Abstract
A 36-kDa diheme c-type cytochrome abundant in Fe(III)-respiring Geobacter sulfurreducens, designated MacA, was more highly expressed during growth with Fe(III) as the electron acceptor than with fumarate. Although MacA has homology to proteins with in vitro peroxidase activity, deletion of macA had no impact on response to oxidative stress. However, the capacity for Fe(III) reduction was greatly diminished, indicating that MacA, which is predicted to be localized in the periplasm, is a key intermediate in electron transfer to Fe(III).
Dissimilatory Fe(III) reduction is an environmentally significant process (22, 23), but the mechanisms of electron transfer to Fe(III) are poorly understood. In contrast to soluble electron acceptors such as oxygen and nitrate, which diffuse into the cell prior to reduction, Fe(III) oxides are insoluble and thus must be reduced at the outer membrane surface. Mechanisms for electron transfer to Fe(III) in Geobacter species are of particular interest because members of the Geobacteraceae are the predominant Fe(III)-reducing microorganisms in a variety of environments in which Fe(III) reduction is an important process. These environments include aquatic sediments (38), aquifers contaminated with organic pollutants (34, 35, 37), and uranium-contaminated subsurface environments in which the growth of dissimilatory metal-reducing microorganisms has been stimulated to promote the reductive precipitation of uranium (3, 17).
In contrast to Shewanella and Geothrix species, which release chelators that solubilize Fe(III) and soluble electron shuttles that alleviate the need for contact with insoluble Fe(III) oxides (30-32), Geobacter species must directly contact Fe(III) oxides in order to reduce them (7, 29). The most abundant electron transport proteins in the genome of Geobacter sulfurreducens are c-type cytochromes (26), and some of these are expected to be important in Fe(III) reduction.
Specific expression of macA during growth on Fe(III).
In order to identify c-type cytochromes that might be specifically involved in Fe(III) reduction, G. sulfurreducens was cultivated as previously described (6, 25), with acetate (20 mM) as the electron donor and either fumarate (27.5 mM) or Fe(III) citrate (55 mM) as the electron acceptor. Cells were harvested by centrifugation during late exponential growth phase, washed with MOPS (morpholinepropanesulfonic acid) buffer (10 mM; pH 7.0) containing MgCl2 (1 mM), resuspended in Tris-HCl (50 mM; pH 7.5), and lysed by passing them twice through a French pressure cell (40,000 kPa). The lysate was centrifuged (20 min; 1,500 × g; 4°C) to remove cell debris, and the supernatant was centrifuged (50 min; 100,000 × g; 4°C) to separate the membrane fraction from the soluble fraction. The membrane pellet was suspended in Tris-HCl (50 mM; pH 7.5), and proteins were separated by Tris-Tricine sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis (SDS-PAGE) (5) and stained with Coomassie blue R-250 (5) to detect total protein or N,N,N′,N′-tetramethylbenzadine (12, 39) to detect heme-binding proteins.
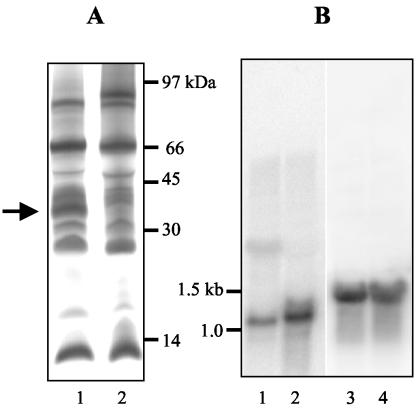
The membrane fractions from both Fe(III)- and fumarate-respiring cells contained abundant c-type cytochromes, but the membranes of Fe(III)-reducing cells had more cytochromes in the region of 35 kDa than those of fumarate-reducing cells (Fig. 1A). These cytochromes, along with several other cytochromes of various sizes, could be extracted from the membranes with weak salt treatment (150 mM NaCl), indicating a possible peripheral association. The 35-kDa cytochromes were further enriched with cation-exchange (SP-Sepharose; Amersham Biosciences, Piscataway, N.J.) and size exclusion chromatography (Superdex 75; Amersham Biosciences). The cytochromes in this enriched fraction were separated with SDS-PAGE, excised, trypsin digested (in the presence of 0.01% n-octylglucopyranoside), and subjected to matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry (Kratos Axima CFR; Kratos Analytical, Manchester, England) (8, 15). Sequence information was obtained from four peptide fragments: RMLFFDPRL, RNSPTVLNAVYNIAQFWDGRA, KSIPGYPPLFR, and RNAPTVLNSVFNTAQFWDGRA. The first three sequences were found within open reading frame GSU0466 (NP_951525) of the G. sulfurreducens genome, designated macA. The fourth fragment matched GSU2813 (NP_953857), which encodes a protein with 68% amino acid identity with, and of similar size to, MacA. Further studies focused on macA.
FIG. 1.
Expression of c-type cytochromes and MacA. (A) SDS-PAGE of insoluble (membrane) protein fractions (50 μg/lane) prepared from Fe(III)-reducing cells (lane 1) and fumarate-reducing cells (lane 2) stained for covalently bound heme. The arrow indicates the 36-kDa cytochrome designated MacA. (B) Northern blot of RNA (7 μg/lane) prepared from fumarate-reducing cells (lanes 1 and 3) or Fe(III)-reducing cells (lanes 2 and 4). The blots were probed with macA (lanes 1 and 2) or 16S rRNA (lanes 3 and 4).
MacA had a predicted molecular mass of 36.2 kDa (4), two heme-binding motifs (CXXCH), and a predicted isoelectric point of pH 9.0 (4). It contained a putative signal peptide (33) and lacked transmembrane regions (42), suggesting a periplasmic location (28, 33). Genes similar to macA are distributed throughout the Proteobacteria (2), with the closest homolog found in Geobacter metallireducens (79% amino acid identity, ZP_00080813) (24). Characterized proteins similar (ca. 60% identity) to MacA include periplasmic diheme c-type cytochromes from Rhodobacter capsulatus and Pseudomonas aeruginosa (10, 13, 36). Both of these proteins have been shown to have cytochrome c-hydrogen peroxide oxidoreductase activity in vitro (10, 11). Comparison of MacA to the cytochrome c peroxidases from P. aeruginosa and other organisms reveals that much of the divergence is due to substitutions in charged residues, with the loss of many acidic residues, though the heme and calcium binding ligands are conserved. G. sulfurreducens also lacks homologs to the putative electron donors to cytochrome c peroxidases of other bacteria (14).
To further evaluate the expression levels of macA and to distinguish macA expression from that of other similarly sized cytochromes, total RNA was isolated from G. sulfurreducens cells reducing Fe(III) or fumarate (RNeasy Mini kit; QIAGEN, Inc.), blotted (Northern Max-Gly kit; Ambion Inc., Austin, Tex.), and screened by using a probe created with primers macANf (CCGAAATCTCGCATGG) and macANr (GGAAAAGGGGAGGGTAAC) (NEBlot kit; New England Biolabs Inc., Beverly, Mass.). Cells grown on Fe(III) had higher levels of mRNA for macA than cells grown on fumarate (Fig. 1B). The macA mRNA was approximately 1.1 kb in length, consistent with monocistronic transcription. Predicted open reading frames near macA in the genome are all hypothetical proteins.
Analysis of the mutant strain deficient in macA.
In order to elucidate the physiological role of MacA, 64% of macA was replaced with a kanamycin resistance cassette with the single-step gene replacement method (20, 21). Briefly, a fragment in which a kanamycin resistance cassette from pBBR1MCS-2 (19) was flanked by the upstream and downstream regions of macA was constructed from three pieces by using recombinant PCR. The following primers were used, with the pBBR1MCS-2 sequence indicated in bold: macA1 (CAGTTCACGCCATCTCTCTATG), macA2 (GATTAAGTGCGAAGCCGAAAGC), macA3 (GTTCTTCGATCCGCGGCTTTCATGAATGTCAGCTACTGG), macA4 (CTTGACGGCGTCCTTCAGTTTCAATCGAAATCTCGTGATGG), macA5 (GCAAGGTCTGGAAACTGAAGG), and macA6 (GACTGCCGGTTCATATCC). Electroporation and mutant isolation were performed as previously described (9). Gene disruption was confirmed by PCR and by Southern blotting, and one clone was chosen as the representative mutant strain. Growth of the mutant was analyzed as previously described (20).
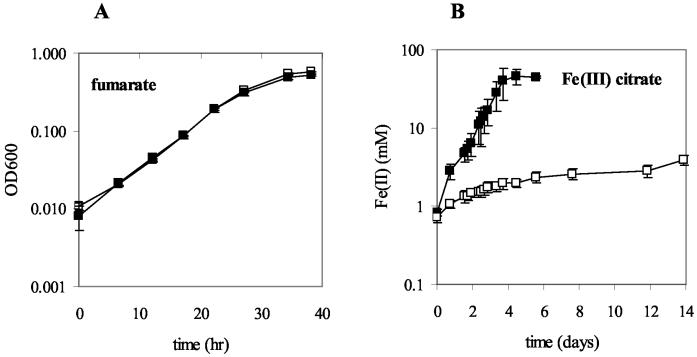
Growth of the macA-deficient strain with acetate as the electron donor and fumarate as the electron acceptor (generation time, 4.9 ± 0.3 h [mean ± standard deviation]; n = 3) was comparable to that of the wild type (5.1 ± 0.2 h) (Fig. 2A). Growth rates and lag times of the macA-deficient strain subjected to air (6 to 12.5% headspace), H2O2 (0.1 to 0.5 mM), or 0.25 to 5 mM Fe(II), which might lead to the production of free radicals in the presence of trace amounts of oxygen (40), were comparable to those for the wild type. This finding suggests that although MacA has homology to c-type cytochromes that have in vitro peroxidase activity, MacA is not required for G. sulfurreducens to tolerate oxidative stress. This finding is consistent with the suggestion that other c-type cytochromes with homology to MacA that have in vitro peroxidase activity have alternative in vivo functions (14, 18, 41).
FIG. 2.
Growth of the wild-type strain (filled squares) compared to that of the macA-deficient strain (open squares). Mid-log-phase acetate-fumarate-grown cultures were inoculated (2%) into fresh media with 20 mM acetate as the electron donor and either 27.5 mM fumarate (A) or 55 mM Fe(III) citrate (B) as the terminal electron acceptor. The data for each curve are the means ± the standard deviations of the results for triplicate cultures.
The primary phenotype of the macA-deficient strain was a significant decrease in the ability to reduce Fe(III). When acetate-fumarate-grown cultures of the mutant were transferred at mid-log phase into acetate-Fe(III) citrate medium, less than 4 mM Fe(III) was reduced after 14 days, whereas the wild-type cells, treated in a similar manner, reduced all 45 mM Fe(III) within 4 days (Fig. 2B).
To further evaluate the impact of the deletion of macA on Fe(III) reduction, acetate-fumarate-grown cells were washed and suspended in a buffer containing (grams per liter): 2.5 NaHCO3, 0.6 NaH2PO4H2O, 0.1 KCl, 0.3 MgSO4, 0.01 CaCl22H2O, and 3.56 NaCl, with Fe(III) citrate (20 mM) added as the electron acceptor and either acetate (20 mM) or hydrogen (a headspace mixture of H2:CO2 at 80:20) as the electron donor. Fe(III) reduction was measured for 3 h in triplicate incubations. The rates of Fe(III) reduction in the mutant were only 13% of the wild-type rate of 1.1 μmol min−1 (mg of protein)−1 with acetate as the electron donor and 11% of the wild-type rate of 0.6 μmol min−1 (mg of protein)−1 with hydrogen as the electron donor. Reintroduction of macA into the mutant strain in trans, on a gentamicin-resistant plasmid derived from pCM66 (25a) using previously described methods (20), restored Fe(III) reduction to a rate that was 36% of the wild-type rate. This is consistent with the low level of macA mRNA expression in the complemented strain, as determined by Northern blotting with primers macANf and macANr. This finding is in accordance with previous results (20) that also reflect the fact that the expression system that is available for complementation does not totally restore wild-type expression (20).
Role of MacA in Fe(III) reduction by G. sulfurreducens.
The phenotype of the macA-deficient strain is very similar to that of the G. sulfurreducens mutant deficient in OmcB, a 12-heme, putative outer membrane-bound c-type cytochrome (20). The predicted location of OmcB suggests that it could be involved in terminal electron transfer to Fe(III). In contrast, MacA is predicted to be localized in the periplasm and thus is unlikely to function as an Fe(III) reductase. A potential role for MacA in electron transfer to Fe(III) could be as an intermediate carrier between electron transfer components in the inner and the outer membrane. Present evidence suggests that several periplasmic c-type cytochromes are involved in electron transfer to Fe(III) in dissimilatory Fe(III) reducers (1, 16, 21, 27), though none have significant homology to MacA. A G. sulfurreducens mutant strain deficient in PpcA, a 9.6-kDa, triheme, periplasmic c-type cytochrome, was also impaired in Fe(III) reduction (21), though less so than the macA-deficient strain. It is not possible with the present information to speculate on how PpcA or MacA may interact with each other, with OmcB, or with other potential electron carriers involved in reduction of Fe(III). However, the discovery of the importance of MacA in the respiration of Fe(III) adds to the evidence that a network of c-type cytochromes is involved in electron transport to extracytoplasmic terminal acceptors in G. sulfurreducens.
Acknowledgments
We thank John Leszyk of the Proteomic Mass Spectrometry Lab, University of Massachusetts Medical School, for performing the MALDI-TOF mass spectrometry.
This research was supported by grants DE-FC02-02ER63446 and DE-FG02-01ER63145 from the Office of Science (BER), U.S. Department of Energy.
REFERENCES
- 1.Afkar, E., and Y. Fukumori. 1999. Purification and characterization of triheme cytochrome c7 from the metal-reducing bacterium, Geobacter metallireducens. FEMS Microbiol. Lett. 175:205-210. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, R. T., H. A. Vrionis, I. Ortiz-Bernad, C. T. Resch, P. E. Long, R. Dayvault, K. Karp, S. Marutzky, D. R. Metzler, A. Peacock, D. C. White, M. Lowe, and D. R. Lovley. 2003. Stimulating the in situ activity of Geobacter species to remove uranium from the groundwater of a uranium-contaminated aquifer. Appl. Environ. Microbiol. 69:5884-5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appel, R. D., A. Bairoch, and D. F. Hochstrasser. 1994. A new generation of information retrieval tools for biologists: the example of the ExPASy WWW server. Trends Biochem. Sci. 19:258-260. [DOI] [PubMed] [Google Scholar]
- 5.Ausubel, F., R. Brent, R. Kingston, D. Moore, J. Seidman, J. Smith, and K. Struhl. 1999. Current protocols in molecular biology, vol. 2. John Wiley & Sons, Inc., New York, N.Y.
- 6.Caccavo, F., Jr., D. J. Lonergan, D. R. Lovley, M. Davis, J. F. Stolz, and M. J. McInerney. 1994. Geobacter sulfurreducens sp. nov., a hydrogen- and acetate-oxidizing dissimilatory metal-reducing microorganism. Appl. Environ. Microbiol. 60:3752-3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Childers, S. E., S. Ciufo, and D. R. Lovley. 2002. Geobacter metallireducens accesses insoluble Fe(III) oxide by chemotaxis. Nature 416:767-769. [DOI] [PubMed] [Google Scholar]
- 8.Clauser, K. R., P. Baker, and A. L. Burlingame. 1999. Role of accurate mass measurement (+/- 10 ppm) in protein identification strategies employing MS or MS/MS and database searching. Anal. Chem. 71:2871-2882. [DOI] [PubMed] [Google Scholar]
- 9.Coppi, M. V., C. Leang, S. J. Sandler, and D. R. Lovley. 2001. Development of a genetic system for Geobacter sulfurreducens. Appl. Environ. Microbiol. 67:3180-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Smet, L., G. W. Pettigrew, and J. J. Van Beeumen. 2001. Cloning, overproduction and characterization of cytochrome c peroxidase from the purple phototrophic bacterium Rhodobacter capsulatus. Eur. J. Biochem. 268:6559-6568. [DOI] [PubMed] [Google Scholar]
- 11.Ellfolk, N., and R. Soininen. 1970. Pseudomonas cytochrome c peroxidase. I. Purification procedure. Acta Chem. Scand. 24:2126-2136. [DOI] [PubMed] [Google Scholar]
- 12.Francis, R. T., Jr., and R. R. Becker. 1984. Specific indication of hemoproteins in polyacrylamide gels using a double-staining process. Anal. Biochem. 136:509-514. [DOI] [PubMed] [Google Scholar]
- 13.Fülöp, V., C. J. Ridout, C. Greenwood, and J. Hajdu. 1995. Crystal structure of the di-haem cytochrome c peroxidase from Pseudomonas aeruginosa. Structure 3:1225-1233. [DOI] [PubMed] [Google Scholar]
- 14.Fülöp, V., N. J. Watmough, and S. J. Ferguson. 2001. Structure and enzymology of two bacterial diheme enzymes: cytochrome cd1 nitrite reductase and cytochrome c peroxidase. Adv. Inorg. Chem. 51:163-204. [Google Scholar]
- 15.Gharahdaghi, F., C. R. Weinberg, D. A. Meagher, B. S. Imai, and S. M. Mische. 1999. Mass spectrometric identification of proteins from silver-stained polyacrylamide gel: a method for the removal of silver ions to enhance sensitivity. Electrophoresis 20:601-605. [DOI] [PubMed] [Google Scholar]
- 16.Gordon, E. H., A. D. Pike, A. E. Hill, P. M. Cuthbertson, S. K. Chapman, and G. A. Reid. 2000. Identification and characterization of a novel cytochrome c(3) from Shewanella frigidimarina that is involved in Fe(III) respiration. Biochem. J. 349:153-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmes, D. E., K. T. Finneran, R. A. O'Neil, and D. R. Lovley. 2002. Enrichment of members of the family Geobacteraceae associated with stimulation of dissimilatory metal reduction in uranium-contaminated aquifer sediments. Appl. Environ. Microbiol. 68:2300-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koutný, M., L. Křiz̆, I. Kuc̆era, and I. Pluhacek. 1999. Evaluation of relative contributions of two enzymes supposed to metabolise hydrogen peroxide in Paracoccus denitrificans. Biochim. Biophys. Acta 1410:71-76. [DOI] [PubMed] [Google Scholar]
- 19.Kovach, M. E., R. W. Phillips, P. H. Elzer, R. M. Roop III, and K. M. Peterson. 1994. pBBR1MCS: a broad-host-range cloning vector. BioTechniques 16:800-802. [PubMed] [Google Scholar]
- 20.Leang, C., M. V. Coppi, and D. R. Lovley. 2003. OmcB, a c-type polyheme cytochrome, involved in Fe(III) reduction in Geobacter sulfurreducens. J. Bacteriol. 185:2096-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lloyd, J. R., C. Leang, A. L. Hodges Myerson, M. V. Coppi, S. Cuifo, B. Methé, S. J. Sandler, and D. R. Lovley. 2003. Biochemical and genetic characterization of PpcA, a periplasmic c-type cytochrome in Geobacter sulfurreducens. Biochem. J. 369:153-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lovley, D. R. 2000. Fe(III) and Mn(IV) reduction, p. 3-30. In D. R. Lovley (ed.), Environmental microbe-metal interactions. ASM Press, Washington, D.C.
- 23.Lovley, D. R., and J. D. Coates. 2000. Novel forms of anaerobic respiration of environmental relevance. Curr. Opin. Microbiol. 3:252-256. [DOI] [PubMed] [Google Scholar]
- 24.Lovley, D. R., S. J. Giovannoni, D. C. White, J. E. Champine, E. J. Phillips, Y. A. Gorby, and S. Goodwin. 1993. Geobacter metallireducens gen. nov. sp. nov., a microorganism capable of coupling the complete oxidation of organic compounds to the reduction of iron and other metals. Arch. Microbiol. 159:336-344. [DOI] [PubMed] [Google Scholar]
- 25.Lovley, D. R., and E. J. Phillips. 1988. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl. Environ. Microbiol. 54:1472-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25a.Marx, C. J., and M. E. Lidstrom. 2001. Development of improved versatile broad-host range vectors for use in methylotrophs and other Gram-negative bacteria. Microbiology 147:2065-2075. [DOI] [PubMed] [Google Scholar]
- 26.Methé, B. A., K. E. Nelson, J. A. Eisen, I. T. Paulsen, W. Nelson, J. F. Heidelberg, D. Wu, M. Wu, N. Ward, M. J. Beanan, R. J. Dodson, R. Madupu, L. M. Brinkac, S. C. Daugherty, R. T. DeBoy, A. S. Durkin, M. Gwinn, J. F. Kolonay, S. A. Sullivan, D. H. Haft, J. Selengut, T. M. Davidsen, N. Zafar, O. White, B. Tran, C. Romero, H. A. Forberger, J. Weidman, H. Khouri, T. V. Feldblyum, T. R. Utterback, S. E. Van Aken, D. R. Lovley, and C. M. Fraser. 2003. Genome of Geobacter sulfurreducens: metal reduction in subsurface environments. Science 302:1967-1969. [DOI] [PubMed] [Google Scholar]
- 27.Myers, C. R., and J. M. Myers. 1997. Cloning and sequence of cymA, a gene encoding a tetraheme cytochrome c required for reduction of iron(III), fumarate, and nitrate by Shewanella putrefaciens MR-1. J. Bacteriol. 179:1143-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakai, K., and P. Horton. 1999. PSORT: a program for detecting sorting signals in proteins and predicting their subcellular localization. Trends Biochem. Sci. 24:34-36. [DOI] [PubMed] [Google Scholar]
- 29.Nevin, K. P., and D. R. Lovley. 2000. Lack of production of electron-shuttling compounds or solubilization of Fe(III) during reduction of insoluble Fe(III) oxide by Geobacter metallireducens. Appl. Environ. Microbiol. 66:2248-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nevin, K. P., and D. R. Lovley. 2002. Mechanisms for accessing insoluble Fe(III) oxide during dissimilatory Fe(III) reduction by Geothrix fermentans. Appl. Environ. Microbiol. 68:2294-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nevin, K. P., and D. R. Lovley. 2002. Mechanisms for Fe(III) oxide reduction in sedimentary environments. Geomicrobiol. J. 19:141-159. [Google Scholar]
- 32.Newman, D. K., and R. Kolter. 2000. A role for excreted quinones in extracellular electron transfer. Nature 405:94-97. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 34.Röling, W. F., B. M. van Breukelen, M. Braster, B. Lin, and H. W. van Verseveld. 2001. Relationships between microbial community structure and hydrochemistry in a landfill leachate-polluted aquifer. Appl. Environ. Microbiol. 67:4619-4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rooney-Varga, J. N., R. T. Anderson, J. L. Fraga, D. Ringelberg, and D. R. Lovley. 1999. Microbial communities associated with anaerobic benzene degradation in a petroleum-contaminated aquifer. Appl. Environ. Microbiol. 65:3056-3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samyn, B., K. Van Craenenbroeck, L. De Smet, I. Vandenberghe, G. Pettigrew, and J. Van Beeumen. 1995. A reinvestigation of the covalent structure of Pseudomonas aeruginosa cytochrome c peroxidase. FEBS Lett. 377:145-149. [DOI] [PubMed] [Google Scholar]
- 37.Snoeyenbos-West, O. L., K. P. Nevin, R. T. Anderson, and D. R. Lovley. 2000. Enrichment of Geobacter species in response to stimulation of Fe(III) reduction in sandy aquifer sediments. Microb. Ecol. 39:153-167. [DOI] [PubMed] [Google Scholar]
- 38.Stein, L. Y., M. T. La Duc, T. J. Grundl, and K. H. Nealson. 2001. Bacterial and archaeal populations associated with freshwater ferromanganous micronodules and sediments. Environ. Microbiol. 3:10-18. [DOI] [PubMed] [Google Scholar]
- 39.Thomas, P. E., D. Ryan, and W. Levin. 1976. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal. Biochem. 75:168-176. [DOI] [PubMed] [Google Scholar]
- 40.Touati, D. 2000. Iron and oxidative stress in bacteria. Arch. Biochem. Biophys. 373:1-6. [DOI] [PubMed] [Google Scholar]
- 41.Turner, S., E. Reid, H. Smith, and J. Cole. 2003. A novel cytochrome c peroxidase from Neisseria gonorrhoeae: a lipoprotein from a Gram-negative bacterium. Biochem. J. 373:865-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tusnády, G. E., and I. Simon. 2001. The HMMTOP transmembrane topology prediction server. Bioinformatics 17:849-850. [DOI] [PubMed] [Google Scholar]