Abstract
Background
The aim of the study was to determine the significance of spinal anesthesia in the suppression of the metabolic, hormonal, and hemodynamic response to surgical stress in elective surgical patients compared to general anesthesia.
Material/Methods
The study was clinical, prospective, and controlled and it involved 2 groups of patients (the spinal and the general anesthesia group) who underwent the same surgery. We monitored the metabolic and hormonal response to perioperative stress based on serum cortisol level and glycemia. We also examined how the different techniques of anesthesia affect these hemodynamic parameters: systolic arterial pressure (AP), diastolic AP, heart rate (HR), and arterial oxygen saturation (SpO2). These parameters were measured before induction on anesthesia (T1), 30 min after the surgical incisions (T2), 1 h postoperatively (T3) and 24 h after surgery (T4).
Results
Serum cortisol levels were significantly higher in the general anesthesia group compared to the spinal anesthesia group (p<0.01). Glycemia was significantly higher in the general anesthesia group (p<0.05). There was a statistically significant, positive correlation between serum cortisol levels and glycemia at all times observed (p<0.01). Systolic and diastolic AP did not differ significantly between the groups (p=0.191, p=0.101). The HR was significantly higher in the general anesthesia group (p<0.01). SpO2 values did not differ significantly between the groups (p=0.081).
Conclusions
Based on metabolic, hormonal, and hemodynamic responses, spinal anesthesia proved more effective than general anesthesia in suppressing stress response in elective surgical patients.
MeSH Keywords: Anesthesia, General; Anesthesia, Spinal; Blood Glucose; Glucocorticoids; Perioperative Period
Background
The system response to surgical trauma includes activation of the sympathetic nervous system, the endocrine “stress response”, and immunological and hematological changes [1].Various kinds of stress cause an increase in the secretion of ACTH from the adenohypophysis, and (within a few minutes) an increase in secretion of cortisol from the adrenal cortex [2,3]. Cortisol is considered to be a hormone significant in stress. Many different, non-specific stimuli can cause a noticeable increase in secretion of cortisol from the adrenal cortex (e.g., trauma, surgery, infection, strong heat or cold, and almost any debilitating disease). Generally, the level and duration of increase in intra-and postoperative concentrations of cortisol are in proportion with the degree of surgical trauma [4,5].
The etiology of hyperglycemia in stress is multi-factorial, but is thought primarily to be the result of activation of the sympathoadrenal system, with contributions from the hypothalamus and adenohypophysis. The stress response triggers an increase in levels of plasma catecholamines and glucocorticoids, which in turn lead to hyperglycemia. Cortisol also has a huge impact on the glucose metabolism in stress, because by increasing glycogenolysis and glucogenesis it can lead to hyperglycemia [6,7].
Clinical implications of stress response involve hypertension, tachycardia, arrhythmia, myocardial ischemia, protein catabolism, suppression of the immune response, and loss of the excretory renal function with retention of electrolytes and water. Stress response is a significant risk factor for an unsatisfactory outcome in patients with cardiovascular disease, in patients with known endocrine, metabolic and immune disorders, as well as in patients with infection and immunosuppression. Thus, the reduction and modulation of stress response during the operation can significantly reduce the incidence of post-operative complications and morbidity [8,9].
The choice of anesthesia technique depends on the surgical disease, the general condition of the patient, the level and extent of the surgical operation, as well as the availability of resources required for anesthesia. Many surgical procedures can be done under spinal anesthesia, which has been used in clinical practice for more than a hundred years. The advantages of regional anesthesia over general anesthesia are documented in many studies (e.g., inhibition of metabolic and hormonal responses to stress, reducing the incidence of post-operative pain, speeding peristalsis after abdominal operations, reducing the incidence of deep vein thrombosis, and shorter hospital stay). Unfortunately, it cannot always be applied. The potential lack of regional anesthesia is that its limited duration has an impact on the management of surgery [10,11].
The advantages of general anesthesia are its simple and easy method of application, rapid sedation of the patient, and increased comfort of surgeons and anesthesiologists when the surgery has to last longer. The disadvantages of general anesthesia are poor control of anesthetics (depends on the individual ability of the organism to degrade and eliminate them) and postoperative adverse effects (e.g., sickness, nausea, vomiting, and pain) [10,12].
The aim of the study was to determine the significance of spinal anesthesia in suppressing the metabolic, hormonal, and hemodynamic response to surgical stress during and after elective operations in abdominal, urological, and orthopedics surgery compared with the technique of general anesthesia.
To monitor the metabolic and hormonal responses to stress, we measured the serum cortisol levels and glycemia, which are most easily detectable and are closely correlated with the intensity of stress.
The secondary objective was to investigate the suppression of the adrenergic response by monitoring hemodynamic parameters: systolic and diastolic arterial pressure (AP) and heart rate (HR).
Material and Methods
The research was conducted at the Kosovska Mitrovica Health Center from September 2013 to March 2014. The prospective, controlled clinical study included 75 patients aged 18 to 85 years. The patients were divided into 2 groups, depending on the anesthetic technique. In the spinal anesthesia group there were 38 patients and in the general anesthesia group were 37 patients undergoing the same surgical procedure. Depending on the performed surgical procedure, patients were classified into several subgroups: general surgery (herniotomy and thrombosaphenectomy), orthopedics (osteosynthesis), and urology (prostatectomy).
The choice of anesthesia technique was made by the anesthesiologist, based on preanesthetic parameters (clinical examination of the patient, laboratory analysis), the type of surgery, and on the patient’s preference.
To assess the operational risk, we used a classification adopted by the American Society of Anesthesiologists (ASA). The study included patients of ASA 1 (patients with no organic pathology or patients in whom the pathological process is localized and does not cause any systemic disturbance or abnormality; excludes very young and very old patients) and ASA 2 (patients with no functional limitations, with well-controlled disease of 1 body system). The study did not include patients with severe and extreme systemic disorders (ASA 3–6).
Serum cortisol levels were measured using the Eleksys 2010 chemiluminescence apparatus and glycemia was measured on the Hitachi 902 biochemical analyzer in the laboratory of the Kosovska Mitrovica Health Center.
Hemodynamic parameters – systolic and diastolic AP, heart rate (HR), arterial oxygen saturation (SpO2) – were monitored in all patients. The values of these parameters were registered using Nihon Kohden monitors.
Serum cortisol levels, glycemia and hemodynamic parameters were measured at the following time points: before induction on anesthesia (T1), 30 min after the surgical incisions (T2), 1 h postoperatively (T3), and 24 h after surgery (T4).
All patients were premedicated with midazolam 5 mg i.m. 30 min prior to surgery. In the general anesthesia group, for the induction of anesthesia we used propofol (2.5 mg/kg b.w.) and for endotracheal intubation we used succinyl-choline (1.5 mg/kg b.w.). To maintain anesthesia, we used propofol boluses with a 2: 1 nitrous oxide: oxygen (N2O-to-O2) ratio. Analgesia was achieved with fentanyl (0.05 mg/kg b.w.) and muscle relaxation with rocuronium bromide (0.6 mg/kg b.w.). For the reversal of neuromuscular blockade, we used 1.5 mg prostigmin and 0.5 mg atropine. In the spinal anesthesia group, we used a 22–26 G spinal needle, local anesthetic (bupivacaine 15 mg 0.5%), and opioids (fentanyl 0.05 mcg).
For the analysis of primary data, descriptive statistical methods and hypothesis testing methods were used. We used the following descriptive statistical methods: measures of central tendency (arithmetic mean), measures of variability (standard deviation), and relative numbers. To test hypotheses about the difference in frequency, the chi-squared test was used. The t-test was used for testing hypothesis about difference of arithmetic means between the 2 groups. ANOVA for repeated measures was used to identify differences between groups in which the data were parametric and normally distributed. To assess correlations between continuous variables, Pearson’s correlation coefficients were calculated. The levels of statistical significance were p<0.05 and 0.01. All data management and statistical analyses were performed using SPSS Statistics 21.
Results
Demographic variables and general characteristics of the patients are shown in Table 1. Spinal anesthesia was implemented significantly more in males. There was no statistically significant difference between the spinal and the general anesthesia groups regarding age, duration of surgery, ASA categories, and type of surgery. For comorbidity and the type of surgery, the frequency is shown by category (Table 1).
Table 1.
Demographic and general characteristics of the patients.
| Parameters | General anesthesia | Spinal anesthesia | ||
|---|---|---|---|---|
| Number of patient n (%) | 37 (49.3%) | 38 (50.7%) | ||
| Age (yr, mean ±SD) | 53.8±18.7 | 61.0±15.4 | p=0.071 | |
| Duration of surgery (min, mean±SD) | 82.7±20.7 | 77.6±21.6 | p=0.304 | |
| Sex n (%) | Male | 27 (73%) | 36 (94.7%) | p=0.01* |
| Female | 10 (27%) | 2 (5.3%) | ||
| ASA n (%) | ASA 1 | 18 (48.6%) | 16 (42.1%) | p=0.569 |
| ASA 2 | 19 (51.4%) | 22 (57.9%) | ||
| Comorbidity n (%) | Previously healthy | 18 (48.6%) | 16 (47.1%) | |
| Hypertensio arterialis | 15 (40.5%) | 11 (42.3%) | ||
| Chronic bronchitis | 2 (5.4%) | 7 (18.4%) | ||
| Diabetes mellitus | 0 (0%) | 1 (2.6%) | ||
| Anemia | 1 (2.7%) | 1 (2.6%) | ||
| Hyperlipoproteinemia | 1 (2.7%) | 2 (5.3%) | ||
| Type of surgery | Herniectomia | 23 (62.2%) | 23 (60.5%) | |
| Osteosynthesis | 7 (18.9%) | 7 (18.4%) | ||
| Trombosaphenectomia | 4 (10.8%) | 1 (2.6%) | ||
| Prostatectomia | 3 (8.1%) | 7 (18.4%) | ||
n – number; SD – standard deviation;
statistical significance.
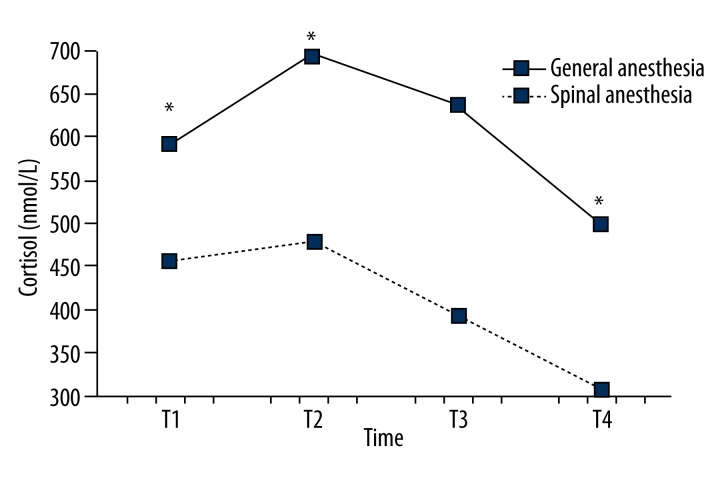
Serum cortisol levels significantly differed between the spinal and the general anesthesia group. Intraoperatively (T2), serum cortisol level (694.7±140.8 vs. 478.7±91.9) increases in both groups, but significantly more in the general anesthesia group. Serum cortisol levels in the general anesthesia group at 1 h after surgery (T3) and 24 h postoperatively (T4) were significantly higher than in the spinal anesthesia group (Figure 1).
Figure 1.
Comparison of serum cortisol levels between the groups in the observed period. Serum cortisol levels were significantly higher in the general anesthesia group compared to the spinal anesthesia group (p<0.01). In the observed time period serum cortisol levels are statistically significantly different (p<0.01) with a significant linear trend of decline of serum cortisol levels (p<0.01) and a significant quadratic trend of increase in serum cortisol levels at T2, which in the further course of the period decreases in value (p<0.01). Changes in serum cortisol levels are statistically significantly different over time for both groups (p<0.01)
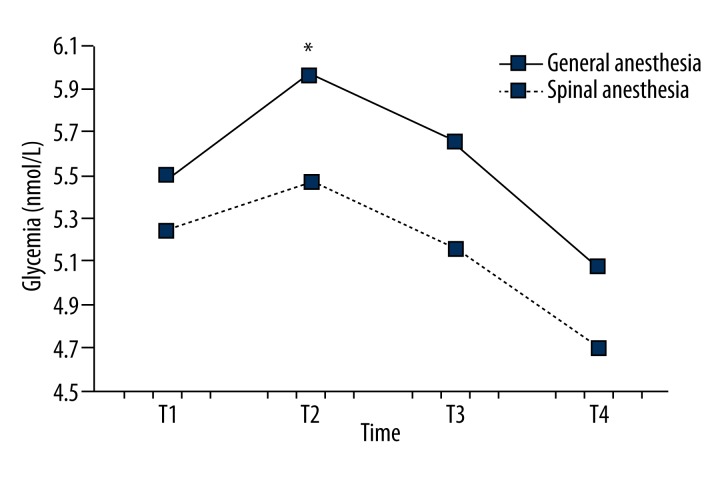
Glycemia values were statistically significantly increases 30 min after surgical incision (T2) in both groups, but at T3 and T4 glycemia decreased (Figure 2).
Figure 2.
Comparison of glycemia between groups during the observed period. Glycemia was significantly higher in the general anesthesia group compared to the spinal anesthesia group (p<0.05). During the observed period serum glucose levels differ significantly (p<0.01) with a significant linear trend of decrease in glycemia (p<0.01) and a significant quadratic trend of increase in serum cortisol levels at T2, which in the further course of the period decreased in values (p<0.01). Glycemia was significantly lower in the spinal anesthesia group at times T2 and T3 (both p<0.05), while the difference was not statistically significant at the times T1 and T4 (both p>0.05). Changes in glycemia did not differ significantly over time for both groups (p=0.60).
There was a statistically significant, positive correlation between serum cortisol levels and glycemia at all times observed. At time T1, there was a statistically significant, weak negative correlation between serum cortisol level and systolic AP, and between serum cortisol level and diastolic AP. During the same period, there was a statistically significant, positive correlation between serum cortisol level and HR. At time T4 there was a statistically significant, weak positive correlation between serum cortisol levels and SpO2 (Table 2).
Table 2.
Correlation between serum cortisol level, glycemia and hemodynamic parameters.
| Time | Parameters | Correlation | Glycemia | Systolic AP | Diastolic AP | HR | SPO2 |
|---|---|---|---|---|---|---|---|
| T1 | Cortisol | r | 0.451 | −0.234 | −0.262 | 0.271 | 0.099 |
| p | 0.000* | 0.043* | 0.023* | 0.019* | 0.396 | ||
| n | 75.0 | 75.0 | 75.0 | 75.0 | 75.0 | ||
| Glycemia | r | −0.040 | −0.133 | 0.077 | 0.153 | ||
| p | 0.732 | 0.254 | 0.511 | 0.191 | |||
| n | 75.0 | 75.0 | 75.0 | 75.0 | |||
| T2 | Cortisol | r | 0.528 | 0.214 | 0.226 | 0.118 | 0.132 |
| p | 0.000* | 0.065 | 0.051 | 0.314 | 0.259 | ||
| n | 75.0 | 75.0 | 75.0 | 75.0 | 75.0 | ||
| Glycemia | r | 0.112 | 0.058 | −0.030 | 0.145 | ||
| p | 0.339 | 0.620 | 0.796 | 0.213 | |||
| n | 75.0 | 75.0 | 75.0 | 75.0 | |||
| T3 | Cortisol | r | 0.595 | 0.052 | 0.195 | 0.226 | 0.226 |
| p | 0.000* | 0.659 | 0.094 | 0.052 | 0.051 | ||
| n | 75.0 | 75.0 | 75.0 | 75.0 | 75.0 | ||
| Glycemia | r | −0.121 | −0.060 | 0.113 | 0.175 | ||
| p | 0.301 | 0.606 | 0.335 | 0.132 | |||
| n | 75.0 | 75.0 | 75.0 | 75.0 | |||
| T4 | Cortisol | r | 0.542 | 0.127 | 0.118 | 0.213 | 0.298 |
| p | 0.000* | 0.279 | 0.315 | 0.067 | 0.009* | ||
| n | 75.0 | 75.0 | 75.0 | 75.0 | 75.0 | ||
| Glycemia | r | 0.101 | 0.145 | 0.067 | 0.200 | ||
| p | 0.390 | 0.214 | 0.568 | 0.086 | |||
| n | 75.0 | 75.0 | 75.0 | 75.0 |
r – Pearson correlation coefficient; n – number of patients; AP – arterial pressure; HR – heart rate;
statistical significance.
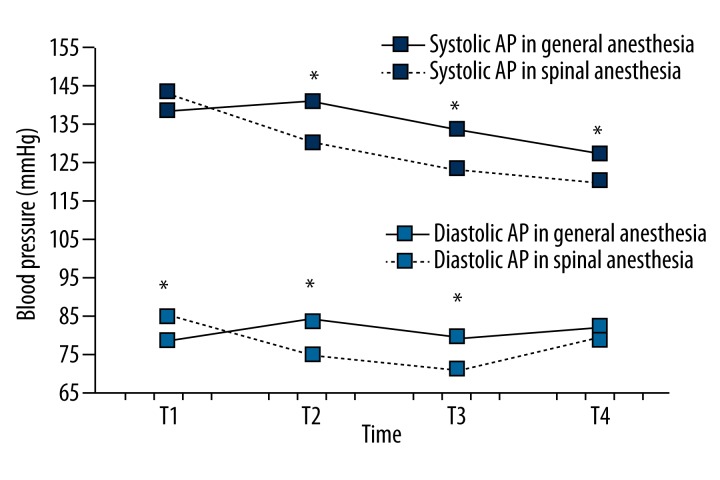
Systolic and diastolic AP did not differ significantly between the groups. In the observed period, systolic and diastolic AP were significantly different, with a significant linear trend of decreasing AP values, but there was no significant difference in the fluctuations at the points of measurement, of the values for systolic and diastolic AP (Figure 3).
Figure 3.
Comparison of systolic and diastolic AP between the groups during the observed period. Systolic AP was significantly higher in the general anesthesia group at times T2, T3 and T4 (p<0.05), while the difference was not statistically significant at time T1 (p=0.303). Diastolic AP in the general anesthesia group was significantly lower at time T1, while at times T2 and T3 diastolic AP is significantly higher compared to the spinal anesthesia group (both p<0.01). The difference in diastolic AP measurements was not statistically significant at time T4 (p=0.246). Changes in values of systolic and diastolic AP are statistically significant in the observed time for both groups (p<0.01). (* p<0.01 or p<0.05).
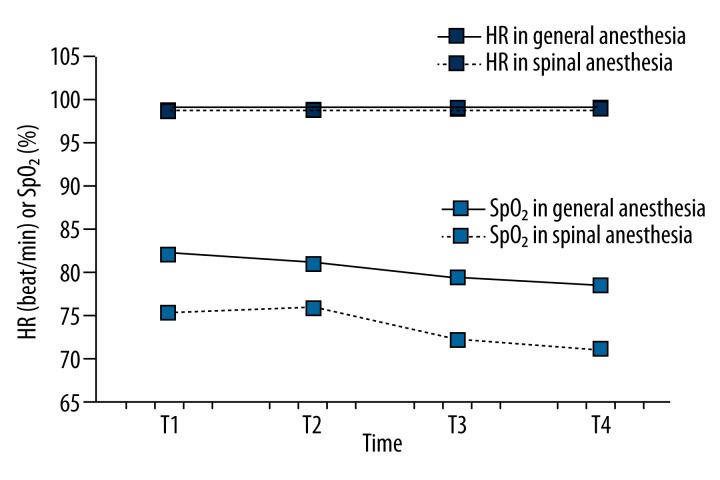
The HR was significantly higher in the general anesthesia group compared to the spinal anesthesia group (p<0.01) (Figure 4).
Figure 4.
Comparison of the HR and SpO2 values between groups during the observed period. During the observed period the HR is significantly different (p<0.01) with a significant linear trend of depreciation of the HR (p<0.01) but there was no significant difference in the fluctuations of the values at different points of measurement (p=0.917). Changes in HR do not vary significantly over time for both group (p=0.456). SpO2 values do not differ significantly between the groups (p=0.081) or in the observed period (p=0.475).
Measurements of the parameters at T3 determined that there was no statistically significant correlation between the duration of surgery and serum cortisol levels or between the duration of surgery and glycemia. Measurements of the parameters at T4 determined that there was no statistically significant correlation between the duration of surgery and serum cortisol levels or between the duration of surgery and glycemia (Table 3).
Table 3.
Correlation between duration of surgery and serum cortisol levels and glycemia.
| Time | Parameters | Correlation | Cortisol | Glycemia |
|---|---|---|---|---|
| T3 | Duration of surgery | r | 0.218 | 0 |
| p | 0.060 | 0.999 | ||
| n | 75.0 | 75.0 | ||
| T4 | Duration of surgery | r | 0.206 | 0.130 |
| p | 0.076 | 0.265 | ||
| n | 75.0 | 75.0 |
r – Pearson Correlation Coefficient; n – number of patients.
There were no significant postoperative complications in any of the groups.
Discussion
Serum cortisol levels are correlated with the intensity, duration, and type of stress that caused them; therefore, plasma cortisol concentration is the most commonly used marker for perioperative examination of stress induced by surgical trauma [13].
Many studies have examined the effect of different anesthesia techniques on the concentration of serum cortisol levels [10,14]. In general, the results of previous studies have shown that the choice of anesthesia technique affects intraoperative stress response, and thus significantly affects the outcome and morbidity of surgical patients and the reduction of postoperative pain [14]. Attenuation of the endocrine metabolic response may reduce the frequency of postoperative complications [15].
In our study, basal serum cortisol levels, which we measured before the start of surgery, were in the range of reference values. Thirty minutes after surgical incision, there was a significant rise in serum cortisol levels in both groups of patients, but significantly higher in the general anesthesia group. Perioperative serum cortisol levels in the spinal anesthesia group were significantly lower compared to those in the general anesthesia group. Postoperative serum cortisol levels were also significantly lower in the spinal anesthesia group, as previously described [16,17].
This difference can be attributed to spinal anesthesia blocking the sensory afferent nerve impulses originating from the surgical trauma. It is known that blocking the efferent and afferent pathways of the sympathetic and somatic nervous system inhibits activation of the neuroendocrine axis during surgical procedures [6].
In our study, we researched the following procedures: prostatectomy, osteosynthesis, herniotomy, and thrombosaphenectomy. These types of surgeries are procedures of intermediate severity and can potentially cause an increase in cortisol secretion in response to surgical stimulation. Increased levels of stress hormones are considered undesirable because they lead to intraoperative and postoperative metabolic catabolism and to hemodynamic instability. Clinical evidence has shown that spinal anesthesia has an effect in modification of stress response by means of modulation or inhibition of the nociceptive afferent signals from the area of surgical trauma.
The changes include alterations in metabolic, hormonal, inflammatory, and immune systems, which can be collectively termed the stress response. Integral to stress responses are the effects of nociceptive afferent stimuli on systemic and pulmonary vascular resistance, heart rate, and blood pressure, which are a combination of efferent autonomic response and catecholamine release via adrenal medulla [18,19]. The magnitude of the response is broadly related to the site of injury (greater in regions with visceral pain afferents, such as the abdomen and thorax) and extent of the trauma [20].
Buyukkocak et al. [17] conducted a comparative study in which they compared the effects of general and spinal anesthesia on serum cortisol levels in anorectal surgery. The results showed that serum cortisol levels were significantly lower in the spinal anesthesia group compared to the general anesthesia group. They concluded that spinal anesthesia can reduce stress response in patients undergoing anorectal surgery.
Davis et al. [21] concluded that unilateral spinal anesthesia more effectively suppresses the metabolic stress response in orthopedic patients undergoing total hip arthroplasty compared with general anesthesia.
In our study, we found a suppression of the hyperglycemic response in both groups. Lower glycemia were observed in the spinal anesthesia group at 1 h and 24 h postoperatively. There was a statistically significant positive correlation between serum cortisol levels and glycemia at all points of measurement, which means that an increase in serum cortisol levels has a direct impact on the increase of glycemia.
Surgery-related metabolic and endocrine derangements lead to adverse effects, including increased oxygen consumption, hypertension, tachycardia, arrhythmia, myocardial ischemia, hemodynamic instability, catabolism, and impaired immune function. These derangements have been associated with poor postoperative course and clinical outcome [22–24].
Anesthesia is performed adequately if the AP and HR do not exceed 20% of the values before induction. In our study, hemodynamic parameters showed that the suppression of the adrenergic response was adequate in both investigated groups. In the general anesthesia group, systolic AP was significantly higher 30 min after the surgical incision, 1 h postoperatively, and 24 h after surgery, but with no substantial oscillation of the values. The diastolic AP was significantly lower in the spinal anesthesia group 30 min after the surgical incision and 1 h postoperatively, but there were no differences in their dynamics. The values of HR were significantly higher in the general anesthesia group, but without significant fluctuations at the different points of measurement, which indicates a satisfactory depth of anesthesia. Wolf [20] reported that the sympathetic block induced by regional anesthesia resulted in a profound suppression of hemodynamic and stress response to pediatric surgery.
Changes in systolic and diastolic AP significantly differed over time for both groups. The systolic and diastolic AP was significantly higher in the general anesthesia group intraoperatively and immediately postoperatively. However, we noted no significant fluctuations in the dynamics of changes in the studied groups. Due to the reduced sensitivity to norepinephrine in patients under stress, despite its elevated levels in plasma, there were no significant changes in AP [25]. Changes in HR during the period did not differ significantly between the groups, although significantly higher values of HR were recorded in the general anesthesia group.
Attari et al. [26] conducted a study in which they compared the effects of spinal and general anesthesia on the hemodynamic stability of patients and the need for postoperative analgesia in elective surgery of the lumbar spine. They concluded that spinal anesthesia is superior in terms of hemodynamic stability and postoperative analgesia compared with general anesthesia. Knezevic et al. [27] concluded that the vast majority of patients who have undergone both general and local anesthesia for dacryocystorhinostomy would choose local anesthesia again.
Our findings are in agreement with other reports in the literature suggesting that regional anesthesia could produce an attenuation of the stress response to surgical trauma [5]. In our study, the number of patients is too small to reach conclusion regarding different types of surgery. Another limitation is that stress response to surgical trauma measured by serum cortisol levels and glycemia could be due to other factors besides type of anesthesia, such as type of surgery, magnitude of surgical injury, duration of operation, and degree of postoperative pain. Different techniques of anesthesia have not been shown to affect clinical outcome, so studies with more sensitive methods should be carried out to further investigate this issue.
Conclusions
Spinal anesthesia showed better efficacy in suppressing cortisol response as compared to the technique of general anesthesia. In the suppression of the hyperglycemic response, both types of anesthesia proved equally effective, although slightly lower glycemia were recorded in patients who underwent spinal anesthesia. Hemodynamic parameters showed that the suppression of the adrenergic response was adequate in both groups, but hemodynamic stability was better in the spinal anesthesia group.
Based on metabolic, hormonal, and hemodynamic responses, spinal anesthesia proved more effective than general anesthesia in suppressing stress response during elective procedures in abdominal, urological, and orthopedic surgery.
Footnotes
Source of support: Departmental source
References
- 1.Lalevic i sar. Anesteziologija. Zavod za udzbenike i nastavna sredstva; Beograd: 1999. pp. 4–5. [in Serbian] [Google Scholar]
- 2.Sapolsky R, Romero M, Muunck A. How do glucocorticoides influence stress responses? Integrating, permissive, suppressive, stimulatory and preparative actions. Endocrine Rev. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 3.Guyton A, Hall JE. Medicinska fiziologija. Savremena administracija; Beograd: 2008. [in Serbian] [Google Scholar]
- 4.Antanaskovic S, Stojanovic-Ilic M, Milic S, Dukic M. Neuroendokrinoloski i metabolicki odgovor bolesnika na preoperativni stres. Medicina Danas. 2012;11(7–9):207–11. [in Serbian] [Google Scholar]
- 5.Desborough JP. The stress response to trauma and surgery. Br J Anaesth. 2000;85(1):109–17. doi: 10.1093/bja/85.1.109. [DOI] [PubMed] [Google Scholar]
- 6.Buyukkocak U, Caglayan O, Oral H, et al. The effects of anesthetic techniques on acute phase response at delivery (anesthesia and acute phase response) Clin Biochem. 2003;36:67–70. doi: 10.1016/s0009-9120(02)00422-8. [DOI] [PubMed] [Google Scholar]
- 7.Vogeser M, Groetzner J, Kupper C, Briegel J. The serum cortisol: cortisone ratio in the postoperative acute-phase response. Horm Res. 2003;59:293–96. doi: 10.1159/000070628. [DOI] [PubMed] [Google Scholar]
- 8.Bochicchio GV, Scalea TM. Glycemic control in the ICU. Adv Surg. 2008;42:261–75. doi: 10.1016/j.yasu.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Laird AM, Miller PR, Kilgo PD, et al. Relationship of early hyperglycemia in trauma patients. J Trauma. 2004;56(5):1058–62. doi: 10.1097/01.ta.0000123267.39011.9f. [DOI] [PubMed] [Google Scholar]
- 10.Malenkovic V, Labus M, Marinković O. Supresija hormonskog odgovora organizma na stres primenom kombinovane spinalne, epiduralne i opšte anestezije u kolorektalnoj hirurgiji. Anestezija i Intenzivna Terapija. 2005;28(1):23–30. [in Serbian] [Google Scholar]
- 11.Covino BG. Rationale for spinal anesthesia. Int Anesthesiol Clin. 1989;27(1):8–12. doi: 10.1097/00004311-198902710-00003. [DOI] [PubMed] [Google Scholar]
- 12.Riquelme J. The safe of use combined general and regional anesthesia for upper abdominal surgery. ESRA. 1997;6:321–24. [Google Scholar]
- 13.Ram E, Vishne TH, Weinstein T, et al. General anesthesia for surgery influences melatonin and cortisol levels. World J Surg. 2005;29:826–29. doi: 10.1007/s00268-005-7724-1. [DOI] [PubMed] [Google Scholar]
- 14.Buyukkocak U, Caglayan O, Daphan C, et al. Similar effects of general and spinal anaesthesia on perioperative stress response in patients undergoing haemorrhoidectomy. Mediators Inflamm. 2006;2006(1):97257. doi: 10.1155/MI/2006/97257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chloropoulou P, Iatrou C, Vogiatzaki T, et al. Epidural anesthesia followed by epidural analgesia produces less inflammatory response than spinal anesthesia followed by intravenous morphine analgesia in patients with total knee arthroplasty. Med Sci Monit. 2013;19:73–80. doi: 10.12659/MSM.883749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hogevold HE, Lyberg T, Kahler H, et al. Changes in plasma IL-1beta, TNF-alpha and IL-6 after total hip replacement surgery in general or regional anaesthesia. Cytokine. 2000;12:1156–59. doi: 10.1006/cyto.2000.0675. [DOI] [PubMed] [Google Scholar]
- 17.Buyukkocak U, Daphan C, Caglayan O, et al. Effects of different anesthetic techniques on serum leptin, C-reactive protein, and cortisol concentrations in anorectal surgery. Croat Med J. 2006;47(6):862–68. [PMC free article] [PubMed] [Google Scholar]
- 18.Bevan DR. Modification of the metabolic response to trauma under extradural analgesia. Anaesthesia. 1971;26:188–91. doi: 10.1111/j.1365-2044.1971.tb04760.x. [DOI] [PubMed] [Google Scholar]
- 19.Aggo AT, Fyneface-Ogan S, Mato CN. The differential impact of two anesthetic techniques on cortisol levels in Nigerian surgical patients. Niger J Clin Pract. 2012;15:68–74. doi: 10.4103/1119-3077.94102. [DOI] [PubMed] [Google Scholar]
- 20.Wolf A. Effects of regional analgesia on stress responses to pediatric surgery. Pediatr Anaesth. 2012;22:19–24. doi: 10.1111/j.1460-9592.2011.03714.x. [DOI] [PubMed] [Google Scholar]
- 21.Davis FM, Laurenson VG, Lewis J, et al. Metabolic response to total hip arthroplasty under hypobaric subarachnoid or general anaesthesia. Br J Anaesth. 1987;59(6):725–29. doi: 10.1093/bja/59.6.725. [DOI] [PubMed] [Google Scholar]
- 22.Marana E, Annetta MG, Meo F, et al. Sevoflurane improves the neuroendocrine stress response during laparoscopic pelvic surgery. Can J Anaesth. 2003;50:348–54. doi: 10.1007/BF03021031. [DOI] [PubMed] [Google Scholar]
- 23.Marrocco-Trischitta MM, Tiezzi A, Svampa MG, et al. Perioperative stress response to carotid endarterectomy: the impact of anesthetic modality. J Vasc Surg. 2004;39:1295–304. doi: 10.1016/j.jvs.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Norman JG, Fink GW. The effects of epidural anesthesia on the neuroendocrine response to major surgical stress: a randomized prospective trial. Am Surg. 1997;63:75–80. [PubMed] [Google Scholar]
- 25.Savic C, Belkic K. Centralni mehanizmi stresa i kardiovaskularni odgovor. Savremena administracija; Beograd: 1995. [in Serbian] [Google Scholar]
- 26.Attari MA, Mirhosseini SA, Honarmand A, Safavi MR. Spinal anesthesia versus general anesthesia for elective lumbar spine surgery: A randomized clinical trial. J Res Med Sci. 2011;16(4):524–29. [PMC free article] [PubMed] [Google Scholar]
- 27.Knezevic M, Vlajkovic G, Stojkovic M, et al. Comparison of postoperative pain and satisfaction after dacryocystorhinostomy in patients operated on under local and general anesthesia. Med Sci Monit. 2012;18(5):CR265–70. doi: 10.12659/MSM.882730. [DOI] [PMC free article] [PubMed] [Google Scholar]