-PCR, and the corresponding RNA was visualized by Northern blotting. A gene fragment homologous to capN of serotypes 33 and 37 suggests that group II strains were derived from encapsulated pneumococci some time ago. Therefore, loss of capsule expression in vivo was found to be associated with the importation of one or two aliB homologues in some nonencapsulated pneumococci.
Streptococcus pneumoniae is an important human pathogen that causes meningitis, sepsis, pneumonia, and upper respiratory tract infections, such as acute otitis. This gram-positive bacterium is usually surrounded by a polysaccharide capsule, which acts as a virulence factor by protecting the bacterium from phagocytosis. The capsule is the main target for protective antibodies, and pneumococcal strains are grouped into serotypes based on the antigenic properties of their capsules. The capsule is also the main target of currently used pneumococcal vaccines.
Some S. pneumoniae strains do not appear to bind anticapsular antibodies and, when cultured on agar, form rough colonies rather than the smooth colonies formed by bacteria with capsules. Nonencapsulated strains are less virulent than encapsulated strains in a mouse model (12), but nonencapsulated strains can also be responsible for disease, as described by Martin et al. (17), who found that an outbreak of conjunctivitis on a college campus was due to a nonencapsulated clone.
Little is known about the epidemiology and population genetics of nonencapsulated pneumococci. Whatmore et al. (30) described two groups of S. pneumoniae which lack typical features of pneumococci: (i) strains which, based on the sequence of housekeeping genes, appear to be genetically distinct from typical encapsulated strains and lack one or more of the three characteristics bile solubility, optochin sensitivity, and capsule expression expected of S. pneumoniae, and (ii) strains which lack capsule expression but genetically appear to be closely related to typical encapsulated strains.
Nonencapsulated strains adhere better to respiratory epithelial cells and have an advantage when the nasopharynx is colonized (1, 6, 27). Also, the lack of a capsule makes them more transformable (20, 28). Encapsulated pneumococci may therefore take advantage of down-regulation or transitory loss of capsule expression while they colonize the nasopharynx (25, 26). A mechanism that has been shown to prevent capsule expression in vitro is inactivation of capsule genes by duplications which can later be excised, causing reversion to the encapsulated phenotype (25, 26). Also, a single mutation can lead to the loss of capsule expression for serotype 3 (3).
In this paper we describe a novel finding. Loss of capsule expression in vivo was observed to be associated with the importation of an aliB homologue in some nonencapsulated pneumococci.
(Some of the data were presented at the 103rd General Meeting of the American Society for Microbiology, Washington, D.C., 18 to 22 May 2003.)
MATERIALS AND METHODS
Bacterial strains.
The colonizing strains used for this study originated from a strain collection (1,980 isolates) obtained from a nationwide surveillance for nasopharyngeal isolates of S. pneumoniae in 1998 and 1999 and in 2001 and 2002 (19). The invasive strains of S. pneumoniae used (an invasive strain was defined as a culture from a sterile body site) originated from nationwide collection of all invasive isolates in 1998 and 1999 (23) and between March and December 2002 (24). S. pneumoniae was grown and identified and antibiotic susceptibility was determined as described previously (19). Bacteria were routinely grown on Columbia sheep blood agar (CSBA) plates at 37°C in a 5% CO2-enriched atmosphere or in brain heart infusion (BHI) broth containing 5% fetal calf serum (FCS) at 37°C in ambient air and were stored at −80°C by using Protect bacterial preservers (TSC, Heywood, United Kingdom).
Serotyping.
Isolates were serotyped by using the Quellung reaction with specific antisera from the Statens Serum Institute (Copenhagen, Denmark). Strains which did not react with any of the pool sera were retyped after growth under anaerobic conditions to enhance capsule expression (29). All nontypeable strains were sent to the Statens Serum Institute for retyping.
DNA methods.
Chromosomal DNA were obtained from S. pneumoniae isolates as described previously (18). Southern blotting was performed essentially as described previously (21), with the following exceptions. Probes were labeled nonradioactively by using the ECL direct nucleic acid labeling and detection system (Amersham Biosciences, Duebendorf, Switzerland) according to the manufacturer's instructions. The same system was used to detect signals. Chromosomal DNA was digested with restriction enzyme PvuII. The primers and probes used for Southern blotting are described in Table 1 and Fig. 1. Probes were amplified by using Taq DNA polymerase (Roche Molecular Biochemicals, Rotkreuz, Switzerland). Restriction enzymes were purchased from New England Biolabs (Frankfurt am Main, Germany).
TABLE 1.
Primers used for characterization of the cap region
| Primera | Sequenceb | Use | Reference |
|---|---|---|---|
| FI3 | TCTTAGTTCCATGGGATGCTTTCTGTGTG | Long-range PCR, Southern blot probe cap110.58, sequencing of cap region, and in vitro translation of aliB-like ORF 1 | 11 |
| FI4 | CGCTGAACTTTTGTAGTTGCTGTCTGGTCAAC | Long-range PCR and sequencing of cap region | 11 |
| aliA_b832 | TGCAGGTTTGCTGGTATCTTGAC | Long-range PCR, Southern blot probe cap110.58, and sequencing of cap region | This study |
| cpsA3 | ATCCTTGTCAGCTCTGTGTC | PCR for Southern blot probe cap111.46 | 14 |
| cpsB2 | TCACTTGCAACTACATGAAC | PCR for Southern blot probe cap111.46 | 14 |
| 104_b832.12 | GTATCTGAACCTGATTGTCCGC | In vitro translation of aliB-like ORF 1 | This study |
| 110_FI3.4 | AACACTTGGAACGGAGAATG | PCR detection of aliB-like ORF 1 and RT-PCR | This study |
| 104_b832.14 | GCCCTTTGTTATACCTAGATGTTTC | PCR detection of aliB-like ORF 1 and RT-PCR | This study |
| 104_FI3.6 | AGATGCCAAATGGTTCACGG | PCR detection of aliB-like ORF 2 and PCR for Southern blot aliB-like ORF 2 probe | This study |
| 104_b832.10 | GAAATCTTTTAACAAATAAGGTCCG | PCR detection of aliB-like ORF 2 and PCR for Southern blot aliB-like ORF 2 probe | This study |
| Rif-399f | GATGAYATCGAYCACCTCGGAAA | RT-PCR, control | 18 |
| Rif-525r | GATGTTAGGTCCTTCAGGTGTCTC | RT-PCR, control | 18 |
| dexB_f145_XbaI | GCTCTAGAGCTGGCTTTCTCCCGTTTATGACA | PCR for mutant construction | This study |
| dexB_b1504_BamHI | CGGGATCCCGAGACAGATTTGACGTTTCCTTC | PCR for mutant construction | This study |
| capN_F1_ClaI | CCATCGATGGTCGTTGGTGCTGGTTTGTCA | PCR for mutant construction | This study |
| capN_B1_XhoI | CCGCTCGAGGCACTGCTTTGAGGTTGTAGATAA | PCR for mutant construction | This study |
| cat_Spn_F1_HindIII | CCCAAGCTTGGATTTTTCGCTACGCTCAA | PCR for mutant construction | This study |
| cat_Spn_B1_HindIII | CCCAAGCTTTCACTTATTCAGGCGTAGCACCAG | PCR for mutant construction | This study |
Primers used for primer walking sequencing of the different cap regions are not shown.
Recognition sites for restriction enzymes within the primers are underlined.
FIG. 1.
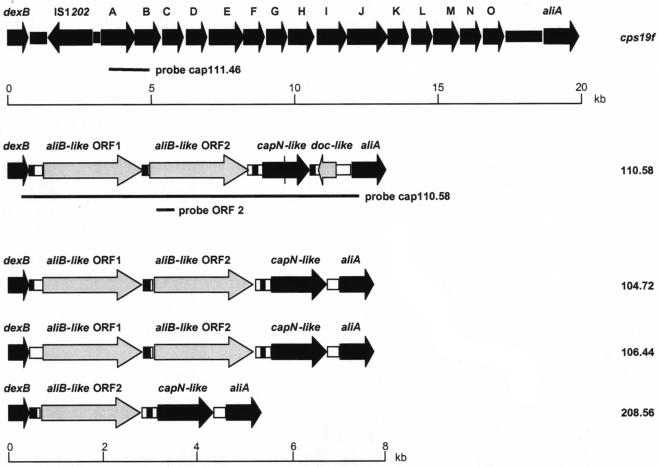
Schematic representation of cap regions of nonencapsulated S. pneumoniae strains. Cap regions (i.e., the DNA sequence between the dexB and aliA genes) were sequenced and analyzed for nonencapsulated S. pneumoniae strains 110.58, 104.72 106.44, and 208.56. For comparison, the cap region of a serotype 19F strain is shown at the top (9). Regions and ORFs found in nonencapsulated strains exhibiting high levels of homology to the cap region (including dexB and aliA) of encapsulated S. pneumoniae strains are indicated by black arrows (ORF) or boxes. The capN-like ORF of strain 110.58 contains a premature stop codon within the ORF represented by a vertical line. The aliB-like ORF 1 and aliB-like ORF 2, as well as the doc-like ORF found in the cap region of the nonencapsulated strains, are indicated by gray arrows. Regions with no homology to database entries are indicated by open boxes. The black boxes indicate complete and incomplete BOX elements found between dexB and aliB-like ORF 1 (strains 110.58 and 104.72), between aliB-like ORF 2 and a capN-like ORF (all nonencapsulated strains), and between a capN-like ORF and aliA (strain 110.58). Probes cap111.46 and cap110.58 and the aliB-like ORF 2 probe used for Southern blot analysis are indicated by lines. The cps19f gene (9) and the cap region of nonencapsulated strains are drawn to different scales; scale bars are indicated beneath the corresponding regions.
Multilocus sequence typing (MLST).
Amplification, sequencing, and analysis of the seven housekeeping genes were carried out as described previously (8).
PCR amplification and sequencing of the cap region.
The cap region of individual strains was amplified with forward primer FI3 and reverse primer FI4 or aliA_b832 (Table 1) by using the Expand Long Template PCR system (Roche) according to the manufacturer's instructions. Amplification was performed by using the following cycling conditions: primary denaturation for 2 min at 92°C, followed by 10 cycles consisting of 92°C for 10 s, 65°C for 30 s, and 68°C for 17 min and then 20 cycles in which each extension cycle was prolonged by 20 s. PCR products were purified with a QIAquick PCR purification kit (QIAGEN, Basel, Switzerland). The entire cap region of strains 104.72, 106.44, 110.58, and 208.56 was sequenced by primer walking performed by Microsynth GmbH (Balgach, Switzerland) or in house with a ABI PRISM 310 genetic analyzer (PE Biosystems, Rotkreuz, Switzerland) by using a Big Dye terminator cycle sequencing Ready Reaction kit (PE Biosystems). DNA sequences were analyzed and aligned by using the Lasergene software (DNASTAR Inc., Madison, Wis.). Open reading frames (ORF) within the cap regions were analyzed further by performing a translated BLAST search (blastx) at the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov/BLAST/). PCRs for detection of aliB-like ORF 1 and aliB-like ORF 2 were carried out by using Taq DNA polymerase (Roche). The DNA molecular weight standard used was Marker X (Roche).
RNA isolation.
Bacteria were subcultured in BHI broth containing 5% FCS and were incubated at 37°C until the optical density at 600 nm was 0.6. Twice the culture volume of RNAprotect (QIAGEN) was added to prevent further transcription and to prevent RNA degradation. After vortexing and 5 min of incubation at room temperature, the bacteria were pelleted by centrifugation for 10 min at 5,000 × g. The pellet was resuspended in 200 μl of TE buffer (10 mM Tris-Cl, 1 mM EDTA; pH 8) containing 600 μg of lysozyme. After incubation for 10 min at room temperature with repeated vortexing, 700 μl of RLT buffer (QIAGEN RNeasy mini kit) containing β2-mercaptoethanol was added, and the tube was vortexed. The mixture was transferred to a 1.5-ml tube containing 0.05 g of 100-μm-diameter acid-washed glass beads (Sigma) and vortexed for 5 min. The mixture was then centrifuged, and the RNA was extracted from the supernatant by using a QIAGEN RNeasy mini kit according to the manufacturer's instructions. The RNA recovered was treated with DNase I (Stratagene Europe, Amsterdam, The Netherlands) used according to the manufacturer's instructions to remove any contaminating DNA.
RT-PCR.
Reverse transcription (RT)-PCR was performed by using a QIAGEN One Step RT-PCR kit according to the manufacturer's instructions or in two steps to allow inclusion of a reverse transcriptase negative control to confirm the absence of contaminating DNA. RT was performed by using an Omniscript kit (QIAGEN) according to the manufacturer's instructions, and the PCR was performed under the following conditions by using Taq DNA polymerase (Roche): 94°C for 3 min and then 30 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min, followed by 10 min at 72°C. The primers used are shown in Table 1. The PCR products were visualized in an agarose gel by staining with ethidium bromide.
Northern blotting.
Northern blotting was performed by standard methods (21), except that the nylon membrane was probed with a chemiluminescently labeled PCR product. Labeling of the probe and visualization of the blot were performed according to the manufacturer's instructions (ECL direct nucleic acid labeling and detection system; Amersham Biosciences). The molecular weight standard used was RNA Ladder (New England Biolabs).
In vitro translation of ORF 1.
PCR amplicons of ORF 1 were generated with primers FI3 and 104_b832.12 (Table 1). PCR products were purified with a QIAquick gel extraction kit (QIAGEN) and were used in an Escherichia coli S30 extract system for linear templates (Promega, Madison, Wis.). Proteins were expressed from 2 μg of PCR-amplified template DNA. Samples of each reaction mixture were resolved by sodium dodecyl sulfate—10% polyacrylamide gel electrophoresis and were blotted onto polyvinylidene difluoride membranes (Immobilon-P; Millipore Corp., Bedford, Mass.). Proteins were detected by using the Transcend chemiluminescent translation detection system (Promega) according to the manufacturer's instructions. The sizes of proteins were estimated by comparison with a prestained marker (Bio-Rad Laboratories, Inc.).
Construction of an aliB-like ORF-deficient mutant of strain 110.58.
Transformation of E. coli and S. pneumoniae was performed as described previously (10, 18). Plasmid pBluescript KSII (Stratagene) was used for plasmid construction.
Within pBluescript KSII the following genes were cloned into the multiple cloning site. First, a 1,381-bp fragment of the dexB ORF of strain 110.58, flanked by the chloramphenicol acetyltransferase (cat) gene from plasmid pJS3copG7 (kindly provided by M. Espinosa, Centro de Investigaciones Biologicas, Madrid, Spain) (13), was inserted. Downstream of cat a 883-bp fragment of the capN-like ORF of strain 110.58 was inserted. The primers used to amplify the individual genes are described in Table 1. The whole dexB-cat-capN construct (3.1 kb) was amplified by PCR by using primers dexB_f145_XbaI and capN_B1_XhoI (Table 1). The dexB-cat-capN amplicon was then used to transform competent cells of S. pneumoniae strain 110.58. Recombinant clones were selected on CSBA plates containing 3 mg of chloramphenicol per liter. Positive recombinants were analyzed by PCR and sequencing to confirm correct deletion of aliB-like ORF 1 and aliB-like ORF 2.
Adherence of S. pneumoniae to human lung epithelial cell line A549.
A549 epithelial cells were inoculated into 24-well tissue culture plates (105 cells/well) in minimum essential medium (MEM) (Gibco, Paisley, United Kingdom) containing 10% FCS, 1% glutamine (Gibco), and 1% penicillin/streptomycin (Biochrom KG, Berlin, Germany) and were cultured until complete confluence was reached at 37°C in the presence of 5% CO2. S. pneumoniae strain 110.58 and an aliB-like ORF-deficient mutant of this strain grown in BHI broth containing 5% FCS to the logarithmic phase were washed in MEM and then resuspended to obtain 107 bacteria in 0.5 ml of MEM, which was added to each well containing washed A549 cells (the concentration of bacteria was confirmed by plating appropriate dilutions of the inoculum). The bacteria were centrifuged onto the A549 cells at 390 × g for 5 min at room temperature. After 1 h of incubation at 37°C in the presence of 5% CO2, the monolayers were washed five times with phosphate-buffered saline, and the cells were detached by 5 min of incubation with 200 μl of trypsin-EDTA (Gibco) diluted 1:5 in phosphate-buffered saline. Appropriate dilutions were plated on CSBA plates for subsequent counting of colonies. A total of eight experiments were performed, and each strain was analyzed in triplicate in each experiment.
RESULTS
Nonencapsulated strains.
A total of 1,980 noninvasive and 215 invasive S. pneumoniae isolates were evaluated for capsule expression. Forty-five isolates (2.1% of the 2,195 isolates examined) produced rough colonies. Of these 45 isolates, 27 (1.2% of all isolates) could not be typed by the Quellung reaction, indicating that they did not express capsule polysaccharide, but the remaining 18 (0.8%) isolates could be assigned to a serotype after repeated typing. Of the 27 truly nonencapsulated isolates, 7 were invasive and 20 colonized the nasopharynx.
MLST analysis.
The 27 nonencapsulated strains were characterized by MLST. Three clusters were identified. Clone A comprised 10 isolates with identical MLST patterns. Three isolates differed from this pattern by one or two alleles (and so were included in clonal complex A) or by five alleles (Table 2). Clone A strains were isolated from patients of different ages and from different geographical locations around Switzerland (data not shown). None of the clone A isolates were susceptible to penicillin, and all of them were resistant to erythromycin and cotrimoxazole (Table 2). Clones B and C each contained two isolates with identical MLST patterns.
TABLE 2.
MLST of 27 nonencapsulated clinical pneumococcal isolates
| Strain | Sourcea | Cloneb | Penicillin MIC (mg/liter) | Allelesd
|
Related cloneh | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| aro | ddl | gdh | gki | spi | rec | xpt | |||||
| 208.80 | NP | Ac | 0.125 | 8 | 53 | 37 | 9 | 2 | 1 | 12 | NT, Norway |
| 207.63 | NP | A | 0.094 | 8 | 53 | 37 | 9 | 2 | 29 | 12 | NT, Norway |
| 101.43 | NP | A | 0.19 | 8 | 53 | 37 | 9 | 2 | 29 | 12 | NT, Norway |
| 104.58 | NP | A | 0.094 | 8 | 53 | 37 | 9 | 2 | 29 | 12 | NT, Norway |
| 104.72 | NP | A | 1.0 | 8 | 53 | 37 | 9 | 2 | 29 | 12 | NT, Norway |
| 106.44 | NP | A | 2.0 | 8 | 53 | 37 | 9 | 2 | 29 | 12 | NT, Norway |
| 110.58 | NP | A | 0.19 | 8 | 53 | 37 | 9 | 2 | 29 | 12 | NT, Norway |
| 111.81 | NP | A | 0.125 | 8 | 53 | 37 | 9 | 2 | 29 | 12 | NT, Norway |
| 202.66 | NP | A | 0.125 | 8 | 53 | 37 | 9 | 2 | 29 | 12 | NT, Norway |
| 203.15 | NP | A | 0.094 | 8 | 53 | 37 | 9 | 2 | 29 | 12 | NT, Norway |
| 205.09 | NP | A | 0.064 | 8 | 53 | 37 | 9 | 2 | 29 | 12 | NT, Norway |
| 305.33 | NP | Ac | 0.094 | 8 | 53e | 37 | 9 | 64f | 29 | 12 | NT, Norway |
| 110.41 | NP | s | 0.012 | 11 | 14 | 3 | 9 | 2 | 29g | 19 | NT, Norway |
| B104.72 | BL | B | <0.06 | 8 | 71 | 5 | 2 | 2 | 27 | 11 | NT, United Kingdom |
| 208.15 | NP | B | <0.06 | 8 | 71 | 5 | 2 | 2 | 27 | 11 | NT, United Kingdom |
| 208.37 | NP | s | <0.06 | NM | NM | NM | NM | NM | NM | NM | |
| 301.77 | NP | s | <0.06 | NM | NM | NM | NM | NM | NM | NM | |
| 208.56 | NP | s | 0.125 | NM | NM | NM | NM | NM | NM | NM | |
| 209.68 | NP | s | <0.06 | NM | NM | NM | NM | NM | ND | NM | |
| 108.46 | NP | C | 0.25 | 2 | 14 | 5 | 9 | 17 | 1 | 21 | 15A |
| 105.81 | NP | C | 0.25 | 2 | 14 | 5 | 9 | 17 | 1 | 21 | 15A |
| B105.04 | BL | s | 0.064 | 2 | 31 | 10 | 9 | 27 | 18 | 1 | 3 |
| B105.27 | BL | s | <0.06 | 7 | 8 | 25 | 8 | 25 | 6 | 6 | 6A |
| B102.34 | BL | s | <0.06 | 10 | 14 | 10 | 9 | 13 | 43 | 1 | 6A |
| B105.28 | BL | s | <0.06 | 7 | 14 | 5 | 1 | 14 | 8 | 11 | 14, Denmark |
| Bil86 | BL | s | <0.06 | NM | NM | NM | NM | ND | NM | NM | |
| Bil700 | BL | s | <0.06 | NM | NM | NM | NM | NM | NM | NM | |
Clinical specimens were collected from the nasopharynx (NP) or blood (BL).
MLST allelic patterns allowed isolates to be grouped into three clusters, designated clones A, B, and C; some of the isolates produced individual patterns (s).
Strains 208.80 and 305.33 belong to clonal complex A.
aroE encodes shikimate dehydrogenase, gdh encodes glucose-6-phosphate dehydrogenase, gki encodes glucose kinase, recP encodes transketolase, spi encodes signal peptidase I, xpt encodes xanthine phosphoribosyltransferase, and ddl encodes d-alanine-d-alanine ligase. The numbers indicate allele types. NM, no match; ND, not determined.
98% homology with this allele.
96% homology with this allele.
98% homology with this allele.
Serotype and/or country of isolation for the nearest match in the MLST database. NT, nontypeable.
Of the 18 difficult-to-type isolates, all but three belonged to one of three clones (data not shown) which were eventually characterized as serotypes 1, 38, and 6A.
Characterization of the cap region.
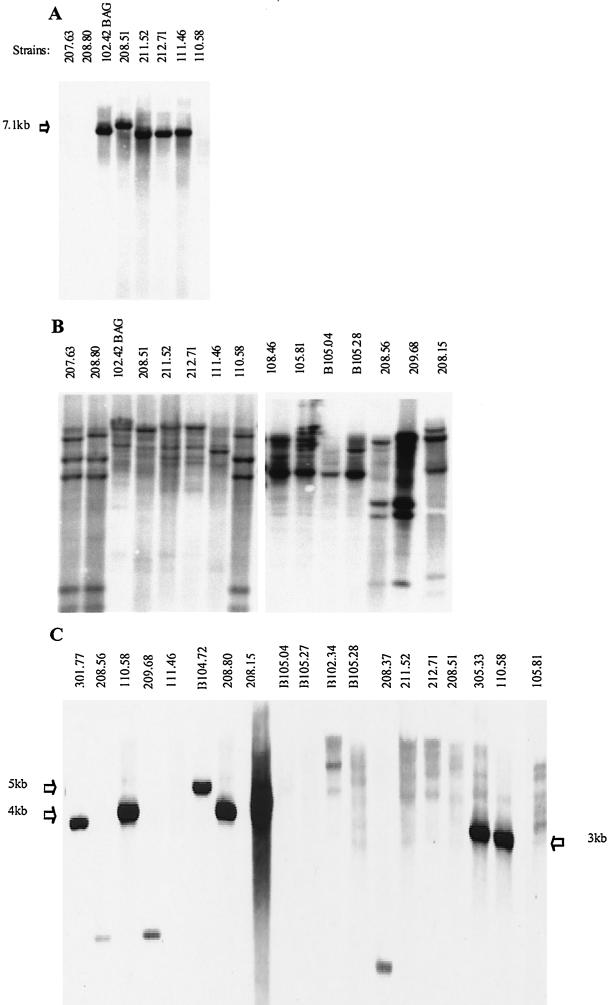
Southern blotting was performed by probing with PCR products representing a conserved region of the cap locus of a typical encapsulated serotype 19F strain, strain 111.46 (probe cap111.46; 1,795 bp) or the cap locus of nonencapsulated strain 110.58 (probe cap110.58; 7.4 kb) (Fig. 1 and 2A and B and Table 3). Probe cap111.46 bound the 18 encapsulated strains (data not shown) and 8 of the 27 nonencapsulated strains, including clone C strains with a single band (Table 3). This indicated that cap genes were present and corresponded to the observation made by Arrecubieta et al. about a serotype 3 strain not expressing the capsule due to a point mutation in the cap operon (3). Probe cap110.58 bound all strains that produced distinctive banding patterns, which correlated with the MLST results (Table 2). Because of the apparent differences in the cap locus, sequence analysis was performed with three representative strains belonging to clone A and strain 208.56. This analysis revealed high homology in this region for all four strains (Fig. 1). BLAST analysis revealed that capsule genes, which are usually found in encapsulated pneumococci, were absent, except for a region with homology to the capN gene of serotypes 33F and 37 (15). However, we found two apparent ORFs which were 1,968 to 2,004 and 1,959 bp long, exhibited 61 to 64.5% homology to the ATP-binding cassette (ABC) transporter gene aliB (and 62% homology to each other), and are not normally found in the capsule-encoding region of encapsulated strains. These homologous regions were designated aliB-like ORF 1 and aliB-like ORF 2. Strain 208.56 lacked aliB-like ORF 1 but possessed aliB-like ORF 2. The sequence of the region between aliB-like ORF 1 and aliB-like ORF 2 also matched the sequence between the dexB and capA genes in serotype 33F (85 to 91% homology), and the sequence following aliB-like ORF 2 also matched the BOX element CBBA sequence (77 to 78% homology over 159 bp). In strains 110.58 and 104.72 there was also a 106-bp region with homology to a BOX AC element.
FIG.2.
(A) Southern blot probed with cap111.46 bound with a single band to DNA of encapsulated pneumococcal isolates (strains 102.42 BAG, 208.51, 211.52, 212.71, and 111.46) but not to DNA of nonencapsulated isolates (strains 207.63, 208.80, and 110.58). (B) Probe cap110.58, representing the capsule region of a nonencapsulated clone A strain, bound to DNA from all strains with several bands. The banding pattern for the nonencapsulated strains belonging to the clone A complex (strains 207.63, 208.80, and 110.58) is identical or very similar. The banding patterns for the five encapsulated strains (strains 102.42 BAG, 208.51, 211.52, 212.71, and 111.46) represented four different hybridization patterns, in accordance with the expected polymorphism of the cap operon in these strains. The banding patterns for nonencapsulated strains representing clone B (strain 208.15) and clone C (strains 108.46 and 105.81) and for strains not belonging to a clone (strains B105.04, B105.28, 208.56, and 209.68) are also shown. (C) Probe specific for aliB-like ORF 2 bound to some of the nonencapsulated strains (strains 301.77, 208.56, 110.58, 209.68, B104.72, 208.80, 208.15, 208.37, 305.33, and 110.58) but not to nonencapsulated strains B105.04, B105.27, B102.34, B105.28, and 105.81 or to encapsulated strains (strains 111.46, 211.52, 212.71, and 208.51). Molecular weights were determined by using a standard run on the gel, which was visualized before blotting.
TABLE 3.
Characterization of the cap region of nonencapsulated pneumococcal isolates by Southern blotting, PCR, and sequencing analysis
| Strain | Clone | Southern blotting with the following probes:
|
PCR for aliB-like ORF 1d | PCR for aliB-like ORF 2e | ||
|---|---|---|---|---|---|---|
| cap111.46a | cap110.58b | aliB-like ORF 2c | ||||
| 104.72 | A | n | T1 | ND | pos | pos |
| 106.44 | A | n | T1 | ND | pos | pos |
| 110.58 | A | n | T1 | 1b | pos | pos |
| 207.63 | A | n | T1 | 1b | pos | pos |
| 208.80 | Af | n | T1 | 1b | pos | pos |
| 305.33 | Af | n | T1 | 1b | pos | pos |
| 110.41 | sg | n | T1 | 1b | pos | pos |
| B104.72 | B | n | T2 | 1b | pos | pos |
| 208.15 | B | n | T2 | 1b | pos | pos |
| 208.37 | s | n | S | 1b | w | pos |
| 301.77 | s | n | S | 1b | w | pos |
| 208.56 | s | n | S | 1b | w | pos |
| 209.68 | s | n | S | 1b | w | pos |
| 108.46 | C | 1b | T3 | n | neg | neg |
| 105.81 | C | 1b | T3 | n | w | neg |
| B105.04 | s | 1b | T4 | n | w | neg |
| B105.27 | s | 1b | S | n | w | neg |
| B102.34 | s | 1b | S | n | neg | neg |
| B105.28 | s | 1b | T4 | n | w | neg |
| Bil86 | s | 1b | S | 1b | neg | neg |
| Bil700 | s | 1b | S | 1b | neg | pos |
Southern blots were probed with a conserved region of the cap locus from encapsulated serotype 19F strain 111.46 (Fig. 1). 1b, 1 band; n, no bands.
Southern blots were probed with the cap locus of nonencapsulated strain 110.58 (Fig. 1). T1, T2, T3, and T4, four different banding patterns; S, sporadic bands.
Southern blots were probed with a region of aliB-like ORF 2. 1b, 1 band; n, no bands; ND, not determined.
PCR was performed with primers binding within aliB-like ORF 1. pos, positive for PCR product; neg, no PCR product; w, weak, probably nonspecific, band.
PCR was performed with primers binding within aliB-like ORF 2. pos, positive for PCR product; neg, no PCR product.
Strains 208.80 and 305.33 belong to clonal complex A.
s, individual pattern.
Having identified these novel ORFs in the cap locus, we used a probe specific for aliB-like ORF 2 (370 bp) for Southern blotting of three clone A strains and all nontypeable strains not belonging to clone A. This probe hybridized only to the isolates which failed to bind to probe cap111.46 (except for strains Bil86 and Bil700) (Table 3 and Fig. 2C). PCRs performed with primers binding within aliB-like ORF 2 (Table 1) confirmed these results. Amplification products were obtained for all strains that reacted with the aliB-like ORF 2 probe except strain Bil86 (Table 3). Therefore, nontypeable strains could be divided into two groups. Group I appeared to have the conserved capsule gene region (binding to probe cap111.46) and not aliB-like ORF 2. Group II seemed to lack typical capsule genes but possessed aliB-like ORF 2. aliB-like ORF 1, however, was restricted to strains in clonal complexes A and B plus strain 110.41; an intense band was produced by PCR performed with aliB-like ORF 1 primers (Table 1) for all six representatives of clonal complex A tested, as well as strain 110.41 (which differs from clone A at five alleles, as determined by MLST) and both members of clone B. All other nonencapsulated isolates either gave a nondetectable or weak amplification product. The weak band was interpreted as a nonspecific band, since it was also observed for strain 208.56, in which no aliB-like ORF 1 region was found by sequencing, and strain 111.46, a typical serotype 19F isolate.
BLAST analysis of aliB-like ORFs.
The closest match (66% nucleotide homology over 1,977 bp) obtained for aliB-like ORF 1 was the match with a sequence fragment of the partially sequenced Streptococcus gordonii genome (http://www.tigr.org/tdb/mdb/mdbinprogress.html), and aliB-like ORF 1 also exhibited 80% homology over 242 bp to the S. gordonii oligopeptide-binding lipoprotein gene hppH. BLAST analysis revealed 93% nucleotide homology over 1,886 bp of aliB-like ORF 2 with a sequence fragment of the Streptococcus mitis genome between dexB and the first capsule gene (unfinished S. mitis strain NCTC 12261 genome [http://www.tigr.org/tdb/mdb/mdbinprogress.html]). In addition, the hypothetical protein gene spr0311, adjacent to dexB in the capsule region of the S. pneumoniae R6 genome, exhibited 92% homology over 163 bp to a region at the 5′ end of aliB-like ORF 2. In a short region before the cps2A gene of R6 30 of 32 bp matched the residues in a region toward the 3′ end of aliB-like ORF 2, which may indicate possible sites of insertion or deletion. The G+C content of aliB-like ORF 1 and aliB-like ORF 2 was around 40%, which is equivalent to the G+C content of the whole S. pneumoniae R6 genome. Genes of S. mitis have a similar average value, making it difficult to determine the sites of possible recombination from differences in the G+C contents. However, the homology between the capsule regions of S. pneumoniae strain 110.58 and S. mitis DNA stops at the 3′ end of aliB-like ORF 2, suggesting that that this may be a site of recombination.
Gene expression analysis of aliB-like ORFs.
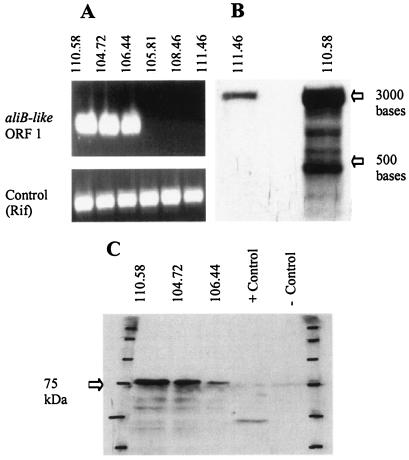
RT-PCR showed that three representatives of clone A (strains 110.58, 104.72, and 106.44) transcribed aliB-like ORF 1 (Fig. 3A). No transcription product was found for clone C isolates 105.81 and 108.46 or the encapsulated serotype 19F strain 111.46 when they were grown under the same conditions. By using Northern blotting (Fig. 3B) an aliB-like ORF 1 probe was found to give multiple bands with nonencapsulated strain 110.58 (clone A) and a particularly strong band around 3,000 bases. There was also a weak band of a similar size for encapsulated isolate 111.46, most likely due to nonspecific binding to a transcript homologous to aliB-like ORF 1. No expression of aliB-like ORF 2 was detected in any of the strains tested by RT-PCR or Northern blotting (data not shown).
FIG. 3.
(A) RT-PCR detected aliB-like ORF 1 expression in nonencapsulated clone A isolates (strains 110.58, 104.72, and 106.44) but not in the nonencapsulated clone C isolates (strains 105.81 and 108.46) or the encapsulated serotype 19F control strain (strain 111.46). The lower panel shows the results for a positive control for RT-PCR performed with equal amounts of PCR product produced for all strains with Rif primers binding to cDNA of rpoB. (B) Northern blot showing expression of RNA recognized by the aliB-like ORF 1 probe in nonencapsulated clone A strain 110.58. There is a weak band for encapsulated serotype 19F control strain 111.46, which may have been due to a cross-reaction. Molecular weights were determined by using a standard run on the gel, which was visualized before blotting. (C) In vitro translation produced a protein from the aliB-like ORF 1 region for all three nonencapsulated clone A strains tested (strains 110.58, 104.72, and 106.44). The position of the protein standard was marked on the blot (first and last lanes); positive and negative controls were produced according to the instructions of the kit manufacturer.
In vitro translation assays showed that translation products for aliB-like ORF 1 were obtained from isolates 110.58, 104.72, and 106.44 representing the major clone A cluster (Fig. 3C).
Adherence of S. pneumoniae to lung epithelial cells.
The mean percentage of cells in the strain 110.58 inoculum which adhered to A549 epithelial cells was 13.5% (standard deviation, 4.8%), compared to 10.6% (standard deviation, 5.9%) for the aliB-like ORF 1- and aliB-like ORF 2-deficient mutant, indicating that there was no significant difference in adherence between these two strains.
DISCUSSION
Just over 1% of the colonizing and invasive S. pneumoniae strains analyzed in this study were rough forms (i.e., nonencapsulated). These nontypeable strains otherwise had the characteristics of typical S. pneumoniae strains (bile soluble, susceptible to optochin, and binding to a species-specific 16S rRNA probe) rather than those of the atypical pneumococci described by Whatmore et al. (30). MLST and analysis of the cap locus was used to divide the nonencapsulated S. pneumoniae strains into two groups. Group I strains appear to be closely related to encapsulated strains, since they have typical capsule genes and produce the MLST patterns of typeable strains. Group II strains are less closely related to known encapsulated pneumococci as determined by MLST and contain, instead of the capsule genes, a sequence with homology to the aliB gene. aliB encodes a peptide-binding molecule associated with an ABC transporter (2, 4). All strains in group II of nonencapsulated pneumococci apparently possess this aliB homologue, which we designated aliB-like ORF 2. However, some strains had, in addition, a second ORF, aliB-like ORF 1 upstream of aliB-like ORF 2. All but one of the strains with aliB-like ORF 1 belonged to clonal complex A or clone B.
The origin of the aliB-like ORFs is not known. Based on BLAST analysis, aliB-like ORF 2 may have been acquired by horizontal gene transfer from S. mitis, and aliB-like ORF 1 may have originated from S. gordonii, similar to the acquisition of penicillin-binding proteins by S. pneumoniae from the oral commensal organisms S. mitis and Streptococcus oralis (7, 22). The simultaneous occurrence of aliB-like ORF 1 and aliB-like ORF 2 in some isolates may be explained by repeated transformation. Duplication within the pneumococcal cap region seems less likely, given the 38% sequence divergence between the two ORFs.
The association of aliB-like ORF 1 with two clones is intriguing. Clone A was more prevalent, and at least one isolate has been found in Norway (http://spneumoniae.mlst.net/). In this study, clone A strains did not cluster by time, age, or geographical region, suggesting that clone A is widely distributed. Strains of this clone were also characterized by reduced susceptibility to penicillin; in fact, this clone contained 10% of the nonsusceptible nasopharyngeal isolates in Switzerland (data not shown). Clone B matched a clone which caused an outbreak of conjunctivitis on an American college campus (17). Therefore, the clones possessing aliB-like ORF 1 may have a selective advantage. Whether such an advantage is related to the presence of aliB-like ORF 1 is a matter for speculation at this time. The function of the putative AliB-like protein is not known, but true AliB has been described as a membrane-bound lipoprotein which binds oligopeptides to deliver them to the Ami permease ABC transporter for uptake into the cell (2, 4). It has been proposed that, in addition to taking up peptide for nutrition, this may be a way of sensing the environment and that this ABC transporter may affect a gene regulator which controls pathways involved in diverse processes, such as induction of competence or adherence (2, 4). Mutation of the ami locus results in a reduced ability of S. pneumoniae to bind to resting human lung pneumocytes, which represent the alveolar site of infection, and to human endothelial cells (5).
aliB-like ORF 1, but not aliB-like ORF 2, was expressed at the RNA level and could be translated in vitro, and so the protein may be expressed and have a function in vivo. No difference in adherence to human lung epithelial cells between a wild-type strain and a mutant of this strain lacking the aliB-like ORFs has been found so far. Also, no difference in transformability has been detected (data not shown). Further experiments to determine possible functions of AliB-like molecules are being performed. The presence of sequences with homology to BOX sequences (106 bp with homology to BOX AC and 159 bp with homology to BOX CBBA) in the capsule region of nonencapsuled strains possessing aliB-like ORF 1 and aliB-like ORF 2 may indicate the presence of functional genes, since it has been proposed that BOX elements are involved in regulation of virulence genes (16). Nontypeable S. pneumoniae strains are often considered to be avirulent. However, the fact that seven of the invasive strains in this study were nonencapsulated and the fact that nontypeable strains can cause outbreaks of disease (17) show that this is not necessarily always the case.
The phylogenetic origin of group II of the nonencapsulated strains carrying aliB-like ORFs in the cap locus is not known. A small region with homology to the capN gene of serotypes 33F and 37 downstream of aliB-like ORF 2 indicates that this group may have been derived from one of these serotypes or a close relative some time ago. Also, one strain which shared two alleles with clone A and had one allele that was closely related to a clone A allele contained aliB-like ORF 1 and aliB-like ORF 2. This indicates that clone A and related strains may have persisted for quite a long time, allowing extensive recombination. Group I of nonencapsulated strains described in this study exhibits a closer phylogenetic relationship to encapsulated pneumococci that have the cap region. This suggests that they may have arisen from encapsulated pneumococci by mutation in the cap region. Different mechanisms for loss of capsule expression have been described. Capsule gene inactivation by duplication has been demonstrated in vitro for serotypes 3, 8, and 37 (25, 26). Arrecubieta et al. (3) described a single mutation in the cap3A gene of serotype 3 that led to a loss of capsule expression. S. pneumoniae may take advantage of such (partially) reversible mechanisms for capsule loss to enhance survival at the mucosal level (25, 27).
In conclusion, invasive and noninvasive nonencapsulated S. pneumoniae strains were identified and could be assigned to two groups based on the structure of the cap region and MLST. Group I was closely related to encapsulated pneumococci. Group II had a clonal population structure and was found to carry genes with homology to the aliB gene in the cap region, a novel feature. Therefore, loss of capsule expression in vivo was found to be associated with the importation of one or two aliB homologues in some nonencapsulated pneumococci.
Acknowledgments
This study was supported by grant 3200-067998from the Swiss National Science Foundation to K. Mühlemann.
We thank Jacques Billes for providing two of the invasive strains used in this study and Thomas Geiser for providing the A549 cell line. We are indebted to Martin Täuber for support and encouragement.
REFERENCES
- 1.Adamou, J. E., T. M. Wizemann, P. Barren, and S. Langermann. 1998. Adherence of Streptococcus pneumoniae to human bronchial epithelial cells (BEAS-2B). Infect. Immun. 66:820-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alloing, G., P. de Philip, and J. P. Claverys. 1994. Three highly homologous membrane-bound lipoproteins participate in oligopeptide transport by the Ami system of the gram-positive Streptococcus pneumoniae. J. Mol. Biol. 241:44-58. [DOI] [PubMed] [Google Scholar]
- 3.Arrecubieta, C., R. Lopez, and E. Garcia. 1994. Molecular characterization of cap3A, a gene from the operon required for the synthesis of the capsule of Streptococcus pneumoniae type 3: sequencing of mutations responsible for the unencapsulated phenotype and localization of the capsular cluster on the pneumococcal chromosome. J. Bacteriol. 176:6375-6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Claverys, J.-P., B. Grossiord, and G. Alloing. 2000. Is the Ami-AliA/B oligopeptide permease of Streptococcus pneumoniae involved in sensing environmental conditions? Res. Microbiol. 151:457-463. [DOI] [PubMed] [Google Scholar]
- 5.Cundell, D. R., B. J. Pearce, J. Sandros, A. M. Naughton, and H. R. Masure. 1995. Peptide permeases from Streptococcus pneumoniae affect adherence to eucaryotic cells. Infect. Immun. 63:2493-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cundell, D. R., J. N. Weiser, J. Shen, A. Young, and E. I. Tuomanen. 1995. Relationship between colonial morphology and adherence of Streptococcus pneumoniae. Infect. Immun. 63:757-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dowson, C. G., T. J. Coffey, C. Kell, and R. A. Whiley. 1993. Evolution of penicillin resistance in Streptococcus pneumoniae; the role of Streptococcus mitis in the formation of a low affinity PBP2B in S. pneumoniae. Mol. Microbiol. 9:635-643. [DOI] [PubMed] [Google Scholar]
- 8.Enright, M., and B. Spratt. 1998. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology 144:3049-3060. [DOI] [PubMed] [Google Scholar]
- 9.Garcia, E., D. Llull, R. Munoz, M. Mollerach, and R. Lopez. 2000. Current trends in capsular polysaccharide biosynthesis of Streptococcus pneumoniae. Res. Microbiol. 151:429-435. [DOI] [PubMed] [Google Scholar]
- 10.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 11.Iannelli, F., B. J. Pearce, and G. Pozzi. 1999. The type 2 capsule locus of Streptococcus pneumoniae. J. Bacteriol. 181:2652-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim, J. O., and J. N. Weiser. 1998. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J. Infect. Dis. 177:368-377. [DOI] [PubMed] [Google Scholar]
- 13.Lacks, S. A., P. Lopez, B. Greenberg, and M. Espinosa. 1986. Identification and analysis of genes for tetracycline resistance and replication functions in the broad-host-range plasmid pLS1. J. Mol. Biol. 192:753-765. [DOI] [PubMed] [Google Scholar]
- 14.Lawrence, E., C. Arias, B. Duke, D. Beste, K. Broughton, A. Efstratiou, R. George, and L. Hall. 2000. Evaluation of serotype prediction by cpsA-cpsB gene polymorphism in Streptococcus pneumoniae. J. Clin. Microbiol. 38:1319-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Llull, D., R. Lopez, E. Garcia, and R. Munoz. 1998. Molecular structure of the gene cluster responsible for the synthesis of the polysaccharide capsule of Streptococcus pneumoniae type 33F. Biochim. Biophys. Acta 1443:217-224. [DOI] [PubMed] [Google Scholar]
- 16.Martin, B., O. Humbert, M. Camara, E. Guenzi, J. Walker, T. Mitchell, P. Andrew, M. Prudhomme, G. Alloing, and R. Hakenbeck. 1992. A highly conserved repeated DNA element located in the chromosome of Streptococcus pneumoniae. Nucleic Acids Res. 20:3479-3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin, M., J. H. Turco, M. E. Zegans, R. R. Facklam, S. Sodha, J. A. Elliott, J. H. Pryor, B. Beall, D. D. Erdmann, Y. Y. Baumgartner, P. A. Sanchez, J. D. Schwartzman, J. Montero, A. Schuchat, and C. G. Whitney. 2003. An outbreak of conjunctivitis due to atypical Streptococcus pneumoniae. N. Engl. J. Med. 348:1112-1121. [DOI] [PubMed] [Google Scholar]
- 18.Meier, P. S., S. Utz, S. Aebi, and K. Muhlemann. 2003. Low-level resistance to rifampicin in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 47:863-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muhlemann, K., H. Matter, M. Tauber, and T. Bodmer. 2002. Nationwide surveillance of nasopharyngeal Streptococcus pneumoniae isolates from children with respiratory infection, Switzerland, 1998-1999. J. Infect. Dis. 187:589-596. [DOI] [PubMed] [Google Scholar]
- 20.Pearce, B. J., F. Iannelli, and G. Pozzi. 2002. Construction of new unencapsulated (rough) strains of Streptococcus pneumoniae. Res. Microbiol. 153:243-247. [DOI] [PubMed] [Google Scholar]
- 21.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 22.Sibold, C., J. Henrichsen, A. Konig, C. Martin, L. Chalkley, and R. Hakenbeck. 1994. Mosaic pbpX genes of major clones of penicillin-resistant Streptococcus pneumoniae have evolved from pbpX genes of a penicillin-sensitive Streptococcus oralis. Mol. Microbiol. 12:1013-1023. [DOI] [PubMed] [Google Scholar]
- 23.Swiss Federal Office of Health. 2002. Invasive Pneumokokken-Infektionen in der Shweiz 1998-1999: Empfindlichkeit auf Antibiotika und Abdeckung durch 7- und 9-valente Konjugat-Impfstoffe. Bundesamt Gesundheit Bull. 31:544-549. [Google Scholar]
- 24.Swiss Federal Office of Health. 2003. Überwachung der Infektionen durch invasive und nicht invasive Pneumokokken im Jahr 2002. Bundesamt Gesundheit Bull. 22: 368-372. [Google Scholar]
- 25.Waite, R. D., J. K. Struthers, and C. G. Dowson. 2001. Spontaneous sequence duplication within an open reading frame of the pneumococcal type 3 capsule locus causes high-frequency phase variation. Mol. Microbiol. 42:1223-1232. [DOI] [PubMed] [Google Scholar]
- 26.Waite, R. D., D. W. Penfold, J. K. Struthers, and C. G. Dowson. 2003. Spontaneous sequence duplications within capsule genes cap8E and tts control phase variation in Streptococcus pneumoniae serotypes 8 and 37. Microbiology 149:497-504. [DOI] [PubMed] [Google Scholar]
- 27.Weiser, J. N., R. Austrian, P. K. Sreenivasan, and H. R. Masure. 1994. Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect. Immun. 62:2582-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiser, J. N., and M. Kapoor. 1999. Effect of intrastrain variation in the amount of capsular polysaccharide on genetic transformation of Streptococcus pneumoniae: implications for virulence studies of encapsulated strains. Infect. Immun. 67:3690-3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiser, J. N., D. Bae, H. Epino, S. B. Gordon, M. Kapoor, L. A. Zenewicz, and M. Shchepetov. 2001. Changes in availability of oxygen accentuate differences in capsular polysaccharide expression by phenotypic variants and clinical isolates of Streptococcus pneumoniae. Infect. Immun. 69:5430-5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whatmore, A. M., A. Efstratiou, A. P. Pickerill, K. Broughton, G. Woodard, D. Sturgeon, R. George, and C. G. Dowson. 2000. Genetic relationships between clinical isolates of Streptococcus pneumoniae, Streptococcus oralis, and Streptococcus mitis: characterization of “atypical” pneumococci and organisms allied to S. mitis harboring S. pneumoniae virulence factor-encoding genes. Infect. Immun. 68:1374-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]