Abstract
BACKGROUND
Understanding the causes of failure in older patients with acute lymphocytic leukemia (ALL) may help improve treatment strategies for patients in this particular age group.
METHODS
The objectives of the current study were to define the causes of death in older patients (aged ≥60 years) with ALL during induction and consolidation-maintenance with a dose-intensive regimen of alternating 8 courses of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD) with high doses of methotrexate and cytarabine followed by maintenance with 6-mercaptopurine, vincristine, methotrexate, and prednisone and to compare their outcomes with the outcomes of older patients who received earlier, less intensive regimens and younger patients who received hyper-CVAD. One hundred twenty-two older patients who received hyper-CVAD were compared with 34 older patients who received less intensive regimens and with 409 younger patients who received hyper-CVAD.
RESULTS
The complete response (CR) rates in older patients receiving hyper-CVAD, older patients receiving other regimens, and younger patients receiving hyper-CVAD were 84%, 59%, and 92%, respectively (P <.001); and the respective induction mortality rates were 10%, 12%, and 2% (P not significant in older patients). The incidence of disease resistance during induction was 5%, 27%, and 2%, respectively (P < .001). The majority of deaths were related to infections. Among patients who achieved a CR, death in CR was noted in 34%, 15%, and 7% of older patients receiving hyper-CVAD, older patients receiving other regimens, and younger patients, respectively (P < .001); and the respective rates of recurrence were 40%, 80%, and 48% (P = .004). The estimated 5-year survival rates were 20%, 9%, and 48%, respectively (P <.001).
CONCLUSIONS
The results of the current study suggested that intensifying the chemotherapy in older patients with ALL reduced the incidence of leukemia resistance but increased the incidence of death in CR from myelosuppression-associated infections. The overall benefit:risk ratio was favorable. Identifying novel, low-intensity agents/regimens for older patients with ALL may improve the results further.
Keywords: acute lymphocytic leukemia, cause of death, complete response, elderly, hyperfractionated chemotherapy, survival
Multiagent, intensive chemotherapy regimens induce complete response (CR) rates of 90% to 100% and cure rates of 80% to 90% in childhood acute lymphocytic leukemia (ALL).1–4 In adult ALL, similarly designed regimens, albeit less dose-intensive with some components (such as vincristine and asparaginase, because of their poor tolerance by adults), result in CR rates of 80% to 90% and long-term survival rates of 20% to 50%.5–10 Overall results vary according to entry criteria and well described prognostic factors. Programs that excluded older patients (ages 50 years to ≥65 years), patients with a poor performance status or organ dysfunctions, and patients with Philadelphia chromosome (Ph)-positive ALL have reported better results11,12 compared with regimens that have more inclusive eligibility.5
Elderly patients with ALL have a worse prognosis than young patients when they receive the same regimens.13–17 In such patients, the CR rates have varied from 35% to 80%, but the long-term survival rates are 5% to 15%.13–17 The definition of an elderly patient varies but most commonly refers to ages 60 to ≥65 years. Defining the causes of failure in elderly patients with ALL may help improve our therapeutic strategies. For example, if the main cause of failure is resistant disease, then novel agents with specific acute-ALL activity in elderly patients would be important. If treatment-associated mortality is predominant, then designing regimens that lower the treatment dose intensity, without compromising anti-ALL efficacy, might prove beneficial. Identifying causes of failure in elderly patients with ALL was the objective of the current analysis.
MATERIALS AND METHODS
All patients with a new diagnosis of ALL who were referred to our institution from 1980 onward and were treated on ALL regimens were reviewed. Elderly patients were defined as patients aged ≥60 years. The analysis focused on elderly patients who were treated with the recently developed hyper-CVAD regimens.5 Hyper-CVAD refers to dose-intensive therapy that alternates 8 total courses of fractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone (C-VAD) with high doses of methotrexate and cytarabine (HDMTX-ara-C) followed by maintenance with 6-mercaptopurine, vincristine, methotrexate, and prednisone (POMP) for 18 to 30 months. The program includes central nervous system prophylaxis with intrathecal therapy, antibiotic prophylaxis, and granulocyte–colony-stimulating factor support. The components of the hyper-CVAD regimen and subsequent modifications have been detailed previously. The analysis compared elderly versus younger patients on hyper-CVAD (implemented since 1992) as well as previous regimens (VAD, C-VAD).18
Criteria for response were standard. A CR required a bone marrow blast count ≤5% in cellular bone marrow with normalization of peripheral blood counts, including a granulocyte count ≥109/L and a platelet count ≥100 × 109/L. Induction death referred to death during the induction course without evidence of ALL. All other patients were considered to have resistant disease. Death in CR described patients who died in remission from any cause without evidence of leukemia. Survival was measured from start of therapy.
RESULTS
Study Group
In total, 122 elderly patients with ALL received hyper-CVAD. These patients were compared with 34 elderly patients who received previous regimens and 409 younger patients who received hyper-CVAD. The characteristics of the 3 groups are shown in Table 1.
TABLE 1.
Characteristics of Elderly and Younger Patients With Acute Lymphoblastic Leukemia
| Characteristic | No. of Patients (%)
|
P | ||
|---|---|---|---|---|
| Aged ≥60 Years
|
Aged <60 Years
|
|||
| Hyper-CVAD | Other Regimens | Hyper-CVAD | ||
| Women | 52 (43) | 12 (35) | 156 (38) | .80 |
| Splenomegaly present | 17 (13) | 10 (29) | 96 (23) | .001 |
| Lymphadenopathy present | 21 (17) | 8 (24) | 140 (34) | <.001 |
| Hemoglobin <10 g/dL | 87 (71) | 25 (74) | 290 (71) | .19 |
| WBC >20×109/L | 34 (28) | 13 (38) | 115 (28) | .37 |
| Platelets <50×109/L | 60 (49) | 18 (53) | 197 (48) | .40 |
| Karyotype | ||||
| Ph-positive | 33 (27) | 8 (24) | 74 (18) | |
| Burkitt | 10 (8) | 3 (9) | 23 (6) | |
| Diploid | 28 (23) | 6 (18) | 123 (30) | .007 |
| Hyperdiploid/≥50 chromosomes | 3 (3) | 3 (9) | 23 (6) | |
| Other | 31 (25) | 6 (17) | 102 (25) | |
| Morphology | ||||
| L1 | 24 (20) | 2 (6) | 120 (29) | |
| L2 | 64 (53) | 25 (74) | 210 (51) | .001 |
| L3 | 18 (15) | 2 (6) | 31 (8) | |
ALL indicates acute lymphocytic leukemia; hyper-CVAD, hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (8 total courses alternating with high doses of methotrexate and cytarabine followed by maintenance with 6-mercaptopurine, vincristine, methotrexate, and prednisone); WBC, white blood cells; Ph, Philadelphia chromosome.
Induction Therapy
The rates of CR, induction mortality, and resistant disease with induction therapy are shown in Table 2. Since 2000, elderly patients have received induction therapy in a protected environment. Induction mortality was observed in 3 of 61 such patients (5%) compared with in 9 of 61 elderly patients (15%) who were treated previously outside the protected environment on hyper-CVAD (P = .069) and 4 of 34 elderly patients (12%) who received other regimens (P = .22). The incidence of resistant disease was 27% with prehyper-CVAD regimens and 5% with hyper-CVAD (P < .001). Among the 102 CRs that were obtained with hyper-CVAD in elderly patients, 82 CRs (80%) were obtained after the first course, and 20 CRs (20%) were obtained after the second course or subsequent courses.
TABLE 2.
Response to Induction Therapy
| Response | No. of Patients (%)
|
P | ||
|---|---|---|---|---|
| Aged ≥60 Years
|
Aged <60 Years
|
|||
| Hyper-CVAD, n=122 | Other Regimens, n=34 | Hyper-CVAD, n=409 | ||
| CR | 102 (84) | 20 (59) | 378 (92) | <.001 |
| PR | 1 (1) | 1 (3) | 0 | |
| CRp | 0 (0) | 0 (0) | 2 (1) | |
| CR at start | 1 (1) | 0 (0) | 12 (3) | |
| Induction mortality | 12 (10) | 4 (12) | 9 (2) | <.001 |
| Resistant disease | 6 (5) | 9 (27) | 8 (2) | <.001 |
Hyper-CVAD indicates hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (8 courses alternating with high doses of methotrexate and cytarabine followed by maintenance with 6-mercaptopurine, vincristine, methotrexate, and prednisone); CR, complete response; PR, partial response; CRp, pathologic complete response.
Among 16 elderly patients who died during induction, all deaths were related to infection (Table 3). Multiorgan failure associated with infections was observed during the induction or by the time of death in 14 of 16 patients.
TABLE 3.
Causes of Death During Induction Therapy
| Predominant Cause of Death | No. of Patients
|
Total, n=35 | |
|---|---|---|---|
| Aged ≥60 Years, n=16 | Aged <60 Years, n=19 | ||
| Infections | |||
| Bacterial | 5 | 6 | 11 |
| Pneumonia | 3 | 4 | 7 |
| Fungal | 2 | 0 | 2 |
| Fungal and bacterial | 6 | 3 | 9 |
| Other | 0 | 1 | 1 |
| Bleeding | 0 | 5 | 5 |
| Organ failure as a component of infection | 14 | 10 | 24 |
Causes of Failure After Remission
Next, we focused on the causes of failure after remission induction, particularly the incidence and cause of death in CR. The hypothesis was that elderly patients would be more susceptible to death in CR from the myelosuppression-associated complications (infections, bleeding) of intensive chemotherapy. We characterized the deaths in CR according to whether they occurred during the hyper-CVAD or HDMTX-ara-C components of the 8 courses or during POMP maintenance.
Among 102 elderly patients with ALL who achieved a CR with hyper-CVAD, 76 patients (75%) experienced subsequent treatment failure. The causes of failure were death in CR in 35 patients and recurrence in 41 patients. Among the 35 deaths in CR, 22 deaths (63%) were caused predominantly by infections, 1 death was caused by bleeding, and 4 deaths were from other causes (in 8 patients, the cause of death was unknown). Seventeen of 35 deaths (49%) occurred during hyper-CVAD therapy, 7 deaths (20%) occurred during HDMTX-araC therapy, 10 deaths (29%) occurred during POMP maintenance, and 1 death occurred after allogeneic stem cell transplantation (SCT) (Table 4). Most of the deaths in elderly patients on hyper-CVAD occurred during the 8 courses of dose-intensive chemotherapy. Table 4 details the causes of death in CR. Two points are important: 1) Younger patients tolerated consolidation-maintenance therapy better and had a lower rate of mortality in CR (7%); and 2) as the treatment intensity increased (prehyper-CVAD to hyper-CVAD), the cause of failure post-CR changed in elderly patients with ALL from disease recurrence (prehyper-CVAD, 80%; hyper-CVAD, 40%) to myelosuppression-related mortality in CR (prehyper-CVAD, 15%; hyper-CVAD, 34%). Overall, the risk:benefit ratio of the more intensive hyper-CVAD regimen was favorable, because it translated into a modest but significant survival improvement (Fig. 1). Among the 35 elderly patients who died in CR, that event was preceded by at least 1 serious or life-threatening infectious complication in a previous dose-intensive course in 28 patients (80%). The survival of elderly patients who received hyper-CVAD is shown in Figure 1 and is compared with elderly patients who were treated on prehyper-CVAD regimens and with younger patients who were treated with hyper-CVAD.
TABLE 4.
Causes of Failure After Disease Remission in Elderly Patients and Younger Patients Receiving the Hyperfractionated Cyclophosphamide, Vincristine, Doxorubicin, and Dexamethasone Regimen and in and Elderly Patients Receiving Other Regimens
| Cause of Failure | No. of Patients (%)
|
P | ||
|---|---|---|---|---|
| Aged ≥60 Years
|
Aged <60 Years
|
|||
| Hyper-CVAD, n=102 | Other Regimens, n=20 | Hyper-CVAD, n=378 | ||
| Leukemia recurrence | 41 (40) | 16 (80) | 82 (48) | .004 |
| Death in CR | 35 (34) | 3 (15) | 25 (7) | <.001 |
| Infections | 21 (21) | 1 (5) | 11 (3) | |
| Bleeding | 1 (1) | 0 | 1 (0.3) | |
| Infection and bleeding | 1 (1) | 0 | 0 | |
| Organ failure | 0 | 0 | 4 (1) | |
| Other | 4 (4) | 1 (5) | 7 (2) | |
| Unknown | 8 (8) | 1 (5) | 2 (0.5) | |
Hyper-CVAD indicates hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (8 total courses alternating with high doses of methotrexate and cytarabine followed by maintenance with 6-mercaptopurine, vincristine, methotrexate, and prednisone); CR, complete response.
FIGURE 1.
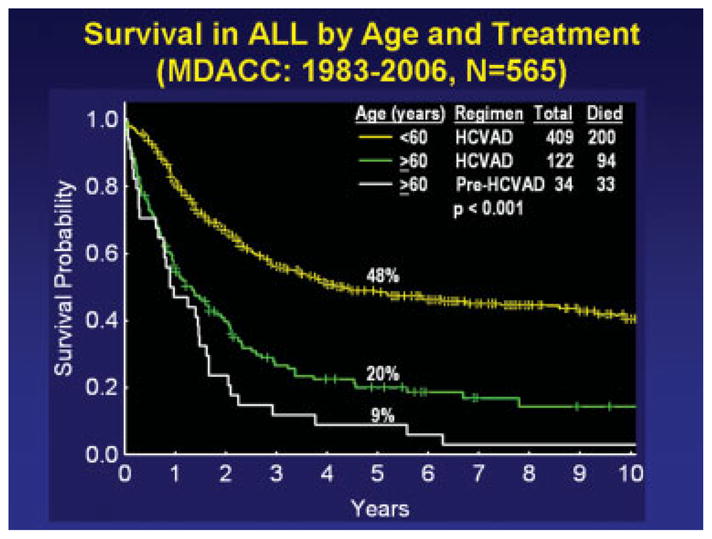
Survival of older and younger patients with acute lymphocytic leukemia (ALL) in different regimens. MDACC indicates the M. D. Anderson Cancer Center; HCVAD indicates hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (8 total courses of HCVAD were alternated with high doses of methotrexate and cytarabine followed by maintenance with 6 mercaptopurine, vincristine, methotrexate, and prednisone).
DISCUSSION
This analysis indicated that, as ALL regimens have become more dose-intensive, outcome in elderly patients with ALL has improved, but the primary cause of failure has changed from recurrence to death in CR because of myelosuppression-associated complications. Among elderly patients with ALL, the CR rate with hyper-CVAD was 84%; the induction mortality rate was 12% but was reduced to 5% with the use of the protective environment. Similarly, the 5-year survival on hyper-CVAD regimen improved to 20% compared with 9% on regimens that were used before hyper-CVAD (P = .046) (Fig. 1). Thus, although dose-intensive regimens improved outcome even in elderly patients with ALL, they shifted (unexpectedly) the cause of failure to complications of therapy. These were the cause of 34% of failures in CR among elderly patients with ALL on hyper-CVAD, or 29% of all failures on the regimens.
How can the outcome in elderly patients with ALL be improved further? Reverting back to less intensive regimens is an option that may result in higher rates of recurrence and overall failure. Because all patients received prophylactic antibiotics and growth factor support, it is unlikely that changes in supportive care will yield substantial improvement. The use of more dose-intense, nonmyelosuppressive agents also is associated with infections (eg, steroids) or extramedullary complications (eg, vincristine, asparaginase). More active and less toxic versions of older agents (eg, liposomal vincristine, pegylated asparaginase) may be 1 way to improve outcomes. A study comparing liposomal vincristine with vincristine as part of induction-consolidation-maintenance therapy in elderly patients with ALL is under consideration. Newer agents with selective anti-ALL activity may improve outcomes further in ALL patient subsets. This may be the case for nelarabine in T-cell ALL19 and for imatinib and dasatinib in Ph-positive ALL.20,21 In addition, monitoring for minimal residual disease using sensitive techniques, such as 4-color flow cytometry or polymerase chain reaction analysis, may allow for the cessation of therapy earlier in patients at high risk for complications.22–26
References
- 1.Pui CH, Evans WE. Acute lymphoblastic leukemia. N Engl J Med. 1998;339:605–615. doi: 10.1056/NEJM199808273390907. [DOI] [PubMed] [Google Scholar]
- 2.Schrappe M, Reiter A, Ludwig WD, et al. Improved outcome in childhood acute lymphoblastic leukemia despite reduced use of anthracyclines and cranial radiotherapy: results of trial ALL-BFM 90. German-Austrian-Swiss ALL-BFM Study Group. Blood. 2000;95:3310–3322. [PubMed] [Google Scholar]
- 3.Silverman LB, Gelber RD, Dalton VK, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood. 2001;97:1211–1218. doi: 10.1182/blood.v97.5.1211. [DOI] [PubMed] [Google Scholar]
- 4.Nachman JB, Sather HN, Sensel MG, et al. Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. N Engl J Med. 1998;338:1663–1671. doi: 10.1056/NEJM199806043382304. [DOI] [PubMed] [Google Scholar]
- 5.Kantarjian H, Thomas D, O’Brien S, et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101:2788–2801. doi: 10.1002/cncr.20668. [DOI] [PubMed] [Google Scholar]
- 6.Faderl S, Jeha S, Kantarjian HM. The biology and therapy of adult acute lymphoblastic leukemia. Cancer. 2003;98:1337–1354. doi: 10.1002/cncr.11664. [DOI] [PubMed] [Google Scholar]
- 7.Gokbuget N, Hoelzer D, Arnold R, et al. Treatment of adult ALL according to protocols of the German Multicenter Study Group for Adult ALL (GMALL) Hematol Oncol Clin North Am. 2000;14:1307–1325. ix. doi: 10.1016/s0889-8588(05)70188-x. [DOI] [PubMed] [Google Scholar]
- 8.Durrant IJ, Richards SM, Prentice HG, Goldstone AH. The Medical Research Council trials in adult acute lymphocytic leukemia. Hematol Oncol Clin North Am. 2000;14:1327–1352. doi: 10.1016/s0889-8588(05)70189-1. [DOI] [PubMed] [Google Scholar]
- 9.Thiebaut A, Vernant JP, Degos L, et al. Adult acute lymphocytic leukemia study testing chemotherapy and autologous and allogeneic transplantation. A follow-up report of the French protocol LALA 87. Hematol Oncol Clin North Am. 2000;14:1353–1366. doi: 10.1016/s0889-8588(05)70190-8. [DOI] [PubMed] [Google Scholar]
- 10.Larson RA. Recent clinical trials in acute lymphocytic leukemia by the Cancer and Leukemia Group B. Hematol Oncol Clin North Am. 2000;14:1367–1379. doi: 10.1016/s0889-8588(05)70191-x. [DOI] [PubMed] [Google Scholar]
- 11.Linker CA, Levitt LJ, O’Donnell M, Forman SJ, Ries CA. Treatment of adult acute lymphoblastic leukemia with intensive cyclical chemotherapy: a follow-up report. Blood. 1991;78:2814–2822. [PubMed] [Google Scholar]
- 12.Garcia-Manero G, Kantarjian HM. The hyper-CVAD regimen in adult acute lymphocytic leukemia. Hematol Oncol Clin North Am. 2000;14:1381–1396. x–xi. doi: 10.1016/s0889-8588(05)70192-1. [DOI] [PubMed] [Google Scholar]
- 13.Kantarjian HM, O’Brien S, Smith T, et al. Acute lymphocytic leukaemia in the elderly: characteristics and outcome with the vincristine-Adriamycin-dexamethasone (VAD) regimen. Br J Haematol. 1994;88:94–100. doi: 10.1111/j.1365-2141.1994.tb04982.x. [DOI] [PubMed] [Google Scholar]
- 14.Delannoy A, Sebban C, Cony-Makhoul P, et al. Age-adapted induction treatment of acute lymphoblastic leukemia in the elderly and assessment of maintenance with interferon combined with chemotherapy. A multicentric prospective study in forty patients. French Group for Treatment of Adult Acute Lymphoblastic Leukemia. Leukemia. 1997;11:1429–1434. doi: 10.1038/sj.leu.2400780. [DOI] [PubMed] [Google Scholar]
- 15.Taylor PR, Reid MM, Bown N, Hamilton PJ, Proctor SJ. Acute lymphoblastic leukemia in patients aged 60 years and over: a population-based study of incidence and outcome. Blood. 1992;80:1813–1817. [PubMed] [Google Scholar]
- 16.Ferrari A, Annino L, Crescenzi S, Romani C, Mandelli F. Acute lymphoblastic leukemia in the elderly: results of 2 different treatment approaches in 49 patients during a 25-year period. Leukemia. 1995;9:1643–1647. [PubMed] [Google Scholar]
- 17.Larson RA. Management of acute lymphoblastic leukemia in older patients. Semin Hematol. 2006;43:126–133. doi: 10.1053/j.seminhematol.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Kantarjian HM, Walters RS, Keating MJ, et al. Results of the vincristine, doxorubicin, and dexamethasone regimen in adults with standard- and high-risk acute lymphocytic leukemia. J Clin Oncol. 1990;8:994–1004. doi: 10.1200/JCO.1990.8.6.994. [DOI] [PubMed] [Google Scholar]
- 19.Kurtzberg J, Ernst TJ, Keating MJ, et al. Phase I study of 506U78 administered on a consecutive 5-day schedule in children and adults with refractory hematologic malignancies. J Clin Oncol. 2005;23:3396–3403. doi: 10.1200/JCO.2005.03.199. [DOI] [PubMed] [Google Scholar]
- 20.Ottmann OG, Druker BJ, Sawyers CL, et al. A phase 2 study of imatinib in patients with relapsed or refractory Philadelphia chromosome-positive acute lymphoid leukemias. Blood. 2002;100:1965–1971. doi: 10.1182/blood-2001-12-0181. [DOI] [PubMed] [Google Scholar]
- 21.Ottmann O, Dombret H, Martinelli G, et al. Dasatinib induces rapid hematologic and cytogenetic responses in adult patients with Philadelphia chromosome positive acute lymphoblastic leukemia with resistance or intolerance to imatinib: interim results of a phase 2 study. Blood. 2007;110:2309–2315. doi: 10.1182/blood-2007-02-073528. [DOI] [PubMed] [Google Scholar]
- 22.Gameiro P, Mortuza FY, Hoffbrand AV, Foroni L. Minimal residual disease monitoring in adult T-cell acute lymphoblastic leukemia: a molecular based approach using T-cell receptor G and D gene rearrangements. Haematologica. 2002;87:1126–1134. [PubMed] [Google Scholar]
- 23.Mortuza FY, Papaioannou M, Moreira IM, et al. Minimal residual disease tests provide an independent predictor of clinical outcome in adult acute lymphoblastic leukemia. J Clin Oncol. 2002;20:1094–1104. doi: 10.1200/JCO.2002.20.4.1094. [DOI] [PubMed] [Google Scholar]
- 24.Vidriales MB, Perez JJ, Lopez-Berges MC, et al. Minimal residual disease in adolescent (older than 14 years) and adult acute lymphoblastic leukemias: early immunophenotypic evaluation has high clinical value. Blood. 2003;101:4695–4700. doi: 10.1182/blood-2002-08-2613. [DOI] [PubMed] [Google Scholar]
- 25.Bruggemann M, Raff T, Fiohr T, et al. Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood. 2006;107:1116–1123. doi: 10.1182/blood-2005-07-2708. [DOI] [PubMed] [Google Scholar]
- 26.Raff T, Gokbuget N, Luschen S, et al. Molecular relapse in adult standard-risk ALL patients detected by prospective MRD monitoring during and after maintenance treatment: data from the GMALL 06/99 and 07/03 trials. Blood. 2007;109:910–915. doi: 10.1182/blood-2006-07-037093. [DOI] [PubMed] [Google Scholar]


